Abstract
The skin epithelium covers our body and serves as a vital interface with the external environment. Here, we review the context-specific interactions between immune cells and the epithelium that underlie barrier fitness and function. We highlight the mechanisms by which these two systems engage each other and how immune–epithelial interactions are tuned by microbial and inflammatory stimuli. Epithelial homeostasis relies on a delicate balance of immune surveillance and tolerance, breakdown of which results in disease. In addition to their canonical immune functions, resident and recruited immune cells also supply the epithelium with instructive signals to promote repair. Decoding the dialogue between immunity and the epithelium therefore has great potential for boosting barrier function or mitigating inflammatory epithelial diseases.
Keywords: DETCs, epithelial immunity, Langerhans cells, regeneration and repair, regulatory T cells
Introduction
The outermost layer of our skin, the epidermis, serves as a primary interface with the external environment. Encasing our body, this physical barrier is reinforced by an immunological arsenal that not only patrols the epithelium to protect from harmful agents but also provides context-specific signals to sustain the barrier’s integrity. Accumulating evidence points to a dynamic immune–epithelial cross-talk that maintains a healthy barrier and rapidly responds in times of duress. This intertwined relationship between the skin epithelium and immunity starts even before birth and evolves throughout life (1, 2), incorporating cues from the external environment to maintain a state of alertness (3).
A stratified immune structure supports the skin in multiple ways. Functionally specialized immune cells reside in the epidermis, dermis and subcutaneous tissue, and engage their surrounding parenchyma in an active cross-talk. Additionally, the formation of productive immune responses requires constant sampling of the epithelial barrier and transmission of this information via lymph or migrating immune cells to the cutaneous lymph nodes. These vital aspects of skin immunity, which are beyond the scope of the present article, have been extensively reviewed by Pasparakis and colleagues (4).
Here, we review recent advances in the field of skin epithelial immunity, starting with a discussion of how the epidermis is established and seeded with immune cells. We then explore studies highlighting the means by which immune cells maintain tissue integrity and conversely the epithelium-derived factors that dictate immune homeostasis. As the skin experiences injurious and pathogenic stimuli, the coordinated efforts of immune and epithelial populations protect the host and aid in regeneration to restore barrier integrity. When such interactions go awry, unchecked responses can lead to inflammatory epithelial diseases and cancers (5, 6). Finally, we end with a discussion on the therapeutic potential of leveraging immune–epithelial interactions to treat skin diseases.
Epidermal development and establishment of immunity
Shortly after gastrulation, the epidermis arises from the surface of the ectoderm as a single layer of epithelial progenitors. These embryonically specified progenitors occupy the lowermost or basal layer of the epidermis and regenerate this tissue throughout an organism’s lifetime (7). As development progresses, mitotically active progenitors repopulate the basal layer and undergo a linear program of stratification and differentiation to give rise to the suprabasal layers of the epidermis (Fig. 1) (8, 9).
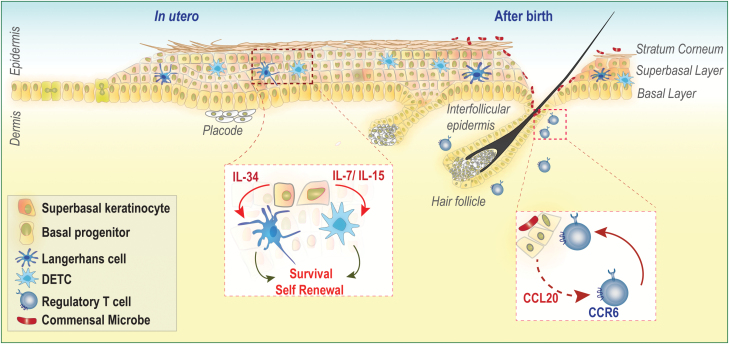
Fig. 1.
Establishment of immunity during epidermal development. Early in embryonic development, the epidermis is specified as a single layer of progenitors that stratify to give rise to the differentiated superbasal layers. The outermost layer of the epidermis is known as the stratum corneum and provides a watertight barrier. LCs and DETCs colonize prior to birth and rely on local survival factors (IL-34 and IL-7/IL-15, respectively) produced by adjacent keratinocytes. Within moments of birth, the skin is colonized by commensal microbes, which inhabit the surface of the skin and invaginations like hair follicles. Commensal colonization induces CCL20-mediated migration of commensal-specific CCR6+ Tregs into neonatal skin.
Essential to epidermal development is acquisition of a watertight epidermal barrier that requires generation of a proteinaceous and lipid layer termed the cornified envelope. Kruppel-like factor 4 is a key orchestrator of barrier formation whose absence results in a catastrophic failure to generate the cornified envelope, a significantly compromised epidermal barrier, and death shortly after birth (10). Loss-of-function variants of filaggrin, an essential cornified envelope protein, have been convincingly identified as a key predisposing factor for atopic dermatitis (11). Similarly, genetic disruptions of not just the cornified layer but any of the stratified epidermal layers result in devastating diseases (12). Thus, embryonic specification of the epidermis and subsequent establishment of a functional barrier are vital to establish health and limit penetration of inflammation-inducing agents (12).
