Abstract
Objectives
With this study, we aimed to develop a mobile technology (mHealth) intervention to improve medication adherence among patients with coronary heart disease (CHD).
Methods
The study was conducted in two phases with CHD patients from a Cardiology Department of a hospital located in China. Each phase was independent from the other. Phase 1 tested the integration of the two apps — “WeChat” and “BB Reminder” — as an mHealth intervention. All participants received the same educational materials via WeChat every two days. Participants in the experimental group received a reminder from BB Reminder for every dose of their medications. The duration of Phase 1 was 30 days for each participant. Phase 2 refined the intervention, in which educational materials were sent every five days rather than every two days, and medication-taking reminders were sent daily rather than every dose.
Results
In Phase 1, an mHealth intervention was developed by integrating two mobile apps. In Phase 2, medication adherence increased at 30-day follow-up in both groups compared to baseline. At the 30-day follow-up, the mean of the decrease in medication non-adherence score in the experimental group (M = −1.35, SD = 2.18, n = 36) was more than the decrease in control group (M = −0.69, SD = 1.58, n = 36), which means the medication adherence improved more in the experimental group.
Conclusion
The feasibility of using mHealth to remind CHD patients to take their medications is high.
Keywords: China, Coronary disease, Medication adherence, mHealth, Mobile applications
1. Introduction
In China, coronary heart disease (CHD) is the second leading cause of death [[1], [2], [3]]. The treatment of CHD and other chronic illnesses typically involves long-term pharmaceutical therapy [4]. Particularly for patients with CHD, taking cardio-protective medications can prevent the enlargement of harmful clots [5], cardiovascular symptoms, and poor therapeutic outcomes, such as uncontrolled high blood pressure, hyperlipidemia, arrhythmia, heart failure [6], and sudden cardiac death [7]. These medications, which include antiplatelet drugs, β-blockers, statins, and angiotensin-converting enzyme inhibitors, can thus reduce the risk of mortality due to CHD [6,8,9]. However, in China, poor adherence to cardio-protective medications has been cited as a public health concern [10,11]. This poor adherence to cardio-protective medications has been linked to increases in healthcare costs due to poor therapeutic outcomes typically requiring major medical interventions, such as coronary angioplasty and coronary artery bypass grafting [12]. In fact, improving the adherence to pharmacotherapy for chronic illnesses, such as hypertension and hyperlipidemia, which are two leading risk factors of CHD, has been shown to yield extensive health and economic benefits [13]. Conversely, neglecting medication adherence could render futile any effort to treat CHD [14], and cause severe adverse health outcomes. Therefore, it is critical to improve medication adherence among patients with CHD in China.
In China, local primary healthcare clinics are often not the first choice for treatment. Instead, patients with serious illnesses such as CHD prefer to utilize hospitals. Consequently, many of the 100 million people diagnosed with CHD in China [15] receive prescriptions and medication-related knowledge in hospitals only without a primary care clinician to monitor their treatment. Under this healthcare utilization model, patients are often not provided with proper treatment maintenance and knowledge regarding their medication-taking behaviors [16]. This lack of follow-up care decreases patients’ awareness of the importance of taking medications the way they were prescribed.
China has 1.28 billion mobile phone users [17], and 81% of total Internet users in China access the Internet by using a mobile phone [18]. These conditions in China are ideal for implementing a mobile technology (mHealth) intervention to improve health and practice. However, the use of mHealth to improve medication adherence among Chinese patients with CHD is in its infancy. In this study, we proposed to use mHealth as a tool to increase CHD patients’ access to care and care delivery. The purpose of this study was to evaluate the feasibility of using mHealth as a tool to assist CHD patients to take their cardio-protective medications.
2. Methods
2.1. Mobile health technologies
In this study, two mobile applications (apps) were used, WeChat and BB Reminder. WeChat is the most widely used cell-phone messaging app in China [19]. On average, WeChat users spend 70 min per day on the app [20]. WeChat's functions include creating group chats, live chats, video calls, and sending messages, files, and voice notes. Every WeChat account has its own account name and QR code that are connected to a mobile phone number. BB Reminder is a medication-reminder app. By adding a patient's WeChat account to the BB Reminder, a clinician can send personalized medication-taking reminders through messages on WeChat to the patient.
2.2. Study design
This study was conducted in two phases with patients from a Cardiology Department in a major university-affiliated hospital, located in southern China. Phase 1 lasted for three months (from June 16, 2016 to September 19, 2016) and was completed to allow for refinement of the intervention. With the adjustment made from information gathered from Phase 1, Phase 2 lasted for two months (from May 17, 2017 to July 18, 2017). The cohorts of participants in the two phases were mutually exclusive.
Both phases included an exploratory randomized controlled trial (RCT; N = 49 in Phase 1, and N = 50 in Phase 2) and qualitative interviews (n = 4 in Phase 1, and n = 10 in Phase 2). In Phase 1 RCT, participants assigned to the experimental group received a reminder for every dose of their medications and educational materials every other day. In the Phase 2 RCT, participants assigned to the experimental group received a daily medication-taking reminder and educational materials every five days. For both phases, participants in the control group only received educational materials, and the duration of the study was 30 days for each participant.
