Abstract
The strictureplasty operation was originally adopted for use in selected patients with Crohn's disease to allow for bowel conservation. The procedure and its usage have evolved over time as experience and confidence with the technique has grown. The short- and long-term outcomes of strictureplasty compared with resection attest to its safety and durable efficacy.
Keywords: bowel conservation, Crohn's disease, short bowel, small bowel, strictureplasty
Crohn's disease is a chronic, unremitting, and incurable disorder that can arise in patients of all ages, involve any location along the intestinal tract, and demonstrate inflammatory, stricturing, or penetrating behavior. Treatment of the disease accordingly focuses on safely providing an acceptable quality of life for the affected individual through management strategies that may include a wide range of medical therapies, endoscopic procedures, and surgical methods. One such operative technique, strictureplasty, entails elimination of luminal narrowing without loss of bowel, and is commonly employed when conservation of the small intestine is critical to the patient's long-term wellness. Otherwise, multiple resections and progressive reductions in bowel length lead to short bowel syndrome, and explain why Crohn's disease is a leading cause of intestinal failure.
Background
Strictures are defined as a constant endoscopic, radiological, or surgical narrowing of the intestinal lumen accompanied by obstructive signs or prestenotic dilatation, and they ultimately appear in nearly one-third of patients with Crohn's disease. 1 These areas of fibrotic stenosis were previously believed to only arise in sections of chronic inflammation and be permanent in nature, but we now understand the converse is likely true. Recent evidence suggests fibrogenesis is triggered early in the inflammatory process and subsequently progresses independent of inflammation. 2 Multiple genetic and nonmodifiable clinical factors are reportedly associated with the development of small bowel strictures, and include the presence of NOD2/CARD15 mutations, ileocolonic disease location, long duration of disease, and severe disease.
Historically, nearly 80% of patients with Crohn's disease required surgery within 10 years of diagnosis. 3 4 A more recent prospective population-based study from Norway reported that same risk to be only 38%, but all the patients with stricturing disease needed an initial operation in their series. 5 Furthermore, others have shown that patients with disease involving the proximal small intestine are at greater risk for demonstrating stricturing behavior, and these individuals are also more likely to require multiple intestinal operations. 6
The extensive and recurrent nature of the disease was appreciated decades ago and operations for patients with extensive jejunoileitis were consequently avoided because of concerns about imminent short bowel syndrome. These patients were instead treated with anti-inflammatory medications and hyperalimentation. However, a few forward thinking surgeons began to consider alternatives to wide resection in these patients. Despite pervasive concerns about a potentially high mortality rate and little long-term benefit, Emanoel Lee of the John Radcliffe Hospital in Oxford, United Kingdom, was the first to perform strictureplasty in a patient with Crohn's disease after observing this novel procedure safely used for tubercular strictures by Katariya of Chandigarh, India. 7 Lee used this technique for a 21-year-old woman with multiple small bowel strictures, and later reported his experience in nine patients with extensive disease of the small intestine; none of the patients suffered a significant complication and only two of them experienced disease recurrence after 8 to 42 months of follow-up. 8 Since that initial account, multiple strictureplasty techniques have been described as our understanding of the procedure for Crohn's disease has evolved. 9
Indications/Contraindications
Resection remains the most commonly performed operation for Crohn's disease, but strictureplasty is a critically important procedure in the armamentarium of surgeons managing complex disease because it is the procedure of choice for patients with obstructive symptoms and nonphlegmonous fibrotic strictures of the small intestine. 10 11 Although the safety and efficacy of strictureplasty have become well established since its initial utilization, 12 the indications and contraindications for strictureplasty have changed little and are as follows:
Indications for strictureplasty:
Diffuse involvement of the small bowel with multiple strictures.
Nonphlegmonous fibrotic stricture.
Rapid recurrence of Crohn's disease manifested as obstruction.
Stricture(s) in a patient who had undergone previous major resection(s) of small bowel (>100 cm).
Stricture in a patient with intestinal failure or short bowel syndrome.
Contraindications to strictureplasty:
Colonic strictures.
Free or contained perforation of the small bowel.
Hypoalbuminemia (<2.0 g/dL).
Multiple strictures within a short segment.
