Abstract
Regenerative medicine is a new and promising medical method aiming at treating patients with defective or dysfunctional tissues by maintaining or enhancing the biological activity of cells. The development of biomaterial-based technologies, such as cell scaffolds and carriers for drug delivery system, are highly required to promote the regenerative research and regenerative therapy. Nucleic acids are one of the most feasible factors to efficiently modify the biological activity of cells. The effective and stable delivery of nucleic acids into cells is highly required to succeed in the modification. Biomaterials-based non-viral carriers or biological carriers, like exosomes, play an important role in the efficient delivery of nucleic acids. This review introduces the examples of regenerative research and regenerative therapy based on the delivery of nucleic acids with biomaterials technologies and emphasizes their importance to accomplish regenerative medicine.
Keywords: Biomaterials, Drug delivery system, Cell scaffold, Nucleic acids, Regenerative research, Regenerative therapy
Abbreviations: CRISPR, clustered regularly interspaced short palindromic repeats; Cas, CRISPR-associated systems; DDS, drug delivery system; ECM, extracellular matrix; lncRNA, long non-coding RNA; miRNA, microRNA; mRNA, messenger RNA; MSC, mesenchymal stem cells; PEG, polyethylene glycol; PLGA, poly(d,l-lactic acid-co-glycolic acid); RISC, RNA-induced silencing complex; RNAi, RNA interferince; siRNA, small interfering RNA; TALEN, transcription activator-like effector nuclease; ZFN, zinc finger nucleases
Highlights
-
•
Modifying the activity of cells is important for regenerative medicine.
-
•
Various nucleic acids regulate gene expression to modify the activity of cells.
-
•
Intracellular delivery system is vital to the nucleic acids-based modification.
-
•
Biomaterials are useful for the intracellular delivery of nucleic acids.
1. Introduction
Regenerative medicine is to regenerate or repair the injured or lost tissues and substitute organ functions by maintain or augment the biological activity of cells in the site to be regenerated and repaired. It is highly expected that the regenerative medicine may resolve the problem of present advanced therapies, such as reconstruction therapy and organ transplantation.
There are two fields in regenerative medicine; regenerative research and regenerative therapy. The regenerative research scientifically supports the next generation of regenerative therapy and basically can be achieved through in vitro induction of cellular activity. Regenerative research composes of two objectives; the biological research of cells to make clear their activity for tissue regeneration and the drug discovery to efficiently evaluate drug action and toxicity with active cells. On the other hand, the objective of regenerative therapy is to treat diseases by the in vivo induction of cellular activity for tissue regeneration. In these contexts, it is important to develop technologies which enable to maintain or augment the biological activities of cells necessary for both the regenerative research and regenerative therapy.
The biological activity of cells is modulated by their interaction with bio-functional molecules and the surrounding microenvironment. For the successful modification of cellular biological activity, it is highly required to allow cells to effectively interact with bio-functional molecules and give good conditions for cells to augment the activities. Technologies based on biomaterials, defined as materials which are used contacting and interacting with the biological components, play an important role in the artificial modification of cellular biological activities for regenerative medicine.
In the case of modification of cellular biological activities by the bio-functional molecules, the biomaterials-based drug delivery system (DDS) technology can be utilized aiming at the effective delivery to the target cells. The objectives of DDS include the controlled release, the life-time extension, the accelerated permeation and absorption, and the targeting of drug. All of DDS objectives are applicable for the modification of cell biological activities based on the bio-functional molecules. The bio-functional molecules include low-molecular-weight substances, immune-stimulators, growth factors, antigens, agonists, antagonists, diagnostic agents, imaging probes, nucleic acids, and so on. The way how to interact with cells for the biological modification depends on the type of bio-functional molecules. For example, bio-signaling molecules of growth factors modify the biological activity of cells through the interaction with the receptors present on the surface of cells, followed by the induction of signaling pathways. On the other hand, several bio-functional molecules are required to be internalized into cells and interacted with intracellular substances to modify the biological activities of cells. Therefore, it is important to develop the DDS technology for the augmentation of cellular biological activities considering the manner of their interaction.
The biological activity of cells is also influenced by the surrounding microenvironment. It is well recognized that cells are present in the living tissue interacting with the extracellular matrix (ECM), which induces various types of cellular behaviors such proliferation, differentiation, and morphogenesis. Therefore, for the successful modification of cellular biological activities, it is indispensable to develop technologies to give cells a local microenvironment similar to the ECM (artificial scaffold) by making use of biomaterials. It has been demonstrated that the biological activity of cells is modified by various characteristics of scaffolds, such as the charge, wettability, roughness, biological affinity of their surface or the stiffness, porosity, and pore size of the bulk property [1], [2], [3]. The cell scaffolds of biomaterials are used alone or by combining with the DDS technology.
In this review, nucleic acids of a bio-functional molecule to modify the biological activity of cells are focused. The strategies and examples of regenerative research and regenerative therapy based on the delivery of nucleic acids with biomaterials technologies are introduced to emphasize the importance of biomaterials-based DDS to achieve regenerative medicine.
2. Nucleic acids for the biological modification of cell activities
There are several types of nucleic acids which are involved in the biological modification of cellular biological activity through the regulation of cellular gene expression. The supplementation of gene or the corresponding messenger RNA (mRNA) into cells can increase the level of gene expression. On the other hand, the phenomenon of RNA sequence-specific suppression of gene expression level, so-called RNA interference (RNAi), has been discovered in mammalian cells [4]. RNAi has been recognized as a phenomenon that mRNA is sequence-specifically degraded by several small double strand RNAs to suppress the biological activity of the corresponding protein. Based on the understanding of RNA interfering, two main types of small RNAs such as small interfering RNAs (siRNA) and microRNAs (miRNA) have been developed to suppress the level of gene expression [5]. These RNAs form the RNA-induced silencing complex (RISC) with Argonaute and other proteins, followed by the binding to the target mRNA. The siRNA binds to the target mRNA with the full complementarity and cleaves at the 10–12 bases from the 5′ end of binding site. The miRNA bind to the 3’ untranslated region to inhibit the translation. Antisense oligonucleotides have two mechanisms to suppress gene expression [6]. One is the hybridization to inhibit the role of ribosome, alter the splicing, and inhibit the activity of ribonucleoprotein. The other is the degradation of mRNA by the enzymes of RNase H and RISC. The level of gene expression is also suppressed by a decoy of nucleic acid with the same sequence for the interaction with the transcription factor [7]. Taken together, various kinds of nucleic acids enable cell to modify their biological activity via the regulation of gene expression level.