2.3. Interventions
In Phase 1, participants in the experimental group received medication-taking reminders and educational materials from the two mobile apps, BB Reminder and WeChat, respectively. All participants in this study received the same educational materials via WeChat. The educational materials were sent every two days at a random time between 8 a.m. and 10 p.m. These educational materials retrieved from the Chinese Cardiovascular Disease-Prevention Information Website were curated into short articles (<500 words) and pictures. The educational materials were screened by a cardiologist and a nurse to assure their accuracy before being sent to participants. Participants in the experimental group also received medication-taking reminders. These reminders were sent through BB Reminder on an unencrypted device (iPhone 5; used only for research purposes). BB Reminder has the function that after inputting a patient's phone number and prescriptions, participants can receive reminders through text messages on WeChat of the name, dosage, function, and administration route for every dose of his/her medication. The reminders were sent to participants 15 min before they were supposed to take their medications.
The intervention of Phase 2 was refined based on Phase 1 (see Table 1). The intervention also involved daily reminders and educational materials sent via BB Reminder and WeChat. All participants in this study received the same educational materials via WeChat, however, the educational materials were not sent every two days, but every five days at a random time between 9 a.m. and 9 p.m. These educational materials retrieved from the World Health Organization website (http://www.who.int/features/qa/27/zh/) and were curated into a library. Like Phase 1, the educational materials in Phase 2 were also screened by a cardiologist and a nurse to assure their accuracy before being sent to participants. In Phase 2, participants in the experimental group received one-daily medication-taking reminders rather than receiving a reminder for every dosage of their medications. These reminders were also sent through BB Reminder on an unencrypted device (iPhone 5; used only for research purposes). The daily reminders were sent to participants every day at a random time between 9 a.m. and 9 p.m.
Table 1.
The comparison of the two phases.
| Phase 1 | Phase 2 | Rationale for change | |
|---|---|---|---|
| Components | An exploratory randomized controlled trial + qualitative interviews. | Same as phase 1. | N/A |
| Duration | 30 days for each participants. | Same as phase 1. | N/A |
| Baseline data collection | A paper-based questionnaire with 23 items. | The same questionnaire was created in REDCap (http://www.project-redcap.org) a secure, web-based application. A hyperlink of the questionnaire was sent to participants on WeChat. | Completing the paper-based questionnaire at hospital was not convenient for many participants. A hyperlink sent to WeChat provided participants more flexible time. Participants could complete the survey at anytime when they were free. |
| Reminder | Participants in the experimental group received a reminder for every dose of medication. | Participants in the experimental group received a daily reminder. | In China, participants had the right to decide when to take each dose of their medications, although the frequency of each medication was prescribed by physicians. Because of this reason, many participants could not tell the researchers when they were going to take their medications at the enrollment. |
| Educational materials | All participants received them every two days at a random time between 8am and 10pm. | All participants received them every five days at a random time between 9am and 9pm. | Receiving educational materials every other day is too frequent for elderly participants to digest the included knowledge. Also, 10pm is a little bit late for many elderly participants to read educational materials. |
| Medication adherence measurement | The number of dosage taken divided by the number of dosage prescribed (percentage). | A standardized three-question scale named Voils Medication Non-Adherence Extent Scale (thereinafter named Voils scale). | Some participants forgot the number of dosage they taken, and others might lie about the number of dosages they taken. |
| Medication adherence collection | Participants reported every 3 days on WeChat. Researchers documented the data on paper. | The Voils scale was created in REDCap. A hyperlink of it was sent to participants on WeChat every 15 days. Participants reported medication adherence by clicking the hyperlink and filling the scale. | Reporting every 3 days is too frequent for many participants. |
| Health outcomes collection | Participants reported every 3 days on WeChat. Researchers documented the data on paper. | Within the Voils scale hyperlink, participants were asked to report their health outcomes. Participants reported them by clicking the hyperlink and filling SBP, DBP, and HR. | Reporting every 3 days is too frequent for many participants. |
| Interview | An interview invitation sent to the participants in the experimental group. Those who accepted were interviewed by phone call. | An interview invitation sent to all participants. Those who accepted were interviewed by phone call. | Given the small sample size, the research team wanted to hear feedback from participants. |
After the intervention, an interview invitation was sent out via WeChat to all the experimental group participants in Phase1 and to all participants in Phase 2. Those who accepted the invitation were interviewed via phone calls by a study coordinator who had been well-trained in conducting interviews. The purpose of the interviews was to obtain the participants’ feedback on the intervention. The interview questions included “What do you think of this mHealth program?”, “What do you think of the reminders? Would they work?”, and “What do you think of the educational materials received on WeChat?”
2.4. Participants
2.4.1. Inclusion criteria
This study gained ethical approval from Duke Health Institutional Review Board (Pro00073395) and was allowed to be conducted in the clinical site located in China (the Cardiology Department of West China hospital). Inclusion criteria for Phase 1 and Phase 2 were the same: (1) a medical diagnosis of CHD; (2) aged 18 years or older; (3) taking an antihypertensive medication that would last for 30 days or more from enrollment; (4) able to read messages through mobile phone; (5) have a mobile phone that can receive messages from WeChat, and can receive reminders from BB Reminder; (6) capable of giving his/her own consent; and (7) have an electronic blood pressure cuff to check blood pressures and heart rates.
