Abstract
Background
Cold stress is the main factor that reduces rice yield in subtropical areas, especially at the seedling stage. Most of the current studies on cold stress focus the responses of rice shoots to cold stress. Limited studies are available on that of rice roots to cold stress. This study aimed to illustrate the biochemical responses of rice root under cold treatment, and subject to the establishment of cold stress-related biochemical traits for rice breeding or cropping-adjustment.
Results
Our results showed that the growth of rice seedling diminished under cold stress with difference extents among eight rice cultivars of most productive in Taiwan. Under cold treatments, the tested cultivars with higher growth rate had a higher level of hydrogen peroxide (H2O2) in the shoots but had a lower level in the roots. In contrast, the tested cultivates with low growth rate had higher levels of H2O2 in the roots but a lower level in the shoots. Meanwhile, higher MDA contents and higher cell-damage related electrolyte leakage were also found in the roots not in the shoots, suggesting that cold stress might induce oxidative stress in the roots, not in the shoots. Furthermore, the activity analysis of four antioxidant enzymes, namely superoxide dismutase (SOD), catalase (CAT), ascorbic peroxidase (APX), and glutathione reductase (GR), revealed that cold stress could increase SOD and CAT activities in the roots.
Conclusions
In summary, low H2O2 and low MDA contents along with lower SOD and CAT activities in rice root could be the biochemical traits of cold responses in rice seedlings. The results are hoping to have a contribution to the rice breeding or cropping-adjustment on cold tolerance.
Electronic supplementary material
The online version of this article (10.1186/s40529-019-0262-1) contains supplementary material, which is available to authorized users.
Keywords: Cold responses, Rice seedling, Root, Oxidative stress
Background
Rice (Oryza sativa L.) is mainly cultivated in tropical and subtropical regions and provides a substantial food resource. Because of climate change and an increase in extreme temperatures, the yield of rice has gradually declined (Solomon et al. 2007). The incidence of low temperature is one factor responsible for the declining yield, especially at the seedling stage (Aghaee et al. 2011; Bhattacharjee 2013; Dashtmian et al. 2014). This cost of cold damage is approximately 75% of all disaster loss in Taiwan (Additional file 1: Figure S1).
In general, cold temperatures of 0–15 °C can reduce the crop survival rate, inhibit photosynthesis, retard growth, and block the synthesis of proteins, lipids, and carbohydrates (Setter and Greenway 1988; Aghaee et al. 2011; Liu et al. 2013). At the seedling stage, rice is more sensitive to low temperatures because low temperatures can inhibit seed germination (Morsy et al. 2006; Baruah et al. 2009) and also retard seedling growth, resulting in leaf curving, shoot shortening, and few tillers (Dashtmian et al. 2014). In addition, low temperatures may cause the accumulation of reactive oxygen species (ROS), such as superoxide anion, singlet oxygen, and hydrogen peroxide (H2O2), which leads to lipid peroxidation, electrolyte leakage, and membrane damage (Kuk et al. 2003; Hung et al. 2008; Bhattacharjee 2013).
Cold temperatures have been found to damage the rice root tissue, resulting in a decrease in water obtained by the roots and upward nutrient transport to the shoot, retarding the growth of rice seedlings (Setter and Greenway 1988; Neilson et al. 2013). However, the biochemical mechanism is not clear, especially with regard to the roles of ROS. Nevertheless, the study of salt stress on rice root by Lin and Kao (2001a) revealed an increase in ionically bound cell-wall peroxidase activity after NaCl treatment in rice roots, which resulted in H2O2 generation and thus inhibited the growth of rice roots. Lin and Kao (1999, 2001a) have found that NaCl treatment inhibited the root growth of rice seedlings and agreed with the theory of H2O2 induced cell-wall stiffening process (Fry 1986; Lin and Kao 2001b). We found that Cd toxicity in rice leaves is due to H2O2 accumulation (Hsu and Kao 2007) and further reported that H2O2 accumulation is responsible for Cd-inhibited root growth of rice seedlings (Cho et al. 2012), where Cd could inhibit the activity of catalase (CAT), which is supposed to break down H2O2 into water and oxygen in rice roots. Thus, Cho et al. (2012) suggested that a decrease in CAT may result in the accumulation of H2O2 in the rice root. In the root cell of Arabidopsis, H2O2 was also found to be involved in the nutrient-deficiency response (Shin and Schachtman 2004; Shin et al. 2005), and it might play a role in the sensing and signaling of N, P, K, and S nutrients (Schachtman and Shin 2007).
Therefore, our study was attempting to analyze mechanisms underlying the responses of the rice root to cold stress, especially with regard to the oxidative status of the root and the activities of four antioxidant enzymes, namely superoxide dismutase (SOD), CAT, ascorbate peroxidase (APX), and glutathione reductase (GR). The accumulation of ROS is the beginning of oxidative stress and results in the lipid peroxidation of the cell membrane, which can be expressed by the increase of malondialdehyde (MDA) contents (Hung et al. 2008; Bhattacharjee 2013). In respond to oxidative stress, plant tissue will increase the activity of SOD to reduce the ROS level and generate H2O2. Since H2O2 is toxic to the cell, the activities of CAT or APX will be strengthened to decrease H2O2 contents (Chao et al. 2010; Chou et al. 2012). The activity of APX can be maintained in couple with the action of GR. These biochemical traits may be used as selecting marker for a rice breeding project or cropping-adjustment in cold tolerance. This study aimed to illustrate the link between rice root responses and cold stress by analyzing these biochemical traits on eight rice cultivars.