Phlegmonous inflammation involving the affected site.
Stricture in close proximity to a site chosen for resection.
In the past, an internal or external fistula was viewed as a contraindication, but strictureplasty can be safely performed in this setting if any associated inflammation is chronic rather than active in appearance.
Preoperative Evaluation and Optimization
Patients being considered for elective operative intervention should be extensively queried about their present illness including specific questions related to constitutional symptoms and pertinent medications. They should also undergo a comprehensive physical examination that includes inspection of the abdomen and anoperineal region. Lastly, all relevant medical records need to be requested and reviewed when available to better understand the results of prior endoscopic/imaging studies, laboratory tests, pathology reports, and intestinal operations. The extent of disease is best ascertained by colonoscopy and imaging with computed tomography (CT) or magnetic resonance (MR) enterography. A recent meta-analysis suggests that both modalities possess a high diagnostic accuracy in detecting small bowel disease, but MR enterography has the advantage of being a radiation-free modality. 13 In multivariate analysis, small stricture luminal diameter and increased stricture wall thickness were predictors of strictures for which medical therapy typically fails. 14
The patients should be educated about their disease and the operative options because they commonly possess only rudimentary insight. Many surgeons feel that biologic agents should be discontinued several weeks prior to an elective procedure because they might interfere with the normal immune response and increase the likelihood of postoperative complications, although the impact of these agents is controversial and no strictureplasty-specific evidence is currently available. Smoking cessation should be facilitated and any physiologic deficits should be corrected during the preoperative period, but malnutrition is usually difficult to reverse, even with the use of hyperalimentation. Patients who might require temporary fecal diversion should be appropriately counseled and preoperatively marked because a major factor influencing satisfactory rehabilitation of an ostomy patient is the correct location and construction of the stoma. Mechanical bowel preparation, perioperative antibiotic therapy, and deep venous thrombosis prophylaxis are commonly prescribed while the need for stress-dose corticosteroids is debated.
Operative Approach
Regardless of whether a conventional open or laparoscopic-assisted approach is used, the entirety of the small bowel should be carefully palpated and visually inspected to identify all areas of disease involvement and guide the operative plan. Some surgeons employ intraoperative endoscopy or devices such as an inflated urethral catheter balloon or steel sphere to help identify subtle strictures, while others solely rely on haptic detection of strictures. The preferred method has not been definitively studied, and likely depends in part on the individual surgeon's experience with complex disease.
Since the time of the initial strictureplasty descriptions, the Heineke-Mikulicz ( Fig. 1 ) and Finney ( Fig. 2 ) strictureplasty techniques are the two most widely utilized methods for short- (<10 cm) and medium-length (10–20 cm) strictures of the small intestine, respectively. A Michelassi side-to-side isoperistaltic strictureplasty ( Fig. 3 ) with or without midstricture resection is indicated for long-length (>20 cm) strictured segments.
Fig. 1.
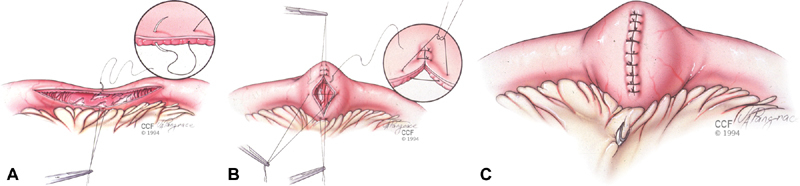
The Heineke-Mikulicz strictureplasty is constructed by creating a longitudinal enterotomy (A) that is transversely closed in one or two layers using interrupted sutures (B). The site is marked by applying a clip to the mesenteric margin (C). Copyright retained by CCF.
Fig. 2A-C.