It is well recognized that the nucleic acids in the naked form cannot effectively exhibit their biological activities in the living systems. A lot of technologies have been developed for the DDS modification of nucleic acids. The general strategies on the design of DDS for nucleic acids have been reviewed in various literature [8], [9], [10], [11]. The DDS strategy of nucleic acids to achieve regenerative therapy and regenerative research is intensively introduced in the following sections.
3. Strategies of regenerative research with nucleic acid
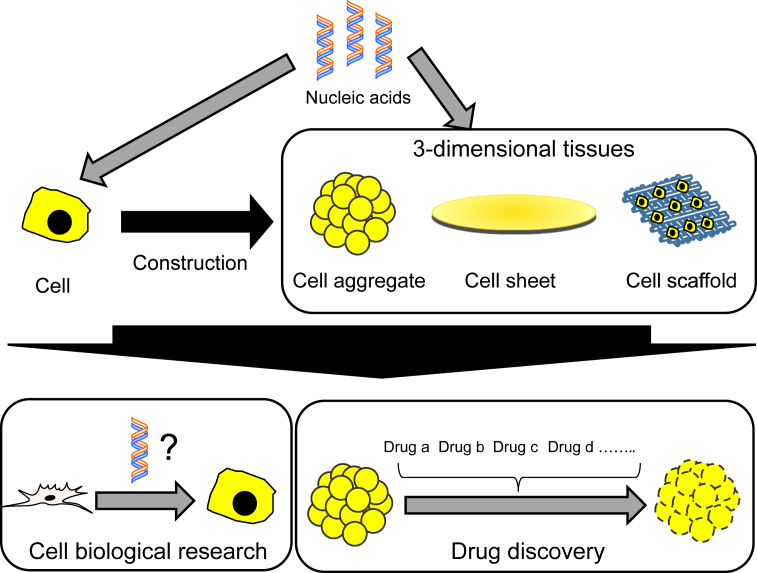
The objective of regenerative research is to create cells or their constructs having high biological activities with biomaterials in vitro and further develop the drug discovery and the basic research of cell biology. Regenerative research with nucleic acids is being proceeded based on the fact that the fate of cells is regulated by nucleic acids which regulate the cellular gene expression (Fig. 1). The efficient delivery of nucleic acids will be able to create cells with interesting activities or elucidate molecular mechanisms underlying the biological behavior of cells. For example, nucleic acids reprogramming and differentiation technologies can genetically engineer to create a disease cell by cell isolated from a disease patient [12]. Personalized drug discovery can be performed by using the disease cells created. In addition, recently, technologies to create a 3-demensional (3D) tissue in vitro by combining cell with the 3D scaffold of biomaterial have been actively developed [13], [14], [15]. It has been demonstrated that the biological activities of 3D tissue-like cell constructs are high compared with those of original cells [16], [17], [18], [19]. There are many approaches for the 3D construction of cells, which include cell aggregates, cell sheets, and cell microfabrication. Combination of DDS-modified nucleic acids with the 3D construct will further develop the research efficacy of drug discovery and cell biology. The imaging technology based on nucleic acids is also promising for the regenerative research. Modification with genes encoding fluorescent or luminescent proteins enables cells to demonstrate the distribution in the 3D construct, which is useful for the validation of 3D construct. When used in the drug discovery, the 3D construction of cells modified with a gene encoding a fusion protein of fluorescent protein and transcription factor, which is responsive to a biological phenomenon, enables the biological evaluation against drug candidates in the non-invasive manner.
Fig. 1.
The objective of regenerative research with nucleic acid is to effectively and stably deliver nucleic acids to cells or their 3D constructs in vitro by biomaterials-based DDS technologies. Cells or their 3D constructs modified with nucleic acids enable to promote the cell biological research and drug discovery.
4. Regenerative research based on nucleic acids of DDS
For the successful regenerative research with nucleic acids, it is indispensable to develop methods to efficient deliver the nucleic acid into cells of interest. Several biomaterials have been proposed for DDS of nucleic acids, which include cationized polymers [20], [21], [22], cationized liposomes [23], [24], [25], and ceramics [26]. However, since the biological expression of nucleic acids based on the carriers is transient, it is not always suitable to effectively carry out the regenerative research which is required to continue for long time period. As one trial, an intracellular controlled release of nucleic acid is considered to be promising.
We have prepared the nanoparticles of cationized gelatin hydrogel to succeed in the controlled release of nucleic acids inside cells [27]. The gene-carrying plasmid DNA incorporated in the nanoparticles was released in the cells with their degradation. When internalized into bone marrow-derived mesenchymal stem cells, the cationized gelatin hydrogel nanoparticles were intracellularly degraded with time and the plasmid DNA incorporated was released in the controlled fashion. The time period of plasmid DNA release depended on the degradation extent of nanoparticles. In addition, this intracellular release technology with the cationized gelatin nanoparticles also extended the duration of gene suppression of siRNA [28].
In addition to the intracellular controlled release technology, we have applied a reverse transfection procedure established by Sabatini et al. [29] for the regenerative research. In the conventional procedure, transfection of nucleic acid is performed by adding nucleic acid complexed with a carrier to the culture medium of cells to be transfected. The reverse transfection is the transfection culture on the substrate immobilized nucleic acid complexed with a carrier. It has been revealed that the reverse transfection procedure has three advantages over the conventional transfection procedures [30]. First, the reverse transfection procedure maintained a high level of biological expression even in the presence of serum, where the level was decreased by the conventional method. Next, the biological expression of nucleic acid was extended by the reverse transfection method to a significantly great extent compared with that of the conventional method. Third, the cellular viability after the reverse transfection method was significantly higher than that by the conventional method. Considering the procedure of reverse transfection, this transfection method can be applied for the 3D culture substrate. We have succeeded in the reverse transfection in/on a non-woven fabric of polyethylene terephthalate [30], poly(d,l-lactic acid-co-glycolic acid) (PLGA) scaffold [31], and gelatin sponge [32]. It is no doubt that the technology of transfection of nucleic acids in the 3D scaffold will contribute the active promotion of regenerative research.
