Abstract
Background and objectives
Proinflammatory cytokines appear to have a central role in the destruction of periodontal tissues. By binding to Receptor Activator of Nuclear Factor κb (RANK) on osteoclast progenitor cells, these cytokines are locally responsible for the activation of bone resorbing osteoclasts differentiation and function. Interleukin-34 (IL-34) is a proinflammatory cytokine derived from the osteoblasts and plays an important role in osteoclastogenesis. The present study was carried out to assess the levels of IL-34 in Gingival Crevicular Fluid (GCF) in periodontally healthy patients, chronic periodontitis and aggressive periodontitis.
Materials and method
A total of 90 patients were recruited in the study and were divided into 3 groups: Periodontally healthy patients (Group I), Chronic periodontitis (Group II) and Aggressive periodontitis (Group III). Clinical Parameters like plaque index, gingival index, probing depth, clinical attachment levels were recorded and GCF samples were collected at baseline for the assessment of IL-34 levels in all the three groups. IL-34 levels were calculated using Enzyme-Linked Immune-Sorbent Assay (ELISA).
Results
The mean GCF levels of IL-34 in Group I was 47.22 ± 18.04 pg/ml, Group II was 103.76 ± 26.61 pg/ml and in Group III was 191.71 ± 49.24 pg/ml. The mean GCF IL-34 level was found to be higher in Group III followed by Group II and Group I. The IL-34 levels correlated with clinical parameters like plaque index, gingival index, probing depth, clinical attachment levels. But correlation with probing depth and clinical attachment level were significantly higher in Group III followed by Group II and Group I.
Interpretation and conclusion
The study demonstrated higher levels of IL-34 in aggressive periodontitis when compared with chronic periodontitis. IL-34, a novel diagnostic marker which was found to be at higher level in GCF of aggressive and chronic periodontitis patients.
Keywords: Interleukin-34, Aggressive periodontitis, Chronic periodontitis, Cytokine
1. Introduction
Advances in science and technology over the last century have gradually expanded our knowledge regarding the pathogenesis of periodontal diseases (Padmanabhan, 2013).
It is widely accepted that the initiation and progression of periodontitis are dependent on the presence of virulent microorganisms which can cause disease. Although bacteria are the initiating agents in periodontitis, the host response to the pathogenic infection is critical to the disease progression (Offenbacher, 1996). An imbalance between the plaque biofilm and the host immune system results in the overexpression of an array of proinflammatory cytokines, propagation of inflammation through the gingival tissues leading to destruction of connective tissue and the subsequent destruction of alveolar bone. This is the hallmark of periodontal disease (Armitage, 2004a, Armitage, 2004b).
Cytokines are low molecular weight signaling proteins. They are produced by a wide range of cells that play important roles in many physiological responses. Cytokines are involved in immunity and inflammation where they regulate the amplitude and duration of the response (Idelgaufts, 1995).
Several proinflammatory and anti-inflammatory cytokines including IL-1, IL-6, IL-8, IL-10, IL-12, IL-17, IL-18, IL-21, TNFα and IFN-γ have been demonstrated to be involved in the pathogenesis of periodontitis (Kjeldsen et al., 1993). The role of IL-1, IL-6, IL-8, and IL-12 has previously been established in periodontitis proving their definitive role in periodontal destruction (Mootha et al., 2016).
Proinflammatory cytokines increases the bactericidal capacity of phagocytes, recruit additional innate cell population to the site of infection, include dendritic cell maturation and direct the subsequent specific immune response to the invading microbes (Graves and Cochran, 2003). These proinflammatory cytokines also appear to have a central role in periodontal tissue degradation. Proinflammatory cytokines such as TNF-α, interferon (IFN), IL-1, IL-6, IL-12 and IL-17 are present in higher levels in chronic and aggressive periodontitis (Nikolopoulos et al., 2008).
IL-34 is a newly discovered proinflammatory cytokine without significant amino acid sequence homology to other cytokines. This cytokine derived from the osteoblasts, shares common receptors and binds to Macrophage Colony stimulating factor (M-CSF) receptor and possesses similar characteristics to M-CSF in promoting and regulating myeloid cell survival, differentiation and proliferation (Bostrom & Lundberg, 2013).
IL-34 is expressed in the synovial tissues and synovial fluid of rheumatoid arthritis (RA) patients, and is expressed by synovial fibroblasts and associated with synovitis severity in RA patients. The formation of IL-34 by RA-synovial fibroblast is stimulated by TNF-α suggestive of role of IL-34 in the pathogenesis of inflammatory diseases (Hwang et al., 2012).