Methods
The most productive cultivars of rice (Oryza sativa L.) in Taiwan are these eight, namely Taitung 30 (TT30), Tainan 11 (TN11), Tainung 71 (TNG 71), Kaohsiung 139 (KH139), Tai-Keng 16 (TK16), Tai-Keng 9 (TK9), Tai-Keng 14 (TK14), and Taichung-Sen 10 (TCS10). This study selected these eight cultivars for cold treatment. Rice seeds were kept in an incubator at 37 °C for 48 h to break seed dormancy. Twelve sprouts of the same size from one cultivate were selected and placed in a covered plate with a 9-cm wet filter paper as one replicate. The plates were kept in a growth chamber at a light/dark cycle of 14/10 h with a light intensity of 200 μmole photons/m2/s. The temperature of the growth chamber was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the chamber temperature was adjusted to 27 °C, and plates were kept uncovered for 3 days and watered every day before harvesting for growth analysis. Each cultivates had four replicates in one experiment. Data from three experiments were collected for statistical analysis. From the 12 sprouts of each replicate, nine seedlings in the middle range of length were collected for the measurement of length, fresh weight, and dry weight of the shoot and root.
Protein and chlorophyll contents were determined according to the methods of Bradford (1976) and Wintermans and de Mots (1965), respectively. The degree of lipid peroxidation was expressed as the content of malondialdehyde (MDA), which was determined using the method reported by Health and Packer (1968). The tissue content of H2O2 was determined using the method reported by Jana and Choudhuri (1982), which has previously been used for rice seedlings (Lin and Kao 2001a, b; Hsu and Kao 2004). The method for measuring electrolyte leakage was modified from that used by Blum and Ebercon (1981) and Dashtmian et al. (2014). The detached roots were soaked in 10 mL of deionized water for 16 h for the first water conductivity measurement (C1) and then soaked in boiling water for 1 h for the second measurement (C2). The degree of electrolyte leakage was calculated as C1/C2 × 100.
The activities of SOD, CAT, APX, and GR were determined according to methods that have been tested in rice seedlings (Chou et al. 2012; Chao et al. 2010). SOD was determined according to the method used by Paoletti et al. (1986). One unit of SOD was defined as the amount of enzyme that inhibits the rate of NADH oxidation observed in the blank by 50%. The CAT activity was assayed by measuring the initial rate of disappearance of H2O2 (Kato and Shimizu 1985). APX was determined according to the methods used by Nakano and Asada (1981). A decrease in the ascorbate concentration was correlated to a decrease in optical density at 290 nm. GR was determined using the method reported by Foster and Hess (1980). One unit of GR was defined as the amount of an enzyme that reduces 1 absorbance of reading on 340 nm/min. Activities of all enzymes expressed on the basis of fresh weight.
Statistical analyses, including standard error, analysis of variance, least significant difference multiple comparisons, t-test, and regression test, were performed using the Statistical Analysis System (SAS 9.4).
Results
Cold stress inhibited the growth of rice seedlings
Eight of the most productive rice cultivars in Taiwan were chosen for cold treatments (Additional file 1: Table S1). According to the pedigree of these cultivars, the ratio of their genome origin was estimated and displayed in Additional file 1: Table S1. In general, most of them contain a high portion of Japonica type genome and less portion of Indica type genome. It was noticed that two cultivars (TN11 and KH139) are 100% from Japonica genotype, and one cultivar (TCS10) is 100% from Indica genotype. The rice origin of Japonica genotype is from the temporal region and that of Indica genotype is from subtropical and tropical regions. The native rice of Taiwan is Indica type. The current Japonica type of rice is introduced from Japan. Thus it was general to be believed that rice of Japonica genotype is more tolerant to cold stress than that of Indica genotype. However, the initial breeding project of Taiwan was focused on heat tolerance and, as a result, more and more new Japonica type rice is able to grow in the south of Taiwan. Less attention had been put on cold tolerant. Thus, even though the current popular rice varieties in Taiwan are Japonica type, Taiwan is still in the suffering of yield loss of rice by cold stress (Additional file 1: Figure S1). We speculated that some of the cold stress-related genomes might be lost during previous breeding selection. Thus, even with a high portion of Japonica type genome in most productive eight rice cultivars, the cost of cold damage was as high as 75% of all disaster loss in the subtropic region of Taiwan. To establish biochemical traits of cold stress-related would be valuable for future rice breeding on cold tolerance. The results in Additional file 1: Figure S1 also showed January and February are the 2 months of having the most loss on cost, during which the rice is on the stage of the seedling. Thus, this study focused on the performance of rice seedlings under cold stress.