Cells of the immune system take residence in the lowermost layers of the epidermis during embryonic development and serve crucial roles in epithelial homeostasis, defense and repair throughout life. First identified in the human epidermis in 1868 by their namesake Paul Langerhans (13), Langerhans cells (LCs) develop from primitive Csfr1+ yolk-sac progenitors and fetal liver monocytes and arrive in the epidermis shortly after specification from the ectoderm (1, 14). LCs are joined by dendritic epidermal T cells (DETCs) at later stages of epidermal development (15). These intra-epithelial lymphocytes were recently shown to be derived from yolk-sac progenitors that develop in the fetal thymus, seed the epidermis in small numbers and clonally expand throughout life (2).
Intriguingly, both LCs and DETCs are sustained independently of adult hematopoiesis and renew locally (1, 16) relying on survival signals from the adjacent epithelium (1, 14). LCs require IL-34, and DETCs are sustained by epithelium-derived IL-7 and IL-15 (17, 18). Adult epidermis is poorly vascularized and largely relies on environmental oxygen (19). In this regard, the poor blood supply may necessitate the maintenance of a self-sustaining immune–epithelial network that is not dependent on bone marrow progenitors. These developmentally established epithelial networks are overridden during inflammation as new blood vessels seep into the epithelium, bringing with them a slew of marrow-derived cells to replenish dying or migrating cells and also provide a broader immune arsenal to defend the barrier. Indeed, during inflammation, monocytes gain entry into the epidermis via hair follicles and replenish depleted LCs (20).
The epidermis also has a remarkable ability to generate appendages, such as the hair follicle, which serves as an epicenter for cutaneous immunity (21). As epidermal development progresses, signals emanating from the underlying mesenchyme induce epidermal invaginations called hair placodes (Fig. 1) (22). Downgrowth of placodes and maturation into functional follicles are controlled by activating Wnts that stabilize the LEF1–β-catenin complex in epithelial progenitors (23, 24). Hair follicle morphogenesis is tightly linked to the homeostasis of regulatory T cells (Tregs) in neonatal skin. Follicular epithelial cells express the chemokine CCL20 to recruit Tregs to the epidermal–dermal interface (Fig. 1). Mice overexpressing the Wnt inhibitor Dkk1 lack hair follicles and fail to accumulate Tregs (25). It is still unknown whether and how DETCs, LCs, Tregs or other dermal resident immune cells may talk back to the developing epithelium and provide instructive developmental cues. Though animals deficient in these cell types do not exhibit overt epidermal barrier defects (26, 27), it remains to be seen if developmental deficiencies in specific immune populations alter the behavior of newly generated skin epithelia.
Balancing immune tolerance and surveillance
On the one hand, as a portal for pathogen invasion and a tissue that is routinely exposed to carcinogens, the epidermis requires constant surveillance by cells of the immune system (28). On the other hand, when left unchecked, these same immune guardians can cause devastating diseases. Epidermal homeostasis, therefore, impinges on a constant negotiation between tolerance and surveillance.
Tregs at the epidermal interface
Tregs, localized in close apposition to the epithelium, represent a dominant mode of tolerance in the skin (29, 30). Microbial colonization of the epidermis shortly after birth results in the accumulation of commensal-specific Tregs that are critical for maintaining tolerance to surface commensals (Fig. 1) (31). Blocking the neonatal wave of Tregs results in severe epithelial inflammation and loss of tolerance to commensals following epidermal scarification (31). Precisely how commensal antigens permeate the neonatal epidermis and are perceived by the immune system remains an open question. One possibility is that generation of commensal-specific Tregs relies on LC-mediated capture of surface antigens and induction of Tregs (32).
Tregs specific for epithelial antigens also occupy this niche and prevent autoimmune responses. LCs were recently shown to capture a keratinocyte cell adhesion molecule, Desmoglein 3 (Dsg3), and to generate Dsg3-specific Tregs (33). Chronic exposure to epidermal antigens in the context of ongoing inflammation also results in generation of Tregs that attenuate autoimmune responses and sustain homeostasis thereafter (34). Similarly, chronic skin pathogens such as Leishmania major also create a regulatory environment to promote the accumulation of IL-10-producing Tregs (35). During inflammation, keratinocytes up-regulate expression of RANK ligand [receptor activator of nuclear factor (NF)-κB ligand] and, via LCs, augment local and systemic Tregs (36). At homeostasis, epidermal LCs provide tonic signals to promote local proliferation of this diverse repertoire of commensal-specific, epithelium-specific and pathogen-specific Tregs at the dermis–epidermis junction (37). Thus, the coordinated and context-specific efforts of epithelial cells and antigen-presenting LCs promote tolerance by generating Tregs both at steady state and during inflammation.
