Abstract
Background
Linezolid-resistant enterococci pose great challenges in clinical practice. The aim of this study is to study the mechanisms underlying the resistance and genetic environment of antimicrobial resistance gene of linezolid-resistant enterococci.
Results
The linezolid MICs of 16 enterococci were 4 mg/L to 16 mg/L. Four strains belonged to multi-drug resistant (MDR) bacteria. The sequence types (STs) of 13 enterococci strains performed WGS were diverse: 3 ST476, 1 ST86, ST116, ST480, ST59, ST416, ST21, ST67, ST16, ST585 and ST18. None of them carried multi-drug resistance gene cfr. Only one strain had the G2658 T mutation of target 23S rRNA gene. Thirteen (13/16, 81.3%) strains harbored the novel oxazolidinone resistance gene optrA. WGS analysis showed that the optrA gene was flanked by sequence IS1216E insertion in 13 strains, and optrA was adjacent to transposons Tn558 in two strains and Tn554 in one strain. The optrA gene was identified to be co-localized with fexA, the resistance genes mediated florfenicol resistance in 13 strains, and ermA1, the resistance genes mediated erythromycin resistance in 9 strains, indicating that linezolid-resistant strains may be selected due to non-oxazolidinone antibiotics (i.e. macrolides and florfenicol) usage.
Conclusion
Our findings demonstrate the high diversity of optrA-carrying genetic platforms. The mobile genetic elements (MGEs) may play an important role in the dissemination of optrA into the enterococci isolates of human origin. The genetic evidence of transferable feature and co-selection of optrA should be gave more attention in clinical practice.
Keywords: optrA, Linezolid resistance, Oxazolidinone, Enterococci, Genetic environment
Background
Linezolid, which belongs to oxazolidinone, is the clinically last resort to treat vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), and other multi-drug Gram-positive bacteria [1]. Linezolid exerts antibacterial effects by inhibiting the binding of mRNA to the ribosome, thereby affecting the synthesis of the protein [1]. It is generally considered that linezolid is a completely synthetic antibiotic, and theoretically, there should be no natural resistance phenomenon. Unfortunately, clinically resistant strains have emerged shortly after use of linezolid in clinical practice [2, 3]. The occurrence of linezolid-resistant strains show an increasing trend, especially in animal husbandry [4], which should attract sufficient attention.
The resistance to linezolid by gram-positive bacteria can be achieved by target-modified 23S rRNA mutations [5], acquiring exogenous chloramphenicol-florfenicol resistance (cfr) [6], optrA [7] or poxtA [8]. Targets 23S rRNA, L3, L4 and L22 mutations usually affect ribosome function and easily reverse in the absence of selective pressure. Therefore, chemical modifications (such as methylation) of rRNA are the more common resistance mechanisms of linezolid. The cfr gene encodes a methyltransferase that modifies the 23S rRNA at position A2503, which confers resistance to phenicols, lincosamide, oxazolidinones, pleuromutilin, and streptogramin A (PhLOPSA phenotype) [9]. The cfr gene has been identified in a variety of genera, including Staphylococcus [10], Bacillus [11], Enterococcus [12], Macrococcus [13], Jeotgalicoccus [13], Streptococcus [14], Proteus [15] and Escherichia [16]. The cfr gene widely disseminates among oxazolidinone-resistant isolates from human [17] and animal [18] origin, which represents a serious threat to public health. Recently, two cfr variants, cfr(B) and cfr(C), have been found in Enterococcus faecium [19], Clostridium difficile [20] and Campylobacter [21]. The cfr gene was often found on a number of different plasmids [7, 15, 22], and integrated into transposons, leading to dissemination of this gene among the same or between different species of bacteria.
The transferable gene, optrA, has been identified, which confers cross-resistance to phenicols and oxazolidinones, including tedizolid [23]. This gene was identified in enterococci and staphylococci from clinical [24], healthy human and animal isolates [25, 26]. The resistance gene optrA can be located either on plasmid or chromosome [26]. Recently, one florfenicol-resistant Staphylococcus sciuri isolate, which carried both optrA and cfr, was identified in pig [27]. In this study, we investigated the oxazolidinones resistance genes among linezolid-resistant isolates in Chinese hospitals and utilized whole-genome sequencing (WGS), and further analyzed the genetic environment surrounding the resistance genes.
