Abstract
Aim
To evaluate the in vivo antibacterial efficacy of 2% chlorhexidine gel (CHX), 2% chitosan (CS) gel and their combination as an intracanal medicament against Enterococcus faecalis during endodontic retreatment procedure, with the use of qPCR.
Method
A total of 45 single rooted permanent teeth were selected from 28 systemically healthy patients (mean age of 43 years). After complete disinfection and access opening as well as gutta-percha (GP) removal, the first microbiological pretreatment sample (S1) was collected. After completion of instrumentation, a post-instrumentation sample (S2) was taken and the teeth were randomly divided into three experimental groups: 2% CHX gel; 2% CS gel; 2% CHX with CS; Fuji IX glass ionomer cement was used to seal the access; after 7 days, the post medication sample (S3) was collected and E. faecalis was quantified using qPCR.
Results
Maximum reduction in bacterial mean CFU (×106) counts was observed between S1 and S2; S2 and S3; S1 and S3 in 2% CHX with chitosan group compared to other groups. Percentage reduction in CFU (×106) counts at different time intervals (S1-S2; S2-S3; S1-S3) was maximum for the 2% CHX with the chitosan group. Comparison of the mean CFU (×106) count within the 2% CHX gel group and the 2% CHX with chitosan group at different time intervals (S1,S2,S3) was found to be highly significant (P = 0.001); whereas in group II, it was significant (P = 0.002).
Conclusion
2% CHX with chitosan group showed the highest microbial reduction against E. faecalis during retreatment of failed endodontic cases.
1. Introduction
Poor coronal seal and persistent microorganisms after root canal therapy are the main reasons for the endodontic failure. Research shows that microorganisms can be found in 35–100% of endodontic failure cases with persistent periradicular inflammatory lesion after treatment (Gomes et al., 2004, Gomes et al., 2006, Kaufman et al., 2005). Enterococcus faecalis (E. faecalis) prevalence in root canal filled teeth associated with a periradicular lesion is 29–77% (Siqueria and Rôças, 2004).
Deeper penetration of E. faecalis into dentinal tubules may be due to its acquired resistance to many antibiotics owing to activation of virulence factors and biofilm formation, thereby, resisting chemo-mechanical preparation. Evidence shows that they are resistant to calcium hydroxide and sensitive to chlorhexidine gluconate (CHX) (Siqueria and Rocas, 2004). Based on its broad-spectrum antibacterial activity and substantivity to dentin (Zehnder, 2006, Stuart et al., 2006), CHX is commonly used during endodontic retreatment procedures (Zehnder, 2006, Stuart et al., 2006). However, the infected dentin must be exposed to CHX for a longer time period to achieve long-term substantivity. To sustain the release of chlorhexidine from the device, a controlled release drug device is more effective. (Heling et al., 1992, Ballal et al., 2009).
To increase the intracanal medicament stability, insolubility chitosan (CS) can be used as a medicament carrier offering the advantage of the slow and controlled release of intracanal medicaments (Ballal et al., 2009). Chitosan is a naturally occurring polysaccharide polymer which consists of copolymers of glucosamine and N-acetyl glucosamine. It has various applications in the preparation of nanoparticles because of its antimicrobial, antifungal, biodegradable and non-toxic properties (Raafat and Sahl, 2009). Yet, there are no sufficient in vivo data on the antimicrobial effect of intracanal medicaments using chitosan itself or as a carrier against E. faecalis in failed endodontic cases.
The conventional polymerase chain reaction cannot differentiate between viable and nonviable microorganisms; but can detect only the presence or absence of microorganisms (Siqueira, 2002). Also, the standard PCR requires post-amplification processing to separate and identify individual PCR products. These limitations are addressed in real-time quantitative PCR (qPCR) (Siqueria and Rôças, 2005). Clinical studies using qPCR in cases of failed endodontic therapy are rare and the antimicrobial effect of CHX plus CS combination against E. faecalis during retreatment procedures has not been fully examined in vivo.