2.4.2. Recruitment
Participants were recruited by using flyers and healthcare provider referral for both phases. Study flyers were available to the public at the clinical site. Additionally, a head nurse and an experienced cardiologist contacted potential participants in the Cardiology department. For those who agreed to participate in the study, a meeting was arranged in the office of the Cardiology Department during dates and times convenient for the patients and eligibility was assessed. For those who met the eligibility, the details of the purpose and procedure of the study were explained. If they agreed to participate, they were asked to sign the informed consent form. The participation was voluntary. Participants were always given the option to consent or decline.
2.5. Demographic and outcome variables
Participants’ demographic characteristics were collected at enrollment by the study coordinator through a paper-based questionnaire and an online questionnaire via WeChat at Phase 1 and Phase 2, respectively. Demographic variables including gender, race, marriage status, job status, educational level, medical insurance, and living area were coded as categorical variables. Numerical baseline variables included the number of prescribed medications, family income, weight, and height.
The four outcome variables in this study were medication non-adherence score, health rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Medication adherence was measured as the number of dosage taken by a patient divided by the number of dosage prescribed by physician (percentage) during Phase 1, and by the Voils Medication Non-Adherence Extent Scale [21], a five-point Likert scale with three items, during Phase 2. For the Voils Medication Non-Adherence Extent Scale, a total score of non-adherence was calculated by averaging responses to the three items. The lower the non-adherence score, the better the medication adherence.
We compared the effects of the mHealth intervention and control condition on medication adherence, systolic (SBP) and diastolic (DBP) blood pressures, and heart rate (HR). Health outcomes (SBP, DBP, HR) were reported by participants via WeChat every three days during Phase 1 and at three time points: enrollment (baseline), 15 days post-enrollment and 30 days post-enrollment during phase 2. The three time points were decided based on the Voils Medication Non-Adherence Extent Scale [21] and the feedback from Phase 1.
2.6. Procedures
Since the procedures of Phase 1 and Phase 2 were similar (see Table 1), in this article we only described the procedures of Phase 2. Following informed consent, participants who completed the baseline assessment were randomly assigned to an experimental group using stratified randomization by gender with a permuted block size of four. This is because Phase 1 of this feasibility study showed that the ratio of male to female participants was 8:3. Thus, we sought to balance the sample size of female participants in experimental group and control group.
The study coordinator sent “friend requests” to participants' WeChat accounts. A “friend request” is an invitation to connect on WeChat. After accepting the requests, participants could communicate with the study coordinator. The intervention was planned for 30 days for each participant. Participants' medication non-adherence score, SBP, DBP, and HR were examined and documented at three time points: at enrollment, on the 15th day, and the 30th day. After the intervention, the study coordinator sent an interview invitation to all participants via WeChat. Those who accepted the invitation were interviewed through a WeChat phone call. Interviews (n = 10) were conducted by the study coordinator to explore participants’ experiences with the mHealth intervention. The interviews were recorded and the audio files were stored on a secure server.
2.7. Data analysis
The purpose of Phase 1 was to test whether the integration of WeChat and BB Reminder could be used as an intervention in a clinical setting. Since improvements to the study were made in Phase 2, in this article, we only described the data analyses and results of Phase 2. Descriptive statistics, frequency (n) and percentage (%) for categorical measures and count, mean, standard deviation (SD), were reported to detail the demographic and clinical characteristics of the entire sample. Independent t-tests and chi-square tests were used to test for between-group difference in sample characteristics as well as medication adherence total scores and patient health measures at baseline. Wilcoxon Two-Sample Test was used if two sample t-test assumption was not met. Fisher's exact test was used as alternative when chi-square test was not applicable due to small expected cells. When a significant between-group difference in a baseline demographic or clinical characteristic was detected, the variable was evaluated as a covariate in the modeling stage. All statistical tests were non-directional and conducted with a 0.05 significance level. Quantitative data were collected and managed by using REDCap (http://www.project-redcap.org), a secure, web-based application. Analyses were performed using SAS 9.4 (Cary, NC).
This study explored whether mHealth intervention improved medication adherence total scores better over the 30-day period when compared to the control intervention in patients with CHD. The mean and standard deviation for all outcomes by different groups at each of the three time points were computed and graphed to show the trend. A two groups t-test was used to test the difference in change of medication adherence score from baseline to either 15 days or 30 days between the intervention and control groups. To account for possible difference in outcomes at baseline and correlations between repeated measures within same participant, a mixed-effects model with a random intercept and a random slope was used to test for between-group differences in the trajectory of change in all four outcomes: medication non-adherence, HR, SBP, and DBP. The fixed effects included in the model were intervention group (Experimental vs Control), time, the group-by-time interaction and other covariates that are significantly different between experimental and control group.
Feasibility was determined by the number of patients enrolled, retention (percent of patients who complete the study), and responses over time. The recorded interviews were transcribed verbatim by the study coordinator in Chinese. The transcriptions were double checked by a doctoral student fluent in Chinese. After the accuracy of the transcriptions was guaranteed, they were read line by line several times by the study coordinator and the doctoral student to explore participants’ perceived benefits of the intervention, technical difficulties and barriers, and how often they read the reminders.
