Abstract
We evaluated the potential sequestration of cesium (Cs+) by microalgae under heterotrophic growth conditions in an attempt to ultimately develop a system for treatment of radioactive wastewater. Thus, we examined the effects of initial Cs+ concentration (100–500 μM), pH (5–9), K+ and Na+ concentrations (0–20 mg/L), and different organic carbon sources (acetate, glycerol, glucose) on Cs+ removal. Our initial comparison of nine microalgae indicated that Desmodesmus armatus SCK had removed the most Cs+ under various environmental conditions. Addition of organic substrates significantly enhanced Cs+ uptake by D. armatus, even in the presence of a competitive cation (K+). We also applied magnetic nanoparticles coated with a cationic polymer (polyethylenimine) to separate 137Cs-containing microalgal biomass under a magnetic field. Our technique of combining bioaccumulation and magnetic separation successfully removed more than 90% of the radioactive 137Cs from an aqueous medium. These results clearly demonstrate that the method described here is a promising bioremediation technique for treatment of radioactive liquid waste.
Subject terms: Biological techniques, Pollution remediation
Introduction
The Fukushima Daiichi Nuclear Power Plant accident of 2011 released large amounts of radioactive nuclides into the environment. In particular, the release of radioactive cesium (137Cs) was a major concern because of its long half-life (30.2 years), high water solubility, and rapid uptake by terrestrial and aquatic organisms due to its chemical similarity to potassium (K+)1,2. This accident has thus led to the search for new methods that can prevent the adverse effects of pollution by radioactive nuclides, especially 137Cs.
Researchers have previously examined the effects of many chemical and biological techniques for removal of Cs+ and/or 137Cs from wastewater effluents. Biological technologies have attracted intense interest because they appear to be less expensive and more ecologically friendly than non-biological methods3. The uptake of radioactive compounds by microorganisms can be a metabolism-independent process (biosorption, a physiochemical process that does not require cellular energy) or a metabolism-dependent process (bioaccumulation, uptake into the cytoplasm by use of cellular energy). Previous research indicated that energy-independent processes play a minor role in Cs+ accumulation by microbes, because Cs+ is a very weak Lewis acid and only has limited interaction with ligands4. However, microorganisms can actively take up Cs+ via endogenous K+ transport systems5 because of the chemical similarity of Cs+ and K+. To the best of our knowledge, only a limited number of reports examined the use of microalgae for the bioaccumulation of environmental Cs+. Conventional separation techniques, such as chemical precipitation and ion exchange, are well-developed, but are expensive and inefficient when the environmental concentration of Cs+ is low. In general, contaminated environments have much lower concentrations of Cs+ than other co-occurring and competing cations. Thus, a biological method that uses microalgae, which can efficiently accumulate low levels of Cs+ in the presence of competing ions, is considered a promising approach2.
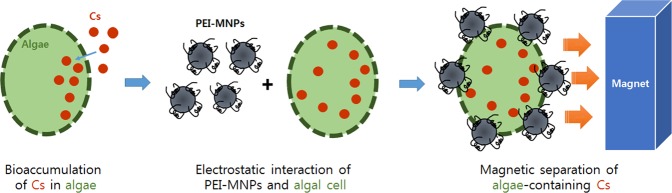
In this study, we examined the feasibility of using microalgae with a magnetic separation system to remove Cs+ and 137Cs from aqueous solutions. Thus, we initially examined the Cs+-uptake capabilities of nine microalgae, including several newly isolated strains, under constant illumination to select the strain that best accumulates Cs+. We then selected the best of these nine microalgae and examined the effect of different environmental factors on Cs+ uptake and sequestration6, including pH, initial concentrations of different ions (Cs+, K+, and Na+), and different organic carbon sources (acetate, glycerol, and glucose). We also used polyethyleneimine (PEI)-coated magnetic nanoparticles (MNPs) to simultaneously recover the microalgae and 137Cs from solution. As a cationic surfactant, polyethlylenimine (PEI), which is known for its high density of positive charge, was introduced onto the surface of Fe3O4 nanoparticles to synthesize PEI-coated magnetic nanocomposites7. The proposed overall approach is summarized in Fig. 1.
