Abstract
Sensitive and specific monoclonal antibodies (mAbs) against not only human but also mouse, rat, rabbit, dog, cat, bovine, pig, and horse podoplanins (PDPNs) have been established in our previous studies. However, anti-goat PDPN (gPDPN) has not been established yet. PDPN has been utilized as a lymphatic endothelial cell marker especially in pathological diagnoses; therefore, mAbs for immunohistochemical analyses using formalin-fixed paraffin-embedded tissues are needed. Although we recently demonstrated that an anti-bovine PDPN mAb, PMab-44 cross-reacted with gPDPN, PMab-44 did not detect lymphatic endothelial cells in immunohistochemistry. In this study, we immunized mice with gPDPN-overexpressing Chinese hamster ovary (CHO)–K1 (CHO/gPDPN) cells, and screened mAbs against gPDPN using flow cytometry. One of the mAbs, PMab-235 (IgG1, kappa), specifically detected CHO/gPDPN cells by flow cytometry. Furthermore, PMab-235 strongly detected lung type I alveolar cells, renal podocytes, and lymphatic endothelial cells of colon by immunohistochemistry. These findings suggest that PMab-235 may be useful as a lymphatic endothelial cell marker for goat tissues.
Keywords: Lymphatic endothelial cells, PMab-235, Goat podoplanin, PDPN
1. Introduction
Podoplanin (PDPN)/T1alpha/Aggrus is a type I transmembrane sialo-glycoprotein, which is expressed in many cell types, such as pulmonary type I alveolar cells, renal podocytes, mesothelial cells, and epithelial cells or lymphatic endothelial cells of many organs [1]. PDPN could induce platelet aggregation by binding to the endogenous receptor of PDPN, C-type lectin-like receptor-2 (CLEC-2) [2]. PDPN has been used to distinguish lymphatic endothelial cells from vascular endothelial cells in pathophysiological studies [3]. The PDPN-CLEC-2 interaction facilitates the separation of lymphatic vessels and embryonic blood [4]. Our previous analyses of glycopeptides produced by Edman degradation and mass spectrometry demonstrated that the disialyl-core1 (NeuAcα2-3Gal β1-3(NeuAcα2-6)GalNAcα1-O-Thr) structure was attached to a glycosylation site at residue Thr52 of human PDPN (hPDPN) [5]. Sialic acid-deficient PDPN recovered its activity after additional sialylation, indicating that the sialylated core1 of Thr52 is critical for PDPN-induced platelet aggregation.
The expression of hPDPN has been reported in many malignant tumors, including malignant oral squamous cell carcinomas [6], lung squamous cell carcinomas [7], esophageal squamous cell carcinomas [8], malignant mesotheliomas [9], osteosarcomas [10], chondrosarcomas [11], brain tumors [12], and seminomas of testicular tumors [13]. Our previous studies demonstrated that PDPN expression in Chinese hamster ovary (CHO)–K1 cells promoted pulmonary metastasis in both an experimental and a spontaneous mouse model [14]. No differences in the size of metastatic foci or in primary tumor growth were found in either set of mice. PDPN-expressing cells, which were covered with platelets, were found to be arrested in the lung microvasculature 30 minutes after injection. Furthermore, lung metastasis resulting from PDPN expression decreased the survival of the mice. We showed that point mutation at the platelet aggregation-stimulating (PLAG) domain of hPDPN lost both platelet aggregation and metastasis. Inhibition of platelets with aspirin reduced the formation of PDPN-promoted metastasis, indicating that PDPN contributes to the establishment of metastasis by promoting platelet aggregation without affecting subsequent growth.
We have developed monoclonal antibodies (mAbs) against not only human [12], mouse [15], rat [16], rabbit [17], but also bovine [18], dog [19], cat [20], pig [21], horse [22], Tasmanian devil [23], alpaca [24], tiger [25], and bear [26] PDPNs. About anti-hPDPN mAbs, we established cancer-specific mAbs (CasMabs), such as LpMab-2 [27] and LpMab-23 [28,29]. An anti-dog PDPN (dPDPN), PMab-38 also showed cancer-specificity [19, 30, 31]. PMab-44 (an anti-bovine PDPN (bovPDPN) mAb), PMab-52 (an anti-cat PDPN (cPDPN) mAb), PMab-213 (an anti-pig PDPN (pPDPN) mAb), PMab-219 (an anti-horse PDPN (horPDPN) mAb), PMab-233 (an anti-Tasmanian devil PDPN (tasPDPN) mAb), PMab-225 (an anti-alpaca PDPN (aPDPN) mAb), PMab-231 (an anti-tiger PDPN (tigPDPN) mAb), and PMab-247 (an anti-bear PDPN (bPDPN) mAb) have been shown to be useful for flow cytometry, Western blot, and immunohistochemical analyses [18, 20, 21, 22, 23, 24, 25, 26]. In this study, we immunized mice with CHO/goat PDPN (gPDPN) cells and established mAbs against gPDPN.
2. Materials and methods
2.1. Cell lines
Ch 1 Es, a fibroblastic cell line from normal fetal goat esophagus was obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). P3X63Ag8U.1 (P3U1) and CHO–K1 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Synthesized DNA (Eurofins Genomics KK, Tokyo, Japan) encoding gPDPN (accession No.: XM_005690821.3) plus an N-terminal MAP16 tag (PGTGDGMVPPGIEDKI), which is recognized by an anti-MAP16 tag mAb (PMab-1: the same mAb against MAP tag [32]), was subcloned into a pCAG-Neo vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). Plasmids were transfected using Lipofectamine LTX with Plus Reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA). Stable transfectants were selected by limiting dilution and cultivation in a medium containing 0.5 mg/ml of G418 (Nacalai Tesque, Inc., Kyoto, Japan).
