Abstract
Background
Both long and short sleep duration increase risk of mortality. Most previous studies have been performed in Europeans and have focused on sleep duration. Thus, we aimed to investigate the association between sleep quality and mortality across three different ethnic groups.
Methods
We used data from the Southall and Brent REvisited Study (SABRE) cohort, which comprises first generation migrant South Asian and African Caribbean men and women, aged 40–69 years, recruited between 1988 and 1991. In sum, 4399 participants provided complete data at baseline and follow-up. Of those, 1656 died by December 2017. Our exposures (eg, difficulty falling asleep, early morning waking and waking up tired in the morning) were self-reported and our primary outcome was mortality. We used Cox proportional hazards models to analyse our data, adjusting for baseline-measured confounders.
Results
None of the sleep measures were strongly associated with all-cause mortality in Europeans or African Caribbeans, whilst in South Asians difficulty falling asleep was related to an increased risk of all-cause mortality (HR = 1.28, 95%CI = 1.01; 1.61). In Europeans, early morning waking was associated with a moderately increased risk of cardiovascular death (HR = 1.31, 95%CI = 1.05; 1.63); alternately, this association was not as strong in the other groups.
Conclusion
Our findings suggest that the relationship between sleep quality and mortality may differ by ethnic group, but formal heterogeneity tests indicated that the strongest difference in HRs was observed for early morning waking and cardiovascular disease (CVD) mortality across the three groups (Cochran's Q test p = 0.036). As such, these results are novel and provide support for ethnic differences in sleep quality and mortality, and may have implications for precision medicine.
Keywords: Sleep quality, Cardiovascular, Mortality, Ethnic differences
Highlights
-
•
We explored the association between sleep quality and cancer, CVD and all-cause mortality.
-
•
We used data from a white Europeans, African Caribbeans and South Asians.
-
•
Early morning waking was associated with greater CVD mortality risk in Europeans.
-
•
Findings suggest ethnic differences in these effects, warranting further investigation.
1. Introduction
Over the past four decades there has been substantial evidence suggesting U [1], [2], [3], [4] or J-shaped [5] associations between sleep duration and subsequent mortality, with the greatest mortality risk observed in those with shortest and longest sleep durations. Currently, the National Sleep Foundation recommends between 7 and 9 h of sleep for young adults and 7 to 8 for older adults [6].
However, time asleep does not fully capture the characteristics of this important physiological trait. Therefore, measures of sleep quality (eg, difficulty falling asleep, snoring, tiredness and early morning waking) irrespective of duration may also affect mortality outcomes. Furthermore, sleep quality may elucidate mechanisms via which sleep influences mortality risk.
Sleep duration and quality may be affected by cultural, economic, psychological and physical influences; where global trends are moving towards longer working hours, shift work and access to commodities 24 h a day, seven days a week. This trend may contribute to shorter sleep and increased daytime fatigue or tiredness [7].
In contrast to sleep duration, the association between sleep quality and mortality has not been studied at length, and thus, is poorly understood. A longitudinal Finnish cohort study with 22 years of follow-up data investigating self-reported sleep behaviour and mortality [8] found that those sleeping ‘fairly well’ or ‘fairly poorly’ had an increased risk of all-cause mortality when compared to those who reported sleeping well. However, the associations no longer remained significant following statistical adjustments for confounding variables, such as demographics, body mass index (BMI), health behaviours and satisfaction with life [5]. A more recent meta-analysis found that poor sleep quality is a risk factor for coronary heart disease but found no effect for mortality [5].
To date, the majority of studies investigating sleep and mortality have focused on Europeans and no previous UK studies have examined this association in other ethnic groups. Moreover, different ethnic groups have been shown to have different associations to risk factors such as obesity or diabetes. For example, South Asians are generally not obese when measuring BMI but have higher central fat obesity, and are more likely to be insulin resistant when compared to Europeans [9], [10]. Therefore, exploring associations between sleep quality and outcomes by ethnic group may help elucidate which, if any, mechanisms are involved. Here we used data from the UK population-based tri-ethnic Southall and Brent Revisited (SABRE) cohort to determine whether there were ethnic differences in the association between sleep quality and mortality.
