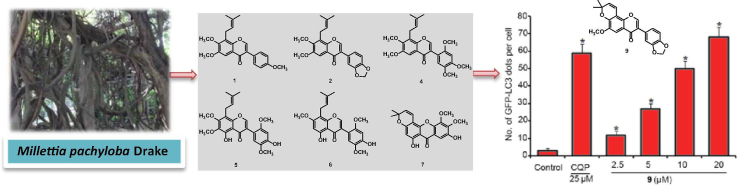
Graphical abstract
Keywords: Millettia pachyloba, Leguminosae, Isoflavones, Cytotoxicity, Autophagy, Apoptosis
Highlights
-
•
Six new natural compounds were isolated from Millettia pachyloba Drake.
-
•
The cytotoxic activities of these new compounds were evaluated.
-
•
Ten (3–5, 9, 12, 17–19, 24, and 25) of 28 isolated compounds showed cytotoxicity.
-
•
The ten cytotoxic compounds induced autophagy in cancer cells.
-
•
Compound 9 induced apoptosis and autophagy, suggesting it could be a potential anticancer drug candidate.
Abstract
In this study, systematic separation and subsequent pharmacological activity studies were carried out to identify cytotoxic natural products from the dried stems of Millettia pachyloba Drake. Five previously undescribed isoflavones, pachyvones A–E; one previously undescribed xanthone, pachythone A; and twenty-two known compounds were obtained. The structures of these compounds were assigned on the basis of 1D/2D NMR data and high-resolution electrospray ionization mass spectroscopy analysis. Preliminary activity screening with HeLa and MCF-7 cells showed that ten compounds (3–5, 9, 12, 17–19, 24, and 25) had potential cytotoxicity. Further in-depth activity studies with five cancer cell lines (HeLa, HepG2, MCF-7, Hct116, and MDA-MB-231) and one normal cell line (HUVEC) revealed that these ten compounds showed specific cytotoxicity in cancer cells, with IC50 values ranging from 5 to 40 μM, while they had no effect on normal cell lines. To investigate whether the cytotoxicity of these ten compounds was associated with autophagy, their autophagic effects were evaluated in GFP-LC3-HeLa cells. The results demonstrated that compound 9 (durmillone) significantly induced autophagy in a concentration-dependent manner and had the best activity as an autophagy inducer among all of the compounds. Therefore, compound 9 was selected for further study. The PI/Annexin V double staining assay and Western blotting results revealed that compound 9 also induced obvious apoptosis in HeLa and MCF-7 cells, which suggests that it mediates cytotoxic activity through activation of both apoptosis and autophagy. Taken together, this study identified ten natural cytotoxic products from the dried stems of Millettia pachyloba Drake, of which compound 9 induced apoptosis and autophagy and could be an anticancer drug candidate.
Introduction
Millettia (Leguminosae) is a genus with approximately 200 species that are primarily distributed in tropical and subtropical regions, such as Africa, Asia, America, and Australia [1]. Historically, Millettia is a traditional medicine used in the treatment of gynecological diseases, dysentery, cardiovascular diseases, intestinal pain, rheumatic arthritis, and skin diseases [2], [3]. Previous phytochemical investigations of this genus have demonstrated the presence of steroids, alkaloids, triterpenoids, and flavonoids [4], [5], [6], [7].
Millettia pachyloba Drake (M. pachyloba) belongs to the Leguminosae family and is a type of semi-evergreen perennial woody vine plant primarily distributed in the Guangdong, Hainan, Guangxi, and Yunnan Provinces of China. The stems of M. pachyloba are often used by locals as a herbal medicine for the treatment of tumors, rheumatic arthritis and removing edema from patients. To date, only three studies have focused on the phytochemical study of M. pachyloba [8], [9], [10], which is far from providing a deep understanding of M. pachyloba. Thus, further intensive phytochemical study of M. pachyloba is needed.
Autophagy is the primary cellular process for protecting cells and organisms from natural stressors such as ER-stress as well as nutrient deficiency. In addition to its function in normal physiology, autophagy also plays a role in cancer [11]. Recently, it was established as a tumor suppression mechanism; loss of autophagy function was required for the initiation of cancer [12]. Because previous research reported that isolated flavonoids from M. pachyloba exhibited initial cytotoxicity against KB cells [8], it was worthwhile screening the autophagy inducer in M. pachyloba and further testing the underlying mechanism. Durmillone is an isoflavone with a dimethyl pyran moiety connected to C6 and C7. It is widespread in the genus of Millettia [13], [14] and Lonchocarpus [15]. However, there is no research reporting its cytotoxic mechanism of action.
This study carried out intensive phytochemical study of M. pachyloba. In addition, the initial cytotoxic mechanism of action of the compounds separated form M. pachyloba were investigated.
Material and methods
General experimental procedures
The following equipment and methods were used in the present study: silica gel column chromatography (200–300 mesh, Qingdao Makall Group Co., Qingdao, China), Sephadex LH-20 column chromatography (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), high-performance liquid chromatography (HPLC, Waters, Milford, USA), a Sunfire C18 column (5 μm, 4.6 mm × 150 mm; Waters, Milford, USA), a semipreparative HPLC instrument (SP-HPLC, NovaSep, Miramas, France), a digital polarimeter for optical rotation measurements (Jasco P-1020, Tokyo, Japan), a UV-2100 spectrophotometer for ultraviolet absorbance detection (Shimadzu, Kyoto, Japan), a Nicolet-6700 FT-IR spectrometer for IR spectral detection (Thermo Scientific, Waltham, USA), an Aviv Model 400 CD spectrometer (Aviv Biomedical, Lakewood, USA), an Avance-400 spectrometer for NMR spectral detection (Bruker, Billerica, USA), and a Q-TOF Premier mass spectrometer coupled with an ESI source (Waters, Milford, USA).
Plant material
Researcher Hua Peng (Kunming Institute of Botany, Chinese Academy of Sciences) collected and identified the stems of M. pachyloba at Pingbian, Yunan, China, in September 2015. A voucher specimen (SKLB-201509) was deposited in the Lab of Natural Product Drugs and Cancer Biotherapy, Sichuan University.
Extraction and isolation
Air-dried stems of M. pachyloba (10 kg) were ground into powder (approximately 20-mesh). The powder was extracted with 60 L 95% aqueous EtOH three times. The EtOH extracts were combined and evaporated to dryness in vacuo to produce 712 g crude sample. It was then suspended in 5 L deionized H2O and successively exhausted with 5 L petroleum ether and 5 L CH2Cl2 to give dried petroleum ether (48 g) and CH2Cl2 (77 g) extracts, respectively, for further separation.
The petroleum ether extract was subjected to silica gel column chromatography (petroleum ether/EtOAc from 100/1 to 1/1, v/v) for rough separation, and eleven fractions (Fr. A1–Fr. A11) were collected. Fr. A5 (1.3 g) was subjected to SP-HPLC (MeOH/H2O, 75/25, v/v) to yield 7.8 mg of compound 18. Fr. A7 (1.7 g) was also subjected to SP-HPLC (MeOH/H2O, 85/15, v/v) to yield 15.7 mg of compound 1. Fr. A8 (3.9 g) was subjected to silica gel column chromatography (petroleum ether/EtOAc from 20/1 to 1/5, v/v) and produced five subfractions (Fr. A8.1–Fr. A8.5). Fr. A8.2 was subjected to SP-HPLC (MeOH/H2O, 80/20, v/v) to produce 135.5 mg compound 6, 5.1 mg compound 7, and 15.4 mg compound 9. Fr. A 8.3 was subjected to Sephadex LH-20 column chromatography (CH2Cl2/MeOH from 10/1 to 1/5, v/v) to yield 83.4 mg compound 22 and 112.7 mg compound 23. Fr. A9 (2.8 g) was subjected to silica gel column chromatography (petroleum ether/EtOAc, from 20/1 to 1/5, v/v) and further purified by SP-HPLC (MeOH/H2O, 80/20 and 85/15, v/v, respectively) to yield 8.7 mg compound 3 and 19.6 mg compound 5.