Therefore, the aim of the present study is to analyze and quantify the antimicrobial effect of 2% CS gel, 2% CHX gel and their combination as an intracanal medicament in the endodontic retreatment procedures against E. faecalis, using RT qPCR.
2. Materials and method
2.1. Patients selection
Twenty eight patients were selected from those who attended the oxford dental college for non-surgical endodontic retreatment. The human volunteer’s research and Ethics committee of the oxford dental college approved the protocol (IEC no.163/15/16), describing sample collection for this investigation and signed informed consent forms were taken from all patients, prior to sample size calculation:
Sample size determination:
Fischer’s formula corrected by Hazoor Bazar’s theoretical correction
(Z = abscissa of normal curve = 1.96, p = level of significance = 0.05; n = odd ratio; confidence limit = 95%).
Inclusion Criteria
-
•
Teeth requiring root canal retreatment; symptomatic teeth with or without lesion
-
•
Root filled teeth which showed radiographic evidence of periradicular disease.
-
•
Root canal treated more than 2–4 years earlier
-
•
Teeth with enough crown structure for adequate isolation with rubber dam.
-
•
Gutta-percha material within 4–7 mm of the radiographic apex and no direct exposure to oral cavity was evident
Exclusion Criteria
-
•
Medically compromised patients or patients who are allergic to drugs
-
•
Existence of periodontal pocket deeper than 4 mm
-
•
Antibiotics use within the past 30 days and patients who had some general disease
-
•
Teeth having intraradicular posts.
A total of 40 incisors were included of which 34 were maxillary, 6 were mandibular and 5 were mandibular premolars.
2.2. Microbial sampling
Retreatment and sampling procedures were performed by the same endodontic specialist. Strict aseptic conditions were used to collect all samples. The tooth and surrounding field were then cleansed with hydrogen peroxide (30% H2O2) and decontaminated with sodium hypochlorite (3% NaOCl) solution followed by isolation with rubber dam. Sterile high-speed endo access bur (Dentsply Maillefer, Ballaigues, Switzerland) was used until the root canal filling was exposed, followed by the above mentioned disinfection protocol. Swabbing of the access cavity was done using sodium thiosulfate solution (5%) to inactivate the NaOCl, so that the remnants of NaOCl would not influence the bacteriologic sampling (Gomes et al., 2004, Möller, 1966). Coronally, gutta-percha was removed using proTaper retreatment files (ProTaper Universal, Dentsply Maillefer), following the manufacturer’s instructions and Hedstrom file or K-file was used to retrieve apical GP. No solvent was used at any time. The filling material removed from the canals was transferred to the sterile tube containing 0.75 ml of reduced transport fluid (RTF). Intra oral periapical radiographs were used to ensure the canal cleanliness after all the filling materials were removed. The working length was determined radiographically as well as with an apex locator (Root ZX mini, J Morita Corp, Tokyo, Japan.). A 2 ml sterile saline solution (Nirlife Nirma ltd) was introduced into the canal with a sterile syringe. A sterile Hedstrom file corresponding to the last instrument used was inserted in the canal at working length and pumped vigorously, with reaming motion, to disrupt canal contents. To soak up the fluid in the canal, 2 sterile paper points were placed to the working length. Each paper point was retained in the canal for 1 min and transferred to the sterile tube containing 0.75 ml RTF, along with dentinal shavings and GP remnants. The retrieved gutta-percha and the paper points were used as Sample 1 (S1); (Siqueria, 2007).
2.3. Preparation of root canal
The root canals were prepared with a minimum size of ISO no. 35 and a maximum of ISO no. 60 kept at 0.5–2 mm from the radiographic apex. The preparation of root canal was then completed using K-files and 3% NaOCl solution for irrigation, using a flexible 30-gauge needle, (Cavities, Ultra dent, South Jordan UT, USA) which was kept at 1–2 mm short of working length. Canals were irrigated for a minute with 17% EDTA (5 ml). Thereafter, the root canals were rinsed with 10 ml 3% NaOCl. 5% Sodium thiosulfate solution was used to inactivate the NaOCl, followed by flushing with saline water. Sterile paper points were used to dry the canal and a second root canal sample (S2) was taken as earlier described.