The P3U1, CHO–K1, CHO/gPDPN, CHO/hPDPN [33], CHO/mouse PDPN (mPDPN) [33], CHO/rat PDPN (rPDPN) [16], CHO/rabbit PDPN (rabPDPN) [17], CHO/dPDPN [19], CHO/bovPDPN [18], CHO/cPDPN [20], CHO/pPDPN [21], CHO/horPDPN [22], CHO/tigPDPN [25], CHO/aPDPN [24], CHO/bPDPN [26], CHO/tasPDPN [23], CHO/sheep PDPN (sPDPN) [34], and CHO/whale PDPN (wPDPN) [35] were cultured in a Roswell Park Memorial Institute (RPMI) 1640 medium (Nacalai Tesque, Inc.), supplemented with 10% of heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). Ch 1 Es was cultured in E-MEM with Earle's Salts, L-Gln and Non-Essential Amino Acids (Nacalai Tesque, Inc.), supplemented with 10% of heat-inactivated FBS (Thermo Fisher Scientific Inc.), 100 units/mL of penicillin, and 100 μg/mL of streptomycin (Nacalai Tesque, Inc.). The cells were grown in an incubator at 37 °C with humidity and 5% CO2 and 95% air atmosphere.
2.2. Hybridoma production
Female BALB/c mice (6 weeks of age) were purchased from CLEA Japan (Tokyo, Japan). The animals were housed under specific pathogen-free conditions. The Animal Care and Use Committee of Tohoku University approved all animal experiments. We used a Cell-Based Immunization and Screening (CBIS) method [21, 22, 23, 24, 25] to develop mAbs against gPDPN. Briefly, one BALB/c mouse was immunized with CHO/gPDPN cells (1 × 108) intraperitoneally (i.p.) together with the Imject Alum (Thermo Fisher Scientific Inc.). The procedure included three additional immunizations, followed by a final booster injection administered i.p. 2 days prior to the harvest of spleen cells. Subsequently, these spleen cells were fused with P3U1 cells using PEG1500 (Roche Diagnostics, Indianapolis, IN, USA), and the hybridomas were grown in an RPMI medium supplemented with hypoxanthine, aminopterin, and thymidine (HAT) for selection (Thermo Fisher Scientific Inc.). The culture supernatants were screened by flow cytometry.
2.3. Flow cytometry