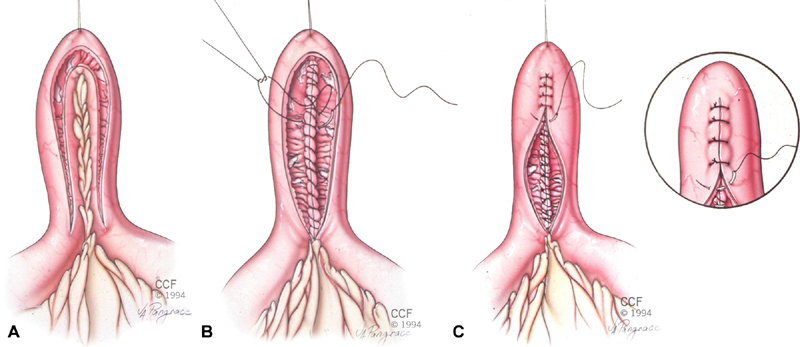
The Finney strictureplasty is constructed by creating a longitudinal enterotomy and folding the bowel on itself (A). The back wall of the enterotomy is closed in two layers using running sutures reinforced with interrupted sutures (B). The front wall is closed in the same manner or with interrupted sutures placed in one or two layers (C). With permission and use by Cleveland Clinic Foundation. Copyright retained by CCF.
Fig. 3.
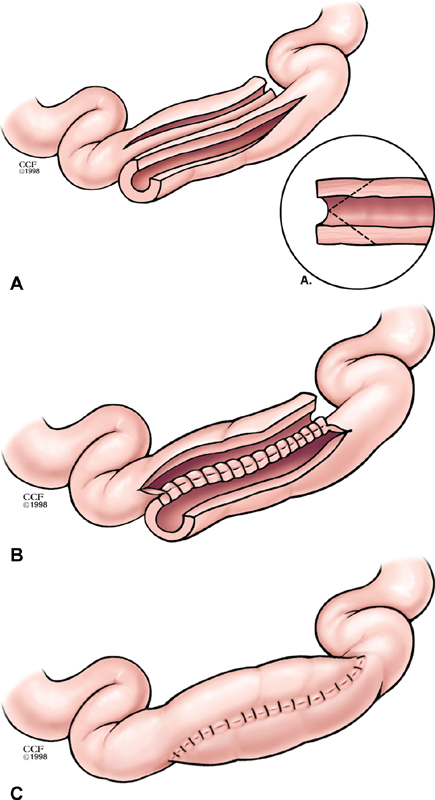
The Michelassi strictureplasty is constructed by creating a longitudinal enterotomy and dividing the strictured segment in its midpoint before sliding the two segments into an isoperistaltic configuration (A). The back wall of the enterotomy is closed in two layers using running sutures reinforced with interrupted sutures (B). The front wall is closed in the same manner or with interrupted sutures placed in one or two layers (C). Copyright retained by CCF.
Other techniques of strictureplasty have been described, but are much less frequently employed. 15 16 17 18 19 The Judd and Moskel–Walske–Neumayer strictureplasties are modified from the Heineke-Mikulicz procedure and used for complex short-length strictures. 15 A Judd strictureplasty may be employed when a fistula accompanies the stricture, whereas the Moskel–Walske–Neumayer strictureplasty might be warranted if the bowel proximal to the stricture is excessively dilated. If a medium-length stricture is encountered, a combination of Heineke-Mikulicz and Finney strictureplasty can be used. 16 Alternatively, a double Heineke-Mikulicz-type strictureplasty 17 or Jaboulay strictureplasty can be performed in this instance.
Strictureplasty has also been used for strictures of the terminal ileum, 18 20 ileocecal valve, 18 and ileocolostomy. 21 22 23 Taschieri et al 18 described a side-to-side ileocecal plasty for terminal ileitis affecting the distal small bowel with relative sparing of the ileocecal valve and ileocolic plasty for severe disease with narrowing of the ileocecal valve. Either a Heineke-Mikulicz or Finney strictureplasty technique can be used to safely remedy an ileocolostomy stricture.
At the time of procedure, only those segments considered to be contributing to the patient's constellation of symptoms deserve resection. However, if strictures are identified during the operation, regardless of whether they seem symptomatic, strictureplasty should augment the primary procedure in appropriately selected patients. Experience justifies this approach because strictureplasty, as will be discussed later, adds little to the morbidity of resection, and reoperation following strictureplasty is more likely to be necessary for new symptomatic strictures than for restricturing of an old strictureplasty site. Interestingly, recent review of a nation-wide database suggested the proportion of operations for Crohn's disease that included strictureplasty has gradually decreased for unknown reasons from 5.1 to 1.7% over an 8-year interval. 24
Operative Technique
Nearly all strictureplasty techniques share a common feature, which is a full-thickness longitudinal incision along the antimesenteric aspect of the bowel wall that extends 1 to 2 cm into nondiseased bowel on either side of the stricture. Closure of the resultant enterotomy discriminates the different techniques from one another. The number of strictures, length of each stricture, and the distance between strictures as well as sites warranting resection primarily dictate the choice of strictureplasty configuration.
