Abstract
Among all tumor types, skin cancers are profoundly sensitive to immunotherapy. Indeed, the recently reported response rates for anti-PD-1 (anti-programmed-death 1) therapy for cutaneous malignant melanomas (MM), Merkel cell carcinomas, basal cell carcinomas, cutaneous squamous cell carcinomas and Kaposi sarcomas are all above 40%. This unique immunogenicity renders skin cancers as a paradigm for tumor–immune interactions and is driven by high mutational burdens, over-expressed tumor antigens and/or viral antigens. However, despite the clear demonstration of immunologic cure of skin cancer in some patients, most tumors develop either early (primary) or late (adaptive) resistance to immunotherapy. Resistance mechanisms are complex, and include contributions of tumor cell-intrinsic, T cell and microenvironment factors that have been recently further elucidated with the advent of single-cell technologies. This review will focus on the exciting progress with immunotherapy for skin cancers to date, and also our current understanding of the mechanisms of resistance to immunotherapy.
Keywords: cutaneous malignancy, immune checkpoint, melanoma, Merkel cell, PD-1
Introduction
Skin cancer is an enormous public health concern, with non-melanoma skin cancer (NMSC) being the most common and melanoma the sixth most common cancer in the USA. The numbers of aggressive skin cancers have rapidly increased, and there are now >90 000 cutaneous malignant melanomas (MM) and nearly 3000 Merkel cell carcinomas (MCC) diagnosed each year in the USA (1, 2). Although basal cell carcinomas (BCC) and cutaneous squamous cell carcinomas (cSCC) are often controlled with local measures, their combined millions of cases are estimated to result in several thousand deaths annually (3, 4).
Despite the discouraging rise in skin cancer incidence, the recent remarkable success of immunotherapies brings great promise to the field. For instance, the 3-year overall survival for metastatic melanoma has improved 5-fold: from 12% with dacarbazine (DTIC) chemotherapy prior to ipilimumab approval in 2011 in to 58% with the combined checkpoint inhibitor immunotherapies ipilimumab and nivolumab in 2017 (5, 6). Indeed, in 2018 James Allison (7) and Tasuku Honjo (8) were awarded the Nobel Prize in Physiology and Medicine for their discovery of the CTLA4 (cytotoxic T-lymphocyte-associated antigen 4) and PD-1 (programmed-death 1) immune checkpoints that led to these treatments.
This review will focus on skin cancers as a paradigm disease for tumor immunotherapy, starting with the reasons for the immunogenicity of skin cancers. Next, the history of immunotherapy for melanoma will be reviewed, with a special focus on how results from these trials elucidate tumor–immune interactions more generally. The use of immunotherapy for other skin cancers will then be discussed, including successes and complications thereof. Despite marked improvements in outcomes with immune therapy, at least half of patients with advanced/metastatic skin cancers are still not cured with these treatments. This is an area of very active research, and substantial progress has been made over the last 5 years. We will thus conclude with a detailed examination of the current understanding of mechanisms of how resistance to immunotherapy arises, including primary and adaptive resistance.
Why are skin cancers so T-cell immunogenic?
The incidence of skin cancer is elevated in patients with T-cell immunosuppression. This reflects both an underlying tumor immunogenicity and the critical importance of T cells in both surveilling against and controlling these diseases. Forms of both CD4+ T-cell immunosuppression (HIV/AIDS) and CD8+ T-cell immunosuppression (solid-organ transplants) have been associated with increased risk and worsened outcomes of cSCC (9, 10), BCC (9), MCC (11–13), Kaposi sarcoma (KS) (14) and MM (14), with the strongest and most consistent effects seen for cSCC, MCC and KS (15). In patients with normal cutaneous immunity, nascent immunogenic skin tumors are eliminated unless the tumor can evade immune destruction. Reversing tumor immune evasion is the goal of anti-cancer immunotherapy.
The profound antigenicity of skin cancers is thought to derive from tumor-associated antigens, neoepitopes and/or viral oncoproteins. Melanomas aberrantly over-express tumor-associated antigens (e.g. MAGE antigens, MART1/MLANA, NY-ESO1) which render them susceptible to T-cell killing because T cells recognizing these antigens escape negative thymic selection (16). High-throughput sequencing approaches have further established that most skin cancers, including MM (17), cSCC (18, 19), BCC (20) and virus-negative MCC (VN-MCC) (21) also have very high tumor mutational burdens (TMB), largely driven by UV-signature mutations, creating new tumor-associated epitopes (22–24). Indeed, cSCC, VN-MCC and melanoma have the highest TMB of all solid tumors profiled thus far (25). TMB has been a consistent predictor of immunotherapy responsiveness, both within and across tumor types, supporting the conclusion of a large contribution of neoantigens to immunogenicity in most cancers (17, 26).
In stark contrast to most skin cancers, which are TMB-high, the virus-positive skin cancers [KS and virus positive-MCC (VP-MCC)] have an extremely low TMB similar to cancers that respond poorly to immunotherapy such as pancreatic cancer or uveal melanoma (17, 21, 27). Despite this low TMB, both KS and VP-MCC are frequently responsive to anti-PD-1 immunotherapies. Instead of mutations, the antigenicity in these cancers is being driven by expression of viral proteins from causative oncoviruses [human herpesvirus 8 (HHV-8) and Merkel cell polyomavirus (MCPyV), respectively] (28, 29) which are CD8+ and CD4+ T-cell antigens (30, 31). This critical clinical observation among skin cancers suggests that immunotherapy responsiveness can be driven by a few high-quality antigens, and as a corollary, that TMB-high cancers are simply more likely to produce reactive antigens than TMB-low tumors.
Further conceptual support for this is provided by melanomas, where PD-1 immunotherapy responsiveness from TMB-low cutaneous melanomas can be abrogated by a loss of a single HLA (32) antigen and where tumor-infiltrating lymphocyte (TIL) regressions can be mediated by T cells restricted to a single epitope (33). Therapeutically, this implies that triggering recognition of solid tumors through a vaccine or adoptive cellular therapy restricted to a few epitopes [T-cell receptor (TCR) transgenic and/or chimeric antigen receptor (CAR)-T transgenic] (34) may be adequate to enable an immune response, particularly when combined with therapies addressing microenvironment and/or tumor-intrinsic resistance mechanisms.
Progress with immunotherapy in melanoma
Cutaneous melanoma arises from mutations in skin melanocytes, specialized pigment cells that originate from neural-crest progenitors. These neural-crest cells are highly motile in embryogenesis, and melanoma retains this high propensity for metastasis/spread (35). Thus, although melanoma represents ~1–2% of all skin cancers (4), it is the largest contributor to deaths caused by skin cancer. Melanoma has a strong UV association (4), and derives substantial immunogenicity from a large number of tumor-associated UV-signature mutations (25).
In the late 1800s, Coley developed an intralesional immunotherapy for melanoma. However, his injections of killed streptococcal and serratial bacteria were far less successful for melanoma than for sarcomas (36, 37), and so for many decades melanoma immunotherapy was not further pursued.