Materials and methods
Bacterial strains
A total of 15 non-duplicable linezolid-resistant enterococci strains and one linezolid intermediate-resistant enterococci strain (13 E. faecalis and 3 E. faecium) (1.5%, 16/1067) were collected from specimens of 16 patients from 9 hospitals between 2009 and 2013 in 6 provinces of China, including 5 samples from Beijing, 4 samples from Guangdong, 3 samples from Zhejiang, 2 samples from Fujian, 1 sample from Jiangsu and 1 sample from Hubei (Table 1.). Among the 16 strains, 6 were recovered from patients with urinary tract infection, 5 from patients with bacteremia, 4 from patients with wound infection and 1 from patients with biliary tract infection. Among the 16 strains, 7 strains (1203_10W003, 1202_13E004, 1202_21W014, 19113, 19677, 19506 and SZ21494) were isolated in our previous study [28], and the 9 remaining strains were isolated in this study. Bacteria were first identified at the species level using the VITEK system (bioMerieux, Crapome, France), followed by a molecular method based on the 16S rRNA gene, and then by sequencing analysis.
Table 1.
Clinical, phenotypic and genotypic data for the linezolid-resistant Enterococci isolates investigated
| Isolate no. | Organism | Isolation year | Hospitalb | Isolation site | STc | MICs (mg/L)a | Linezolid resistance genes | 23S rRNA gene mutations | Other resistance genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LNZ | P | AMP | VAN | TEC | DAP | TGC | LVX | ERY | HLG | Antibiotic resistance profiles | |||||||||
| 29462 | E. faecalis | 2009 | ZRYH | urine | 86 | 8 | 4 | <=2 | 2 | <=0.125 | 0.5 | 0.06 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, ANT(6)-Ia, AAC(6′)-Ie-APH(2″)-Ia, dfrG, dfrE, lsaA, fexA, cat, efrB, efrA, ermB, tetM, tet(L) |
| ZJ11066 | E. faecalis | 2011 | ZJFY | blood | 116 | 8 | 2 | <=2 | 1 | 0.125 | 0.5 | 0.12 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, APH(3′)-IIIa, AAC(6′)-Ie-APH(2″)-Ia, dfrF, dfrG, dfrE, lnuG, lsaA, fexA, efrA, efrB, ermB, ermA1, tet(L), tetM |
| JS11041 | E. faecium | 2011 | JSRM | urine | ND | 8 | > = 64 | > = 32 | 0.5 | 0.25 | 2 | 0.06 | 8 | > 4 | R | P, AMP, LVX, ERY | – | – | ND |
| 19113 | E. faecalis | 2011 | SZRM | bile | ND | 8 | > = 64 | > = 32 | 2 | <=0.125 | 0.5 | 0.06 | 1 | > 4 | R | P, AMP, LVX, ERY | – | – | ND |
| ZJLRE1 | E. faecium | 2011 | ZJFE | blood | ND | 16 | > = 64 | > = 32 | 1 | 0.5 | 1 | 0.06 | 8 | > 4 | R | P, AMP, LVX, ERY | – | G2658 T | ND |
| 1207_26W003 | E. faecalis | 2012 | BJRM | urine | 476 | 4 | 2 | <=2 | 1 | 0.12 | 0.5 | 0.06 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, APH(3′)-IIIa, AAC(6′)-Ie-APH(2″)-Ia, aad(6), ANT(9)-Ia, dfrG, dfrE, lnuB, lsaE, lsaA, mdtF, SAT-4, cat, fexA, efrB, efrA, ermA1, ermB, tet(L), tetM |
| 1203_10W003 | E. faecalis | 2012 | BJRM | urine | 480 | 8 | 2 | <=2 | 1 | 0.12 | 0.5 | 0.06 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, AAC(6′)-Ie-APH(2″)-Ia, APH(3′)-IIIa, aad(6), ANT(6)-Ia, dfrG, dfrE, lnuB, lsaE, lsaA, SAT-4, cat, fexA, efrA, efrB, ermB, ermA1, tetM, tet(L) |