Figure 1.
Overall process for removal of radioactive Cs via bioaccumulation by microalgae and magnetic separation.
Results and Discussion
Screening of Cs+-accumulating microalgae
We initially grew the nine different microalgae under constant illumination in Cs+-containing TAP medium, following 3 days of growth in K+-starved TAP medium (Fig. 2a). Measurements of OD680nm indicated Chlorella sp. Arm 0029B had the highest cell density, followed by M. inermum F014, C. vulgaris, and then D. armatus SCK. Measurements of Cs+ uptake by these microalgae indicated that D. armatus SCK removed the greatest amount of Cs+ (63.9 μmol/g DCW), followed by Ettlia sp. YC001 (28.6 μmol/g DCW), Chlorella sp. Arm 0029B (22.2 μmol/g DCW), and M. inermum F014 (16.7 μmol/g DCWM) (Fig. 2a). We also examined the cellular uptake of K+ from TAP medium without Cs+ (Fig. 2b). It is well known that cells take up Cs+ using their K+ transport systems, such as the K+/K+ and K+/H+ exchanger5,8,9.
Figure 2.
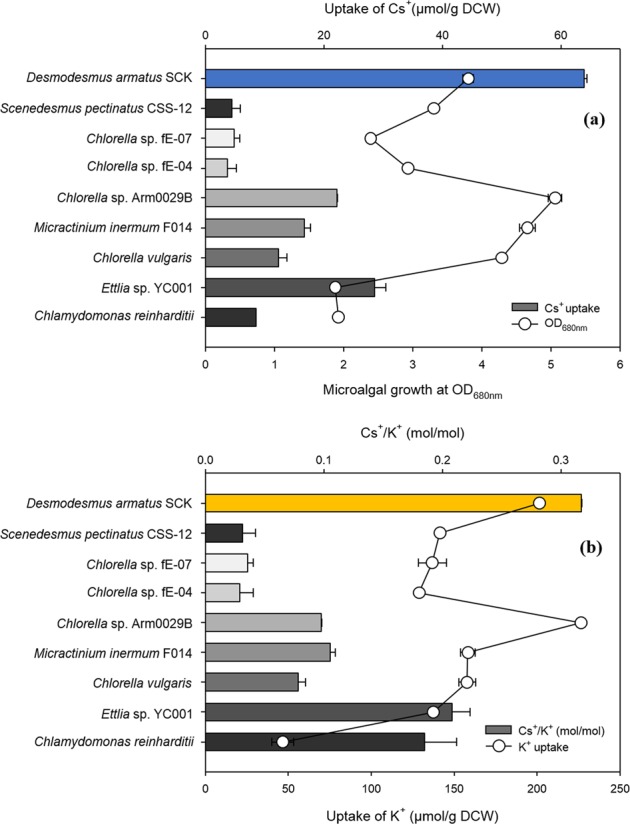
(a) Relationship of Cs+ uptake (bars) and algal growth (circles) and (b) relationship of the Cs+/K+ ratio (bars) and K+ accumulation (circles) by nine species of microalgae under constant illumination.
However, our results indicated the uptake of K+ was not always proportional to uptake of Cs+. Based on uptake of K+, D. armatus SCK had the greatest Cs+ uptake at a Cs+/K+ molar ratio of 0.33. This indicated that uptake of Cs+ does not necessarily correlate with uptake of K+ or cell growth. Trans-membrane movement of monovalent cations like Cs generally occurs against a concentration gradient and thus energy is consumed to drive it. Additionally, it likely has to do with the plasma membrane-bound H+-ATPase, which acts to generate a transmembrane electrochemical proton gradient4,5. It is therefore possible that cation transports are coupled with H+ movements by either symport or antiport. Besides, the monovalent cation uptake may be mediated rather directly by K+-ATPase4,5. All this led to a mechanistic hypothesis that an apparent Cs+ uptake capacity is could be different for each cell type whose monovalent cation transport system has varied affinities toward Cs.
