Abstract
Aims
Paediatric pressure ulcers are a serious problem to healthcare service. Thus, effective and early identification of the risk of developing pressure ulcer is essential. The Braden Q scale is a widely used tool in the risk assessment of paediatric pressure ulcer, but its predictive power is controversial. Hence, we performed a meta-analysis to evaluate the predictive power of the Braden Q scale for pressure ulcer in hospitalised children and offer recommendations for clinical decision.
Methods
Studies that evaluated the predictive power of the Braden Q scale were searched through databases in English and Chinese, including Medline, Cochrane Library, Embase, CINAHL, SinoMed, CNKI, Wangfang and VIP. The studies were screened by two independent reviewers. QUADAS-2 was used to assess the risk of bias of eligible studies. Demographic data and predictive value indices were extracted. The pooled sensitivity, specificity and receiver operating characteristics (ROC) were calculated by MetaDiSc 1.4 using random-effects models.
Results
Cochran Q = 26.13 (P = 0.0036) indicated the existence of heterogeneity; the I2 for pooled DOR was 61.7%, suggesting significant heterogeneity among the included studies. The pooled sensitivity and specificity were 0.73 (95% CI: 0.67–0.78) and 0.61 (95% CI: 0. 59–0.63), respectively, yielding a combined DOR of 3.47 (95% CI: 2–6.01). The area under the ROC curve was 0.7078 ± 0.0421, and the overall diagnostic accuracy (Q*) was 0.6591 ± 0.0337. Sensitivity analysis showed the results were robust.
Conclusion
The Braden Q scale has moderate predictive validity with medium sensitivity and low specificity for pressure ulcers in hospitalised children. Further development and modification of this tool for use in paediatric population are warranted.
Keywords: Braden Q scale, Child, Pressure injury, Pressure ulcer, Risk assessment, Sensitivity and specificity
1. Introduction
Paediatric patients, regardless of age or developmental level, are at risk for pressure ulcers (PUs) [1]. A PU is a localised injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure or pressure in combination with the shear [2]. PU imposes physical and psychological burden on patients and their caregivers. The injury causes discomfort and pain, impairs quality of life, prolongs hospital length of stay and is associated with high morbidity and mortality [[3], [4], [5]]. Recent surveys have reported that the prevalence rates of PU in the paediatric population range from 1.4% to 35% [[6], [7], [8]] and that the incidence rates of hospital-acquired PU rates range from 1.1% to 66% [6,9]. Increasing incidence of PUs results in increasing medical expenditure on patients and the healthcare system. The estimated daily expenditure on PU treatment ranges from € 1.71 to € 470.49 across different settings [10]. In the UK, the treatment cost depends on severity and varies from £ 1214 to £ 14,108 [11].
Avoidance of pressure-related injuries and maintenance of skin and tissue integrity are the key focus of many healthcare institutions worldwide. Effective implementations of PU prevention protocols require early identification of at-risk patients. According to a systematic review, 12 paediatric PU assessment tools are available [12], but only the Braden Q scale, the Glamorgan scale, and the neonatal skin risk assessment scale (NSRAS) have a sensitivity and specificity test [13]. Relative to the Glamorgan scale developed in 2009 [14] and the NSRAS in 1997 that is only suitable for neonates [15], Braden Q has a longer age span. The Braden scale is a widely used PU risk prediction tool in adult-based clinical settings. In 1996, Quigley and Curley adapted this tool for use in the paediatric population and named it ‘Braden Q’ [16]. Braden Q contains the original six subscales of the Braden scale. A seventh subscale according to the risk factor in the paediatric population was added [16]. Thus, Braden Q has seven subscales: mobility, activity, sensory perception, skin moisture, friction and sheer, nutrition and tissue perfusion/oxygenation [16]. All seven subscales are mutually exclusive. The minimum score for each subscale is 1 (high risk), and the maximum score is 4 (low risk). Potential scores range from 7 to 28 points; the lower the score, the higher is the patient's risk for PUs [17].