The cells were harvested following a brief exposure to 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). The cells were washed with 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and treated with primary mAbs for 30 min at 4 °C. Thereafter, the cells were treated with Alexa Fluor 488-conjugated anti-mouse IgG (1:2000; Cell Signaling Technology, Inc., Danvers, MA, USA) or Oregon Green anti-rat IgG (1:2000; Thermo Fisher Scientific Inc.). Then, fluorescence data were collected using the SA3800 Cell Analyzer (Sony Corp., Tokyo, Japan).
2.4. Determination of binding affinity by flow cytometry
CHO/gPDPN was suspended in 100 μL of serially diluted PMab-235. Then, Alexa Fluor 488-conjugated anti-mouse IgG (1:200; Cell Signaling Technology, Inc.) was added. Fluorescence data were collected using the EC800 Cell Analyzer (Sony Corp.). The dissociation constant (KD) was calculated by fitting the binding isotherms to built-in one-site binding models in the GraphPad PRISM 6 (GraphPad Software, Inc., La Jolla, CA, USA).
2.5. Immunohistochemical analyses
Goat tissues were collected in NIPPON ZENYAKU KOGYO CO., LTD. (Fukushima, Japan), fixed in 4%-Paraformaldehyde Phosphate Buffer Solution (Nacalai Tesque, Inc.). Histological sections (4-μm thick) were directly autoclaved in a citrate buffer (pH 6.0; Nichirei Biosciences, Inc., Tokyo, Japan) for 20 min, and were blocked using the SuperBlock T20 (PBS) Blocking Buffer (Thermo Fisher Scientific Inc.), incubated with PMab-235 (1 μg/mL) or PMab-44 (1 μg/mL) for 1 h at the room temperature, and then treated with the Envision + Kit for mouse (Agilent Technologies Inc. Santa Clara, CA, USA) for 30 min. Color was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Agilent Technologies Inc.) for 2 min, and counterstaining was performed using hematoxylin (FUJIFILM Wako Pure Chemical Corporation).
3. Results
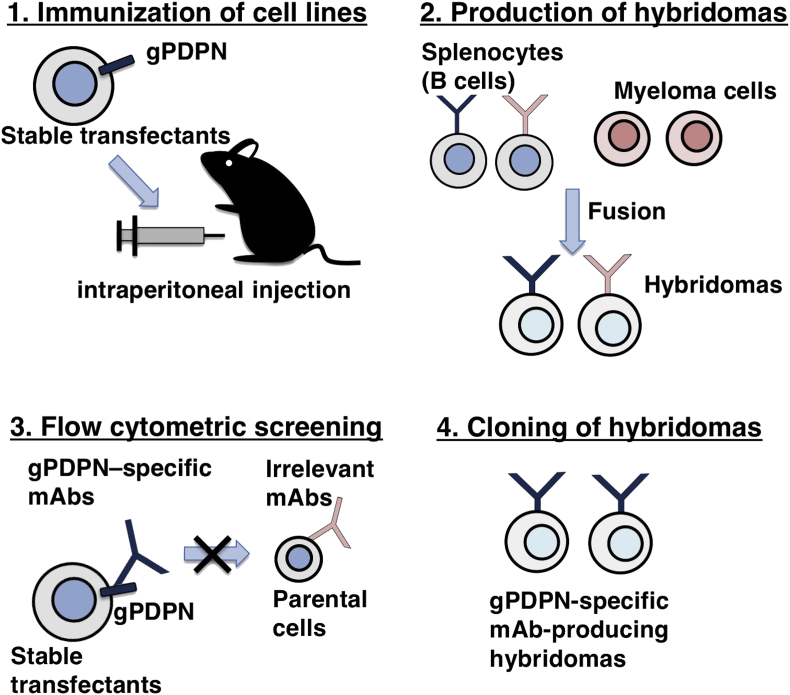
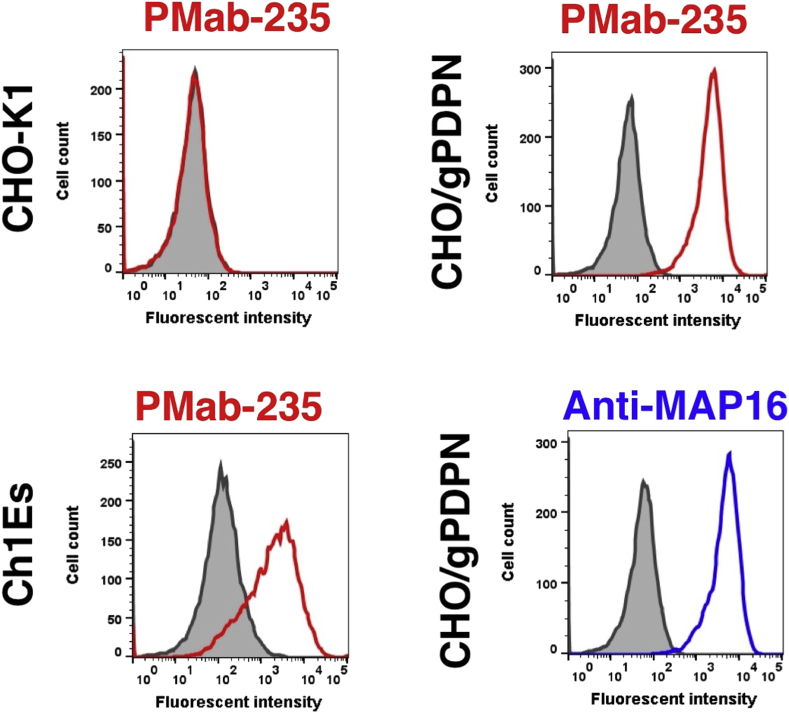