In the early 1970s, interest again arose in melanoma immunotherapy. High-dose intralesional Bacillus Calmette–Guérin (BCG) was injected into patients with dermal melanoma metastases, resulting in many local and some distant tumor regressions and renewed enthusiasm for the concept of immunotherapeutic cure of solid tumors (38, 39). However, the high toxicity of this approach and the contemporaneous approval of DTIC chemotherapy dampened widespread use of BCG for melanoma (40), although BCG immunotherapies were adopted for bladder cancer. In 2017, the mechanisms of BCG response in melanoma were elucidated and include a complex set of tumor cell-intrinsic (increased class I HLA expression), T cell (increased CD8+ T-cell infiltration and activation) and microenvironment (macrophage repolarization) changes that augment anti-tumor immunity (41).
The next set of immunotherapies to be tested in melanoma included the cytokine therapies. Type I interferons are polyfunctional and not only activate T cells, but also enhance presentation of tumor-intrinsic antigens and impact other cells in the environment such as polarizing macrophages and inhibiting T-regulatory cells (Tregs) (42). Interferons were most effective in an adjuvant setting (i.e. additional therapies given after surgery), particularly in patients with ulcerated tumors (43). However, low response rates and high toxicity limited the benefits. Interleukin 2 (IL-2) is a cytokine growth factor that promotes expansion of T cells and other lymphocytes. Dosed at toxic levels, IL-2 produced some durable responses in the metastatic setting with a response rate on the order of 10–20% (44). This finding was later appreciated to be due, in part, to the paradoxical nature of IL-2, which promotes not only expansion of CD8+ T-effector cells, but also expansion of Tregs. The dual nature of IL-2 was demonstrated by knockout experiments in mice, where IL-2-deficient animals developed autoimmunity (45).
A number of adoptive cellular therapies have been tested for melanoma. These include therapies using TILs (where tumor lymphocytes are expanded in vitro and infused), endogenous cell therapies (where circulating T cells recognizing specific tumor antigens are expanded) and more recently transgenic T-cell therapies (where CD8+ and CD4+ cells are transduced with either a TCR or a CAR to confer tumor specificity) (46–52). Some impressive durable responses have been observed, including ongoing remissions lasting for >5 years; however, most of the treated patients have not had durable benefit to date. There is thus strong interest in combining cell therapy with other factors to improve T-cell recognition of tumor cells, overcome ‘exhaustion’ of immune cell responses and/or promote a more favorable microenvironment.
In 2015, the first intralesional oncolytic virus was approved. Talimogene laherparepvec (T-vec) is a gene-modified herpes virus that is non-pathogenic and preferentially replicates in tumor cells, leading to death of melanoma cells by multiple mechanisms including increased antigen expression. T-vec further expresses granulocyte/macrophage colony-stimulating factor (GM-CSF), to promote maturation and function of professional antigen-presenting cells (APCs), including dendritic cells, in the microenvironment. T-vec is associated with an overall response rate of 26%, with a higher response at injected as compared with non-injected lesions (53). Additional oncolytic viruses are under study, as are combinations of T-vec with other immunotherapies.
Multiple therapeutic vaccine approaches have been attempted for melanoma. Recently, tumor-specific neoantigens have been targeted with personalized vaccines in early phase trials (54, 55) and have encouragingly demonstrated the generation of tumor-specific T-cell immune responses and early evidence of clinical activity. The efficacy and optimal timing of vaccine treatments is currently being tested in clinical trials. The recent definition of the melanoma HLA-ligandome—i.e. tumor-specific peptides (neoantigens and/or over-expressed tumor antigens) that are presented/shown by melanoma tumors to T cells—offers substantial promise for further vaccine refinement (56).
By far the greatest success in melanoma with immunotherapy has been with immune checkpoint inhibitors. The first to reach the clinic was ipilimumab, a monoclonal antibody against CTLA4 that targets the interaction between APCs and T cells. During antigen-specific lymphocyte activation, one of the B7 proteins (also termed CD80 and CD86) on the APC binds to CD28 on the T cell which stimulates activation and induces CTLA4 expression. CTLA4 on T cells then competes with CD28 for B7 binding, and thus inhibits T-cell activation (7, 57, 58). Although ipilimumab monotherapy for metastatic disease has a response rate <20%, the potential for durable responses and improved overall survival led to its Food and Drug Administration (FDA) approval in 2011 (6, 59, 60).
In 2014, the FDA approved two PD-1 inhibitors, which target PD-1/PD-L1 (PD-1 ligand 1) signaling between T cell and tumor cell, or T cell and APC (8). Both pembrolizumab and nivolumab as monotherapy are associated with response rates of ~40–45% (6, 61). The addition of ipilimumab to pembrolizumab or nivolumab improves the response rate by ~10%, but comes at a cost of substantially higher toxicity including a markedly increased rate of significant immune-mediated adverse events (6).
In addition to immune checkpoint inhibitors, BRAF inhibitors (BRAFi) and MEK inhibitors have also recently been approved for cutaneous melanoma with BRAF V600 mutations (62). Consistent with the findings after use of tyrosine kinase inhibitors (TKI) for other indications, melanoma typically develops resistance to BRAF/MEK inhibition. Interestingly, there is some suggestion that these inhibitors have immunologic impacts in addition to direct anti-tumor effects (63), with specific improvements on tumor-specific antigen presentation (64), cytolytic T-cell function and microenvironment polarization (65, 66) in the context of BRAFi treatment.
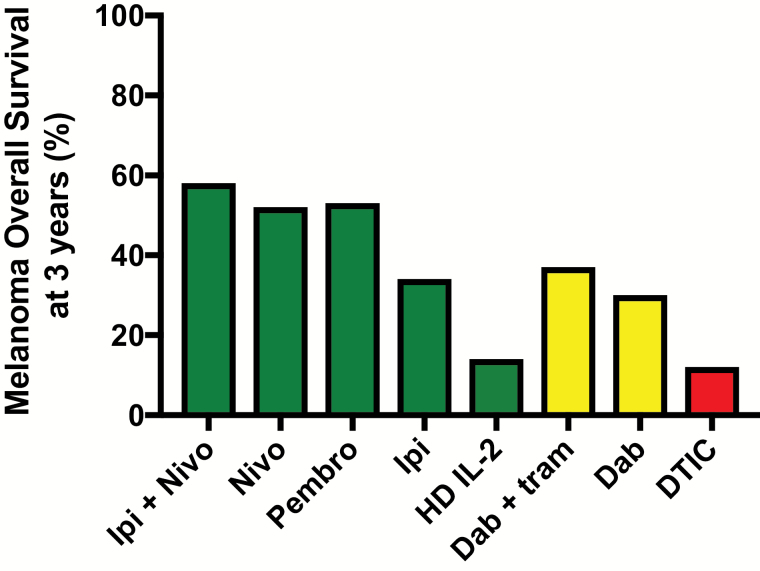
The introduction of immune checkpoint inhibitors and TKI has changed the natural history of advanced melanoma. In the DTIC era, extending from 1974 to 2011, the 3-year overall survival for clinical trial participants with metastatic melanoma from start of first-line treatment was ~13% (5). In 2018, overall survival at 3 years has improved to >50% (Fig. 1).
Fig. 1.