| 19677 | E. faecalis | 2012 | SZRM | blood | 59 | 8 | 2 | <=2 | 0.5 | 0.12 | 0.5 | 0.12 | 0.03 | > 4 | R | ERY | optrA | – | emeA, dfrE, lsaA, fexA, efrA, efrB, ermA1, tetM, tet(L) |
| 19506 | E. faecium | 2012 | SZRM | wound | 18 | 16 | > = 64 | > = 32 | 0.5 | 0.25 | 2 | 0.06 | 8 | > 4 | S | P, AMP, LVX, ERY | optrA | – | AAC(6′)-Ii, dfrG, efmA, msrC, fexA, ermA1 |
| 1202_13E004 | E. faecalis | 2012 | BJRM | wound | 416 | 16 | 8 | <=2 | 2 | <=0.125 | 0.5 | 0.12 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, ANT(6)-Ia, AAC(6′)-Ie-APH(2″)-Ia, dfrG, dfrE, lsaA, fexA, efrB, efrA, ermB, ermA1, tet(L), tetM |
| 1202_21W014 | E. faecalis | 2012 | BJRM | urine | 21 | 8 | 4 | <=2 | 2 | <=0.125 | 0.5 | 0.12 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, AAC(6′)-Ie-APH(2″)-Ia, aad(6), ANT(6)-Ia, dfrG, dfrE, lnuG, lsaA, SAT-4, fexA, cat, efrA, efrB, ermB, tet(L) |
| SZ21494 | E. faecalis | 2012 | SZRM | wound | 67 | 8 | 4 | <=2 | 1 | <=0.125 | 1 | 0.06 | 1 | > 4 | S | ERY | optrA | – | emeA, dfrE, dfrG, lnuG, lsaA, fexA, cat, efrA, efrB, ermB, ermA1, tetM, tet(L) |
| XM2013_71028 | E. faecalis | 2013 | XMDY | wound | 16 | 8 | 2 | <=2 | 1 | <=0.125 | 1 | 0.06 | 0.5 | > 4 | R | ERY | optrA | – | emeA, APH(3′)-IIIa, AAC(6′)-Ie-APH(2″)-Ia, ANT(9)-Ia, aad(6), dfrG, dfrE, lnuB, lsaE, lsaA, SAT-4, fexA, cat, efrB, efrA, ermB, ermA1, tetM |
| XM2013_42321 | E. faecalis | 2013 | XMDY | urine | 585 | 16 | 4 | <=2 | 1 | <=0.125 | 0.5 | 0.06 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, APH(3′)-IIIa, AAC(6′)-Ie-APH(2″)-Ia, aad(6), ANT(9)-Ia, dfrE, dfrG, lmrD, lnuB, lsaE, lsaA, SAT-4, cat, fexA, efrB, efrA, ermB, tetM, tet(L) |
| TZ2 | E. faecalis | 2013 | TZSY | blood | 476 | 8 | 2 | <=2 | 1 | <=0.125 | 0.5 | 0.12 | 8 | > 4 | R | LVX, ERY | optrA | – | emeA, AAC(6′)-Ie-APH(2″)-Ia, APH(3′)-IIIa, aad(6), ANT(6)-Ia, dfrG, dfrE, lsaA, SAT-4, fexA, cat, efrB, efrA, ermA1, ermB, tet(L), tetM |
| WHXH | E. faecalis | 2013 | WHDS | blood | 476 | 8 | 4 | <=2 | 2 | <=0.125 | 0.5 | 0.12 | 8 | > 4 | S | LVX, ERY | optrA | – | emeA, AAC(6′)-Ie-APH(2″)-Ia, APH(3′)-IIIa, aad(6), ANT(9)-Ia, dfrE, dfrG, lnuB, lsaE, lsaA, SAT-4, cat, fexA, efrB, efrA, ermB, tetM, tet(C), tet(L) |
aMICs, the minimal inhibitory concentrations; LNZ, linezolid, susceptible (S): ≤ 2 mg/L, intermediate (I): 4 mg/L, resistant (R): ≥ 8 mg/L; P, penicillin, S: ≤ 2 mg/L, R: ≥ 8 mg/L; AMP, ampicillin, S: ≤ 2 mg/L, R: ≥ 8 mg/L; VAN, vancomycin, S: ≤ 4 mg/L, I: 8–16 mg/L, R: ≥ 32 mg/L; TEC, teicoplanin, S: ≤ 8 mg/L, I: 16 mg/L, R: ≥ 32 mg/L; DAP, S: ≤ 1 mg/L, susceptible-dose dependent (SDD): 2–4 mg/L, R: ≥ 8 mg/L; TGC, tigecycline, no breakpoint in CLSI M100; LVX, levofloxacin, S: ≤ 2 mg/L, I: 4 mg/L, R: ≥ 8 mg/L; ERY, erythromycin, S: ≤ 0.5 mg/L, I: 1–4 mg/L, R: ≥ 8 mg/L; HLG, high-level gentamycin (500 mg/L); −, negative; ND, not determined