The CH2Cl2 extract was subjected to silica gel column chromatography (petroleum ether/EtOAc, from 50/1 to 1/5, v/v), and 15 fractions (Fr. B1–Fr. B15) were produced. Fr. B3 (1.3 g) was subjected to SP-HPLC (MeOH/H2O, 85/15, v/v) to yield 5.9 mg compound 19. Fr. B5 (4.6 g) was subjected to silica gel column chromatography using petroleum ether/EtOAc (from 20/1 to 1/5, v/v), and six subfractions were obtained (Fr. B5.1–Fr. B5.6). Fr. B5.3 and Fr. B5.5 were subjected to SP-HPLC (MeOH/H2O, 85/15, and 70/30, respectively) to produce 12.5 mg compound 2 and 16.3 mg compound 25. Fr. B6 (1.7 g) was purified using SP-HPLC (MeOH/H2O, 65/35, v/v) to obtain 61.4 mg compound 20. Fr. B7 (4.8 g) was subjected to silica gel column chromatography (petroleum ether/EtOAc from 20/1 to 1/5, v/v) and further purified using Sephadex LH-20 column chromatography (CH2Cl2/MeOH, from 10/1 to 1/5, v/v) to produce 9.3 mg compound 8, 23.1 mg compound 13 and 17.2 mg compound 21. Fr. B8 (2.4 g) was subjected to SP-HPLC (MeOH/H2O, 75/25, v/v) to yield 7.1 mg compound 10. Fr. B9 (3.6 g) was also subjected to SP-HPLC (MeOH/H2O, 80/20, and 75/25, respectively) to yield 38.5 mg compound 4 and 21.5 mg compound 11. Fr. B10 (4.1 g) was separated on a silica gel column (petroleum ether/EtOAc, from 20/1 to 1/5, v/v) and by SP-HPLC (MeOH/H2O, 75/25, and 65/35, respectively) to yield 47.8 mg compound 12 and 13.7 mg compound 15. Fr. B11 (4.5 g) was purified in the same manner as Fr. B10 to produce 10.2 mg compound 14, 36.3 mg compound 24 and 38.1 mg compound 27. Fr. B12 (2.3 g) underwent Sephadex LH-20 column chromatography (CH2Cl2/MeOH, from 10/1 to 1/5, v/v) to yield 18.5 mg compound 16 and 16.1 mg compound 17. Fr. B13 (3.7 g) underwent Sephadex LH-20 column chromatography (H2O/MeOH, from 10/1 to 1/5, v/v) to yield 48.3 mg compound 26 and 53.9 mg compound 28. In total, 28 compounds with purities>98% analyzed by HPLC (Waters, Milford, USA) were isolated from the ethanol extract of the stems of M. pachyloba.
Pachyvone A (1)
White powder; ultraviolet (UV) (MeOH) λmax (log ε) 262 (4.18), 326 (2.97) nm; Infrared (IR) (KBr) vmax 3028, 2910, 1673, 1456, 834 cm−1. Both 1H nuclear magnetic resonance (NMR) and 13C NMR data are shown in Table 1; high-resolution electrospray ionization mass spectroscopy (HRESIMS) m/z 381.1707 [M+H]+ (calcd for C23H25O5, 381.1702).
Table 1.
1H and 13C NMR spectroscopic data for compounds 1, 2, 4–6a (400 and 100 MHz for 1H and 13C NMR, CDCl3).
| Position |
1 |
2 |
4 |
5 |
6 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 152.3 | 8.02, s | 152.5 | 8.01, s | 154.5 | 8.05, s | 155.4 | 7.97, s | 155.2 | 7.94, s |
| 3 | 123.9 | 124.1 | 120.9 | 119.8 | 119.9 | |||||
| 4 | 176.1 | 175.9 | 176.0 | 181.8 | 181.4 | |||||
| 4a | 120.7 | 120.7 | 120.8 | 108.6 | 105.8 | |||||
| 5 | 103.8 | 7.60, s | 103.9 | 7.59, s | 103.9 | 7.59, s | 152.5 | 160.9 | ||
| 6 | 151.1 | 151.2 | 151.0 | 136.6 | 95.1 | 6.41, s | ||||
| 7 | 151.9 | 151.9 | 151.8 | 157.1 | 162.7 | |||||
| 8 | 124.7 | 124.7 | 124.7 | 113.0 | 107.8 | |||||
| 8a | 150.0 | 149.9 | 149.9 | 150.4 | 154.6 | |||||
| 1′ | 124.5 | 125.9 | 112.5 | 110.0 | 109.9 | |||||
| 2′ | 130.1 | 7.52, d (8.8) | 122.3 | 7.00, dd (8.0, 1.6) | 151.9 | 152.4 | 152.5 | |||
| 3′ | 113.9 | 6.98, d (8.8) | 108.4 | 6.87, d (8.0) | 98.3 | 6.63, s | 100.0 | 6.67, s | 100.0 | 6.66, s |
| 4′ | 159.5 | 147.6 | 149.8 | 146.9 | 146.8 | |||||
| 5′ | 113.9 | 6.98, d (8.8) | 147.6 | 143.1 | 140.4 | 140.4 | ||||
| 6′ | 130.1 | 7.52, d (8.8) | 109.8 | 7.12, d (1.6) | 115.3 | 6.96, s | 114.4 | 6.87, s | 114.4 | 6.88, s |
| 7′ | 101.1 | 5.99, s | ||||||||
| 1″ | 22.9 | 3.59, d (7.2) | 22.9 | 3.59, d (7.2) | 23.0 | 3.60, d (7.2) | 22.2 | 3.46, d (7.2) | 21.5 | 3.42, d (7.2) |
| 2″ | 121.5 | 5.21, t (7.2) | 121.5 | 5.21, t (7.2) | 121.6 | 5.23, t (7.2) | 122.2 | 5.18, t (7.2) | 122.1 | 5.17, t (7.2) |
| 3″ | 132.7 | 132.7 | 132.6 | 132.1 | 131.9 | |||||
| 4″ | 17.9 | 1.84, s | 17.9 | 1.84, s | 17.9 | 1.84, s | 17.8 | 1.81, s | 17.8 | 1.79, s |
| 5″ | 25.8 | 1.69, s | 25.8 | 1.69, s | 25.8 | 1.70, s | 25.8 | 1.70, s | 25.8 | 1.68, s |
| 6-OCH3 | 56.0 | 3.96, s | 56.1 | 3.96, s | 56.0 | 3.96, s | 60.7 | 3.93, s | ||
| 7-OCH3 | 61.1 | 3.93, s | 61.1 | 3.93, s | 61.1 | 3.93, s | 61.4 | 4.01, s | 56.1 | 3.90, s |
| 2′-OCH3 | 56.9 | 3.79, s | 56.5 | 3.74, s | 56.5 | 3.75, s | ||||
| 4′-OCH3 | 55.3 | 3.84, s | 56.2 | 3.94, s | ||||||
| 5′-OCH3 | 56.6 | 3.86, s | 56.7 | 3.86, s | 56.7 | 3.86, s | ||||
Chemical shifts are given in ppm. J values (Hz) are given in parentheses. Assignments were made based on the analysis of 1H–1H COSY, HSQC, and HMBC data.
PachyvoneB (2)
White powder; UV (MeOH) λmax (log ε) 263 (3.64), 326 (3.07) nm; IR (KBr) vmax 3019, 2917, 1682, 1462, 863, 845 cm−1. Both 1H NMR and 13C NMR data are shown in Table 1; HRESIMS m/z 395.1506 [M+H]+ (calcd for C23H23O6, 395.1495).
PachyvoneC (4)
White powder; UV (MeOH) λmax (log ε) 255 (3.38), 298 (2.87) nm; IR (KBr) vmax 3025, 2921, 1679, 1443, 865 cm−1. Both 1H NMR and 13C NMR data are shown in Table 1; HRESIMS m/z 441.1914 [M+H]+ (calcd for C25H29O7, 441.1913).
PachyvoneD (5)
Yellowish powder; UV (MeOH) λmax (log ε) 266 (3.76) nm; IR (KBr) vmax 3593, 3016, 2934, 1688, 1459, 827 cm−1. Both 1H NMR and 13C NMR data are shown in Table 1; HRESIMS m/z 443.1705 [M+H]+ (calcd for C24H27O8, 443.1706).
PachyvoneE (6)
Yellowish powder; UV (MeOH) λmax (log ε) 264 (3.58), 294 (2.92) nm; IR (KBr) vmax 3598, 3023, 2928, 1663, 1466, 831 cm−1. Both 1H NMR and 13C NMR data are shown in Table 1; HRESIMS m/z 413.1605 [M+H]+ (calcd for C23H25O7, 413.1600).
PachythoneA (7)
Yellow powder; UV (MeOH) λmax (log ε) 287 (4.18), 340 (2.17) nm; IR (KBr) vmax 3604, 2894, 1664, 1456, 848, 715 cm−1. Both 1H NMR and 13C NMR data are shown in Table 2; HRESIMS m/z 371.1137 [M+H]+ (calcd for C20H19O7, 371.1131).
