Abstract
As phytochemical-enriched edible greens, sweet potato (Ipomoea batatas L.) leaves have become popular. However, the profile and content of phytochemicals in sweet potato leaves are mostly unknown. We previously bred a purple-fleshed sweet potato P40 that demonstrated cancer prevention due to high levels of anthocyanins in the tuberous roots. The objectives of this study were to identify and quantify anthocyanins in P40 leaves when compared with the white-fleshed Bonita and orange-fleshed Beauregard. The mature leaves of P40 at 6-week vine stage were collected and extracted for anthocyanin analysis by HPLC-MS/MS. Fourteen anthocyanins, including a novel anthocyanin (peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside), were identified and quantitated. The contents of anthocyanins in P40 leaves (32.7 ± 2.9 mg/kg DW) were much lower than that in the root (13,100 ± 70 mg/kg DW). Furthermore, anthocyanin contents in P40 leaves were even lesser than those of the orange-fleshed Beauregard (334 ± 60.9 mg/kg DW) and white-fleshed Bonita (563 ± 50.4 mg/kg DW). Total phenolic contents as measured by Folin-Ciocalteu were 36.8 ± 4.8 mg GAE/g DW in the leaves of P40, but 41.2 ± 5.0 mg GAE/g DW in Beauregard and 46.7 ± 2.1 mg GAE/g DW in Bonita. No anthocyanin was detectable in the stem of these three sweet potato varieties. Taken together, this study reports for the first time the profile and content of anthocyanins in the leaves of three sweet potato varieties with a new anthocyanin identified. The unexpected lower levels of anthocyanins in the purple-fleshed sweet potato leaves when compared with either the counterpart tuberous roots or the control white-fleshed and orange-fleshed sweet potato varieties advanced our existing knowledge and also validated a diverse phenotype of anthocyanin biosynthesis between sweet potato leaves and roots.
Keywords: Food Science, Chemistry, Food Chemistry, Anthocyanin, Sweet potato leaves, P40, Bonita, Beauregard, HPLC MS/MS
1. Introduction
As the world's sixth most important crop, sweet potato (Ipomoea batatas L.) is a staple food in many developing countries due to high productivity but low input requirements (Bovell-Benjamin, 2007; Claessens et al., 2008). Sweet potato is mainly cultivated for tuberous roots, while huge mass of sweet potato vines (stems and leaves) is discarded or uses as feedstuff for livestock (Nyaata et al., 2000; Fu et al., 2016). While the tubers of the sweet potato are nutritious and commonly consumed, sweet potato leaves are also considered as an edible green in African and Asian countries, containing protein, essential amino acids, antioxidants, vitamin B, minerals, and dietary fiber (Islam, 2006; Ishiguro et al., 2004; Yoshimoto et al., 2003; Woolfe, 1992). Although sweet potato leaves are not part of the typical Western diet, sweet potato greens are becoming popular due to a high level of bioactive phytochemicals such as anthocyanins and phenolic acids which may provide numerous health-promoting benefits (Islam, 2006; Islam et al., 2009; Meyer et al., 1998; Yan-Hwa et al., 2000; Wang et al., 2016).
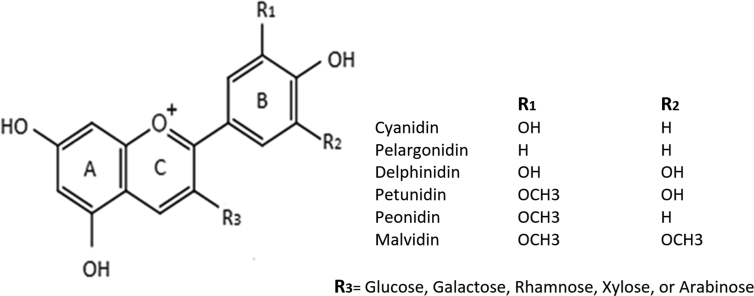
Most sweet potato cultivars have white or yellow flesh, but some have orange flesh that contains carotenoids or purple flesh that contains anthocyanins. Anthocyanins are the predominant subclass of colored flavonoids that consist of red, purple, or blue pigmentations in various plants (Xu and Howard, 2012). Among over 600 types of anthocyanins (Wang and Stoner, 2008), the majority of anthocyanin aglycones found in nature comprise with six anthocyanidins, i.e., cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin with a 2-phenylbenzopyrilium (flavylium) skeleton hydroxylated in 3, 5, and 7 positions by different substitutions at R1 and R2 (Fig. 1). In comparison with other flavonoids, anthocyanins contain a positive charge on its C-ring (Lim et al., 2013).