bZRYH, China-Japan Friendship Hospital; ZJFY, 1st Affiliated Hospital of Zhejiang University; JSRM, Jiangsu Province Hospital; SZRM, Shenzhen People’s Hospital; ZJFE, 2nd Affiliated Hospital of Zhejiang University; BJRM, Peking University People’s Hospital; XMDY, 1st Affiliated Hospital of Xiamen University; TZSY, Taizhou Hospital of Zhejiang Province; WHDS, Wuhan Fourth Hospital
cST sequence type, ND not determined
Antimicrobial susceptibility testing
The minimal inhibitory concentrations (MICs) of 8 antimicrobial agents were determined by the agar dilution method, and tigecycline and daptomycin by broth microdilution. The antimicrobial agents tested included linezolid (Sigma Chemical Co., St. Louis, MO, USA), vancomycin (Sigma), teicoplanin (Sigma), levofloxacin (Sigma), erythromycin (Sigma), tigecycline (Pfizer, NY, USA), daptomycin (Cubist Pharmaceuticals, MA, USA), penicillin (Sigma), ampicillin (Sigma) and gentamycin (Sigma). E. faecalis ATCC 29212 was used for quality control in antimicrobial susceptibility testing. The results of susceptibility testing were interpreted according to CLSI guideline M100-S27. Isolates resistant to three or more antibiotics of different families were considered to be multi-drug resistant (MDR).
Molecular detection of resistance genes and mutations
The resistance genes cfr and optrA were determined by PCR as described previously. The mutation of domain V of the 23S rRNA gene was determined by PCR combined with sequencing as described previously [29]. Nucleotide sequences were compared with the linezolid-susceptible E. faecalis and E. faecium from Peking University People’s Hospital during the same period. The mutation was identified by the E. coli numbering.
Whole-genome sequencing (WGS)
Total genomic DNA of 13 enterococci strains carrying optrA gene was extracted by the standard phenol/chloroform method. The whole-genome sequencing was performed using Illumina technology. The sequences with read length of 150 bases were assembled into contigs using SPAdes (v.3.9.0) [30]. Plasmid content associated with optrA was analyzed using the contigs obtained by plasmidSPAdes. The assembled contigs were annotated by the Prokka v1.12 [31]. Insertion sequences (IS) were identified using ISFinder [32]. Multilocus sequence types (MLST) were assigned using the silico tool hosted by Center for Genomic Epidemiology (CGE) (www.genomicepidemiology.org). The resistance genes were identified by ResFinder 3.0 [33]. Maximum likelihood phylogenetic analysis of the core genome was performed using RAxML (Linux version v7.2.8) [34]. The sequences of the optrA-containing regions of 13 enterococci strains have been deposited at GenBank under the following accession numbers MH225413 (1202_13E004), MH225414 (1202_21W014), MH225415 (1203_10W003), MH225416 (1207_26W003), MH225417 (19506), MH225418 (19677), MH225419 (29462), MH225420 (SZ21494), MH225421 (TZ2), MH225422 (WHXH), MH225423 (XM2013_42321), MH225424 (XM2013_71028) and MH225425 (ZJ11066).