The Heineke-Mikulicz strictureplasty is constructed by transversely closing the longitudinal enterotomy in one or two layers using interrupted sutures. If the enterotomy is too long or the bowel wall is too rigid, the closure site can bowstring across the mesenteric margin of the bowel lumen and cause a relative obstruction. In this instance or strictures measuring 10 to 20 cm, a Finney strictureplasty is likely required. This form of strictureplasty requires the bowel to be folded upon itself such that the two ends of the enterotomy are opposed. The bowel is usually closed in a two-layer manner using running suture. The outer layer of the back wall begins at the middle of the enterotomy, incorporates the seromuscular layers of the bowel wall, and stops at the end of the enterotomy. The inner layer begins at the same site as the outer layer, but includes all layers of the bowel. Once both back wall layers have been completed, the inner layer is continued onto the front wall in a Connell pattern. Lastly, the back outer layer is carried onto the front wall while incorporating only the seromuscular layers.
The Michelassi strictureplasty requires division of both the bowel and a portion of its mesentery to allow the two limbs to slide against one another so that a tension-free isoperistaltic anastomosis can be constructed. In some cases, a portion of the bowel generally measuring less than one-quarter of the total enterotomy length must be resected from the middle of the affected segment to allow for a tension-free anastomosis. The anastomosis is typically constructed in a two-layer manner with the outer layer including the seromuscular layers and the inner layer incorporating all layers of the bowel wall.
After completing all strictureplasties and resections, a small metal clip is placed on the mesentery near the most proximal anastomosis. An additional clip is placed on the mesentery of each additional anastomosis as one moves distal along the intestinal tract. In this manner, a bleeding strictureplasty site can be readily localized by angiography in the event that laparotomy is required for postoperative hemorrhage.
Outcomes
Many centers have conducted comprehensive studies of patients undergoing strictureplasty. Campbell et al 25 performed the most recent meta-analysis of 32 articles that supplied detailed and specific discussion pertaining to premorbid patient history, intraoperative findings, utilized techniques, and postoperative follow-up. Collectively, the studies included 1,616 patients who underwent a total of 4,538 strictureplasties. The Heineke-Mikulicz (79%) and Finney (10%) techniques were the most commonly performed types of strictureplasty with all other configurations accounting for a minority (11%) of the procedures.
According to an earlier analysis, the typical patient treated by strictureplasty presents with symptoms related to small bowel obstruction unresponsive to appropriate medical therapy. 26 More than one-half of patients will undergo synchronous resection, most have three separate segments treated by strictureplasty, and more than 250 cm of small bowel remains at the procedure's conclusion. The most commonly treated small bowel strictures involve the jejunoileal region (40%) followed by the ileum alone (35%) and isolated jejunum (25%).
Short-Term Outcomes
The operation has proven to be quite safe with a morbidity rate of 13% and rare mortalities reported from the major series. 25 Of patients undergoing strictureplasty, 2.7% experience a recurrent small bowel obstruction, 3.1% develop gastrointestinal bleeding, and 4.2% manifest sepsis, but only 2.8% require reoperation during the postoperative period.
Most of the complications are linked to sepsis and include enterocutaneous fistula (23%), abscess (11%), wound infection (8%), abdominal sepsis (5%), and anastomotic leak (3%). 26 Prior to the widespread use of biologic agents, the likelihood of septic complications was primarily influenced by recent weight loss and hypoalbuminemia, and was nearly tripled when the preoperative serum albumin level was less than 2.5 g/dL. 27 Therefore, a diverting stoma proximal to the sites of strictureplasty should be carefully considered in instances where the patient's albumin value is less than 2.5 g/dL. Contrarily, steroid dosage, length, number, and site of strictureplasties and the need for synchronous resection do not significantly impact the patient's risk for experiencing a septic complication. 12
Hemorrhage, while uncommon, can be particularly challenging to manage. Most instances of strictureplasty site bleeding will spontaneously cease without intervention. If hemorrhage persists, however, it can usually be controlled by therapeutic mesenteric angiography. In the rare event that bleeding is uncontrolled by the interventional radiologist or recurs, laparotomy is typically required to control the hemorrhage. If reoperation is necessary, it can be difficult to be certain of the specific bleeding site without opening the several strictureplasties.