Fig. 1.
Chemical structures of common anthocyanidins and anthocyanins.
Our previous studies demonstrated a purple-fleshed sweet potato P40 that showed cancer prevention against colorectal cancer in both in vitro cell culture and in vivo animal model due to high levels of anthocyanins in the tuberous roots (Lim et al., 2013). The acylated anthocyanins in a unique cyaniding-predominated purple-fleshed sweet potato P40 as well as the stability of individual anthocyanin in response to various cooking onditions were further reported (Xu et al., 2015). However, the profile and content of anthocyanins in P40 leaves, although it might be relevant to the roots, are not fully elucidated. Considering the increasing consumption and the promising health benefits, there is a need to discover and clarify the anthocyanins in the sweet potato leaves.
To better understand and cover the knowledge gap, the profile and content of anthocyanins in the leaves of the purple-fleshed sweet potato P40 were identified and quantitated in this study. The results were also compared not only with the counterpart tuberous roots but also with white-fleshed and orange-fleshed sweet potato varieties. In addition, total phenolic contents in all the three sweet potato varieties were further evaluated.
2. Materials and methods
2.1. Materials
Acetonitrile, methanol, and formic acid used in this study at either HPLC grade or analytic grade were purchased from Thermal Fisher Scientific (Suwanee, GA 30024, USA). Water used was purified through Barnstead E-Pure Deionization System (Dubuque, IA 52001, USA) and filtered by Millpore 0.45 μm membrane (Bedford, MA 01730, USA). A standard of Peonidin-3-glucoside chloride, Folin-Ciocalteu reagent, and gallic acid were purchased from Sigma-Aldrich (St. Louis, MO 63118, USA).
2.2. Sample preparation and anthocyanin extraction
Three cultivars of sweet potato [Ipomoea batatas (L.) Lam.], ‘Bonita’, ‘Beasuregard’ (B14), and ‘P40’, were selected and planted at the John C. Pair Horticultural Center, Kansas State University (Haysville, KS 67060, USA). The mature leaves at vine stage were pruned at six-weeks of growth cycle. The leaves and stems were separated and washed with tap water, chopped into approximately 2 cm slices, freeze-dried, and ground by a food processor into powder. The counterpart sweet potato root tubers harvested in the end of season were also collected and prepared according to our previous publication (Xu et al., 2015). The powders of leaves, stems, and roots were then stored at -80 °C until further extraction.
For preparation of anthocyanin extraction, 0.5 g of the powder was extracted in 20 mL of acidified water (1 N of formic acid). The tubes containing powder solvent mixture were wrapped with an aluminum foil to avoid light exposure. After a 24-h extraction at room temperature on a rotary shaker, the samples were centrifuged at 4000 rpm for 45 min and then the supernatant was collected and dried by vacuum drier at 25 °C overnight. One mL of the acidified methanol was added and then the dissolved extract was filtered by a Whatman syringe filter at 0.45μm PVDF for next HPLC-MS/MS analysis.
