Abstract
The cell adhesion molecule neuroligin2 (NLGN2) regulates GABAergic synapse development, but its role in neural circuit function in the adult hippocampus is unclear. We investigated GABAergic synapses and hippocampus-dependent behaviors following viral-vector-mediated overexpression of NLGN2. Transducing hippocampal neurons with AAV-NLGN2 increased neuronal expression of NLGN2 and membrane localization of GABAergic postsynaptic proteins gephyrin and GABAA Rγ2, and presynaptic vesicular GABA transporter protein (VGAT) suggesting trans-synaptic enhancement of GABAergic synapses. In contrast, glutamatergic postsynaptic density protein-95 (PSD-95) and presynaptic vesicular glutamate transporter (VGLUT) protein were unaltered. Moreover, AAV-NLGN2 significantly increased parvalbumin immunoreactive (PV+) synaptic boutons co-localized with postsynaptic gephyrin+ puncta. Furthermore, these changes were demonstrated to lead to cognitive impairments as shown in a battery of hippocampal-dependent mnemonic tasks and social behaviors.
Keywords: Neuroligin, gephyrin, VGAT, Sociability, Social Dominance, Spatial Memory
Graphical Abstract

1. Introduction
Of the neuroligin (NLGN) family of synaptic proteins (NLGN1-4) only NLGN2 is selectively found at inhibitory synapses [1–4]. The NLGN proteins are integral membrane adhesion molecules that bind to presynaptic neurexins [5–8]. As mutations in NLGNs have been identified in patients with autism [9–11], schizophrenia [12], and other neurological disorders [13, 14], the role of NLGN2 in inhibitory synapse formation, maturation, and function is of considerable interest. The expression of NLGN2 in vitro in non-neuronal cells induces putative synaptic contacts [8, 15–17] and in vivo, binding between presynaptic neurexin and postsynaptic NLGN2 enhances inhibitory synaptic transmission [18]. NLGN2 tethers GABA receptors within a scaffold on the postsynaptic neuron through molecular interactions with gephyrin and collybistin [3]. By recruiting gephyrin and GABAARs, NLGN2 shapes mature electrophysiological response properties [19, 20].
Several studies have utilized genetic deletion or viral-vector-mediated overexpression to test whether dysregulation of NLGN2 causes GABAergic circuit dysfunction or cognitive changes with conflicting results. While genetically-induced NLGN2 deficiency or overexpression increase anxiety-like behaviors [21–24], limiting NLGN2 overexpression to the adult mouse dorsal hippocampus did not alter anxiety phenotypes [25]. Furthermore, regardless of whether NLGN2 expression was experimentally increased or decreased, preference ratios in the three-chamber sociability test were abolished [22, 26, 27]. Others found that constitutive NLGN2 knockout in the whole brain [23] or selective overexpression of NLGN2 in the adult rat hippocampus [25] did not alter social behavior. Aggression, as measured by the resident intruder test, also showed variable results between overexpression studies [25, 27].
As a step toward addressing gaps in our understanding of the role of NLGN2 in inhibitory synapse formation and function in the adult brain, we increased NLGN2 expression in the DG and hippocampal subfields CA3 and CA1 in normal, naïve mice through adeno-associated viral (AAV) vectors and studied recruitment of synapse-specific presynaptic and postsynaptic molecules using immunohistochemistry, confocal microscopy, and biochemistry. Given the distinct functional roles for GABAergic cell types in the DG and CA subfields of the hippocampus [28] and the significant roles of GABAergic connections with hippocampal principle cells in regulating social behaviors [29, 30], spatial memory [31, 32], and brain rhythms [33–38], we also investigated whether NLGN2 overexpression altered social dominance, social memory, spatial memory, novel object recognition, object location, and anxiety.
2. Materials and Methods
2.1. Experimental Animals
All animal use followed protocols approved by the Wesleyan University’s Institutional Animal Care and Use Committee. Male wild-type C57BL/6NHsd mice (Envigo; 8-10 weeks old, approximately 24-30 grams) and VGAT-ChR2-eYFP mice (Jackson; 8-10 weeks old, approximately 24-30 grams) were pair-housed in our animal facility in self-ventilating cage units, maintained on a 12-hour light/dark cycle, and provided with food and water ad libitum. A total of 51 mice were used for this study; 29 received stereotaxic injections into the hippocampus of the AAV-NLGN2 vector and 22 received stereotaxic injections of the control vector, AAV-mCherry. C57BL/6NHsd adult males were used for all behavioral and biochemistry experiments and both C57BL/6NHsd and VGAT-ChR2-eYFP adult male mice [39] were used for immunohistochemical studies. Behavioral testing was performed on 44 adult male C57BL/6NHsd mice; 24 received AAV-NLGN2 and 20 received AAV-mCherry injections. Prior to behavioral testing, mice were pair-housed with like-treated littermates and received handling for a week.
2.2. Viral constructs for overexpression ofNLGN2
AAV/DJ mixed serotype viruses with CMV promoter-driven gene expression were used (Vector Biolabs). The NLGN2 vector contained the mouse NLGN2 gene (NM_198862) and an mCherry fluorescent reporter. The resulting custom plasmid (pAAV-cDNA6-mCherry-NLGN2) was packaged into AAV/DJ (Addgene; AAV/DJ-cDNA6-mCherry-NLGN2; titer: 1.3 × 1013 GC/mL). The control vector contained the CMV promoter driving expression of mCherry minus NLGN2 (AAV/DJ-CMV-mCherry; titer: 1.8 × 1013 GC/ml). Both vectors resulted in strong transduction of hippocampal neurons and robust expression for at least 4-6 weeks, the longest period that we tested.
2.3. Stereotaxic surgery.
Mice were initially injected with Medicam (0.3mL, s.c., Henry Schein) and stereotaxic injections of virus were made under isoflurane gas anesthesia (VetEquip, Harvard Apparatus). Each mouse received 0.5 μ1 of AAV vector/site (0.2 μ1/minute) into 5 sites per hippocampus by means of an automated stereotaxic injection system (Stoelting Quintessential), equipped with a 5 μ1 glass syringe (Hamilton 7633–01) and bevel-tip needle (Hamilton, 30°, 30 gauge, 0.5”). The coordinates for anterior hippocampal injections were determined as follows: AP (Bregma – Lambda)/2; ML +/− 1.25 mm; DV 2.2, 2.0, and 1.3 mm [40]. The coordinates for posterior injections were determined as follows: AP −3.0 – (−1.9 – (Bregma – Lambda)/2), ML ± 3.5, DV −4.0 and −3.0.
2.4. Behavioral assays
We conducted behavioral analyses 2–3 weeks post-AAV injections, then euthanized the mice for either biochemistry or immunohistochemistry. Each mouse was acclimated in its home cage in the behavioral testing facility 30 minutes prior to behavioral testing and apparatuses were wiped down with 75% ethanol between tests. The order of behavioral testing was: Open Field, Zero Maze, Novel Object Location, Novel Object Recognition, Sociability test, Social Dominance test, and the Morris Water Maze (Table S1). Testing was conducted under 16–22 lux illumination with ambient white noise while video recording, and later analyzed with AnyMaze software by a trained-observer blind to the treatment group.
Open Field testing to evaluate exploration and anxiety was performed in a white plastic circular arena, elevated approximately 86 cm from the ground, with 46 cm high sides and 91.4 cm inner diameter. The mouse was placed in the center of the arena and allowed to explore for 10 minutes. The distance traveled and time in the central area (designated by a circle of approximately 60 cm) were measured.
We also measured anxiety behavior with the Zero maze. As described previously, performance in this maze is relatively stable over time, as compared to the elevated plus maze [41]. The Zero maze consisted of an elevated circular platform with two open and two enclosed zones (inside diameter 61cm; outside diameter 86.4 cm; platform elevation above the floor 77.5 cm; wall height 8.1 cm; camera height 223.5 cm). To initiate testing, the mice were placed facing an enclosed zone then allowed to explore the apparatus for 5 minutes. Mice the fell off the open arms of the apparatus were placed back on the maze and retested. The total distance travelled and time spent in each zone were quantified.
To test to test spatial memory, we used to two behavioral tests; the novel object recognition test and the object new location test. Both were performed in a square plastic arena (30.5 cm2). Each mouse was acclimated to the arena for 10 min the day prior to testing. For novel object recognition, the mice were allowed to investigate 2 objects placed into opposite corners of the arena for 10 minutes. Objects included: 50mL conical tubes filled with marbles, three 15mL conical tubes taped together, and 100mL plastic bottles with varying labels, all weighted to prevent displacement. One hour following the initial exposure, one of the objects was replaced with a different object of similar size, and the mice were allowed to investigate the objects for 10 minutes. For the object new location test, two objects were placed into opposite corners of the apparatus and the mice were allowed to investigate for 10 minutes. One hour later, one of the objects was relocated and the mice were allowed to investigate for 10 minutes. An area extending 1 inch in all directions around each object was designated as the area of interaction. The number of entries and the duration of time that the mouse’s head was within this area were computed.
Social interactions were measured with Crawley’s three-chambered sociability test in clear Plexiglass apparatus with three 20 cm by 40.5 cm interconnected chambers. Circular cages were placed in the two outer chambers (diameter 10 cm) and testing was conducted as previously described [42–44]. The mouse to be tested was placed in the central chamber with the doors shut and allowed to acclimate for 1 minute. For the first round of testing, one cage contained a stranger mouse and the other was empty. The doors were then opened, and the mouse explored the apparatus freely for 10 minutes. The mouse was moved back into the central chamber and the doors were shut. A novel stranger mouse was placed in the previously empty cage and the experimental mouse was again allowed to explore the apparatus for 10 minutes. Interaction times and number of interactions were quantified. The area of interaction was designated as the area containing the social interaction cage area with a 3-cm border. The number of entries and the duration of time that the mouse’s head was within this area were computed.