2. Methods
2.1. Sample
The Southall and Brent REvisited (SABRE) Study is a community-based, tri-ethnic cohort of European, South Asian and African Caribbean individuals residing in the West London areas of Southall and Brent in the UK at recruitment. Full cohort details have been published elsewhere [9]. In brief, from 1988 to 1991, men and women aged between 40 and 69 years were randomly selected from primary care lists (n = 4063) and workplaces (n = 795), stratified by sex and age. This form of sampling ensured that the cohort is representative because primary care registration is free in the UK and enables users to access all health services. Apart from being unable to provide informed consent, exclusions were made on the basis of having a cancer diagnosis, a severe disability or psychiatric disturbance. Ethnicity was self-assigned and checked against country of birth of parents. All South Asians and African Caribbeans were first generation migrants, reflecting national migration patterns to the UK from these ethnic groups. Respondents were asked to come into the clinic after having fasted and abstained from alcohol, smoking and caffeine for at least 12 h prior to attendance. A questionnaire for self-completion was administered, which asked for educational attainment, health behaviours, details of medication and medical history. Resting blood pressure was measured, in addition to an oral glucose tolerance test, with fasting and post-load blood samples performed. Ethical approval was obtained from Ealing, Hounslow and Spelthorne, Parkside and University College London research ethics committees.
2.2. Measures
2.2.1. Exposures
Our main exposure of interest in this study was self-reported sleep quality at visit 1. Using three questions adapted from the validated Jenkins Sleep Questionnaire (JSEQ), participants were asked how well they slept in the past 30 days [11]. These questions were about whether participants felt that they had been waking up too early, had difficulty falling asleep and woke up feeling tired in the past 30 days. Possible answers to the sleep questions were ‘No’ (0) or ‘Yes’ (1).
2.2.2. Outcome
Deaths were reported by the Office for National Statistics (ONS). Participants were followed up for all-cause mortality from the baseline study date (1988) until December, 2017. To date, there were total of 1656 deaths in participants who also had complete exposure and covariate data (total N = 4399 across all three ethnic groups).
2.2.3. Covariates
Covariates were selected on the basis that they may have an important role in the relationship between measures of sleep quality and mortality, as per previous literature. These included age, sex, years of education, smoking status (ex-smoker, current smoker, never smoked), total alcohol units per week, waist-hip-ratio (WHR), hypertension medication (No/Yes), cardiovascular disease (CVD) (No/Yes) and type 2 diabetes (No/Yes) and were all measured at baseline. CVD diagnosis was determined via self-reported doctor diagnosis of stroke or coronary heart disease (CHD), which was also the case for baseline diabetes diagnosis.
2.2.4. Statistical analyses
StataCorp. 2015. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP was used to perform the statistical analyses. The nominal p-value was set at 5% (0.05). Differences across ethnic groups were assessed using Chi-squared tests for categorical variables and one-way analysis of variance (ANOVA) for continuous measures. Cox proportional hazard models were used for survival analysis. Initially, we ran Cox models in which all three ethnic groups were included, but the proportional hazards assumption was not met and thus, we performed the analyses separately by group. Three models (one for each ethnicity with all relevant covariates) were constructed as follows: Model 1 was our baseline model and included adjustments for age, sex and education; Model 2 was additionally adjusted for health behaviours (smoking, physical activity and alcohol consumption); Model 3 was adjusted (in addition to age, sex and education) for comorbidities [CVD, T2DM, obesity (WHR) and anti-hypertensive medication)]; and Model 4 was fully adjusted: Model 1 + Model 2 + Model 3. Furthermore, we performed Cochran's Q heterogeneity tests across the three ethnic groups and sleep quality measures to determine whether there were any differences in the effect sizes (hazard ratios) in our final fully-adjusted model for all-cause and cause-specific mortality outcomes.
Model checking. Cox proportional hazard models assume that the explanatory variables act on survival in such a way that the hazard ratio is constant over time. We assessed this assumption using the Global Test evaluating the interaction of each covariate with time, as well as the Schoenfeld residuals.
Assessment of model fit. The model performance was evaluated using Cox-Snell residuals. If any of the covariates suggested that the proportional hazard assumption could be affected, we compared the predictions of the Cox model with the Kaplan–Meier estimate of the survival data; and if they yielded similar results, then the Cox model was deemed appropriate.