Previous studies reported that Chlorella salina and Synechocystis PCC 6803 accumulated Cs+ in the range of 0.8 to 0.49 nmol per 106 cells when grown in BG-11 medium under constant illumination10,11. We found that D. armatus SCK sequestrated 2.08 nmol Cs+ per 106 cells under our growth conditions, so this microalga appears to be a promising option for the removal of environmental Cs+. Thus, all of our subsequent experiments focused on this species.
Effects of initial Cs+ concentration and pH on Cs+ removal
Cs+ is a hard metal that is generally non-toxic to microorganisms because of its weak coordinating ability. Moreover, several hard metals are essential nutrients for microbial growth, and so they are readily accumulated4. Thus, we examined the effect of the initial Cs+ concentration and pH on Cs+ uptake by D. armatus SCK (Fig. 3). The results show that Cs+ uptake increased with an increasing initial Cs+ concentration, with an apparent saturation near 400 to 500 μM. The maximum equilibrium uptake of Cs+ was 280 μmol/g DCW and the removal efficiency was 70% at 400 μM. Tomioka et al.12 reported that several strains of Rhodococcus accumulated Cs+ in the range of 98.3 to 395 μmol/g cells weight for initial Cs+ concentrations of 0.01 to 1 mM, but that the Cs+ uptake did not increase when the extracellular Cs+ concentration was greater than 100 μM12.
Figure 3.
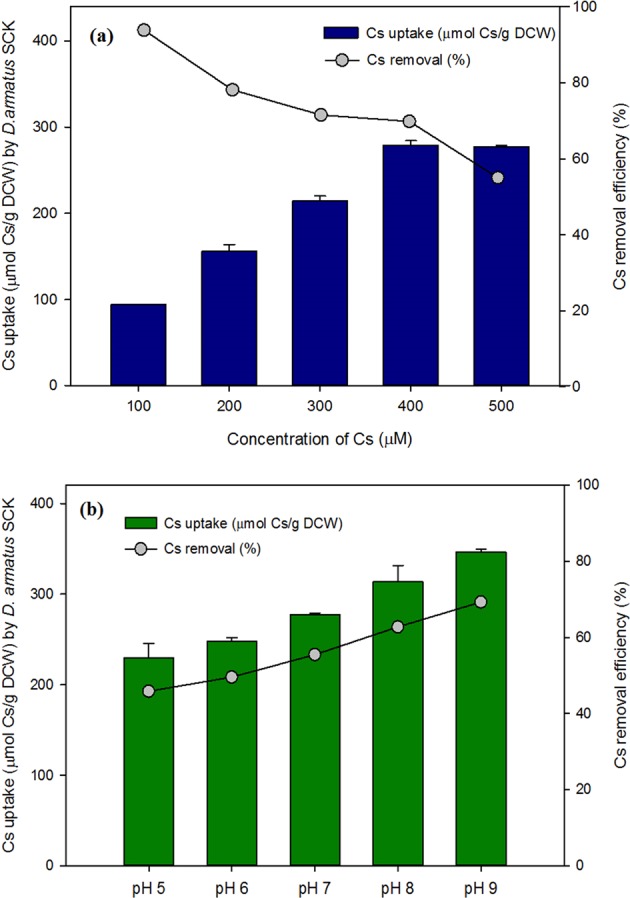
(a) Effect of initial Cs+ concentration on Cs+ uptake (bars) and removal efficiency (circles) at pH 7, and (b) effect of pH on Cs+ uptake (bars) and removal efficiency (circles) at 400 μM Cs+ by D. armatus SCK cells grown in TAP medium.
The pH of the growth medium can impact the bioaccumulation or adsorption of Cs+. Our results show that the bioaccumulation of Cs+ by D. armatus SCK was efficient at pH values between 5 and 9, and the greatest uptake and removal efficiency were at pH 9. This result is similar to those reported for cyanobacterial removal of Cs+. In particular, optimal Cs+ accumulation by Synechocystis PCC 6803 and Rhodococcus strain occurred under alkaline conditions (pH 9)1,10, probably because plasma membrane depolarization at low pH inhibits Cs+ uptake10.