5. Strategies of regenerative therapy with nucleic acids
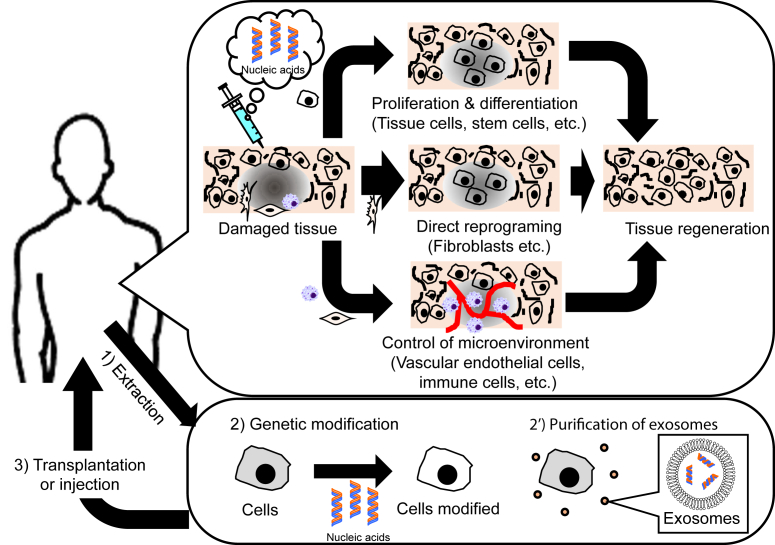
When the nucleic acid is applied for the regenerative therapy, the effective and stable delivery into the target cells in vivo is highly desired. There are two approaches for the regenerative therapy with nucleic acid (Fig. 2). One is to directly deliver nucleic acids to cells present in the body. Types of cells to be delivered and nucleic acids to deliver depend on the strategy of regenerative therapy. To deliver the nucleic acids to tissue or stem cells which should be proliferated or differentiated for cell-based tissue regeneration, genes encoding the corresponding growth factors are used as a nucleic acid [33]. Recently, it has been demonstrated that one or several transcription factors or miRNA can control the fate of cells. Especially, a biological phenomenon, called as “direct reprograming”, has been paid much attention. The direct reprogramming is the direct conversion from a terminally-differentiated cells to ones of different lineages. Many transcription factors and miRNA which enable cells to induce the direct reprogramming, have been discovered in various lineages [34]. The induction of “in vivo” direct reprogramming allows fibroblasts of a body cell which do not always contribute to the cell-based tissue regeneration.
Fig. 2.
There are two approaches for the regenerative therapy with nucleic acid; i) the direct delivery of nucleic acids to cells in vivo and ii) the transplantation of cells after extraction and modification with nucleic acids or the injection of exosomes from cells extracted.
As described in the Introduction section, the regulation of cellular microenvironment is crucial for cellular activity-based tissue regeneration. For example, angiogenesis plays an important role in tissue regeneration, because it enables cells to supply the oxygen and nutrient to the tissue as well as remove the wastes from the tissue. Many researches have been reported that the delivery of genes encoding angiogenic growth factors promotes the tissue regeneration [35]. In addition, recently much attention has been paid in the regulation of inflammatory environment governed by immune cells for the tissue regeneration [36]. Various types of immune cells, such as dendritic cells, macrophages, monocytes, neutrophils, and T cells etc., have been transfected with gene encoding factors affecting anti-inflammatory environments [37], [38], [39].
Cells transplantation is one of the most feasible approaches for the regeneration therapy since cells themselves have good therapeutic potentials in terms of their inherent targetability to the site injured or biological properties. However, the therapeutic efficacy of transplanted cells is not always as high as expected, which is one of the largest problems in cell therapy. This is because the survival ratio of transplanted cells is low, and consequently the biological activities of cells are not always achieved in the body as strongly as those expected from the in vitro activities. There are two approaches to tackle the problem by making use of nucleic acids. One is to create an angiogenic or anti-inflammatory microenvironment by the transfection of nucleic acids to resident cells as described above. The other is to prepare cells biologically modified with nucleic acids before their transplantation. Genes encoding humoral factors to promote the tissue regeneration or reduce the suppression of cell biological activity associated with transplantation, have been selected for nucleic acids [40], [41], [42].
The exosome is one of the extracellular vesicles secreted from cells and includes various intracellular biomolecules, such as proteins, lipids, and nucleic acids including non-coding RNAs, such as miRNA and long non-codingRNAs (lncRNAs) [43], [44]. The exosome is advantageous over other genetic substances in terms of the biological stability and specific information reflected to secreting cells. In addition, recently the intercellular communication by the exosome has been demonstrated [45], [46], [47]. In this context, the exosome can be expected to be a genetic substances used for gene-related therapeutic approaches [48]. Recently, trails on regenerative therapy have been performed by making use of exosomes obtained from somatic stem cells [49], [50].
For successful cell therapy, it is indispensable to elucidate the therapeutic mechanism of cells transplanted by utilizing imaging technologies. Delivery of genes encoding fluorescent or luminescent proteins and transporter proteins for the uptake of radioactive substances has been performed to visualize and trace of cells without any invasiveness [51], [52].
6. Regenerative therapy based on nucleic acids of DDS
Nanoparticle-based carriers, biodegradable polymer-based carriers, and biological carriers, such as cells and exosomes [53], [54], [55], [56], [57], [58], have been mainly used to achieve regenerative therapy based on nucleic acids. In this section, material design of these carriers and concrete examples of regenerative therapy are introduced.
Since naked nucleic acids cannot always function in biological systems, the active usage of DDS is highly required for the nucleic acid-based regenerative therapy. There are two types of administration procedures in regenerative medicine; local and systemic administrations. So far, few researches have been performed on the regenerative therapy based on the systemic administration of nucleic acids combined with DDS. In the case of local administration of nucleic acid, it is necessary to consider the technology how to avoid the diffusion away from the injection site and the degradation by nucleases. Complexation based on the electrostatic interaction or encapsulation of nucleic acids with biomaterial carriers has been explored as one trial to tackle these problems. The large size of complexes and the steric hindrance of biomaterial chains present on the surface of complexes can avoid the diffusion away from the injection site and the degradation by nucleases, respectively. For example, Uchida et al. have developed a nanomicelle carrier for mRNA [59]. The nanomicelle has an mRNA-containing inner core which is surrounded by the layer of polyethylene glycol (PEG). The PEG chain of nanomicelle makes mRNA stable even in the body. This nanomicelle system has been applied for the regenerative therapy in the brain as well as cartilage [60], [61]. In addition, polymeric nanoparticles and liposomes have been used for the in vivo effective delivery of nucleic acid to achieve the tissue regeneration [33], [62], [63], [64].