The social dominance apparatus consisted of an elevated clear Plexiglas tube (3 cm above table) [45]. Pairs of mice were placed in the opposite ends of the tube and allowed to meet in the center. The first mouse backing out of the tube was considered the “loser” of the bout, while the mouse remaining in the tube was considered the “winner”. Testing lasted until one mouse backed out of the tube, or until 3 minutes elapsed; if neither mouse backed out, it was considered a draw. Bouts within experimental group were used to confirm that the mice exhibited normal dominance behavior and then we quantified bout between the AAV-NLGN2 vs. AAV-mCherry groups of mice. Any mice showing <0.2 or >0.8 ratios within experimental group were excluded (2 AAV-mCherry mice, 0 AAV-NLGN2 mice).
Spatial memory was tested in a circular Morris Water Maze pool (122 cm diameter, 76 cm depth) filled with water (20°C) made opaque with non-toxic white paint and enclosed by white curtains with visual cues. Training to find a submerged platform (diameter 16 cm) was for five days, followed by a probe trial on the sixth day. Training consisted of four training trials/day/mouse, lasting 60 seconds/trial, or until the mouse climbed on the platform. The mice remained on the platform for 10 seconds; if they failed to reach the platform, they were guided there. During the first two days of training only, a visual cue was placed on the platform. During the 60-sec probe trial on the sixth day, the platform was removed, and the swim speed, escape latency to the platform during training, latency to first platform area crossing in the probe trial, the number of platform area crossings in the probe trail, and the quadrant dwell time were measured.
2.5. Western blot analyses of adult mouse hippocampi
To harvest hippocampal tissue, the mice were euthanized using cervical dislocation and the left and right hippocampi were extracted and frozen at −80°C. Membrane fractions were prepared by thawing the tissue in 400 μ1 of ice-cold TEVP buffer (10 mM Tris, pH 7.4, 5 mM NaF, 1 mM EDTA, and 1.0 mM EGTA, 1 mM NasVOf) containing 320 mM sucrose with protease inhibitor and homogenizing with a dounce homogenizer. Samples were centrifuged for 10 minutes at 2500 rpm (Eppendorf 5415 C), the supernatant was extracted, and centrifuged for 15 minutes at 10000 rpm to obtain the P2 pellet. The P2 fraction was suspended in TEVP buffer and solubilized by 10 freeze-thaw cycles, then frozen in aliquots and stored at −80°C. Protein concentrations were calculated with a BCA assay kit (Thermo Scientific 23227).
SDS-PAGE was performed with a mini-gel electrophoresis system (Bio-Rad 525BR) with 4–20% precast gradient gels (Bio-Rad 4561091S). The membrane fractions from control and experimental mice run together on the same gels for comparison (20μg protein/lane) and proteins were separated at a constant voltage (150V) for 120 minutes and transferred onto PVDF membranes (Bio-Rad 153BR) with constant voltage (100V) for 90 minutes. The transferred proteins were visualized by staining the PVDF membranes in Ponceau S, then the blots were cut into horizontal strips at 150, 75, 50 and 37 kDa, based on the migration of protein standards (Precision Plus Protein™ Dual Color Standards, BioRad #161–0374), to allow each lane to be probed with antibodies (see Table S1). The following antibodies were used: mouse anti-actin (1:2000), mouse anti-gephyrin (1:2000), rabbit anti-NLGN2 (1:4000), mouse anti-GABAARγ2 (1:4000), mouse anti-NR2B (1:1000), mouse anti-PSD95 (1:4000), and mouse anti-VGAT (1:8000). After washing the blots, the bound antibodies were detected by incubating in HRP-conjugated goat anti-mouse IgG (1:5000) or HRP-conjugated anti-rabbit IgG (1:10000) followed by a 1-minute incubation in SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific 34096). The blots were imaged with GeneSnap software (SynGene 7.12) in a G:Box apparatus (Syngene G:Box Chemi XX6). Blot images were viewed in Image J software, inverted, and optical densities for each protein band were calculated. All values were normalized to actin within the same lane. Statistical analyses were performed using Student’s T-Test function, using each lane as a separate input.
2.6. Immunohistochemistry
For histology, the mice were euthanized (sodium pentobarbital, Henry Schein, 100 mg/kg, i.p.), and perfused transcardially (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4). Cryostat sections were cut at 12 μm, mounted and immunostained on Superfrost Plus glass slides. Incubations were overnight at room temperature in primary antibodies diluted in 0.1 M sodium phosphate buffered saline containing 10% normal goat serum and 1% Triton-X 100. The following primary antibodies were used (see Table S1): chicken anti-GFP (1:1000), mouse anti-mCherry (1:1000), rabbit anti-mCherry (1:1000), chicken anti-mCherry (1:1000), rabbit anti-NLGN2 (1:1000), rabbit anti-parvalbumin (1:1000), guinea pig anti-VGAT (1:500), mouse anti-vGlut (1:500), and mouse anti-gephyrin (1:1000). Detection was performed with secondary antibodies (Life Technologies) and sections were mounted in Prolong Diamond with DAPI (Life Technologies).
2.7. Synaptic protein density analyses
Immunofluorescently labeled synaptic puncta were imaged by confocal microscopy at an optical slice interval of 0.33 μm (Zeiss LSM 510 Meta confocal microscope, C-Apochromat 63× objective 1.2 N.A.). The confocal images were analyzed in ImageJ with Puncta Analyzer [46]. We selected regions of interest (ROI) containing mCherry+ neurons and then quantified co-localized synaptic protein labeling. Values were normalized based on mCherry expression within the ROI.
2.8. Quantification of putative synapses onto individual neurons
We quantified sites of putative synapses on granule and pyramidal cell somas by identifying puncta immunolabeled for presynaptic proteins (e.g. VGAT) that were in alignment with clusters of immunolabeled postsynaptic proteins (e.g. gephyrin). To ensure unbiased selection of AAV-labeled neurons, the images were viewed in the red channel and individual somas outlined, then the red channel was hidden, and the green and infrared channels were visualized. To quantify the number of putative synapses, we identified sites with alignment between immunolabeled presynaptic and postsynaptic proteins, and used Zeiss Zen software to generate a fluorescence histograms for these puncta. We set the background level based on the fluorescent intensity of an adjacent region without immunofluorescent staining in the same channel. Putative fluorescently labeled synaptic puncta were identified as peaks of fluorescently labeled pre- and postsynaptic markers that were both above background and in close proximity and alignment.
2.9. Statistical Analysis and Graphs
Statistical analyses were performed in the Statistical Package for Social Science (SPSS)(IBM). The graphs were generated in Graph Pad Prism (https://www.graphpad.com/scientific-software/prism/). Box and whisker plots show the maximum and minimum values, with the upper and lower bounds of the box encompassing the third and first quartiles, respectively. All data points are shown on each box and whisker plot. Further information regarding statistical analyses is available in Table S2.
3. Results
3.1. AAV/DJ vector-mediated NLGN2 gene expression increases NLGN2 in the hippocampus
Western blot experiments confirmed that neuronal transduction with the AAV-NLGN2 vector significantly increased levels of NLGN2 in membrane fractions by approximately 55% (Figure 1A), compared to the AAV-mCherry vector (Figure 1B; p=0.0002, Student’s t-test; n=18 hippocampi from 9 AAV-mCherry+ mice, 20 hippocampi from 10 AAV-NLGN2+ mice).
Figure 1. Increased NLGN2 expression following neuronal transduction with AAV-NLGN2 vector in the adult mouse hippocampus.
A, Top panels, representative Western blots showing NLGN2 protein in membrane fractions from hippocampal neurons transduced with the AAV-mCherry vector (mCh) or the AAV-NLGN2 vector (NL2). Beneath, the same blot probed for actin as a loading control. B, Box and whisker plot showing average optical density of membranes probed for NLGN2/Actin. Membrane fractions from hippocampus transduced with the AAV-NLGN2 vector contained significantly more NLGN2 protein compared to AAV-mCherry vector controls (***p=0.0002, Student’s t-test). C-J, The AAV vectors (red) transduced multiple neuronal cell types in the dentate gyrus (DG). C, AAV-transduced GABAergic interneurons (red) in VGAT-ChR2-eYFP (green) transgenic mouse brain showing co-labeling of GABAergic neurons in the hilus (HIL) and granule cell layer (GCL). D-F, Magnified views indicated in boxed region in C’. E, AAV-mCherry transduced GC receiving GABAergic synaptic contacts (green) and F, the merged image of this cell. G-I, Higher-magnification views indicated in boxed region in C”. G, AAV-transduced GABAergic intemeuron (red) and H, VGAT-ChR2-eYFP co-expression (green). I, the merged view of cell. J, AAV-NLGN2-vector transduced CA1 pyramidal neurons. Labeled neurons in the stratum pyramidale (PYR) with dendrites extending into stratum oriens (ORI). K-M, AAV-mCherry+ pyramidal cells (box) receiving VGAT-ChR2-eYFP labeled synaptic contacts. K, mCherry; L, eYFP, M, merged image. N-U, Triple-labeled CA3 pyramidal neuron with O, vector driven expression of mCherry (red). P, EYFP+GABAergic inputs on this neuron (green). Q, Punctate cell-surface immunostaining for Neuroligin2 (NLGN2, white). R, Triple-labeled neurons in a mouse transduced with the AAV-NLGN2 vector; CA1 pyramidal neurons (red) expressed high levels of NLGN2 (white), in association with GABAergic synapses (VGAT-ChR2-eYFP positive boutons; green). Boxed region magnified in S-U. S, mCherry-labeling; T, eYFP+ GABAergic terminals; U, NLGN2-immunoreactive puncta. C, J Scale bars equal 20 μm; N, R scale bars equal 10 μm.
To determine whether the AAV/DJ vector was transduced in both excitatory and inhibitory neurons, we injected the AAV-mCherry or the AAV-NLGN2 vectors into the hippocampus of transgenic mice expressing VGAT-ChR2-eYFP to identify GABAergic interneurons based on green fluorescence. As shown in Figure 1 C–I, GABAergic interneurons in the hilus and the molecular layer (MOL) of the DG were transduced by the vectors, as well granule cells in the DG granule cell layer (GCL), and spiny (putative) mossy cells in the hilus of DG (Figure 1, C–M) and CA1 pyramidal neurons, based on the location of the cell bodies, their morphology, and the presence of punctate GABAergic terminals covering the somas (Figure 1 J–M). Additionally, we found that neurons transduced with AAV-mCherry vector had lower immunoreactivity for NLGN2 (Figure 1N–Q) compared to neurons transduced with the AAV-NLGN2 vector (Figure 1 R–U). These experiments confirmed that the vectors transduced the principal cells of the hippocampus as well as hippocampal GABAergic interneurons. Moreover, AAV-NLGN2 vector transduction resulted in substantial increases in the expression of the NLGN2 protein in hippocampal neurons.