Overall survival from selected first-line therapies for metastatic melanoma. The graph shows 3-year overall survival rates for selected immunotherapies, targeted therapies and chemotherapies (5, 6, 44, 62, 127, 128). As there are differences between the selected studies in terms of entry criteria, as well as change over time in availability of second-line therapies, this graph is intended to illustrate the marked improvement in overall survival with recently FDA-approved immunotherapies and not intended for treatment decision-making. Ipi, ipilimumab; Nivo, nivolumab; Pembro, pembrolizumab; HD IL-2, high-dose IL-2; Dab, dabrafenib; tram, trametinib; DTIC = dacarbazine. Green indicates immune therapy; yellow indicates targeted therapy; red indicates chemotherapy.
Potential for the immune system to cure metastatic solid tumors
With rare exceptions (e.g. testicular cancer), chemotherapy as isolated agents or combination regimens have been insufficient to cure metastatic solid tumors. Similarly, although often effective for months or years, targeted therapies do not typically result in cure. This is due to the large number of cancer cells in a metastatic cancer at the time of diagnosis (~1 billion per gram), and thus the statistical likelihood that a treatment-resistant sub-clone already exists at the time of treatment start. However, the adaptive immune system has potential to engage with tumor diversity, and mediate tumor sterilization/cure. Indeed, this has been demonstrated with melanoma and the high-dose IL-2 therapies, where 5–10% of patients were cured with no further treatment, and the longest complete responses now extend decades (67).
Although the ultimate durability of responses to anti-CTLA4 and anti-PD-1 in melanoma remains to be demonstrated, recent data suggest that survival has a plateau at 2–3 years with ongoing responses at >8 years and >3.5 years after stopping therapy, respectively, supporting the curative potential of these approaches (68, 69). Thus, the experience in melanoma clearly demonstrates the potential for a properly engaged immune system to cure metastatic solid tumors.
Treatment of a low-tumor burden may be associated with improved outcomes
Adjuvant immunotherapy with ipilimumab was associated with improved melanoma-specific and overall survival as compared with placebo control for patients with resected stage III (nodal) melanoma (43). More recently, adjuvant immunotherapy with nivolumab or pembrolizumab has been associated with improved progression-free survival for resected stage III (or for nivolumab III/IV) disease. This supports the likely effectiveness of immunotherapy when the disease burden is small/microscopic, even without a large tumor to ‘prime’ the immune system (70, 71). Among patients who have metastatic melanoma treated with anti-PD-1, an increased burden pre-treatment is associated with a lower response rate and with worsened overall survival, supporting the potential for improved responsiveness if treatment is initiated in a low-burden context (72).
There are multiple hypotheses as to why immunotherapy might be more effective in terms of both responses and cures at a time of lesser disease burden. The first is mutation based: more tumor present means more statistical chance of having a sub-clone that bears immunotherapy resistance, such as a β-2-microglobulin mutation abrogating presentation of a tumor-specific antigen (73). The second is microenvironment based: larger tumors are more likely to have a microenvironment that is more suppressive to CD8+ T cells, and have less oxygen, more lactate (74, 75) and more fibroblasts and M2-like macrophages (76) that pose both biochemical and physical (77) barriers to T-cell function.
Although it appears there is benefit to treating patients when the tumor burden is low, the optimal timing of anti-PD-1 initiation related to tumor and nodal removal for stage III patients remains to be established. One viewpoint suggests that for resectable disease, neoadjuvant (i.e. given before surgery) initiation of immunotherapy may be more effective. Neoadjuvant checkpoint inhibition may have a greater ability to trigger epitope spreading with the tumor serving as its own ‘vaccine’ with intact draining lymph nodes to potentiate responses to anti-PD-1 antibody therapy; however, clinical data in support of this are limited to small case series without comparators (78, 79). It remains unclear whether these benefits outweigh the microenvironment benefit of debulking, or the risk to the patient of complications from immunotherapy potentially delaying complete surgical cure. The current standard of care is to perform surgical resection prior to immunotherapy implementation; however, this may change pending the results of further studies.
Application of anti-PD-1 to other skin tumors
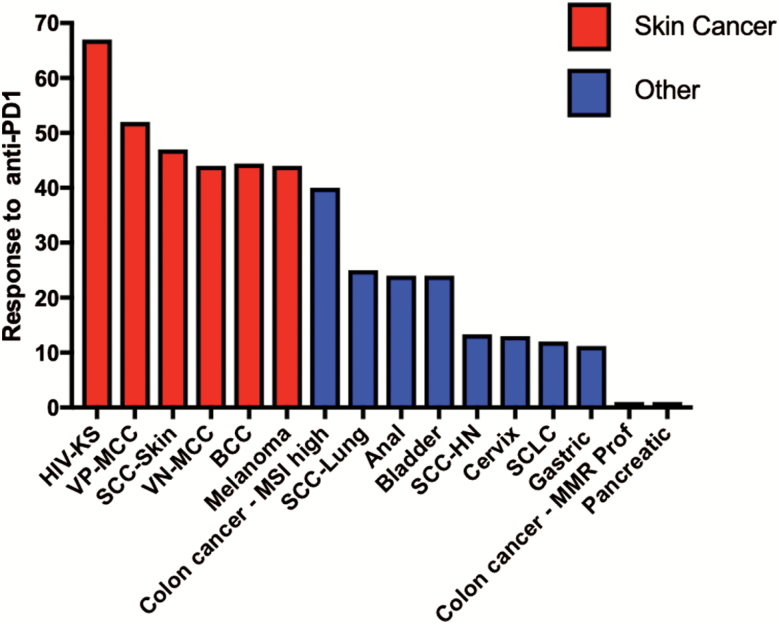
Given the stellar outcomes for many melanoma patients treated with immune checkpoint inhibitors, as well as the established immunogenicity of other skin cancers, immune checkpoint inhibition with PD-1 and/or PD-L1 inhibitors has been attempted on many other skin cancers. These studies support the observed high response rates across skin cancers, which are consistently superior to the experience in other solid tumors, as shown in Fig. 2.
Fig. 2.
Response to anti-PD-1 blockade in advanced skin cancers and other solid tumors. Data from representative trials of anti-PD1 agents in skin cancers (6, 20, 27, 80, 82) and non-skin cancers are shown (17, 32, 129–133); for selected gastrointestinal malignancies [DNA mismatch repair (MMR)-proficient colon cancer, pancreatic cancer, gastric cancer] summary data are as reported from Yarchoan et al. (17). Where possible data are from first-line therapy; however, for some cancers only 2L+ data are available. Data include all comers irrespective of PD-L1 tumor status. Only anti-PD-1 monotherapy is included. Reported response rates to anti-PD-1 monotherapy in skin cancers are consistently >30–40% and are substantially higher than reported response rates for most other solid tumors. SCC-HN, squamous cell carcinoma of the head and neck; SCLC, small cell lung cancer.
Among NMSCs, phase II trials have been performed on cSCC and MCC. For advanced cSCC, the 47% objective response rate to the anti-PD-1 agent cemiplimab (47%) (80) led to its recent FDA approval. For MCC, responses to pembrolizumab (56%) (81, 82) and avelumab (anti-PD-L1; 62%) (83) both exceed 40% as first-line treatments, and overall survival is substantially improved with these agents as compared with historical data derived from the use of chemotherapy (82, 84). The response to pembrolizumab is similar irrespective of whether the MCC is virally induced or UV-induced (82).