When injected into the body, a nucleic acid in the solution form rapidly diffused and disappeared from the injection site, resulting in the decreased biological activity. Considering the regenerative therapy based on the nucleic acid, the expression of biological activity induced by the nucleic acid in the target cells is highly required to maintain over several weeks or more. One of the promising ways is to make the nucleic acid to remain around the injection site and continuously act on the target cells. The controlled release technology of DDS enables a nucleic acid to maintain and release at the injection site in the controlled manner. This technology can reduce the adverse effects by the single bolus administration and the repeated administration. There are three types of carriers for controlled release; non-biodegradable polymers, biodegradable polymers, and ceramics. There are several studies on controlled release of drug with non-biodegradable materials [65], [66]. However, they are required to exclude after the complete release of incorporated drug. On the other hand, various biodegradable polymers have been used as carriers for the controlled release of nucleic acids, such as poly(lactic acid) [67], polyanhydride [68], poly(orthoester) [69], PLGA [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], poly(d,l-lactide-co-4-hydroxy-l-proline) [81], poly(1,8-octanediol-co-citrate) [82], oligo(poly(ethylene glycol) fumarate) [83], poly(2-aminoethyl propylene phosphate) [84], polypseudorotaxane [85], polysaccharide [86], silk elastin-like polymer [87], and atelocollagen [88], [89], [90]. It has been reported that some ceramics, such as calcium phosphate and calcium carbonates, are used for the controlled release of nucleic acid [91], [92]. By using the release system of nucleic acids with the biodegradable polymers, the regenerative therapy for various tissues has been tried, such as the bone formation [80], [93], the recovery of spinal cord [78], and angiogenesis [86], [94].
We have developed a cationized gelatin hydrogel as a carrier for the controlled release of nucleic acid. Cationization of gelatin was performed to electrostatically interact with the negatively-charged nucleic acids. In the same fashion as other gelatin hydrogels previously reported [95], [96], [97], the in vivo degradation of gelatin hydrogels can be controlled by their crosslinking extents, while the time profile of nucleic acid release was well corresponded to that of hydrogel degradation [98]. Different from other controlled release systems, nucleic acids are released from the hydrogels in the state of complex with the cationized gelatin fragments, which prevents the enzymatic degradation by nucleases. Nucleic acids complexed with the cationized gelatin fragments can be easily internalized into cells by the electrostatic interaction with the negatively charged cell surface. As a result, the augmented and extended biological expression of nucleic acid was achieved by the cationized gelatin hydrogel. We have confirmed that the profile of the hydrogel degradation and release of nucleic acid is not influenced by the shape and size of hydrogel [99]. So far, the controlled release system of nucleic acids with the cationized gelatin hydrogels has succeeded in the regeneration therapies for fibrosis [73], [100], [101], [102], [103], aortic aneurysms [104], and autoimmune alopecia [105].
The genetic engineering of somatic stem cells is also one of the promising approaches for regenerative therapy. Various methods to genetically engineer somatic stem cells have been reported [40], [41], [42]. Mesenchymal stem cells (MSC) of somatic stem cell, is a promising cell source and has been used for the regeneration of various tissues [106]. We have designed and prepared cationized pullulan as a non-viral carrier of nucleic acid for MSC [107]. The complexation with the cationized pullulan enabled a plasmid DNA to augment the expression level of MSC to a significantly high extent compared with that of LipofectAmine 2000® commercially available. The genetic engineering of MSC by cationized pullulan has demonstrated the efficient therapeutic effect for myocardiac infarction [108], brain diseases [109], liver fibrosis [110], cartilage regeneration [111], and wound repair [112], [113].
7. Closing remarks and future perspectives
In this review, materials, technologies, and methodologies for regenerative research and regenerative therapy by utilizing nucleic acids were summarized. There have been reported on several materials and methodologies based on nucleic acids, which will be useful and utilized in the future for regenerative research and regenerative therapy.
Genome editing is a methodology to achieve the deletion and insertion of specific sequence in the genome through the repair mechanisms (non-homologous end joining, homologous recombination, etc.) after DNA double strand break by the artificial restriction enzymes [114]. The restriction enzymes include zinc-finger nuclease (ZFN) [115], transcription activator-like effector nuclease (TALEN) [116], and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated systems (Cas) (CRISPR/Cas) [117]. It is no doubt that the genome editing methods will strongly contribute to the regenerative research.
Optogenetics are methodologies to control the biological activities of cells based on a light by the cellular expression of gene encoding the light-responsive protein. It has been demonstrated that the control of ion channel, second messenger, actin polymerization, and gene expression was achieved by this method [118]. The spatial control of biological activity for cells by light is advantageous over other methods. By utilizing this method, the preparation of 3D construct for the regenerative research, where biological activities of cells are spatially regulated by light, might be achieved.
With the progress of biology and chemistry on nucleic acid, various functional nucleic acids, which can regulate or detect the biological activities of cells through the specific interaction with target endogenous nucleic acids (mRNA or miRNA), have been easily designed and prepared. For example, Saito et al. have developed a technology to regulate or detect the biological activities by artificially creating functional RNA or its complex of protein [119], [120]. The technology to estimate the sequence and structure of nucleic acid necessary to interact with target nucleic acids has been already established. By this advantage, the functional nucleic acids to any target nucleic acid can be easily created. The versatile functional nucleic acids will strongly support the regenerative research.
In future, the technological fusion of genome editing, optogenetics, and functional nucleic acids with DDS will achieve a new strategy of regenerative medicine. It is no doubt that the DDS for nucleic acids is crucial to promote regenerative research and regenerative therapy. However, the DDS for nucleic acids is not always perfect and has some rooms to be improved technologically and methodologically. In fact, most of regenerative research and regenerative therapy with nucleic acids have been carried out by using viral carriers so far. For the promotion of regeneration research and regeneration therapy to the clinical level, it would be necessary to actively utilize the carriers of biomaterials which are being used in clinics and derived from the body component, such as extracellular vesicles [53], [54], [55], [56], [57], [58], may become required. In addition, it is important for several researchers with scientific backgrounds of materials, chemicals, physics, biology, and informatics to develop a new research field of regenerative medicine by efficient and substantial fusion to each other based on their own specialty.