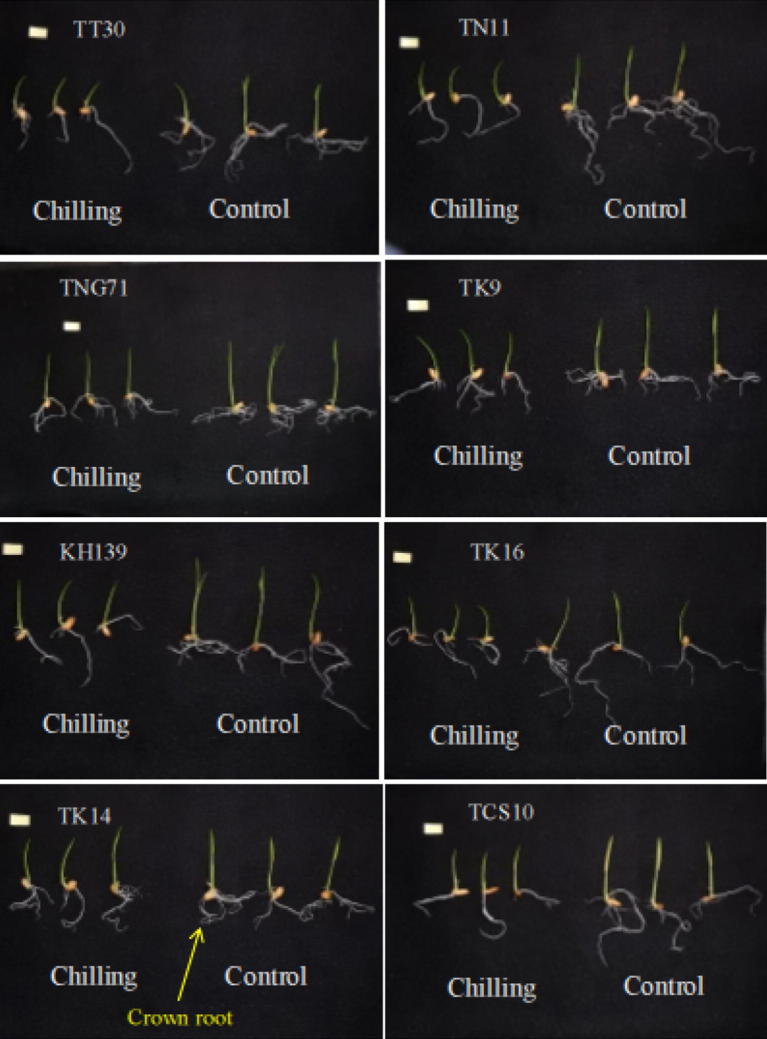
The rice seedlings of eight cultivars were treated with a cold temperature at 15 °C for 4 days that is the frequent duration of cold stress in Taiwan. After the cold treatment, the seedlings were re-warmed at 27 °C for 3 days and followed with harvesting and growth analysis. The treatment of 27 °C to 27 °C for 7 days was considered as the control. The results of cold treatment showed that the growth of shoots decreased in all testing cultivars (Fig. 1) as compared with the control in regarding the fresh weight, dry weight, and length (Table 1). The results were similar to that of roots (Table 2 and Fig. 1). Moreover, the variance analysis on shoot growth (Table 1) demonstrated that the extents of growth were significantly different within genotypes and between treatments, meanwhile the interactions of variance on genotypes and cold treatment (G × T) were also differing significantly. Similar to the results obtained for the shoot, the extents of root growth were differing significantly within genotypes and between treatments, and also to the interactions of variance on genotypes and cold treatment (G × T). In addition, the results of Table 1 showed that cultivar TCS10 (100% Indica genotype) was the most affected by cold treatment, with a 41.6% reduction on shoot length. Among the other seven cultivars which contain more or less of Japonica type genome, cultivar TK14 (12.5% Indica genotype) was the least affected by cold treatment with a 10.6% reduction on shoot length. In Table 2, the root length of cultivar TCS10 (100% Indica genotype) was the least affected by cold treatment, with a 24% reduction in root length. The next was cultivar TK9 (50% Indica genotype) with a 28.4% reduction in root length. Cultivar TT30 (93.75% Japonica genotype) was the most affected by cold treatment with 51.3% reduction in root length. The next two cultivars of vulnerable to cold treatment were TN11 and KH139, both were 100% Japonica genotype, Thus, it was likely that the more of portion containing with Japonica type genome in testing cultivars, the less of shoot length reduction corresponding to cold stress. In opposition with the results in shoot length, the more of portion containing with Japonica genotype genome in testing cultivars, the more of root length reduction corresponding to cold stress. Seemingly, the cultivars mainly with a high portion of Japonica genotype genome might keep their shoot to grow under cold stress. The cultivars mainly with a high portion of Indica genotype genome might keep their root to grow under cold stress.
Fig. 1.
The cold stress response of rice seedlings. The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting. Bar = 1 cm
Table 1.
The cold stress response of rice seedling in the shoot
| Genotype | Temperature (°C) | Fresh weight (g) | Dry weight (g) | Height (cm) | Height reduction (%) |
|---|---|---|---|---|---|
| TT30 |
27 → 27 15 → 27 |
#0.101bc 0.071fg |
0.0184b 0.0093ef |
3.22def 2.53ghij |
21.5 |
| TN11 |
27 → 27 15 → 27 |
0.077ef 0.055h |
0.0139c 0.0068g |
3.57cd 2.12ij |
30.6 |
| TNG71 |
27 → 27 15 → 27 |
0.120a 0.068fg |
0.0226a 0.0123cd |
4.82a 3.45de |
28.4 |
| TK9 |
27 → 27 15 → 27 |
0.099cd 0.064gh |
0.0190b 0.0106de |
3.50de 2.67ghi |
23.7 |
| KH139 |
27 → 27 15 → 27 |
0.103bc 0.066fgh |
0.0192b 0.0078fg |
4.00bc 2.85fgh |
28.7 |
| TK16 |
27 → 27 15 → 27 |
0.088de 0.055h |
0.0140c 0.0065g |
3.35de 2.35ij |
29.1 |
| TK14 |
27 → 27 15 → 27 |
0.096cd 0.063gh |
0.0183b 0.0073fg |
3.02defg 2.70ghi |
10.6 |
| TCS10 |
27 → 27 15 → 27 |
0.114ab 0.053h |
0.0201b 0.0066g |
4.25b 2.48hij |
41.6 |
| $ANOVA analysis | |||||
| Genotype | ** | ** | ** | ||
| Temperature | ** | ** | ** | ||
| G × T | ** | ** | ** | ||
The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
#Different letters represent the significant difference with LSD tests among cultivars or treatments (p < 0.05)
$G × T: effect of genotype and temperature interaction; *p < 0.05; **p < 0.01
Table 2.