Homeostatic effectors and resident memory
Implicit in the need for regulation is the persistence of active immune effectors and resident memory cells that patrol the barrier even in conditions of health (3, 38). Intriguingly, dysbiosis of skin commensals and translocation of surface microbes to regional lymph nodes are observed at the steady state in Rag-deficient mice (39). Thus, even in the absence of overt barrier disruptions, adaptive immune effectors provide constitutive signals to limit penetrance of resident bacteria.
In contrast to neonatal commensal colonization, interactions with surface commensals in the adult epidermis lead to enhancement of local effector αβ and γδ T cells (40–42). Specific commensal species have the ability to elicit unique subsets of local effector cells. For instance, commensals belonging to the Corynebacterium genus promote activation of dermal IL-17A+ Vγ4+ γδ T cells, whereas certain strains of the ubiquitous skin commensal Staphylococcus epidermidis induce S. epidermidis-specific IL-17A+ CD8+ T cells (Tc17s) that persist in the basal layer of the epidermis (40, 42). Upon epicutaneous challenge with the fungal pathogen Candida albicans, commensally elicited Tc17s rapidly produce IL-17A to stimulate epithelial anti-microbial S100A8 and S100A9 production and limit the establishment of infection (40).
Infections of the skin by viruses also generate resident memory CD103+ CD8+ T cells (TRMs) that localize to the epidermis and confer rapid protection upon subsequent infections (43). Intra-epithelial TRMs are formed from circulating effectors elicited in the tissue by local inflammation and can persist in the absence of local antigen presentation (44). TRMs tether to E-cadherin on adjacent keratinocytes via αvβ6 and αvβ8 integrins, whose expression is dependent upon transforming growth factor β (TGF-β) signaling (45). Like DETCs, TRM homeostasis is tightly controlled by epithelium-derived IL-7 and IL-15 (46). It is thus no surprise that pathogen-induced TRMs displace DETCs in the epithelial niche, suggesting that they are better equipped to compete for finite survival factors (47).
Unchecked immune activation
Protective factors that reinforce the barrier in health are co-opted in the context of inflammatory diseases such as psoriasis, typified by epithelial hyperplasia and hyperproliferation (5). For instance, aberrant IL-17A signaling in keratinocytes results in up-regulation of anti-microbial peptides such as defensins, S100A8 and S100A9 and can perpetuate disease by recruiting and/or activating inflammatory immune cells (48, 49). IL-22 produced by Th17 and innate lymphoid cells induces hyperproliferation and hyper-thickening of the epidermis (50, 51). Analogous to responses to pathogens, relapsing–remitting inflammatory epithelial diseases also generate localized memory pools that are thought to drive recurrent disease flares (52). TRMs intercalate within the epithelium or line up at the epidermal–dermal border awaiting a trigger. Subsequent perturbations presumably result in reactivation of the immune–epithelial disease circuit.
Epithelial sensing and immunomodulatory functions
Just as immune cells can signal to the epithelium to drive their activation, so, too, can epithelial cells act as generals in controlling immunity. In this regard, epithelial cells sense environmental cues and integrate this information to direct immune homeostasis and instigate inflammation.
Epithelial sensing molecules
The skin epithelium forms the front line of defense against invading pathogens and noxious agents. It is also routinely subjected to damage-causing injurious stimuli. As such, epithelial cells are equipped with a myriad of pattern-recognition receptors (PRRs) that sense pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (53). PRRs, such as Toll-like receptors (TLRs), NOD-like receptors (NLRs) and various nucleic acid-sensing molecules, are expressed not only on the surface of keratinocytes but also in various subcellular compartments such as the endosome (Fig. 2) (54). Engagement of surface TLRs by exogenous stimuli induces activation of classical inflammatory programs such as NF-κB and interferon regulatory factor (IRF) and thus spawns a pro-inflammatory insult (Fig. 2).