One mouse was immunized with CHO/gPDPN cells (Fig. 1). The developed hybridomas were seeded into 96-well plates and cultivated for 10 days. Wells positive for CHO/gPDPN and negative for CHO–K1 were selected by flow cytometry. The screening approach identified strong signals from CHO/gPDPN cells and weak or no signals from CHO–K1 cells in 29 of the 480 wells (6.0%). PMab-235 (IgG1, kappa) was finally selected via flow cytometry against Ch 1 Es, a fibroblastic cell line from normal fetal goat esophagus (Fig. 2). PMab-235 recognized CHO/gPDPN cells, but showed no reaction with CHO–K1 cells, as assessed by flow cytometry (Fig. 2). As a positive control, an anti-MAP16 tag (clone PMab-1) reacted with CHO/gPDPN.
Fig. 1.
Schematic illustration of the Cell-Based Immunization and Screening (CBIS) method. Stable transfectants expressing the protein of interest are used as an immunogen with no purification procedure. The selection of hybridomas secreting specific mAbs is performed by flow cytometry using parental and transfectant cells.
Fig. 2.
Detection of gPDPN by flow cytometry using PMab-235. CHO/gPDPN, CHO–K1, and Ch 1 Es cells were treated with PMab-235 (red line) or anti-MAP16 tag (PMab-1; blue line) at a concentration of 1 μg/mL or 0.1% BSA in PBS (gray) for 30 min, followed by incubation with secondary antibodies.
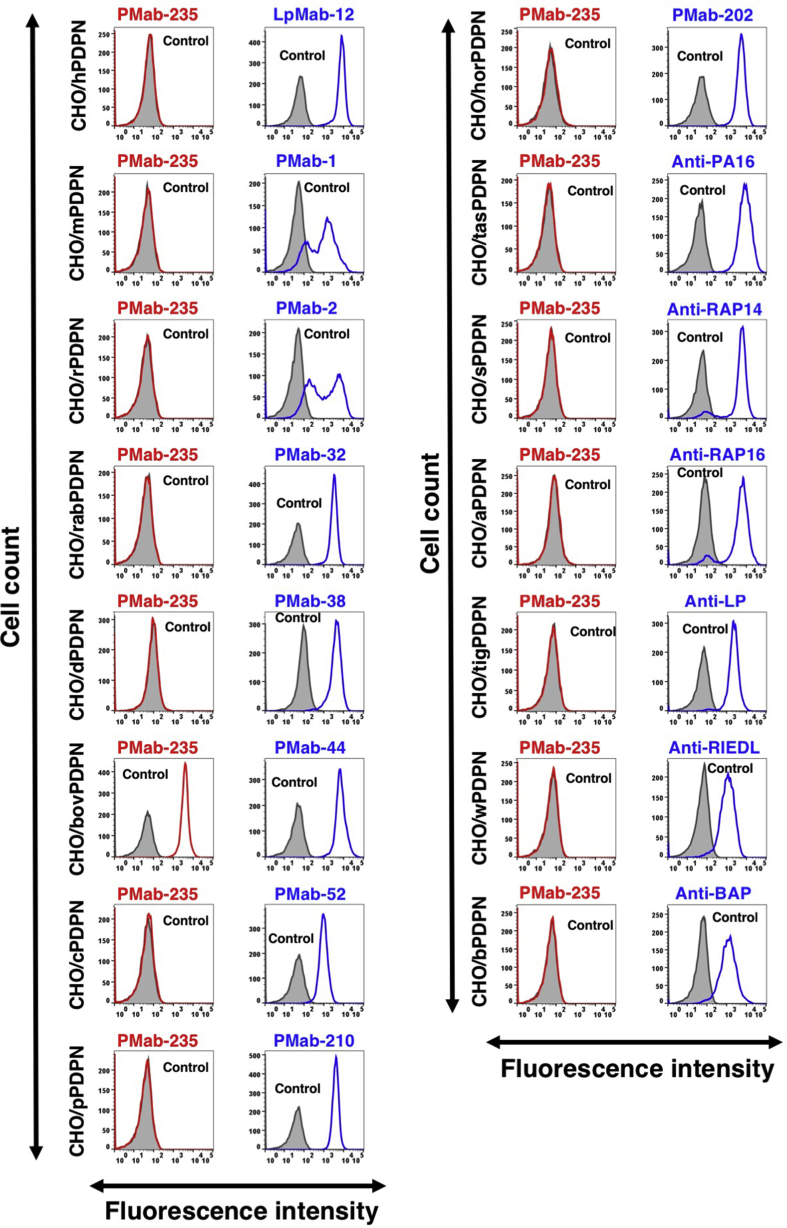
We next investigated the crossreactivity of PMab-235 with PDPNs of the other species. PDPN expression levels in CHO cell lines were confirmed by positive controls, such as anti-PDPN mAbs or anti-peptide tag mAbs. We used clone LpMab-12 [36] as an anti-hPDPN mAb; clone PMab-1 [15] as an anti-mPDPN mAb; clone PMab-2 [16] as an anti-rPDPN mAb; clone PMab-32 [17] as an anti-rabPDPN mAb; clone PMab-38 [19] as an anti-dPDPN mAb; clone PMab-44 [18] as an anti-bovPDPN mAb; clone PMab-52 [20] as an anti-cPDPN mAb; clone PMab-210 [35] as an anti-pPDPN mAb; and clone PMab-202 [37] as an anti-horPDPN mAb. We also used clone NZ-1 as an anti-PA16 tag (GLEGGVAMPGAEDDVV) mAb; clone PMab-2 as an anti-RAP14 tag (DMVNPGLEDRIEDL) mAb; clone PMab-2 as an anti-RAP16 tag (GPGDDMVNPGLEDRIE) mAb; clone LpMab-17 as an anti-LP tag (NSVTGIRIEDLPTSES) mAb; clone LpMab-7 as an anti-RIEDL tag (RIEDL) mAb; and clone PMab-44 as an anti-BAP tag (EKTTLGVEDYTTTPAA) mAb. All positive control mAbs showed high reactivity for each PDPN-overexpressed CHO cell lines, such as human, mouse, rat, rabbit, dog, bovine, cat, pig, horse, Tasmanian devil, sheep, alpaca, tiger, whale, or bear PDPNs by flow cytometry (Fig. 3). PMab-235 crossreacted with only bovine PDPN, and did not react with human, mouse, rat, rabbit, dog, cat, pig, horse, Tasmanian devil, sheep, alpaca, tiger, whale, or bear PDPNs by flow cytometry (Fig. 3).
Fig. 3.
Cross-reaction of PMab-235 to PDPNs of the other species by flow cytometry. CHO–K1 cells transfected with PDPNs of the other species were treated with PMab-235 (red line) or each positive control (blue line) at a concentration of 1 μg/mL or 0.1% BSA in PBS (gray) for 30 min, followed by incubation with secondary antibodies.
In addition, kinetic analysis conducted by flow cytometry was employed to assess the interaction of PMab-235 with CHO/gPDPN cells. KD of PMab-235 for CHO/gPDPN cells was determined to be 1.5 × 10−8 M, indicating a moderate affinity of PMab-235 for CHO/gPDPN cells.
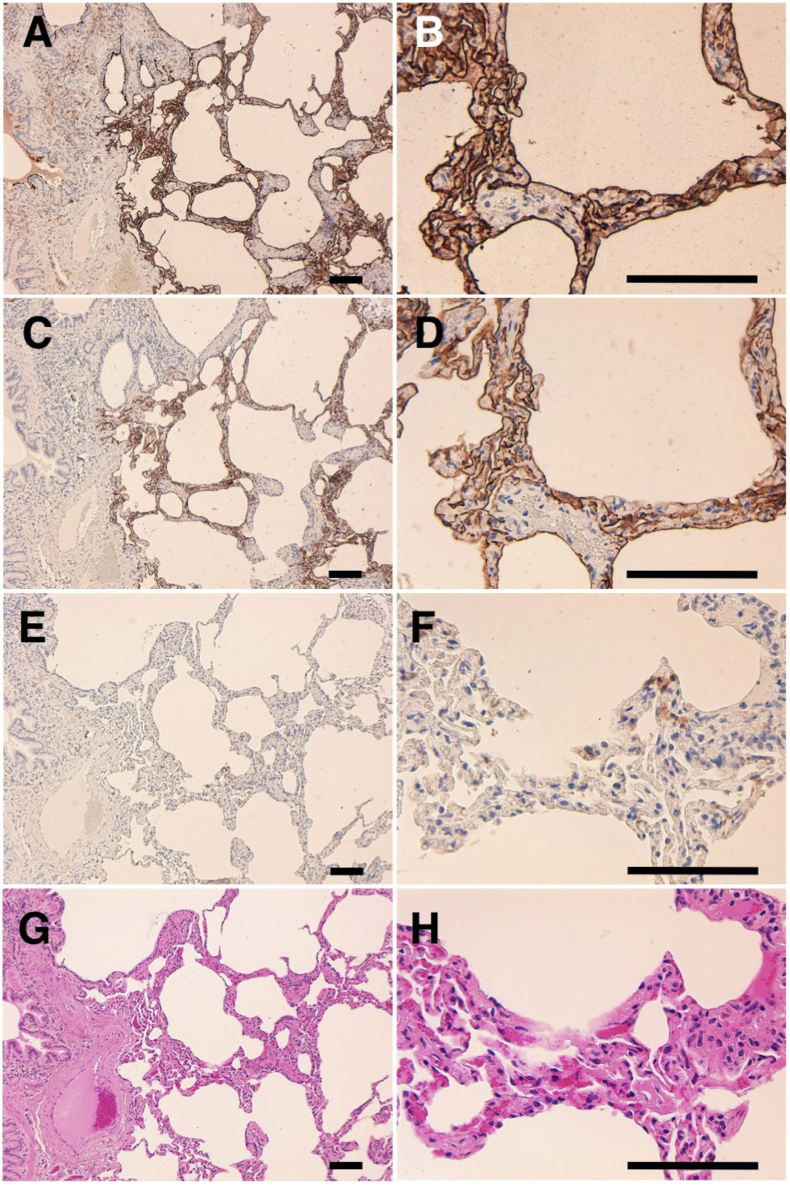
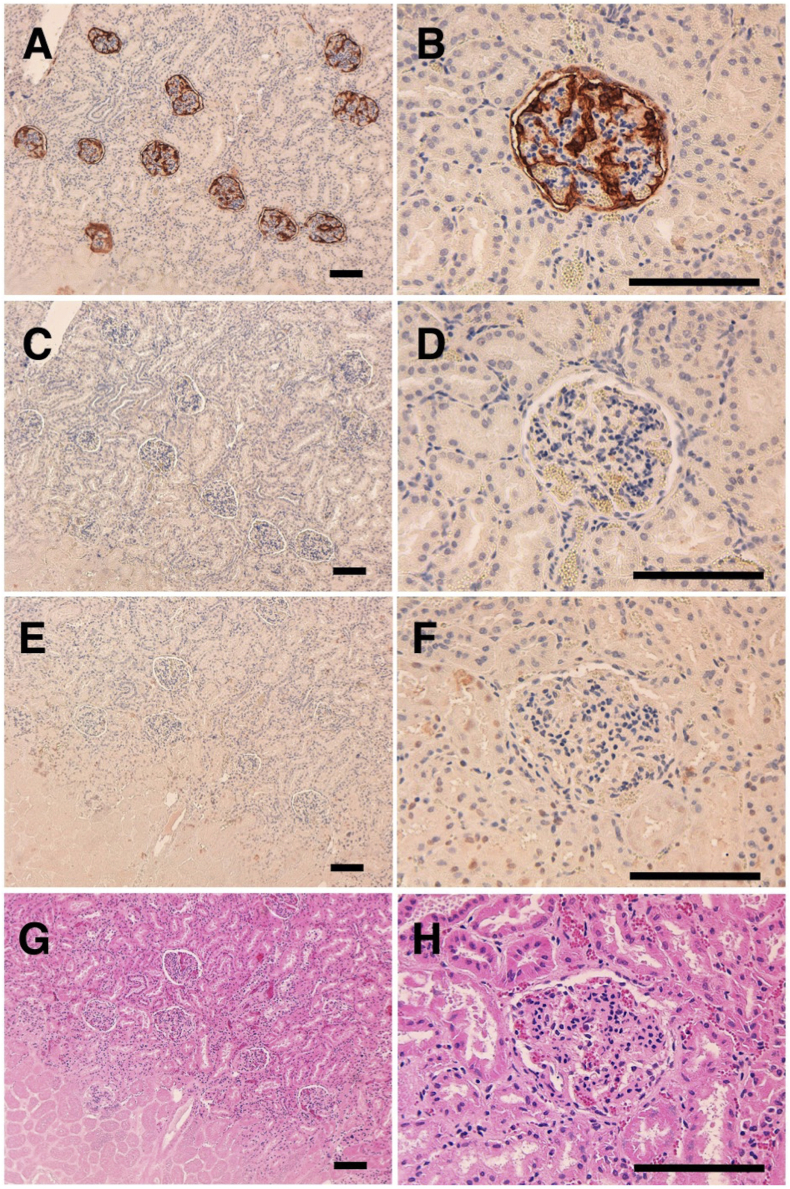
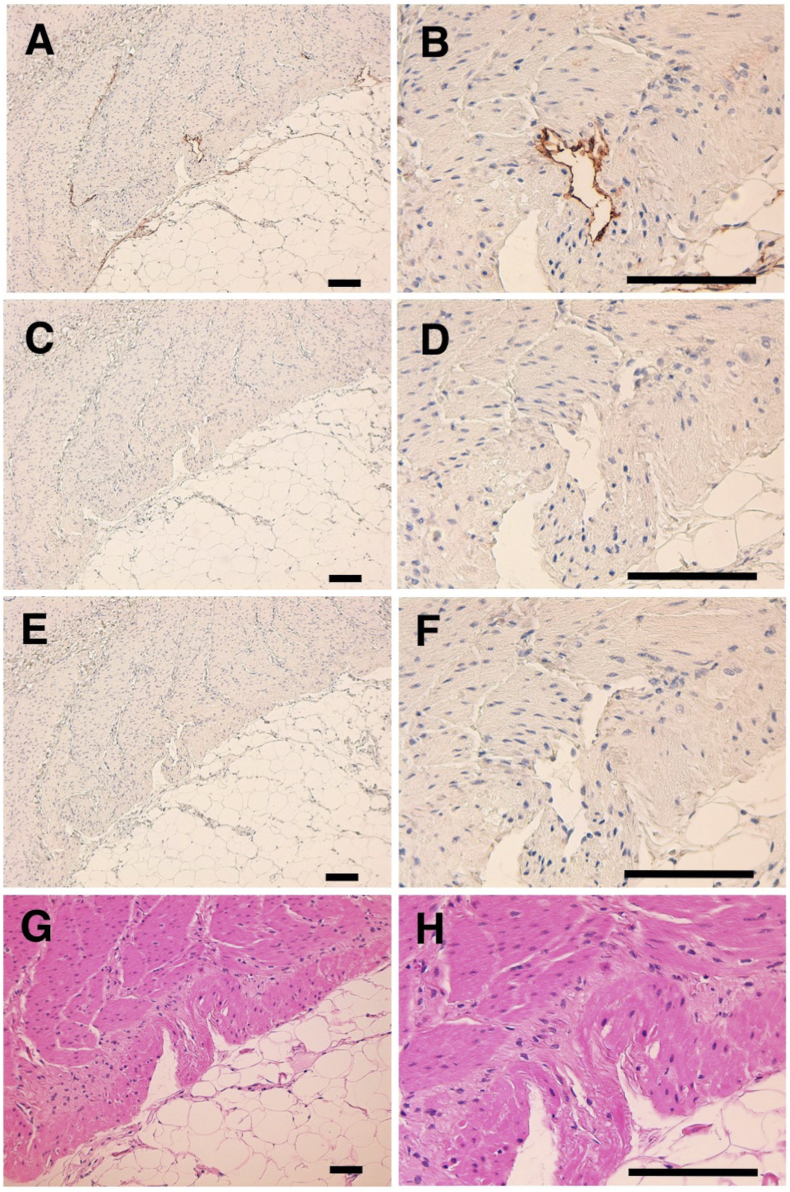
The immunohistochemical analyses revealed that PMab-235 strongly stained type I alveolar cells of lung (Fig. 4A and B), podocytes of kidney (Fig. 5A and B), and lymphatic endothelial cells of colon (Fig. 6A and B). We used PMab-44 as a positive control to stain gPDPN because PMab-44 was shown to crossreact with gPDPN in our previous study [38]. Although PMab-44 strongly stained type I alveolar cells of lung (Fig. 4C and D), it did not stain podocytes of kidney (Fig. 5C and D) and lymphatic endothelial cells of colon (Fig. 6C and D). No staining was observed without primary antibodies against type I alveolar cells of lung (Fig. 4E and F), podocytes of kidney (Fig. 5E and F), and lymphatic endothelial cells of colon (Fig. 6E and F).