2.3. Identification and analysis of anthocyanins by HPLC-MS/MS
HPLC coupled Electrospray Ionization tandem Mass Spectrometry (HPLC-MS/MS) was used to carry out anthocyanin identification and quantification according to our previous publications (Xu et al., 2015; Su et al., 2016). Briefly, a Shimadzu HPLC system (Kyoto, Japan) with LabSolutions Workstation Software was used for chromatographic analysis and separation. This system employed a DGU-20A3 built in degasser, a LC-20AB solvent delivery pump, a SIL-20ACHT auto-sampler, a CTO-20AC column holding oven, a CBM-20A communicator module, and a SPDM20A Photodiode Array Detector. A Waters C18 (250 × 4.6 mm i.d.) reversed-phase column (Milford, MA 01757, USA) was used for anthocyanin separation. Data was analyzed using HPLC solution software (Kyoto, Japan). Elution was performed with mobile phase A (5% formic acid in de-ionized water) and mobile phase B (5% formic acid in acetonitrile/water 1:1 v: v). An optimum column temperature was set at 25 °C. At a flow rate of 0.8 mL/min, the gradient conditions were set as follows: solvent B volume at 20–40% for 30 min, 40–50% in following 5 min and held at 50% for 10 min before returning to 20%. The detector performed a full spectrum scan between 190 and 800 nm, where 520 nm was used for monitoring anthocyanins. Peonidin-3-glucoside was used as an internal standard for quantitation of extraction recovery, and the anthocyanin contents were expressed as peonidin 3-glucoside equivalent (PN3GE) per g dry weight (DW). It should be noted that the spectral properties of peonidin 3-glucoside could differ from acylated anthocyanins, but the relevant contents and content comparision should be valid. Based on a signal-to-noise ratio of 3:1 and the standard deviation of the lowest concentration of PN3G/slope of the calibration line, the detection limit was estimated to be 5 μmol.
Mass spectrometric scan was performed on a Bruker Esquire 3000 in positive mode with a scanning interval 500–1200 m/z. Nebulization was conducted at 350 °C aided by concurrent N2 flow at 10 psi; capillary and cone voltages were set at 3.5 kV and 40 V; drying gas flow rate was 5 L/min. Mass of precursor ions and reactions of fragments loss were evaluated. Data were analyzed using Bruker Hystar Post Processing software (Bruker Daltonics, GmbH, Billerica, MA 01821, USA). The ESI/MS data was used to confirm the mass of each anthocyanin HPLC peak. The mass spectrometry instrument was controlled by the esquire control 5.3 software (Bruker Daltonics, GmbH, Billerica, MA 01821, USA) and the data were processed with Data analysis 3.3 software (Bruker Daltonics, GmbH, Billerica, MA 01821, USA). Identification of each anthocyanin was accomplished by comparison of HPLC retention time, absorbance spectra, and MS spectra with our previously published database (Lim et al., 2013; Xu et al., 2015; Su et al., 2016) and the National Institute of Standards and Technology Mass Spectra Library data (NIST08, National Institute of Standards and Technology, Gaithersburg, MD, 20899, USA).
2.4. Total phenolic content
Total phenolics in the extract of each sweet potato variety were measured by Folin-Ciocalteu method according to the published method by Singleton and Rossi (1965) with a slight modification. Briefly, a stock solution of gallic acid at 1 mg/mL in distilled water was prepared. Then the stock solution was diluted to 12.5–200 μg/mL in 70% acetone for a standard curve. To each of the 96 wells, 75 μl distilled water was added, followed by 25 μl either aliquot of extract or various concentrations of gallic acid solution. Folin-Ciocalteu regent diluted by 1:1 with distilled water was then added to each well. The reaction was then allowed to stand for 10 min at room temperature, and then 100 μl of Na2CO3 solution at 7.5% (w/v) was added to each well. Plate was covered and kept in dark for 90 min before measuring. Absorbance was read in a microplate reader Synergy HT (BioTek Inc., Winnoski, VT 05404, USA) with Gen5TM2.0 data analysis software. Result were expressed as mg gallic acid equivalent (GAE) per g DW.
2.5. Statistical analysis
Data were analyzed using SAS statistical software version 9.3 (SAS Institute, Cary, NC 27511, USA). Results were evaluated by one way ANOVA using a general linear model procedure followed by Tukey's post-hoc test. The results were presented as means ±SD, and a probability at p ≤ 0.05 was considered significant.