3.2. Increased gephyrin+ and VGAT+ puncta at putative GABAergic synaptic sites in neurons transduced with AA V-NLGN2
Prior work has established the parvalbumin-expressing (PV+) GABAergic interneurons form inhibitory synapses preferentially at perisynaptic sites containing NLGN2 homomeric dimers. This is in contrast to somatostatin-expressing (SOM+) intemeurons, which form NLGN2-NLGN3 heterodimers at synaptic contacts [47]. These prior findings suggested that neurons overexpressing NLGN2 might show increased PV+ GABAergic synapses. To investigate whether transduction with AAV-NLGN2 led to an increase in PV+ GABAergic synapses, we utilized immunostaining and high-resolution confocal microscopy. We addressed this question by quantifying putative synaptic boutons expressing PV+ that co-localized with postsynaptic gephyrin+ puncta in virally-transduced hippocampal neurons (Figure 2A–J). Overall, we found a 23% increase in PV+ synaptic endings aligned with gephyrin+ puncta in AAV-NLGN2+ pyramidal neurons, compared to neurons transduced with AAV-mCherry (Figure 2K; AAV-NLGN2+ neurons: 20.27 ± 1.22 PV+/gephyrin+ puncta/cell vs. AAV-mCherry+ neurons: 16.48 ± 1.20 puncta/cell, p=0.04, Student’s t-test). Similarly, by counting the number of PV+/gephyrin+ appositions per micron of labeled cell membrane; we found −23% more appositions per micron in AAV-NGLN2+neurons (0.49 appositions/μm) compared to AAV-mCherry+ neurons (0.40 appositions/μm; data not shown; p=0.01, Student’s t-test; n=31 cells from 4 AAV-mCherry+ control mice and 51 cells from 5 AAV-NGLN2+mice). However, the lengths of contacts between PV+ axons and AAV-NLGN2+ pyramidal neurons was not significantly different from controls (Figure 2L; p=0.26, Student’s t-test; n=27 cells from 4 AAV-mCherry+ mouse brains and n=33 cells from 5 AAV-NLGN2+ brains). These results suggest that transduction with the AAV-NLGN2 vector resulted in high expression of NLGN2 in hippocampal neurons and increased trafficking of inhibitory postsynaptic proteins to sites of parvalbumin+ GABAergic synapses, in agreement with previous in vitro studies [3, 15, 17].
Figure 2. Increased gephyrin+ synaptic puncta at parvalbumin+ synaptic endings in AAV-NLGN2-expresing neurons.
A, CA1 pyramidal neurons (red) transduced with control AAV that were contacted by inhibitory neurons co-expressing parvalbumin (PV+) (green) exhibited sparse gephyrin puncta (white). B-E, Higher magnified views of the mCherry+ neuron from A, with orthogonal views in the Z-plane. Crosshairs indicate a putative synaptic site where mCherry (B), PV (C), and gephyrin (D) all colocalize (E). F, CA1 pyramidal neurons (red) infected with experimental AAV-NLGN2 virus that were contacted by PV+ (green) inhibitory interneurons exhibited increased density of punctate gephyrin (white). G-J, Higher resolution view of an mCherry+ neuron from F, with orthogonal views in the Z-plane. Crosshairs indicate a putative synaptic site where mCherry (G), PV (H), and gephyrin (I) all colocalize in the same z-axis (J). K, Gephyrin puncta that co-localized with PV+ contacts to AAV-NLGN2-neurons were significantly increased (***p=0.01, Student’s t-test). L, The length of PV+ contacts onto AAV-labeled pyramidal cells was unaltered by AAV-NLGN2 (p=0.26, Student’s t-test). Scale bars equal 20 μm.
The finding that AAV-NLGN2 transduction led to a preponderance of PV+/gephyrin+ putative synapses led us to next ask whether AAV-NLGN2 led to a significant increase in GABAergic synapses. To address this, we identified putative GABAergic synapses onto virally transduced neurons. As shown in Figure 3 (A, B), AAV-NLGN2+ neuronal cell bodies and proximal dendrites (identified by mCherry expression) were outlined by VGAT+ GABAergic synaptic boutons (Figure 3C). Many of these putative presynaptic boutons co-localized with postsynaptic gephyrin+ puncta in neurons transduced with the AAV (Figure 3D, E, arrowheads). Based on counts of sites with co-alignment of VGAT+ boutons and gephyrin+ puncta (VGAT+/gephyrin+puncta, Figure 3F, arrows), AAV-NLGN2+ neurons showed significantly more co-localized puncta (21.769 ± 0.5829 in n=52 AAV-NLGN2+ neurons) as compared to controls (16.897 ± 0.5771 co-localized puncta in n=39 AAV-mCherry+ neurons) (p =0.0000001; Student’s t-test). To control for possible sampling biases, we also analyzed the density of co-labeled puncta per micron of cell surface and found that, on average, AAV-NLGN2+ neurons had 0.69 VGAT+ /gephyrin+ puncta /μm whereas AAV-mCherry+ neurons had approximately 0.58 VGAT+/gephyrin+ puncta/μm (Figure 3G; p=0.0003, Student’s t-test; n=39 neurons from 3 AAV-mCherry+ control mice and 52 neurons from 5 AAV-NLGN2+ mice). Taken together, these findings suggest that AAV-NLGN2 transduction substantially increased putative GABAergic synapses containing gephyrin, a key postsynaptic inhibitory protein responsible for anchoring GABA receptors and other molecules at the postsynaptic density.
Figure 3. AAV-NLGN2 expression increased gephyrin-immunoreactive puncta at putative GABAergic synapses.
A, AAV-NLGN2+ GCs (red) with presynaptic VGAT (green) and postsynaptic gephyrin (white) immunoreactivity. B-D, A representative GC (yellow dashed line, arrow) is shown with co-localization of immunoreactivity for mCherry (red), VGAT (green), and gephyrin (white) in X, Y, and Z-axes in confocal Z-stacks. E, Merged image of cell from B-D, F, Representative fluorescence histogram generated from the GC shown in B-E. For this cell, the VGAT immunofluorescence is represented by the green histogram, the gephyrin immunofluorescence is represented by the white histogram, and the dashed lines indicate baseline values. Arrows indicate sites along the membrane where peaks of fluorescence detected for presynaptic VGAT and postsynaptic gephyrin were above baseline levels and colocalized, indicating putative pre and postsynaptic sites. G, Quantification of putative pre- and postsynaptic sites using histogram analyses showed that AAV-NLGN2+ cells had significantly more apposed VGAT and gephyrin (*p=0.0003, Student’s t-test). H, AAV-NLGN2+ cells also had significantly also significantly more VGAT puncta per μm2 of mCherry (***Student’s t-test, p=0.0057). I, AAV-NLGN2+ cells had significantly more gephyrin puncta per μm2 of mCherry (***Student’s t-test, p=0.0195). A-D, E; Scale bars equal 20μm.
We next asked whether AAV-NLGN2 transduction altered the overall level of VGAT localized to putative GABAergic synaptic terminals, as increased VGAT could resulted in enhanced synaptic release of GABA from vesicular stores. To address this question, we analyzed larger regions of interest (ROI) within the hippocampal subfields, while controlling for variations in AAV transduction, using mCherry expression as a proxy (Figure 3A–B). We found an increase of ~39% in the number of VGAT+ puncta (17.26 puncta/μm2 mCherry expression ± 2.10) in the hippocampus of mice transduced with the AAV-NLGN2 vector, compared to mice injected with the control AAV-mCherry vector (6.69 puncta/μm2 mCherry expression ± 2.47, Figure 3L; p = 0.00057; Students t-test; n=8 GCL subfields from 3 AAV-mCherry+ mice and 8 GCL subfields from 5 AAV-NLGN2+ mice). Similarly, we found −25% increase in the overall density of gephyrin puncta (30.80 puncta/μm2 mCherry expression ± 8.15), compared to controls (7.76 puncta/μm2 mCherry expression ± 2.93, Figure 3M; p=0.01; Student’s t-test; n=8 GCL sections from 3 AAV-mCherry+ mice and 8 GCL sections from 5 AAV-NLGN2+ mice). Thus, transducing hippocampal neurons with the AAV-NLGN2 vector not only increased recruitment, retention or membrane trafficking of gephyrin but also had transynaptic effects by increasing VGAT.
3.3. AAV-NLGN2 transduction is linked to elevated levels of GABAergic synaptic proteins
Prior studies showed that a key role of NLGNs is to provide a linkage between the extracellular and intracellular proteins at synapses [8, 18, 48, 49] and our results support this view by showing that NLGN2 overexpression increased NLGN2, VGAT, and gephyrin at putative GABAergic synapses. As VGAT specifically localizes to GABAergic synaptic vesicles, and gephyrin localizes to the postsynaptic specialization at GABAergic synapses, our findings could indicate that AAV-NLGN2’s effects include increasing or enhancing efficacy of GABAergic synaptic transmission, however testing this hypothesis would require either quantitative immuno-electron microscopic studies or electrophysiology or both. As an alternative, we chose a biochemical approach to compare the extent of membrane recruitment of inhibitory synaptic proteins VGAT, gephyrin, and the GABAA receptor-specific GABAARγ2 subunit [50, 51] in mice transduced with either the AAV-NLGN2 vector or the AAV-mCherry vector (Figure 4A). AAV-NLGN2 transduction significantly increased hippocampal membrane-associated gephyrin by 1.3-fold (Figure 4B; p=0.0326, Student’s t-test; n=18 hippocampi from 9 AAV-mCherry+ mice and n=20 hippocampi from 10 AAV-NLGN2+ mice). Similarly, AAV-NLGN2 transduction significantly increased VGAT in membrane fractions ~1.5-fold increase (Figure 4C; p=0.0097, Student’s t-test; n=18 hippocampi from 9 AAV-mCherry+ mice and 20 hippocampi from 10 AAV-NLGN2+ mice). Moreover, overexpression of NLGN2 increased membrane levels of the GABA receptor subunit, GABAAγ2 by ~2.1-fold (Figure 4D; p=0.016, Student’s t-test; n= 8 hippocampi from 4 AAV-mCherry+ mice, 10 hippocampi from 7 AAV-NLGN2+ mice).