The cold stress response of rice seedling in the root
| Genotype | Temperature (°C) | Fresh weight (g) | Dry weight (g) | Length (cm) | Length reduction (%) |
|---|---|---|---|---|---|
| TT30 |
27 → 27 15 → 27 |
#0.211b 0.107fg |
0.0316a 0.0143ef |
8.12a 3.95e |
51.3 |
| TN11 |
27 → 27 15 → 27 |
0.134e 0.070h |
0.0206d 0.0083h |
6.68b 4.43de |
33.7 |
| TNG71 |
27 → 27 15 → 27 |
0.246a 0.121ef |
0.0344a 0.0156e |
8.22a 5.57c |
32.2 |
| TK9 |
27 → 27 15 → 27 |
0.182cd 0.094g |
0.0240bc 0.0118fg |
6.63b 4.75cde |
28.4 |
| KH139 |
27 → 27 15 → 27 |
0.196bc 0.088gh |
0.0254bc 0.0097gh |
8.03a 4.53de |
43.6 |
| TK16 |
27 → 27 15 → 27 |
0.182cd 0.083gh |
0.0266b 0.0116fg |
7.97a 5.45c |
31.6 |
| TK14 |
27 → 27 15 → 27 |
0.169d 0.092gh |
0.0241bc 0.0112fgh |
6.58b 4.60de |
30.1 |
| TCS10 |
27 → 27 15 → 27 |
0.161d 0.098fg |
0.0229cd 0.0139ef |
6.82b 5.18cd |
24.0 |
| $ANOVA analysis | |||||
| Genotype | ** | ** | ** | ||
| Temperature | ** | ** | ** | ||
| G × T | ** | ** | ** | ||
The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
#Different letters represent the significant difference with LSD tests among cultivars or treatments (p < 0.05)
$G × T: effect of genotype and temperature interaction; *p < 0.05; **p < 0.01
Cold stress affect the contents of the protein and chlorophyll
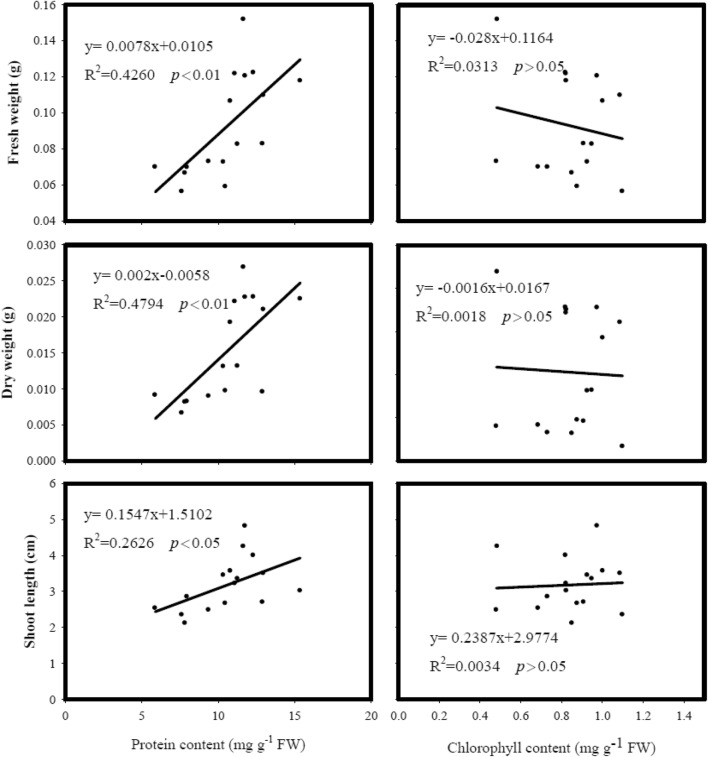
The results of Table 3 showed a decrease in protein content in both roots and shoots of eight cultivars after cold treatment. Additionally, the results of the regression analysis (Fig. 2) revealed that the protein content was positively correlated with shoot growth including fresh weight, dry weight, and length, suggesting the more of protein content, the more of shoot growth. On the contrary, the results in Table 3 showed that the chlorophyll contents were not affected in most cultivars. In addition, the results of the regression analysis (Fig. 2) revealed that the chlorophyll contents were not correlated with shoot growth among all tested cultivars. Therefore, the results of the above suggested that the rice chlorophyll content was less sensitive to cold stress than the protein content (Table 3).
Table 3.
The cold stress response of rice seedlings
| Genotype | Temperature (°C) | Root | Shoot | |
|---|---|---|---|---|
| Protein content (mg/gFW) | Protein content (mg/g FW) | Chlorophyll content (mg/g FW) | ||
| TT30 |
27 → 27 15 → 27 |
#6.36e 11.16bcd |
11.1cdef 5.9h |
0.82bcd 0.69d |
| TN11 |
27 → 27 15 → 27 |
5.98e 10.45cd |
10.8def 7.9g |
1.00ab 0.85bcd |
| TNG71 |
27 → 27 15 → 27 |
6.86e 10.56cd |
11.8bcde 10.3ef |
0.97ab 0.93abc |
| TK9 |
27 → 27 15 → 27 |
7.52e 11.69abc |
13.0b 10.5ef |
1.09a 0.88bcd |
| KH139 |
27 → 27 15 → 27 |
6.34e 10.62cd |
12.3bcd 8.0g |
0.82bcd 0.73cd |
| TK16 |
27 → 27 15 → 27 |
6.92e 12.46ab |
11.3bcde 7.6gh |
0.95ab 1.10a |
| TK14 |
27 → 27 15 → 27 |
9.71d 12.92a |
15.4a 12.9bc |
0.82bcd 0.91abc |
| TCS10 |
27 → 27 15 → 27 |
6.73e 11.65abc |
11.7bcde 9.4fg |
0.49e 0.48e |
| $ANOVA test | ||||
| Genotype | ** | ** | ** | |
| Temperature | ** | ** | ns | |
| G × T | ns | ns | ns | |
The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
ns non-significant
#Different letters represent the significant difference with LSD tests among cultivars or treatments (p < 0.05)
$G × T: effect of genotype and temperature interaction; *p < 0.05; **p < 0.01
Fig. 2.