Data for effects of anti-PD-1 monotherapy on metastatic BCC, KS and cutaneous angiosarcoma are emerging. For BCC, a prospective study included nine patients of whom four (44%) responded (20). For KS, a retrospective review included nine consecutive patients with HIV-associated KS (six objective responders; 67%) (27) and a second case series of two responding endemic KS patients has also been reported. For cutaneous angiosarcoma, a single case report describes a positive response (85). Although the data are emerging in these cancers, early evidence supports a high response rate consistent with other skin cancers.
Skin toxicities of checkpoint inhibitor immunotherapy
Toxicity from immune checkpoint inhibitors is largely driven by a broad spectrum of immune-related adverse events (iRAEs) related to autoimmune attack of healthy tissues. The iRAEs for immune checkpoint inhibitors given for skin cancers appear similar to those for other malignancies, with the exception of increased cutaneous toxicities. The national comprehensive cancer network (NCCN) has recently published a guideline to iRAEs and their management (86). As iRAEs can affect many organ systems (cutaneous, gastrointestinal tract, pulmonary, endocrine, hematologic, nervous and others), a comprehensive detailing of all iRAEs is beyond the scope of this review and we will instead focus on cutaneous toxicities.
Cutaneous iRAEs occur in between one-third and half of patients treated with immune checkpoint inhibitors (86). Rashes are the most common; however, these represent a wide variety of clinical presentations from focal to widespread/diffuse, with eczematous, psoriatic, lichenoid, erythrodermic and other variants. Rashes are often pruritic. More severe cutaneous toxicities have occurred including bullous dermatidites, Stevens–Johnson syndrome/toxic epidermal necrolysis, Sweet’s syndrome and DRESS (drug rash with eosinophilia and systemic symptoms) syndrome (87–89).
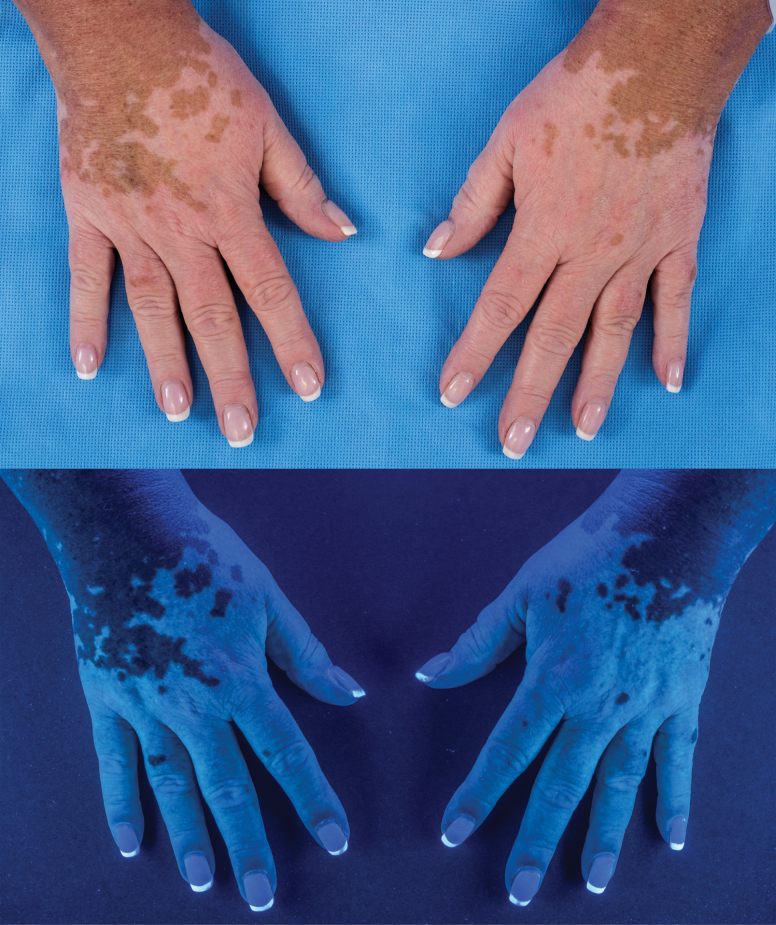
Interestingly, immune checkpoint inhibitors in melanoma have been specifically associated with the development of hypopigmentation/vitiligo ranging from 3 to 25% of cases (Fig. 3) (90, 91). Hypopigmentation has been very rarely reported in immune checkpoint inhibitors use for other solid tumors, but appears to be much higher in melanoma, occurring exclusively in melanoma in several case series (92–94). This is thought to represent on-target, off-tissue immune recognition of over-expressed melanoma antigens on healthy melanocytes. Indeed, vitiligo development has been associated with improved melanoma survival after immunotherapy treatment in a number of small trials and a large meta-analysis (95).
Fig. 3.
Development of vitiligo-like hypopigmentation in a patient being treated with immunotherapy. Top panel: standard photograph. Bottom photograph: Wood’s lamp. Vitiligo/hypopigmentation arose in a patient on immune checkpoint inhibitor immunotherapy.
Determinants of skin cancer response/resistance to anti-PD-1 and other immunotherapies
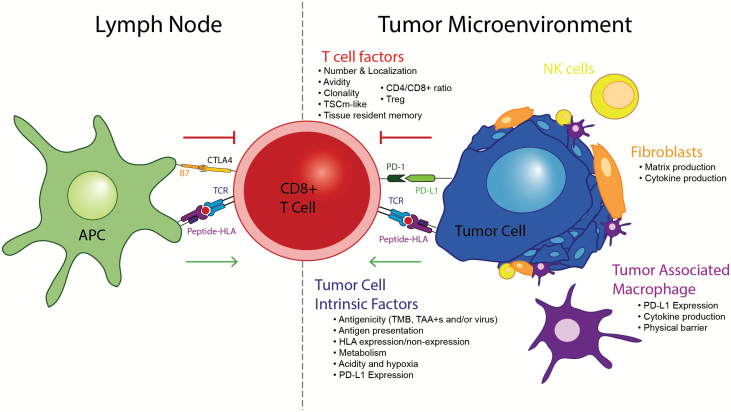
Determining the mechanisms of resistance to immunotherapies and especially immune checkpoint blockade, including both early/primary and late/acquired resistance (73), is imperative to improving immunotherapy outcomes in skin cancers and increasing the fraction of patients cured. It is increasingly evident that responsiveness and resistance to immune checkpoint blockade and immunotherapy does not seem to be readily predicted by a single biomarker and there has been dramatic recent progress in understanding these mechanisms (Fig. 4).
Fig. 4.
Contributors to immunotherapy response/resistance. A number of factors contribute to immunotherapy response/resistance, which broadly fall into three major categories: tumor cell-intrinsic, T cell and microenvironment. Please see text for additional details. TAA+s indicate positivity for tumor-associated antigens.
Tumor cell-intrinsic factors
Many tumor cell-intrinsic features are appreciated to contribute to immunotherapy responsiveness and resistance.
Antigens.
The underlying presence of antigen visible to T cells is critical to immunotherapy success, again highlighting the importance of T-cell recognition as a component of an effective anti-tumor immune response. TMB-low tumors are less likely to respond to immunotherapy (17), and tumor escape from immunotherapy has been mediated by de-differentiation and other forms of antigen loss (96).