Results
Susceptibility profiles of linezolid-resistant enterococci isolates
The susceptible breakpoint of enterococci to linezolid is defined as less than or equal to 2 mg/L, and the resistant breakpoint is defined as greater than or equal to 8 mg/L. The linezolid MICs of 16 enterococci were 4 mg/L to 16 mg/L, respectively. There were no significant differences in the linezolid MICs between optrA-positive strains (4–16 mg/L) and optrA-negative strains (8–16 mg/L). Most of the optrA-positive strains also exhibited resistance to erythromycin (16/16, 100%), levofloxacin (12/16, 75%) and high-level gentamycin (500 mg/L) (13/16, 81.3%). All strains were susceptible to vancomycin, teicoplanin, daptomycin and tigecycline. Three E.faecium and one E. faecalis strains (4/16, 25%) were resistant to penicillin and ampicillin, and all of 16 enterococci strains didn’t possess beta-lactamase. Four strains (4/16, 25%) belonged to MDR organism (Table 1).
Distribution of antimicrobial resistance genes
None of 16 linezolid-resistant enterococci strains contained cfr gene. Only one strain had the G2658 T mutation in 23S rRNA gene with linezolid MIC of 16 mg/L. Most of the linezolid-resistant enterococci strains (n = 13) carried optrA gene (Table 1).
In addition to optrA genes, all optrA-positive strains harbored phenicols resistance gene fexA (13/13, 100%), erythromycin resistance genes of different erm gene classes (ermA1, ermB) (13/13, 100%), trimethoprim resistant dihydrofolate reductase different dfr gene classes (dfrE, dfrG) (13/13, 100%), ATP-binding cassette (ABC) antibiotic efflux pump different gene classes (lsaA, lsaE, efrA, efrB) (13/13, 100%). Further, majority optrA-positive strains carried tetracycline resistance genes of different tet gene classes (tet[C], tet[L], tetM) (12/13, 92.3%), multidrug and toxic compound extrusion (MATE) transporter emeA gene (12/13, 92.3%) and aminoglycosides inactivating enzyme different gene classes (AAC(6′)-Ii, AAC[6′]-Ie-APH[2″]-Ia, APH[3′]-IIIa, aad [6], ANT[6]-Ia, ANT[9]-Ia) (10/13, 76.9%). Various additional resistance genes were identified including cat, lnuB, lnuG, mdtF, SAT-4 and efmA.
Core-genome phylogenetic analysis
The 12 E. faecalis isolates performed WGS were classified into 10 sequence types (STs): 3 ST476, 1 ST86, ST116, ST480, ST59, ST416, ST21, ST67, ST16 and ST585, respectively. One E. faecium isolate belonged to ST18.
The phylogenetic tree of 12 E. faecalis isolates harboring optrA gene showed that two of these isolates (29462 and XM2013_42321) were genetically unrelated with the rest isolates. Importantly, 1207_26W003 (Beijing), TZ2 (Zhejiang) and WHXH (Hubei) were recovered from different cities, were found very closely related (99.9%), and all of 3 strains belonged to ST476. In addition, strain 19677 recovered from Guangdong was closely related (99.4%) to strain 1202_13E004 recovered from Beijing. Further, strain 1203_10W003 isolated from Beijing and strain XM2013_71028 isolated from Fujian was closely related (99.3%) (Fig. 1).
Fig. 1.