Figure 4. GABAergic synaptic proteins gephyrin, VGAT and GABAAγ2 were significantly increased after transduction of hippocampal neurons with AAV-NLGN2.
A, Western blots showing bands for gephyrin, VGAT, GABAAγ2, and actin in hippocampal membrane fractions from mice receiving stereotaxic injections of the AAV-NLGN2 or AAV-mCherry vectors into the hippocampus. B, Total membrane levels of gephyrin were significantly increased after transduction with the AAV-NLGN2 vector, compared to the AAV-mCherry vector (p=0.032, Student’s t-test). C, Total membrane levels of VGAT protein were significantly increased after transduction with the AAV-NLGN2 vector, compared to the AAV-mCherry vector (p=0.0097, Student’s t-test). D, Total membrane levels of GABAAγ2 were significantly increased after transduction with AAV-NLGN2, compared to the AAV-mCherry vector (p=0.016, Student’s t-test).
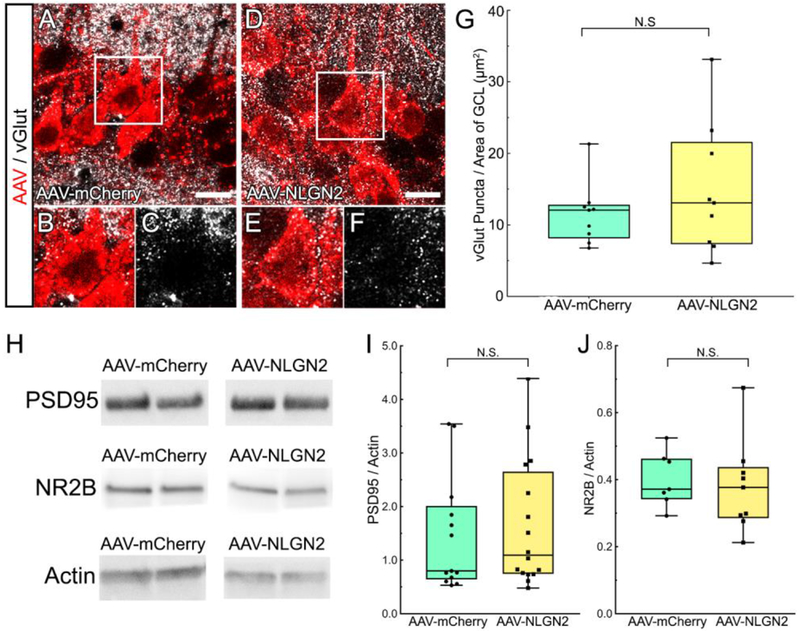
3.4. AAV-NLGN2 vector transduction did not increase excitatory synaptic proteins
Our results showing that AAV-NLGN2 transduction increased levels of inhibitory synaptic proteins in hippocampal synaptic membranes, led us to ask whether these effects were specific for proteins normally trafficked to GABAergic synapses. To address this, we assayed whether AAV-NLGN2 transduction increased excitatory synapse-specific proteins at putative synaptic sites in neuronal membranes [13, 52–55]. As shown in Figure 5 (A–F) vesicular glutamate transporter (vGlut) immunoreactive puncta were localized on the surfaces of apical dendrites of pyramidal neurons and on somas. When we quantified excitatory synapses onto pyramidal neurons and dentate GCs, we found that the density of vGlut+ puncta did not differ between neurons transduced with AAV-NLGN2 or AAV-mCherry (Figure 5G; 15.81 puncta/μm mCherry expression ± 3.26 in n=5 AAV-NLGN2+ mice vs. 13.09 puncta/pm mCherry expression ± 2.34 n=3 AAV-mCherry+ control mice; Students t-test, p=0.56).
Figure 5. Excitatory synaptic proteins vGlut, PSD-95, and NR2B were not altered after transduction of hippocampal neurons with AAV-NLGN2.
A, Representative confocal photomicrographs showing putative sites of synaptic contacts indicated by vGlut+ puncta (white) on AAV-mCherry+ pyramidal neurons (red). B, C, Magnified views of selected cells. D, Confocal images of vGlut+ puncta (white) apposed to AAV-NLGN2+ pyramidal neurons (red). E, F, Magnified views of selected cells. G, Quantification for vGlut+ puncta/μm2 of mCherry immunofluorescence showing that vGlut+ puncta were not increased in neurons transduced with the AAV-NLGN2 vector, compared to AAV-mCherry vector (p=0.5631, Student’s t-test). H, Representative Western blots comparing PSD95 and NR2B protein bands in hippocampal membrane fractions after transduction with the AAV-NLGN2 or the AAV-mCherry vectors. I, Quantification of protein bands revealed that PSD95 was not significantly different in the tissue transduced with the two different vectors (p=0.6622, Student’s t-test). J, Membrane levels of NR2B were also comparable in hippocampal tissue transduced with the AAV-NLGN2 or AAV-mCherry vectors (p=0.7113, Student’s t-test). A, D, Scale bars equal 20 microns.
To further extend these immunohistochemical data, we next used biochemical approaches to examine levels of the excitatory postsynaptic density protein 95 (PSD95) and N-Methyl-D-aspartate receptor (NMDA) subunit NR2B protein in membrane fractions from the hippocampus of mice receiving AAV-NLGN2 vector vs. the control vector (Figure 5H). Neither the level of PSD95 (Figure 5I; p=0.33, Student’s t-test; n=16 hippocampi from AAV-NLGN2+ mice vs. n=13 hippocampi from AAV-mCherry+ control mice) nor NR2B differed (Figure 5J; p=0.35, Student’s t-test; n=9 hippocampi from AAV-NLGN2+ mice vs. n=7 hippocampi from AAV-mCherry+ control mice). These results suggest that AAV-NLGN2 vector transduction of hippocampal neurons selectively increased GABAergic synaptic proteins, but not excitatory synaptic proteins.
3.5. NLGN2 overexpression in the hippocampus is associated with altered social dominance and social preference
Our finding that NLGN2 increased putative GABAergic synapses led us to examine the effects on behavior. We first tested for social dominance and compared wins and losses of experimental mice receiving hippocampal injections of the AAV-NLGN2 vector when they were matched against control mice receiving hippocampal injections of the AAV-mCherry vector (Figure 6A). Our initial analysis, using Fisher’s exact test, indicated that group scores were significantly different than what would be expected by chance (p=0.02). Next, we constructed a 95% confidence interval to analyze score distributions in bouts between groups. AAV-NLGN2+ mice had an upper confidence bound of 0.4881, which skewed their group results significantly below the 0.5 chance score. Comparatively, control mice with AAV-mCherry vector transduction showed a lower confidence interval of 0.6, indicating a win value of greater than chance (1-sample t-interval test). Direct comparisons of wins/bouts between groups showed that mice with AAV-NLGN2 transduction had significantly lower dominance ratios (Figure 6B; p<0.01, Student’s t-test; n=18 AAV-mCherry+ and 24 AAV-NLGN2+ mice).
Figure 6. Altered social behavior but not anxiety following neuronal transduction with the AAV-NLGN2 vector.
A, Schematic of the tube test apparatus for investigating social dominance. B, Mice transduced with the AAV-NLGN2 vector showed reduced dominant aggressive behavior compared to mice transduced with the AAV-mCherry vector (***p<0.01, ANOVA). C, Schematic of the three-chambered sociability test apparatus. D, Mice transduced with the AAV-NLGN2 vector showed reduced preference for novel interactions (discrimination index), compared to controls (p=0.0201, Student’s t-test). E, Schematic of the elevated zero maze apparatus. F, Mice transduced with the AAV-NLGN2 vector showed anxiety levels comparable to AAV-mCherry transduced mice, based on time spent in the open arms of the zero maze (p=0.8420, Student’s t-test).
We next compared social preference and social memory for conspecific mice with the three-chambered sociability test (Figure 6C). In the test of preference for a novel object versus a novel mouse, both AAV-NLGN2+ and AAV-mCherry+ animals showed a preference for social interaction, with no significant differences between groups (data not shown; p=0.84, Student’s t-test). When comparing preference for social novelty, as expected, control mice exhibited normal novelty preference and spent significantly more time with a novel mouse than the familiar one (p<0.01, Student’s t-test; n=20). In contrast, AAV-NLGN2+ mice did not spend more time with the novel vs. familiar mouse (p=0.25, Student’s t-test; n=24). Similarly, the novelty discrimination index was significantly greater for control vs. AAV-NLGN2+ mice (discrimination index = time with novel mouse – time with familiar mouse/total interaction time) (Figure 6D; p=0.03, Student’s t-test).
3.6. NLGN2 overexpression in the hippocampus does not alter anxiety
Given that the hippocampus and amygdala are highly interconnected, we reasoned that hippocampal NLGN2 overexpression might impact anxiety-like behaviors, as assessed in the Open Field test and elevated Zero Maze (Figure 6E). In the Open Field, both AAV-NLGN2+ and AAV-mCherry+ mice did not differ significantly in time spent in the center of the arena (182.34 ± 12.02s in n=16 AAV-mCherry+ controls vs. 182.32 ± 14.26s in n=17 AAV-NLGN2+; p=0.99, Student’s t-test). Distance travelled was also equivalent (65.82 ± 3.50m in AAV-mCherry+ mice, 62.83 ± 2.97 in AAV-NLGN2+ mice; p=0.52, Student’s t-test). We also found no significant differences in the time spent in the open arms of the Zero Maze (Figure 6F; 39.96 ± 7.5s in n=13 AAV-mCherry+ controls vs. 37.96 ± 6.51s in n=17 AAV-NLGN2+ mice; p=0.84, Student’s t-test). Furthermore, the experimental and control animals travelled the same distance; AAV-mCherry+ mice travelled on average 10.34 ± 1.16m vs. AAV-NLGN2+ mice which travelled 9.05 ± 0.82m, suggesting equivalent motor activity levels (p=0.36, Student’s t-test). Therefore, our data suggest that mice with hippocampal transduction of AAV-NLGN2 do not show increased anxiety, compared to AAV-mCherry+ controls.