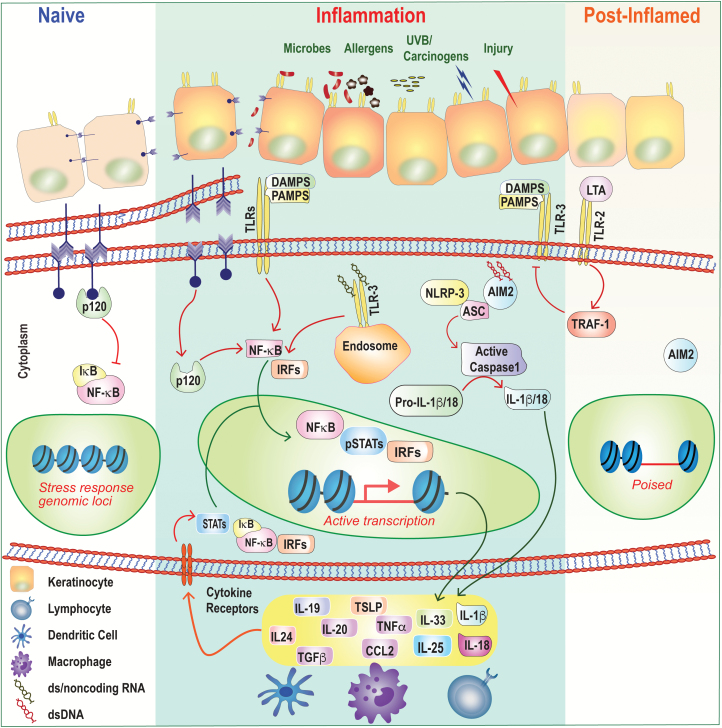
Fig. 2.
Epithelial inflammation and memory. Under homeostatic or ‘naive’ conditions, epithelial cells tether to each other by forming adherens junctions via E-cadherin and p120, which keeps inflammatory NF-κB activation at bay. These adherens junctions in the plasma membranes of the cells at the top-left of the figure (purple arrow heads) are shown in more detail below them. Barrier disruption and loss of cell-to-cell contact result in p120-mediated NF-κB nuclear translocation and in expression of inflammatory cytokines and chemokines. Epithelial cells express a number of PRRs that sense PAMPs and DAMPs including TLRs, NLRP3 and AIM2. Ligation of these receptors also induces downstream inflammatory transcriptional programs and/or activation of the inflammasome and processing of cytokines from the ‘pro’ to active forms. In some instances, ligation of TLRs can also be anti-inflammatory. Following skin injury, LTA from the commensal bacterium S. epidermidis dampens TLR3-mediated inflammation in a TRAF1-dependent manner. Inflammatory cytokines secreted by epithelial cells modulate immune cell function and can also signal autonomously into the epithelium to activate inflammatory transcription factors such as STATs, NF-κB and IRFs. Following resolution, epithelial progenitors retain a memory of inflammation by maintaining chromatin accessibility at key stress-response genes. These ‘poised’ loci enable a more rapid transcriptional response to secondary stimuli.
UVB-mediated injury serves as an example of where cytosolic sensing of self-non-coding RNAs by TLR3 induces an inflammatory signaling cascade (Fig. 2) (55). Similarly, inflammation and tissue damage result in cytoplasmic double-stranded (ds) DNA, which can be sensed by absent in melanoma 2 (AIM2), leading to activation of the inflammasome and secretion of IL-1β and/or IL-18 (Fig. 2) (56). Underscoring the importance of the inflammasome in epithelial biology, a number of cutaneous autoimmune conditions are associated with perturbations in this pathway (57, 58). Familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal-onset multisystem inflammatory disease, which are catalogued as cryopyrin-associated periodic syndromes and caused by autosomal-dominant mutations in the NLR-family pyrin domain-containing 3 (NLRP3) gene. These gain-of-function mutations in the NLRP3 gene result in increased inflammasome activation and overproduction of IL-1β, resulting in skin inflammation (58).
The expression and function of sensing molecules on keratinocytes can be dynamically regulated by inflammatory cytokines such as tumor necrosis factor-α, and ligation of PRRs can serve not only to signal immunity but also to enhance the physical barrier by reinforcing cell adhesion molecules (59, 60). Sensing molecules on the epithelium can also dampen inflammation. Lipoteichoic acid (LTA) from S. epidermidis down-regulates the TLR3-mediated keratinocyte injury response by signaling via TLR2 (Fig. 2) (61). Thus, far from being static cells of the barrier, the skin epithelium has intrinsic means of monitoring its environment and is dynamically tuned by exogenous stimuli.
Epithelial inflammatory programs
Engagement of PRRs, breaches in the barrier and/or inflammatory signaling in the epithelium induce programs of inflammation. Not only are epithelial cells the recipients of inflammatory signals, they can also lead the charge and incite inflammation. Overexpressing inflammatory factors such as activated signal transducers and activators of transcription (STATs) or cytokines under the control of keratinocyte-specific promoters has illustrated that their expression in epithelial cells is sufficient to elicit disease (62, 63). Keratinocyte-derived cytokines not only activate immune cells but also can autonomously feed back on keratinocytes to potentiate disease. IL-19, IL-20 and IL-24 are potent STAT3-inducing pro-inflammatory cytokines that are produced by and act on keratinocytes themselves to trigger disease (Fig. 2) (64, 65).
Highlighting their possible inductive role in psoriasis, epidermal-specific deletion of JunB was sufficient to induce psoriasis-like inflammation (66). Indeed, genome-wide association studies have identified JunB as a susceptibility factor (66), indicating that genomic variations in inflammatory disease may mediate their effect via the epithelium. The anti-microbial and immune-activating factors S100A8 and S100A9, mapped to susceptibility loci PSORS4, are found to be strongly induced in psoriatic keratinocytes (48), suggesting that epithelium-produced factors are essential for potentiating disease.