The similarities between RA and periodontitis are the tissue and bone degenerations that are caused by inflammation and cells like fibroblasts that increases the inflammation by producing inflammatory mediators. Both diseases are considered to be chronic disorders that run in relapses (Berthelot and LeGoff, 2010).
Therefore, the present study is designed to evaluate the levels of IL-34 in GCF of chronic periodontitis (CP) and aggressive periodontitis (AP) patients and to correlate between the levels of IL-34 in GCF and the clinical parameters. This is the first study of its kind correlating levels of IL-34 in GCF of chronic and aggressive periodontitis.
The objectives of the study were to estimate the levels of IL-34 in GCF of periodontally healthy patients, chronic periodontitis and aggressive periodontitis and to compare the IL-34 levels in GCF of periodontally healthy patients, chronic periodontitis and aggressive periodontitis.
2. Methodology
The study was conducted for a period of 1 year between June 2015 to 2016. The patients were recruited from The Department of Periodontics, at a Dental Institution. Before the starting of the study, ethical clearance was obtained from the ethical committee of the same institute. An informed consent was obtained from all the patients.
2.1. Sample size
Samples from ninety patients were subjected to GCF sampling. The sample size was determined based on 1% significant level and the power of the study was 90% (p < 0.05).
Patients in the age group of 20–60 years, presenting to the outpatient unit of Department of Periodontics, with at least 20 natural teeth and diagnosed with chronic or aggressive periodontitis, and had not received periodontal therapy, within preceding six months were included in the study.
Patients who smoked or consumed alcohol, women who were pregnant and/or lactating, patients with Rheumatoid arthritis, Diabetes mellitus, liver disorders or any other systemic disease which could alter the course of periodontal disease and those who had received any anti-inflammatory drugs and antibiotics in the previous three months were excluded from the study.
2.2. Study design
Patients were selected based on inclusion and exclusion criteria and categorized into 3 groups.
Ninety patients were divided into three groups with 30 patients in each group: Group I (Healthy periodontium), Group II (Chronic periodontitis) and Group III (Aggressive periodontitis) based on American Academy of Periodontitis, 2004 (Armitage, 2004a).
2.3. Method of collection of data
Plaque index (Silness and Loe, 1964), gingival index (Loe and Silness, 1963), probing depth, clinical attachment level, bleeding on probing were assessed for all the patients.
The GCF samples from deepest probing depth were collected for IL-34 assessment. The GCF samples from all the patients were collected after 24 h following baseline examination to avoid contamination of the sample with blood. Gingival crevicular fluid samples were obtained from the sites with deepest probing depth. Supragingival plaque of the intended tooth was removed with U 15/30 scaler before sampling. The tooth was air dried prior to obtaining the sample. The GCF collection was done using micropipettes with proper isolation of the site with cotton rolls.
Detection of IL-34 levels was done by using Enzyme Linked Immuno-sorbent Assay (ELISA). Anti-IL-34 antibody was pre-coated into 96-well plates and the biotin conjugated anti- IL-34 antibody was used as detection antibodies. The standards, test samples and biotin conjugated detection antibody were added to the wells subsequently, and washed using wash buffer. Horseradish peroxidise (HRP)-Streptavidin was added and unbound conjugates were washed away with wash buffer. Tetramethylbenzidine (TMB) substrates were used to visualize the HRP enzymatic reaction. TMB was catalyzed by HRP to produce a blue coloured product that changed into yellow after adding acidic stop solution. The density of yellow was proportional to the IL-34 amount of sample captured in plate. Reading of the optical density (O.D.) absorbance was recorded at 450 nm in a microplate reader, and then the concentration of IL-34 was calculated.
GCF samples were stored at −80 °C till the assay procedure was carried out. It was made clear to the potential patients that participation will be voluntary and written informed consent was obtained from those who agreed to participate (Preiano et al., 2016).
3. Results
The study included a total number of 90 patients, comprising of 54 males and 36 females. Patients were categorized into three groups with 30 patients in each group.
Group I – Periodontally healthy
Group II – Chronic Periodontitis
Group III – Aggressive Periodontitis.
In this age and gender matched study, the age of patients ranged from 20 to 60 years. The mean age of patients in Group I was 27.00 ± 2.82 years, in Group II was 40.80 ± 7.65 years and Group III was 30.00 ± 5.27 years.