Table 2.
1H and 13C NMR spectroscopic data for compound 7a (400 and 100 MHz for 1H and 13C NMR, CDCl3).
| position |
7 |
|
|---|---|---|
| δC | δH | |
| 1 | 156.9 | |
| 2 | 95.1 | 6.40, s |
| 3 | 160.5 | |
| 4 | 104.7 | |
| 4a | 157.5 | |
| 5 | 139.8 | |
| 6 | 145.6 | |
| 7 | 145.5 | |
| 8 | 103.6 | 7.47, s |
| 8a | 116.5 | |
| 9 | 180.1 | |
| 9a | 103.3 | |
| 10a | 145.1 | |
| 4′ | 115.5 | 6.73, d (10.0) |
| 5′ | 127.5 | 5.60, d (10.0) |
| 6′ | 78.2 | |
| 7′ | 28.4 | 1.48, s |
| 8′ | 28.4 | 1.48, s |
| 5-OCH3 | 61.9 | 4.05, s |
| 6-OCH3 | 61.5 | 4.16, s |
Chemical shifts are given in ppm. J values (Hz) are given in parentheses. Assignments were made based on the analysis of 1H–1H COSY, HSQC, and HMBC data.
Cell culture and transfection
GFP-LC3-HeLa (HeLa cells stably expressing GFP-LC3) were established as previously reported [16], [17]. HeLa, HepG2, MCF-7, Hct116, MDA-MB-231, and HUVECs were obtained from KeyGEN Biotech Co. (Nanjing, China) and cultured with Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were cultured at 37 °C in a humidified atmosphere, and the concentration of CO2 was set at 5%.
Cytotoxicity assay
The cytotoxic effects of the isolated compounds were investigated using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Briefly, cells were plated in 96-well plates with 1 × 104 cells per well. Cells were cultured for 24 h before treatment with different compounds at various concentrations (0–40 μM) for 72 h. Then, 20 μL MTT solution (5 mg/mL) was added to each well and incubated for another 4 h. The supernatants were discarded, and 150 μL dimethyl sulfoxide (DMSO) was added to each well and incubated for 10 min. The absorbance at 570 nm was detected using a microplate reader (BioTek, Winooski, USA). The cytotoxicity (IC50, half maximal inhibitory concentration) of each compound was calculated using GraphPad Prism 5.
Autophagy detection using GFP-LC3 expression in HeLa cells
The effects of compound-induced autophagy were determined in GFP-LC3-HeLa cells [16], [17]. The GFP-LC3-HeLa cells were plated in 24-well plates and incubated with the tested compounds at various concentrations. Chloroquine phosphate treatment-induced LC3 dots were used as an observation control. Plates were incubated for 24 h, and the GFP-LC3 puncta were detected and imaged under a fluorescence microscope (Olympus, Tokyo, Japan) with Olympus Stream software.
Detection of apoptotic cells using flow cytometry
HeLa and MCF-7 cells seeded on six-well plates for 24 h were incubated with 0, 2.5, 5, 10, and 20 μM compound 9 or colchicine (positive control) for 48 h. Cells were collected, digested with ethylenediaminetetraacetic acid-free trypsin for 3 min and centrifuged (170 × g, 3 min) before being washed with phosphate buffered saline (PBS) buffer twice and centrifuged (170 × g, 3 min). Then, the cells were resuspended and stained with reagents from the Annexin V/propidium iodide (PI) Apoptosis Detection Kit (Invitrogen) for approximately 30 min according to the manufacturer’s instructions. The stained cells were subjected to flow cytometry (Attune NxT, Life Technology, Waltham, USA) for analysis. Data and image analyses were conducted using FlowJo 7.6 software. The PI−/Annexin V−, PI+/Annexin V−, PI−/Annexin V+, and PI+/Annexin V+ cells were considered viable cells, necrotic cells, early apoptotic cells, and late apoptotic cells, respectively. In this study, the early apoptotic cells and late apoptotic cells were combined and counted as the total number of apoptotic cells.
Western blotting analysis
HeLa cells were collected and washed twice with PBS before being lysed with protein lysis radioimmunoprecipitation assay buffer for 30 min at 4 °C. Samples were subjected to centrifugation at 18894g for 30 min at 4 °C. The supernatants were collected, and the protein concentration was determined using a bicinchoninic acid assay (Thermo Scientific, Waltham, USA). Proteins were denatured in 1 × loading buffer in boiling water for 10 min. Equal amounts (20 μg) of samples were loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis for the ionophoretic separation of proteins for 1 h using a constant voltage of 120 V. Proteins in the gel were transferred to polyvinylidene difluoride (PVDF) membranes using 260 mA constant current for 2 h. Transferred PVDF membranes were blocked with a 5% milk solution (in 1 × PBST buffer (0.1% Tween® 20 in PBS buffer)) for 1 h at room temperature before incubation with primary antibodies at 4 °C overnight. PVDF membranes were washed three times (10 min each) with 1 × PBST buffer. Secondary antibodies were incubated with PVDF membranes for 45 min at room temperature, and the membranes were washed three times (10 min each) with 1 × PBST. The membranes were stained with enhanced chemiluminescence reagents (Millipore, Burlington, USA) and imaged using a chemiluminescence image analysis system (Tianneng, Shanghai, China) with Tanon-5200 Multi software (Tianneng, Shanghai, China).
Results and discussion
Isolation of compounds 1–28.
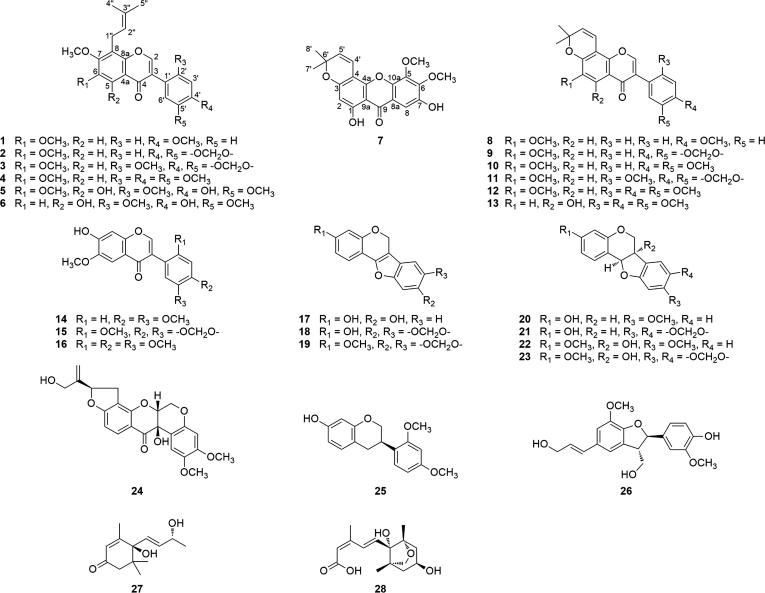
Approximately 10 kg dry stems of M. pachyloba were shattered into powder (approximately 20-mesh) and extracted with 95% aqueous EtOH three times. The EtOH extracts were combined, evaporated to dryness, suspended in H2O and successively extracted with petroleum ether and CH2Cl2. The petroleum ether and CH2Cl2 extracts were further separated using column chromatography (silica gel and Sephadex LH-20) as well as reverse-phase SP-HPLC to obtain compounds 1–28 (see Fig. 1).
Fig. 1.
Structures of compounds 1–28.
Chemical structure identification of the isolated compounds.