The patients were divided randomly into three groups. Intra-canal dressings were put inside the canals as follows: Group I (n = 15): 2% chlorhexidine gluconate gel [(CHX), Univac Chemicals and Pharmaceuticals, Mumbai, India)]; Group II (n = 15): 2% chitosan gel; Group III (n = 15): 2% CHX gel and 2% chitosan gel (1:1) (Everest Biotic, Bangalore, India).
2.4. Intracanal medicament placement
Canal were dried with paper points after which intracanal medicaments in Group I, II and III were placed in gel form using a lentil spiral (Mani Inc) up to a working length. 3-mm thick layer of Coltosol (Coltene Whaledent, Cuyahoga Falls, OH, USA) and a second layer of Fuji IX glass ionomer (Dentsply Int. Inc. York, PA) were used to seal the access cavity.
During subsequent appointment (after 7 days), the tooth was isolated with rubber dam and disinfected according to the procedure used during the 1st appointment. All restorations were checked for integrity and teeth with dislodged fillings were excluded from the study. The remaining restoration was removed using a sterile bur. After further disinfection and inactivation as mentioned previously, residual intracanal medicament was flushed out with sterile saline and removed using the master apical file.
In Group I, to neutralize CHX, 3% Tween 80 (3 ml) and 0.3% α lecithin were used as shown previously by Zamany and Spangberg (2002) for 5 min followed by saline irrigation. In Group II: 16% sodium carbonate was used to neutralize the chitosan, and in Group III, a combination of the above mentioned neutralizers was used, along with serial dilution using saline water. Root canals were dried to evaluate the residual burden of E. faecalis microorganism, thereafter, the post medicament sample (S3) was taken as earlier described. After sample collection, the root canal treatment was completed. None of the patients in this study needed intervention during treatment due to flare up. All restorations were intact in patients after 7 days recall, hence, no sample was excluded.
All the samples (S1, S2, and S3) were transferred to a microbiology laboratory (Credora Life Sciences, Bangalore, India), for evaluation of E. faecalis count using real- time qPCR within few hours of collection of samples.
2.5. DNA isolation protocol
The DNA from clinical samples was isolated by N- Cetyl- N, N, N-trimethyl- ammonium bromide (CTAB) method. The concentration and purity of DNA was assessed using a spectrophotomer Sartorius (Göttingen Germany).
2.6. Primer synthesis
The primers for quantification analysis were designed using Perkin Elmer Primer Express® software (Massachusetts, USA). The Melting temperature (Tm) was calculated and the synthesized primers were purified by HPLC (Kyoto, Japan). Primers used for quantification included a Forward Primer 5′ GTGTAGCGGTGAAATGCGTAG 3′ and a reverse primer, 5′ GGAAACCCTCCAACACTTAGC 3′ Real-time PCR was performed using a Step One, Applied Biosystems (Foster City, California, USA) with the SYBR Green Chemistry. Each PCR was performed using a total volume of 20 µl, containing 2 µl of 10X FastStart DNA Master SYBR Green. A total of 0.5 µM each of HPLC purified forward and reverse primers were used as well as 1 µl of template DNA. Real-time PCR was carried out with an initial incubation period of 10 min at 95 °C, followed by 35 cycles consisting of denaturing at 95 °C for 10 s; annealing was done at 60 °C for 5 s followed by amplification at 72 °C for 30 s. A melting curve analysis was performed after amplification to determine the specificity of the PCR products. For melting curve analysis, PCR products were incubated for 15 s at 5 °C below the annealing temperature for the respective primers and the temperature was increased to 95 °C at a ramp rate of 0.1/s. All reactions were performed in duplicates against a serially diluted standard. Threshold cycle (Ct) analysis of all samples were either set at 0.5 relative fluorescence units or left to automatic detection by the system. The standard curve was plotted as Ct versus log 10 concentrations (Fig. 1).