Effects of K+ and Na+ on Cs+ removal
Cs+ is an alkali monovalent cation that cells can transport because of its similarity to K+. Thus, we examined the effects of K+ and Na+ concentration on Cs+ uptake by D. armatus SCK (Fig. 4). The results show that the Na+ concentration had little or no effect on Cs+ uptake in the concentration range tested, but that Cs+ uptake declined as the K+ concentration was above 10 mg/L. Similar to other microbes, our results show that Cs+ accumulation may occur through sharing K+-transport channel, not Na+-migration route5. Similarly, Cs+ accumulation by Synechocystis sp. Strain PCC 6803 and C. emersonii declined as the concentration of K+ increased5,10. These results also suggest that the K+-transport system(s) of phototrophic microalgae, which are not specific to K+, play an important role in accumulation of Cs+12. The K+ concentrations in natural fresh water and ground water systems typically range from 0.5 to 3 mg/L, levels that have no apparent impact on Cs+ uptake by D. armatus SCK2. However, our results indicate that when this strain is used in laboratory or bioengineering studies, the K+ concentration should be 10 mg/L or less.
Figure 4.
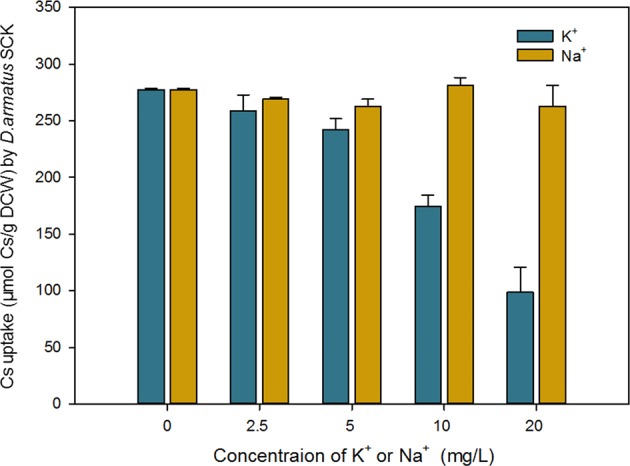
Effect of K+ and Na+ concentration on Cs+ uptake by D. armatus SCK grown in TAP medium containing 400 μM Cs+.
Effects of different organic carbon sources on Cs+ removal
We investigated the effects of three organic carbon sources (acetate, glycerol, and glucose; concentration: 1 g-Carbon/L) on Cs+ uptake by D. armatus SCK with or without 500 μM K+ (Fig. 5). The results show that each carbon source notably increased Cs+ uptake in the presence or absence of K+, and that glucose had the greatest effect. In agreement, a previous study also reported increased net K+ uptake by heterotrophic Rhodococcus cells when glucose was added to the growth medium13.
Figure 5.
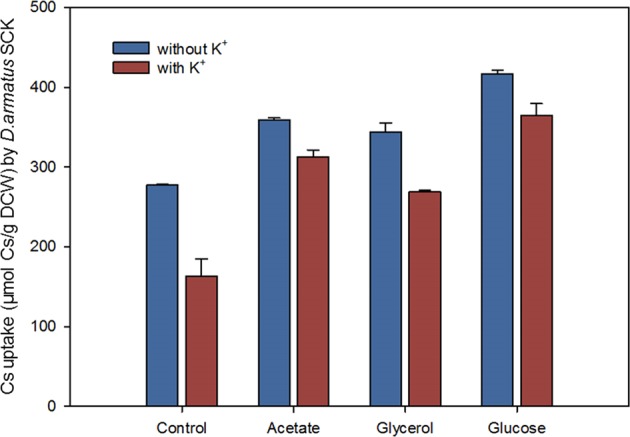
Effect of 500 μM K+ and three different carbon sources on uptake of Cs+ by D. armatus SCK when grown in liquid medium containing 400 μM Cs+.