Diagnostic tests or tools must identify a condition correctly. PU risk scale scores must indicate when a risk really exists (sensitivity) and when no risk exists (specificity) [18]. Currently, many studies evaluated the diagnostic role of the Braden Q scale in the early prediction of pressure sores. However, the predictive ability and best cut-off value of the tool vary because of variation among subjects, sample size and cut-off value. The predictive ability of the Braden Q scale for PUs is controversial. Hence, we performed a meta-analysis to determine the overall predictive accuracy of the Braden Q scale in hospitalised children and offer recommendations for clinical decision.
2. Methods
2.1. Search strategy
A Search strategy was developed by the research team through literature reading, repeatedly searching and consulting with relevant experts. We searched both English (Cochrane Library, Medline (via PubMed), Embase, CINAHL (via EBSCO)) and Chinese (SinoMed, CNKI, Wangfang and VIP) databases to explore relevant papers about the predictive validity of the Braden Q scale for PU assessment in paediatric patients published from January 1996 to July 2018. Search terms were as follows: (ʻchild’ or ʻinfant’ or ʻpaediatric’), (ʻpressure ulcer’ or ʻpressure sore’ or ʻbed sore’ or ʻdecubitus’ or ʻpressure injury’) and (ʻassess*’ or ʻpredict*’ or ʻscale’). Mesh terms and free words were combined for use according to different databases. Additional studies were identified through hand-searching references of the identified studies. Publication language was limited to Chinese and English. Paper publication should be between 1996 and 2018 because Braden Q was first described by Quigley and Curley in 1996. We conducted our search on July 25, 2018. The detailed full search strategy is described in Appendix 1.
2.2. Eligibility criteria
The selection criteria for the studies were as follows: (1) assessed the predictive accuracy of Braden Q scale for PUs in paediatric patients, including children, infant or newborns; (2) provided sufficient information to construct two-by-two contingency tables for individual study subjects or included sufficient data to calculate these factors. The study was excluded if (1) participants were older than 18 years old; (2) review, duplicate or expert opinions; (3) modified Braden Q was used and not the complete original Braden Q; (4) the reported outcome include pressure ulcers and other wounds that can not know the exact incidence of pressure ulcer. Two reviewers independently assessed the study eligibility by screening the title, abstract and full-text in accordance with an ordered guideline. When the results were controversial, the third investigator was responsible for reconciling.
2.3. Quality assessment
Two reviewers individually assessed the quality of studies using QUADAS-2 [19], which was designed to assess the quality of primary diagnostic accuracy studies. QUADAS-2 consists of four key domains (11 items) that discuss patient selection, index test, reference standard and flow of patients through the study and timing of the index tests. Risk of bias was judged as ʻlow’, ʻhigh’ or ʻunclear’ according to the answers ʻyes’, ʻno’ or ʻunclear’ to all signalling questions in each domain. Disagreements were resolved by a third reviewer.
2.4. Data extraction
The two reviewers extracted independently the following information: name of first author, year of publication, country, study setting, sample size, cut-off value, PU staging system, mean age of participants and predictive validity index, such as sensitivity, specificity, TP, FP, TN and FN. In the presence of multiple cut-off values in a study, the values that have the best sensitivity and specificity were chosen.
2.5. Data analysis
Data were processed using MetaDiSc version 1.4. Threshold effect was determined through the Spearman correlation coefficient of sensitivity logarithm and 1-specificity logarithm; a positive relationship indicates heterogeneity resulting from threshold effect [20]. Q-test of diagnostic odds ratio was used to determine heterogeneity caused by non-threshold effect; P ≤ 0.1 indicates significant heterogeneity. The degree of heterogeneity was also measured by I2 (I2 < 25%, 25% < I2 < 50% and I2 > 50% indicate low, moderate and high heterogeneity, respectively) [21]. Meta-regression was performed to explore the causes of heterogeneity. Pooled sensitivity and specificity and area under curve (AUC) of summary receiver operating characteristics (SROC) were calculated to assess the predictive accuracy of the Braden Q scale. In addition, publication bias was inspected via Deeks’ funnel plot of the diagnostic odds ratio against the study size using Stata software version 14.0.