Antigen presentation.
Tumors must not only contain T-cell antigens, they must also properly process and present these antigens bound to the major histocompatibility complex (MHC). Genetic (32, 97) and adaptive/transcriptional (98–100) mechanisms resulting in poor antigen processing and/or presentation are frequently employed by skin cancers and have been identified as mediating multiple forms of immunotherapy, including resistance to anti-PD-1, to anti-CTLA4 and to adoptive T-cell therapy. Active research is ongoing into means to improve antigen presentation.
Tumor metabolism.
Highly metabolic tumors both consume a large amount of glucose and release lactate. This simultaneously starves CD8+ effector T cells and promotes Treg function (101). Another tumor cell-intrinsic factor is expression of T-cell suppressive surface markers: melanomas and MCC have been described to express molecules known to inhibit CD8+ T cells, including PD-L1 (6) and CD200 (102, 103) among others.
T-cell factors
Multiple T-cell factors have been proposed as biomarkers to predict immunotherapy responsiveness and patient outcome.
CD8+ T cells
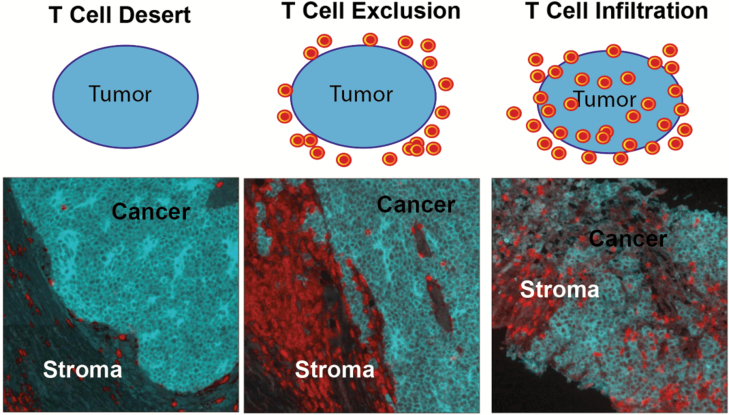
The number and pattern of localization of CD8+ T cells (absent/desert, excluded or infiltrated; Fig. 5) modestly predict anti-PD-1 responsiveness in melanoma, other skin cancers and other solid tumors (82, 104). Results on T-cell clonality are mixed and this has not emerged as a consistent predictor (105, 106). CD8+ T-cell avidity against MART-1 (107) and viral (108) antigens has been positively associated with improved patient outcomes in small series of patients with melanoma and VP-MCC, respectively. In November 2018, investigators used single-cell technologies to interrogate CD8+ T-cell populations in responding and non-responding melanoma patients. A subset of CD8+ T cells expressing the transcription factor TCF7, which is implicated in development of TSCm (memory stem T cells) (109) was identified, and predicted immunotherapy responsiveness in a second test set of patients (110). Finally, it is apparent that the tissue restriction/specificity of CD8+ T cells may contribute to tumor-specific immunity. Indeed, recent studies in animal models demonstrate the importance of tissue-resident (in the case of melanoma skin-resident) CD8+ T cells in controlling melanoma progression (111); the impact on these on immunotherapy response in humans is an area of active investigation.
Fig. 5.
Patterns of T-cell recognition of skin tumors. Three major patterns of T-cell recognition are observed across tumor types including melanomas and MCC: T-cell desert, T-cell exclusion and T-cell infiltration (134, 135). These patterns partially predict anti-PD-1 immunotherapy responsiveness (104). Top row: schematic indicating the localization pattern of T cells (red) compared with tumor (blue). Bottom row: three VP-MCC tumors (blue) exhibiting each of the three described patterns; in the bottom row, blue staining indicates CD56 (marks MCC) and red staining indicates anti-CD3 (marks T cells).
CD4+ T cells
Fundamental experiments in the 1980s demonstrated that CD4+ T cells mediate protective immunity against cancer (112). Given the difficulties in studying CD4+ T cells in human tumors, the role of CD4+-restricted T-cell functions in responses to PD-1 therapy remains poorly defined. CD4+ T-cell infiltration alone failed to predicted outcomes (113). However, in melanoma patients who received neoantigen-specific vaccines followed in some cases by immune checkpoint inhibitors, tumor-specific CD4+ T-cell responses were predominant, supporting the conclusion that this subset has a role (55). More research is needed in this area.
Tregs.
Not unexpectedly, Tregs have been anticorrelated with PD-1 response. The improved clinical outcomes in aged melanoma patients treated with PD-1 have been recently correlated with the relative paucity of Tregs in these patients (114).
Other checkpoints.
A number of other ligand–receptor interactions have reported as modulators of tumor-specific T cells, including those involving ICOS, TIM3, BTLA, A2aR and others (115).
Microenvironment factors
Macrophages.
Macrophages are adversely polarized in melanoma and are believed to contribute to CD8+ T-cell inhibition through PD-L1–PD-1 interactions, CTLA4 signaling, cytokine production and other factors (116). The role of macrophages in anti-PD-1 resistance and susceptibility remains to be determined. Macrophage contributions are likely complex in that patients with high numbers of PD-L1-expressing macrophages may be more likely to respond to anti-PD-1 therapy, whereas macrophage compartments expand and repolarize during responses to immunotherapy in murine models (117, 118). The role of macrophages in other skin cancers is less well defined. Macrophages have been reported to be both immunosuppressive and to correlate with positive T-cell responses in MCC, and the impact of macrophages on PD-1 responsiveness in MCC and cSCC has not been reported (103, 118).
Fibroblasts.
Cancer-associated fibroblasts (CAF) make up a significant portion of the stromal environment in most skin cancers, including melanomas. These fibroblasts have been demonstrated to contribute to an immunosuppressive microenvironment through chemokine production and extracellular matrix (ECM) production (119, 120). Pre-clinical studies have recently implicated a significant CAF contribution to melanoma PD-1 resistance (121); however, human studies have not yet been reported.
Dendritic cells and Langerhans cells.
Dendritic cells and Langerhans cells promote tumor-specific immunity through antigen cross-presentation. They are specifically targeted by topical imiquimod (a TLR7 agonist) that has been FDA-approved for BCC (122). Recently, these cells have been demonstrated to positively contribute to anti-PD-1 responsiveness in both mouse models and human melanomas (123).
Endothelial cells.
Endothelial cells may limit immune responses by restricting CD8+ T-effector entry.
Natural killer (NK) cells.
Peripheral and microenvironment signatures of NK responses have been associated with clinical efficacy for therapies using IL-2, interferon α (124) and PD-1 inhibitors in human melanoma (123). In addition, murine melanomas lacking the murine equivalent of HLA-E, an inhibitor of NK function over-expressed by human melanoma (125), do not develop PD-1 resistance (126), and an MCC was demonstrated to over-express HLA-E at the time of immunotherapy escape (99). This supports a model wherein antigen-specific T cells and NK cells cooperate to clear tumors, with NK cells being essential for eradicating tumor clonotypes that have lost class I HLA expression.