The relationships of shoot protein contents and of chlorophyll contents with rice seedling fresh weight, dry weight and length under cold treatment among eight cultivars. The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
Cold stress-induced oxidative stress on the root
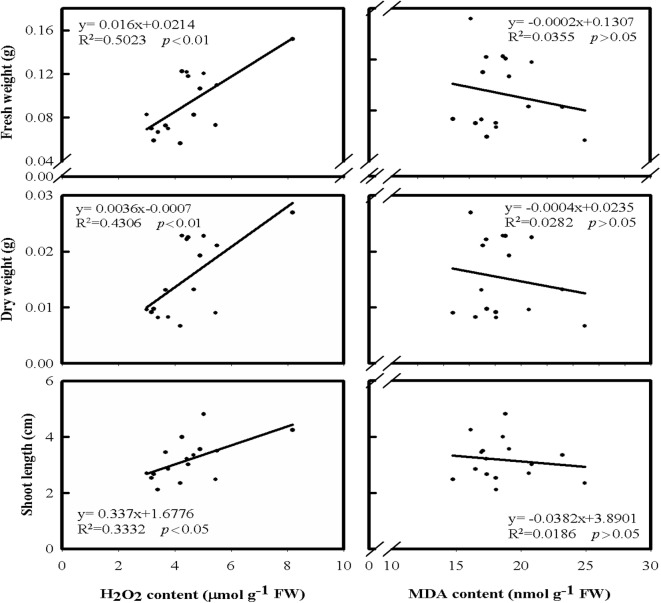
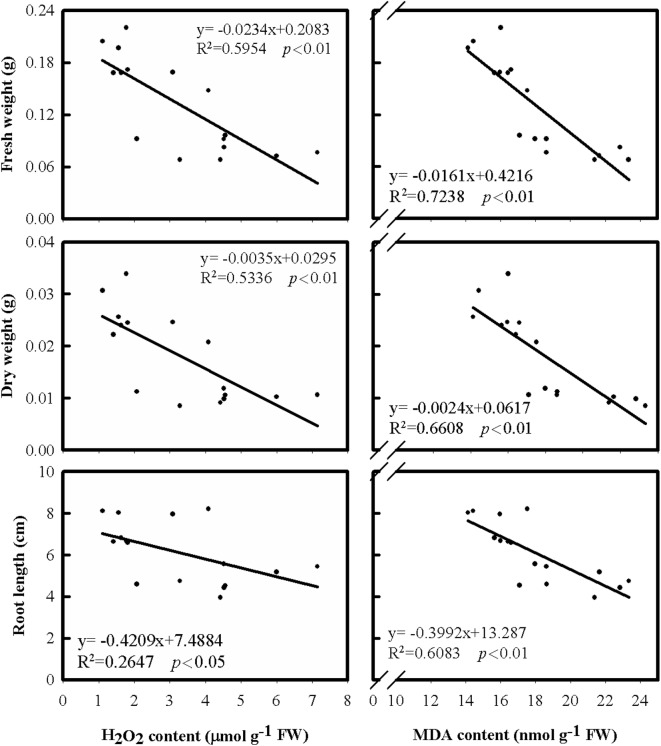
Since the varieties tested in this study had different extents in response to cold stress, the regression analysis was performed and seeking cold stress-related biochemical traits. The biochemical traits for analysis included the contents of H2O2 and MDA, and the activities of four antioxidant enzymes, SOD, CAT, APX, and GR. The contents of H2O2 and MDA were monitored in both shoots and roots of rice after cold treatment (Figs. 3 and 4). The results of this study revealed that the H2O2 content in shoots was positively correlated with shoot growth (Fig. 3). In contrast, the H2O2 content in roots was negatively correlated with root growth (Fig. 4), suggesting that the increase of H2O2 content in shoots might favor the growth of shoot, but the increase of H2O2 content in roots might reduce the growth of roots. Furthermore, the analysis of MDA contents (Fig. 3), an indicator of cell damage, showed that the MDA contents in shoots only had a weak correlation with shoot growth. Thus, the fluctuation of the H2O2 contents in shoots under cold treatment found in this study might not be related to the damage of the cells in rice shoots. In contrast, the MDA contents in roots, as well as H2O2 contents, were negatively correlated with root growth (Fig. 4), suggesting the cells of roots might be damaged by elevated H2O2 content. Moreover, an increase in electrolyte leakage in roots was found when the MDA content increased (Fig. 5) revealing that the cell damage could occur in the roots under cold treatment.
Fig. 3.
The relationships of leaf H2O2 and MDA contents with shoot fresh weight, dry weight and length under cold treatment among eight rice cultivates. The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
Fig. 4.
The relationships of root H2O2 and MDA contents with root fresh weight, dry weight and length under cold treatment among eight rice cultivates. The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
Fig. 5.