3. Results
3.1. Results of the search
A total of 1731 articles (1239 in English and 490 in Chinese) were identified, of which 589 were excluded due to duplication determined through the citation management software Noteexpress. After screening the titles and abstracts, 1080 articles were excluded due to topic irrelevancy and 31 for not being published in Chinese or English. The full manuscripts of the remaining 31 articles were reviewed in detail. Eight studies did not use the Braden Q scale. Four were excluded due to insufficient data for calculating sensitivity. Another two were excluded for using a modified form of Braden Q. Four studies were excluded for being reviews and two for being duplicates. Thus, 11 studies (4 in English and 7 in Chinese) with 2508 patients were eligible for the meta-analysis. The detailed selection process is shown in Fig. 1.
Fig. 1.
Flow chart of study selection.
3.2. Information on the eligible studies
The eligible studies included a total of 2508 participants and 248 developed PUs. The incidence rate ranged from 3.3% to 26.7%. The 11 studies were conducted in three countries: eight in China, two in England and one in the United States. Majority of the participants were in intensive care units; only two studies focused on all hospitalised children. PU diagnoses were based on the National Pressure Ulcer Advisory Panel (NPUAP) and European Pressure Ulcer Advisory (EPUA) guidelines and included Stage I through Stage IV. Cut-off ranged from 15 to 21. Sensitivity ranged from 0.47 to 0.88, and specificity ranged from 0.17 to 0.98. Detailed information on each study is listed in Table 1.
Table 1.
Characteristics of 11 included studies.
| First author | Year | Country | Setting | Sample size | Event | Mean age /age range |
PU Staging system | Blindb | Cut-off score | Sensitivity | Specificity | TP | FP | FN | TN | AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu [22] | 2010 | China | ICU | 145 | 9 | 1–15 Y | NPUAP1989 | yes | 17 | 0.667 | 0.348 | 6 | 89 | 3 | 47 | 0.481 |
| Li [23] | 2016 | China | Alla | 372 | 21 | 6.37 ± 5.64 Y | EPUAP2009 | no | 16 | 0.826 | 0.759 | 17 | 85 | 4 | 266 | 0.9226 |
| Gu [24] | 2009 | China | ICU/PICU/CICU/SICU | 133 | 7 | 1.5Y | NPUAP1989 | no | 15 | 0.23 | 0.98 | 2 | 3 | 5 | 123 | none |
| Feng [25] | 2010 | China | ICU | 113 | 8 | 43.15 ± 52.03 M | NPUAP1989 | yes | 19 | 0.625 | 0.476 | 5 | 55 | 3 | 50 | 0.502 |
| Jiang [26] | 2017 | China | NICU | 452 | 15 | none | none | no | 16 | 0.585 | 0.601 | 8 | 174 | 7 | 263 | 0.577 |
| Shen [27] | 2014 | China | ICU | 80 | 7 | 6.4 ± 1.6 Y | NPUAP1989 | yes | 17 | 0.667 | 0.346 | 5 | 48 | 2 | 25 | 0.478 |
| Wang [28] | 2008 | China | PICU/CICU | 145 | 11 | 28 D–8 Y | none | no | 21 | 0.818 | 0.172 | 9 | 110 | 2 | 24 | 0.557/0.597 |
| Curley [29] | 2003 | USA | PICU | 322 | 86 | 3 Y | NPUAP1989 | yes | 16 | 0.88 | 0.58 | 76 | 99 | 10 | 137 | 0.83 |
| Willock [30] | 2009 | England | All | 336 | 61 | 1 D–17 Y 11 M | EPUAP2009 | no | 21 | 0.672 | 0.648 | 41 | 97 | 20 | 178 | 0.697 |
| Lu [31] | 2014 | China | PICU | 198 | 14 | 4 Y | NPUAP1989 | yes | 19 | 0.71 | 0.53 | 10 | 86 | 4 | 98 | 0.57 |
| Habib [32] | 2013 | England | PICU/NICU/GIMU/GICU | 212 | 9 | 0–17 Y | EPUAP2009 | no | 16 | 0.47 | 0.83 | 9 | 32 | 10 | 161 | 0.8 |
aAll hospitalised children were included except those in the department of neonate, surgery in outpatient and operation room.
bBlind means two nurses performed the assessment individually; D means day; Y means year; M means month.