3. Results
By December 2017 there were 1656 deaths; of which 914 were Europeans, 552 were South Asians and 190 were African Caribbeans. Table 1 presents sample characteristics at baseline, by ethnic group. South Asians were more likely to report early morning waking, whilst African Caribbeans were more likely to report difficulty falling asleep, in comparison to Europeans. However, there was no difference between the groups in morning tiredness. South Asians had the greatest prevalence of type-2 diabetes, whilst African Caribbeans were the most likely to be on anti-hypertensive medication. Europeans were the most likely to have CVD at baseline.
Table 1.
Sleep quality, demographics, lifestyle factors and comorbidities by ethnic group.
| Total N = 4399 |
P | |||
|---|---|---|---|---|
| Europeans (n = 2200) | South Asians (n = 1468) | African Caribbeans (n = 731) | ||
| Age (years) | 52.9 (7.1) | 50.7 (6.9) | 53.4 (5.9) | <0.001 |
| Males | 1678 (76.3) | 1264 (86.1) | 414 (56.6) | <0.001 |
| Years of education | 10.5 (2.5) | 11.9 (3.8) | 10.6 (3.0) | <0.001 |
| BMI (kg/m2) | 26.2 (4.1) | 26.0 (3.6) | 27.6 (4.2) | <0.001 |
| Alcohol frequency | ||||
| Less than daily | 656 (28.3) | 332 (19.5) | 116 (15.1) | <0.001 |
| Daily/almost daily | 807 (34.9) | 351 (20.6) | 241 (31.4) | |
| Rarely | 710 (30.7) | 328 (19.3) | 336 (43.8) | |
| Never | 142 (6.1) | 693 (40.7) | 75 (9.8) | |
| Smoking status | ||||
| Never | 691 (31.4) | 1125 (76.6) | 481 (65.8) | <0.001 |
| Ever smoked | 1509 (68.6) | 343 (23.4) | 250 (34.2) | |
| CVD | 198 (9.0) | 128 (8.7) | 45 (6.2) | 0.050 |
| Diabetes | 143 (6.5) | 292 (19.9) | 139 (13.1) | <0.001 |
| Antihypertensives | 249 (11.3) | 208 (14.2) | 165 (22.6) | <0.001 |
| Morning tiredness | 835 (38.0) | 525 (35.8) | 271 (37.1) | 0.404 |
| Difficulty falling asleep | 408 (17.5) | 342 (20.1) | 189 (24.0) | <0.001 |
| Early morning waking | 806 (36.6) | 707 (48.2) | 285 (39.0) | <0.001 |
Note. Values represent means (SDs) or n (%), BMI = body mass index, CVD = cardiovascular disease, P = p-value from either one-way ANOVA or chi-squared analysis.
The leading cause of death for the entire sample was neoplasms (28.07%). When broken down by ethnic group, the main cause of death was neoplasm in Europeans (34.47%) and African Caribbeans (33.15%). Meanwhile, main cause of death for South Asians was CHD (33.41%). There were 320, 95 and 66 cancer deaths, respectively. Furthermore, there were 372, 308 and 89 CVD deaths in Europeans, South Asians and African Caribbeans, respectively. Thus, we investigated all-cause mortality, as well as cancer and CVD mortality.