3. Results and discussion
3.1. Chromatographic separation
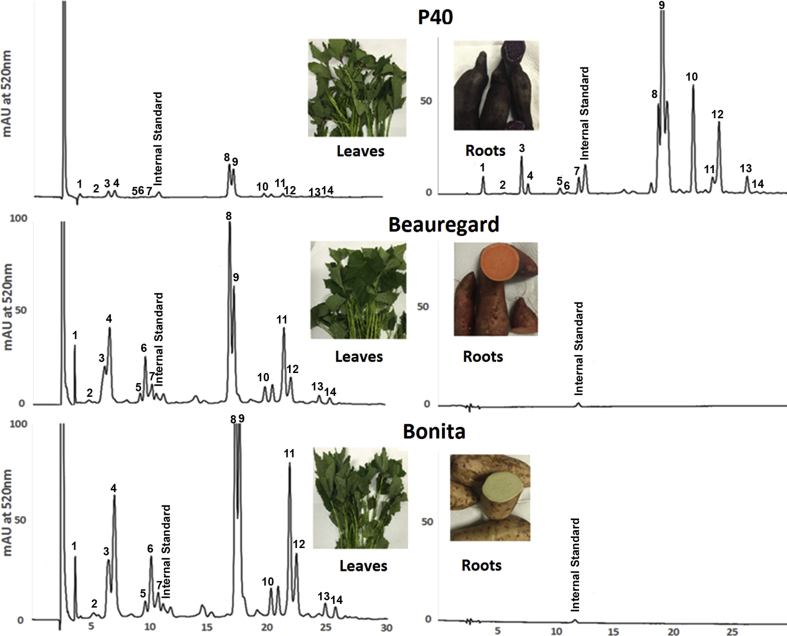
The objectives of this study were focused on characterizing the anthocyanin profile and quantifying the anthocyanin contents in each varieties of the sweet potato leaves using HPLC-MS/MS. The profile of the anthocyanin peaks from sweet potato leaves versus counterpart roots were shown by representative HPLC chromatograms in Fig. 2. In addition to the internal standard, fourteen anthocyanins were eluted and detected in the leaves or roots of three sweet potato varieties. Of these, peaks 8 and 9 appeared to be the major anthocyanins and their peak areas were more than half of the total anthocyanin peak areas. No anthocyanin peaks were detectable in the extract of sweet potato stems in all of the three varieties (data not shown).
Fig. 2.
Representative HPLC chromatograms of anthocyanins in sweet potato leaves (left) versus roots (right). The peak number corresponding to each anthocyanin is shown in Table 1.
3.2. Mass spectrometric identification
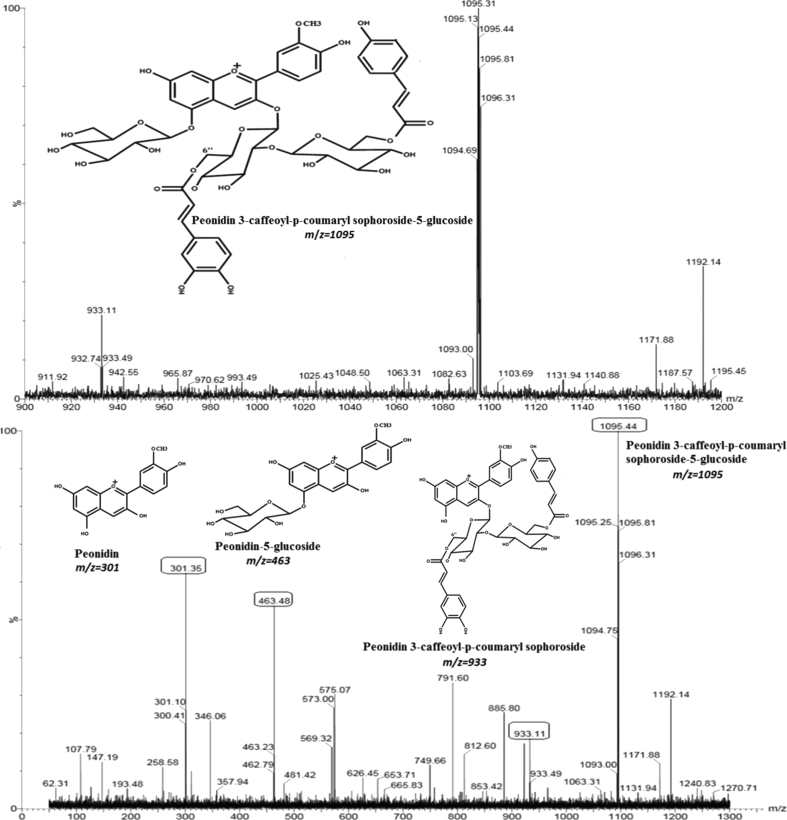
Following HPLC separation, HPLC-MS/MS data were characterized by monitoring the molecular ion characteristics for each peak. The m/z ratio of each intact anthocyanin and its fragment ions are listed in Table 1. As shown in Table 1, cyanidin (m/z 287) and peonidin (m/z 301) were the only two types of anthocyanidins found in the leaves of all the three sweet potato varieties. Thirteen of the fourteen anthocyanins including cyanidins (peaks 1, 3, 4, 7–10) and peonidin (peaks 2, 5, 6, 11–13) have been reported previously (Islam et al., 2002a,b; Lee et al., 2013; Xu et al., 2015), but one peonidin (peak 14) was newly found in the leaves of three sweet potato varieties for the first time. As shown in Fig. 3, the m/z ratio of molecular ion with fragment ion for peak 4 was captured as an intact molecular of peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside (m/z 1095) and three major fragments of peonidin 3-caffeoyl-p-coumaryl sophoroside (m/z 933), peonidin-5-glucoside (m/z 463), and peonidin (m/z 301). Transitions from m/z 1095 to 933 and m/z 1095 to 463 implied the loss of glucose (m/z 162) and 3-caffeoyl-p-coumaryl sophoroside (m/z 632), respectively. Transition from m/z 1095 to 301 produced peonidin (m/z 301) by the loss of glucose and 3-caffeoyl-p-coumaryl sophoroside.