Long-Term Outcomes
Yamamoto et al 12 conducted a systematic review and meta-analysis one decade ago, prior to the aggressive use of postoperative prophylactic therapy, to determine long-term outcomes following all forms of strictureplasty. Overall, clinical recurrence developed in 39% of patients and operative recurrence occurred in 30%. In 18 studies, a 28% 5-year recurrence rate was reported after metaregression analysis for variable study follow-up periods. More importantly, only 10% of patients developed surgical recurrence at a previous strictureplasty site giving a site-specific reoperation rate of 3.3%.
The Cleveland Clinic reported their experience with 314 persons undergoing an index strictureplasty with 1124 strictureplasties followed up for a median of 7.5 years. 28 The 5- and 10-year operative recurrence rates were 20 and 44%, respectively. Yamamoto et al reviewed 111 patients who underwent 285 primary strictureplasties. 29 After a median follow-up of nearly 9 years, clinical recurrence occurred in 60 patients (54%), and 49 of these (44%) required reoperation.
At Oxford University, 479 strictureplasties were performed during 159 operations in 100 patients. 30 The complication rate associated with strictureplasty performed at the site of a previous anastomosis or strictureplasty was similar to the rate witnessed in patients undergoing routine strictureplasty. Moreover, operative recurrence rates were similar following the first (52%) and second strictureplasty (56%) operations after a median follow-up of 85 months.
Greenstein and colleagues 31 from Mount Sinai Medical Center reported on their outcomes with 339 strictureplasties performed in 88 patients at an initial operation. Their 5-year operative recurrence rate was 14% for patients with ≤8 strictures compared with 31% for those with >8 strictures. Moreover, the 5-year rates were 14% for patients with ≤4 strictureplasties contrasted to 33% for those with >4 strictureplasties. In multivariate regression, both the number of strictures and number of strictureplasties were independently associated with recurrence. The risk for operative recurrence increased to 7 and 23% for each additional stricture and strictureplasty, respectively.
Long-term outcome studies specific to the Michelassi side-to-side isoperistaltic strictureplasty suggest a 5-year operative recurrence rate of 23% in 184 patients undergoing operation at six centers. 32 The recurrence occurred at the site of the side-to-side strictureplasty in 34% of these patients. In a separate series of 91 patients from a single center, multivariate analysis suggested that age at diagnosis, age at surgery, family history, and smoking habit were independent risk factors for recurrence. 33
Strictureplasty for fibrotic strictures of the terminal ileum, ileocecal valve, or ileocolostomy is just as safe and efficacious as resection with anastomosis. Although strictureplasty has been described for colonic narrowing, 0.8 to 6.8% of colonic strictures are complicated by an underlying malignancy that is difficult to discriminate from inflammatory disease and colon strictureplasty offers no better quality of life over resection. 34 35 Therefore, symptomatic colonic strictures generally should be resected rather than treated by strictureplasty.
Special Considerations
Duodenal Strictureplasty
The incidence of symptomatic Crohn's disease of the stomach and duodenum is low, but inflammation is commonly discovered when upper endoscopy is performed in patients with disease noted elsewhere. Most symptomatic patients who fail medical therapy and require operative management of their foregut disease complain of obstructive symptoms stemming from duodenal strictures that persist despite endoscopic dilatation. These strictures tend to be short in length and amenable to Heineke-Mikulicz strictureplasty if the stricture is isolated in the second or third portions of the duodenum. Alternatively, a Jaboulay strictureplasty is used if the stricture involves the second portion of the duodenum and the bowel is too rigid to permit transverse closure.