Fig. 1.
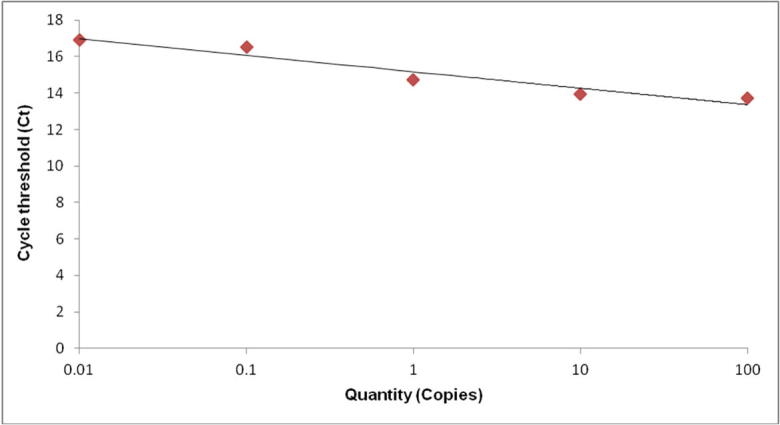
Cycle threshold (CT) values determined by qPCR (log10 copies/µl) E. faecalis standard curve generated by qPCR; the figure shows a representative experiment plotting the log 10 cycle threshold value (Ct) of each qPCR reaction with its corresponding input log10 DNA concentration.
2.7. Statistical analysis
Statistical analysis was performed using the statistical package SPSS (Statistical Presentation System Software, version 23.0 (SPSS Inc.; Chicago, IL, USA).
Quantitative data obtained for all groups were compared. After performing test of normality (Shapiro Wilk test), the data was found to be abnormally distributed and hence, the non-parametric Kruskal Wallis test was used. The Kruskal Wallis test was chosen to determine the percentage reduction after each treatment step (S1, S2 & S3) in all three groups, based on the quantitative data obtained from samples (Fig. 2).
Fig. 2.

Percentage reduction of CFU (×106) count in Group I, Group II and Group III from S1, S2 and S3.
Kruskal Wallis test was used to compare the mean CFU (x106) counts between the groups at S1, S2 and S3 time intervals. (Table 1, Table 2). Friedman’s repeated measures were applied to compare the mean CFU (×106) count within the groups at different time intervals, S1, S2 and S3 (Table 3). Wilcoxon sign rank test was used for pair wise comparison in all three groups at S1-S2; S1-S3; and S2-S3 (Table 4).
Table 1.
Mean CFU (×106) count of the three groups at different time intervals.
| Time interval | Group I Mean (SD) |
Group II |
Group III Mean (SD) |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | |
| S1 | 2.27 ± 2.96 | 984,200 | 2.2 ± 3.16 | 894,300 | 2.16 ± 4.61 | 61,716 |
| S2 | 0.78 ± 1.6 | 116,098 | 0.96 ± 2.22 | 436,900 | 0.76 ± 1.57 | 32,472 |
| S3 | 0.61 ± 1.34 | 86,945 | 0.59 ± 8.52 | 520,401 | 0.35 ± 0.69 | 21,454 |
Table 2.
Comparison of mean CFU (×106) count between the groups at S1, S2 & S3 time period.
| Time period | Groups | N | Median | Mean Rank | χ2 value | p value |
|---|---|---|---|---|---|---|
| S1 | GROUP I | 15 | 984,200 | 26.93 | 7.66 | 0.02* |
| GROUP II | 15 | 894,300 | 26.73 | |||
| GROUP III | 15 | 61,716 | 15.33 | |||
| S2 | GROUP I | 15 | 116,098 | 19.23 | 17.63 | 0.001** |
| GROUP II | 15 | 436,900 | 34.40 | |||
| GROUP III | 15 | 32,472 | 15.37 | |||
| S3 | GROUP I | 15 | 86,945 | 23.63 | 6.141 | 0.04* |
| GROUP II | 15 | 520,401 | 28.60 | |||
| GROUP III | 15 | 21,454 | 16.77 | |||
Kruskal – Wallis test applied.
p < 0.05.
p < 0.001.