Loss of cell-to-cell contact results in autonomous epithelial activation of inflammatory programs. Epithelium-specific loss of the adherens junction protein p120 leads to autonomous epithelial NF-κB activation, expression of chemokines and immune infiltration (Fig. 2) (67). Atopic dermatitis, a disease strongly associated with barrier defects, is characterized by epidermal expression of the alarmin thymic stromal lymphopoietin (TSLP) (68, 69). Consistently, mouse models of epidermal overexpression of TSLP recapitulate the development of atopic dermatitis (62). The aforementioned barrier breaches result in microbes penetrating into the lower epidermal and dermal layers, which contributes to the phenotype. However, barrier-deficient mice reared under germ-free conditions or treated with antibiotics also have cutaneous inflammatory responses (70, 71). Thus, immune activation programs are hardwired into the epithelial barrier and do not require exogenous microbial signals.
Epithelial progenitors remember inflammation
As inflammation resolves, it imprints the tissue with a memory. Basal epithelial progenitors were recently shown to bear this memory, which enhanced their response to subsequent injuries (72). Progenitors retained this memory at the level of chromatin, maintaining accessibility of key inflammatory genes. These inflammation-poised progenitors rapidly increase expression of transcripts of genes governed by memory domains, allowing them to regenerate tissue faster than naive counterparts (Fig. 2). Several questions remain. How is memory encoded in the epithelium and passed on from progenitor to progeny? Does memory always confer a regenerative advantage, or can it have deleterious implications for disease? Relapsing–remitting epithelial diseases often afflict the same skin site (5). Understanding how epithelial memory contributes to the site specificity of these conditions may help devise novel interventions to treat disease.
Sanctuary from immunity
Epithelia have evolved clever ways of shielding themselves from the surveilling immune system by hiking up expression of immunosuppressive factors and down-regulating immune-activating molecules. The hair follicle and in particular the stem cells of the follicle exhibit several properties of immune privilege including an enrichment of immune-dampening factors such as TGF-β1 and also reduced expression of the antigen presentation molecule, major histocompatibility complex class I (MHCI) (73, 74). Agudo et al. recently linked the activation status of hair follicle stem cells to their immune-evasive capacities. Quiescent stem cells down-regulate expression of MHCI, suggesting that these precious follicle-generating cells couple cell cycling with immune detection as a possible way to limit hyperplasia (74). Privilege, however, is not a permanent state, as inflammatory cues can override these programs with devastating consequences. Alopecia areata, a hair-loss disorder, has strong associations with genetic loci responsible for immune regulation, suggesting that such pathways are vital to preserve this appendage from autoimmune onslaught (75).
Regeneration and repair
Owing to its function as a barrier tissue, the epidermis has extraordinary capacity for regeneration and repair. Cells of the interfollicular epidermis are constantly regenerating; basal progenitors continually replenish the tissue as differentiated cells are sloughed off the skin’s surface (76). By contrast, hair follicles undergo cyclical bouts of rest and regeneration (76). Whether and how immune mechanisms contribute to the basal regenerative state of the interfollicular epidermis remain to be examined.
Observing the dynamic changes in immune composition around cycling hair follicles has provided important clues about the immune cell types that govern hair cycling (30, 77). Castellana et al. found that, whereas populations of epidermal LCs are constant during the hair cycle, macrophage subsets in the dermis fluctuate dramatically (77). In telogen (the resting phase), macrophages are enriched around the follicle but are lost during the active cycling phase (anagen). Perifollicular macrophages undergo apoptosis towards the end of telogen, releasing activating Wnts to promote hair cycling during the natural cycle.
Ali et al. similarly found that Tregs are also enriched in the vicinity of hair follicle stem cells in telogen and promote anagen after chemical depilation. The effect is mediated by Treg expression of the Notch ligand, Jagged 1 (JAG1) (30). Accordingly, depletion of Tregs delays onset of depilation-induced anagen. Tregs are also enriched in the hair follicle stem cell niche when the follicular barrier is disrupted by genetic ablation of E-cadherin (71), suggesting that these cells can sense damage. Indeed, in wounded skin, Tregs up-regulate expression of epidermal growth factor receptor and attenuate macrophage inflammation to facilitate healing (78).