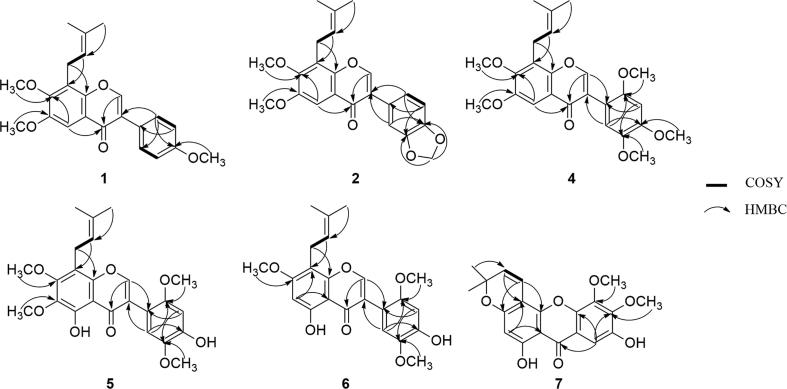
Compound 1 was obtained as a white powder and assigned a molecular formula of C23H24O5 by HRESIMS at m/z 381.1707 ([M+H]+, calcd for 381.1702), indicating twelve double bond equivalents. The UV spectrum absorption at 256 and 326.4 nm, 1H (δH 8.02 for H-2) and 13C (δC 152.3 for C-2) NMR spectra and 1H detected heteronuclear multiple bond correlation (HMBC) correlations (Fig. 2) of H-2 (δH 8.02) to C-3 (δC 123.9), C-8a (δC 150.0) and C-4 (δC 176.1) showed this compound to be an isoflavone-type skeleton [18], [19]. Analysis of the 1H NMR (Table 1) and homonuclear chemical shift correlation spectroscopy (COSY) correlations revealed a γ, γ-dimethylallyl unit [δH 3.59 (2H, d, J = 7.2 Hz), 5.21 (1H, t, J = 7.2 Hz), 1.84 (3H, s), 1.69 (3H, s)], a 1,4-disubstituted benzene ring [δH 7.52 (2H, d, J = 8.8 Hz), 6.98 (2H, d, J = 8.8 Hz)], three methoxy groups [δH 3.96 (3H, s), 3.93 (3H, s), 3.84 (3H, s)], an aromatic proton [δH 7.60 (1H, s)] and a vinyl proton [δH 8.02 (1H, s)]. The 13C NMR spectra of 1 indicated 23 signals, including three methoxy groups [δC 55.3, 56.0 and 61.1] and one γ, γ-dimethylallyl unit [δC 17.9, 22.9, 25.8, 121.5 and 132.7]. Comparison of the NMR data of 1 and millesianin H [2] revealed similar carbon and proton resonances, except that 1 contained one more methoxy group. A further HMBC correlation study (Fig. 2) showed that this methoxy group (δH 3.93) was attached to C-7 (δC 151.9). Therefore, the chemical structure of compound 1 was identified as 8-(γ, γ-dimethylallyl)-6,7,4′-trimethoxyisoflavone, and it was named pachyvone A.
Fig. 2.
Key COSY and HMBC correlations of compounds 1, 2, and 4–7.
Compound 2 was isolated as a white powder. Its formula of C23H22O6 was deduced by HRESIMS at m/z 395.1506 ([M+H]+, calcd for 395.1495). In the 1H and 13C NMR spectra of 2, an olefinic proton at δH 8.01 (s, H-2), an oxygenated carbon resonance at δC 152.5 (C-2) and a carbonyl carbon resonance at δH 175.9 (C-4) suggested an isoflavone skeleton [18], [19]. The 1H NMR spectrum showed (Table 1) the presence of a γ, γ-dimethylallyl unit [δH 3.59 (2H, d, J = 7.2 Hz), 5.21 (1H, t, J = 7.2 Hz), 1.84 (3H, s), 1.69 (3H, s)], an ABX-type benzene ring [δH 6.87 (1H, d, J = 8.0 Hz), 7.00 (1H, dd, J = 8.0 Hz, J = 1.6 Hz), 7.12 (1H, d, J = 1.6 Hz)], two methoxy groups [δH 3.96 (3H, s), 3.93 (3H, s)], a methylenedioxy group [δH 5.99 (2H, s)], an aromatic proton [δH 7.59 (1H, s)] and a vinyl proton [δH 8.01 (1H, s)]. The 13C NMR spectra of 2 indicated 23 signals, including two methoxy groups [δC 56.1 and 61.1], one γ, γ-dimethylallyl unit [δC 17.9, 22.9, 25.8, 121.5, and 132.7] and a methylenedioxy functionality [δC 101.1]. These signals were similar to the resonances of predurmillone [20], except that the hydroxyl group in predurmillone was replaced by a methoxyl group (δH 3.93) in 2. These results were further directly supported by the HMBC correlation (Fig. 2) from the proton signal at δH 3.93 to C-7 (δC 151.96) and indirectly demonstrated by the HMBC correlations (Fig. 2) from the proton signals at δH 7.59 (s) to C-7 (δC 151.96) and C-4 (δC 175.9) because this proton was not substituted and the methoxyl group (δH 3.93) should be attached to C-7. Therefore, the structure of compound 2 was identified as 8-(γ, γ-dimethylallyl)-6,7-dimethoxy-4′,5′-methylenedioxyisoflavone, and it was named pachyvone B.
Compound 4 was isolated as a white powder and assigned the molecular formula C25H28O7, as indicated by the HRESIMS at m/z 441.1914 ([M+H]+, calcd for 441.1913), which suggested twelve double bond equivalents. A singlet at δH 8.05 (H-2) in the 1H NMR spectrum and the 13C NMR signals at δC 154.5 (C-2), 120.9 (C-3), and 176.0 (C-4) were consistent with an isoflavone core structure that was further corroborated by its UV spectrum (λmax at 255.4 and 297.9 nm) [18], [19]. Compound 4 and millesianin I [2] had similar 1H and 13C NMR data (Table 1) because both compounds contained a γ, γ-dimethylallyl unit, a 1,2,4,5-tetrasubstituted benzene ring, four methoxy groups, an aromatic proton and a vinyl proton, except that compound 4 contained one additional methoxy group signal at δC 61.11 and δH 3.93. HMBC correlation (Fig. 2) of the proton resonance at δH 3.93 with C-7 (δC 151.81) demonstrated that the additional methoxy group was attached to C-7. Accordingly, the structure of compound 4 was established as 8-(γ, γ-dimethylallyl)-6,7,2′,4′,5′-pentamethoxyisoflavone, and it was named pachyvone C.
Compound 5 was isolated as a yellowish powder. Its molecular formula was determined to be C24H26O8 by HRESIMS at m/z 443.1705 ([M+H]+, calcd for 443.1706), suggesting twelve double bond equivalents. The UV maxima at λmax 266.0 and 300.0 nm as well as the specific proton signal at δH 7.97 (1H, s, H-2) that was correlated with δC 155.4 (C-2), as shown by the heteronuclear single quantum coherence spectrum, suggested that compound 5 possessed an isoflavone-type skeleton [18], [19]. The 1H NMR (Table 1) and COSY correlations of compound 5 revealed signals for a γ, γ-dimethylallyl unit [δH 3.46 (2H, d, J = 7.2 Hz), 5.18 (1H, t, J = 7.2 Hz), 1.81 (3H, s), 1.70 (3H, s)], a 1,2,4,5-tetrasubstituted benzene ring [δH 6.67 (1H, s), 6.87 (1H, s)], four methoxy groups [δH 4.01 (3H, s), 3.93 (3H, s), 3.86 (3H, s), 3.74 (3H, s)] and a hydroxyl group [δH 12.88 (1H, s)]. The 13C NMR spectrum of 5 indicated 23 signals, including four methoxy groups [δC 56.5, 56.7, 60.7 and 61.4] and one γ, γ-dimethylallyl unit [δC 17.8, 22.2, 25.8, 122.2 and 132.1]. Compound 5 exhibited NMR data very similar to those of compound 4. However, 5 had a chelated hydroxyl group at δH 12.88 (1H, s, OH-5), which is absent in 4. Moreover, there is one more methoxyl group in 5 compared to 4. The HMBC correlations of the hydroxyl group (δH 12.88) with C-4a (δC 108.60), C-5 (δC 152.47) and C-6 (δC 136.64) suggested a hydroxy group at the C-5 position. HMBC correlations from the proton signals of four methoxy groups [δH 4.01 (3H, s), 3.93 (3H, s), 3.86 (3H, s), 3.74 (3H, s)] demonstrated that these four methoxy groups were attached to C-7, C-6, C-5′, and C-2′, respectively. The chemical shift of C-4′ (δC 146.89) combined with the molecular formula C24H26O8 showed that compound 5 had a hydroxy group at the C-4′ position. The key HMBC correlations are shown in Fig. 2. On the basis of the evidence obtained, the structure of compound 5 was determined to be 8-(γ, γ-dimethylallyl)-5,4′-dihydroxy-6,7,2′,5′-tetramethoxyisoflavone, and it was named pachyvone D.