Conclusion
Skin cancers are highly immunogenic, due to tumor-associated antigens, mutations and/or viral gene expression. As a consequence, skin tumors must evade immune clearance and thus immunotherapies that derepress immune responses are extremely effective. Melanoma is the paradigm solid tumor for immunotherapy development and has demonstrated the potential for the immune system to cure solid tumors. Other skin cancers are being treated in a similarly successful way with anti-PD-1 immune checkpoint inhibitors, supporting their underlying immunogenicity. However, despite great progress most patients are not cured by these treatments. Barriers to the efficacy of immunotherapy are being actively delineated and include tumor cell-intrinsic, T cell and microenvironment obstacles. Promising research on these mechanisms is ongoing, and combination regimens offer the hope of increasing response rates, extending response durability and affording cures to a significantly higher fraction of patients.
Funding
SITC-Merck Fellowship (to K.G.P.), Immunotherapy Integrated Research Center/FHCRC (to K.G.P. and A.G.C.), NIH T32CA009515 (to K.G.P.) and T32GM095421 (to M.L.).
Conflicts of interest statement: K.G.P. receives research support to her institution from Merck, EMD Serono and Bluebird Biosciences, and her institution holds intellectual property related to T-cell receptors for treatment of Merkel cell carcinoma. A.G.C. receives research support to her institution from Juno Therapeutics, EMD Serono and Bluebird Biosciences, and holds intellectual property related to T-cell receptors for treatment of multiple cancers and other diseases. This work was supported by the NIH Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1. Siegel R. L., Miller K. D. and Jemal A. 2018. Cancer statistics, 2018. CA Cancer J. Clin. 68:7. [DOI] [PubMed] [Google Scholar]
- 2. Paulson K. G., Park S. Y., Vandeven N. A. et al. 2018. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 78:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palyca P., Koshenkov V. P., and Mehnert J. M. 2014. Developments in the treatment of locally advanced and metastatic squamous cell carcinoma of the skin: a rising unmet need. Am. Soc. Clin. Oncol. Educ. Book 34:e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogers H. W., Weinstock M. A., Feldman S. R. and Coldiron B. M. 2015. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 151:1081. [DOI] [PubMed] [Google Scholar]
- 5. Robert C., Thomas L., Bondarenko I. et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517. [DOI] [PubMed] [Google Scholar]
- 6. Wolchok J. D., Chiarion-Sileni V., Gonzalez R. et al. 2017. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leach D. R., Krummel M. F. and Allison J. P. 1996. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734. [DOI] [PubMed] [Google Scholar]
- 8. Ishida Y., Agata Y., Shibahara K. and Honjo T. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11:3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao H., Shu G. and Wang S. 2016. The risk of non-melanoma skin cancer in HIV-infected patients: new data and meta-analysis. Int. J. STD AIDS 27:568. [DOI] [PubMed] [Google Scholar]
- 10. Tessari G. and Girolomoni G. 2012. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol. Surg. 38:1622. [DOI] [PubMed] [Google Scholar]
- 11. Engels E. A., Frisch M., Goedert J. J., Biggar R. J. and Miller R. W. 2002. Merkel cell carcinoma and HIV infection. Lancet 359:497. [DOI] [PubMed] [Google Scholar]
- 12. Heath M., Jaimes N., Lemos B. et al. 2008. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J. Am. Acad. Dermatol. 58:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paulson K. G., Iyer J. G., Blom A. et al. 2013. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J. Invest. Dermatol. 133:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grulich A. E., van Leeuwen M. T., Falster M. O. and Vajdic C. M. 2007. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370:59. [DOI] [PubMed] [Google Scholar]
- 15. Lanoy E., Costagliola D. and Engels E. A. 2010. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int. J. Cancer 126:1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ilyas S. and Yang J. C. 2015. Landscape of tumor antigens in T cell immunotherapy. J. Immunol. 195:5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yarchoan M., Hopkins A. and Jaffee E. M. 2017. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377:2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashford B. G., Clark J., Gupta R., Iyer N. G., Yu B. and Ranson M. 2017. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck 39:1462. [DOI] [PubMed] [Google Scholar]
- 19. Jacob J. M., Ferry E. K., Gay L. M. et al. 2018. Comparative genomic profiling of refractory/metastatic penile and non-penile cutaneous squamous cell carcinoma: implications for selection of systemic therapy. J. Urol. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 20. Chang A. L. S., Tran D. C., Cannon J. G. D. et al. 2019. Pembrolizumab for advanced basal cell carcinoma: an investigator-initiated, proof-of-concept study. J. Am. Acad. Dermatol. 80:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harms P. W., Collie A. M., Hovelson D. H. et al. 2016. Next generation sequencing of cytokeratin 20-negative Merkel cell carcinoma reveals ultraviolet-signature mutations and recurrent TP53 and RB1 inactivation. Mod. Pathol. 29:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodis E., Watson I. R., Kryukov G. V. et al. 2012. A landscape of driver mutations in melanoma. Cell 150:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pickering C. R., Zhou J. H., Lee J. J. et al. 2014. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 20:6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goh G., Walradt T., Markarov V. et al. 2016. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 7:3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chalmers Z. R., Connelly C. F., Fabrizio D. et al. 2017. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodman A. M., Kato S., Bazhenova L. et al. 2017. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 16:2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galanina N., Goodman A. M., Cohen P. R., Frampton G. M. and Kurzrock R. 2018. Successful treatment of HIV-associated Kaposi sarcoma with immune checkpoint blockade. Cancer Immunol. Res. 6:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore P. S., Gao S. J., Dominguez G. et al. 1996. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcomae. J. Virol. 70:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng H., Shuda M., Chang Y. and Moore P. S. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robey R. C., Mletzko S. and Gotch F. M. 2010. The T-cell immune response against Kaposi’s sarcoma-associated herpesvirus. Adv. Virol. 2010:340356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iyer J. G., Afanasiev O. K., McClurkan C. et al. 2011. Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 17:6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chowell D., Morris L. G. T., Grigg C. M. et al. 2018. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veatch J. R., Lee S. M., Fitzgibbon M. et al. 2018. Tumor-infiltrating BRAFV600E-specific CD4+ T cells correlated with complete clinical response in melanoma. J. Clin. Invest. 128:1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson L. A. and June C. H. 2017. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 27:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gray-Schopfer V., Wellbrock C. and Marais R. 2007. Melanoma biology and new targeted therapy. Nature 445:851. [DOI] [PubMed] [Google Scholar]
- 36. Coley W. B. 1991. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 262:3. [PubMed] [Google Scholar]
- 37. McCarthy E. F. 2006. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 26:154. [PMC free article] [PubMed] [Google Scholar]
- 38. Morton D. L., Eilber F. R., Holmes E. C. et al. 1974. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann. Surg. 180:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenberg S. A. 1975. Future prospects for immunotherapy. Cancer 36:821. [DOI] [PubMed] [Google Scholar]
- 40. Comis R. L. 1976. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat. Rep. 60:165. [PubMed] [Google Scholar]