The outcomes following strictureplasty for duodenal disease are limited and somewhat controversial for uncertain reasons. Worsey and colleagues 36 reported on 13 patients, and their experience suggested the procedure was safe and durably effective. Conversely, Yamamoto and associates 37 noted 9 of 13 patients needed a repeat operation for either persistent or recurrent symptoms following duodenal strictureplasty. Lastly, Tonelli et al 38 performed strictureplasty in eight patients with duodenal stricture, and found two recurrences after a mean follow-up of 11 years.
Development of Malignancy
The incidence of small bowel adenocarcinoma complicating Crohn's disease is low, but significantly higher than the rate seen in the general population. 39 Although the intraoperative features are similar to those seen with benign disease, a relatively rigid obstruction in a patient with long-standing disease should raise the question of malignancy especially if the patient experienced a sudden exacerbation of symptoms on a background of previously quiescent disease.
Over the past few years, a handful of cases of adenocarcinoma arising in a prior strictureplasty site have been reported, 40 41 42 43 44 and this has prompted some to recommend frozen-section analysis of all suspicious strictures at the time of strictureplasty. 10
Conclusions
Resection remains the procedure of choice for patients undergoing operative treatment of their Crohn's disease. However, concerns of eventual short bowel syndrome have led to bowel conservation with limited resection margins and liberal use of strictureplasty. Nearly all series attest to the short-term safety and efficacy of strictureplasty with long-term studies reporting competitive clinical and operative recurrence rates.
References
- 1.Bharadwaj S, Fleshner P, Shen B. Therapeutic armamentarium for stricturing Crohn's disease: medical versus endoscopic versus surgical approaches. Inflamm Bowel Dis. 2015;21(09):2194–2213. doi: 10.1097/MIB.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 2.Bettenworth D, Rieder F. Reversibility of stricturing Crohn's disease - fact or fiction? Inflamm Bowel Dis. 2016;22(01):241–247. doi: 10.1097/MIB.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer R G, Whelan G, Fazio V W. Long-term follow-up of patients with Crohn's disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88(06):1818–1825. doi: 10.1016/0016-5085(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 4.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg. 2000;231(01):38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solberg I C, Vatn M H, Høie O et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Lazarev M, Huang C, Bitton A et al. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108(01):106–112. doi: 10.1038/ajg.2012.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katariya R N, Sood S, Rao P G, Rao P L. Stricture-plasty for tubercular strictures of the gastro-intestinal tract. Br J Surg. 1977;64(07):496–498. doi: 10.1002/bjs.1800640713. [DOI] [PubMed] [Google Scholar]
- 8.Lee E CG, Papaioannou N. Minimal surgery for chronic obstruction in patients with extensive or universal Crohn's disease. Ann R Coll Surg Engl. 1982;64(04):229–233. [PMC free article] [PubMed] [Google Scholar]
- 9.Ambe R, Campbell L, Cagir B. A comprehensive review of strictureplasty techniques in Crohn's disease: types, indications, comparisons, and safety. J Gastrointest Surg. 2012;16(01):209–217. doi: 10.1007/s11605-011-1651-2. [DOI] [PubMed] [Google Scholar]