Fig. 4.
Immunohistochemical analyses for goat lung. Lung sections were incubated with 1 μg/mL of PMab-235 (A, B), 1 μg/mL of PMab-44 (C, D) or with blocking buffer (E, F), followed by that with the Envision + Kit. (G, H) Hematoxylin and eosin staining. Scale bar = 100 μm.
Fig. 5.
Immunohistochemical analyses for goat kidney. Kidney sections were incubated with 1 μg/mL of PMab-235 (A, B), 1 μg/mL of PMab-44 (C, D) or with blocking buffer (E, F), followed by that with the Envision + Kit. (G, H) Hematoxylin and eosin staining. Scale bar = 100 μm.
Fig. 6.
Immunohistochemical analyses for goat colon. Colon sections were incubated with 1 μg/mL of PMab-235 (A, B), 1 μg/mL of PMab-44 (C, D) or with blocking buffer (E, F), followed by that with the Envision + Kit. (G, H) Hematoxylin and eosin staining. Scale bar = 100 μm.
4. Discussion
In 1979, Magari et al. reported morphological studies on liver lymphatics in human, pig, calf, dog, rabbit, and goat [39]. They studied form, distribution, and structure of liver lymphatics using both light and electron microscopes. Further, Ezeasor et al. reported the distribution and characteristics of lymph vessels in caprine (goat) hemal nodes after glutaraldehyde fixation and epoxy resin embedding [40]. Because anti-gPDPN mAbs, which are useful for immunohistochemical analysis to detect lymphatic endothelial cells, have not been reported, specific detection of lymphatic endothelial cells was difficult. Previously, we immunized mice with a synthesized peptide of gPDPN, such as 37-KNEQTTLGVEDYMT-49, which is corresponding to platelet aggregation-stimulating (PLAG) domain. Unfortunately, we could not establish specific mAbs for immunohistochemical analysis against gPDPN (data not shown). Although an anti-bovine PDPN mAb PMab-44 crossreacted with gPDPN in immunohistochemistry for goat lung tissues, it did not react with lymphatic endothelial cells of goat tissues [38]. In the present study, we employed the CBIS method to develop sensitive and specific mAbs against gPDPN to facilitate the immunohistochemical analysis of paraffin-embedded tissue sections. Established PMab-235 reacted with endogenous gPDPN of a fibroblastic goat cell line as well as CHO/gPDPN cells (Fig. 2). The immunohistochemical analyses revealed that PMab-235 strongly stained type I alveolar cells of lung (Fig. 4), podocytes of kidney (Fig. 5), and lymphatic endothelial cells of colon (Fig. 6), indicating that PMab-235 is useful for the detection of gPDPN by immunohistochemistry. PMab-235 cross-reacted with bovine PDPN not only in flow cytometry (Fig. 3) but also in immunohistochemistry (data not shown). Unfortunately, PMab-235 did not react with gPDPN in western blot analysis (data not shown). In conclusion, we established PMab-235 against gPDPN, which is suitable for use in flow cytometry and immunohistochemical analyses using CBIS method. The epitope of PMab-235 needs further investigation to clarify the sensitivity and specificity of PMab-235 against gPDPN.
Declarations
Author contribution statement
Yoshikazu Furusawa: Performed the experiments; Wrote the paper.
Shinji Yamada, Takuro Nakamura: Performed the experiments.
Masato Sano, Shunsuke Itai, Junko Takei: Analyzed and interpreted the data.
Hiroyuki Harada, Masato Fukui: Contributed reagents, materials, analysis tools or data.
Mika K. Kaneko, Yukinari Kato: Conceived and designed the experiments; Wrote the paper.
Funding statement
Yukinari Kato was supported in part by AMED (Grant numbers: JP18am0101078, JP18am0301010, and JP18ae0101028). Yukinari Kato was supported by JSPS KAKENHI (Grant Number 19K07705). Mika K.Kaneko was supported by JSPS KAKENHI (Grant Number 17K07299).
Competing interest statement
Yukinari Kato received research funding from ZENOAQ RESOURCE CO., LTD.
Additional information
No additional information is available for this paper.