Table 1.
Mass Spectrometric Data of Anthocyanins in the Leaves of Purple-fleshed Sweet Potato P40, orange-fleshed Beauregard, or white-fleshed Bonita.
| Peak # | Retention (min) | Anthocyanins | [M + H] (m/z) | Fragment ions (m/z) |
|---|---|---|---|---|
| 1 | 3.61 | Cyanidin 3-sophoroside-5-glucoside | 773 | 661,449,287 |
| 2 | 5.28 | Peonidin 3-sophoroside-5-glucoside | 787 | 595,433,271 |
| 3 | 6.23 | p-hydroxybenzoylated (Cyanidin 3-sophoreside-5-glucoside) | 893 | 731,449,287 |
| 4 | 6.96 | Caffeoylated (Cyanidin 3-sophoroside-5-glucoside) | 935 | 773,449,287 |
| 5 | 9.75 | p-hydroxybenzoylated (Peonidin 3-sophoroside-5-glucoside) | 907 | 745,463,301 |
| 6 | 10.29 | Caffeoylated (Peonidin 3-sopheroside-5-glucoside) | 949 | 787,463,301 |
| 7 | 11.31 | Feruloylated (Cyanidin 3-sophoroside-5-glucoside) | 949 | 787,449,287 |
| 8 | 17.90 | Cyanidin 3-(6,6′-dicaffeoyl sophoroside)-5-glucoside | 1097 | 935,449,287 |
| 9 | 17.57 | Cyanidin 3-(6,6′-caffeoyl-p-hydroxybenzoyl sophoroside)-5-glucoside | 1055 | 893,449,287 |
| 10 | 21.26 | Cyanidin 3-(6,6′-caffeoyl-feruloyl-sophoroside)-5-glucoside | 1111 | 949,449,287 |
| 11 | 22.23 | Peonidin 3-(6,6′-dicaffeoyl-sophoroside)-5-glueoside | 1111 | 949,463,301 |
| 12 | 22.85 | Peonidin 3-(6,6′-caffeoyl-p-hydroxybenzoyl sophoreside)-5-glucoside | 1069 | 907,463,301 |
| 13 | 25.30 | Peonidin 3-(6,6′-caffeoyl-feruloyl sophoroside)-5-glucoside | 1125 | 963,463,301 |
| 14 | 26.09 | Peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside | 1095 | 933,463,301 |
Fig. 3.
Mass spectra data of peak 14 as a new anthocyanin identified in sweet potato leaves: (Top Panel) molecular ion spectra of peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside; (Bottom Panel) fragment ion spectra of peonidin, peonidin-5-glucoside, and peonidin-3-caffeoyl-p-coumaryl sophoroside, respectively.