Compound 6 was obtained as a yellowish powder with the molecular formula C23H24O7 deduced by HRESIMS at m/z 413.1605 (([M+H]+, calcd for 413.1600)). The UV maxima at λmax 263.7 and 294.4 nm along with the IR absorptions (νmax) at 1663 and 1466 cm−1 showed this compound to be an isoflavonoid, as supported by the characteristic 1H and 13C NMR resonances at δH-2 7.94 and δC-2 155.2 for this type of natural product [18], [19]. The NMR data (Table 1) were very similar to those of compound 5, except for the loss of one methoxy group signal and the appearance of one additional aromatic proton signal at δC 95.12 and δH 6.41, which indicates that one methoxy group of compound 5 may be replaced by an aromatic proton to obtain compound 6. The HMBC correlations (Fig. 2) from the aromatic proton signal at δH 6.41 to C-4a (δC 105.83), C-5 (δC 160.93), C-7 (δC 162.72) and C-8 (δC 107.82) suggested that the aromatic proton was attached to C-6. Therefore, compound 6 was determined to be 8-(γ, γ-dimethylallyl)-5,4′-dihydroxy-7,2′,5′-trimethoxyisoflavone, and it was named pachyvone E.
The physical nature of isoflavones has a close relationship with their structure. In these newly isolated isoflavones, compounds 1, 2, and 4 were reported as white powders, while compounds 5 and 6 were reported as yellow powders. Careful investigation of the differences in structures revealed that the presence of a 4-OH group in the yellow-colored compounds (5 and 6) could verify the extended conjugation system, while this 4-OH moiety is absent or replaced in the white-colored compounds.
Compound 7 was obtained as a yellow powder, and its molecular formula was assigned as C20H18O7 from the positive ion peak at m/z 371.1137 ([M+H]+, calcd for 371.1131) in the HRESIMS, which corresponded to twelve double bond equivalents. The 1H NMR spectrum (Table 2) showed similar signals to nigrolineaxanthone F [21]: two aromatic protons [δH 6.40 (1H, s) and 7.47 (1H, s)] and dimethylchromene protons [δH 5.60 (1H, d, J = 10.0 Hz), 6.73 (1H, d, J = 10.0 Hz) and 1.48 (6H, s)]. These protons were located at the same positions as nigrolineaxanthone F according to their HMBC correlations. The major difference between compound 7 and nigrolineaxanthone F was that compound 7 exhibited two more methoxy group signals at δH 4.05 and 4.16 and nigrolineaxanthone F had two more vinyl signals at δH 7.39 (1H, d, J = 8.5 Hz) and 7.28 (1H, dd, J = 8.5 Hz, J = 3.0 Hz), which suggests that the vinyl protons were substituted by these two methoxy groups. This result was corroborated by the HMBC correlations (Fig. 2) from the proton resonance at δH 7.47 (H-8) to C-6 (δC 145.6). Therefore, the structure of compound 7 was determined to be 1,7-dihydroxy-5,6-dimethoxy-6′,6′-dimethylpyrano (2′,3′:3,4) xanthone, and it was named pachythone A.
Based on the spectroscopic data and comparisons with the data found in the literature, the known compounds were identified as 8-prenylmilldurone (3) [22], 6-methoxycalpogonium isoflavone A (8) [23], durmillone (9) [24], durallone (10) [25], ichthynone (11) [8], millesianin C (12) [26], toxicarol isoflavone (13) [27], cladrastin (14) [28], dalpatein (15) [29], 7-hydroxy-2′,4′,5′,6-tetramethoxyisoflavone (16) [30], 3,9-dihydroxypterocarp-6a-en (17) [31], dehydromaackiain (18) [32], flemichapparin B (19) [33], (−)-medicarpin (20) [34], (−)-maackiain (21) [35], (−)-variabilin (22) [36], (−)-pisatin (23) [37], dalbinol (32) [38], (−)-sativin (25) [39], (−)-dehydrodiconiferyl alcohol (26) [40], (+)-vomifoliol (27) [41], and dihydrophaseic acid (28) [42].
Primary screening for cytotoxic compounds
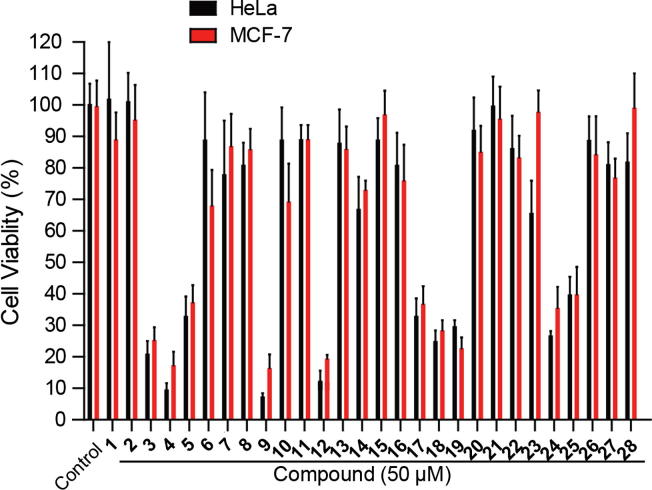
Flavones have shown cytotoxic activity toward cancer cells such as HeLa and MCF-7 cell lines [43], [44]. Therefore, the primary cytotoxic activities of 28 compounds were tested on HeLa and MCF-7 cells by MTT assay. As shown in Fig. 3, ten compounds (3–5, 9, 12, 17–19, 24, and 25) showed growth inhibition of HeLa and MCF-7 cells at a 50 μM concentration, while the other compounds possessed no activity. Notably, compounds 4, 9, and 12 are the most active compounds.
Fig. 3.
Preliminary screening of active compounds on HeLa and MCF-7 cells. HeLa and MCF-7 cells were treated with 50 μM for 72 h, and then cell viability was tested by MTT assay.
Cytotoxic activities of selected compounds on cancer cells and normal cells
Isoflavones are known to be phytoestrogens, and thus, an estrogen receptor-positive cell line (MCF-7) and estrogen receptor-negative cell line (MDA-MB-231) together with other cancer cell lines were used in this study. The cytotoxic activities of the ten active compounds were evaluated in five cancer cell lines (HeLa, HepG2, MCF-7, Hct116, and MDA-MB-231) and one normal cell line (HUVEC) using doxorubicin as a positive control. Cancer cells were treated with increasing concentrations of the compounds (0, 2.5, 5, 10, 20, and 40 μM), and normal cells were treated with the compounds at 50 μM for 72 h. Cell viability was examined by the MTT assay. The IC50 values of the ten compounds were calculated and are presented in Table 3. Compounds 4, 9, and 12 showed better anticancer activities than the other compounds. These compounds showed no selectivity on estrogen receptor-negative and estrogen receptor-positive cells, implying that these compounds exhibit no activity on estrogen receptors. Notably, all of these compounds had no activity against normal cells, suggesting that these compounds are safe anticancer candidate compounds.
Table 3.
Cytotoxicity of selected compounds against five cancer cell lines and a normal cell lines (HUVEC).a
| Compound | IC50 (μM) |
|||||
|---|---|---|---|---|---|---|
| HeLa | HepG2 | MCF-7 | HCT-116 | MDA-MB-231 | HUVEC | |
| 3 | 14.56 ± 0.54 | 15.97 ± 0.67 | 19.61 ± 0.66 | 23.21 ± 1.22 | 20.78 ± 2.35 | >50 |
| 4 | 7.86 ± 1.21 | 8.74 ± 0.83 | 18.46 ± 0.51 | 8.61 ± 0.72 | 15.85 ± 2.15 | >50 |
| 5 | 35.67 ± 3.91 | 31.61 ± 2.06 | 35.05 ± 1.44 | 25.91 ± 0.85 | 27.64 ± 4.54 | >50 |
| 9 | 6.09 ± 1.09 | 17.85 ± 1.60 | 11.08 ± 0.68 | 15.14 ± 0.61 | 12.89 ± 3.10 | >50 |
| 12 | 14.82 ± 2.12 | 8.05 ± 0.90 | 14.37 ± 1.84 | 19.78 ± 1.29 | 11.09 ± 0.91 | >50 |
| 17 | 36.15 ± 7.34 | 34.25 ± 1.87 | 30.34 ± 1.32 | 39.66 ± 2.06 | 36.78 ± 5.61 | >50 |
| 18 | 22.50 ± 1.09 | 13.39 ± 1.41 | 21.21 ± 0.93 | 21.90 ± 1.73 | 25.45 ± 2.09 | >50 |
| 19 | 30.19 ± 0.54 | 25.38 ± 1.92 | 21.10 ± 1.65 | 27.03 ± 1.64 | 22.76 ± 3.54 | >50 |
| 24 | 19.89 ± 2.09 | 32.61 ± 1.84 | 33.12 ± 1.93 | 16.65 ± 0.82 | 27.16 ± 3.25 | >50 |
| 25 | 40.12 ± 4.32 | 28.61 ± 2.90 | 36.42 ± 2.08 | 31.90 ± 1.52 | 33.45 ± 2.33 | >50 |
| Doxorubicin | 0.03 ± 0.001 | 0.02 ± 0.002 | 0.02 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.002 | 0.04 ± 0.005 |
Results are presented as means ± SEM (n = 3).
