Abstract
In the present study, we evaluated the effects of biological factors, lifestyle factors, and environmental conditions on the induction of DNA damage in exfoliated cells of the oral mucosa. Age, sex, medication use, and environmental conditions were analyzed in individuals residing in the cities of Caarapó and Itaporã. The individuals were assessed by a questionnaire, and oral mucosa cells were collected and subjected to mutagenicity analysis. We observed no statistical differences in DNA damage related to sex. However, the mutagenic effect was found to be proportional to age, with higher frequencies of DNA damage observed in individuals between the ages of 46 and 65 years. In addition, higher frequencies of DNA damage were found in individuals who continuously used medication and for prolonged periods, and greater DNA damage was observed in individuals who used antihypertensive drugs than those who took antidepressants. In terms of environmental conditions, Caarapó residents had a significantly higher frequency of DNA damage than that of residents from Itaporã. Based on the analysis of land use and occupation, this result can be attributed to the smaller fraction of forest fragments and the higher proportion of buildings in Caarapó than Itaporã. We concluded that age, continued medication use, and environmental conditions can lead to greater DNA damage.
Keywords: Karyorrhexis, Land use and coverage, Mutagenesis, Micronucleus, Pharmaceutical drugk, Genetics
1. Introduction
An individual's lifestyle and exposure to various environmental factors can cause adverse health effects, including damage to DNA integrity [1]. The term “exposure” refers to all types of exposures throughout an individual's life and the manner in which such exposures interfere with health [2].
In addition to analyzing exposure to different contaminants on a daily basis, it is necessary to consider relevant biological factors, such as age and sex [3]. Several studies have indicated that the extent of genetic damage in older individuals is greater than that observed in younger individuals, which could be attributed to the accumulation of unrepaired DNA and/or a decrease in DNA repair capacity, which in turn promotes genetic instability [4].
In addition to age, studies have indicated that sex can contribute to the occurrence of genetic damage [5, 6]. However, the differences in genetic alterations between men and women are very small, displaying only point aspects [7, 8]. Furthermore, the frequency of DNA damage is also influenced by lifestyle factors. For instance, smoking habits, alcohol consumption, long working hours, few hours of sleep, sedentary lifestyle, diet, and psychological stress are known to contribute to DNA damage [6].
Drug use also contributes to DNA damage. Continuous, generalized, and long-term drug use, as well as the consumption of multiple types of drugs, such as antidepressants [9, 10] and antihypertensives [11], may further promote DNA damage [12]. In addition to biological factors and lifestyle habits, the exposure of a population to contaminated environments should be considered. For example, individuals who reside in areas that are close to agricultural industries or regions experience greater exposure to contaminants present in the air, water [13], or food [14].
Micronucleus testing (MN), which is employed to detect mutagenic damage and evaluate metanuclear abnormalities, can be used to measure genotoxic damage in exfoliated cells of the oral mucosa and is considered an effective method for evaluating genetic damage [15]. The present study aimed to evaluate whether age, sex, drug use, and environmental conditions influence micronucleus frequencies and nuclear alterations.
2. Materials and methods
The present study was based on a study conducted by Maran et al. [16], which reported the prevalence of agropastoral activities in the municipalities of Itaporã and Caarapó that are located in the south of the State of Mato Grosso do Sul, Brazil. Based on the questionnaire data, which evaluated biological parameters and lifestyle habits of the residents, 35 volunteers were selected from the cities of Caarapó and Itaporã. The sample group was divided according to gender (male and female), age (15–75 years), and medication use (antidepressants and antihypertensives). We excluded individuals who used commercial mouthwash or orthodontic appliances, as well as those who recently underwent oral surgery or presented lesions in the oral cavity. Individuals who smoked and/or consumed alcohol did not participate in the study.
Informed consent was obtained from all volunteers in accordance with the Declaration of Helsinki. The study protocol was approved by the Human Research Ethics Committee of the UFGD, with the approval certificate in Platform Brazil 417.582/2013.