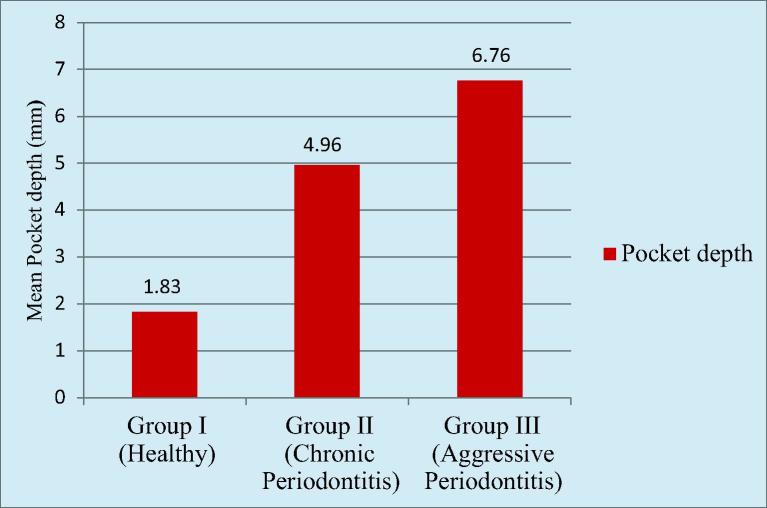
The mean probing depth in Group I was 1.83 ± 0.32 mm. The mean probing depth in Group II was 4.96 ± 1.14 mm. The mean probing depth in Group III was 6.76 ± 0.88 mm. Higher mean PD was recorded in Group III followed by Group II and Group I respectively. There was highly statistically significant difference found between Group I and Group II, Group II and Group III as well as Group I and Group III (Graph 1).
Graph 1.
Comparison of probing depth (pd) among the groups.
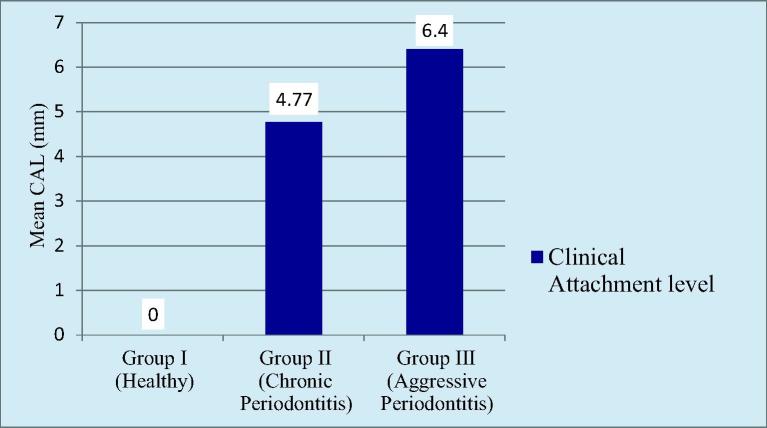
There was no loss of clinical attachment level in Group I. The mean clinical attachment level in Group II was 4.77 ± 1.07 mm. The mean clinical attachment level in Group III was 6.40 ± 0.88 mm. Higher loss in mean clinical attachment level was recorded in Group III followed by Group II. There was highly statistically significant difference found between Group I and Group II, Group II and Group III as well as Group I and Group III (Graph 2).
Graph 2.
Comparision of cal among the groups.
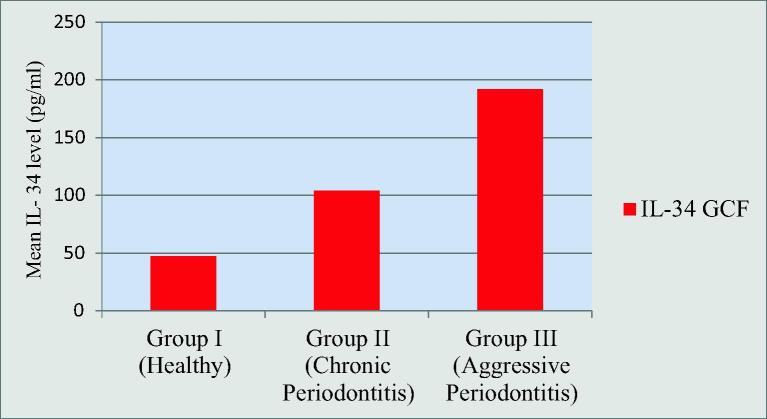
The mean GCF levels of IL-34 in Group I was 47.22 ± 18.04 pg/ml, Group II was 103.76 ± 26.61 pg/ml and in Group III was 191.71 ± 49.24 pg/ml. Higher mean GCF IL-34 value was recorded in Group III followed by Group II and Group I respectively. There was highly statistically significant difference found between Group I and Group II, Group II and Group III as well as between Group I and Group III (Graphs 3).