Maximum-likelihood phylogenetic tree of E. faecalis (n = 12)
Genetic environment of optrA on plasmids or chromosome
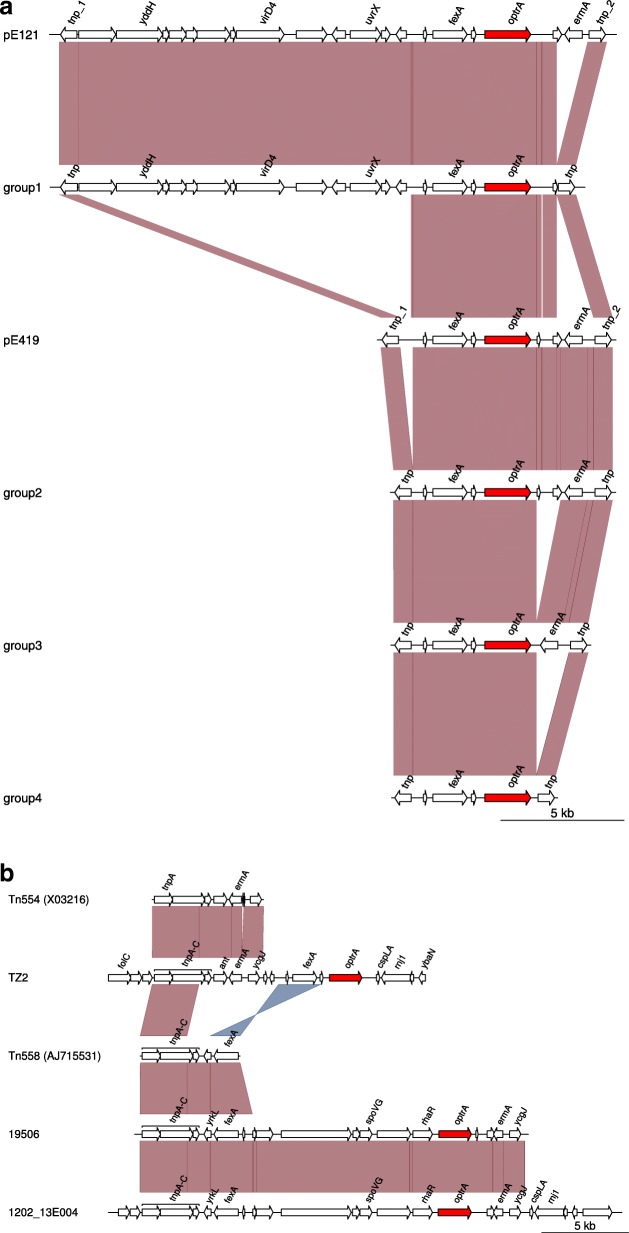
Thirteen contigs containing the optrA gene were blasted in the GenBank database, and 10 contigs were mapped against the plasmids (pE121 [GenBank accession number KT862776] and pE419 [KT862777]). The size of these 10 contigs was between 6372 bp and 21568 bp. According to the gene arrangements, the 10 contigs were divided into 4 groups: group 1 (29462 [MH225419], 1202_21W014 [MH225414]), group 2 (1203_10W003 [MH225415], SZ21494 [MH225420], ZJ11066 [MH225425]), group 3 (1207_26W003 [MH225416], 19677 [MH225418], XM2013_71028 [MH225424]), group 4 (WHXH [MH225422], XM2013_42321 [MH225423]). The genetic environment of optrA in Group 1 was similar to that of plasmid pE121 (KT862776). Compared to the plasmid pE121, ermA1 gene was absent and the rest of the sequences were almost identical. The genetic environment of optrA from Group 2 to Group 4 resembled that of plasmid pE419 (KT862777). Compared with pE419, the intergenic region between the left IS1216E and the first hypothetical protein was truncated in Group 2, two hypothetical proteins between optrA gene and the right IS1216E were missing in Group 3, and ermA1 gene and two hypothetical proteins were missing in Group 4. The common feature of genetic environment of optrA from Group 1 to Group 4 was flanked by IS1216E, and all of them carried phenicol resistance gene fexA and erythromycin resistance gene ermA1 (Fig. 2a.).
Fig. 2.
a Schematic presentation of the genetic environment of optrA-containing contigs mapped on plasmids in 10 enterococci isolates investigated in this study. b Schematic presentation of optrA-containing contigs mapped on chromosome in three enterococci isolates. Arrows indicate the positions and directions of transcription of the different genes. Genes with unknown functions are not marked. According to the gene arrangement, the 10 contigs mapped on plasmids were divided into 4 groups-group 1 (29462 [MH225419], 1202_21W014 [MH225414]), group 2 (1203_10W003 [MH225415], SZ21494 [MH225420], ZJ11066 [MH225425]), group 3 (1207_26W003 [MH225416], 19677 [MH225418], XM2013_71028 [MH225424]), group 4 (WHXH [MH225422], XM2013_42321 [MH225423])
The contigs containing optrA gene of 1202_13E004 (MH225413) (29141 bp), 19506 (MH225417) (22720 bp) and TZ2 (MH225421) (75117 bp) were mapped on chromosomal (CP008816). The strains 1202_13E004 and 19506 contained a transposon Tn558 (AJ715531) with three transposases and the resistance gene fexA, and the resistance gene optrA was adjacent to resistance gene ermA1. The strain TZ2 carried another transposon Tn554 (X03216) with three transposases and the resistance gene ermA1, and optrA was adjacent to resistance gene fexA (Fig. 2b.).