3.3. Methodological quality of included studies
Quality assessment results are shown in Fig. 2. None of the 11 studies fulfilled all the quality criteria. The most frequent risks of bias were patient selection and index test. Of three studies with a high risk in the patient selection domain, one (Li 2016) enrolled participants through convenience sampling, the other one (Lu 2010) excluded patients with a low risk of developing PU and another one (Willock 2009) enrolled patients existing PU before risk assessment. Four studies were judged high risk in the index test domain because the text reader was not blinded to the clinical data; the person who completed the Braden Q scale was the same person who did the skin assessment. One study was deemed unclear in standard reference because it did not specify the PU staging system.
Fig. 2.
Risk of bias and applicability concerns summary.
3.4. Study heterogeneity
The Spearman correlation coefficient of sensitivity logarithm and 1-specificity logarithm was 0.506 (P = 0.113), which indicated no threshold effect. Cochran Q = 26.13 (P = 0.0036) indicated the existence of heterogeneity of non-threshold effect; I2 for pooled DOR was 61.7%, indicating significant heterogeneity among the included studies. Possible sources of heterogeneity across the studies were explored using meta-regression analysis with the following covariates as predictor variables: country (China = 0, England and USA = 1); blind (yes = 1, no = 0, if two nurses independently assessed skin condition and completed Braden Q scale assessment, then yes; if only one nurse completed the assessment, then no); cut-off score (≤16 = 0, >16 = 1). Results suggested that these predictors were not associated with accuracy (Table 2). Information in some original studies was limited; thus, other variables such as participants’ age and enrolment method were not included in the analysis. On the basis of heterogeneity, the random-effects model was used for the pooled analysis.
Table 2.
Meta-regression analysis results.
| Meta-Regression(Inverse Variance weights)(1) | |||||
|---|---|---|---|---|---|
| Var | Coeff. | Std. Err. | P value | RDOR | [95%CI] |
| Cte. | 1.495 | 0.5111 | 0.0265 | – | – |
| S | −0.112 | 0.1960 | 0.5874 | – | – |
| country | 0.551 | 0.5470 | 0.3523 | 1.74 | (0.46; 6.62) |
| cutoff | −1.016 | 0.5904 | 0.1362 | 0.36 | (0.09; 1.54) |
| blind |
0.241 |
0.6080 |
0.7052 |
1.27 |
(0.29; 5.63) |
|
Meta-Regression(Inverse Variance weights)(2) | |||||
| Var | Coeff. | Std. Err. | P value | RDOR | [95%CI] |
| Cte. | 1.572 | 0.4302 | 0.0081 | – | – |
| S | −0.070 | 0.1681 | 0.6882 | – | – |
| country | 0.549 | 0.5024 | 0.3106 | 1.73 | (0.53; 5.68) |
| cutoff |
−0.984 |
0.5394 |
0.1109 |
0.37 |
(0.10; 1.34) |
|
Meta-Regression(Inverse Variance weights)(3) | |||||
| Var | Coeff. | Std. Err. | P value | RDOR | [95%CI] |
| Cte. | 1.845 | 0.3709 | 0.0011 | – | – |
| S | −0.080 | 0.1768 | 0.6634 | – | – |
| cutoff | −1.096 | 0.5680 | 0.0897 | 0.33 | (0.09; 1.24) |
3.5. Predictive validity
The pooled sensitivity of the studies was 0.73 (95%CI: 0.67–0.78; χ2 = 29.19, P = 0.0012) (Fig. 3a). The pooled specificity was 0.61 (95% CI: 0.59–0.63; χ2 = 359.12, P = 0.000), and the pooled DOR was 3.47 (95% CI: 2 to 6.01) (Fig. 3b, Fig. 3cb and c, respectively). The overall weighted AUC was 0.7078 ± 0.0421, and the Q* value was 0.6591 ± 0.0337. The SROC curve is shown in Fig. 3d. The pooled sensitivity and specificity were 0.745 and 0.579, respectively, indicating that the results were robust.
Fig. 3a.
Forest plot of pooled sensitivity.
Fig. 3b.
Forest plot of pooled specificity.
Fig. 3c.