3. Results
3.1. Baseline characteristics
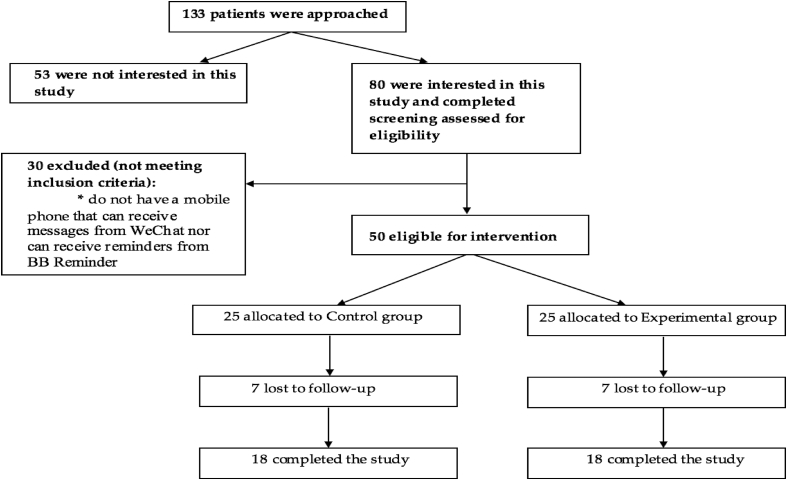
Participant characteristics are summarized in Table 2. Overall, 50 participants were recruited in Phase 2, 25 each in experimental group and control group. Thirty-six participants completed the study, 18 each in experimental group and control group(see Fig. 1). The data analysis is based on the 36 participants. Of the characteristics examined, educational level (P = 0.04) and body weight (P = 0.02) were statistically different between the control and experimental groups. Most participants were Han people, married, and living in urban area. At baseline, 66.7% of the participants were prescribed at least five medications. Likewise, most participants reported their health statuses were fair or good. Eighty percent of participants reported using WeChat daily and had used WeChat for more than a year before the study.
Table 2.
Baseline characteristics of samples in Phase 2 at enrollment [n (%)].
| Variable | All participants (n = 36) | Experimental (n = 18) | Control (n = 18) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 29 (80.6) | 14 (77.8) | 15 (83.3) | 1.00 |
| Female | 7 (19.4) | 4 (22.2) | 3 (16.7) | |
| Race | ||||
| Han | 34 (94.4) | 16 (88.9) | 18 (100) | 0.49 |
| Zang | 1 (2.8) | 1 (5.6) | 0 | |
| Yi | 1 (2.8) | 1 (5.6) | 0 | |
| Marital status | ||||
| Married | 32 (88.9) | 15 (83.3) | 17 (94.4) | 0.74 |
| Divorced | 2 (5.6) | 2 (11.1) | 0 | |
| Widowed | 2 (5.6) | 1 (5.6) | 1 (5.6) | |
| Job status | ||||
| Employed | 8 (22.2) | 4 (22.2) | 4 (22.2) | 0.42 |
| Self-employed | 2 (5.6) | 0 | 2 (11.1) | |
| Farmer | 1 (2.8) | 0 | 1 (5.6) | |
| Retired | 23 (63.9) | 12 (66.7) | 11 (61.1) | |
| Other | 2 (5.6) | 2 (11.1) | 0 | |
| Education | ||||
| < High school | 10 (27.8) | 1 (5.6) | 9 (50) | 0.04 |
| High school – Undergraduate | 17 (47.2) | 12 (66.7) | 5 (27.8) | |
| Undergraduate – Master's | 9 (25) | 5 (27.8) | 4 (22.2) | |
| Number of prescribed medications | ||||
| <5 | 12 (33.3) | 6 (33.3) | 6 (33.3) | 0.70 |
| 5 – < 10 | 23 (63.9) | 11 (61.1) | 12 (66.7) | |
| ≥10 | 1 (2.8) | 1 (5.6) | 0 | |
| Having medical insurance* | 35 (97.2) | 17 (94.4) | 18 (100) | 1.00 |
| Medications were covered by medical insurance | ||||
| All covered | 11 (35.6) | 5 (27.8) | 6 (33.3) | 1.00 |
| Some covered | 19 (52.8) | 10 (55.6) | 9 (50) | |
| Not covered at all | 1 (2.8) | 0 | 1 (5.6) | |
| Do not know | 5 (13.9) | 3 (16.7) | 2 (11.1) | |
| Living area | ||||
| Urban | 33 (91.7) | 17 (94.4) | 16 (88.9) | 1.00 |
| Rural | 3 (8.3) | 1 (5.6) | 2 (11.1) | |
| Living with others | ||||
| Living alone | 3 (8.3) | 2 (11.1) | 1 (5.6) | 0.79 |
| With spouse | 29 (80.6) | 15 (83.3) | 14 (77.8) | |
| With child | 2 (5.6) | 0 | 2 (11.1) | |
| With others | 2 (5.6) | 1 (5.6) | 1 (5.6) | |
| The years of using WeChat* | ||||
| <1 | 7 (20) | 3 (16.7) | 4 (22.2) | 0.77 |
| ≥1 | 28 (80) | 14 (77.8) | 14 (77.8) | |
| The frequency of using WeChat before participating in the study* | ||||
| Daily | 28 (80) | 14 (77.8) | 14 (77.8) | 0.86 |
| Weekly | 2 (5.7) | 1 (5.6) | 1 (5.6) | |
| Monthly | 1 (2.9) | 1 (5.6) | 0 | |
| Never | 2 (5.7) | 1 (5.6) | 1 (5.6) | |
| Other | 2 (5.7) | 0 | 2 (11.1) | |
| Yearly family income (US dollars) | ||||
| <8147 | 16 (44.4) | 9 (50) | 7 (38.9) | 0.85 |
| 8147–13,575 | 8 (22.2) | 2 (11.1) | 6 (33.3) | |
| 13,575–18,100 | 4 (11.1) | 3 (16.7) | 1 (5.6) | |
| ≥18,100 | 5 (13.9) | 3 (16.7) | 2 (11.1) | |
| Refuse to answer | 3 (8.3) | 1 (5.6) | 2 (11.1) | |
| General health status | ||||
| Good | 13 (36.1) | 6 (33.3) | 7 (38.9) | 0.92 |
| Fair | 18 (50) | 10 (55.6) | 8 (44.4) | |
| Bad | 5 (13.9) | 2 (11.1) | 3 (16.7) | |
| Weight (kg, Mean ± SD) | 66.8 ± 9.7 | 63.1 ± 7.4 | 70.4 ± 10.4 | 0.02 |
| Height (cm, Mean ± SD) | 165.0 ± 6.6 | 163.8 ± 6.5 | 166.1 ± 6.6 | 0.31 |
Note: * One participant's information is missing.