References
- 1.Retzbach E.P., Sheehan S.A., Nevel E.M., Batra A., Phi T., Nguyen A.T.P., Kato Y., Baredes S., Fatahzadeh M., Shienbaum A.J., Goldberg G.S. Podoplanin emerges as a functionally relevant oral cancer biomarker and therapeutic target. Oral Oncol. 2018;78:126–136. doi: 10.1016/j.oraloncology.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki-Inoue K., Kato Y., Inoue O., Kaneko M.K., Mishima K., Yatomi Y., Yamazaki Y., Narimatsu H., Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J. Biol. Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 3.Jung E., Gardner D., Choi D., Park E., Jin Seong Y., Yang S., Castorena-Gonzalez J., Louveau A., Zhou Z., Lee G.K., Perrault D.P., Lee S., Johnson M., Daghlian G., Lee M., Jin Hong Y., Kato Y., Kipnis J., Davis M.J., Wong A.K., Hong Y.K. Development and characterization of A novel prox1-EGFP lymphatic and schlemm's canal reporter rat. Sci. Rep. 2017;7:5577. doi: 10.1038/s41598-017-06031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertozzi C.C., Schmaier A.A., Mericko P., Hess P.R., Zou Z., Chen M., Chen C.Y., Xu B., Lu M.M., Zhou D., Sebzda E., Santore M.T., Merianos D.J., Stadtfeld M., Flake A.W., Graf T., Skoda R., Maltzman J.S., Koretzky G.A., Kahn M.L. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko M.K., Kato Y., Kameyama A., Ito H., Kuno A., Hirabayashi J., Kubota T., Amano K., Chiba Y., Hasegawa Y., Sasagawa I., Mishima K., Narimatsu H. Functional glycosylation of human podoplanin: glycan structure of platelet aggregation-inducing factor. FEBS Lett. 2007;581:331–336. doi: 10.1016/j.febslet.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa-Alvarez J.A., Krishnan H., Pastorino J.G., Nevel E., Kephart D., Lee J.J., Retzbach E.P., Shen Y., Fatahzadeh M., Baredes S., Kalyoussef E., Honma M., Adelson M.E., Kaneko M.K., Kato Y., Young M.A., Deluca-Rapone L., Shienbaum A.J., Yin K., Jensen L.D., Goldberg G.S. Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms. Oncotarget. 2015;6:9045–9060. doi: 10.18632/oncotarget.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato Y., Kaneko M., Sata M., Fujita N., Tsuruo T., Osawa M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumor Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]
- 8.Schoppmann S.F., Jesch B., Riegler M.F., Maroske F., Schwameis K., Jomrich G., Birner P. Podoplanin expressing cancer associated fibroblasts are associated with unfavourable prognosis in adenocarcinoma of the esophagus. Clin. Exp. Metastasis. 2013;30:441–446. doi: 10.1007/s10585-012-9549-2. [DOI] [PubMed] [Google Scholar]
- 9.Abe S., Kaneko M.K., Tsuchihashi Y., Izumi T., Ogasawara S., Okada N., Sato C., Tobiume M., Otsuka K., Miyamoto L., Tsuchiya K., Kawazoe K., Kato Y., Nishioka Y. Antitumor effect of novel anti-podoplanin antibody NZ-12 against malignant pleural mesothelioma in an orthotopic xenograft model. Cancer Sci. 2016;107:1198–1205. doi: 10.1111/cas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko M.K., Oki H., Ogasawara S., Takagi M., Kato Y. Anti-podoplanin monoclonal antibody LpMab-7 detects metastatic legions of osteosarcoma. Monoclon. Antibodies Immunodiagn. Immunother. 2015;34:154–161. doi: 10.1089/mab.2014.0091. [DOI] [PubMed] [Google Scholar]
- 11.Ariizumi T., Ogose A., Kawashima H., Hotta T., Li G., Xu Y., Umezu H., Sugai M., Endo N. Expression of podoplanin in human bone and bone tumors: new marker of osteogenic and chondrogenic bone tumors. Pathol. Int. 2010;60:193–202. doi: 10.1111/j.1440-1827.2009.02510.x. [DOI] [PubMed] [Google Scholar]
- 12.Kato Y., Kaneko M.K., Kuno A., Uchiyama N., Amano K., Chiba Y., Hasegawa Y., Hirabayashi J., Narimatsu H., Mishima K., Osawa M. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem. Biophys. Res. Commun. 2006;349:1301–1307. doi: 10.1016/j.bbrc.2006.08.171. [DOI] [PubMed] [Google Scholar]
- 13.Kato Y., Sasagawa I., Kaneko M., Osawa M., Fujita N., Tsuruo T. Aggrus: a diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene. 2004;23:8552–8556. doi: 10.1038/sj.onc.1207869. [DOI] [PubMed] [Google Scholar]
- 14.Kunita A., Kashima T.G., Morishita Y., Fukayama M., Kato Y., Tsuruo T., Fujita N. The platelet aggregation-inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am. J. Pathol. 2007;170:1337–1347. doi: 10.2353/ajpath.2007.060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaji C., Tsujimoto Y., Kato Kaneko M., Kato Y., Sawa Y. Immunohistochemical examination of novel rat monoclonal antibodies against mouse and human podoplanin. Acta Histochem. Cytoc. 2012;45:227–237. doi: 10.1267/ahc.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oki H., Honma R., Ogasawara S., Fujii Y., Liu X., Takagi M., Kaneko M.K., Kato Y. Development of sensitive monoclonal antibody pmab-2 against rat podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2015;34:396–403. doi: 10.1089/mab.2015.0041. [DOI] [PubMed] [Google Scholar]
- 17.Honma R., Fujii Y., Ogasawara S., Oki H., Liu X., Nakamura T., Kaneko M.K., Takagi M., Kato Y. Establishment of a novel monoclonal antibody PMab-32 against rabbit podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:41–47. doi: 10.1089/mab.2015.0066. [DOI] [PubMed] [Google Scholar]
- 18.Honma R., Ogasawara S., Kaneko M., Fujii Y., Oki H., Nakamura T., Takagi M., Konnai S., Kato Y. PMab-44 detects bovine podoplanin in immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:186–190. doi: 10.1089/mab.2016.0016. [DOI] [PubMed] [Google Scholar]
- 19.Honma R., Kaneko M.K., Ogasawara S., Fujii Y., Konnai S., Takagi M., Kato Y. Specific detection of dog podoplanin expressed in renal glomerulus by a novel monoclonal antibody pmab-38 in immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:212–216. doi: 10.1089/mab.2016.0022. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S., Itai S., Nakamura T., Yanaka M., Saidoh N., Chang Y.W., Handa S., Harada H., Kagawa Y., Ichii O., Konnai S., Kaneko M.K., Kato Y. PMab-52: specific and sensitive monoclonal antibody against cat podoplanin for immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:224–230. doi: 10.1089/mab.2017.0027. [DOI] [PubMed] [Google Scholar]