Graph 3.
Comparison of il-34 levels in gcf among the groups.
Pearson’s correlation coefficient test was used to observe the correlation between clinical parameters i.e. PI, GI, PD, CAL and IL-34 concentration in GCF and Serum. There was gradual increase in the clinical parameters from group I followed by group II and group III and correlation was considered significant at p < 0.01 Table 1, Table 2, Table 3.
Table 1.
Comparison of plaque index among the groups.
| Group | Mean | Standard deviation | 95% Confidence interval for mean |
p-value | Significant difference between groups | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Group I | 0.3600 | 0.09856 | 0.3054 | 0.4146 | <0.001** | I vs II (p < 0.001**) |
| Group II | 1.8933 | 0.26040 | 1.7491 | 2.0375 | <0.001** | II vs III (p < 0.001**) |
| Group III | 1.0600 | 0.23543 | 0.9296 | 1.1904 | <0.001** | I vs III (p < 0.001**) |
Statistically significant.
Table 2.
Comparison of gingival index among the groups.
| Group | Mean | Standard deviation | 95% Confidence interval for mean |
p-value | Significant difference between groups | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Group I | 0.3400 | 0.12421 | 0.2712 | 0.4088 | <0.001** | I vs II (p < 0.001**) |
| Group II | 1.9000 | 0.19272 | 1.7933 | 2.0067 | <0.001** | II vs III (p < 0.001**) |
| Group III | 1.3867 | 0.25033 | 1.2480 | 1.5253 | <0.001** | I vs III (p < 0.001**) |
Statistically significant.
Table 3.
Correlation between PI, GI, PD and Cal with GCF il-34 levels in group I, II and III.
| IL-34 levels |
||||||
|---|---|---|---|---|---|---|
| Group I |
Group II |
Group III |
||||
| Serum | GCF | Serum | GCF | Serum | GCF | |
| PI | 0.091 | 0.393 | 0.874 | 0.217 | 0.9 | 0.883 |
| GI | 0.6 | 0.03 | 0.760 | 0.197 | 0.128 | 0.476 |
| PD | 0.975 | 0.62 | 0.858* | 0.815* | 0.43* | 0.77* |
| CAL | – | – | 0.934* | 0.546* | 0.994* | 0.6* |
4. Discussion
In periodontitis, the initial host response to bacterial infection is a local inflammatory reaction that activates the innate immune system. An imbalance between the plaque biofilm and the host immune system results in the overexpression of an array of proinflammatory cytokines, propagation of inflammation through the gingival tissues and the subsequent destruction of alveolar bone (Armitage, 2004a, Armitage, 2004b).
IL-34 is a newly discovered proinflammatory cytokine without significant amino acid sequence homology to other cytokines. IL-34 is expressed in synovial tissue and synovial fluid of rheumatoid arthritis (RA) patients and is expressed by synovial fibroblasts and is associated with synovitis severity in RA patients (Hwang et al., 2012).
The similarities between RA and periodontitis are the tissue and bone degenerations that are caused by inflammation and cells like fibroblasts that trigger the inflammation by producing inflammatory mediators. Both states are considered to be chronic disorders that run-in relapse (Berthelot and LeGoff, 2010).
Therefore, this study is designed to evaluate the levels of IL-34 in GCF of chronic periodontitis (CP) patients and aggressive periodontitis (AP) patients and to correlate between the levels of IL-34 in GCF and the clinical parameters. This is the first study of its kind correlating levels of IL-34 in GCF in chronic and aggressive periodontitis.
The molecular weight of IL-34 is 22 kDa which is below 1000KDa for passing through gingival cells to appear in gingival crevicular fluid (Hwang et al., 2012). Proinflammatory cytokine, in Gingival Crevicular Fluid (GCF) or tissues adjacent to periodontitis affected sites in patients, has been suggested as a sensitiveand important aid in monitoring the clinical severity of the disease. Cytokine are produced locally inthe periodontal tissues and they canbe readily detected in the Gingival Crevicular Fluid (GCF), which is also an inflammatory exudate that can be easily collected clinically. A study conducted by Rasmussen et aldemonstrated that by collecting and analyzing GCF the detection and quantification of inflammatory cytokines present in the crevice’s fluid can be achieved (Rasmussen et al., 2000). Therefore, this method was used in this study to investigate IL-34 protein levels in GCF from healthy and inflamed gingival sulcus.