Forest plot of DOR.
Fig. 3d.
SROC curve.
3.6. Sensitivity analysis
Sensitivity analysis was conducted by removing one study at a time. Results are presented in Table 3. Compared with the original results of all included studies, the consequence of sensitivity and AUC reduced slightly after the removal of Curley's study. Thus, the meta-analysis results are robust.
Table 3.
Sensitivity analysis results.
| Study to remove | Sensitivity | Specificity | DOR | P value | I2(%) | AUC |
|---|---|---|---|---|---|---|
| Lu 2010 | 0.731 | 0.627 | 4.49 | 0.007 | 60.1 | 0.72 |
| Li 2016 | 0.722 | 0.582 | 3.80 | 0.011 | 58.0 | 0.69 |
| Gu 2009 | 0.741 | 0.588 | 4.14 | 0.004 | 62.9 | 0.70 |
| Feng 2010 | 0.732 | 0.616 | 4.39 | 0.004 | 63.0 | 0.72 |
| Jiang 2017 | 0.741 | 0.612 | 4.55 | 0.006 | 61.2 | 0.72 |
| Shen 2014 | 0.729 | 0.619 | 4.37 | 0.004 | 63.1 | 0.71 |
| Wang 2008 | 0.725 | 0.637 | 4.44 | 0.006 | 60.8 | 0.71 |
| Curley 2003 | 0.651 | 0.613 | 3.17 | 0.042 | 48.5 | 0.68 |
| Willock 2009 | 0.746 | 0.605 | 4.35 | 0.002 | 65.4 | 0.71 |
| Lu 2014 | 0.730 | 0.617 | 4.32 | 0.002 | 65.1 | 0.71 |
| Allah 2013 | 0.749 | 0.589 | 4.17 | 0.002 | 65.5 | 0.71 |
| origin | 0.729 | 0.610 | 4.20 | 0.004 | 61.7 | 0.71 |
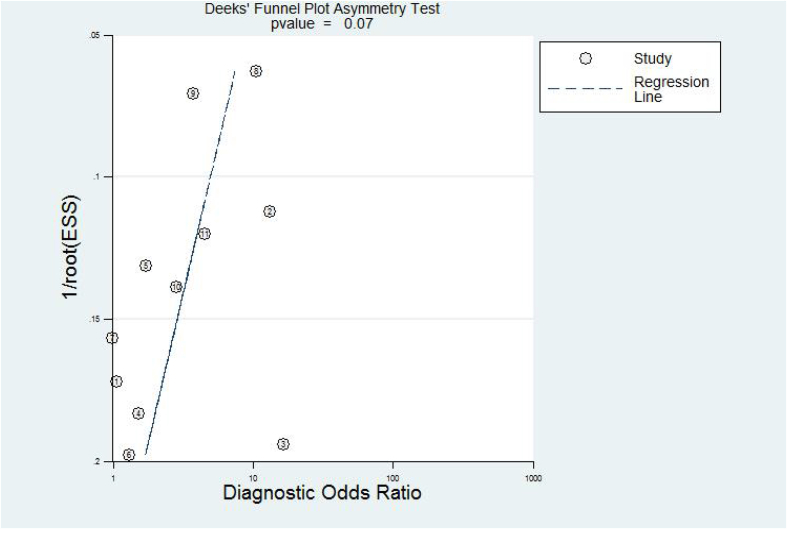
3.7. Publication bias
Fig. 4 shows that all included studies were symmetrically distributed. Deeks’ funnel plot asymmetry test with a P-value of 0.07 revealed no significant difference in the publication biases among the pooled study.
Fig. 4.
Deeks' funnel plot.