- 41. Lardone R. D., Chan A. A., Lee A. F. et al. 2017. Mycobacterium bovis Bacillus Calmette-Guérin alters melanoma microenvironment favoring antitumor T cell responses and improving M2 macrophage function. Front. Immunol. 8:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanlorenzo M., Vujic I., Carnevale-Schianca F. et al. 2017. Role of interferon in melanoma: old hopes and new perspectives. Expert Opin. Biol. Ther. 17:475. [DOI] [PubMed] [Google Scholar]
- 43. Eggermont A. M., Suciu S., Rutkowski P. et al. ; EORTC Melanoma Group. 2016. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: ulceration of primary is key determinant for IFN-sensitivity. Eur. J. Cancer 55:111. [DOI] [PubMed] [Google Scholar]
- 44. Davar D., Ding F., Saul M. et al. 2017. High-dose interleukin-2 (HD IL-2) for advanced melanoma: a single center experience from the University of Pittsburgh Cancer Institute. J. Immunother. Cancer 5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nelson B. H. 2004. IL-2, regulatory T cells, and tolerance. J. Immunol. 172:3983. [DOI] [PubMed] [Google Scholar]
- 46. Dudley M. E., Yang J. C., Sherry R. et al. 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26:5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yee C., Thompson J. A., Byrd D. et al. 2002. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA 99:16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chodon T., Comin-Anduix B., Chmielowski B. et al. 2014. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 20:2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chapuis A. G., Lee S. M., Thompson J. A. et al. 2016. Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. J. Exp. Med. 213:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chapuis A. G., Thompson J. A., Margolin K. A. et al. 2012. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc. Natl Acad. Sci. USA 109:4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Merhavi-Shoham E., Itzhaki O., Markel G., Schachter J. and Besser M. J. 2017. Adoptive cell therapy for metastatic melanoma. Cancer J. 23:48. [DOI] [PubMed] [Google Scholar]
- 52. Moore T., Wagner C. R., Scurti G. M. et al. 2018. Clinical and immunologic evaluation of three metastatic melanoma patients treated with autologous melanoma-reactive TCR-transduced T cells. Cancer Immunol. Immunother. 67:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andtbacka R. H., Kaufman H. L., Collichio F. et al. 2015. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33:2780. [DOI] [PubMed] [Google Scholar]
- 54. Sahin U., Derhovanessian E., Miller M. et al. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547:222. [DOI] [PubMed] [Google Scholar]
- 55. Ott P. A., Hu Z., Keskin D. B. et al. 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalaora S., Wolf Y., Feferman T. et al. 2018. Combined analysis of antigen presentation and T-cell recognition reveals restricted immune responses in melanoma. Cancer Discov. 8:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hurwitz A. A., Sullivan T. J., Krummel M. F., Sobel R. A. and Allison J. P. 1997. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J. Neuroimmunol. 73:57. [DOI] [PubMed] [Google Scholar]
- 58. Chambers C. A., Sullivan T. J. and Allison J. P. 1997. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7:885. [DOI] [PubMed] [Google Scholar]
- 59. Hodi F. S., O’Day S. J., McDermott D. F. et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolchok J. D., Rollin L. and Larkin J. 2017. Nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377:2503. [DOI] [PubMed] [Google Scholar]
- 61. Schachter J., Ribas A., Long G. V. et al. 2017. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390:1853. [DOI] [PubMed] [Google Scholar]
- 62. Long G. V., Eroglu Z., Infante J. et al. 2018. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J. Clin. Oncol. 36:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reddy S. M., Reuben A. and Wargo J. A. 2016. Influences of BRAF inhibitors on the immune microenvironment and the rationale for combined molecular and immune targeted therapy. Curr. Oncol. Rep. 18:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sumimoto H., Imabayashi F., Iwata T. and Kawakami Y. 2006. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203:1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang X., Zhou J., Giobbie-Hurder A., Wargo J. and Hodi F. S. 2013. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin. Cancer Res. 19:598. [DOI] [PubMed] [Google Scholar]
- 66. Frederick D. T., Piris A., Cogdill A. P. et al. 2013. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rosenberg S. A. 2012. Raising the bar: the curative potential of human cancer immunotherapy. Sci. Transl. Med. 4:127ps8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schadendorf D., Hodi F. S., Robert C. et al. 2015. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33:1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robert C., Ribas A., Hamid O. et al. 2018. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. 36:1668. [DOI] [PubMed] [Google Scholar]
- 70. Eggermont A. M. M., Blank C. U., Mandala M. et al. 2018. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378:1789. [DOI] [PubMed] [Google Scholar]
- 71. Weber J., Mandala M., Del Vecchio M. et al. ; CheckMate 238 Collaborators. 2017. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377:1824. [DOI] [PubMed] [Google Scholar]
- 72. Joseph R. W., Elassaiss-Schaap J., Kefford R. et al. 2018. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin. Cancer Res. 24:4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sharma P., Hu-Lieskovan S., Wargo J. A. and Ribas A. 2017. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Menk A. V., Scharping N. E., Moreci R. S. et al. 2018. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scharping N. E. and Delgoffe G. M. 2016. Tumor microenvironment metabolism: a new checkpoint for anti-tumor immunity. Vaccines (Basel) 4:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Huber R., Meier B., Otsuka A. et al. 2016. Tumour hypoxia promotes melanoma growth and metastasis via high mobility group box-1 and M2-like macrophages. Sci. Rep. 6:29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hotblack A., Holler A., Piapi A., Ward S., Stauss H. J. and Bennett C. L. 2018. Tumor-resident dendritic cells and macrophages modulate the accumulation of TCR-engineered T cells in melanoma. Mol. Ther. 26:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Blank C. U., Rozeman E. A., Fanchi L. F. et al. 2018. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24:1655. [DOI] [PubMed] [Google Scholar]
- 79. Fransen M. F., Schoonderwoerd M., Knopf P. et al. 2018. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 3. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Migden M. R., Rischin D., Schmults C. D. et al. 2018. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 379:341. [DOI] [PubMed] [Google Scholar]
- 81. Nghiem P. T., Bhatia S., Lipson E. J. et al. 2016. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 374:2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nghiem P., Bhatia S., Lipson E. J., Sharfman W. H., Kudchadkar R. R. and Friedlander P. A. 2018. Durable tumor regression and overall survival (OS) in patients with advanced Merkel cell carcinoma (aMCC) receiving pembrolizumab as first-line therapy. J. Clin. Oncol. 36:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. D’Angelo S. P., Russell J., Lebbé C. et al. 2018. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 4:e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kaufman H. L., Russell J. S., Hamid O. et al. 2018. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immunother. Cancer 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sindhu S., Gimber L. H., Cranmer L., McBride A. and Kraft A. S. 2017. Angiosarcoma treated successfully with anti-PD-1 therapy - a case report. J. Immunother. Cancer 5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brahmer J. R., Lacchetti C., Schneider B. J. et al. ; National Comprehensive Cancer Network. 2018. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 36:1714.29442540 [Google Scholar]