Acknowledgement
The research is partly supported by the National Natural Science Foundation of China (81620108028).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Jafari M., Paknejad Z., Rad M.R., Motamedian S.R., Eghbal M.J., Nadjmi N. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater. 2017;105(2):431–459. doi: 10.1002/jbm.b.33547. [DOI] [PubMed] [Google Scholar]
- 2.Ghasemi-Mobarakeh L., Prabhakaran M.P., Tian L., Shamirzaei-Jeshvaghani E., Dehghani L., Ramakrishna S. Structural properties of scaffolds: crucial parameters towards stem cells differentiation. World J Stem Cell. 2015;7(4):728–744. doi: 10.4252/wjsc.v7.i4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carletti E., Motta A., Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol Biol. 2011;695:17–39. doi: 10.1007/978-1-60761-984-0_2. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Davidson B.L., McCray P.B., Jr. Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12(5):329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braasch D.A., Corey D.R. Novel antisense and peptide nucleic acid strategies for controlling gene expression. Biochemistry (Mosc) 2002;41(14):4503–4510. doi: 10.1021/bi0122112. [DOI] [PubMed] [Google Scholar]
- 7.Hecker M., Wagner A.H. Transcription factor decoy technology: a therapeutic update. Biochem Pharmacol. 2017;144:29–34. doi: 10.1016/j.bcp.2017.06.122. [DOI] [PubMed] [Google Scholar]
- 8.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 9.Wang W., Li W., Ma N., Steinhoff G. Non-viral gene delivery methods. Curr Pharmaceut Biotechnol. 2013;14(1):46–60. [PubMed] [Google Scholar]
- 10.Ni R., Zhou J., Hossain N., Chau Y. Virus-inspired nucleic acid delivery system: linking virus and viral mimicry. Adv Drug Deliv Rev. 2016;106(Pt A):3–26. doi: 10.1016/j.addr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Bakhtiar A., Sayyad M., Rosli R., Maruyama A., Chowdhury E.H. Intracellular delivery of potential therapeutic genes: prospects in cancer gene therapy. Curr Gene Ther. 2014;14(4):247–257. doi: 10.2174/1566523214666140612152730. [DOI] [PubMed] [Google Scholar]
- 12.Papp B., Plath K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Res. 2011;21(3):486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng W., Datta P., Ayan B., Ozbolat V., Sosnoski D., Ozbolat I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017;57:26–46. doi: 10.1016/j.actbio.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Katakam P., Dey B., Assaleh F.H., Hwisa N.T., Adiki S.K., Chandu B.R. Top-down and bottom-up approaches in 3D printing technologies for drug delivery challenges. Crit Rev Ther Drug Carrier Syst. 2015;32(1):61–87. doi: 10.1615/critrevtherdrugcarriersyst.2014011157. [DOI] [PubMed] [Google Scholar]
- 15.Peck Y., Wang D.A. Three-dimensionally engineered biomimetic tissue models for in vitro drug evaluation: delivery, efficacy and toxicity. Expert Opin Drug Deliv. 2013;10(3):369–383. doi: 10.1517/17425247.2013.751096. [DOI] [PubMed] [Google Scholar]
- 16.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103(5):389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda J., Sakai Y., Nakazawa K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials. 2006;27(7):1061–1070. doi: 10.1016/j.biomaterials.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Enriquez S., Gallardo-Perez J.C., Aviles-Salas A., Marin-Hernandez A., Carreno-Fuentes L., Maldonado-Lagunas V. Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol. 2008;216(1):189–197. doi: 10.1002/jcp.21392. [DOI] [PubMed] [Google Scholar]
- 19.Chen D.Y., Wei H.J., Lin W.W., Lin K.J., Huang C.C., Wu C.T. Intramuscular delivery of 3D aggregates of HUVECs and cbMSCs for cellular cardiomyoplasty in rats with myocardial infarction. J Control Release. 2013;172(2):419–425. doi: 10.1016/j.jconrel.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Liu C., Zhang N. Nanoparticles in gene therapy principles, prospects, and challenges. Prog Mol Biol Transl Sci. 2011;104:509–562. doi: 10.1016/B978-0-12-416020-0.00013-9. [DOI] [PubMed] [Google Scholar]
- 21.Itaka K., Kataoka K. Progress and prospects of polyplex nanomicelles for plasmid DNA delivery. Curr Gene Ther. 2011;11(6):457–465. doi: 10.2174/156652311798192879. [DOI] [PubMed] [Google Scholar]
- 22.Singha K., Namgung R., Kim W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Therapeut. 2011;21(3):133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong F., Mi Z., Gu N. Cationic liposomes as gene delivery system: transfection efficiency and new application. Pharmazie. 2011;66(3):158–164. [PubMed] [Google Scholar]
- 24.Tros de Ilarduya C., Sun Y., Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Ewert K.K., Zidovska A., Ahmad A., Bouxsein N.F., Evans H.M., McAllister C.S. Cationic liposome-nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNA. Top Curr Chem. 2010;296:191–226. doi: 10.1007/128_2010_70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas S.C., Harshita, Mishra P.K., Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharmaceut Des. 2015;21(42):6165–6188. doi: 10.2174/1381612821666151027153246. [DOI] [PubMed] [Google Scholar]
- 27.Doi N., Jo J.I., Tabata Y. Preparation of biodegradable gelatin nanospheres with a narrow size distribution for carrier of cellular internalization of plasmid DNA. J Biomater Sci Polym Ed. 2011;23(8):991–1004. doi: 10.1163/092050611X568214. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa H., Nakamura Y., Jo J., Tabata Y. Gelatin nanospheres incorporating siRNA for controlled intracellular release. Biomaterials. 2012;33(35):9097–9104. doi: 10.1016/j.biomaterials.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Ziauddin J., Sabatini D.M. Microarrays of cells expressing defined cDNAs. Nature. 2001;411(6833):107–110. doi: 10.1038/35075114. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki A., Jo J.I., Tabata Y. A reverse transfection technology to genetically engineer adult stem cells. Tissue Eng. 2007;13(2):245–251. doi: 10.1089/ten.2006.0185. [DOI] [PubMed] [Google Scholar]
- 31.Miao P.H., He C.X., Hu Y.L., Tabata Y., Gao J.Q., Hu Z.J. Impregnation of plasmid DNA into three-dimensional PLGA scaffold enhances DNA expression of mesenchymal stem cells in vitro. Pharmazie. 2012;67(3):229–232. [PubMed] [Google Scholar]