3.7. NLGN2 overexpression correlates with disruptions in hippocampal-dependent spatial memory
GABAergic inhibitory interneurons fine tune spatial memory in the hippocampus and entorhinal cortex [31,35, 56, 57]. An altered excitatory/inhibitory balance in the hippocampus could disrupt the ability to form normal place fields. To determine whether AAV-NLGN2 induced enhancement of hippocampal GABAergic synapses correlated with altered spatial memory performance, we evaluated memory using the novel object location test and the Morris water maze. In the novel object location test, a mouse must recognize that one of two previously known objects has been displaced from its original location (Figure 7A). On average, controls spent 33.4 seconds longer with the novel-located object, whereas AAV-NLGN2+ animals only spent 4.8 seconds longer with the novel object. When expressed as a discrimination index for novelty, mCherry controls showed a significantly higher discrimination index for novelty compared to AAV-NLGN2+ mice (Figure 7C; p<0.01, Student’s t-test; n=13 AAV-mCherry+ control mice vs. n=13 AAV-NLGN2+ mice).
Figure 7. Impaired performance in spatial memory tasks but not in tasks that did not depend upon the hippocampus following neuronal transduction with the AAV-NLGN2 vector.
A, Schematic of the apparatus used for testing recall of a novel object’s location. B, Mice transduced with the AAV-NLGN2 vector showed reduced discrimination between objects in the same vs. novel locations, compared to mice that were transduced with the AAV-mCherry vector (p=0.0358, Student’s t-test). C, Schematic of the apparatus for testing hippocampal-independent novel object recognition. D, Mice showed a similar preference for the new object, indicating that they were able to recall the previously explored object (p=0.9231, Student’s t-test). E, Schematic and timeline for the Morris water maze test of spatial memory. F, Mice transduced with the AAV-NLGN2 or AAV-mCherry vectors performed similarly during cued learning, but the mice transduced with the AAV-NLGN2 vector showed significant impairments in learning the spatial navigation task during days 3–5, after the platform cue was removed (p=0.043, Two-way repeated measures ANOVA). G, During probe trials, AAV-NLGN2 transduced mice showed a trend toward longer latencies to initially crossing the platform, compared to AAV-mCherry controls (p=0.0815, Student’s t-test). H, Mice transduced with the AAV-NLGN2 vector crossed the platform area significantly fewer times than mice transduced with the AAV-mCherry vector (p=0.0220, Student’s t-test).
To confirm that the cognitive deficits were not due to impaired vision or novelty preferences, we compared performance in the novel object recognition test. This task requires that the mice distinguish between a familiar and unfamiliar object after an hour of memory consolidation. We found that both AAV-NLGN2+ and control animals showed a significantly greater preference for the novel object compared to the familiar one (Figure 7B; p<0.01 AAV-NLGN2+ mice vs. p<0.05 AAV-mCherry+ control mice; Student’s t-test) with similar discrimination indices for novelty (Figure 7D; p>0.05, Student’s t-test; n=13 AAV-NLGN2+ mice and n=13 AAV-mCherry+ control mice). Taken together, these findings suggest that transducing hippocampal neurons with the AAV-NLGN2 vector resulted in mnemonic deficits.
To further examine hippocampal-dependent spatial memory deficits, we tested these two groups of mice in the Morris water maze (Figure 7E). Control mice showed normal, average escape latencies under 15 seconds for all three days of uncued learning, while the AAV-NLGN2+ mice were significantly worse (Figure 7F; p<0.05, two-way repeated measures ANOVA). In the probe trial, AAV-NLGN2+ mice showed a non-significant trend towards longer latencies for crossing the platform area (Figure 7G; p=0.08, Student’s t-test) and crossed through the platform area significantly fewer times, compared to controls (Figure 8H p=0.02, Student’s t-test; n=12 AAV-mCherry+ control mice vs. 16 AAV-NLGN2+ mice). However, quadrant dwell time in the probe trial was unaffected by NLGN2 overexpression, with both groups spending significantly more time in the target quadrant compared to the opposite quadrant (p>0.05, ANOVA). Deficits in learning could not be correlated with motor or visual impairments, as both groups performed identically when they were tested in cued learning trials with a visible, marked platform (Figure 7F). Taken together, these results suggest that AAV-NLGN2+ mice showed significant cognitive impairments in hippocampal-dependent tasks, including social dominance, social memory, and some measures of performance in spatial memory, while performing normally on tests of anxiety, motor behavior, and visually cued learning.
4. Discussion
Previous in vitro studies showed that NLGN2 recruits synaptic proteins to synaptic locations [15, 17, 58] in accordance with in vivo studies suggesting that NLGN2 is critical in assembling components of GABAergic synapses [3, 8, 18, 59, 60]. Studies in adult mice examining the effects of altered NLGN2 expression on behavior have given conflicting results. However, mutations in synaptic adhesion molecules in human patients, including the NLGN family, are commonly associated with neuropsychiatric disorders [61–63]. Mutations in NLGN3 and NLGN4 are well characterized in autism patients [10, 64] and recent case studies in schizophrenia and autism patients have linked missense mutations NLGN2 to disease phenotypes [9, 12]. While prior studies investigated mRNA expression followed experimentally increased NLGN2 [25], our work is the first to examine whether NLGN2 overexpression resulted in increased localization of GABAergic synaptic proteins at inhibitory synapses. We utilized viral-mediated gene delivery to overexpress NLGN2 in the adult mouse dentate gyrus and CA1-CA3 regions of the hippocampus and quantified the number of PV+ GABAergic synaptic endings apposed to gephyrin puncta in neurons expressing NLGN2 by confocal microscopy.
Our results show that AAV-NLGN2 transduction in adult hippocampal neurons increased putative GABAergic synapses in the adult mouse dentate gyrus and CA1. NLGN2 overexpression resulted in a significant increase at putative GABAergic terminals in VGAT and gephyrin, a molecule localized to the postsynaptic scaffold at GABAergic synapses. Both VGAT, the presynaptic vesicular transporter that packages GABA [65] and gephyrin, a protein essential to postsynaptic GABAAR clustering [50, 66, 67] are necessary components of the GABAergic synapse, and their appositions are most likely found at GABAergic synapses, although definitive proof would require additional electron microscopic studies. Our results do suggest however that AAV-NLGN2 may increase inhibitory neurotransmission in the adult hippocampus by recruiting molecules involved in neurotransmitter uptake in presynaptic vesicles and GABAergic receptor trafficking.
Our results expand upon a previous biochemical study that examined genetic overexpression of NLGN2 in mice; this prior study demonstrated increased VGAT, syntaxin, and NLGN3, but they did not examine different brain regions nor did they analyze neuronal membrane fractions, where synaptic proteins are localized [21]. Our Western blot analyses of gephyrin, VGAT, and GABAARγ2, the gephyrin-associated GABAA subunit [51, 68, 69], demonstrated that mice receiving the AAV-NLGN2 vector showed significant increases in GABAergic synapse-specific molecules in hippocampal membrane fractions, compared to controls. The accumulation of GABAARγ2 with AAV-NLGN2 overexpression may be due to increased GABAA receptor trafficking to synaptic sites and/or reduced endocytosis and these possibilities should be investigated in future studies. We did not find an effect of AAV-NLGN2 on excitatory synaptic proteins, suggesting that NLGN2 overexpression-induced changes are specific to GABAergic synapses. Thus, at least after 3–4 weeks, AAV-NLGN transduction increased recruitment or expression of GABAergic synaptic molecules, without concomitant changes in excitatory synaptic proteins.
Our results showing an increase in both structural and functional components of GABAergic synapses with NLGN2 overexpression concur with a previous genetic knockout study demonstrating that NLGN2 deficiency reduced GABAARγ2 and gephyrin clustering and also increased neuronal excitation [70]. Thus, NLGN2 overexpression might increase inhibitory postsynaptic currents in mature neurons or the quantal release of GABA, and it would be of interest to directly test this in future studies.
An important area for future work would be to determine how NLGN2 overexpression alters the functional properties of hippocampal circuits. Our AAV/DJ vector transduced excitatory granule cells, pyramidal cells, putative hilar mossy cells, and inhibitory GABAergic interneurons. Further electrophysiological studies are required to dissect apart the effects of altering GABAergic neurotransmission onto different hippocampal cell types. This brain regions contains multiple subtypes of interneurons, including some that specifically target and inhibit other GABAergic interneurons, causing disinhibition [71–73]. NLGN2 overexpression in these cells could lead to disinhibition which might cause cognitive deficits and altered social behavior. Alternatively, NLGN2 overexpression in interneurons could enhance excitation, as suggested by a recent study of the long range GABAergic projections from the parvalbumin+ interneurons in the entorhinal cortex, which are regulated by NLGN2 homomeric dimers [47] and serve an excitatory rather than inhibitory role in the hippocampus [74]. Therefore, limiting NLGN2 overexpression to subsets of GABAergic or glutamatergic neuron populations using an intersectional approach could provide additional insights into NLGN2’s role in regulating different types of GABAergic synapses and the effects on behavior and cognition.