DETCs are another skin lymphocyte population that plays a prominent role in wound healing (79). These epidermal residents are rapidly activated by damaged keratinocytes and, in turn, supply neighboring epithelial progenitors with insulin growth factor (IGF) and keratinocyte growth factor to promote their proliferation (80). Intriguingly, aging results in breakdown of this DETC–epithelium cross-talk and a delay in wound repair (81). Although human epidermis does not contain DETCs, resident αβ T cells from human epidermis can produce IGF upon activation (82). Thus, epithelial repair is highly conserved across species, relying on instructive cues from resident lymphocytes (82). Like their epidermal counterparts, dermal resident γδ T cells also produce growth factors that are essential for generation of new hair follicles following wounding. Dermal γδ T cells express high levels of fibroblast growth factor 9 and trigger Wnt expression by fibroblasts to induce follicle neogenesis (83).
The question of whether resident lymphocytes produce growth factors in response to damage signals and/or if they are hardwired with repair programs during their specification was recently addressed by Harrison et al. (84). Examining commensally induced epidermal Tc17s shortly after skin injury revealed their rapid responsiveness to epithelium-induced alarmins such as IL-18 and IL-33 and subsequent production of the pro-healing cytokine IL-13 to promote epithelial cell migration, either directly or indirectly via accessory cells (84). Thus, residence at the barrier demands that lymphocytes maintain flexible functional programs, making them poised to respond acutely to local signals.
Conclusions and perspectives
We have discussed historical and recent findings highlighting the complex interdependencies between epithelial cell populations and resident immune cells. Although it is now well accepted that optimal barrier function requires such cross-talk as a means of rapidly adapting to varying environmental conditions, much remains to be learned about the precise mechanisms by which immune–epithelial interactions are established and fluctuate over time. For instance, the functional interactions between surface microbes and the epithelium upstream of epidermal immunity are incompletely understood. Defining the mechanisms of epithelial sensing of microbes and damage, the precise downstream programs induced and the consequences for immunity are areas of active investigation.
Many questions remain. Which receptors are involved? Is there functional specialization in populations of epithelial cells that sense damage and microbial signals? Does each layer of the epidermis, basal versus superbasal, maintain distinct sensing abilities and immune activation programs? Such mechanistic understanding of environmental sensing by the epithelium and its immune residents and the downstream effector programs that arise from this cross-talk may provide novel interventional strategies to boost barrier function and repair. Inflammatory and hyperproliferative diseases such as psoriasis and epithelial cancers are rooted in aberrant damage and microbial sensing leading to unchecked inflammation and loss of tolerance. Restoring health in this setting will require not just an interruption of the aberrant immune–epithelial dialogue but also reprograming of a healthy cross-talk. Eavesdropping on the ever-changing conversation between immunity and the skin epithelium is certain to reveal intriguing new ways in which these two systems interface that can be leveraged to promote skin health and mitigate disease.
Funding
This work is supported by an Arnold O. Beckman Postdoctoral Fellowship (to A.F.M.) and grants from the National Institute of Allergy and Infectious Diseases (NIAID; 6.90 1K22AI135099-01 to S.N.) and the Damon Runyon Cancer Research Foundation (DFS-30-18 to S.N.)
Acknowledgement
We apologize to our colleagues in immunology and skin biology whose work we could not cite due to space constrains.
Conflicts of interest statement: The authors declared no conflicts of interest.
References
- 1. Gomez Perdiguero E.,, Klapproth K.,, Schulz C., et al. 2015. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gentek R.,Ghigo C., Hoeffel G.et al. 2018. Epidermal γδ T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J. Exp. Med. 215:2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mueller S. N. and Mackay L. K. 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16:79. [DOI] [PubMed] [Google Scholar]
- 4. Pasparakis M.,Haase I. and Nestle F. O. 2014. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 14:289. [DOI] [PubMed] [Google Scholar]
- 5. Lowes M. A.,Bowcock A. M. and Krueger J. G. 2007. Pathogenesis and therapy of psoriasis. Nature 445:866. [DOI] [PubMed] [Google Scholar]
- 6. Naik S.,Larsen S. B.,Cowley C. J. and Fuchs E. 2018. Two to tango: dialog between immunity and stem cells in health and disease. Cell 175:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs E. 2008. Skin stem cells: rising to the surface. J. Cell Biol. 180:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lechler T. and Fuchs E. 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koster M. I., Dai D., Marinari B.et al. 2007. p63 induces key target genes required for epidermal morphogenesis. Proc. Natl Acad. Sci. USA 104:3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Segre J. A.,Bauer C. and Fuchs E. 1999. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 22:356. [DOI] [PubMed] [Google Scholar]
- 11. Palmer C. N.,, Irvine A. D.,, Terron-Kwiatkowski A., et al. 2006. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 38:441. [DOI] [PubMed] [Google Scholar]