Table 3.
Comparison of the mean CFU (×106) count within the groups at different time intervals.
| Group | Time Interval | Mean (SD) | Median | χ2 | p value |
|---|---|---|---|---|---|
| GROUP I | S1 | 2.27 ± 2.96 | 984,200 | 30 | 0.001** |
| S2 | 0.78 ± 1.6 | 116,098 | |||
| S3 | 0.61 ± 1.34 | 86,945 | |||
| GROUP II | S1 | 2.2 ± 3.16 | 894,300 | 12.9 | 0.002** |
| S2 | 0.96 ± 2.22 | 436,900 | |||
| S3 | 0.59 ± 8.52 | 520,401 | |||
| GROUP III | S1 | 2.16 ± 4.61 | 2.16 ± 4.61 | 30 | 0.001** |
| S2 | 0.76 ± 1.57 | 0.76 ± 1.57 | |||
| S3 | 0.35 ± 0.69 | 0.35 ± 0.69 | |||
Friedman’s repeated measures applied.
*p < 0.05.
p < 0.001.
Table 4.
Comparison of mean CFU (×106) count in the groups from S1, S2 and S3.
| Groups | Reference Time | p value | |
|---|---|---|---|
| GROUP I | S1 | S2 | 0.001** |
| S3 | 0.001** | ||
| S2 | S3 | 0.001** | |
| GROUP II | S1 | S2 | 0.03* |
| S3 | 0.008** | ||
| S2 | S3 | 0.004** | |
| GROUP III | S1 | S2 | 0.001** |
| S3 | 0.001** | ||
| S2 | S3 | 0.001** | |
Wilcoxon sign rank test applied.
p < 0.05.
p < 0.001.
3. Results
Mean CFU in group I (CHX group) at S1 2.27 + 2.96 × 106 CFU/ml ; at S2 0.78 + 1.6 × 106 CFU/ml CFU/ml at S3 0.61 + 1.34 × 106 CFU/ml; Mean CFU in group II (CS) at S1 2.2 + 3.16 × 106 CFU/ml; S2 0.96 + 2.22 × 106 CFU/ml; S3 0.59 + 8.52 × 106 CFU/ml; in group III (CHX + CS) at S1 2.16 + 4.61 × 106 CFU/ml; at S2 0.76 + 1.57 × 106 CFU/ml; at S3 0.35 + 0.69 × 106 CFU/ml; a significant reduction in bacterial count was observed in Group III (CHX + CS) compared to other groups; (Table 1).
Percentage reduction along three time periods, S1-S2; S2-S3 and S1-S3 were done in all groups. At S2-S3, group I (CHX) showed 21%; group II (CS) showed 38% and Group III (CHX + CS) showed 52%. At S1-S3, CHX group showed mean reduction of 73%; CS group showed mean reduction of 72% and CHX + CS group showed mean reduction of 83%.; which recorded the highest among all groups (Fig. 2). Comparison between the mean CFU (x106) count within group I and group III at different time intervals (S1,S2,S3) was found to be highly significant (P = 0.001); (Tables 1 & 3), whereas in group II, it was significant (P = 0.002); pair wise comparison at different time intervals from S1-S2; S1- S3; S2-S3 for groups I and III was highly significant (P = 0.001) (Table 4) compared to group II.
On intergroup comparisons, mean rank at S1 time period for group I was 26.93, group II 26.73, group III 15.33; mean rank at S2 time period for group I was 19.23; Group II was 34.14; for Group III was 15.37; mean rank at S3 time period for group I was 23.63, for group II was 28.60, for group III was 16.77; group III showed the lowest mean rank which was significant at all three time periods (Table 2); in all the groups, the mean CFU count difference at S2 time period was significant (P = 0.001) (Table 3).