- 10.Strong S, Steele S R, Boutrous M et al. Clinical practice guideline for the surgical management of Crohn's disease. Dis Colon Rectum. 2015;58(11):1021–1036. doi: 10.1097/DCR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 11.Gionchetti P, Dignass A, Danese S et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 2: surgical management and special situations. J Crohn's Colitis. 2017;11(02):135–149. doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Fazio V W, Tekkis P P. Safety and efficacy of strictureplasty for Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50(11):1968–1986. doi: 10.1007/s10350-007-0279-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Liu J, Xiao W, Luo G. A diagnostic accuracy meta-analysis of CT and MRI for the evaluation of small bowel Crohn disease. Acad Radiol. 2017;24(10):1216–1225. doi: 10.1016/j.acra.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Campos C, Perrey A, Lambert C et al. Medical therapies for stricturing Crohn's disease: efficacy and cross-sectional imaging predictors of therapeutic failure. Dig Dis Sci. 2017;62(06):1628–1636. doi: 10.1007/s10620-017-4572-4. [DOI] [PubMed] [Google Scholar]
- 15.Gaetini A, De Simone M, Resegotti A. Our experience with strictureplasty in the surgical treatment of Crohn's disease. Hepatogastroenterology. 1989;36(06):511–515. [PubMed] [Google Scholar]
- 16.Fazio V W, Tjandra J J. Strictureplasty for Crohn's disease with multiple long strictures. Dis Colon Rectum. 1993;36(01):71–72. doi: 10.1007/BF02050305. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki I, Funayama Y, Naito H, Fukushima K, Shibata C, Matsuno S. Extended strictureplasty for multiple short skipped strictures of Crohn's disease. Dis Colon Rectum. 1996;39(03):342–344. doi: 10.1007/BF02049479. [DOI] [PubMed] [Google Scholar]
- 18.Taschieri A M, Cristaldi M, Elli M et al. Description of new “bowel-sparing” techniques for long strictures of Crohn's disease. Am J Surg. 1997;173(06):509–512. doi: 10.1016/s0002-9610(97)00003-2. [DOI] [PubMed] [Google Scholar]
- 19.Selvaggi F, Sciaudone G, Giuliani A, Limongelli P, Di Stazio C. A new type of strictureplasty for the treatment of multiple long stenosis in Crohn's disease. Inflamm Bowel Dis. 2007;13(05):641–642. doi: 10.1002/ibd.20056. [DOI] [PubMed] [Google Scholar]
- 20.Sampietro G M, Cristaldi M, Maconi Get al. A prospective, longitudinal study of nonconventional strictureplasty in Crohn's disease J Am Coll Surg 2004199018–20., discussion 20–22 [DOI] [PubMed] [Google Scholar]
- 21.Tjandra J J, Fazio V W.Strictureplasty for ileocolic anastomotic strictures in Crohn's disease Dis Colon Rectum 199336121099–1103., discussion 1103–1104 [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Keighley M R. Long-term results of strictureplasty for ileocolonic anastomotic recurrence in Crohn's disease. J Gastrointest Surg. 1999;3(05):555–560. doi: 10.1016/s1091-255x(99)80112-7. [DOI] [PubMed] [Google Scholar]
- 23.Tonelli F, Ficari F. Strictureplasty in Crohn's disease: surgical option. Dis Colon Rectum. 2000;43(07):920–926. doi: 10.1007/BF02237351. [DOI] [PubMed] [Google Scholar]
- 24.Geltzeiler C B, Young J I, Diggs B S et al. Strictureplasty for treatment of Crohn's disease: an ACS-NSQIP database analysis. J Gastrointest Surg. 2015;19(05):905–910. doi: 10.1007/s11605-015-2749-8. [DOI] [PubMed] [Google Scholar]
- 25.Campbell L, Ambe R, Weaver J, Marcus S M, Cagir B. Comparison of conventional and nonconventional strictureplasties in Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55(06):714–726. doi: 10.1097/DCR.0b013e31824f875a. [DOI] [PubMed] [Google Scholar]
- 26.Tichansky D, Cagir B, Yoo E, Marcus S M, Fry R D. Strictureplasty for Crohn's disease: meta-analysis. Dis Colon Rectum. 2000;43(07):911–919. doi: 10.1007/BF02237350. [DOI] [PubMed] [Google Scholar]
- 27.Ozuner G, Fazio V W, Lavery I C, Church J M, Hull T L.How safe is strictureplasty in the management of Crohn's disease? Am J Surg 19961710157–60., discussion 60–61 [DOI] [PubMed] [Google Scholar]
- 28.Dietz D W, Fazio V W, Laureti S et al. Strictureplasty in diffuse Crohn's jejunoileitis: safe and durable. Dis Colon Rectum. 2002;45(06):764–770. doi: 10.1007/s10350-004-6294-x. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Bain I M, Allan R N, Keighley M R. An audit of strictureplasty for small-bowel Crohn's disease. Dis Colon Rectum. 1999;42(06):797–803. doi: 10.1007/BF02236939. [DOI] [PubMed] [Google Scholar]