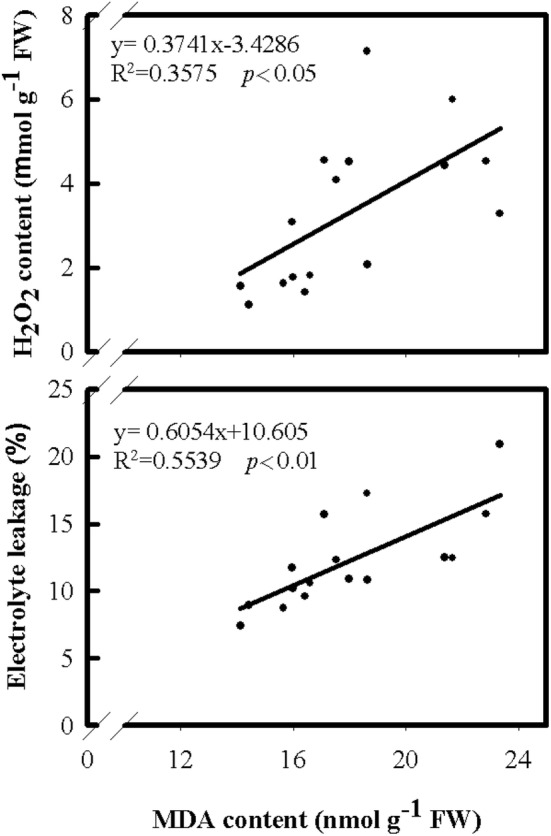
The relationships of root MDA contents with rice root H2O2 contents and rate of electrolyte leakage under cold treatment among eight cultivars. The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
Cold stress induces the change of the antioxidant enzymes on the root
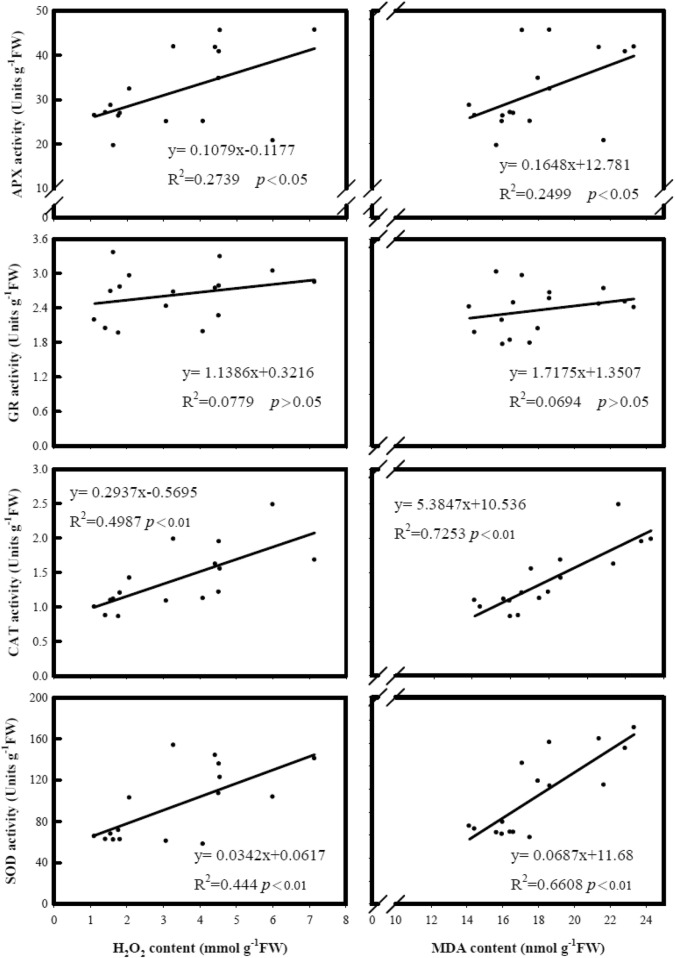
In order to reveal the possible mechanisms on the regulation of the H2O2 content in rice roots under cold treatment, this study analyzed the activities of four antioxidant enzymes (Fig. 6), SOD, CAT, APX, and GR. The results of Fig. 6 showed the activities of SOD and CAT correlated well with the increases of H2O2 and MDA content. However, the activities of APX and GR had a weaker correlation with the increases in H2O2 and MDA content as compared with that of SOD and CAT.
Fig. 6.
The relationships of root H2O2 and MDA contents with root superoxide dismutase (SOD), catalase (CAT), ascorbic peroxidase (APX), glutathione reductase (GR) activities under cold treatment among eight cultivars. The temperature was set at 15 °C for cold treatment and 27 °C for control. After 4 days of treatment, the temperature was adjusted to 27 °C for 3 days before harvesting
Discussion
The reduction of rice yield in subtropical areas due to cold stress is getting attention. This study showed, even with a high portion of temporal region genome, Japonica type, most of the productive rice cultivars have the cost-loss with cold damage as high as 75% of all disaster in the subtropical region. Since heat adaptation and tasty were more concerned in previous rice breeding project, the establishment of cold stress-related biochemical traits would be valuable for future rice breeding. In general, most of the current studies on cold stress emphasis the responses of rice shoots to cold treatments. Indeed, this studies showed cold stress induced a high level of H2O2 content in the shoots of cultivars with higher growth rate (Fig. 3); however, their MDA content was not affected, suggesting the absence of oxidative stress in shoots. The other studies were shown similar results (Morsy et al. 2006; Bonnecarrère et al. 2011). The results of Table 3 showed a decrease in protein content in all tested cultivars after cold treatment. Additionally, the results of the regression analysis (Fig. 2) revealed that the protein content was positively correlated with shoot growth including fresh weight, dry weight, and length, suggesting the more of protein content, the more of shoot growth. There were certain reports showed similar results to our results (Setter and Greenway 1988; Knox 2008; Burton et al. 2010; Neilson et al. 2013; Sampathkumar et al. 2014). Thus, the results above agree with the founding that the reduction of protein turnover under cold treatment could be a result of shoot growth inhibition and leaf development retarding (Setter and Greenway 1988; Yan et al. 2006; Neilson et al. 2013). On the contrary, the results in Table 3 showed that the chlorophyll contents were not affected in most cultivars. In addition, the results of the regression analysis (Fig. 2) revealed that the chlorophyll contents were not correlated with shoot growth among all tested cultivars. Therefore, the results of the above suggested that the rice chlorophyll content was less sensitive to cold stress than the protein content (Table 3). Nevertheless, it should be considered that if cold stress is prolonged, the decrease in chlorophyll could be found as well as the inhibition of shoot growth, that has been reported in many documents. Thus, what the treatment of old temperature at 15 °C had initiated in this study could be just the early responses of rice seedling to cold stress.