4. Discussion
The sensitivity of the molecular method is excellent due to its ability to detect yet uncultivated bacteria compared to the culture technique. Unculturability can be related to the viable but non-cultivable (VBNC) state (Lleo et al., 2007). Williams et al. (2006) detected VBNC E faecalis in 4 samples among a total of 7 E. faecalis positive samples. Thus, molecular techniques seem to be more useful in detecting bacteria after antimicrobial therapy.
On intergroup comparison, all three groups showed significance at S1 and S2 time periods (P = 0.001); the result is in accordance with various clinical studies that have reported that chemo mechanical procedures with alternating irrigation (3% NaOCl and 17% EDTA) reduce the population of microorganisms in the root canal system (Siqueira, 2002, Stuart et al., 2006).
Even though the present protocol reduced E. faecalis counts, the bacterium was not totally eliminated from the treated root canals. Intracanal medicaments significantly reduced the micro flora and play a significant role in multi-visit endodontic retreatment (Barbosa et al. (1997)). In the present study, after instrumentation and irrigation, the intracanal medicament like 2% CHX, 2% chitosan and 2% CHX + CS was placed in group I, II and group III respectively for 7 days and Fuji IX glass ionomer cement restoration was placed to prevent root canal reinfection.
In group I, the mean number of CFUs in post instrumentation (S2) was 0.78 + 1.6, post-medication sample (S3) was 0.61 + 1.34 CFU/ml, and percentage reduction in group I at S2-S3 was 21%; and S1-S3 was 73%; (Fig. 2) Similar results were found in a clinical study which showed 78% negative culture after 7 days (Barbosa et. al. (1997). Some in vitro studies (Zerella et al., 2005, Siqueria et al., 2007, Ballal et al., 2009) showed that CHX was effective in retreatment cases against resistant organisms like E. faecalis, anaerobic bacteria and Candida albicans. Fouad and Barry (2005), in their investigation demonstrated that 2% concentration is nearly 17 times stronger than 0.12% concentration. Present in vivo study, used 2% CHX gel along with instrumentation for better mechanical cleansing of the anatomical complexities of the root canal which acts as a lubricant, thereby reducing stress on the files used (Gomes et al., 2001). The results of the present study showed that there was a significant reduction of bacteria in the CHX group. Comparison of the mean CFU (×106) count within the group I at different time intervals (S1-S2; S1-S3 and S2-S3) was also found to be highly significant [Table 3(P = 0.001)]; This has been explained by the fact that CHX, has immediate antibacterial effects as it increases membrane permeability, leading to leakage of low molecular weight ions like potassium ions and phosphorus ions at low concentration and precipitation of cytoplasmic contents at higher concentration. It may be absorbed by dental tissues and impart substantive antimicrobial activity when used as intracanal medicament (Basrani et al., 2002). Ercan et al also in his study, found a higher reduction with E-faecalis and Candida after a 7-day application (Ercan et al., 2006).
Comparison of the mean CFU(×106) count within group II at different time intervals was found to be significant [Table 3 (P = 0.002)]; Antimicrobial action of chitosan might be due to the positively charged NH3+ groups of glucosamine that interact with negatively charged surface components of bacteria, resulting in extensive cell surface attraction, leakage of intracellular substances, which causes damage to vital bacterial activities (Raafat and Sahl, 2009). The biodegradable property of chitosan is also an added advantage for its intracanal use. Studies have proved its antifungal effect (Staroniewiez et al., 1994), but its antimicrobial action against E. faecalis in endodontic retreatment was not known. So in the present study, 2% chitosan was used alone as an intracanal medicament and it showed a significant reduction in bacterial count from S2 to S3 and S1 to S3. Furthermore, due to the complexity of root canal infection, a single irrigant or a medicament could not result in effective sterilization of the root canal. In vitro studies have shown that the combination of two medicaments may produce additive or synergistic effect and thus, the antimicrobial action might last longer and also sustain the release of medicaments, thereby decreasing the development of resistant bacterial strains, as reported by earlier studies (Heling et al., 1992, Basrani et al., 2002, Ballal et al., 2009).