- 30.Fearnhead N S, Chowdhury R, Box B, George B D, Jewell D P, Mortensen N J. Long-term follow-up of strictureplasty for Crohn's disease. Br J Surg. 2006;93(04):475–482. doi: 10.1002/bjs.5179. [DOI] [PubMed] [Google Scholar]
- 31.Greenstein A J, Zhang L P, Miller A T et al. Relationship of the number of Crohn's strictures and strictureplasties to postoperative recurrence. J Am Coll Surg. 2009;208(06):1065–1070. doi: 10.1016/j.jamcollsurg.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Michelassi F, Taschieri A, Tonelli F et al. An international, multicenter, prospective, observational study of the side-to-side isoperistaltic strictureplasty in Crohn's disease. Dis Colon Rectum. 2007;50(03):277–284. doi: 10.1007/s10350-006-0804-y. [DOI] [PubMed] [Google Scholar]
- 33.Fazi M, Giudici F, Luceri C, Pronestì M, Tonelli F. Long-term results and recurrence-related risk factors for Crohn disease in patients undergoing side-to-side isoperistaltic strictureplasty. JAMA Surg. 2016;151(05):452–460. doi: 10.1001/jamasurg.2015.4552. [DOI] [PubMed] [Google Scholar]
- 34.Broering D C, Eisenberger C F, Koch A et al. Strictureplasty for large bowel stenosis in Crohn's disease: quality of life after surgical therapy. Int J Colorectal Dis. 2001;16(02):81–87. doi: 10.1007/s003840000278. [DOI] [PubMed] [Google Scholar]
- 35.Kristo I, Riss S, Argeny S, Maschke S, Chitsabesan P, Stift A. Incidental adenocarcinoma in patients undergoing surgery for stricturing Crohn's disease. World J Gastroenterol. 2017;23(03):472–477. doi: 10.3748/wjg.v23.i3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsey M J, Hull T, Ryland L, Fazio V. Strictureplasty is an effective option in the operative management of duodenal Crohn's disease. Dis Colon Rectum. 1999;42(05):596–600. doi: 10.1007/BF02234132. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Allan R N, Keighley M R. An audit of gastroduodenal Crohn disease: clinicopathologic features and management. Scand J Gastroenterol. 1999;34(10):1019–1024. doi: 10.1080/003655299750025138. [DOI] [PubMed] [Google Scholar]
- 38.Tonelli F, Alemanno G, Bellucci F, Focardi A, Sturiale A, Giudici F. Symptomatic duodenal Crohn's disease: is strictureplasty the right choice? J Crohn's Colitis. 2013;7(10):791–796. doi: 10.1016/j.crohns.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 39.von Roon A C, Reese G, Teare J, Constantinides V, Darzi A W, Tekkis P P. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007;50(06):839–855. doi: 10.1007/s10350-006-0848-z. [DOI] [PubMed] [Google Scholar]
- 40.Marchetti F, Fazio V W, Ozuner G. Adenocarcinoma arising from a strictureplasty site in Crohn's disease. Report of a case. Dis Colon Rectum. 1996;39(11):1315–1321. doi: 10.1007/BF02055130. [DOI] [PubMed] [Google Scholar]
- 41.Jaskowiak N T, Michelassi F. Adenocarcinoma at a strictureplasty site in Crohn's disease: report of a case. Dis Colon Rectum. 2001;44(02):284–287. doi: 10.1007/BF02234306. [DOI] [PubMed] [Google Scholar]
- 42.Menon A M, Mirza A H, Moolla S, Morton D G. Adenocarcinoma of the small bowel arising from a previous strictureplasty for Crohn's disease: report of a case. Dis Colon Rectum. 2007;50(02):257–259. doi: 10.1007/s10350-006-0771-3. [DOI] [PubMed] [Google Scholar]
- 43.Partridge S K, Hodin R A. Small bowel adenocarcinoma at a strictureplasty site in a patient with Crohn's disease: report of a case. Dis Colon Rectum. 2004;47(05):778–781. doi: 10.1007/s10350-003-0101-y. [DOI] [PubMed] [Google Scholar]
- 44.Tonelli F, Bargellini T, Leo F, Nesi G. Duodenal adenocarcinoma arising at the strictureplasty site in a patient with Crohn's disease: report of a case. Int J Colorectal Dis. 2009;24(04):475–477. doi: 10.1007/s00384-008-0582-1. [DOI] [PubMed] [Google Scholar]