Fig. 1.
Study sample flow chart of Phase 2.
3.2. Medication adherence and health outcomes
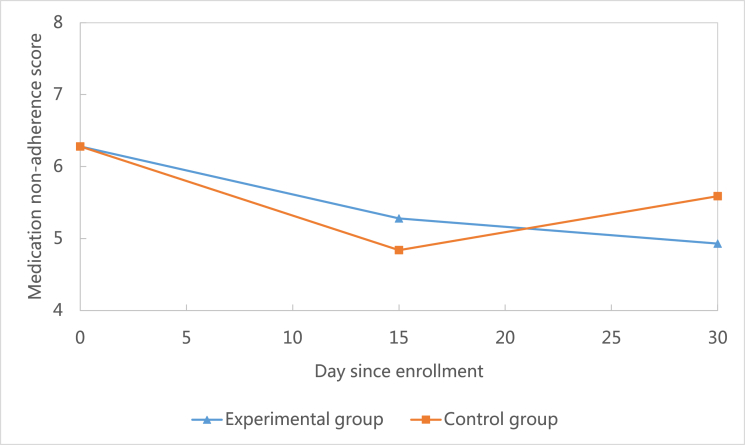
Medication adherence increased at 30-day follow-up in both groups compared to baseline(see Fig. 2). At the 30-day follow-up, the mean of the decrease in medication non-adherence score in the experimental group (M = −1.35, SD = 2.18, N = 36) was more than the decrease in control group (M = −0.69, SD = 1.58, N = 36) (Table 3), which means the medication adherence improved more in the experimental group. However, this difference between the two groups was not statistically significant (t = 1, df = 31, P = 0.33).
Fig. 2.
Comparison of changes of medication non-adherence between groups.
Table 3.
Compare the baseline and unadjusted changes in medication adherence and health outcomes between two arms (Mean ± SD).
| Variable | Experimental (n = 18) | Control (n = 18) | t-value (df) | P-value |
|---|---|---|---|---|
| Medication non-adherence | ||||
| Baseline | 6.28 ± 2.14 | 6.28 ± 1.99 | 0 (34) | 1.00 |
| 15 days-baseline | −1.00 ± 2.67 | −1.44 ± 1.59 | −0.56 (29) | 0.58 |
| 30 days-baseline | −1.35 ± 2.18 | −0.69 ± 1.58 | 1.00 (31) | 0.33 |
| Heart rate (bpm) | ||||
| Baseline | 67.13 ± 9.23 | 76.82 ± 14.32 | 2.24 (30) | 0.03 |
| 15 days-baseline | 4.92 ± 13.42 | −6.12 ± 12.14 | −2.36 (28) | 0.03 |
| 30 days-baseline | 4.64 ± 11.89 | −8.06 ± 13.66 | −2.73 (29) | 0.01 |
| Systolic blood pressure (mm Hg) | ||||
| Baseline | 125.7 ± 11.80 | 123.60 ± 14.27 | −0.47 (33) | 0.64 |
| 15 days-baseline | −1.33 ± 18.51 | −0.83 ± 19.93 | 0.07 (31) | 0.94 |
| 30 days-baseline | 0.93 ± 10.40 | −3.76 ± 25.72 | −0.66 (30) | 0.51 |
| Diastolic blood pressure (mm Hg) | ||||
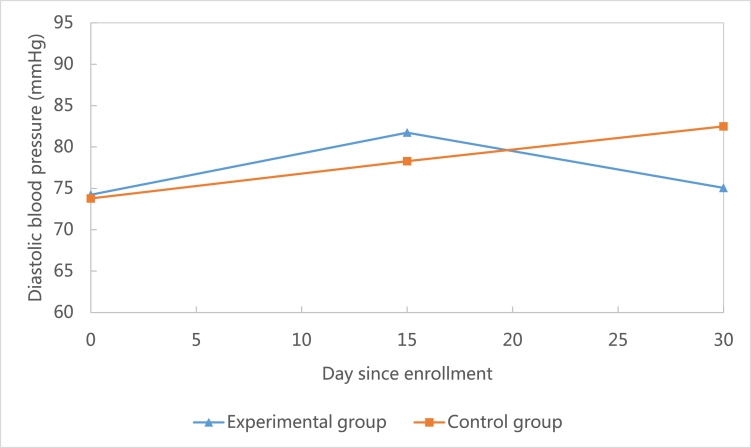
| Baseline | 74.24 ± 10.07 | 73.78 ± 8.45 | −0.15 (33) | 0.88 |
| 15 days-baseline | 7.50 ± 17.43 | 4.50 ± 16.78 | −0.51 (32) | 0.62 |
| 30 days-baseline | 0.81 ± 10.38 | 8.71 ± 21.43 | 1.33 (31) | 0.19 |