Considering the role of GABAergic interneurons in hippocampal-dependent behaviors, increased inhibitory synaptic transmission in the hippocampus in animal models might be predicted to alter social behavior, social dominance, aggression, anxiety, and/or learning and memory. Considerable interest in this topic is supported by studies that have linked the development of autism, comorbid with social deficits, to deficits in members of the NLGN family, particularly NLGN2–4 [9, 10, 14, 61, 62, 64, 75]. Regarding hippocampal-dependent social behaviors, projections between the hippocampus and the amygdala coordinate the formation of emotional contexts necessary for social memory [76], while social behavior and aggression are regulated by multiple regions in the hippocampus, including the dentate, CA1, CA2, and CA3 [29, 30, 77–83]. Several previous studies examined the effects of NLGN2 overexpression in the hippocampus on novelty preference, anxiety, social behavior, and aggression in both rats and mice, but the results were contradictory [25, 27].
To study the effects of overexpressing NLGN2 on social interactions, we used Crawley’s three-chambered sociability test and found deficits in preference for social novelty. Our results in mice NLGN2 overexpression throughout the hippocampus are in agreement with a previous study in mice [27]. However a second study from this group performed in rats with NLGN2 overexpression limited to the dorsal hippocampus failed to show a deficit in preference for social novelty, emphasizing the importance of ventral hippocampal circuits for regulating social behaviors in mice [25].
Conflicting results have also been obtained regarding NLGN2’s effect on social dominance or aggressive behavior [25, 27, 84]. As prior work had examined aggression in the resident intruder test [30, 32], we examined the formation of dominance hierarchies with the tube test of social dominance. This quick, simple and common assay for assessing social dominance behavior in mice has been used extensively for over 50 years [45]. Abnormal tube test dominance behavior is typically observed in mice that display other deficits in social interactions. Importantly, new findings in the present study is that mice with NLGN2 overexpression in the hippocampus show significant changes in social interactions and dominance behavior; mice with AAV-NLGN2 overexpression exhibited reduced dominance in the tube test when matched against the controls. Although they were significantly less likely to push a control mouse out of the tube, they showed normal performance within their treatment group.
In contrast to the effects of overexpressing NLGN2 on social behaviors, we did not find alterations in anxiety-related behaviors, as assessed with the Zero maze or the Open Field test, in agreement with a prior study in which NLGN2 was overexpressed in the rat dorsal hippocampus followed by testing for anxiety in the elevated plus maze [25]. The elevated Zero Maze appears to be more sensitive to changes in GABAergic neurotransmission than the elevated plus maze and studies have reported that performance in the elevated zero maze is relatively stable over several trials whereas mice adapt rapidly to the elevated plus maze over time [41]. Our results are also consistent with prior studies in mice that examined the effects of genetically-induced NLGN2 deficiency and showed that this manipulation increased anxiety-like behaviors, not only in the elevated plus maze, but also in the light-dark box and open field test [28, 29]. Taken together these findings suggest that while NLGN2 may be necessary to establish inhibitory circuits involved in anxiety-related behaviors, increasing NLGN2 above normal levels in the adult brain may not have a measurable effect on anxiety.
Based on a prior study showing deficits in contextual fear memory acquisition following conditional knockout of NLGN2 in the rodent prefrontal cortex [85, 86], and work showing deficits in spatial memory in mice expressing the human R215H loss of function mutation in NLGN2 [87], we predicted that AAV-NLGN2-mediated overexpression would significantly alter memory formation. As object investigation in the open field had been studied following NLGN2 overexpression [25], we chose to focus on acquisition of spatial memory in two different paradigms - Morris Water Maze and the novel object location test, which requires that the mouse distinguish between an object in a familiar location and that same object after it is moved to a new location [88–90]. When compared with controls, the mice with NLGN2 overexpression showed impairments in their ability to discriminated between objects in familiar vs. new locations. To eliminate visual/motor deficits or loss of novelty preference as possible explanations for the apparent spatial memory deficits, we also tested whether these mice were impaired in an object-based novelty task involving novel object recognition. When spatial cues are relevant for identifying novel objects, this task requires normal hippocampal function [89, 91, 92], although hippocampal involvement in this task may be more related to long-term memory, rather than the type of short-term memory that we tested [90, 93–97]. Although both the control and experimental groups of mice showed equivalent performance in the test for object novelty, the experimental mice that received the NLGN2 vector were impaired in their performance on tasks involving object-location spatial memory. Furthermore, these mice showed spatial memory deficits when compare to controls in the Morris water maze. On the first two days of training, when the escape platform location was visible using a local cue, both groups performed equivalently, demonstrating equivalent abilities to locate and escape out of the water onto the platform. However, removal of the local cue on the platform on day 3, forced the mice to navigate using memory and distal visual cues. With this more difficult spatial navigation task, the mice that received the AAV-NLGN2 vector were significantly worse than controls, suggesting that overexpression of NGLN2 interferes with spatial memory acquisition (Figure 8F). Interestingly, these deficits in the probe trial were modest; while the mice with AAV-NLGN2 crossed the platform area significantly fewer times and showed a trend towards higher latencies to first platform crossing, they did not exhibit significant deficits in the time spent in the correct quadrant. Thus, overexpression of AAV-NLGN2 may impair the acquisition of object-based spatial memories, rather than the recall of those memories. One possible explanation for our findings is that increased expression of NLGN2 may alter the receptive field properties of hippocampal place cells, which form a finely tuned “mental map” composed of discrete receptive fields that activate and deactivate as an animal moves through space [98–104]. Given the evidence that GABAergic neurons in the hippocampus and entorhinal cortex play key roles in defining hippocampal place cell receptive field properties [105–107] and regulating theta rhythms [37, 107, 108], future experiments could investigate whether NLGN2 overexpression in the hippocampus selectively disrupts the receptive field properties or firing rates of place cells during spatial memory acquisition tasks, while leaving other types of object recognition memory intact [109–114].
Conclusions
This study showed that NLGN2 overexpression may induce de novo synaptic changes in the adult mouse hippocampus, which functionally impact hippocampal-dependent behaviors. Our studies showed that hippocampal transduction of AAV-NLGN2 increased localization of inhibitory synaptic proteins to putative pre and postsynaptic sites. These findings correlated with deficits in hippocampal-dependent behavior and memory formation.
Supplementary Material
Acknowledgements
We would like to thank Jyoti Gupta, Muhammad Nauman Arshad, and Swecchya Shrestha for their insightful comments on this project and technical help. We also wish to thank Dan Lawrence, Luis Gonzalez, Kevin Cobbol, and Jaye Jeong for help with data analyses, and Wesleyan University Quantitative Analysis Center for assistance with statistical analyses.
Funding
This work was supported by grants from CURE (JRN), NINDS 1R15NS072879 (JRN), the CT Regenerative Medicine Fund (JRN), and pilot grant funding from Wesleyan University.
Abbreviations:
- CA3
Cornu Ammonis 3
- CA1
Cornu Ammonis 1
- NLGN2
Neuroligin2
- GABA
Gamma-Aminobutyric Acid
- VGAT
vesicular GABA transporter
- GCL
granule cell layer
- MOL
molecular layer
- DG
dentate gyrus
- AAV
adeno-associated virus
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Varoqueaux F, Jamain S, Brose N, Neuroligin 2 is exclusively localized to inhibitory synapses, European journal of cell biology 83(9) (2004) 449–456. [DOI] [PubMed] [Google Scholar]
- [2].Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Südhof TC, Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2, Neuron 54(6) (2007) 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F, Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin, Neuron 63(5) (2009) 628–642. [DOI] [PubMed] [Google Scholar]
- [4].Levinson JN, Li R, Kang R, Moukhles H, El-Husseini A, Bamji SX, Postsynaptic scaffolding molecules modulate the localization of neuroligins, Neuroscience 165(3) (2010) 782–793. [DOI] [PubMed] [Google Scholar]
- [5].Siddiqui TJ, Craig AM, Synaptic organizing complexes, Current opinion in neurobiology 21(1)(2011) 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nguyen T, Südhof TC, Binding properties of neuroligin 1 and neurexin 1 reveal function as heterophilic cell adhesion molecules, Journal of Biological Chemistry 272(41) (1997) 26032–26039. [DOI] [PubMed] [Google Scholar]
- [7].Lisé MF, El-Husseini A, The neuroligin and neurexin families: from structure to function at the synapse, Cellular and Molecular Life Sciences 63(16) (2006) 1833–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Futai K, Doty CD, Baek B, Ryu J, Sheng M, Specific trans-synaptic interaction with inhibitory interneuronal neurexin underlies differential ability of neuroligins to induce functional inhibitory synapses, The Journal of neuroscience : the official journal of the Society for Neuroscience 33(8) (2013) 3612–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parente DJ, Garriga C, Baskin B, Douglas G, Cho MT, Araujo GC, Shinawi M, Neuroligin 2 nonsense variant associated with anxiety, autism, intellectual disability, hyperphagia, and obesity, American Journal of Medical Genetics Part A 173(1) (2017) 213–216. [DOI] [PubMed] [Google Scholar]