Glucose-induced net uptake of K+ and Cs+ is likely due to stimulation of an electro-neutral ATP-dependent K+/H+ exchange, because glucose metabolism acidifies the cell interior. Ohunki et al.14 also found that glucose stimulated the accumulation of Cs+ in a fungal strain14. Our results also indicate that glucose and other carbon sources can act as energy sources that increase Cs+ accumulation by microalgae under heterotrophic conditions. In contrast, other research indicated that Chlorella accumulated 2-fold less Cs+ in chemoheterotrophic conditions relative to photoautotrophic conditions5. Few previous studies have examined the effects of other organic substrates, such as acetate and glycerol, on the uptake of Cs+ by phototrophic microalgae. Our results (Fig. 5) indicate that acetate and glycerol significantly increased the uptake of Cs+, although glucose had a stronger effect. Acetate is a volatile fatty acid that microalgae can directly convert into acetyl-CoA (an intermediate in the synthesis of cellular fatty acids) via the pyruvate pathway in the absence of glucose15, so this may explain its effect (Fig. 5). Although glycerol was not as effective as glucose, it can be considered as an alternative to improve the accumulation of Cs+ by D. armatus SCK. Therefore, from an economic perspective, these two low-cost organic carbon sources (acetate and glycerol) have potential for enhancing the accumulation of Cs+ during the heterotrophic growth of D. armatus SCK.
Removal of 137Cs using bioaccumulation and magnetic separation
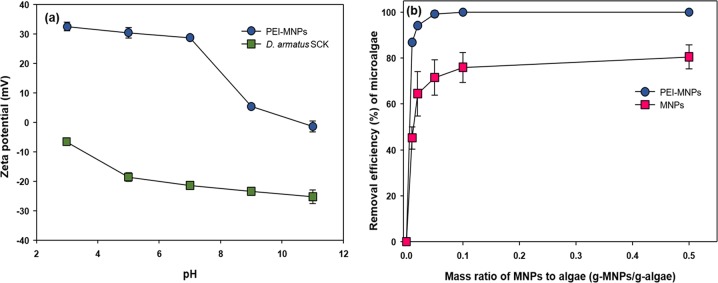
We measured the separation of microalgae containing Cs+ from liquid medium by use of PEI-MNPs. In an aqueous solution, the surface of metal oxide nanoparticles contains hydroxyl groups, which undergo pH-dependent protonation/deprotonation. Fe3O4 nanoparticles are generally negatively charged at pH 716,17. Therefore, the negative surface potential of algal cells (D. armatus SCK: −21.4 ± 0.92 mV at pH 7) makes them strongly attracted to Fe3O4 nanoparticles that are coated with PEI, which have a high-density cationic charge (+28.7 ± 0.64 mV at pH 7) (Fig. 6a). As reported in previous studies, the addition of cationic functional groups, such as PEI, to the surface of coated particles increases the effectiveness of separation18–20. As shown in Fig. 6b, the maximum recovery efficiency (~100%) was achieved when the mass ratio exceeds 0.05 g-PEI-MNPs/g-algae, which showed the improved magnetic separation than that of naked Fe3O4 particles. We also found that the complex of magnetic nanocomposites and microalgal cells were easily separated within 3 min in a magnetic field (Fig. 7). Thus, this magnetic harvesting method has potential for the efficient separation of microalgae containing 137Cs because it is simple, rapid, and consumes very little energy.
Figure 6.
(a) Zeta potentials of PEI-MNPs and D. armatus SCK at different pHs, (b) Recovery efficiency of D. armatus SCK according to the mass ratio of MNPs to algae.
Figure 7.
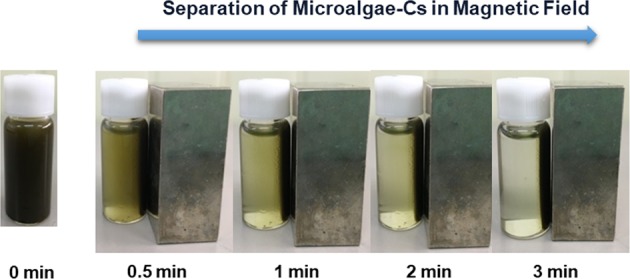
Time course of separation of Cs+-containing microalgae using PEI-MNPs in a magnetic field.
We also examined the use of D. armatus SCK cells followed by magnetic separation for removal of 137Cs from a medium with glucose (1 g/L) and 10 Bq/mL or 100 Bq/mL of 137Cs (Fig. 8). Measurement of radioactivity in the liquid medium before and after treatment indicated these cells sequestered 91.4% and 97.1% of 137Cs at 10 and 100 Bq/mL of 137Cs, respectively (Fig. 8). These data are meaningful because low levels of 137Cs are often difficult to remove using conventional methods in aqueous systems.