3.1. Associations between sleep quality and all-cause mortality
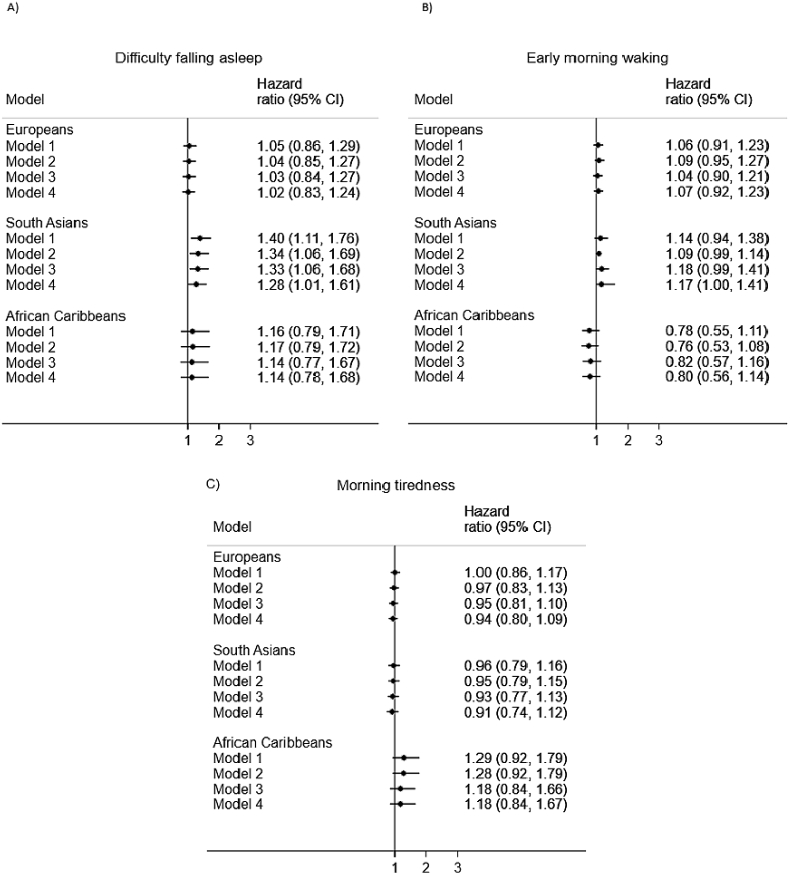
In Europeans, in a minimally-adjusted model there was a weak association (HR ∼1) between the three sleep quality measures and all-cause mortality (Fig. 1). This remained the case on multi-variable adjustment (Fig. 1). A similar pattern of results was observed for the African Caribbeans for sleep quality and all-cause mortality, such that the associations appeared to be weak and had wide confidence intervals (Fig. 1). In South Asians, associations were also weak between early morning waking, morning tiredness and all-cause mortality (Fig. 1). The pattern of results was, however, different in South Asians for difficulty falling asleep. A model adjusted for age, sex and years of education revealed that those who reported that they had experienced difficulty falling asleep in the previous 30 days were 1.41 times more likely to have died by December 2017 (Fig. 1). This effect was slightly attenuated to 1.33 in a model adjusted for health behaviours (smoking and alcohol consumption), but remained identical in a model adjusted for comorbidities (CVD, antihypertensives and diabetes at baseline). In a final, fully-adjusted model difficulty falling asleep remained associated with an increased risk of all-cause mortality (HR = 1.28). Yet, the results of the heterogeneity test showed that in fact, this difference in HRs between the ethnic groups was not particularly strong (p = 0.18). This test also showed that there was little heterogeneity across the groups in terms of early morning waking (p = 0.18) and morning tiredness (p = 0.43) and all-cause mortality.
Fig. 1.
Associations between sleep quality and all-cause mortality by ethnicity. Note. Model 1 = adjusted for age, sex and years of full-time education; Model 2 = Model 1 + smoking, alcohol consumption; Model 3 = Model 1 + WHR, anti-hypertensive medication, CVD, diabetes status; Model 4 = Model 1 + Model 2 + Model 3. In all models all three of the sleep quality exposures were entered simultaneously; 95%CI = 95% confidence interval.