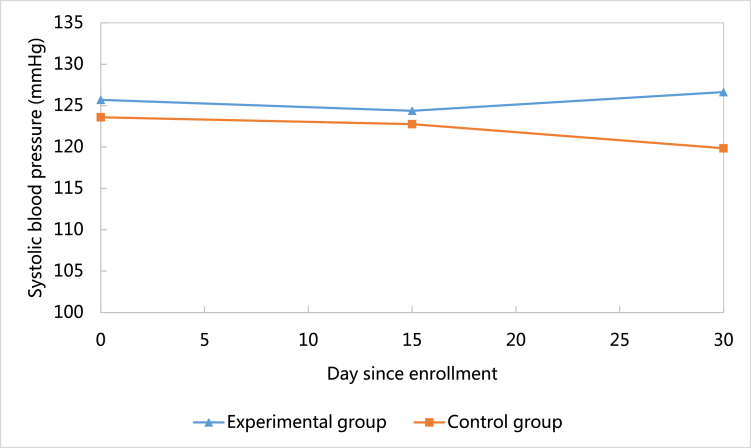
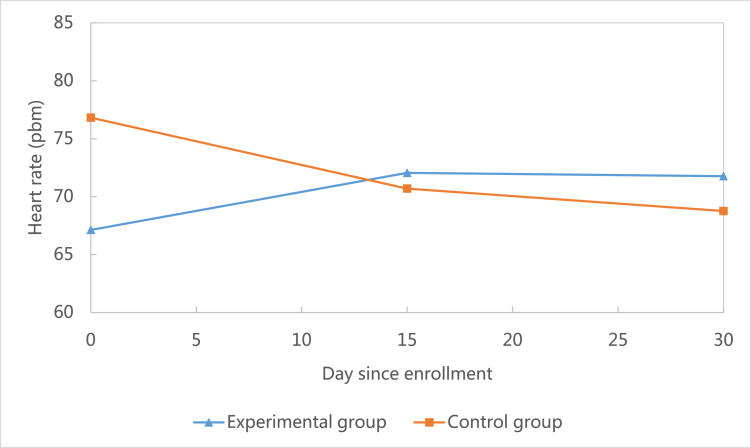
This study showed that when compared to the baseline, systolic blood pressure decreased at 30-day follow-up in the control group (M = −3.76, SD = 25.72), but increased in the experimental group (M = 0.93, SD = 10.40).(see Fig. 3) However, this difference was not statistically significant (P = 0.51). Similarly, heart rate decreased at the 30-day follow-up in the control group (M = −8.06, SD = 13.66), but increased in the experimental group (M = 4.64, SD = 11.89).(see Fig. 4) This difference was statistically significant (P = 0.01). Unlike systolic blood pressure and heart rate, diastolic blood pressure increased at 30-day follow-up in both the control group (M = 8.71, SD = 21.43) and the experimental group (M = 0.81, SD = 10.38).(see Fig. 5) However, this difference was not statistically significant (P = 0.19).
Fig. 3.
Comparison of changes of systolic blood pressure between groups.
Fig. 4.
Comparison of changes of heart rate between groups.
Fig. 5.
Comparison of changes of diastolic blood pressure between groups.
Body weight was associated with heart rate and blood pressure. After controlling the effects of baseline body weight (kg), time, group, and the group-by-time interaction in the mixed-effects model, this study found that the difference in rate of change of medication non-adherence between the two groups were not statistically significant (β = −0.03, P = 0.28) (Table 4). The difference in rate of change of the systolic blood pressure (β = 0.17, P = 0.46) and diastolic blood pressure (β = −0.27, P = 0.17) between the two groups were not statistically significant either. However, the difference in change of heart rate between the two groups were statistically significant (P = 0.02).
Table 4.
Results of the mixed-effects model with a random intercept and slope for between-group differences in the trajectory of change of outcomes.