On comparison of plaque index among the groups, the mean plaque index value was found to be statistically highly significant between Group I and Group II, Group II and Group III as well as Group I and Group III. Plaque index score was higher in chronic periodontitis group compared to aggressive periodontitis and healthy individual and similarly aggressive periodontitis group showed increased plaque index score on comparison with healthy individuals.
The amount of plaque accumulation in aggressive periodontitis is minimal on the affected teeth but the plaque that is present contains elevated levels of specific microorganism like Aggregatibacter actinomycetumcomitans (A.a) and Porphyromonas gingivalis (P.g). Thus, this minimal qualitative plaque is associated with aggressive periodontitis whereas chronic periodontitis is associated with increased plaque accumulation with various plaque retentive factors such as calculus etc. (Newman et al., 2011). This finding is supported by study carried out by Tobon-Arroyave et al., 2008, Mengel et al., 2002 demonstrated higher plaque index in chronic periodontitis as compared to aggressive periodontitis.
Gingival index is used to assess the degree and severity of gingival inflammation in individual patients. On comparison of mean gingival index between the groups, the mean gingival index value was found to be statistically highly significant between Group I and Group II, Group II and Group III as well as Group I and Group III. Gingival inflammation was found to be higher in the chronic periodontitis group compared to aggressive periodontitis and healthy individual and similarly aggressive periodontitis group showed increased gingival inflammation score on comparison with healthy individuals. This finding is supported by a study conducted by Kurtis et al (2005), which demonstrated increase gingival inflammation in chronic periodontitis as compared to aggressive periodontitis due to increase plaque accumulation and plaque retentive factors.
The difference in mean PD and CAL value was found to be statistically significant between Group I and Group II, Group II and Group III as well as Group I and Group III. PD and loss of CAL values obtained were higher in aggressive periodontitis group compared to chronic periodontitis and healthy individual. Similarly, periodontitis group showed increased PD and CAL value on comparison with healthy individuals. This can be attributed to severe and rapid rate of destruction of the periodontal tissues in aggressive periodontitis as compared to chronic periodontitis (Duarte et al., 2015).
The mean GCF levels of IL-34 in Group I was 47.22 ± 18.04 pg/ml, Group II was 103.76 ± 26.61 pg/ml and in Group III was 191.71 ± 49.24 pg/ml. Higher mean GCF IL-34 value was recorded in Group III followed by Group II and Group I respectively. There was gradual rise in levels of IL-34 in GCF, from Group I followed by Group II and Group III. This might suggest a potential cellular hyperactivity that may favor periodontal destruction in aggressive periodontitis.
A study carried out by Ertugrul et al. (2013) demonstrated that the aggressive periodontitis patients presented with higher GCF levels of cytokines than patients with chronic periodontitis and this rise was due to gene polymorphisms coding for the synthesis of inflammatory mediators.
There was a positive correlation of IL-34 GCF levels with the clinical parameters like plaque index, gingival index, probing depth, clinical attachment levels. But correlation with probing depth and clinical attachment level were significantly higher in Group III followed by Group II and Group I. This can be attributed to a rapid rate of destruction of the periodontal tissues resulting in deeper periodontal pocket and advanced bone loss in aggressive periodontitis as compared to chronic periodontitis as demonstrated by Kurtis et al. (2005).
The limitation of the present study is, it did not include intervention with non-surgical therapy which would have altered the inflammatory status and reduced the morbidity of periodontitis.
5. Conclusion
The levels of IL-34 in GCF shows an increase in level from Group I (Healthy) followed by Group II (Chronic periodontitis) and Group III (Aggressive periodontitis).
There was a positive correlation of IL-34 level with the clinical parameters like plaque index, gingival index, probing depth, clinical attachment level. But correlation with probing depth and clinical attachment level was significantly higher in Group III followed by Group II and Group I.
IL-34, a novel diagnostic marker which was found to be at higher level in GCF of aggressive and chronic periodontitis patients, can be valuable for screening of large population and detecting high risk individuals with aggressive and chronic periodontitis patients. In the future, a greater number of multi-centric, interventional prospective studies with a large sample size should be conducted to evaluate and standardize the levels of Interleukin-34 before and after periodontal therapy.