- 32.Kido Y., Jo J., Tabata Y. A gene transfection for rat mesenchymal stromal cells in biodegradable gelatin scaffolds containing cationized polysaccharides. Biomaterials. 2011;32(3):919–925. doi: 10.1016/j.biomaterials.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Fang Y.L., Chen X.G., W T.G. Gene delivery in tissue engineering and regenerative medicine. J Biomed Mater Res B Appl Biomater. 2015;103(8):1679–1699. doi: 10.1002/jbm.b.33354. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava D., DeWitt N. In Vivo cellular reprogramming: the next generation. Cell. 2016;166(6):1386–1396. doi: 10.1016/j.cell.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90(3):315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 36.Fatima F., Nawaz M. Nexus between extracellular vesicles, immunomodulation and tissue remodeling: for good or for bad? Ann Transl Med. 2017;5(6):139. doi: 10.21037/atm.2017.03.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eming S.A., Wynn T.A., Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 38.Smith T.D., Nagalla R.R., Chen E.Y., Liu W.F. Harnessing macrophage plasticity for tissue regeneration. Adv Drug Deliv Rev. 2017;114:193–205. doi: 10.1016/j.addr.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Dumont C.M., Park J., Shea L.D. Controlled release strategies for modulating immune responses to promote tissue regeneration. J Control Release. 2015;219:155–166. doi: 10.1016/j.jconrel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowakowski A., Walczak P., Lukomska B., Janowski M. Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cell Int. 2016;2016:4956063. doi: 10.1155/2016/4956063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng L.H., Fung K.P., Leung P.C., Gao J.Q. Genetically manipulated adult stem cells for wound healing. Drug Discov Today. 2011;16(21–22):957–966. doi: 10.1016/j.drudis.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Hodgkinson C.P., Gomez J.A., Mirotsou M., Dzau V.J. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21(11):1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ela S., Mager I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 44.Fatima F., Vesiculated M.N. Long non-coding RNAs: offshore packages deciphering trans-regulation between cells, cancer progression and resistance to therapies. Non-Coding RNA. 2017;3(1) doi: 10.3390/ncrna3010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mateescu B., Kowal E.J., van Balkom B.W., Bartel S., Bhattacharyya S.N., Buzas E.I. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles. 2017;6(1):1286095. doi: 10.1080/20013078.2017.1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nawaz M., Fatima F., Vallabhaneni K.C., Penfornis P., Valadi H., Ekstrom K. Extracellular vesicles: evolving factors in stem cell biology. Stem Cell Int. 2016;2016:1073140. doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fatima F., Ekstrom K., Nazarenko I., Maugeri M., Valadi H., Hill A.F. Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front Genet. 2017;8:161. doi: 10.3389/fgene.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Jong O.G., Van Balkom B.W., Schiffelers R.M., Bouten C.V., Verhaar M.C. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan A., Rajadas J., Seifalian A.M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65(3):357–367. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Allport J.R., Weissleder R. In vivo imaging of gene and cell therapies. Exp Hematol. 2001;29(11):1237–1246. doi: 10.1016/s0301-472x(01)00739-1. [DOI] [PubMed] [Google Scholar]
- 52.Kircher M.F., Gambhir S.S., Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8(11):677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 53.Seow Y., Wood M.J. Biological gene delivery vehicles: beyond viral vectors. Mol Ther. 2009;17(5):767–777. doi: 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeo R.W., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh B.J. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Lee Y., El Andaloussi S., Wood M.J. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 56.van der Meel R., Fens M.H., Vader P., van Solinge W.W., Eniola-Adefeso O., Schiffelers R.M. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 57.Fatima F., Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin J Canc. 2015;34(12):541–553. doi: 10.1186/s40880-015-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roma-Rodrigues C., Pereira F., Alves de Matos A.P., Fernandes M., Baptista P.V., Fernandes A.R. Smuggling gold nanoparticles across cell types - a new role for exosomes in gene silencing. Nanomedicine. 2017;13(4):1389–1398. doi: 10.1016/j.nano.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Uchida S., Itaka K., Uchida H., Hayakawa K., Ogata T., Ishii T. In vivo messenger RNA introduction into the central nervous system using polyplex nanomicelle. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin C.Y., Perche F., Ikegami M., Uchida S., Kataoka K., Itaka K. Messenger RNA-based therapeutics for brain diseases: an animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J Control Release. 2016;235:268–275. doi: 10.1016/j.jconrel.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Aini H., Itaka K., Fujisawa A., Uchida H., Uchida S., Fukushima S. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci Rep. 2016;6:18743. doi: 10.1038/srep18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galvez-Gastelum F.J., Garcia-Banuelos J.J., Beas-Zarate C., Segura-Flores A., Gonzalez H., Chaparro-Huerta V. Combinatorial gene therapy induces regression of hepatic encephalopathy. Gene Ther. 2011;18(1):88–94. doi: 10.1038/gt.2010.107. [DOI] [PubMed] [Google Scholar]
- 63.Chu Z., Sun Y., Kuan C.Y., Grabowski G.A., Qi X. Saposin C: neuronal effect and CNS delivery by liposomes. Ann N Y Acad Sci. 2005;1053:237–246. doi: 10.1196/annals.1344.021. [DOI] [PubMed] [Google Scholar]
- 64.Huang Y.C., Simmons C., Kaigler D., Rice K.G., Mooney D.J. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12(5):418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 65.Kajihara M., Sugie T., Mizuno M., Tamura N., Sano A., Fujioka K. Development of new drug delivery system for protein drugs using silicone (I) J Control Release. 2000;66(1):49–61. doi: 10.1016/s0168-3659(99)00257-6. [DOI] [PubMed] [Google Scholar]