- [10].Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg CI, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T, Gillberg C, Nydén A, Söderström H, Philippe A, Cohen D, Chabane N, Mouren-Siméoni M-C, Brice A, Sponheim E, Spurkland I, Skjeldal OH, Coleman M, Pearl PL, Cohen IL, Tsiouris J, Zappella M, Menchetti G, Pompella A, Aschauer H, Maldergem L, Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism, Nature Genetics 34(1) (2003) 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig A, Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development, The Journal of Cell Biology 200(3) (2013) 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun C, Cheng M-C, Qin R, Liao D-L, Chen T-T, Koong F-J, Chen G, Chen C-H, Identification and functional characterization of rare mutations of the neuroligin-2 gene (NLGN2) associated with schizophrenia, Human Molecular Genetics 20(15) (2011) 3042–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maćkowiak M, Mordalska P, Wędzony K, Neuroligins, synapse balance and neuropsychiatric disorders, Pharmacological Reports 66(5) (2014) 830–835. [DOI] [PubMed] [Google Scholar]
- [14].Südhof TC, Neuroligins and neurexins link synaptic function to cognitive disease, Nature 455(7215) (2008) 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scheiffele P, Fan J, Choih J, Fetter R, Serafini T, Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons, Cell 101(6) (2000) 657–669. [DOI] [PubMed] [Google Scholar]
- [16].Graf ER, Zhang X, Jin S-XX, Linhoff MW, Craig AM, Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins, Cell 119(7) (2004) 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dong N, Qi J, Chen G, Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors, Molecular and Cellular Neuroscience 35(1) (2007) 14–23. [DOI] [PubMed] [Google Scholar]
- [18].Li J, Han W, Pelkey KA, Duan J, Mao X, Wang Y-XX, Craig MT, Dong L, Petralia RS, McBain CJ, Lu W, Molecular Dissection of Neuroligin 2 and Slitrk3 Reveals an Essential Framework for GABAergic Synapse Development, Neuron 96(4) (2017) 808–995621376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N, Neuroligins determine synapse maturation and function, Neuron 51(6) (2006) 741–754. [DOI] [PubMed] [Google Scholar]
- [20].Fu Z, Vicini S, Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells, Molecular and Cellular Neuroscience 42(1) (2009) 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, Singaraja RR, Cao X, Sammler E, Hormuzdi SG, Zhuo M, El-Husseini A, Synaptic Imbalance, Stereotypies, and Impaired Social Interactions in Mice with Altered Neuroligin 2 Expression, The Journal of Neuroscience 28(24) (2008) 6055–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dachtler J, Glasper J, Cohen RN, Ivorra JL, Swiffen DJ, Jackson AJ, Harte MK, Rodgers RJ, Clapcote SJ, Deletion of α-neurexin II results in autism-related behaviors in mice, Translational psychiatry 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Südhof TC, Powell CM, Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2, Genes, brain, and behavior 8(1) (2009) 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Babaev O, Botta P, Meyer E, Müller C, Ehrenreich H, Brose N, Lüthi A, Krueger-Burg D, Neuroligin 2 deletion alters inhibitory synapse function and anxiety-associated neuronal activation in the amygdala, Neuropharmacology 100 (2016) 56–65. [DOI] [PubMed] [Google Scholar]
- [25].Kohl C, Riccio O, Grosse J, Zanoletti O, Fournier C, Schmidt MV, Sandi C, Hippocampal Neuroligin-2 Overexpression Leads to Reduced Aggression and Inhibited Novelty Reactivity in Rats, PLoS ONE 8(2) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, Singaraja RR, Cao X, Sammler E, Hormuzdi SG, Zhuo M, El-Husseini A, Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(24) (2008) 6055–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kohl C, Wang X-DD, Grosse J, Fournier C, Harbich D, Westerholz S, Li J-TT, Bacq A, Sippel C, Hausch F, Sandi C, Schmidt MV, Hippocampal neuroligin-2 links early-life stress with impaired social recognition and increased aggression in adult mice, Psychoneuroendocrinology 55 (2015) 128–143. [DOI] [PubMed] [Google Scholar]
- [28].Danglot L, Triller A, Marty S, The development of hippocampal interneurons in rodents, Hippocampus 16(12) (2006) 1032–1060. [DOI] [PubMed] [Google Scholar]
- [29].Hitti FL, Siegelbaum SA, The hippocampal CA2 region is essential for social memory, Nature 508(7494) (2014) 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Leroy F, Brann DH, Meira T, Siegelbaum SA, Input-Timing-Dependent Plasticity in the Hippocampal CA2 Region and Its Potential Role in Social Memory, Neuron 95(5) (2017) 1089–110200000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, Yoon S, Bien-Ly N, Ring K, Zwilling D, Potter GB, Rubenstein JLR, Kreitzer AC, Huang Y, Hilar GABAergic Interneuron Activity Controls Spatial Learning and Memory Retrieval, PLoS ONE 7(7) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Paulsen O, Moser E, A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity, Trends in Neurosciences 21(7) (1998) 273–278. [DOI] [PubMed] [Google Scholar]
- [33].Borhegyi Z, Varga V, Szilágyi N, Fabo D, Freund TF, Phase Segregation of Medial Septal GABAergic Neurons during Hippocampal Theta Activity, The Journal of Neuroscience 24(39) (2004) 8470–8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chauvière L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C, Early Deficits in Spatial Memory and Theta Rhythm in Experimental Temporal Lobe Epilepsy, The Journal of Neuroscience 29(17) (2009) 5402–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cutsuridis V, Hasselmo M, GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations, Hippocampus 22(7) (2012) 1597–1621. [DOI] [PubMed] [Google Scholar]
- [36].Gan J, Weng S.-m., Pernía-Andrade AJ, Csicsvari J, Jonas P, Phase-Locked Inhibition, but Not Excitation, Underlies Hippocampal Ripple Oscillations in Awake Mice In Vivo, Neuron 93(2) (2017) 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Richard GR, Titiz A, Tyler A, Holmes GL, Scott RC, Lenck-Santini PP, Speed modulation of hippocampal theta frequency correlates with spatial memory performance, Hippocampus 23(12) (2013) 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Amilhon B, Huh C, Manseau F, Ducharme G, Nichol H, Adamantidis A, Williams S, Parvalbumin Interneurons of Hippocampus Tune Population Activity at Theta Frequency, Neuron 86(5) (2015) 1277–1289. [DOI] [PubMed] [Google Scholar]
- [39].Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G, Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function, Nature methods 8(9) (2011) 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huang Y, Yang S, Hu Z-Y, Lin G, Zhou W-X, Zhang Y-X, A new approach to location of the dentate gyrus and perforant path in rats/mice by landmarks on the skull, Acta Neurobiologiae Experimentalis 72 (2012). [DOI] [PubMed] [Google Scholar]
- [41].Tucker LB, McCabe JT, Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze, Frontiers in Behavioral Neuroscience 11 (2017) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Silverman JL, Yang M, Lord C, Crawley JN, Behavioural phenotyping assays for mouse models of autism, Nature Reviews Neuroscience 11(7) (2010) 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kaidanovich-Beilin O, Lipina I, Vukobradovic I, Roder J, Woodgett JR, Assessment of Social Interaction Behaviors, Journal of Visualized Experiments : JoVE (48) (2011) 2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN, Sociability and preference for social novelty in five inbred strains: an approach to assess autistic- like behavior in mice, Genes, Brain and Behavior 3(5) (2004) 287–302. [DOI] [PubMed] [Google Scholar]
- [45].Lindzey G, Winston H, Manosevitz M, Social Dominance in Inbred Mouse Strains, Nature 191(4787) (1961) 474–476. [DOI] [PubMed] [Google Scholar]
- [46].Ippolito DM, Eroglu C, Quantifying Synapses: an Immunocytochemistry-based Assay to Quantify Synapse Number, Journal of Visualized Experiments (45) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Horn ME, Nicoll RA, Somatostatin and parvalbumin inhibitory synapses onto hippocampal pyramidal neurons are regulated by distinct mechanisms, Proceedings of the National Academy of Sciences 115(3) (2018) 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gangwar S, Zhong X, Seshadrinathan S, Chen H, Machius M, Rudenko G, Molecular Mechanism of MDGA1: Regulation of Neuroligin 2:Neurexin Trans-synaptic Bridges, Neuron 94(6)(2017) 1132–11410000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hu X, Luo J.-h., Xu J, The Interplay between Synaptic Activity and Neuroligin Function in the CNS, BioMed Research International 2015 (2015) 498957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B, Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin, Nature neuroscience 1(7) (1998) 563–571. [DOI] [PubMed] [Google Scholar]
- [51].Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, Bias AL, Luscher B, Chen G, γ-Aminobutyric Acid Type A (GABAA) Receptor α Subunits Play a Direct Role in Synaptic Versus Extrasynaptic Targeting, Journal of Biological Chemistry 287(33) (2012) 27417–27430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt K-FF, Sassoè-Pognetto M, Löwel S, Moser T, Taschenberger H, Brose N, Varoqueaux F, Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina, Proceedings of the National Academy of Sciences of the United States of America 108(7) (2011) 3053–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Polepalli JS, Wu H, Goswami D, Halpern CH, Südhof TC, Malenka RC, Modulation of excitation on parvalbumin interneurons by neuroligin-3 regulates the hippocampal network, Nature Neuroscience 20(2) (2017) 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bemben MA, Shipman SL, Nicoll RA, Roche KW, The cellular and molecular landscape of neuroligins, Trends in Neurosciences 38(8) (2015) 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chanda S, Hale WD, Zhang B, Wernig M, Südhof TC, Unique vs. Redundant Functions of Neuroligin Genes in Shaping Excitatory and Inhibitory Synapse Properties, The Journal of neuroscience : the official journal of the Society for Neuroscience (2017) 125–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Miao C, Cao Q, Moser M-B, Moser EI, Parvalbumin and Somatostatin Interneurons Control Different Space-Coding Networks in the Medial Entorhinal Cortex, Cell 171(3) (2017) 507–1574567936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Murray AJ, Sauer J-F, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P, Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory, Nature Neuroscience 14(3) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fuchs C, Abitbol K, Burden JJ, Mercer A, Brown L, Iball J, Stephenson AF, Thomson AM, Jovanovic JN, GABAA receptors can initiate the formation of functional inhibitory GABAergic synapses, European Journal of Neuroscience 38(8) (2013) 3146–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Panzanelli P, Fruh S, Fritschy J-M, Differential role of GABAA receptors and neuroligin 2 for perisomatic GABAergic synapse formation in the hippocampus, Brain Structure and Function (2017) 1–13. [DOI] [PubMed] [Google Scholar]