| Item | Medication non-adherence |
Diastolic blood pressure |
Systolic blood pressure |
Heart rate |
||||
|---|---|---|---|---|---|---|---|---|
| Parameter estimation | P-value | Parameter estimation | P -value | Parameter estimation | P -value | Parameter estimation | P -value | |
| Intercept | 6.75 | 0.003 | 76.99 | <0.0001 | 118.24 | <0.0001 | 85.03 | <0.0001 |
| Group (reference group = control) | 0.14 | 0.84 | 2.37 | 0.52 | 1.78 | 0.72 | −8.43 | 0.05 |
| Weight | −0.01 | 0.68 | −0.04 | 0.81 | 0.08 | 0.71 | −0.14 | 0.46 |
| Time | −0.02 | 0.26 | 0.29 | 0.04 | −0.13 | 0.40 | −0.27 | 0.01 |
| Group × time | −0.03 | 0.28 | −0.27 | 0.17 | 0.17 | 0.46 | 0.39 | 0.02 |
3.3. Feasibility and acceptability
Overall, 50 participants were recruited. Thirty-six finished the study and were responsive over the 30-day period, resulting in a retention rate of 72%. Ten participants were randomly interviewed after the study intervention. All interviewed participants reported that they were able to see the reminders and that receiving reminders on WeChat did not increase their cellphone costs. Five major themes arose from the participants’ impression of the intervention (Table 5). Participants stated they enjoyed receiving medication-taking reminders on their mobile phones because “receiving the reminders makes me feel that someone is caring about me.” In addition, all interviewed participants reported the reminders they received were helpful to remember to take their medications. For example, one participant stated, “Every morning I took my anti-hypertensives after waking up, but sometimes I forgot to take my other medications.” Similarly, all patients showed appreciation and acceptability of receiving educational materials via the mobile app.
Table 5.
Qualitative themes (n = 10).
| Theme | Description | Verbatim Exemplars |
|---|---|---|
| 1. Cue to action | The intervention reminded participants to take medications. | “After receiving the reminder, I immediately check whether I have taken today's medications.” |
| 2. Useful | Participants felt the reminders were a useful tool for taking medication on time. | “The reminders you sent are helpful because sometimes I will forget taking my medications at noon.” |
| 3. Cost-effective | Participants felt this mHealth program would not increase costs. | “I have Wi-Fi at home.” “Receiving information from you does not increase costs at all. You don't need to worry.” |
| 4. Tailoring | Participants wanted to receive tailored reminders and educational materials. | “I want to receive specific information about my disease.” |
| 5. Caring | Participants felt they were cared by others. | “Receiving the reminders makes me feel that someone is caring about me.” “Receiving reminders from a healthcare provider is very good, …, it makes me feel that I am cared by professionals. It strengthened my confidence of fighting against the heart disease.” |
4. Discussion
The purpose of this study was to evaluate the feasibility of a phone-based medication reminder program aimed at improving medication adherence among patients with coronary heart disease. Phase 1 of the study tested the integration of WeChat and BB Reminder, and demonstrated that they can be used as a clinical intervention in clinical setting. Results from Phase 2 of the study demonstrated that participants in the experimental group improved medication adherence better than their counterparts in the control group during a 30-day period. However, this difference was not statistically significant. This non-significance might be due to the small sample size and short duration of the study.
Although the improvement in medication adherence was not statistically significant, this study does provide significant impact for the future. First, it demonstrated that using mHealth to provide health services and manage patient information is feasible among patients with coronary heart disease. Study results showed that 80% of participants were using WeChat daily and had used WeChat for more than a year before the study. In addition, 72% of participants completed the study and were responsive over the 30-day study period. The interviews of participants showed that the mHealth intervention was acceptable for all interviewed participants. These results demonstrated that a larger scale study of using mHealth to solve the prevalent issue of medication non-adherence among patients with coronary heart disease is feasible.
Analyzing health outcomes including systolic blood pressure, diastolic blood pressure, and heart rate was not the primary goal of this feasibility study (see Fig. 3, Fig. 4, Fig. 5). However, the research team did analyze all outcomes and found that at 30-day follow-up, systolic blood pressure and heart rate were controlled better in the control group than the experimental group. These results contradicted our expectation that the mHealth intervention in this study could improve health outcomes. However, after taking a close look at the trajectory of the two outcomes (Fig. 4, Fig. 5), we found that at 30-day follow-up the two outcomes were within normal clinical range. In addition, this study found that participants in the experimental group were more likely to have a lower level of diastolic blood pressure during the study period (Fig. 3). This indicated that the mHealth intervention in this study could potentially decrease diastolic blood pressure among CHD patients.
Despite the feasibility of the mHealth intervention and its possibility to improve health outcomes in clinical settings, limitations exist. For example, all participants in this study were recruited from one single hospital and most participants (91.7%) were from urban areas. In general, people living in urban areas have more access to the Internet and are more likely to use smart phones. Therefore, if this study was conducted in a rural area, the retention rate and acceptability rate might be different. Furthermore, this study is a feasibility study with a small sample size and short duration. Only 50 participants were recruited in Phase 2 and the mHealth intervention only lasted for 30 days. These could be the reasons that contributed to the non-significance of medication adherence improvement. Likewise, the non-significance of systolic blood pressure, diastolic blood pressure, and heart rate between the experimental group and control group could be due to this limitation. To validate our findings and generate more robust estimates of the effects of mHealth intervention on medication adherence and health outcomes, a larger randomized controlled trial with a longer duration is needed.
One important lesson we learned from this study that should be considered in other mHealth research is that mHealth interventions should be preprogramed and automated. In this study, the coordinator manually sent medication-taking reminders to every participant in the experimental group daily. When the sample size increased, the workload of manually sending reminders increased as well. Although in this feasibility study the study coordinator successfully sent all reminders to participants on time, this may not be the case in a larger scale study or in real clinical settings. It is possible that the heavy workload will interrupt researchers sending every participant a reminder daily. If this happened, the integrity and validity of the mHealth intervention would be damaged. To avoid this methodological issue, schedules for sending reminders should be preprogramed and automated, and message delivery, receipt, and responses should be tracked automatically. To achieve this, inviting a third-party software company to design a reminder management system is necessary, particularly when an mHealth intervention is applied in real clinical settings.