Compound 9 induced autophagy in HeLa and MCF-7 cells
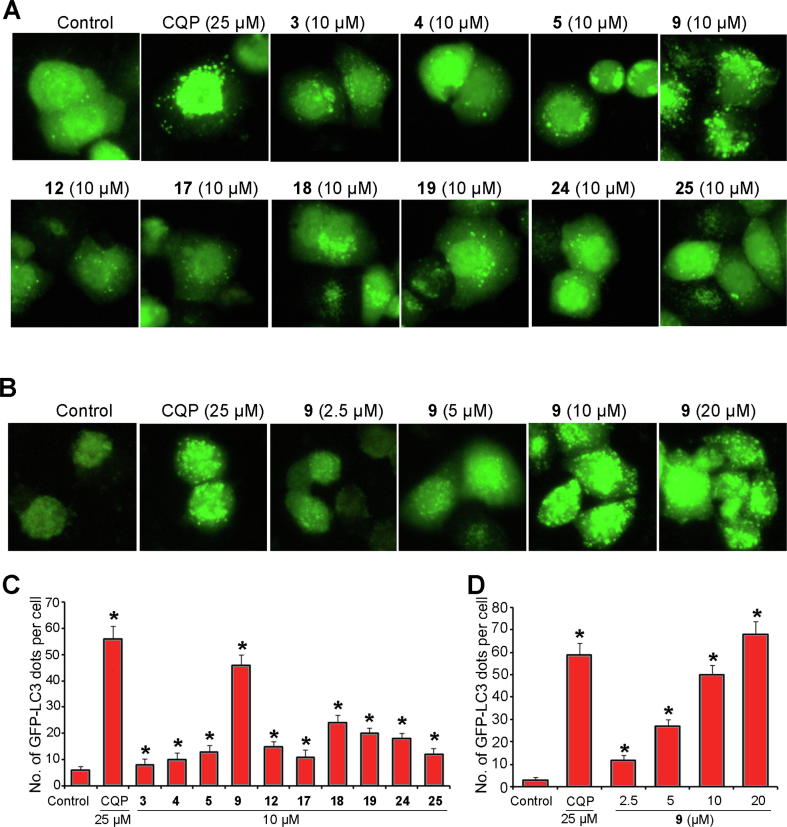
Numerous flavonoids mediate cell death using an autophagy-dependent pathway [45], [46], [47]. Here, a GFP-LC3-HeLa cell line was used to investigate whether the cytotoxicity of the ten compounds was associated with autophagy. LC3 is an autophagy marker protein that forms autophagosomes during autophagy induction [48]. Autophagosome dots, indicating aggregated LC3 protein, were directly observed in GFP-LC3-HeLa cells stably expressing GFP-labeled LC3 proteins using a fluorescence microscope. GFP-LC3-HeLa cells were treated with the ten compounds for 24 h at 10 μM. Chloroquine phosphate treatment-induced LC3 dots were used as the observation control. Fig. 4 shows that chloroquine phosphate induced an obvious increase in the GFP-LC3 dots, which indicates the appearance of autophagosomes. All the tested compounds produced an increase in GFP-LC3 dots, and compound 9 (durmillone) showed the best activity. Durmillone (9) induced GFP-LC3 punctation in a dose-dependent manner. These results suggest that the ten compounds induce autophagy and that compound 9 exhibits the best activity.
Fig. 4.
Selected compounds isolated from M. pachyloba induced autophagy. A. HeLa cells stably expressing GFP-LC3 (GFP-LC3-HeLa) were treated with ten compounds (3–5, 9, 12, 17–19, 24, and 25) at 10 μM or with chloroquine phosphate (CQP) at 25 μM for 24 h. B. GFP-LC3-HeLa cells were treated with compound 9 at 2.5, 5, 10, and 20 μM or chloroquine phosphate at 25 μM for 24 h. C and D. The number of GFP-LC3 dots/cell was quantified.
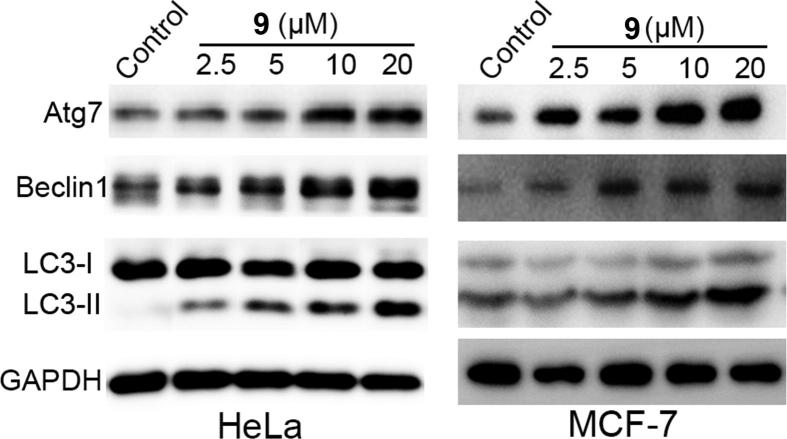
The expression of autophagy-associated proteins, such as LC3-II, Beclin1, and Atg7 [48], was detected in HeLa and MCF-7 cells treated with compound 9 using Western blotting analysis, further verifying this result. The results indicated that compound 9 remarkably and dose-dependently upregulated the expression levels of LC3-II, Beclin1, and Atg7 in HeLa and MCF-7 cells (Fig. 5). Taken together, these results demonstrate that compound 9 induced obvious autophagy in HeLa cells. As compound 9 exhibited the best activity in inducing autophagy, it was chosen for further study.
Fig. 5.
Compound 9 induced autophagy in HeLa and MCF-7 cells. HeLa and MCF-7 cells were treated with the indicated concentrations of compound 9 for 24 h. Western blottings were used to measure the protein levels of LC3, Beclin1, and Atg7. GADPH was used as a loading control.
Compound 9 induced apoptosis in HeLa and MCF-7 cells.