3.2. Association between sleep quality and CVD mortality
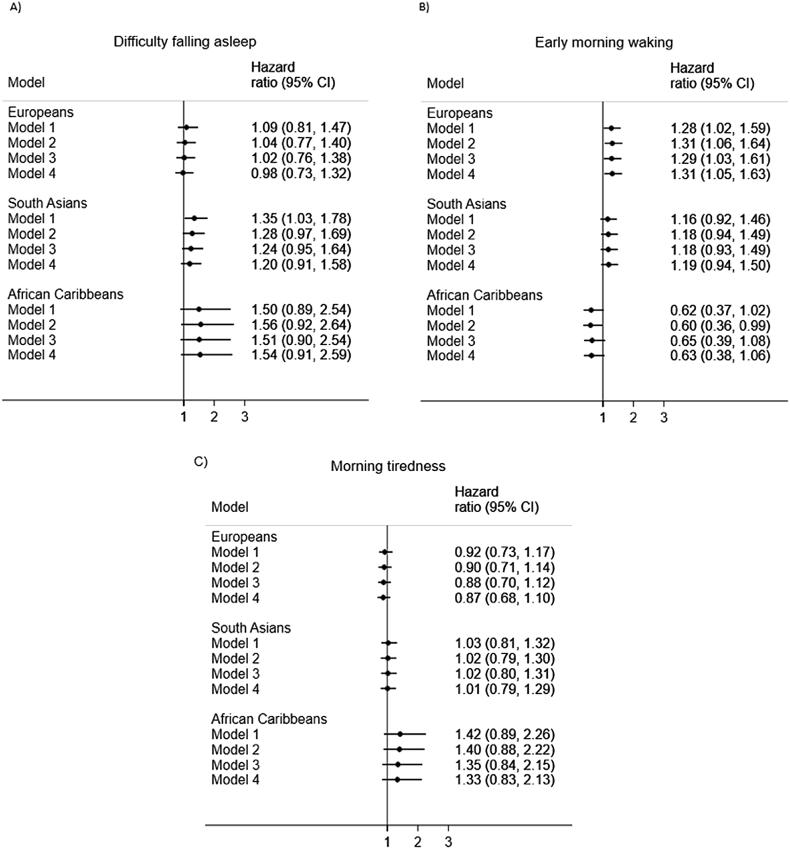
The relationship between sleep quality measures and CVD mortality was particularly weak in both African Caribbeans and South Asians (Fig. 2), but effects were largely directionally consistent. However, in Europeans there was a stronger association between early morning waking and increased risk of CVD mortality, an effect that persisted in models that were multiply-adjusted for baseline-measured confounders (HR = 1.31) (Fig. 2B). The heterogeneity test result confirmed that the HRs for early morning waking differed across the three groups (p = 0.036), but not for difficulty falling asleep (p = 0.30) or morning tiredness (p = 0.27) in relation to CVD mortality.
Fig. 2.
Associations between sleep quality and CVD mortality by ethnicity. Note. Model 1 = adjusted for age, sex and years of full-time education; Model 2 = Model 1 + smoking, alcohol consumption; Model 3 = Model 1 + WHR, anti-hypertensive medication, diabetes status; Model 4 = Model 1 + Model 2 + Model 3. In all models all three of the sleep quality exposures were entered simultaneously; 95%CI = 95% confidence interval.
3.3. Association between sleep quality and cancer mortality
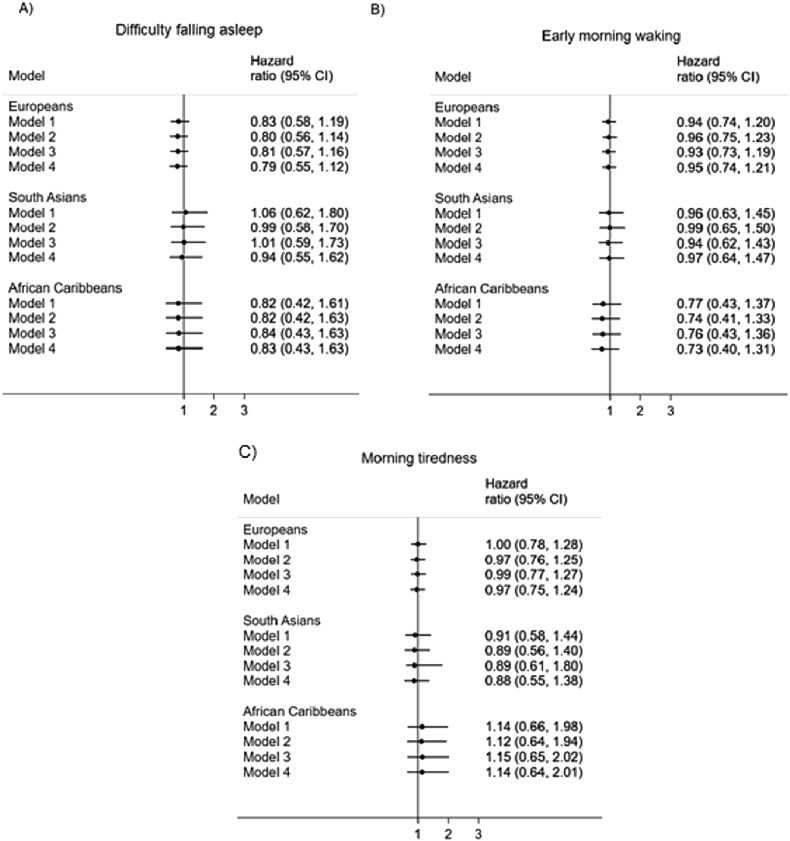
The association between all three sleep quality measures and mortality from cancer was weak in all three ethnic groups (Fig. 3). Largely, the direction of effects suggests, in fully-adjusted models, that those who responded ‘yes’ to having difficulty falling asleep, waking up too early and waking up feeling tired had a slightly lower risk of death from cancer. However, these effects were very small and the associations weak. This was supported by a heterogeneity test, which showed that the HRs did not differ by group for.