Figure 8.
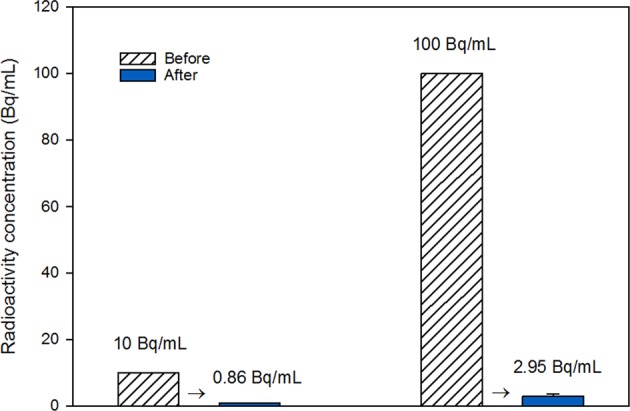
Change of 137Cs concentration in the liquid medium following bioaccumulation by D. armatus SCK and magnetic separation.
Materials and Methods
Screening of Cs-accumulating microalgae
Four microalgae that accumulate Cs+ were isolated from a local sewage treatment plant (Desmodesmus armatus SCK) and a lake (Scenedesmus pectinatus CSS-12, Chlorella sp. fE-04, and Chlorella sp. fE-07) in Daejeon, Republic of Korea. An additional five microalgae from the culture collections of the Korea Research Institute of Bioscience and Biotechnology (KRIBB) in Dajeon, Korea (Chlorella sp. ArM0029B, Ettlia sp. YC001, and Chlamydomonas reinhardtii CC124), Pusan National University in Pusan, Korea (Micractinium inermum F014), and University of Texas (UTEX), Austin, TX, USA (Chlorella vulgaris UTEX265). For screening of Cs-accumulating microalgae, each strain was grown in 1 L vessel in Tris-acetate phosphate (TAP) medium (pH = 7) with 100-μmol/L of CsCl and the following additional components: 25 mL of TAP salts, 0.375 mL of phosphate solution, 1 mL of Hunter’s trace elements, 1 mL of glacial acetic acid, and 2.42 g of Tris15. The initial cell concentration was adjusted to an optical density of 0.2 at 680 nm. These algal strains were cultivated for 7 days in baffled hybrid flasks on a shaker (120 rpm) at 25 °C.
Effects of various parameters on Cs removal by microalgae
To evaluate the maximum uptake capability of Cs+ by microalgae, cultivated cells were transferred to K+-depleted TAP medium and maintained for 3 days (Supplementary Fig. S1). Then, cells in the early stationary growth phase were collected by centrifugation at 7000 rpm for 10 min, and the bio-pellets were suspended in a 20-mM Tris buffer solution containing CsCl, with the biomass adjusted to 1 g/L dry cell weight (DCW). Cell suspensions were then incubated at 25 °C for 24 h with rotary shaking (120 rpm) under continuous illumination. The effects of the initial concentrations of Cs+, K+ and Na+, and various organic carbon sources (acetate, glycerol, and glucose) were examined at 25 °C under continuous illumination. The removal efficiency of non-radioactive Cs+ was calculated as:
| 1 |
where Ci and Cf are the initial (i) and final (f) concentrations of Cs+.
Synthesis of PEI-Fe3O4 nanoparticles and magnetic separation
PEI (polyethyleneimine)-coated magnetic nanoparticles (MNPs) were synthesized according to a previously reported procedure7. First, iron salts (0.99 g FeCl2∙4H2O and 2.7 g FeCl3∙6H2O) were dissolved in 100 mL of deionized water, and deoxygenated with nitrogen gas at 80 °C. Subsequently, 10 mL of NH4OH (25% by wt.) was added and stirred for 0.5 h. After cooling to room temperature, the precipitated nanoparticles were separated with a magnet and washed four times with deionized water. Then, the Fe3O4 nanoparticles were added to a PEI solution (MW = 2 kDa) in phosphate buffer at pH 7.3 (10% by vol.). Finally, the PEI-MNPs were collected using a magnetic field and washed three times with deionized water. After synthesis of the nanoparticles, the zeta potentials of PEI-MNPs and prepared microalgae were measured by a Zetasizer instrument (Nano-ZS, Malvern, UK).