- 12. Segre J. A. 2006. Epidermal barrier formation and recovery in skin disorders. J. Clin. Invest. 116:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langerhans P. 1868. Ueber die Nerven der menschlichen Haut. Arch. Pathol. Anat. Physiol. Klin. Med. 44:325. [Google Scholar]
- 14. Hoeffel G.,, Wang Y.,, Greter M., et al. 2012. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209:1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergstresser P. R.,Tigelaar R. E.,Dees J. H. and Streilein J. W. 1983. Thy-1 antigen-bearing dendritic cells populate murine epidermis. J. Invest. Dermatol. 81:286. [DOI] [PubMed] [Google Scholar]
- 16. Merad M.,, Manz M. G.,, Karsunky H., et al. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y.,, Szretter K. J.,, Vermi W., et al. 2012. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edelbaum D.,Mohamadzadeh M.,Bergstresser P. R.,Sugamura K. and Takashima A. 1995. Interleukin (IL)-15 promotes the growth of murine epidermal gamma delta T cells by a mechanism involving the beta- and gamma c-chains of the IL-2 receptor. J. Invest. Dermatol. 105:837. [DOI] [PubMed] [Google Scholar]
- 19. Boutin A. T.,, Weidemann A.,, Fu Z., et al. 2008. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 133:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagao K.,, Kobayashi T.,, Moro K., et al. 2012. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 13:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christoph T.,, Müller-Röver S.,, Audring H., et al. 2000. The human hair follicle immune system: cellular composition and immune privilege. Br. J. Dermatol. 142:862. [DOI] [PubMed] [Google Scholar]
- 22. Andl T.,Reddy S. T.,Gaddapara T. and Millar S. E. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2:643. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt-Ullrich R. and Paus R. 2005. Molecular principles of hair follicle induction and morphogenesis. Bioessays 27:247. [DOI] [PubMed] [Google Scholar]
- 24. Gat U.,DasGupta R.,Degenstein L. and Fuchs E. 1998. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95:605. [DOI] [PubMed] [Google Scholar]
- 25. Scharschmidt T. C.,, Vasquez K. S.,, Pauli M. L., et al. 2017. Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe. 21:467.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan D. H.,Jenison M. C.,Saeland S.,Shlomchik W. D. and Shlomchik M. J. 2005. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23:611. [DOI] [PubMed] [Google Scholar]
- 27. Itohara S.,, Mombaerts P.,, Lafaille J., et al. 1993. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell 72:337. [DOI] [PubMed] [Google Scholar]
- 28. Kupper T. S. and Fuhlbrigge R. C. 2004. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma R.,Sung S. S.,Fu S. M. and Ju S. T. 2009. Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice. J. Biomed. Sci. 16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ali N.,, Zirak B.,, Rodriguez R. S., et al. 2017. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169:1119.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scharschmidt T. C.,, Vasquez K. S.,, Truong H. A., et al. 2015. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kubo A.,Nagao K.,Yokouchi M.,Sasaki H. and Amagai M. 2009. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206:2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitashima D. Y.,, Kobayashi T.,, Woodring T., et al. 2018. Langerhans cells prevent autoimmunity via expansion of keratinocyte antigen-specific regulatory T cells. EBioMedicine 27:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenblum M. D.,Gratz I. K.,Paw J. S.,Lee K.,Marshak-Rothstein A. and Abbas A. K. 2011. Response to self antigen imprints regulatory memory in tissues. Nature 480:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belkaid Y.,Piccirillo C. A.,Mendez S.,Shevach E. M. and Sacks D. L. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502. [DOI] [PubMed] [Google Scholar]
- 36. Loser K.,, Mehling A.,, Loeser S., et al. 2006. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 12:1372. [DOI] [PubMed] [Google Scholar]
- 37. Seneschal J.,Clark R. A.,Gehad A.,Baecher-Allan C. M. and Kupper T. S. 2012. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belkaid Y. and Harrison O. J. 2017. Homeostatic immunity and the microbiota. Immunity 46:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen W., Li W., Hixon J. A.et al. 2014. Adaptive immunity to murine skin commensals. Proc. Natl Acad. Sci. USA 111:E2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naik S.,, Bouladoux N.,, Linehan J. L., et al. 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naik S.,, Bouladoux N.,, Wilhelm C., et al. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ridaura V. K.,, Bouladoux N.,, Claesen J., et al. 2018. Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 215:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang X.,Clark R. A.,Liu L.,Wagers A. J.,Fuhlbrigge R. C. and Kupper T. S. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mackay L. K., Stock A. T., Ma J. Z.et al. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA 109:7037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mohammed J.,, Beura L. K.,, Bobr A., et al. 2016. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat. Immunol. 17:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adachi T.,, Kobayashi T.,, Sugihara E., et al. 2015. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 21:1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zaid A., Mackay L. K., Rahimpour A.et al. 2014. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl Acad. Sci. USA. 111:5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schonthaler H. B.,, Guinea-Viniegra J.,, Wculek S. K., et al. 2013. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 39:1171. [DOI] [PubMed] [Google Scholar]