- 66.Thakral S., Thakral N.K., Majumdar D.K. Eudragit: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–149. doi: 10.1517/17425247.2013.736962. [DOI] [PubMed] [Google Scholar]
- 67.Egilmez N.K., Jong Y.S., Sabel M.S., Jacob J.S., Mathiowitz E., Bankert R.B. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60(14):3832–3837. [PubMed] [Google Scholar]
- 68.Mathiowitz E., Jacob J.S., Jong Y.S., Carino G.P., Chickering D.E., Chaturvedi P. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen D.N., Raghavan S.S., Tashima L.M., Lin E.C., Fredette S.J., Langer R.S. Enhancement of poly(orthoester) microspheres for DNA vaccine delivery by blending with poly(ethylenimine) Biomaterials. 2008;29(18):2783–2793. doi: 10.1016/j.biomaterials.2008.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D., Robinson D.R., Kwon G.S., Samuel J. Encapsulation of plasmid DNA in biodegradable poly(D, L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. J Control Release. 1999;57(1):9–18. doi: 10.1016/s0168-3659(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 71.Tinsley-Bown A.M., Fretwell R., Dowsett A.B., Davis S.L., Farrar G.H. Formulation of poly(D,L-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. J Control Release. 2000;66(2–3):229–241. doi: 10.1016/s0168-3659(99)00275-8. [DOI] [PubMed] [Google Scholar]
- 72.Cohen H., Levy R.J., Gao J., Fishbein I., Kousaev V., Sosnowski S. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000;7(22):1896–1905. doi: 10.1038/sj.gt.3301318. [DOI] [PubMed] [Google Scholar]
- 73.Stern M., Ulrich K., Geddes D.M., Alton E.W. Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Ther. 2003;10(16):1282–1288. doi: 10.1038/sj.gt.3301994. [DOI] [PubMed] [Google Scholar]
- 74.Chun K.W., Cho K.C., Kim S.H., Jeong J.H., Park T.G. Controlled release of plasmid DNA from biodegradable scaffolds fabricated using a thermally-induced phase-separation method. J Biomater Sci Polym Ed. 2004;15(11):1341–1353. doi: 10.1163/1568562042368103. [DOI] [PubMed] [Google Scholar]
- 75.Diez S., Tros de Ilarduya C. Versatility of biodegradable poly(D,L-lactic-co-glycolic acid) microspheres for plasmid DNA delivery. Eur J Pharm Biopharm. 2006;63(2):188–197. doi: 10.1016/j.ejpb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Intra J., Salem A.K. Rational design, fabrication, characterization and in vitro testing of biodegradable microparticles that generate targeted and sustained transgene expression in HepG2 liver cells. J Drug Target. 2011;19(6):393–408. doi: 10.3109/1061186X.2010.504263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen M., Gao S., Dong M., Song J., Yang C., Howard K.A. Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery. ACS Nano. 2012;6(6):4835–4844. doi: 10.1021/nn300106t. [DOI] [PubMed] [Google Scholar]
- 78.Lowry N., Goderie S.K., Lederman P., Charniga C., Gooch M.R., Gracey K.D. The effect of long-term release of Shh from implanted biodegradable microspheres on recovery from spinal cord injury in mice. Biomaterials. 2012;33(10):2892–2901. doi: 10.1016/j.biomaterials.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 79.Needham C.J., Shah S.R., Mountziaris P.M., Kasper F.K., Mikos A.G. Modulation of polyplex release from biodegradable microparticles through poly(ethylenimine) modification and varying loading concentration. Pharm Res (N Y) 2014;31(1):77–85. doi: 10.1007/s11095-013-1133-1. [DOI] [PubMed] [Google Scholar]
- 80.Qiao C., Zhang K., Jin H., Miao L., Shi C., Liu X. Using poly(lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo. Int J Nanomed. 2013;8:2985–2995. doi: 10.2147/IJN.S45184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Z., Huang L. Sustained delivery and expression of plasmid DNA based on biodegradable polyester, poly(D,L-lactide-co-4-hydroxy-L-proline) J Control Release. 2004;98(3):437–446. doi: 10.1016/j.jconrel.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X.Q., Tang H., Hoshi R., De Laporte L., Qiu H., Xu X. Sustained transgene expression via citric acid-based polyester elastomers. Biomaterials. 2009;30(13):2632–2641. doi: 10.1016/j.biomaterials.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasper F.K., Seidlits S.K., Tang A., Crowther R.S., Carney D.H., Barry M.A. In vitro release of plasmid DNA from oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2005;104(3):521–539. doi: 10.1016/j.jconrel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Wang J., Zhang P.C., Mao H.Q., Leong K.W. Enhanced gene expression in mouse muscle by sustained release of plasmid DNA using PPE-EA as a carrier. Gene Ther. 2002;9(18):1254–1261. doi: 10.1038/sj.gt.3301794. [DOI] [PubMed] [Google Scholar]
- 85.Li Z., Yin H., Zhang Z., Liu K.L., Li J. Supramolecular anchoring of DNA polyplexes in cyclodextrin-based polypseudorotaxane hydrogels for sustained gene delivery. Biomacromolecules. 2012;13(10):3162–3172. doi: 10.1021/bm300936x. [DOI] [PubMed] [Google Scholar]
- 86.Kong H.J., Kim E.S., Huang Y.C., Mooney D.J. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res (N Y) 2008;25(5):1230–1238. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 87.Gustafson J., Greish K., Frandsen J., Cappello J., Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: from molecular definition to controlled gene expression. J Control Release. 2009;140(3):256–261. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ochiya T., Nagahara S., Sano A., Itoh H., Terada M. Biomaterials for gene delivery: atelocollagen-mediated controlled release of molecular medicines. Curr Gene Ther. 2001;1(1):31–52. doi: 10.2174/1566523013348887. [DOI] [PubMed] [Google Scholar]
- 89.Ochiya T., Takahama Y., Nagahara S., Sumita Y., Hisada A., Itoh H. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat Med. 1999;5(6):707–710. doi: 10.1038/9560. [DOI] [PubMed] [Google Scholar]
- 90.Sano A., Maeda M., Nagahara S., Ochiya T., Honma K., Itoh H. Atelocollagen for protein and gene delivery. Adv Drug Deliv Rev. 2003;55(12):1651–1677. doi: 10.1016/j.addr.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 91.Choi S., Murphy W.L. Sustained plasmid DNA release from dissolving mineral coatings. Acta Biomater. 2010;6(9):3426–3435. doi: 10.1016/j.actbio.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uskokovic V., Uskokovic D.P. Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J Biomed Mater Res B Appl Biomater. 2011;96(1):152–191. doi: 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- 93.Chew S.A., Kretlow J.D., Spicer P.P., Edwards A.W., Baggett L.S., Tabata Y. Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model. Tissue Eng. 2011;17(5–6):751–763. doi: 10.1089/ten.tea.2010.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kasahara H., Tanaka E., Fukuyama N., Sato E., Sakamoto H., Tabata Y. Biodegradable gelatin hydrogel potentiates the angiogenic effect of fibroblast growth factor 4 plasmid in rabbit hindlimb ischemia. J Am Coll Cardiol. 2003;41(6):1056–1062. doi: 10.1016/s0735-1097(02)03007-3. [DOI] [PubMed] [Google Scholar]