4. Discussion
In the current study, we included 11 papers and extracted enough data from the enrolled 2508 participants to conduct a meta-analysis. The consequences of the meta-analysis were a summary AUC of 0.71, a summary estimate of 0.73 for sensitivity and 0.61 for specificity. Thus, the results indicated the moderate predictive ability of the Braden Q scale for PUs in hospitalised children, medium sensitivity and low specificity. These results differ from the results of Li's 2016 study with an ideal AUC of 0.92 and Curley's 2003 study with AUC of 0.83. However, the results are consistent with Willock's findings which showed a summary AUC of 0.70, a sensitivity of 0.67 and a specificity of 0.65. Sensitivity analysis results confirmed the credibility of the results. Furthermore, meta-regression analysis found no heterogeneity among country, blind and cut-off score. These results were not encouraging, and some undetected factors could have caused heterogeneity and influenced the predictive validity of the Braden Q scale. Hence, we analysed the reasons as follows:
Firstly, except for one study that did not provide any age information, the other 10 studies had different age inclusion standards for children. Only three studies (Gu 2009, Curley 2003 and Lu 2014) included children aged 21 days after birth to 8 years and provided reasonable explanations. The authors of the three studies believed the infant's skin is not mature until 3 weeks of age [33] and patients over the age of 8 years were excluded because they were considered adults in terms of medical treatment, according to the regulation of American Heart Association [34]. Relative to the NSRAS [35] and the Neonatal/Infant Braden Q Risk Assessment Scale [36] developed specially for neonates, the Braden Q scale has a longer age span. To date, no clear age limitation of the Braden Q scale in children was confirmed. Secondly, a study showed that the Braden Q scale is better than the other tools for paediatric samples in general units with AUC = 0.82 [37]. However, in the current study, 2 of the 11 studies included almost all hospitalised children. Nine of the 11 studies were conducted in intensive care units where seriously ill patients are inclined to develop PUs. No study tested the predictive ability in general wards. Therefore, the predictive accuracy of the Braden Q scale in the normally ill paediatric population is unclear. Thirdly, the Braden Q scale evaluates skin breakdown in seven domains: mobility, activity, sensory perception, skin moisture, friction and sheer, nutrition and tissue perfusion/oxygenation. However, newly discovered risk factors influencing the development of PU were not included in the Braden Q scale. From the included studies, we found that the Braden Q scale cannot assess skin breakdown caused by pressure of a medical device. Recently, medical devices have become indigenous to the care environment. Studies found that infants and children who require mechanical ventilation, non-invasive ventilation, or extracorporeal membrane oxygenation [1,38] and have multiple medical devices in place [39]are more likely to develop PUs compared with their counterparts. In summary, further investigation on the use of the Braden Q scale on different age spans of children and different hospital settings is needed. Furthermore, the scale should be appropriately modified to adapt to the changing medical environment.
5. Limitation
The current meta-analysis has limitations. Firstly, all studies used PU development as a reference standard to investigate the predictive validity of PU risk scales; however, being at risk does not mean getting a PU [18]. Nearly all obtained sensitivity and specificity estimates in PU risk scale research are biased due to the influence of PU preventive measures [18]. Secondly, significant heterogeneity was found among the studies. However, our meta-regression analysis results did not show heterogeneity among the variables in the studies, including country, blind and cut-off score. The included studies provided limited information; thus, other variables could not be analysed via subgroup analysis. Lastly, even though we evaluated studies through QUADAS-2, some items remain unclear due to limited information. These limitations should be considered in the evaluation of the results.
6. Conclusion
The Braden Q Scale has moderate predictive validity with medium sensitivity and low specificity for PUs in hospitalised children. Cautions should be taken by nurses when applying this scale to neonates or non-critically ill children. We suggest further development and modification on the basis of current medical environment to improve the effectiveness of this tool.
Interest conflicts
The authors have no conflicts of interest to declare.
Funding
None declared.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2018.08.003.