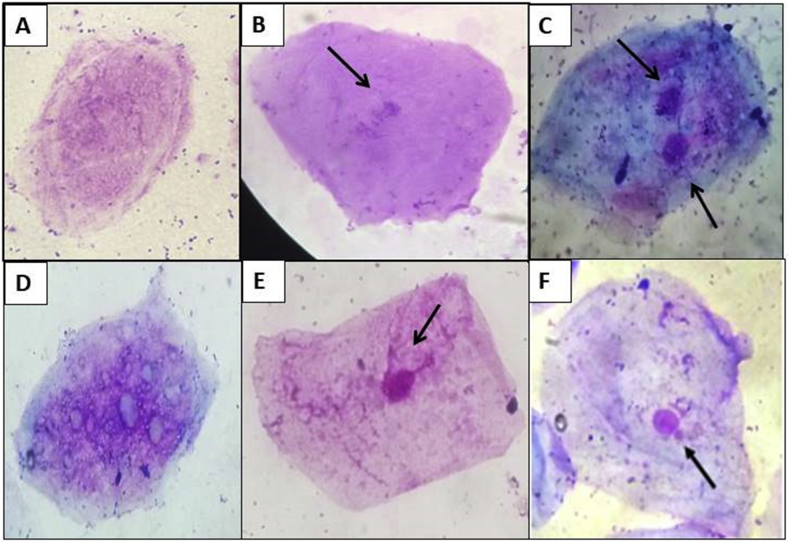
Genetic alterations were evaluated based on the presence of micronuclei and metanuclear alterations in desquamated cells of the buccal mucosa. Preparation, staining, and analysis of slides of exfoliated buccal mucosa cells were conducted following the protocol described by Thomas [15]. The cellular patterns related to the nuclear alterations (micronucleus, pyknosis, chromosomal bridge, karyorrhexis, binucleate cells, and karyolysis) were observed under an optical microscope (Nikon). Images captured at 400× magnification are shown in Fig. 1.
Fig. 1.
Micrographs of the nuclear alterations analyzed at 400× magnification. (A) karyolysis, (B) pyknosis, (C) binucleate cell, (D) karyorrhexis, (E) chromosomal bridge, and (F) micronucleus.
The use and coverage of land was mapped based on aerial images obtained from Google Earth Pro® software with a cell size of 10 m × 10 m. For the study limit, a 1-km buffer radius was generated around each sampling site, considering the pressure exerted by an area of this magnitude on the cities. Land use forms were classified under the following categories: agriculture, forest fragments, forest plantations, exposed soil, water bodies, building areas, pasture land, or secondary vegetation. For the interpretation of the images, visual classification was performed using the digitalization tools provided by ArcGIS 10.4® software, which was used to calculate the areas and percentages of each category of land occupation.
For the statistical analysis, the frequencies of the mutagenic and genotoxic alterations were analyzed by the Kruskall-Wallis non-parametric test (p = 0.05) using R software [17].
3. Results
Results showed no significant difference in nuclear abnormalities between men and women (p > 0.05). Karyorrhexis was the most frequently observed genetic alteration. Compared to other factors (city, age, and drug use), significant differences in karyorrhexis were observed between men and women, indicating genotoxicity (Fig. 2). Individuals aged between 46 and 65 years showed higher frequencies of karyorrhexis (p < 0.01) in. In addition, individuals taking antihypertensive drugs showed significantly higher frequencies of karyorrhexis compared to individuals taking antidepressants or those who do not consume drugs.
Fig. 2.
Frequency of karyorrhexis in relation to cities (A), age (B), and drug use (C).
In terms of location, higher frequencies of nuclear alterations were observed in individuals residing in Caarapó (p < 0.05). This result could be related to the land use and land cover of these cities, considering that Caarapó has a smaller fraction of forest fragments and a higher proportion of buildings (8% and 55%) compared to Itaporã (31.8% and 20%). Itaporã has a larger agricultural area (49%) than Caarapó (36%) (Fig. 3).
Fig. 3.
Map of land use and coverage of the cities analyzed.
4. Discussion
Studies on human exposure to various factors, such as biological conditions, environmental exposure, and lifestyle habits, are fundamental for obtaining a more detailed understanding of the causes of genetic alterations. Our findings showed no significant differences in genetic alterations between men and women. Previous studies showed that men and women have very small differences in MN, corresponding to single base pair differences [7]. Evidence in the literature indicated that older age is associated with increased damage to the genetic material [6, 18, 19]. According to Nassour et al. [16], these results can be explained by the association between aging and genetic instability. Thus, the effects of aging appear to be a combination of genetically programmed processes and genetic changes induced by exogenous and endogenous factors. During aging, enzyme deficiencies, such as deficiencies in DNA repair enzymes, occur progressively, which increases the susceptibility of cells to the effects of genotoxic agents [20]. Kirsch-Volders et al. [21] suggested that failures in cell defense systems and impaired DNA repair efficacy can lead to the accumulation of mutations that, either alone or in combination with other age-related changes, can contribute to aging and the development of age-related diseases. Individuals taking antihypertensive and antidepressant medications have been reported to have higher frequencies of MN compared to individuals who do not take any medication. These results are consistent with the studies conducted in Lima et al. [12], wherein significant differences in DNA damage were observed among individuals who use these same type of medication. Other studies also reported a positive relationship between genetic alterations and the use of antihypertensive drugs, such as losartan, irbesartan [22], captopril, and enalapril maleate [23]. Cells in the buccal mucosa are constantly exposed to various agents with genotoxic and/or mutagenic potential, and the damage caused by these substances depends on the intensity of the mutagenic effect of the chemical agent. Damaged DNA can be repaired when the mutagenic effects are minimal; however, in cases of moderate aggression, the damage cannot be repaired, and the cells are eliminated through mechanisms of cell death, as evidenced by the nuclear alterations [24], such as karyorrhexis. Regarding the analyses of land use and cover, individuals from Caarapó presented significantly higher frequencies of karyorrhexis than Itaporã individuals, which could be associated with the smaller fraction of forest fragments and higher proportion of buildings. Thus, urban and industrial areas also contribute to the reduction of native vegetation cover that act as natural filters for the purification of soil contaminants [25, 26]. The frequency of nuclear alterations in the buccal mucosal cells of the participants can be related to soil conditions near their residence. The two cities have significant proportions of arable land, which could have contributed to the increase in the frequency of alterations found in these individuals. According to Ergene et al. [27], residence in places close to plantations where complex pesticide mixtures are used could present a potential risk to the genetic material, which was observed in the individuals analyzed in the present study. Based on the current findings, we concluded that age, use of antihypertensive and antidepressants drugs, and environmental conditions can induce DNA damage. The high frequency of karyorrhexis found in individuals analyzed in our study warrants public attention. Our findings provided the basis for future research on the detrimental effects of various endogenous and exogenous factors on the DNA.