Acknowledgement
We thank Guiying You, the head nurse of West China Hospital Cardiology Department for her support of this study. We thank nurses working in West China Hospital Cardiology Department for their assistance in recruiting participants. Also, we thank Shaoqing Ge, a PhD student from Duke University, for her assistance in transcribing interview records. Finally, we express our gratitude to Dr. Susan Silva for her advice on designing the data analysis plan.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijnss.2018.09.003.
Conflicts of interest
None declared.
Funding
This work was supported by the Duke University School of Nursing [PhD Student Pilot Study Fund]; and the Duke University Graduate School [International Dissertation Research Travel Award].
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organization China: WHO statistical profile. 2015. http://www.who.int/gho/countries/chn.pdf?ua=1 Retrieved on September 10, 2017.
- 2.Zhang X.H., Lu Z.L., Liu L. Coronary heart disease in China. Heart. 2008;94(9):1126–1131. doi: 10.1136/hrt.2007.132423. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Benjamin E.J., MacMahon S. Prevention and control of cardiovascular disease in the rapidly changing economy of China. Circulation. 2016;133(24):2545–2560. doi: 10.1161/CIRCULATIONAHA.115.008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health How to prevent and control coronary heart disease risk factors. 2016. https://www.nhlbi.nih.gov/health/health-topics/topics/hd/prevent#Medicines Retrieved on December 13, 2016.
- 5.American Heart Association Cardiac medications. 2017. http://www.heart.org/HEARTORG/Conditions/HeartAttack/TreatmentofaHeartAttack/Cardiac-Medications_UCM_303937_Article.jsp#.Wesy0jBrz2U Retrieved on February 13, 2018 from.
- 6.Zhang H., Yuan X., Zhang H., Chen S., Zhao Y., Hua K. Efficacy of long-term β-blocker therapy for secondary prevention of long-term outcomes after coronary artery bypass grafting surgery. Circulation. 2015;131(25):2194–2201. doi: 10.1161/CIRCULATIONAHA.114.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S. Sudden cardiac death in China. Pacing Clin Electrophysiol. 2009;32(9):1159–1162. doi: 10.1111/j.1540-8159.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamm C.W., Bassand J.P., Agewall S., Bax J., Boersma E., Bueno H. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(23) doi: 10.1093/eurheartj/ehr236. 2999-54. [DOI] [PubMed] [Google Scholar]
- 9.Ho P.M., Bryson C.L., Rumsfeld J.S. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 10.Bi Y., Gao R., Patel A., Su S., Gao W., Hu D. Evidence-based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: results from the Clinical Pathways for Acute Coronary Syndromes in China (CPACS) study. Am Heart J. 2009;157(3):509–516. doi: 10.1016/j.ahj.2008.09.026. e1. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J., Hong T., Yu R., Zhang Y., Liu Z., Huo Y. Knowledge of secondary prevention guidelines for coronary heart disease: results from a physicians' survey in China. Eur J Prev Cardiol. 2012;19(5):991–998. doi: 10.1177/1741826711421299. [DOI] [PubMed] [Google Scholar]
- 12.Iuga A.O., McGuire M.J. Adherence and health care costs. Risk Manag Healthc Pol. 2014;7:35–44. doi: 10.2147/RMHP.S19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabaté E., editor. Adherence to long-term therapies: evidence for action. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 14.World Health Organization Chronic diseases and health promotion. 2017. http://www.who.int/chp/knowledge/publications/adherence_report/en/ Retrieved on December 10, 2017 from.
- 15.World Health Organization Cardiovascular disease. 2017. Retrieved on December 11, 2017 from http://www.wpro.who.int/china/mediacentre/factsheets/cvd/en/
- 16.Zhao S., Zhao H., Wang L., Du S., Qin Y. Education is critical for medication adherence in patients with coronary heart disease. Acta Cardiol. 2015;70(2):197–204. doi: 10.1080/ac.70.2.3073511. [DOI] [PubMed] [Google Scholar]
- 17.Daily China. China's mobile users hit 1.3 billion in 2015. 2017. Retrieved on March 15, 2017 from http://www.chinadaily.com.cn/business/2016-01/26/content_23261207.htm.
- 18.China Internet Network Information Center The 33th report on the development of internet in China. 2014. http://www1.cnnic.cn/IDR/ReportDownloads/201404/U020140417607531610855.pdf Retrieved on February 6, 2016, from.
- 19.Li X., Xu Z.R., Tang N., Ye C., Zhu X.L., Zhou T., Zhao Z.H. Effect of intervention using a messaging app on compliance and duration of treatment in orthodontic patients. Clin Oral Invest. 2015;20(8):1849–1859. doi: 10.1007/s00784-015-1662-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Wen D., Liang J., Lei J. How the public uses social media WeChat to obtain health information in China: a survey study. BMC Med Inf Decis Making. 2017;(Suppl 2):66. doi: 10.1186/s12911-017-0470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voils C.I., Maciejewski M.L., Hoyle R.H., Reeve B.B., Gallagher P., Bryson C.L., Yancy W.S., Jr. Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care. 2012;50(12):1013–1019. doi: 10.1097/MLR.0b013e318269e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.