The learning of the responses of rice roots to cold stress should not be omitted, accordingly. In fact, the location of the rice root is near the soil surface at the stage of the seedling. At that stage, both rice shoot and root could be facing the same cold temperature at night time. Neilson et al. (2013) indicate when the roots of rice seedlings were exposed to low temperatures, water absorption, and nutrients uptake were reduced from the soil through the root. The results of this study showed that the growth of both shoots and roots decreased in all testing cultivars as compared with the control in regarding the fresh weight, dry weight, and length (Tables 1, 2). It could be considered that the inhibition of root growth under cold stress might be related to water uptake, but not nutrients. Because the materials we used were 8 days-old seedlings could consume nutrients from seeds.
Previous literature showed that salt-induced H2O2 generation resulted in an increase in ionically bound cell-wall peroxidase activity and followed with the induction of cell-wall stiffening process and the inhibition of root growth (Lin and Kao 2001a, b). Furthermore, a similar result of H2O2 accumulation was found along with Cd-inhibited root growth of rice seedlings (Cho et al. 2012). The Cd-induced H2O2 accumulation was responsible for the inhibition of CAT activity (Cho et al. 2012). The results of this research showed an accumulation of H2O2 content in rice shoots under cold stress (Fig. 3). However, the indication of oxidative stress was found in rice roots only (Figs. 3 and 4). It is likely that the decrease in shoot growth under cold treatment could be a result of the damage on roots, which reduced transportation of water and nutrients to shoots. Moreover, an increase in electrolyte leakage was found in roots under cold treatment (Fig. 5). Thus, the involvement of oxidative stress in rice roots could be the key role of causing cold damage, especially the generation of H2O2. Therefore, the H2O2 content decreased in roots under cold treatment can be one of the factors associated with cold-stress tolerance in rice seedlings and could be used for biochemical markers in improving cold tolerance of rice seedling.
The elevation of ROS, such as superoxide anion, singlet oxygen, and H2O2, has been found in the plant under cold stress (Kuk et al. 2003; Hung et al. 2008; Bhattacharjee 2013) and associated with an increase in lipid peroxidation and damage of the plasma membrane. As we knew, in responding to oxidative stress, plant tissue will increase the activity of SOD to reduce the ROS level and generate H2O2 (Conklin and Barth 2004; Zhang et al. 2010; Faize et al. 2011, Diaz-Vivancos et al. 2013). Since H2O2 is toxic to the cell, the activities of CAT or APX will be strengthened to decrease H2O2 contents (Chou et al. 2012; Chao et al. 2010). Besides, Lin et al. (2016) have shown that the increase of SOD and CAT activity could accelerate ROS reduction. However, our result showed the SOD and CAT activities were positively correlated with the increases of H2O2 and MDA content (Fig. 6), suggesting the increase of SOD activities promoted H2O2 accumulation, and the increase of CAT activity was not enough to catalyzed H2O2. Nevertheless, if CAT or SOD enzyme activities can be enhanced more and able to reduce H2O2 and MDA content, their role in reducing damage under cold stress could not be ruled out. In summary, lower H2O2 and MDA contents along with lower SOD activity in rice root could be subjected for rice cold tolerant breeding. Certain cultivars with stronger CAT or SOD activities merit further cold testing.
Additional file
Additional file 1: Figure S1. The average monthly loss of rice yield in Taiwan with different climate disaster during 1999–2009. The monthly loss is expressed by the estimation of the cost of money loss (data from Yao and Chen 2009). Arrows are indicating the occurrence of different climate disaster. Table S1. The genetic background of selected rice cultivars for cold treatment.
Acknowledgements
Not applicable.
Abbreviations
- APX
ascorbic peroxidase
- CAT
catalase
- GR
glutathione reductase
- H2O2
hydrogen peroxide
- MDA
malondialdehyde
- SOD
superoxide dismutase
Authors’ contributions
CHH and YTH planned and designed the research; CHH performed the experiments. CHH and YTH analyzed and interpreted the data. YTH wrote the manuscript. Both authors read and approved the final manuscript.
Funding
This work was supported by the Council of Agriculture, Executive Yuan, Taiwan. The Grants Numbers are 106AS-17.1.1-ST-a3(1) and 107AS-7.8.1-ST-a3(1).
Availability of data and materials
All data generated during this study are included in this published article and its additional information file.
Ethics approval and consent to participate
Not applicable.
Consent for publication
We agree to the terms of the Springer Open Copyright and License Agreement.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ching Hsin Hsu, Email: cin511167@gmail.com.
Yi Ting Hsu, Phone: +886-422840777, Email: agroting@dragon.nchu.edu.tw.