On intergroup comparison, mean rank at S2 time period for group I was 19.23; Group II was 34.40; for Group III was 15.37; mean rank at S3 time period for group I was 23.63, for group II was 28.60, for group III was 16.77; group III showed the lowest mean rank which made it significant at all three time periods (Table 2). Comparison of the mean CFU(×106) count within the group I and group III at different time intervals (S1, S2, S3) was found to be highly significant (P = 0.001) compared to group II (P = 0.004). Pair wise comparison at different time intervals from S1-S2; S1-S3; S2-S3 for groups I and III was highly significant compared to group II (Table 4). Percentage reduction at S2-S3 and S1-S3 in group III was 52% and 83%, respectively which was highest among the groups (Fig. 2). This is in accordance with some in vitro studies which showed that the combination of two medicaments may produce additive or synergistic effect and thus the antimicrobial action might last longer and also sustain the release of medicaments, thereby decreasing the development of resistant bacterial strains (Ballal et al., 2009). There is the possibility that CHX combined with chitosan inhibit the growth of E. faecalis and affects their re-entry and recolonization. Moreover, chitosan as a drug carrier has the advantages of slow and controlled drug release, which improves drug solubility and stability, thereby enhancing efficacy and reduced toxicity (Raafat and Sahl, 2009). The higher reduction found in this study was possible due to better compaction of medicaments and radio opacity. Also, the use of large apical sizes (size 35 to size 60) may have removed more infected dentin and allowed irrigation and intracanal medication to be more effective (Card et al., 2002).
5. Conclusion
In conclusion, with in the limitations of the present study, 2% CHX was effective against E. faecalis. Whereas, combination of CHX with Chitosan had the highest synergistic antibacterial effect against E. faecalis, and can be used as an intracanal medicament in failed endodontic procedures. Future investigations are required using better qualitative and quantitative isolation methods of various microorganisms and alternate intracanal medicaments should be tested against individual root canal microorganisms.
Declaration of interest
None.
Acknowledgement
The study was supported by grants from the Rajiv Gandhi University Of Health Sciences, Bangalore (ORDER NO. RGU: Adv. Res.: Proposal-D-08:2015-16). The authors wish to thank Dr. Megha, Dr. Rijesh, Dr. Asha, Dr. Archana, Dr. Shwetha, Dr. Shruti, Dr. Kevin, for their assistance in the research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Barbosa C.A., Goncalves R.B., Siqueira J.F., Jr, De Uzeda M. Evaluation of the antibacterial activities of calcium hydroxide, chlorhexidine, and camphorated paramonochlorophenol as intracanal medicament. A clinical and laboratory study. J. Endod. 1997;23:297–300. doi: 10.1016/S0099-2399(97)80409-8. [DOI] [PubMed] [Google Scholar]
- Basrani B., Santos J.M., Tjӓderhane L., Grad H., Gorduysus O., Huang J. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002;94:240–245. doi: 10.1067/moe.2002.124002. [DOI] [PubMed] [Google Scholar]
- Ballal N.V., Kundabala M., Bhat K.S., Acharya S., Ballal M., Kumar R. Susceptibility of Candida albicans and Enterococcus faecalis to chitosan, chlorhexidine gluconate and their combination in vitro. Aust. Endod. J. 2009;35:29–33. doi: 10.1111/j.1747-4477.2008.00126.x. [DOI] [PubMed] [Google Scholar]
- Card S.J., Sigurdsson A., Orstavik D., Trope M. The effectiveness of increased apical enlargement in reducing intracanal bacteria. J. Endod. 2002;28:779–783. doi: 10.1097/00004770-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Ercan E., Dalli M., Dulgergil C.T. in vitro assessment of effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;96:618–624. doi: 10.1016/j.tripleo.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Fouad A.F., Zerella J., Barry J. The effect of antibiotics endodontic antimicrobials on the polymerase chain reaction. J. Endod. 2005;96:618–624. doi: 10.1097/01.don.0000152899.54247.4e. [DOI] [PubMed] [Google Scholar]