- [60].Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, Strata P, Varoqueaux F, Brose N, Fritschy J-M, Sassoè-Pognetto M, Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors, Proceedings of the National Academy of Sciences 105(35) (2008) 13151–13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Betancur C, Sakurai T, Buxbaum JD, The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders, Trends in Neurosciences 32(7) (2009) 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafrenière RG, Fathalli F, Joober R, Krebs M-O, DeLisi LE, Mottron L, Fombonne É, Michaud JL, Drapeau P, Carbonetto S, Craig A, Rouleau GA, Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia, Human Genetics 130(4) (2011) 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li J, Yoshikawa A, Brennan MD, Ramsey TL, Meltzer HY, Genetic predictors of antipsychotic response to lurasidone identified in a genome wide association study and by schizophrenia risk genes, Schizophrenia Research 192 (2018). [DOI] [PubMed] [Google Scholar]
- [64].Lawson-Yuen A, Saldivar J-S, Sommer S, Picker J, Familial deletion within NLGN4 associated with autism and Tourette syndrome, European Journal of Human Genetics 16(5) (2008) 5202006. [DOI] [PubMed] [Google Scholar]
- [65].Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu C-C, Kopke RD, Wu JY, Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu C-C, Kopke RD, Wu J-Y, Demonstration of functional coupling between γ-aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles, Proceedings of the National Academy of Sciences (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ, Gephyrin Regulates the Cell Surface Dynamics of Synaptic GABAA Receptors, The Journal of Neuroscience 25(45) (2005) 10469–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H, Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice, The Journal of neuroscience : the official journal of the Society for Neuroscience 19(21) (1999) 9289–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Essrich C, Lorez M, Benson JA, Fritschy J-M, Lüscher B, Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin, Nature Neuroscience 1(7) (1998) 563–571. [DOI] [PubMed] [Google Scholar]
- [69].Saiepour L, Fuchs C, Patrizi A, Sassoè-Pognetto M, Harvey RJ, Harvey K, Complex Role of Collybistin and Gephyrin in GABAA Receptor Clustering, Journal of Biological Chemistry 285(38) (2010) 29623–29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jedlicka P, Hoon T, Papadopoulos T, Vlachos A, Winkels R, Poulopoulos A, Betz H, Deller T, Brose N, Varoqueaux F, Schwarzacher SW, Increased Dentate Gyrus Excitability in Neuroligin-2-Deficient Mice in Vivo, Cerebral Cortex 21(2) (2011) 357–367. [DOI] [PubMed] [Google Scholar]
- [71].Artinian J, Lacaille J-C, Disinhibition in learning and memory circuits: new vistas for somatostatin interneurons and long-term synaptic plasticity, Brain Research Bulletin (2017). [DOI] [PubMed] [Google Scholar]
- [72].Acsady L, Göres TJ, Freund TF, Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus, Neuroscience 73(2) (1996) 317–334. [DOI] [PubMed] [Google Scholar]
- [73].Basu J, Zaremba JD, Cheung SK, L. H-F … Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition, … (2016). [DOI] [PMC free article] [PubMed]
- [74].Bao H, Asrican B, Li W, Gu B, Wen Z, Lim S-A, Haniff I, Ramakrishnan C, Deisseroth K, Philpot B, Song J, Long-Range GABAergic Inputs Regulate Neural Stem Cell Quiescence and Control Adult Hippocampal Neurogenesis, Cell Stem Cell 21(5) (2017) 604–61700000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N, Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism, Proceedings of the National Academy of Sciences 105(5) (2008)1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Richardson MP, Strange BA, Dolan RJ, Encoding of emotional memories depends on amygdala and hippocampus and their interactions, Nature neuroscience 7(3) (2004) 278–285. [DOI] [PubMed] [Google Scholar]
- [77].Kogan JH, Franklandand PW, Silva AJ, Long- term memory underlying hippocampus-dependent social recognition in mice, Hippocampus 10(1) (2000) 47–56. [DOI] [PubMed] [Google Scholar]
- [78].Stevenson EL, Caldwell HK, Lesions to the CA2 region of the hippocampus impair social memory in mice, European Journal of Neuroscience 40(9) (2014) 3294–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S, Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory, The Journal of neuroscience : the official journal of the Society for Neuroscience 37(15) (2017) 4103–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mercer A, Trigg HL, Thomson AM, Characterization of Neurons in the CA2 Subfield of the Adult Rat Hippocampus, The Journal of Neuroscience 27(27) (2007) 7329–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A, Hippocampal oxytocin receptors are necessary for discrimination of social stimuli, Nature Communications 8(1) (2017) 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S, Ventral CA1 neurons store social memory, Science 353(6307) (2016) 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chiang M-C, Huang A, Wintzer ME, Ohshima T, McHugh TJ, A role for CA3 in social recognition memory, Behavioural Brain Research (2018). [DOI] [PubMed] [Google Scholar]
- [84].van der Kooij MA, Fantin M, Kraev I, Korshunova I, Grosse J, Zanoletti O, Guirado R, Garcia-Mompó C, Nacher J, Stewart MG, Berezin V, Sandi C, Impaired Hippocampal Neuroligin-2 Function by Chronic Stress or Synthetic Peptide Treatment is Linked to Social Deficits and Increased Aggression, Neuropsychopharmacology 39(5) (2014) 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Katzman A, Alberini CM, NLGN1 and NLGN2 in the prefrontal cortex: their role in memory consolidation and strengthening, Current opinion in neurobiology 48 (2018) 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liang J, Xu W, Hsu YT, Yee AX, Chen L, Südhof TC, Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments, Molecular Psychiatry 20(7) (2015) 850–859. [DOI] [PubMed] [Google Scholar]
- [87].Chen C-H, Lee P-W, Liao H-M, Chang P-K, Neuroligin 2 R215H Mutant Mice Manifest Anxiety, Increased Prepulse Inhibition, and Impaired Spatial Learning and Memory, Frontiers in Psychiatry 8 (2017) 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ennaceur A, Neave N, Aggleton JP, Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix, Experimental Brain Research 113(3) (1997) 509–519. [DOI] [PubMed] [Google Scholar]
- [89].Antunes M, Biala G, The novel object recognition memory: neurobiology, test procedure, and its modifications, Cognitive Processing 13(2) (2012) 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dere E, Huston JP, De Souza Silva MA, The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents, Neuroscience and biobehavioral reviews 31(5) (2007) 673–704. [DOI] [PubMed] [Google Scholar]
- [91].Broadbent NJ, Gaskin S, Squire LR, Clark RE, Object recognition memory and the rodent hippocampus, Learning & Memory 17(1) (2010) 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cohen SJ, Jr RW, Assessing rodent hippocampal involvement in the novel object recognition task. A review, Behavioural Brain Research 285 (2015) 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Oliveira A, Hawk JD, Abel T, Havekes R, Post-training reversible inactivation of the hippocampus enhances novel object recognition memory, Learning & Memory 17(3) (2010) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Barker GRI, Warburton EC, When Is the Hippocampus Involved in Recognition Memory?, The Journal of Neuroscience 31(29) (2011) 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mumby DG, Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats, Behavioural Brain Research 127(1–2) (2001) 159–181. [DOI] [PubMed] [Google Scholar]
- [96].Winters BD, Bussey TJ, Transient Inactivation of Perirhinal Cortex Disrupts Encoding, Retrieval, and Consolidation of Object Recognition Memory, The Journal of Neuroscience 25(1) (2005) 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sawangjit A, Oyanedel CN, Niethard N, Salazar C, Born J, Inostroza M, The hippocampus is crucial for forming non-hippocampal long-term memory during sleep, Nature (2018) 1–5. [DOI] [PubMed] [Google Scholar]
- [98].Hafting T, Fyhn M, Molden S, Moser M-BB, Moser EI, Microstructure of a spatial map in the entorhinal cortex, Nature 436(7052) (2005) 801–806. [DOI] [PubMed] [Google Scholar]
- [99].O’Keefe J, Dostrovsky J, The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat, Brain research 34(1) (1971) 171–175. [DOI] [PubMed] [Google Scholar]
- [100].Rolls ET, Spatial view cells and the representation of place in the primate hippocampus, Hippocampus 9(4) (1999) 467–480. [DOI] [PubMed] [Google Scholar]
- [101].Nadel L, The hippocampus and space revisited, Hippocampus 1(3) (1991) 221–229. [DOI] [PubMed] [Google Scholar]
- [102].Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I, Cellular networks underlying human spatial navigation, Nature 425(6954) (2003) 184–188. [DOI] [PubMed] [Google Scholar]
- [103].Ulanovsky N, Moss CF, Hippocampal cellular and network activity in freely moving echolocating bats, Nature neuroscience 10(2) (2007) 224–233. [DOI] [PubMed] [Google Scholar]
- [104].Zhang S-JJ, Ye J, Couey JJ, Witter M, Moser EI, Moser M-BB, Functional connectivity of the entorhinal-hippocampal space circuit, Philosophical transactions of the Royal Society of London. Series B, Biological sciences 369(1635) (2014) 20120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wilent WB, Nitz DA, Discrete place fields of hippocampal formation interneurons, Journal of neurophysiology 97(6) (2007) 4152–4161. [DOI] [PubMed] [Google Scholar]
- [106].Ego-Stengel V, Wilson MA, Spatial selectivity and theta phase precession in CA1 interneurons, Hippocampus 17(2) (2007) 161–174. [DOI] [PubMed] [Google Scholar]
- [107].Hangya B, Li Y, Muller RU, Czurkó A, Complementary spatial firing in place cell-interneuron pairs, The Journal of physiology 588(Pt 21) (2010) 4165–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hangya B, Borhegyi Z, Szilágyi N, Freund TF, Varga V, Hangya B, Borhegyi Z, Szilágyi N, Freund TF, Varga V, GABAergic Neurons of the Medial Septum Lead the Hippocampal Network during Theta Activity, The Journal of Neuroscience (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Deshmukh SS, Knierim JJ, Representation of Non-Spatial and Spatial Information in the Lateral Entorhinal Cortex, Frontiers in Behavioral Neuroscience 5 (2011) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fyhn M, Molden S, Hollup S, Moser M-BB, Moser E, Hippocampal neurons responding to first-time dislocation of a target object, Neuron 35(3) (2002) 555–566. [DOI] [PubMed] [Google Scholar]
- [111].Jeffery KJ, Gilbert A, Burton S, Strudwick A, Preserved performance in a hippocampal dependent spatial task despite complete place cell remapping, Hippocampus 13(2) (2003) 175–189. [DOI] [PubMed] [Google Scholar]
- [112].Knierim JJ, Neunuebel JP, Deshmukh SS, Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local–global reference frames, Philosophical Transactions of the Royal Society of London B: Biological Sciences 369(1635) (2014) 20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jones MW, Wilson MA, Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task, PLoS biology 3(12) (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wilson D, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA, Lateral entorhinal cortex is critical for novel object- context recognition, Hippocampus 23(5) (2013) 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.