- 49. Peric M.,, Koglin S.,, Kim S. M., et al. 2008. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 181:8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng Y.,, Danilenko D. M.,, Valdez P., et al. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648. [DOI] [PubMed] [Google Scholar]
- 51. Perera G. K.,, Ainali C.,, Semenova E., et al. 2014. Integrative biology approach identifies cytokine targeting strategies for psoriasis. Sci. Transl. Med. 6:223ra22. [DOI] [PubMed] [Google Scholar]
- 52. Cheuk S.,, Wikén M.,, Blomqvist L., et al. 2014. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 192:3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miller L. S. 2008. Toll-like receptors in skin. Adv. Dermatol. 24:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Di Meglio P.,Perera G. K. and Nestle F. O. 2011. The multitasking organ: recent insights into skin immune function. Immunity 35:857. [DOI] [PubMed] [Google Scholar]
- 55. Bernard J. J.,, Cowing-Zitron C.,, Nakatsuji T., et al. 2012. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 18:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dombrowski Y.,, Peric M.,, Koglin S., et al. 2011. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci. Transl. Med. 3:82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tervaniemi M. H.,, Katayama S.,, Skoog T., et al. 2016. NOD-like receptor signaling and inflammasome-related pathways are highlighted in psoriatic epidermis. Sci. Rep. 6:22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gurung P. and Kanneganti T. D. 2016. Autoinflammatory skin disorders: the inflammasomme in focus. Trends Mol. Med. 22:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miller L. S.,, Sørensen O. E.,, Liu P. T., et al. 2005. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J. Immunol. 174:6137. [DOI] [PubMed] [Google Scholar]
- 60. Yuki T.,Yoshida H.,Akazawa Y.,Komiya A.,Sugiyama Y. and Inoue S. 2011. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J. Immunol. 187:3230. [DOI] [PubMed] [Google Scholar]
- 61. Lai Y.,, Di Nardo A.,, Nakatsuji T., et al. 2009. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 15:1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoo J.,, Omori M.,, Gyarmati D., et al. 2005. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 202:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sano S.,, Chan K. S.,, Carbajal S., et al. 2005. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 11:43. [DOI] [PubMed] [Google Scholar]
- 64. Kunz S.,, Wolk K.,, Witte E., et al. 2006. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp. Dermatol. 15:991. [DOI] [PubMed] [Google Scholar]
- 65. Kumari S.,, Bonnet M. C.,, Ulvmar M. H., et al. 2013. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity 39:899. [DOI] [PubMed] [Google Scholar]
- 66. Zenz R.,, Eferl R.,, Kenner L., et al. 2005. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437:369. [DOI] [PubMed] [Google Scholar]
- 67. Perez-Moreno M.,Davis M. A.,Wong E.,Pasolli H. A.,Reynolds A. B. and Fuchs E. 2006. p120-catenin mediates inflammatory responses in the skin. Cell 124:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li M., Hener P., Zhang Z., Kato S., Metzger D. and Chambon P. 2006. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl Acad. Sci. USA 103:11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luo Y.,Zhou B.,Zhao M.,Tang J. and Lu Q. 2014. Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin. Exp. Dermatol. 39:48. [DOI] [PubMed] [Google Scholar]
- 70. Natsuga K.,Cipolat S. and Watt F. M. 2016. Increased bacterial load and expression of antimicrobial peptides in skin of barrier-deficient mice with reduced cancer susceptibility. J. Invest. Dermatol. 136:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lay K., Yuan S., Gur-Cohen S.et al. 2018. Stem cells repurpose proliferation to contain a breach in their niche barrier. eLife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Naik S.,, Larsen S. B.,, Gomez N. C., et al. 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Paus R.,Ito N.,Takigawa M. and Ito T. 2003. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 8:188. [DOI] [PubMed] [Google Scholar]
- 74. Agudo J.,, Park E. S.,, Rose S. A., et al. 2018. Quiescent tissue stem cells evade immune surveillance. Immunity 48:271.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Petukhova L.,, Duvic M.,, Hordinsky M., et al. 2010. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 466:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blanpain C. and Fuchs E. 2006. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Castellana D.,Paus R. and Perez-Moreno M. 2014. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 12:e1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nosbaum A.,, Prevel N.,, Truong H. A., et al. 2016. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J. Immunol. 196:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jameson J.,, Ugarte K.,, Chen N., et al. 2002. A role for skin gammadelta T cells in wound repair. Science 296:747. [DOI] [PubMed] [Google Scholar]
- 80. Havran W. L. and Jameson J. M. 2010. Epidermal T cells and wound healing. J. Immunol. 184:5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Keyes B. E.,, Liu S.,, Asare A., et al. 2016. Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell 167:1323.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Toulon A.,, Breton L.,, Taylor K. R., et al. 2009. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gay D.,, Kwon O.,, Zhang Z., et al. 2013. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 19:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Harrison O. J., Linehan J. L., Shih H.-Y.et al. 2018. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 18:6280. [DOI] [PMC free article] [PubMed] [Google Scholar]