- Gomes B.P., Ferraz C.C., Vianna M.E., Berber V.B., Teixeria F.B., Souza-Filho F.J. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus Faecalis. Int. Endod. Ontic. J. 2001;34:424–428. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- Gomes B.P., Pinheiro E.T., Gadȇ- Neto C.R., Sousa E.L., Ferraz C.C., Zaia A.A. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004;19:71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- Gomes B.P., Pinheiro E.T., Sousa E.L. E. faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006;102:247–253. doi: 10.1016/j.tripleo.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Heling I., Steinberg D., Kenig S., Gavrilovich I., Sela M.N., Friedman M. efficacy of sustained release device containing chlorhexidine and calcium hydroxide in preventing secondary infection of dentinal tubules. Int. Endod. Ontic J. 1992;25:20–24. doi: 10.1111/j.1365-2591.1992.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Kaufman B., Spangberg L., Barry J., Fouad A.F. Enterococcus spp. in endodontically treated teeth with and without periradicular lesions. J. Endod. 2005;31:851–856. doi: 10.1097/01.don.0000164133.04548.26. [DOI] [PubMed] [Google Scholar]
- Lleo M., Bonato B., Tafi M. Adhesion to medical device materials and biofilm formation capability of some species of enterococci in different physiological states. FEMS Microbiol. Lett. 2007;274:232–237. doi: 10.1111/j.1574-6968.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- Möller Å.J. Microbiological examination of root canals and periapical tissues of human teeth Methodological studies. Odontol. Tidskr J. 1966;74:1–380. [PubMed] [Google Scholar]
- Raafat D., Sahl H.G. Chitosan and its antimicrobial potential – a critical literature survey. Microb. Biotechnol. 2009;2:186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staroniewiez, Z., Ramisz, A., Wojtosz-Pajak, A., Brzeski, M.M., 1994. Studies on antibacterial and antifungal activity of chitosan. In: Karnicki et al. (Ed.), NW-Verlag Furneve, pp. 374–377.
- Siqueira J.F., Jr. Endodontic infections: concepts, paradigms, and perspectives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;94:281–293. doi: 10.1067/moe.2002.126163. [DOI] [PubMed] [Google Scholar]
- Siqueria J.F., Rôças I.N. Polymerase chain reaction based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- Siqueria J.F., Jr, Rôças I.N. Exploiting molecular methods to explore endodontic infections: part 1 –current molecular technologies for microbiological diagnosis. J. Endod. 2005;31:411–423. doi: 10.1097/01.don.0000157989.44949.26. [DOI] [PubMed] [Google Scholar]
- Stuart C.H., Schwartz S.A., Beeson T.J., Owatz C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006;32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Siqueria J.F., Paiva S.S.M., Rôcas I.N. Reduction in the cultivable bacterial populations infected root canals by a chlorhexidine -based antimicrobial protocol. J. Endod. 2007;33:541–547. doi: 10.1016/j.joen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Williams J.M., Trope M., Caplan D.J., Shugars D.C. Detection and quantitation of E. faecalis by real-time PCR (qPCR), Reverse Transcription-PCR (RT-PCR), and cultivation during endodontic treatment. J. Endod. 2006;32:715–721. doi: 10.1016/j.joen.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Zamany A., Spångberg L. The effective method of inactivating chlorhexidine. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;93:617–620. doi: 10.1067/moe.2002.122346. [DOI] [PubMed] [Google Scholar]
- Zerella J.A., Fouad A.F., Spangberg L.S. effectiveness of a calcium hydroxide and chlorhexidine gluconate mixture as disinfectant during retreatment of failed endodontic cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005;100:756–761. doi: 10.1016/j.tripleo.2005.05.072. [DOI] [PubMed] [Google Scholar]
- Zehnder M. Root canal irrigants. J. Endod. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]