Fig. 3.
Association between sleep quality and cancer mortality by ethnicity. Note. Model 1 = adjusted for age, sex and years of full-time education; Model 2 = Model 1 + smoking, alcohol consumption; Model 3 = Model 1 + WHR, anti-hypertensive medication, diabetes status; Model 4 = Model 1 + Model 2 + Model 3. In all models all three of the sleep quality exposures were entered simultaneously; 95%CI = 95% confidence interval.
4. Discussion
We show associations between sleep quality and mortality outcomes differ by ethnicity. Overall, the association between all three sleep quality measures and all-cause mortality were largely weak in African Caribbeans and Europeans. Alternately, South Asians who had difficulty falling asleep had around a 30% excess risk of all-cause mortality, even after adjustment for demographic characteristics (age, sex and education), health behaviours and comorbidities (obesity, CVD, hypertension and diabetes). There were only weak associations between the three sleep quality measures and CVD mortality in South Asians and African Caribbeans, whilst in Europeans, those who reported early morning waking were more likely to die from CVD (HR = 1.31). In relation to cancer mortality, there were only very weak associations between the three sleep quality exposures and this outcome, across all three ethnic groups.
Currently, few studies have investigated the relationship between sleep quality and mortality. Our lack of associations between sleep quality measures and all-cause mortality in Europeans is in line with previous epidemiological evidence. Specifically, our findings in Europeans are in agreement with the Finnish study by Hublin and colleagues [8], as well as analyses of the Whitehall II study [12], given that neither of these studies found an association between sleep quality and all-cause mortality. One previous study observed that in non-frail men aged over 67 years at baseline, unadjusted poor sleep quality was associated with greater odds of death (OR: 1.26; 95%CI 1.01–1.58) at 3.7-year follow-up [13]. However when adjusting for age, sex, health status and BMI, associations attenuated to the null [13].
In South Asians we observed that difficulty falling asleep was associated with increased risk of all-cause – but not CVD or cancer – mortality after adjustment for demographics, health behaviours and comorbidities (HR = 1.28). Difficulty falling asleep is likely to be a proxy for insomnia and as such, evidence suggests that there are ethnic differences in reporting of this symptom [14]. It has been shown that African Americans and Asians may be less likely than White Americans to report difficulty falling asleep [15]. On the other hand, these studies focused largely on East Asian populations, rather than South Asians and therefore, may not be completely generalisable to our findings. Given this, the present study investigates ethnic differences in a UK population and thus generalisations to data from American study populations may be impaired due to cultural and environmental influences affecting sleep and sleep quality in respective study settings. Moreover, African Americans have equivalent or higher rates of smoking in comparison to White Americans, yet in the UK smoking rates in African Caribbeans are much lower than in Europeans [16], [17].
Whilst our study is the first to suggest that difficulty falling asleep is related to greater risk of all-cause mortality in South Asians, this finding ought to be interpreted with caution, as formal heterogeneity tests indicated that the difference in effect size between the ethnic groups was weak. It may be possible that South Asians may experience more of what is known as ‘sleep reactivity’, that is the degree to which stress may disrupt sleep and manifests itself as difficulty falling and staying asleep [18]. Highly reactive sleepers are those whose sleep systems are more sensitive to stress and thus, one possibility is that a greater proportion of the South Asian group were in this category and this may have influenced their ability to fall asleep.