It should be noted there were a few peaks separated by HPLC but not identified by MS spectral database. A new anthocyanin detected in the sweet potato leaves might be attributed by the effective extraction and improved HPLC method, which allowed for a distinct peak separation. When compared with our previous studies (Xu et al., 2015; Su et al., 2016), the composition of mobile phase and the flow rate have been adjusted in this study for better peak separation. Moreover, 5% of formic acid has been added to the water before extraction, creating a low pH environment for anthocyanin stabilization as suggested by Sang et al. (2017) that the peak area would be increased and the detection would be more sensitive under acid condition due to the increased percentage of the flavylium cation.
3.3. Anthocyanin contents in sweet potato leaves
Table 2 lists the contents of individual and total anthocyanins in the leaves and roots of three sweet potato varieties. The predominant two anthocyanins, i.e., cyanidin 3-(6,6′-dicaffeoyl-sophoroside)-5-glucoside and cyanidin3-(6,6′-caffeoyl-p-hydroxybenzoyl sophoroside)-5-glucoside, comprised up to more than half of total anthocyanins, while cyanidin 3-p-hydroxybenzoyl sophoroside-5-glucoside exhibited the highest level in both leaves and roots of P40. The newly identified anthocyanin (peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside) made up approximately 1% of the total anthocyanins.
Table 2.
The contents of individual and total anthocyanins in the leaves and roots of purple-fleshed sweet potato P40, orange-fleshed Beauregard, and white-fleshed Bonita (mg PN3GE/kg DM)∗.
| Anthocyanins | P40 |
Beauregard |
Bonita |
|||
|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | |
| Cyanidin 3-sophoroside-5-glucoside | 3.8 ± 0.7a | 312.1 ± 19.8c | 6.0 ± 2.8b | UD | 9.4 ± 1.0b | UD |
| Peonidin 3-sophoroside-5-glucoside | 0.8 ± 0.1a | 52.9 ± 0.8d | 1.7 ± 0.3b | UD | 4.9 ± 2.3c | UD |
| p-hydroxybenzoylated (Cyanidin 3-sophoreside-5-glucoside) | 2.7 ± 0.1a | 604.6 ± 51.7d | 7.6 ± 1.4b | UD | 24.4 ± 2.4c | UD |
| Caffeoylated (Cyanidin 3-sophoroside-5-glucoside) | 3.3 ± 0.4a | 180.1 ± 11.9d | 30.9 ± 8.8b | UD | 48.0 ± 3.9c | UD |
| p-hydroxybenzoylated (Peonidin 3-sophoroside-5-glucoside) | 1.0 ± 0.1a | 132.8 ± 5.0d | 1.6 ± 0.3b | UD | 7.4 ± 0.5c | UD |
| Caffeoylated (Peonidin 3-sopheroside-5-glucoside) | 1.0 ± 0.1a | 46.6 ± 0.6d | 8.7 ± 2.2b | UD | 23.5 ± 0.9c | UD |
| Feruloylated (Cyanidin 3-sophoroside-5-glucoside) | 1.1 ± 0.1a | 297.0 ± 12.6d | 3.8 ± 0.7b | UD | 6.6 ± 1.1c | UD |
| Cyanidin 3-(6,6′-dicaffeoyl-sophoroside)-5-glucoside | 11.6 ± 1.3a | 1481.4 ± 18.4c | 82.1 ± 15.4b | UD | 86.1 ± 7.6b | UD |
| Cyanidin 3-(6,6′-caffeoylphydroxybenzoyl sophoroside)-5-glucoside | 9.8 ± 1.3a | 5667.9 ± 34.1d | 37.7 ± 6.3b | UD | 65.8 ± 15.3c | UD |
| Cyanidin 3-(6,6′-caffeoylferuloylsophoroside)-5-glucoside | 1.9 ± 0.4a | 1877.3 ± 19.0d | 8.6 ± 2.2b | UD | 11.7 ± 0.8c | UD |
| Peonidin 3-(6,6′-dicaffeoylsophoroside)-5-glueoside | 2.3 ± 0.6a | 381.6 ± 9.2d | 30 ± 7.8b | UD | 55.4 ± 4.2c | UD |
| Peonidin 3-(6,6′-caffeoylphydroxybenzoyl sophoreside)-5-glucoside | 1.3 ± 0.1a | 1620.9 ± 9.1d | 8.9 ± 1.9b | UD | 24.5 ± 3.1c | UD |
| Peonidin 3-(6,6′-caffeoylferuloylsophoroside)-5-glucoside | 1.0 ± 0.1a | 344.3 ± 7.1d | 4.4 ± 1.4b | UD | 7.1 ± 1.2c | UD |
| Peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside | 1.1 ± 0.2a | 59.3 ± 8.5d | 2.8 ± 0.8b | UD | 5.4 ± 0.3c | UD |
| Total Anthocyanins | 38 ± 2.9a | 13100 ± 70d | 240 ± 60.9b | UD | 448 ± 50.4c | UD |
Data are expressed as mean ± SD (n = 3). Values marked by different letters within same row indicate significant difference (p < 0.05); UD: Undetectable.