For the magnetic separation and removal of radioactive 137Cs, D. armatus SCK cells were cultivated in a Tris buffer solution containing 10 or 100 Bq/mL of 137Cs, and the PEI-MNPs were mixed with the cultures for 1 min. Then, the microalga-nanoparticle aggregates containing 137Cs were separated from the medium using an external permanent magnet within 3 min. After separation, the radioactivity concentration of the solution was measured to calculate the removal efficiency, as described above.
Analytical methods
To quantify cell growth, the optical density of each sample was measured at 680 nm using a UV-visible spectrophotometer (UV-1800; Shimadzu, Japan). Cell counts were performed using an optical microscope (DM2500; Leica, Switzerland) with a hemocytometer.
After the Cs removal experiments, the supernatant was filtered through a PVDF membrane filter (0.2 μm), and the amount of non-radioactive Cs+ remaining in the filtrate was quantified using inductively coupled plasma-mass spectrometry (ICP-MS; ELAN DRC II, Perkin-Elmer). Thus, the cellular uptake of Cs+ was determined by measuring the change in Cs+ concentration of the growth medium. The levels of K+ and Na+ were determined by ion chromatography (883 Basic IC plus; Metrohm AG, Switzerland) using an anionic column (Metrosep A Supp 5–150/4.0; Metrohm AG, Switzerland). The 137Cs concentration was determined using γ-spectrometry (Canberra, Genie 2000).
Conclusion
The aim of this work was to evaluate the ability of microalgae to remove Cs+ and 137Cs from aqueous solutions. Initial experiments indicated that a novel strain, D. armatus SCK, was the most effective of nine tested strains in the removal of Cs+. Our results also showed that D. armatus SCK accumulated high levels of Cs+ in the presence of competitive cations (Na+ and K+), that acetate and glycerol (inexpensive carbon sources) enhanced the uptake of Cs+, and that uptake was greater at a higher pH. Use of D. armatus SCK for bioaccumulation and PEI-MNPs for magnetic separation of cells led to highly effective removal of 137Cs from aqueous solutions. The use of microalgae-magnetic particles with an inexpensive organic substrate appears to have great potential for bioremediation of 137Cs-polluted environments.
Electronic supplementary material
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government, Ministry of Science and ICT (No. 2017M2A8A5015148). Also, this work was also supported by a grant of the Nakkonggang National Institute of Biological Resources (NNIBR), funded by the Ministry of Environment (NNIBR201902102), Republic of Korea.
Author Contributions
B.G. Ryu designed the research and supervised the experiments. I. Kim conducted the most of experiments and wrote the manuscript. H.M. Yang synthesized the PEI-Fe3O4 nanoparticles and assisted Ilgook Kim in performing magnetic separation tests. C.W. Park prepared radioactive cesium solution and assisted in analyzing data. I.H. Yoon, B.K. Seo and E.K. Kim gave B.G. Ryu a number of critical ideas and suggestions for the study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-46586-x.