Our study had certain limitations. First, self-report questionnaire responses about sleep quality from participants are inherently subjective. Second, as our baseline measures were taken over 30 years ago (1988) we were unable to analyse any change in these over the follow-up period, as SABRE does not have sleep quality measured at 20-year follow-up. Finally, any association we observe in the present study may be explained by residual confounding by factors that are not included in our analyses.
In conclusion, our novel findings suggest that sleep quality measures do not affect all-cause mortality in individuals of European descent, yet early morning waking appeared to increase risk of mortality from CVD. Difficulty falling asleep was associated with moderately increased risk of all-cause mortality in South Asians, but a heterogeneity test showed that this difference in effect sizes was not strong. Thus, the findings presented here contribute to, and support current evidence for ethnic differences in sleep and mortality and may have important implications for precision medicine and clinicians working with people who present with these sleep problems. Finally, further research is needed to specifically understand exactly how early morning waking might increase the risk of death from CVD in Europeans.
Acknowledgements
SABRE was funded at baseline by the UK Medical Research Council and Diabetes UK. Follow-up studies have been funded by the Wellcome Trust (WT 082464), British Heart Foundation (SP/07/001/23603 and CS/13/1/30327) and Diabetes UK (13/0004774). NC received support from the National Institute for Health Research University College London Hospitals Biomedical Research Centre. Support has also been provided at follow-up by the North and West London and Central and East London National Institute of Health Research Clinical Research Networks. We are grateful to the UK Office for National Statistics for the provision of mortality data and we declare that those who carried out the original collection and analysis of those data bear no responsibility for their further analysis or interpretation in this manuscript.
Conflict of interest
The following is the supplementary data to this article:
Multimedia component 1
References
- 1.Kronholm E., Laatikainen T., Peltonen M. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. Mar. 2011;12(3):215–221. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Gallicchio L., Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. Jun. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M., Wakai K., Kawamura T. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 4.Grandner M.A., Hale L., Moore M. Mortality associated with short sleep duration: thCe evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok C.S., Kontopantelis E., Kuligowski G. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J Am Heart Assoc. 2018;7(15):e008552. doi: 10.1161/JAHA.118.008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirshkowitz M., Whiton K., Albert S.M. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio F.P., D'Elia L., Strazzullo P. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. Feb. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hublin C., Partinen M., Koskenvuo M. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30(10):1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillin T., Forouhi N.G., McKeigue P.M. Southall and Brent REvisited: cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. 2012;41(1):33–42. doi: 10.1093/ije/dyq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillin T., Hughes A.D., Godsland I.F. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall and Brent Revisited (SABRE) cohort. Diabetes Care. 2013;36(2):383–393. doi: 10.2337/dc12-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins C.D., Stanton B.A., Niemcryk S.J. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. Jan. 1988;41(4):313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 12.Rod N.H., Kumari M., Lange T. The joint effect of sleep duration and disturbed sleep on cause-specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensrud K.E., Blackwell T.L., Ancoli-Israel S. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. doi: 10.1016/j.sleep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldwin C.M., Ervin A., Mays M. Sleep disturbances, quality of life, and ethnicity: the sleep heart health study. J Clin Sleep Med. 2010;6(2):176–183. [PMC free article] [PubMed] [Google Scholar]
- 15.Grandner M.A., Williams N.J., Knutson K.L. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.C. O. on S. and Health . 2018. Smoking and tobacco use; fact sheet; adult cigarette smoking in the United States. [Google Scholar]
- 17.Kozlowski L.T. Pack size, reported cigarette smoking rates, and public health. Am J Public Health. Nov. 1986;76(11):1337–1338. doi: 10.2105/ajph.76.11.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalmach D., Cuamatzi-Castelan A.S., Tonnu C.V. Nature and Science of Sleep Dovepress Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep. 2018;10:193–201. doi: 10.2147/NSS.S138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1