It should be noted that the total contents of anthocyanins in P40 leaves (32.7 ± 2.9 mg/kg DW) were much lower than that in the roots (13,100 ± 70 mg/kg DW), implying an exceedingly diverse phenotype of anthocyanin biosynthesis between sweet potato leaves and roots. The remarkably diverse phenotype seems due to the varied genotype as reported by Mano et al. (2007) that one member of transcriptional factors, i.e., MYB that was sufficient for induction of all structural anthocyanin biosynthesis genes, was predominantly expressed in the purple-flesh roots but not in other related-tissues such as stems, leaves, and flowers. Mano et al. (2007) also reported MYB was only expressed in the roots of purple-fleshed sweet potatoes but not in the roots of orange-, yellow-, or white-fleshed varieties, which is in agreement with our finding of undetectable anthocyanins in the roots of orange-fleshed Beauregard and white-fleshed Bonita. It is unexpected that the total anthocyanin contents in P40 leaves were even lesser than those of the orange-fleshed Beauregard and white-fleshed Bonita. A future study by confirming the genotype of anthocyanin biosynthesis genes in response to different phenotype of anthocyanin contents among different sweet potato varieties may be warrant.
3.4. Total phenolic content
In addition to anthocyanins, there are many other phenolics presented in sweet potato leaves. To estimate other phenolic agents in the extracts, total phenolic contents were measured by Folin-Ciocalteu in three sweet potato varieties, resulting in 36.8 ± 4.8 mg GAE/g DW in the leaves of P40, but 41.2 ± 5.0 mg GAE/g DW in Beauregard and 46.7 ± 2.1 mg GAE/g DW in Bonita (Table 3). A significant lower level of total phenolics was found in purple-fleshed P40 leaves when compared with orange-fleshed Beauregard and white-fleshed Bonita. Considering a similar trend of phenolic levels with anthocyanin contents in the leaves of three sweet potato varieties, it may suggest anthocyanins be contributed as a majority of phenolics in the extracts tested in addition to other minor flavonoids and phenolic acids, etc.
Table 3.
Total phenolic contents in the leaves of sweet potatoes (mg GAE/g DW)∗.
| P40 | Beauregard | Bonita |
|---|---|---|
| 36.8 ± 4.8a | 41.2 ± 5.0b | 46.7 ± 2.1c |
Data are expressed as mean ± SD (n = 3). Values marked by different letters indicate significant difference (p < 0.05).
In conclusion, this study reports for the first time the profile and content of anthocyanins in the leaves of three sweet potato varieties with a new anthocyanin identified. The unexpected lower levels of anthocyanins in the purple-fleshed sweet potato leaves when compared with either the counterpart tuberous roots or the control white-fleshed and orange-fleshed sweet potato varieties advanced our existing knowledge and also validated a diverse phenotype of anthocyanin biosynthesis between sweet potato leaves and roots.
Declarations
Author contribution statement
Xiaoyu Su: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jason Griffin: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jingwen Xu: Performed the experiments; Analyzed and interpreted the data.
Ping Ouyang: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Zhihui Zhao: Analyzed and interpreted the data; Wrote the paper.
Weiqun Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This study was supported in part by a USDA Cooperative Project KS511-1001903 (WW).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This study was supported in part by a USDA Cooperative Project KS511-1001903 from the Agricultural Experiment Station, Kansas State University (Contribution #18-187-J from the Kansas Agricultural Experiment Station).