Declarations
Author contribution statement
Deborah Navit de Carvalho Cavalcante, Bruno do Amaral Crispim: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Beatriz Barufatti Grisolia: Performed the experiments.
Lucilene Finoto Viana: analyzed and interpreted the data; Wrote the paper.
Nayara Halimy Maran, Julio César Jut Solórzano: Analyzed and interpreted the data.
Kelly Mari Pires De Oliveira: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Alexeia Barufatti: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by FUNASA, FUNDECT, CNPQ and CAPES.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nassour J., Martien S., Martin N., Deruy E., Tomellini E., Malaquin N., Bouali F., Sabatier L., Wernert N., Pinte S., Gilson E., Pourtier A., Pluquet O., Abbadie C. Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells. Nat. Commun. 2016;7:10399. doi: 10.1038/ncomms10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC – Exposome and Exposomics - NIOSH Workplace Safety and Health Topic, (n.d.). https://www.cdc.gov/niosh/topics/exposome/default.html (Accessed February 12, 2019).
- 3.Cavalcante D.N.D.C., Sposito J.C.V., Crispim B.D.A., Nascimento A.V.D., Grisolia A.B. Genotoxic and mutagenic effects of passive smoking and urban air pollutants in buccal mucosa cells of children enrolled in public school. Toxicol. Mech. Methods. 2017;27:346–351. doi: 10.1080/15376516.2017.1288767. [DOI] [PubMed] [Google Scholar]
- 4.Zietkiewicz E. Cytogenetic perspective of ageing and longevity in men and women. Artic. J. Appl. Genet. 2009;50(3):261–273. doi: 10.1007/BF03195682. [DOI] [PubMed] [Google Scholar]
- 5.Fenech M., Bonassi S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis. 2011;26:43–49. doi: 10.1093/mutage/geq050. [DOI] [PubMed] [Google Scholar]
- 6.Kažimírová M., Barančoková Z., Džupinková L., Wsólová M. Dušinská. Micronuclei and chromosomal aberrations, important markers of ageing: possible association with XPC and XPD polymorphisms. Mutat. Res. Mol. Mech. Mutagen. 2009;661:35–40. doi: 10.1016/j.mrfmmm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Bonassi S., El-Zein R., Bolognesi C., Fenech M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: evidence from human studies. Mutagenesis. 2011;26:93–100. doi: 10.1093/mutage/geq075. [DOI] [PubMed] [Google Scholar]
- 8.Trzeciak A.R., Barnes J., Ejiogu N., Foster K., Brant L.J., Zonderman A.B., Evans M.K. Age, sex, and race influence single-strand break repair capacity in a human population. Free Radic. Biol. Med. 2008;45:1631–1641. doi: 10.1016/j.freeradbiomed.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lale Dönbak O.T.D.M.Ç. Genotoxic effects of antidepressant reboxetine in human peripheral lymphocytes. Adv. Lab. Med. Int. 2014;4:93–102. http://www.scopemed.org/?mno=166576 [Google Scholar]
- 10.Norizadeh Tazehkand M., Topaktas M. The in vitro genotoxic and cytotoxic effects of remeron on human peripheral blood lymphocytes. Drug Chem. Toxicol. 2015;38:266–271. doi: 10.3109/01480545.2014.947425. [DOI] [PubMed] [Google Scholar]
- 11.Attia S.M., Ashour A.E., Bakheet S.A. Comet-FISH studies for evaluation of genetic damage of citalopram in somatic cells of the mouse. J. Appl. Toxicol. 2013;33:901–905. doi: 10.1002/jat.2859. [DOI] [PubMed] [Google Scholar]
- 12.Lima C.F., Oliveira L.U., Cabral L.A.G., Brandão A.A.H., Salgado M.Â.C., Almeida J.D. Cytogenetic damage of oral mucosa by consumption of alcohol, tobacco and illicit drugs. J. Oral Pathol. Med. 2010;39:441–446. doi: 10.1111/j.1600-0714.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- 13.WHO – World Health Organization., UNICEF . 2014. Progress on Sanitation and Drinking-Water. [Google Scholar]