Appendices. Supplementary data
The following are the supplementary data to this article:
References
- 1.Schindler C.A., Mikhailov T.A., Kuhn E.M. Protecting fragile skin: nursing interventions to decrease development of pressure ulcers in pediatric intensive care. Am J Crit Care: Offic Publ, Am Assoc Crit-Care Nurses. 2011;20(1):26–34. doi: 10.4037/ajcc2011754. [DOI] [PubMed] [Google Scholar]
- 2.National Pressure Ulcer Advisory Panel . 2nd ed. vol. 12. Cambridge Media: Oshome Park; Western Australia: 2014. (European pressure ulcer advisory and Pan pacific pressure injury alliance. Prevention and treatment of pressure ulcers: quick reference guide). [Google Scholar]
- 3.Sibbald R.G., Goodman L., Norton L. Prevention and treatment of pressure ulcers[J] Skin Therapy Lett. 2012;17(8):4–7. [PubMed] [Google Scholar]
- 4.Dreyfus J., Gayle J., Trueman P. Assessment of risk factors associated with hospital-acquired pressure injuries and impact on health care utilization and cost outcomes in US hospitals. Am J Med Qual. 2017;(5):1–11. doi: 10.1177/1062860617746741. [DOI] [PubMed] [Google Scholar]
- 5.Payne D. Strategies to support prevention, identification and management of pressure ulcers in the community. Br J Community Nurs. 2016;21(6):10–18. doi: 10.12968/bjcn.2016.21.Sup6.S10. [DOI] [PubMed] [Google Scholar]
- 6.Razmus I., Bergquistberinger S. Pressure injury prevalence and the rate of hospital-acquired pressure injury among pediatric patients in acute care. J Wound Ostomy & Cont Nurs Offic Publ Wound Ostomy Cont Nurses Soc. 2017;44(2):110–117. doi: 10.1097/WON.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 7.Schluer A.B., Halfens R.J., Schols J.M. Pediatric pressure ulcer prevalence: a multicenter, cross-sectional, point prevalence study in Switzerland. Ostomy/Wound Manag. 2012;58(7):18–31. [PubMed] [Google Scholar]
- 8.Ab S., E C., M M. The prevalence of pressure ulcers in four paediatric institutions. J Clin Nurs. 2009;18(23):3244–3252. doi: 10.1111/j.1365-2702.2009.02951.x. [DOI] [PubMed] [Google Scholar]
- 9.Mclane K.M., Bookout K., Mccord S. The 2003 national pediatric pressure ulcer and skin breakdown prevalence survey: a multisite study. J Wocn. 2016;31(4):168–178. doi: 10.1097/00152192-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Demarre L., Van Lancker A., Van Hecke A. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud. 2015;52(11):1754–1774. doi: 10.1016/j.ijnurstu.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Dealey C., Posnett J., Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care. 2012;21(6) doi: 10.12968/jowc.2012.21.6.261. 261-262, 264, 266. [DOI] [PubMed] [Google Scholar]
- 12.Kottner J., Hauss A., Schlüer A.B. Validation and clinical impact of paediatric pressure ulcer risk assessment scales: a systematic review. Int J Nurs Stud. 2013;50(6):807–818. doi: 10.1016/j.ijnurstu.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Baharestani M.M., Ratliff C.R. Pressure ulcers in neonates and children: an NPUAP white paper. Adv Skin Wound Care. 2007;20(4):218–220. doi: 10.1097/01.ASW.0000266646.43159.99. [DOI] [PubMed] [Google Scholar]
- 14.Willock J., Baharestani M.M., Anthony D. The development of the Glamorgan paediatric pressure ulcer risk assessment scale. J Wound Care. 2009;18(1):17–21. doi: 10.12968/jowc.2009.18.1.32135. [DOI] [PubMed] [Google Scholar]
- 15.Logsdon M.C. The neonatal skin risk assessment scale for predicting skin breakdown in neonates. Issues Compr Pediatr Nurs. 1997;20(2):103–114. doi: 10.3109/01460869709026881. [DOI] [PubMed] [Google Scholar]
- 16.Quigley S.M., Curley M.A. Skin integrity in the pediatric population: preventing and managing pressure ulcers. J Spec Pediatr Nurs (JSPN) 1996;1(1):7–18. doi: 10.1111/j.1744-6155.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 17.Curley M.A., Razmus I.S., Roberts K.E. Predicting pressure ulcer risk in pediatric patients: the Braden Q Scale. Nurs Res. 2003;52(1):22–33. doi: 10.1097/00006199-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kottner J., Balzer K. Do pressure ulcer risk assessment scales improve clinical practice? J Multidiscip Healthc. 2010;3(default):103–111. doi: 10.2147/jmdh.s9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting P.F., Rutjes A.W., Westwood M.E. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Tian-Song Z.W. Meta-disc software in meta-analysis of diagnostic test. J Evid Base Med. 2008;8(2):97–100. [Google Scholar]