References
- Aghaee A, Moradi F, Zare-Maivan H, Zarinkamar F, Pour Irandoost H, Sharifi P. Physiological responses of two rice (Oryza sativa L.) genotypes to chilling stress at the seedling stage. Afr J Biotechnol. 2011;10:7617–7621. [Google Scholar]
- Baruah AR, Ishigo-Oka N, Adachi M, Oguma Y, Tokizono Y, Onishi K, Sano Y. Cold tolerance at the early growth stage in wild and cultivated rice. Euphytica. 2009;165:459–470. [Google Scholar]
- Bhattacharjee S. Heat and chilling induced disruption of redox homeostasis and its regulation by hydrogen peroxide in germinating rice seeds (Oryza sativa L., Cultivar Ratna) Physiol Mol Biol Plants. 2013;19:199–207. doi: 10.1007/s12298-012-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981;21:43–47. [Google Scholar]
- Bonnecarrère V, Borsani O, Díaz P, Capdevielle F, Blanco P, Monza J. Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Science. 2011;180(3):726–732. doi: 10.1016/j.plantsci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biol. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB. Heterogeneity in the chemistry, structure, and function of plant cell walls. Nat Chem Biol. 2010;6:724–732. doi: 10.1038/nchembio.439. [DOI] [PubMed] [Google Scholar]
- Chao YY, Hong CY, Kao CH. The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol Biochem. 2010;48:374–381. doi: 10.1016/j.plaphy.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Cho SC, Chao YY, Hong CY, Kao CH. The role of hydrogen peroxide in cadmium inhibited root growth of rice seedlings. Plant Growth Regul. 2012;66:27–35. [Google Scholar]
- Chou TS, Chao YY, Kao CH. Involvement of hydrogen peroxide in heat shock- and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J Plant Physiol. 2012;169:478–486. doi: 10.1016/j.jplph.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004;27:959–970. [Google Scholar]
- Dashtmian PF, Hosseini MK, Esfahani M. Alleviating harmful effects of chilling stress on rice seedling via application of spermidine as seed priming factor. Afr J Agric Res. 2014;9:1412–1418. [Google Scholar]
- Diaz-Vivancos P, Faize M, Barba-Espin G, Faize L, Petri C, Hernández JA, Burgos L. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol J. 2013;11:976–985. doi: 10.1111/pbi.12090. [DOI] [PubMed] [Google Scholar]
- Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, Clemente-Moreno MJ, Alcobendas R, Artlip T, Hernandez JA. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599–2613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- Foster JG, Hess JL. Superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980;66:482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. Cross-linking of matrix polymers in the growing cells of angiosperms. Annu Rev Plant Physiol. 1986;37:165–186. [Google Scholar]
- Health RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Kao CH. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul. 2004;42:227–238. [Google Scholar]
- Hsu YT, Kao CH. Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil. 2007;298:231–241. [Google Scholar]
- Hung KT, Cheng DG, Hsu YT, Kao CH. Abscisic acid-induced hydrogen peroxide is required for anthocyanin accumulation in leaves of rice seedlings. J Plant Physiol. 2008;165:1280–1287. doi: 10.1016/j.jplph.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jana S, Choudhuri MA. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot. 1982;12:345–354. [Google Scholar]
- Kato M, Shimizu S. Chlorophyll metabolism in higher plants VI. Involvement of peroxidase in chlorophyll degradation. Plant Cell Physiol. 1985;26:1291–1301. [Google Scholar]
- Knox JP. Revealing the structural and functional diversity of plant cell walls. Curr Opin Plant Biol. 2008;11:308–313. doi: 10.1016/j.pbi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Kuk YI, Shin JS, Burgos NR, Hwang TE, Han O, Cho BH, Jung S, Guh JO. Antioxidative enzymes offer protection from chilling damage in rice plants. Crop Sci. 2003;43:2109–2117. [Google Scholar]
- Lin CC, Kao CH. NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil. 1999;216:147–155. [Google Scholar]
- Lin CC, Kao CH. Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil. 2001;230:135–143. doi: 10.1016/s0168-9452(00)00396-4. [DOI] [PubMed] [Google Scholar]
- Lin CC, Kao CH. Abscisic acid-induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci. 2001;160:323–329. doi: 10.1016/s0168-9452(00)00396-4. [DOI] [PubMed] [Google Scholar]
- Lin J, Zou Y, Cao K, Ma C, Chen Z. The impact of heterologous catalase expression and superoxide dismutase overexpression on enhancing the oxidative resistance in Lactobacillus casei. J Ind Microbiol Biotechnol. 2016;43(5):703–711. doi: 10.1007/s10295-016-1752-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Shuai J, Wang P, Shi W, Tao F, Chen Y. Impact of chilling injury and global warming on rice yield in Heilongjiang Province. J Geogr Sci. 2013;23:85–97. [Google Scholar]
- Morsy MR, Jouve L, Hausman J, Hoffmann L, Stewart JM. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J Plant Physiol. 2006;162:157–167. doi: 10.1016/j.jplph.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Neilson KA, Scafaro AP, Chick JM, George IS, Sluyter SCV, Gygi SP, Atewell BJ, Haynes PA. The influence of signals from chilled roots on the proteome of shoot tissues in rice seedlings. Proteomics. 2013;13:1922–1933. doi: 10.1002/pmic.201200475. [DOI] [PubMed] [Google Scholar]
- Paoletti F, Aldinucci D, Mocali A, Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem. 1986;153:36–541. doi: 10.1016/0003-2697(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Sampathkumar A, Yan A, Krupinski P, Meyerowitz EM. Physical forces regulate plant development and morphogenesis. Curr Biol. 2014;24:R475–R483. doi: 10.1016/j.cub.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- Setter TL, Greenway H. Growth reductions of rice at low root temperature decreases in nutrient uptake and development of chlorosis. J Exp Bot. 1988;39:811–819. [Google Scholar]
- Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, Miller HL, Cheng Z. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. New York: Cambridge University Press; 2007. [Google Scholar]
- Wintermans IFGM, De Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochem Biophys Acta. 1965;109:448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Yan SP, Zhang QY, Tang ZC, Su WA. Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteom. 2006;5:484–496. doi: 10.1074/mcp.M500251-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, Wang T, Li H, Ye Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2010;30:389–398. doi: 10.1007/s00299-010-0939-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The average monthly loss of rice yield in Taiwan with different climate disaster during 1999–2009. The monthly loss is expressed by the estimation of the cost of money loss (data from Yao and Chen 2009). Arrows are indicating the occurrence of different climate disaster. Table S1. The genetic background of selected rice cultivars for cold treatment.
Data Availability Statement
All data generated during this study are included in this published article and its additional information file.