- 21.Kato Y., Yamada S., Furusawa Y., Itai S., Nakamura T., Yanaka M., Sano M., Harada H., Fukui M., Kaneko M.K. PMab-213: a monoclonal antibody for immunohistochemical analysis against pig podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:18–24. doi: 10.1089/mab.2018.0048. [DOI] [PubMed] [Google Scholar]
- 22.Kato Y., Yamada S., Itai S., Kobayashi A., Konnai S., Kaneko M.K. Anti-horse podoplanin monoclonal antibody pmab-219 is useful for detecting lymphatic endothelial cells by immunohistochemical analysis. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:272–274. doi: 10.1089/mab.2018.0044. [DOI] [PubMed] [Google Scholar]
- 23.Furusawa Y., Yamada S., Itai S., Nakamura T., Takei J., Sano M., Harada H., Fukui M., Kaneko M.K., Kato Y. Establishment of a monoclonal antibody PMab-233 for immunohistochemical analysis against Tasmanian devil podoplanin. Biochem Biophys Rep. 2019;18:100631. doi: 10.1016/j.bbrep.2019.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y., Furusawa Y., Yamada S., Itai S., Takei J., Sano M., Kaneko M.K. Establishment of a monoclonal antibody PMab-225 against alpaca podoplanin for immunohistochemical analyses. Biochem Biophys Rep. 2019;18:100633. doi: 10.1016/j.bbrep.2019.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furusawa Y., Kaneko M.K., Nakamura T., Itai S., Fukui M., Harada H., Yamada S., Kato Y. Establishment of a monoclonal antibody pmab-231 for tiger podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:89–95. doi: 10.1089/mab.2019.0003. [DOI] [PubMed] [Google Scholar]
- 26.Furusawa Y., Takei J., Sayama Y., Yamada S., Kaneko M.K., Kato Y. Development of an anti-bear podoplanin monoclonal antibody PMab-247 for immunohistochemical analysis. Biochem Biophys Rep. 2019;18:100644. doi: 10.1016/j.bbrep.2019.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato Y., Kaneko M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci. Rep. 2014;4:5924. doi: 10.1038/srep05924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada S., Kaneko M.K., Kato Y. LpMab-23: a cancer-specific monoclonal antibody against human podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:72–76. doi: 10.1089/mab.2017.0001. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko M.K., Nakamura T., Kunita A., Fukayama M., Abe S., Nishioka Y., Yamada S., Yanaka M., Saidoh N., Yoshida K., Fujii Y., Ogasawara S., Kato Y. ChLpMab-23: cancer-specific human-mouse chimeric anti-podoplanin antibody exhibits antitumor activity via antibody-dependent cellular cytotoxicity. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:104–112. doi: 10.1089/mab.2017.0014. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko M.K., Honma R., Ogasawara S., Fujii Y., Nakamura T., Saidoh N., Takagi M., Kagawa Y., Konnai S., Kato Y. PMab-38 recognizes canine podoplanin of squamous cell carcinomas. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:263–266. doi: 10.1089/mab.2016.0036. [DOI] [PubMed] [Google Scholar]
- 31.Ogasawara S., Honma R., Kaneko M.K., Fujii Y., Kagawa Y., Konnai S., Kato Y. Podoplanin expression in canine melanoma. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:304–306. doi: 10.1089/mab.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii Y., Kaneko M.K., Kato Y. MAP tag: a novel tagging system for protein purification and detection. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:293–299. doi: 10.1089/mab.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato Y., Fujita N., Kunita A., Sato S., Kaneko M., Osawa M., Tsuruo T. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J. Biol. Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 34.Kato Y., Yamada S., Itai S., Kobayashi A., Konnai S., Kaneko M.K. Immunohistochemical detection of sheep podoplanin using an antibovine podoplanin monoclonal antibody pmab-44. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:265–268. doi: 10.1089/mab.2018.0036. [DOI] [PubMed] [Google Scholar]
- 35.Furusawa Y., Yamada S., Itai S., Sano M., Nakamura T., Yanaka M., Fukui M., Harada H., Mizuno T., Sakai Y., Takasu M., Kaneko M.K., Kato Y. PMab-210: a monoclonal antibody against pig podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:30–36. doi: 10.1089/mab.2018.0038. [DOI] [PubMed] [Google Scholar]
- 36.Kato Y., Ogasawara S., Oki H., Goichberg P., Honma R., Fujii Y., Kaneko M.K. LpMab-12 established by CasMab Technology specifically detects sialylated O-glycan on Thr52 of platelet aggregation-stimulating domain of human podoplanin. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furusawa Y., Yamada S., Itai S., Sano M., Nakamura T., Yanaka M., Handa S., Mizuno T., Maeda K., Fukui M., Harada H., Kaneko M.K., Kato Y. Establishment of monoclonal antibody pmab-202 against horse podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:233–237. doi: 10.1089/mab.2018.0030. [DOI] [PubMed] [Google Scholar]
- 38.Yamada S., Kaneko M.K., Furusawa Y., Itai S., Sano M., Nakamura T., Yanaka M., Handa S., Hisamatsu K., Nakamura Y., Koyanagi M., Fukui M., Harada H., Kato Y. Anti-bovine podoplanin monoclonal antibody pmab-44 detects goat podoplanin in immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:96–99. doi: 10.1089/mab.2018.0031. [DOI] [PubMed] [Google Scholar]
- 39.Magari S., Fujikawa K., Mizutani Y., Nishi A. Morphological studies on liver lymphatics. Lymphology. 1979;12:14–17. [PubMed] [Google Scholar]
- 40.Ezeasor D.N., Singh A. Morphologic features of lymph vessels in caprine hemal nodes. Am. J. Vet. Res. 1990;51:1139–1143. [PubMed] [Google Scholar]