- 21.Higgins J.P.T., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yefeng L., Jianhua L., Xiuwen L. Research in application of two kinds of pressure ulcer assessment scale in children patients. Chin J Pract Nurs. 2010;26(11):41–43. [Google Scholar]
- 23.Shuangzi L., Ping L., Xianlan Z. High risk cut-off point of Braden-Q pediatric pressure ulcer risk assessment scale. J Chongqing Med Univ. 2016;(6):636–640. [Google Scholar]
- 24.Xiaorong G., Xiulan K., Caifeng W. Applicability of Braden-Q scale for the prediction of pressure UIcers development in children in mainland China. J Nurs. 2009;24(4):6–8. [Google Scholar]
- 25.Sheng F., Xiuwen L., Ha L. Study of two pressure ulcer risk assessment scales utilizing in pediatric patients. J Nurs. 2010;17(13):50–53. [Google Scholar]
- 26.Chun J., Yuqion W., Guofang T. Study of two pressure ulcer risk assessment scales utilizing in neonatal intensive care unit. Chin J Reprod Health. 2017;(6):549–551. [Google Scholar]
- 27.Ling S., Guoxiu Z., Lingling Z. Study of two pressure ulcer assessment tools in children. Int Med Health Guid News. 2014;20(11):1491–1493. [Google Scholar]
- 28.Caifeng W. Shanghai Jiao Tong University; 2008. Research on risk factors and risk assessment scales of pressure ulcer among hospitalized elderly and children. [Google Scholar]
- 29.Curley M.A., Razmus I.S., Roberts K.E. Predicting pressure ulcer risk in pediatric patients: the Braden Q Scale. Nurs Res. 2003;52(1):22–33. doi: 10.1097/00006199-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Willock J., Baharestani M.M., Anthony D. The development of the Glamorgan paediatric pressure ulcer risk assessment scale. J Wound Care. 2009;18(1):17–21. doi: 10.12968/jowc.2009.18.1.32135. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y.F., Yang Y., Wang Y. Predicting pressure ulcer risk with the Braden Q scale in Chinese pediatric patients in ICU. Chin Nurs Res. 2015;2(1):28–34. [Google Scholar]
- 32.Allah H., Laila . De Montfort University; 2013. Pressure ulcer: prevalence, incidence, risk factors and the predictive validity of the Braden Q and the Glamorgan Risk Assessment Scales in paediatrics. [Google Scholar]
- 33.Malloy M.B., Perez-Woods R.C. Neonatal skin care: prevention of skin breakdown[J] Pediatr Nurs. 1991;17(1):41–48. [PubMed] [Google Scholar]
- 34.Textbook of pediatric advanced life support. American Heart Association; 1994. [Google Scholar]
- 35.Huffines B., Logsdon M.C. The neonatal skin risk assessment scale for predicting skin breakdown in neonates. Issues Compr Pediatr Nurs. 1997;20(2):103–114. doi: 10.3109/01460869709026881. [DOI] [PubMed] [Google Scholar]
- 36.de Lima E.L., de Brito M.J., de Souza D.M. Cross-cultural adaptation and validation of the neonatal/infant Braden Q risk assessment scale. J Tissue Viability. 2016;25(1):57–65. doi: 10.1016/j.jtv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Willock J., Habiballah L., Long D. A comparison of the performance of the Braden Q and the Glamorgan paediatric pressure ulcer risk assessment scales in general and intensive care paediatric and neonatal units. J Tissue Viability. 2016;25(2):119–126. doi: 10.1016/j.jtv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Miske L.J., Stetzer M., Garcia M. Airways and injuries: protecting our pediatric patients from respiratory device-related pressure injuries. Crit Care Nurs Clin North Am. 2017;29(2):187–204. doi: 10.1016/j.cnc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Manning M.J., Gauvreau K., Curley M.A. Factors associated with occipital pressure ulcers in hospitalized infants and children. Am J Crit Care: Offic Publ, Am Assoc Crit-Care Nurses. 2015;24(4):342–348. doi: 10.4037/ajcc2015349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.