References
- Bovell-Benjamin A.C. Sweet potato: a review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007;52:1–59. doi: 10.1016/S1043-4526(06)52001-7. [DOI] [PubMed] [Google Scholar]
- Claessens L., Stoorvogel J.J., Antle J.M. Ex ante assessment of dual-purpose sweet potato in the crop-livestock system of western Kenya: a minimum-data approach. Agric. Syst. 2008;99(1):13–22. [Google Scholar]
- Fu Z.F., Tu Z.C., Zhang L., Wang H., Wen Q.H., Huang T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016;15:11–18. [Google Scholar]
- Ishiguro K., Toyama J., Islam M.S., Yoshimoto M., Kumagai T., Kai Y., Yamakawa O. vol. 637. 2004. Suioh, a new sweet potato cultivar for utilization in vegetable greens; pp. 339–345. (Acta Horticulturae). [Google Scholar]
- Islam M.S., Yoshimoto M., Terahara N., Yamakawa O. Anthocyanin compositions in sweet potato (Ipomoea batatas L.) leaves. Biosc. Biotech. Biochem. 2002;66(11):2483–2486. doi: 10.1271/bbb.66.2483. [DOI] [PubMed] [Google Scholar]
- Islam M.S., Yoshimoto M., Yahara S., Okuno S., Ishiguro K., Yamakawa O. Identification and characterization of foliar polyphenolic composition in sweet potato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002;50(13):3718–3722. doi: 10.1021/jf020120l. [DOI] [PubMed] [Google Scholar]
- Islam S. Sweet potato (Ipomoea batatas L.) leaf: its potential effect on human health and nutrition. J. Food Sci. 2006;71(2):13–21. [Google Scholar]
- Islam I., Shaikh A.U., Shahidul I.M. Antioxidative and antimutagenic potentials of phytochemicals from Ipomoea batatas (L.) Lam. Int. J. Cancer Res. 2009;5(1811–9727):83–94. [Google Scholar]
- Lee M.J., Park J.S., Choi D.S., Jung M.Y. Characterization and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in Korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS. J. Agric. Food Chem. 2013;61(12):3148–3158. doi: 10.1021/jf3055455. [DOI] [PubMed] [Google Scholar]
- Lim S., Xu J., Kim J., Chen T.Y., Su X., Standard J., Wang W. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013;57(11):1908–1917. doi: 10.1002/mnfr.201300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H., Ogasawara F., Sato K., Higo H., Minobe Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 2007;143(3):1252–1268. doi: 10.1104/pp.106.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A.S., Heinonen M., Frankel E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998;61(1–2):71–75. [Google Scholar]
- Nyaata O.Z., Dorward P.T., Keatinge J.D.H., O’Neill M.K. Availability and use of dry season feed resources on smallholder dairy farms in central Kenya. Agrofor. Syst. 2000;50(3):315–331. [Google Scholar]
- Sang J., Sang J., Ma Q., Hou X.fang, Li C.qin. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017;218:386–395. doi: 10.1016/j.foodchem.2016.09.093. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. [Google Scholar]
- Su X., Xu J., Rhodes D., Shen Y., Song W., Katz B., Tomich J., Wang W. Identification and quantification of anthocyanins in transgenic purple tomato. Food Chem. 2016;202:184–188. doi: 10.1016/j.foodchem.2016.01.128. [DOI] [PubMed] [Google Scholar]
- Wang L.-S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Nie S., Zhu E. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016;89(1):90–116. doi: 10.1016/j.foodres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- Woolfe J.A. Cambridge Univ; Cambridge, U.K.: 1992. Sweet Potato. An Untapped Food Resource. 118-7. [Google Scholar]
- Xu J., Su X., Lim S., Griffin J., Carey E., Katz B., Wang W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015;186:90–96. doi: 10.1016/j.foodchem.2014.08.123. [DOI] [PubMed] [Google Scholar]
- Xu Z., Howard L.R. 2012. Analysis methods of anthocyanins; pp. 149–180. Analysis of Antioxidant-Rich Phytochemicals (5) [Google Scholar]
- Yan-Hwa C., Chang C.L., Hsu H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000;80(5):561–566. [Google Scholar]
- Yoshimoto M., Okuno S., Islam M.S., Kurata R.A., Yamakawa O. vol. 628. 2003. Polyphenolic content and antimutagenicity of sweet potato leaves in relation to commercial vegetables; pp. 677–685. (Acta Horticulturae). [Google Scholar]