- 14.Mandal P., Rai A., Mishra S., Tripathi A., Das M. Mutagenicity: Assays and Applications. Elsevier; 2018. Mutagens in food. [Google Scholar]
- 15.Thomas P., Holland N., Bolognesi C., Kirsch-Volders M., Bonassi S., Zeiger E., Knasmueller S., Fenech M. Buccal micronucleus cytome assay. Nat. Protoc. 2009;4:825–837. doi: 10.1038/nprot.2009.53. [DOI] [PubMed] [Google Scholar]
- 16.Maran N., Crispim B., Iahnn S., Araújo R., Grisolia A., Oliveira K. Depth and well type related to groundwater microbiological contamination. Int. J. Environ. Res. Public Health. 2016;13:1036. doi: 10.3390/ijerph13101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team R, R: The R Project for Statistical Computing, (n.d.). https://www.r-project.org/(accessed February 12, 2019).
- 18.Wojda E., Zietkiewicz M.Witt. Effects of age and gender on micronucleus and chromosome nondisjunction frequencies in centenarians and younger subjects. Mutagenesis. 2007;22:195–200. doi: 10.1093/mutage/gem002. [DOI] [PubMed] [Google Scholar]
- 19.Nefic H., Handzic I. The effect of age, sex, and lifestyle factors on micronucleus frequency in peripheral blood lymphocytes of the Bosnian population. Mutat. Res. Toxicol. Environ. Mutagen. 2013;753:1–11. doi: 10.1016/j.mrgentox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z., Xu X.S., Yang J., Wang G. Defining the function of XPC protein in psoralen and cisplatin-mediated DNA repair and mutagenesis. Carcinogenesis. 2003;24:1111–1121. doi: 10.1093/carcin/bgg051. [DOI] [PubMed] [Google Scholar]
- 21.Kirsch-Volders M., Mateuca R.A., Roelants M., Tremp A., Zeiger E., Bonassi S., Holland N., Chang W.P., Vande Aka P., Deboeck M., Godderis L., Haufroid V., Ishikawa H., Laffon B., Marcos R., Migliore L., Norppa H., Teixeira J.P., Zijno A., Fenech M. The effects of GSTM1 and GSTT1 polymorphisms on micronucleus frequencies in human lymphocytes in vivo. Cancer Epidemiol. Biomark. Prev. 2006;15:1038–1042. doi: 10.1158/1055-9965.EPI-05-0487. [DOI] [PubMed] [Google Scholar]
- 22.Arranz Gutiérrez P. 2015. Evaluación de la posible capacidad genotóxica de fármacos antihipertensivos: losartán e irbesartán.https://addi.ehu.es/handle/10810/18025 [Google Scholar]
- 23.de Moura Leão M.F., Duarte J.A., Sauzen P.D., Piccoli J. da C.E., de Oliveira L.F.S., Machado M.M. Cytotoxic and genotoxic effects of antihypertensives distributed in Brazil by social programs: are they safe? Environ. Toxicol. Pharmacol. 2018;63:1–5. doi: 10.1016/j.etap.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Farhadi S., Mohamadi M., Mohamadi M. Repair index in examination of nuclear changes in the buccal mucosa of smokers: a useful method for screening of oral cancer. Asian Pac. J. Cancer Prev. APJCP. 2017;18:3087–3090. doi: 10.22034/APJCP.2017.18.11.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorion C.M., Kennedy B.P. Riparian forest buffers mitigate the effects of deforestation on fish assemblages in tropical headwater streams. Ecol. Appl. 2009;19:468–479. doi: 10.1890/08-0050.1. http://www.ncbi.nlm.nih.gov/pubmed/19323203 [DOI] [PubMed] [Google Scholar]
- 26.Burrell T.K., O’Brien J.M., Graham S.E., Simon K.S., Harding J.S., McIntosh A.R. Riparian shading mitigates stream eutrophication in agricultural catchments. Freshw. Sci. 2014;33:73–84. [Google Scholar]
- 27.Ergene S., Çavaş T., Çelik A., Köleli N., Kaya F., Karahan A. Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicology. 2007;16:385–391. doi: 10.1007/s10646-007-0142-4. [DOI] [PubMed] [Google Scholar]