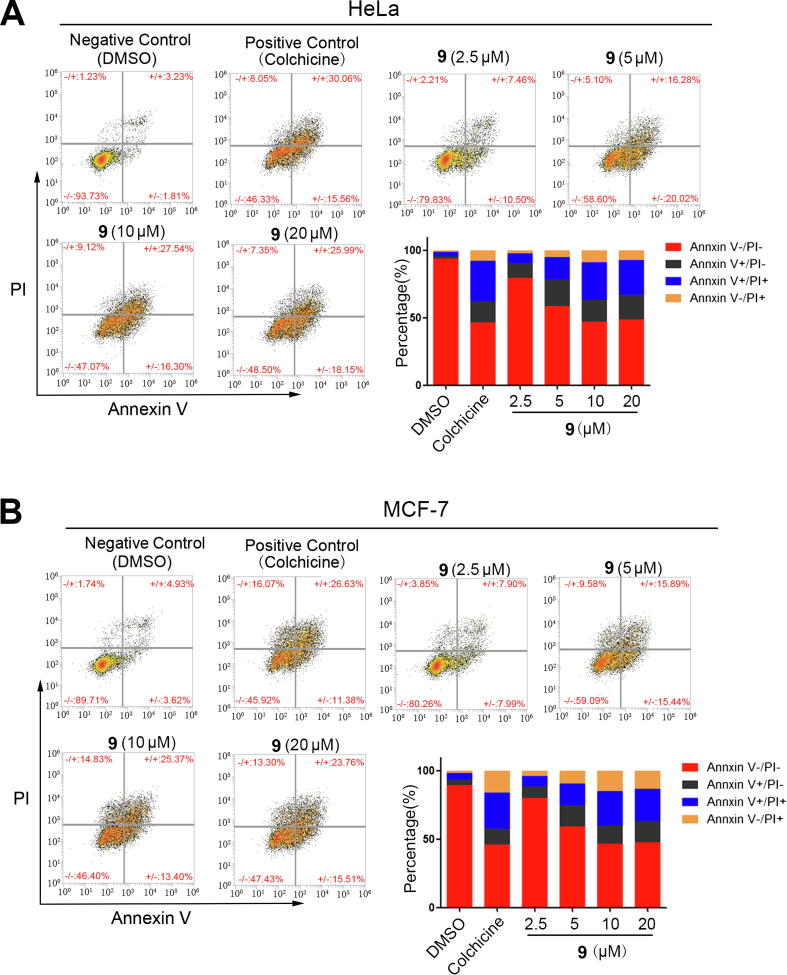
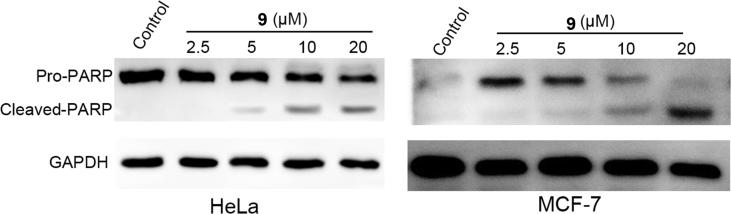
Plasma membrane surface Annexin V is a marker of apoptosis, and propidium iodide (PI) is used to detect late apoptotic cells, so the combined PI/Annexin V double staining method is a classic method of apoptosis detection. In the present study, the PI/Annexin V double staining flow cytometric assay was used to further investigate compound 9 induction of apoptosis in cancer cells. Colchicine, a tubulin inhibitor, was employed as a positive control. The results revealed that compound 9 induced apoptosis in HeLa and MCF-7 cells in a concentration-dependent manner. The apoptosis rates were 5.04%, 45.62%, 17.96%, 36.30%, 43.84%, and 44.14% for HeLa cells treated with the negative control (DMSO), positive control (colchicine), and 2.5, 5, 10 and 20 μM compound 9, respectively. Additionally, the apoptosis rates in MCF-7 cells were 8.55%, 38.01%, 15.89%, 31.33%, 38.77%, and 39.27% for the negative control (DMSO), positive control (colchicine), and 2.5, 5, 10 and 20 μM compound 9, respectively (Fig. 6). To further verify the induction of apoptotic events by compound 9 in cancer cells, the level of poly ADP-ribose polymerase (PARP) cleavage, which is a marker of late apoptotic events, was determined using Western blotting in HeLa and MCF-7 cells. Cells treated with 2.5, 5, 10 and 20 μM compound 9 for 48 h induced obvious PARP cleavage in a concentration-dependent manner (Fig. 7), which further demonstrates that compound 9 also induces apoptosis in cancer cells.
Fig. 6.
Quantitative analysis of apoptosis using the Annexin V/PI double-staining assay and flow cytometry calculations. (A) HeLa cells were treated with compound 9 at different concentrations (0, 2.5, 5.0, 10.0, and 20.0 μM) or 1 μM colchicine for 48 h; the histogram shows the percentages of viable cells (PI−/Annexin V−), necrotic cells (PI+/Annexin V−), early apoptotic cells (PI−/Annexin V+), and late apoptotic cells (PI+/Annexin V+). (B) MCF-7 cells were treated with compound 9 at different concentrations (0, 2.5, 5.0, 10.0, and 20.0 μM) or 1 μM colchicine for 48 h; the histogram shows the percentages of viable cells (PI−/Annexin V−), necrotic cells (PI+/Annexin V−), early apoptotic cells (PI−/Annexin V+), and late apoptotic cells (PI+/Annexin V+).
Fig. 7.
Compound 9 induced apoptosis in HeLa and MCF-7 cells. HeLa and MCF-7 cells were treated with the indicated concentrations of compound 9 for 48 h. Western blottings were used to measure protein levels of PARP. GADPH was used as a loading control.
In summary, the results of the present study suggest that compound 9 mediates cytotoxic activity through the combined action of apoptosis and autophagy.
Conclusions
In this study, systematic separation and subsequent pharmacological activity studies were carried out to obtain cytotoxic natural products from the dried stems of M. pachyloba. Ten cytotoxic natural products (3–5, 9, 12, 17–19, 24, and 25) from the dried stems of Millettia pachyloba Drake were obtained, and compound 9 exhibited the highest cytotoxic activity through the combined action of apoptosis and autophagy.
Phytochemical investigation of the stems of Millettia pachyloba led to the isolation of five previously undescribed isoflavones (1, 2, and 4–6), one previously undescribed xanthone (7), and twenty-two known compounds. These findings enrich the diversity of chemical components of the genus Millettia. Biological assays to examine the cytotoxic effects of ten compounds (3–5, 9, 12, 17–19, 24, and 25) showed that these compounds produced cytotoxic effects in HepG2, MCF-7, and HeLa cell, with IC50 values ranging from 5 to 40 μM. Notably, durmillone (9) induced cytotoxicity through the combined action of apoptosis and autophagy in HeLa cells, which suggests that flavonoids are responsible for the cytotoxicity of M. pachyloba.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
This study acknowledges grant support from the National Natural Science Foundation of China (81874297, 81803021 and 81527806), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University, Post-doctoral Research Project, West China Hospital, Sichuan University (2018HXBH027), and China Postdoctoral Science Foundation (2019M650248).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2019.06.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Schneekloth A.R., Pucheault M., Tae H.S., Crews C.M. Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Le. 2008;18(22):5904–5908. doi: 10.1016/j.bmcl.2008.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye H., Wu W., Liu Z., Xie C., Tang M., Li S. Bioactivity-guided isolation of anti-inflammation flavonoids from the stems of Millettia dielsiana Harms. Fitoterapia. 2014;95(2):154–159. doi: 10.1016/j.fitote.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Tang H., Pei H.Y., Wang T.J., Chen K., Wu B., Yang Q.N. Flavonoids and biphenylneolignans with anti-inflammatory activity from the stems of Millettia griffithii. Bioorg Med Chem Lett. 2016;26(18):4417–4422. doi: 10.1016/j.bmcl.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Ngamga D., Fanso Free S.N., Tane P., Fomum Z.T. Millaurine A, a new guanidine alkaloid from seeds of Millettia laurentii. Fitoterapia. 2007;78(3):276–277. doi: 10.1016/j.fitote.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Deyou T., Gumula I., Pang F., Gruhonjic A., Mumo M., Holleran J. Rotenoids, Flavonoids, and Chalcones from the Root Bark of Millettia usaramensis. J Nat Prod. 2015;78(12):2932–2939. doi: 10.1021/acs.jnatprod.5b00581. [DOI] [PubMed] [Google Scholar]
- 6.Yenesew A., Midiwo J.O., Waterman P.G. Rotenoids, isoflavones and chalcones from the stem bark of Millettia usaramensis subspecies usaramensis. Phytochemistry. 1998;47(2):295–300. [Google Scholar]
- 7.Rajemiarimiraho M., Banzouzi J.T., Rakotonandrasana S.R., Chalard P., Benoit-Vical F., Rasoanaivo L.H. Pyranocoumarin and triterpene from Millettia richardiana. Nat Prod Commun. 2013;8(8):1099–1100. [PubMed] [Google Scholar]
- 8.Mai H.D.T., Nguyen T.T.O., Pham V.C., Litaudon M., Guéritte F., Tran D.T. Cytotoxic prenylated isoflavone and bipterocarpan from Millettia pachyloba. Planta Med. 2010;76(15):1739–1742. doi: 10.1055/s-0030-1249834. [DOI] [PubMed] [Google Scholar]
- 9.Na Z., Fan Q.F., Song Q.S., Hu H.B. Three new flavonoids from Millettia pachyloba. Phytochem Lett. 2017;19:215–219. [Google Scholar]
- 10.Na Z., Song Q.S., Fan Q.F. New Xanthone from Millettia pachyloba Drake. Rec Nat Prod. 2019 [Google Scholar]
- 11.Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassour J., Radford R., Correia A., Fusté J.M., Schoell B., Jauch A. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565(7741):659–663. doi: 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yankep E., Njamen D., Fotsing M.T., Fomum Z.T., Mbanya J.C., Giner R.M. Griffonianone D, an isoflavone with anti-inflammatory activity from the root bark of Millettia griffoniana. J Nat Prod. 2003;66(9):1288–1290. doi: 10.1021/np0205912. [DOI] [PubMed] [Google Scholar]
- 14.Derese S., Barasa L., Akala H.M., Yusuf A.O., Kamau E., Heydenreich M. 4′-Prenyloxyderrone from the stem bark of Millettia oblata ssp. teitensis and the antiplasmodial activities of isoflavones from some Millettia species. Phytochemistry. 2014;8:69–72. [Google Scholar]