- 87. Pintova S., Sidhu H., Friedlander P. A. and Holcombe R. F. 2013. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 23:498. [DOI] [PubMed] [Google Scholar]
- 88. Hwang S. J., Carlos G., Wakade D., Sharma R. and Fernandez-Penas P. 2016. Ipilimumab-induced acute generalized exanthematous pustulosis in a patient with metastatic melanoma. Melanoma Res. 26:417. [DOI] [PubMed] [Google Scholar]
- 89. Collins L. K., Chapman M. S., Carter J. B. and Samie F. H. 2017. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 41:125. [DOI] [PubMed] [Google Scholar]
- 90. Larsabal M., Marti A., Jacquemin C. et al. 2017. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J. Am. Acad. Dermatol. 76:863. [DOI] [PubMed] [Google Scholar]
- 91. Hua C., Boussemart L., Mateus C. et al. 2016. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 152:45. [DOI] [PubMed] [Google Scholar]
- 92. Sanlorenzo M., Vujic I., Daud A. et al. 2015. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 151:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kosche C., Mohindra N. and Choi J. N. 2018. Vitiligo in a patient undergoing nivolumab treatment for non-small cell lung cancer. JAAD Case Rep. 4:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Uenami T., Hosono Y., Ishijima M. et al. 2017. Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: a case report. Lung Cancer 109:42. [DOI] [PubMed] [Google Scholar]
- 95. Teulings H. E., Limpens J., Jansen S. N. et al. 2015. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J. Clin. Oncol. 33:773. [DOI] [PubMed] [Google Scholar]
- 96. Mehta A., Kim Y. J., Robert L. et al. 2018. Immunotherapy resistance by inflammation-induced dedifferentiation. Cancer Discov. 8:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zaretsky J. M., Garcia-Diaz A., Shin D. S. et al. 2016. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rodig S. J., Gusenleitner D., Jackson D. G. et al. 2018. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 10:eear3342. [DOI] [PubMed] [Google Scholar]
- 99. Paulson K. G., Voillet V., McAfee M. S. et al. 2018. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat. Commun. 9:3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jerby-Arnon L., Shah P., Cuoco M. S. et al. 2018. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cascone T., McKenzie J. A., Mbofung R. M. et al. 2018. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 27:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Love J. E., Thompson K., Kilgore M. R. et al. 2017. CD200 expression in neuroendocrine neoplasms. Am. J. Clin. Pathol. 148:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gaiser M. R., Weis C. A., Gaiser T. et al. 2018. Merkel cell carcinoma expresses the immunoregulatory ligand CD200 and induces immunosuppressive macrophages and regulatory T cells. Oncoimmunology 7:e1426517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cristescu R., Mogg R., Ayers M. et al. 2018. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362:eear3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Johnson D. B., Frampton G. M., Rioth M. J. et al. 2016. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol. Res. 4:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hogan S. A., Courtier A., Cheng P. F. et al. 2019. Peripheral blood TCR repertoire profiling may facilitate patient stratification for immunotherapy against melanoma. Cancer Immunol. Res. 7:77. [DOI] [PubMed] [Google Scholar]
- 107. Simon S., Vignard V., Varey E. et al. 2017. Emergence of high-avidity melan-A-specific clonotypes as a reflection of anti-PD-1 clinical efficacy. Cancer Res. 77:7083. [DOI] [PubMed] [Google Scholar]
- 108. Miller N. J., Church C. D., Dong L. et al. 2017. Tumor-infiltrating Merkel cell polyomavirus-specific T cells are diverse and associated with improved patient survival. Cancer Immunol. Res. 5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gattinoni L., Zhong X. S., Palmer D. C. et al. 2009. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sade-Feldman M., Yizhak K., Bjorgaard S. L. et al. 2018. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Park S. L., Buzzai A., Rautela J. et al. 2019. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature 565:366. [DOI] [PubMed] [Google Scholar]
- 112. Greenberg P. D., Cheever M. A. and Fefer A. 1981. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1 + 2- lymphocytes. J. Exp. Med. 154:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tumeh P. C., Harview C. L., Yearley J. H. et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kugel C. H. III, Douglass S. M., Webster M. R. et al. 2018. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin. Cancer Res. 24:5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pardoll D. M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ruffell B. and Coussens L. M. 2015. Macrophages and therapeutic resistance in cancer. Cancer Cell 27:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Vilain R. E., Menzies A. M., Wilmott J. S. et al. 2017. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin. Cancer Res. 23:5024. [DOI] [PubMed] [Google Scholar]
- 118. Kervarrec T., Gaboriaud P., Berthon P. et al. 2018. Merkel cell carcinomas infiltrated with CD33+ myeloid cells and CD8+ T cells are associated with improved outcome. J. Am. Acad. Dermatol. 78:973. [DOI] [PubMed] [Google Scholar]
- 119. Turley S. J., Cremasco V. and Astarita J. L. 2015. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15:669. [DOI] [PubMed] [Google Scholar]
- 120. Kraman M., Bambrough P. J., Arnold J. N. et al. 2010. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330:827. [DOI] [PubMed] [Google Scholar]
- 121. Zhao F., Evans K., Xiao C. et al. 2018. Stromal fibroblasts mediate anti-PD-1 resistance via MMP-9 and dictate TGFβ inhibitor sequencing in melanoma. Cancer Immunol. Res. 6:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Beutner K. R., Geisse J. K., Helman D., Fox T. L., Ginkel A. and Owens M. L. 1999. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J. Am. Acad. Dermatol. 41:1002. [DOI] [PubMed] [Google Scholar]
- 123. Barry K. C., Hsu J., Broz M. L. et al. 2018. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Atzpodien J., Kirchner H., Körfer A. et al. 1993. Expansion of peripheral blood natural killer cells correlates with clinical outcome in cancer patients receiving recombinant subcutaneous interleukin-2 and interferon-alpha-2. Tumour Biol. 14:354. [DOI] [PubMed] [Google Scholar]
- 125. Tremante E., Ginebri A., Lo Monaco E. et al. 2014. A melanoma immune response signature including human leukocyte antigen-E. Pigment Cell Melanoma Res. 27:103. [DOI] [PubMed] [Google Scholar]
- 126. Manguso R. T., Pope H. W., Zimmer M. D. et al. 2017. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 547:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Carlino M. S., Long G. V., Schadendorf D. et al. 2018. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: a randomised clinical trial. Eur. J. Cancer 101:236. [DOI] [PubMed] [Google Scholar]
- 128. Long G. V., Flaherty K. T., Stroyakovskiy D. et al. 2017. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 28:1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Le D. T., Uram J. N., Wang H. et al. 2015. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Balar A. V., Castellano D., O’Donnell P. H. et al. 2017. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18:1483. [DOI] [PubMed] [Google Scholar]
- 131. Chung H. C., Schellens J. H. M., Delord J. P. et al. 2018. Pembrolizumab treatment of advanced cervical cancer: updated results from the phase 2 KEYNOTE-158 study. J. Clin. Oncol. 36:1. [DOI] [PubMed] [Google Scholar]
- 132. Ready N., Farago A. F., de Braud F. et al. 2019. Third-line nivolumab monotherapy in recurrent SCLC: checkmate 032. J. Thorac. Oncol. 14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Garon E. B., Rizvi N. A., Hui R. et al. ; KEYNOTE-001 Investigators. 2015. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372:2018. [DOI] [PubMed] [Google Scholar]
- 134. Chen D. S. and Mellman I. 2013. Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1. [DOI] [PubMed] [Google Scholar]
- 135. Paulson K. G., Iyer J. G., Tegeder A. R. et al. 2011. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J. Clin. Oncol. 29:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]