- 95.Ozeki M., Tabata Y. In vivo degradability of hydrogels prepared from different gelatins by various cross-linking methods. J Biomater Sci Polym. 2005;16(5):549–561. doi: 10.1163/1568562053783731. [DOI] [PubMed] [Google Scholar]
- 96.Young S., Wong M., Tabata Y., Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1–3):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 97.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6(Suppl 3):S311–S324. doi: 10.1098/rsif.2008.0448.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fukunaka Y., Iwanaga K., Morimoto K., Kakemi M., Tabata Y. Controlled release of plasmid DNA from cationized gelatin hydrogels based on hydrogel degradation. J Control Release. 2002;80(1–3):333–343. doi: 10.1016/s0168-3659(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 99.Kushibiki T., Matsumoto K., Nakamura T., Tabata Y. Suppression of the progress of disseminated pancreatic cancer cells by NK4 plasmid DNA released from cationized gelatin microspheres. Pharm Res (N Y) 2004;21(7):1109–1118. doi: 10.1023/b:pham.0000032996.95518.dc. [DOI] [PubMed] [Google Scholar]
- 100.Aoyama T., Yamamoto S., Kanematsu A., Ogawa O., Tabata Y. Local delivery of matrix metalloproteinase gene prevents the onset of renal sclerosis in streptozotocin-induced diabetic mice. Tissue Eng. 2003;9(6):1289–1299. doi: 10.1089/10763270360728206. [DOI] [PubMed] [Google Scholar]
- 101.Lin X., Jo H., Ishii T.M., Fujita M., Fu M., Tambara K. Controlled release of matrix metalloproteinase-1 plasmid DNA prevents left ventricular remodeling in chronic myocardial infarction of rats. Circ J. 2009;73(12):2315–2321. doi: 10.1253/circj.cj-09-0379. [DOI] [PubMed] [Google Scholar]
- 102.Wu T.H., Hsu S.H., Chang M.H., Huang Y.Y. Reducing scar formation by regulation of IL-1 and MMP-9 expression by using sustained release of prednisolone-loaded PDLL microspheres in a murine wound model. J Biomed Mater Res A. 2013;101(4):1165–1172. doi: 10.1002/jbm.a.34413. [DOI] [PubMed] [Google Scholar]
- 103.Xia Z., Abe K., Furusu A., Miyazaki M., Obata Y., Tabata Y. Suppression of renal tubulointerstitial fibrosis by small interfering RNA targeting heat shock protein 47. Am J Nephrol. 2008;28(1):34–46. doi: 10.1159/000108759. [DOI] [PubMed] [Google Scholar]
- 104.Zhong H., Matsui O., Xu K., Ogi T., Sanada J., Okamoto Y. Gene transduction into aortic wall using plasmid-loaded cationized gelatin hydrogel-coated polyester stent graft. J Vasc Surg. 2009;50(6):1433–1443. doi: 10.1016/j.jvs.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 105.Nakamura M., Jo J., Tabata Y., Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (Alopecia Areata) in a C3H/HeJ mouse model. Am J Pathol. 2008;172(3):650–658. doi: 10.2353/ajpath.2008.061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohban R., Pieber T.R. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cell Int. 2017;2017:5173732. doi: 10.1155/2017/5173732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jo J., Tabata Y. Non-viral gene transfection technologies for genetic engineering of stem cells. Eur J Pharm Biopharm. 2008;68(1):90–104. doi: 10.1016/j.ejpb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 108.Jo J., Nagaya N., Miyahara Y., Kataoka M., Harada-Shiba M., Kangawa K. Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial infarction: benefit of a novel nonviral vector, cationized dextran. Tissue Eng. 2007;13(2):313–322. doi: 10.1089/ten.2006.0133. [DOI] [PubMed] [Google Scholar]
- 109.Huang B., Tabata Y., Gao J.Q. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Control Release. 2012;162(2):464–473. doi: 10.1016/j.jconrel.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 110.Ishikawa H., Jo J., Tabata Y. Liver anti-fibrosis therapy with mesenchymal stem cells secreting hepatocyte growth factor. J Biomater Sci Polym Ed. 2012;23(18):2259–2272. doi: 10.1163/156856211X614761. [DOI] [PubMed] [Google Scholar]
- 111.He C.X., Zhang T.Y., Miao P.H., Hu Z.J., Han M., Tabata Y. TGF-beta1 gene-engineered mesenchymal stem cells induce rat cartilage regeneration using nonviral gene vector. Biotechnol Appl Biochem. 2012;59(3):163–169. doi: 10.1002/bab.1001. [DOI] [PubMed] [Google Scholar]
- 112.Peng L.H., Mao Z.Y., Qi X.T., Chen X., Li N., Tabata Y. Transplantation of bone-marrow-derived mesenchymal and epidermal stem cells contribute to wound healing with different regenerative features. Cell Tissue Res. 2013;352(3):573–583. doi: 10.1007/s00441-013-1609-7. [DOI] [PubMed] [Google Scholar]
- 113.Nakamura Y., Ishikawa H., Kawai K., Tabata Y., Suzuki S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials. 2013;34(37):9393–9400. doi: 10.1016/j.biomaterials.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 114.Hu J.H., Davis K.M., Liu D.R. Chemical biology approaches to genome editing: understanding, controlling, and delivering programmable nucleases. Cell Chem Biol. 2016;23(1):57–73. doi: 10.1016/j.chembiol.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 115.Bibikova M., Beumer K., Trautman J.K., Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300(5620):764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 116.Bogdanove A.J., Voytas D.F. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333(6051):1843–1846. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- 117.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 119.Karagiannis P., Fujita Y., Saito H. RNA-based gene circuits for cell regulation. Proc Jpn Acad Ser B Phys Biol Sci. 2016;92(9):412–422. doi: 10.2183/pjab.92.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ohno H., Saito H. RNA and RNP as building blocks for nanotechnology and synthetic biology. Prog Mol Biol Transl Sci. 2016;139:165–185. doi: 10.1016/bs.pmbts.2015.12.004. [DOI] [PubMed] [Google Scholar]