Conflict of interest
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Armitage G.C. Periodontal diagnosis and classification of periodontal diseases. Periodontol. 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Armitage G.C. The complete periodontal examination. J. Periodontol. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. [DOI] [PubMed] [Google Scholar]
- Berthelot J.M., LeGoff B. Rheumatoid arthritis and periodontal disease. Joint Bone Spine. 2010;7:537–541. doi: 10.1016/j.jbspin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Bostrom E.A., Lundberg P. The newly discovered cytokine IL-34 is expressed in gingival fibroblasts, shows enhanced expression by pro inflammatory cytokines, and stimulates osteoclast differentiation. PLos One. 2013;8:e81665. doi: 10.1371/journal.pone.0081665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte P.M., Bastos M.F., Fermiano D. Do subjects with aggressive and chronic periodontitis exhibit a different cytokine/ chemokine profile in the gingival crevicular fluid? A systematic review. J. Periodontal. Res. 2015;50:18–27. doi: 10.1111/jre.12180. [DOI] [PubMed] [Google Scholar]
- Ertugrul A.S., Sahin H., Dikilitas A., Alpaslan N., Bozoglan A. Comparison of CCL28, interleukin-8, interleukin-1β and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J. Periodontal. Res. 2013;48:44–51. doi: 10.1111/j.1600-0765.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- Graves D.T., Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003;74(3):391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- Hwang S., Choi B., Kang S. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res. Ther. 2012;14:10–14. doi: 10.1186/ar3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelgaufts H. first ed. VCH; Germany: 1995. Dictionary of cytokine; pp. 56–78. [Google Scholar]
- Kjeldsen M., Holmstrup P., Bendtzen K. Marginal periodontitis and cytokines: a review of the literature. J. Periodontol. 1993;64:1013–1022. doi: 10.1902/jop.1993.64.11.1013. [DOI] [PubMed] [Google Scholar]
- Kurtis B., Teuter G., Serdar M., Pinar S., Demirel I., Toyman U. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J. Periodontol. 2005;76:1849–1855. doi: 10.1902/jop.2005.76.11.1849. [DOI] [PubMed] [Google Scholar]
- Loe H., Silness J. Periodontal disease in pregnancy. Prevalence and severity. Acta Odontol. Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Mengel R., Bacher M., Flores-De-Jacoby L. Interactions between stress, interleukin-1beta, interleukin-6 and cortisol in periodontally diseased patients. J. Clin. Periodontol. 2002;29:1012–1022. doi: 10.1034/j.1600-051x.2002.291106.x. [DOI] [PubMed] [Google Scholar]
- Mootha A., Malaiappan S., Jayakumar N.D., Varghese S.S., Thomas J.T. The effect of periodontitis on expression of Interleukin-21: A systematic review. Int. J. Inflam. 2016:1–8. doi: 10.1155/2016/3507503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M., Takei H., Klokkevold P., Carranza F. 11. Elseiver; Missouri: 2011. Carranza’s clinical periodontology; pp. 388–392. [Google Scholar]
- Nikolopoulos G.K., Dimou N.L., Hamodrakas S.J., Bagos P.G. Cytokine gene polymorphisms in periodontal disease: a meta-analysis of 53 studies including 4178 cases and 4590 controls. J. Clin. Periodontol. 2008;35(9):754–767. doi: 10.1111/j.1600-051X.2008.01298.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S. Periodontal diseases: pathogenesis. Ann. Periodontol. 1996;1(1):821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- Padmanabhan P. Is there any link between cardiovascular disease, obesity and periodontal disease? J. Dent. Med. Res. 2013;3(6):04–06. [Google Scholar]
- Preiano G., Maggisano N., Lombardo T., Montalcini S., Paduano G. Pelaia et al. Influence of storage conditions on MALDI-TOF MS profiling of gingival crevicular fluid: Implications on the role of S100A8 and S100A9 for clinical and proteomic based diagnostic investigations. J. Proteomics. 2016;16:1033–1045. doi: 10.1002/pmic.201500328. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Hanstrom L., Lerner U. Characterization of bone resorbing activity in gingival crevicular fluid from patients with periodontitis. J. Clin. Periodontol. 2000;27:41–52. doi: 10.1034/j.1600-051x.2000.027001041.x. [DOI] [PubMed] [Google Scholar]
- Silness J., Loe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. ActaOdontolScand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Tobon-Arroyave S.I., Jaramillo-González P.E., Isaza-Guzmán D.M. Correlation between salivary IL-1beta levels and periodontal clinical status. Arch. Oral Biol. 2008;53:346–352. doi: 10.1016/j.archoralbio.2007.11.005. [DOI] [PubMed] [Google Scholar]