References
- 1.Tomioka N, Tanaka K, Uchiyama H, Yagi O, Kokufuta E. Recovery of 137Cs by a bioaccumulation system using Rhodococcus erythropolis CS98. J. Ferment. Bioeng. 1998;85:604–608. doi: 10.1016/S0922-338X(98)80013-5. [DOI] [Google Scholar]
- 2.Fukuda S, et al. Global searches for microalgae and aquatic plants that can eliminate radioactive cesium, iodine and strontium from the radio-polluted aquatic environment: a bioremediation strategy. J. Plant. Res. 2014;127:79–89. doi: 10.1007/s10265-013-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalali-Rad R, et al. Biosorption of cesium by native and chemically modified biomass of marine algae: introduce the new biosorbents for biotechnology applications. J. Hazard. Mater. 2004;116:125–134. doi: 10.1016/j.jhazmat.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Avery SV. Caesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J. Ind. Microbiol. 1995;14:76–84. doi: 10.1007/BF01569888. [DOI] [PubMed] [Google Scholar]
- 5.Avery SV, Codd GA, Gadd GM. Replacement of cellular potassium by caesium in Chlorella emersonii: differential sensitivity of photoautotrophic and chemoheterotrophic growth. J. Gen. Microbiol. 1992;138:69–76. doi: 10.1099/00221287-138-1-69. [DOI] [Google Scholar]
- 6.Samadani M, Perreault F, Oukarroum A, Dewez D. Effect of cadmium accumulation on green algae Chlamydomonas reingardtii and acid-tolerant Chlamydomonas CPCC 121. Chemosphere. 2018;191:174–182. doi: 10.1016/j.chemosphere.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Hu YR, Guo C, Wang SK, Pan F, Liu CZ. Improvement of microalgae harvesting by magnetic nanocomposites coated with polyethylenimine. Chem. Eng. J. 2014;242:341–347. doi: 10.1016/j.cej.2013.12.066. [DOI] [Google Scholar]
- 8.Kannan S. Plasmalemma: the seat of dual mechanisms of ion absorption in Chlorella pyrenoidosa. Science. 1971;173:927–929. doi: 10.1126/science.173.4000.927. [DOI] [PubMed] [Google Scholar]
- 9.Raven JA. Nutrient transport in microalgae. Adv. Microb. Physiol. 1981;21:47–226. doi: 10.1016/S0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- 10.Avery SV, Codd GA, Gadd GM. Caesium accumulation and interactions with other monovalent cations in the cyanobacterium Synechocystis PCC 6803. J. Gen. Microbiol. 1991;137:405–413. doi: 10.1099/00221287-137-2-405. [DOI] [Google Scholar]
- 11.Avery SV, Codd GA, Gadd GM. Salt-stimulation of caesium accumulation in the euryhaline green microalga Chlorella salina: potential relevance to the development of a biological Cs-removal process. Microbiology. 1993;139:2239–2244. [Google Scholar]
- 12.Tomioka N, Uchiyama H, Yagi O. Cesium accumulation and growth characteristics of Rhodococcus erythropolis CS98 and Rhodococcus sp. Strain CS402. Appl. Environ. Microbiol. 1994;60:2227–2231. doi: 10.1128/aem.60.7.2227-2231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tromballa HW. The effect of glucose on potassium transport by Chlorella fusca. Z. Pflanzenphysiol. 1981;105:1–10. doi: 10.1016/S0044-328X(81)80002-5. [DOI] [Google Scholar]
- 14.Ohnuki T, et al. Effect of minerals on accumulation of Cs by fungus Saccaromyces cerevisiae. J. Environ. Radioact. 2015;144:127–133. doi: 10.1016/j.jenvrad.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Ryu BG, et al. Advanced treatment of residual nitrogen from biologically treated coke effluent by a microalga-mediated process using volatile fatty acids (VFAs) under stepwise mixotrophic conditions. Bioresour. Technol. 2015;191:488–495. doi: 10.1016/j.biortech.2015.03.112. [DOI] [PubMed] [Google Scholar]
- 16.Nassar NN. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010;184:538–546. doi: 10.1016/j.jhazmat.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Guo C, Wang F, Zheng S, Liu CZ. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour. Technol. 2011;102:10047–100513. doi: 10.1016/j.biortech.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Wang SK, Stiles AR, Guo C, Liu CZ. Harvesting microalgae by magnetic separation: A review. Algal Res. 2015;9:178–185. doi: 10.1016/j.algal.2015.03.005. [DOI] [Google Scholar]
- 19.Hu YR, Wang F, Wang SK, Liu CZ, Guo C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013;138:387–390. doi: 10.1016/j.biortech.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Prochazkova G, Podolova N, Safarik I, Zachleder V, Branyik T. Physicochemical approach to freshwater microalgae harvesting with magnetic particles. Colloids Surf. B. 2013;112:213–218. doi: 10.1016/j.colsurfb.2013.07.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.