- 15.Adem F.A., Kuete V., Mbaveng A.T., Heydenreich M., Koch A., Ndakala A. Cytotoxic flavonoids from two Lonchocarpus species. Nat Prod Res. 2018;16:1–9. doi: 10.1080/14786419.2018.1462179. [DOI] [PubMed] [Google Scholar]
- 16.Ding W.X., Ni H.M., Gao W., Hou Y.F., Melan M.A., Chen X. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282(7):4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 17.Ding W., Ni H., Li M., Liao Y., Chen X., Stolz D.B. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem. 2010;285(36):27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren Y., Benatrehina P.A., Muñoz Acuña U., Yuan C., Chai H.B., Ninh T.N. Isolation of Bioactive Rotenoids and Isoflavonoids from the Fruits of Millettia caerulea. Planta Med. 2016;82(11–12):1096–1104. doi: 10.1055/s-0042-108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen R., Lv H., Jiang Y., Tu P. Anti-inflammatory isoflavones and isoflavanones from the roots of Pongamia pinnata (L.) Pierre. Bioorg Med Chem Lett. 2018;28(6):1050–1055. doi: 10.1016/j.bmcl.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Dagne E., Bekele A. C -prenylated isoflavones from Millettia ferruginea. Phytochemistry. 1990;29(8):2679–2682. [Google Scholar]
- 21.Rukachaisirikul V., Ritthiwigrom T., Pinsa A., Sawangchote P., Taylor W.C. Xanthones from the stem bark of Garcinia nigrolineata. Phytochemistry. 2003;64(6):1149–1156. doi: 10.1016/s0031-9422(03)00502-8. [DOI] [PubMed] [Google Scholar]
- 22.Deyou T., Marco M., Heydenreich M., Pan F., Gruhonjic A., Fitzpatrick P.A. Isoflavones and rotenoids from the leaves of Millettia oblata ssp. teitensis. J Nat Prod. 2017;80(7):2060–2066. doi: 10.1021/acs.jnatprod.7b00255. [DOI] [PubMed] [Google Scholar]
- 23.Yenesew A., Midiwo J.O., Waterman† PG. 6-Methoxycalpogonium isoflavone a: a new isoflavone from the seed pods of Millettia dura. J Nat Prod. 1997;60(8):806–807. [Google Scholar]
- 24.Dagne E., Bekele A., Waterman P.G. The flavonoids of Millettia ferruginea subsp. ferruginea and subsp. darassana in ethiopia. Phytochemistry. 1989;28(7):1897–1900. [Google Scholar]
- 25.Yenesew A., Midiwo J.O., Waterman P.G. Four isoflavones from seed pods of Millettia dura. Phytochemistry. 1996;41(3):951–955. [Google Scholar]
- 26.Gong T., Wang D.X., Chen R.Y., Liu P., Yu D.Q. Novel benzil and isoflavone derivatives from Millettia dielsiana. Planta Med. 2009;75(3):236–242. doi: 10.1055/s-0028-1112203. [DOI] [PubMed] [Google Scholar]
- 27.Dagne E., Mammo W., Sterner O. Flavonoids of Tephrosia polyphylla. Phytochemistry. 1992;31(10):3662–3663. [Google Scholar]
- 28.Gong T., Zhang T., Wang D.X., Chen R.Y., Liu P., Yu D.Q. Two new isoflavone glycosides from the vine stem of Millettia dielsiana. J Asian Nat Prod Res. 2014;16(2):181–186. doi: 10.1080/10286020.2013.860967. [DOI] [PubMed] [Google Scholar]
- 29.Chuankhayan P., Hua Y., Svasti J., Sakdarat S., Sullivan P.A., Ketudat Cairns J.R. Purification of an isoflavonoid 7-O-beta-apiosyl-glucoside beta-glycosidase and its substrates from Dalbergia nigrescens Kurz. Phytochemistry. 2005;66(16):1880–1889. doi: 10.1016/j.phytochem.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Chuankhayan P., Rimlumduan T., Tantanuch W., Mothong N., Kongsaeree P.T., Metheenukul P. Functional and structural differences between isoflavonoid beta-glycosidases from Dalbergia sp. Arch Biochem Biophys. 2007;468(2):205–216. doi: 10.1016/j.abb.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Miyase T., Sano M., Nakai H., Muraoka M., Nakazawa M., Suzuki M. Antioxidants from Lespedeza homoloba. (I) Phytochemistry. 1999;52(2):303–310. doi: 10.1016/s0031-9422(99)00195-8. [DOI] [PubMed] [Google Scholar]
- 32.Malan E., Swinny E. Flavonoids and isoflavonoids from the heartwood of Virgilia oroboides. Phytochemistry. 1990;29(10):3307–3309. [Google Scholar]
- 33.Nayak M., Jung Y., Kim I. Syntheses of pterocarpenes and coumestans via regioselective cyclodehydration. Org Biomol Chem. 2016;14(34):8074–8087. doi: 10.1039/c6ob01451h. [DOI] [PubMed] [Google Scholar]
- 34.Feng Z.G., Bai W.J., Pettus T.R. Unified total syntheses of (-)-medicarpin, (-)-sophoracarpan A, and (±)-kushecarpin A with some structural revisions. Angew Chem Int Ed Engl. 2015;54(6):1864–1867. doi: 10.1002/anie.201408910. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Kader M.S. Phenolic constituents of Ononis vaginalis roots. Planta Med. 2001;67(4):388–390. doi: 10.1055/s-2001-14325. [DOI] [PubMed] [Google Scholar]
- 36.Calter M.A., Li N. Asymmetric total syntheses of (-)-variabilin and (-)-glycinol. Org Lett. 2011;13(14):3686–3689. doi: 10.1021/ol201332u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato-Noguchi H. Isolation and identification of an allelopathic substance in Pisum sativum. Phytochemistry. 2003;62(7):1141–1144. doi: 10.1016/s0031-9422(02)00673-8. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.S., Ryu Y.B., Curtis-Long M.J., Yuk H.J., Cho J.K., Kim J.Y. Flavanones and rotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem Toxicol. 2011;49(8):1849–1856. doi: 10.1016/j.fct.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 39.Fechner J., Kaufmann M., Herz C., Eisenschmidt D., Lamy E., Kroh L.W., Hanschen F.S. The major glucosinolate hydrolysis product in rocket (Eruca sativa L.), sativin, is 1,3-thiazepane-2-thione: Elucidation of structure, bioactivity, and stability compared to other rocket isothiocyanates. Food Chem. 2018;261:57–65. doi: 10.1016/j.foodchem.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Della G.M., Molinaro A., Monaco P., Previtera L. Neolignans from Arum italicum [rhizomes] Phytochemistry. 1994;35(3):777–779. [Google Scholar]
- 41.Jerković I., Hegić G., Marijanović Z., Bubalo D. Organic extractives from Mentha spp. honey and the bee-stomach: methyl syringate, vomifoliol, terpenediol I, hotrienol and other compounds. Molecules. 2010;15(4):2911–2924. doi: 10.3390/molecules15042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park E., Kim M.C., Choi C.W., Kim J., Jin H.S., Lee R. Effects of dihydrophaseic acid 3'-O-β-d-glucopyranoside isolated from Lycii radicis cortex on osteoblast differentiation. Molecules. 2016;21(9) doi: 10.3390/molecules21091260. pii : E1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alina U., Stefanie S., Philipp G., Corina I., Cristina A.I., Michael L. The Impact of soy isoflavones on MCF-7 and MDA-MB-231 breast cancer cells using a global metabolomic approach. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091443. pii: E1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao J.X., Huang G.Q., Geng X., Qiu X.W. Soy-derived isoflavones inhibit HeLa cell growth by inducing apoptosis. Plant Foods Hum Nutr. 2011;66(2):122–128. doi: 10.1007/s11130-011-0224-6. [DOI] [PubMed] [Google Scholar]
- 45.Huang H.C., Syu K.Y., Lin J.K. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J Agric Food Chem. 2010;58(15):8699–8708. doi: 10.1021/jf101003v. [DOI] [PubMed] [Google Scholar]
- 46.Kai J., Wei W., Xin J., Zhaoyang W., Zhiwei J., Guanmin M. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep. 2015;33(6):2711–2718. doi: 10.3892/or.2015.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson J., Dwyer J. Flavonoids: Dietary occurrence and biochemical activity. Nutr Res. 1998;18(12):1995–2018. [Google Scholar]
- 48.Klionsky D.J., Cuervo A.M., Seglen P.O. Methods for Monitoring Autophagy from Yeast to Human. Autophagy. 2007;3(3):181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.