Discussion
This study indicates that the transferable resistance gene optrA is very prevalent among linezolid-resistant enterococci strains isolated from human. Much more optrA gene is located on plasmid than chromosome. The optrA gene located on plasmid is flanked by IS1216E, while that located on chromosome is mediated by transposons.
In this study, none of linezolid-resistant enterococci strains carried cfr, while most of them harbored optrA. This suggests that acquiring optrA is the main resistant mechanism in linezolid-resistant enterococci from human origin. The presence of optrA was limited to a few species of the genus Enterococcus [35] and only rare species of Staphylococcus [4]. The surveillance studies indicated that only 3.9–6.2% of staphylococci strains were positive for optrA [4, 25], which suggests a low prevalence of this oxazolidinone resistance gene in the genus Staphylococcus.
In present study, the optrA gene was located on plasmids in most of enterococci strains. The optrA gene is often surrounded by insertion sequences when located on plasmids from enterococci strains. Our data showed that all of optrA found on plasmids were flanked by IS1216E, which was similar to a previous study [26]. Other studies also found that co-localization of optrA and cfr was close to IS21–558 and IS257 in S. sciuri [4, 27]. IS1216E belongs to the IS6 family which among other mediates transmission of the vancomycin resistance gene vanA in E. faecium, the oxazolidinone resistance gene cfr in E. faecalis [36], the macrolide-lincosamide-streptogramin B resistance genes erm(B) and erm(T) in E. hirae [37] and Streptococcus gallolyticus subsp. pasteurianus [38], respectively, and the tetracycline resistance gene tet(S) in Streptococcus infantis [39]. This indicates that optrA can be transferred between different genus bacteria by IS-mediated recombination events. Our study found that the optrA gene was located on chromosome in a few of enterococci strains. The optrA gene was adjacent to transposon Tn558 in two strains and to Tn554 in one strain. Tn558 was also detected upstream of optrA gene in S. sciuri and E. faecalis. The functionally active Tn558 and Tn554 could excise from their host DNA and produce circular forms which precede the integration of the transposon into a new target sequence [40]. The similar genetic arrangement of Tn554 and optrA was identified in both of staphylococci and enterococci, which suggest optrA can be disseminated mediated by transposon between different genus bacteria. The optrA gene was flanked by insertion sequences or transposons, indicating that mobile genetic elements mediate horizontal transfer of optrA among different genus bacteria, which should be given more attention to avoid this novel oxazolidinone resistance gene dissemination in hospitals.
Our data showed the co-localization of resistance genes fexA (n = 13) and ermA1 (n = 9) with optrA. The gene fexA mediates resistance to fluorinated and non-fluorinated phenicols, which are widely used in livestock, but not in humans. The fexA gene was prevalent in florfenicol-resistant staphylcococci [4] and enterococci [23] from animal origin. The evidence of co-localization of fexA, ermA1 and optrA indicates that linezolid-resistant strains may be selected due to non-oxazolidinone antibiotics usage, such as macrolides (often used in hospital), florfenicol (often used in livestock) and et al.. The widespread use of florfenicol in livestock has exerted selective pressure on environmental bacteria and poses a significant public health threat to the increased resistance of the novel antibiotic linezolid.
In summary, optrA was found in most of linezolid-resistant enterococci. The high diversity of optrA-carrying genetic platforms was found even in a limited number of analyzed isolates. The role of optrA in enterococci resistance to linezolid requires further investigation. The optrA gene was often flanked by insertion sequences or transposons, which might mediate the spread of optrA between different species or strains. The co-localization of fexA, ermA1 and optrA suggests that linezolid-resistant enterococci can be selected by other antibiotics such as macrolides and so on, which should be given more attention in clinical practice.
Conclusion
We discovered the high diversity of optrA-carrying genetic platforms in our limited number of analyzed isolates. MGE mediated the dissemination of optrA between different species or strains. The optrA gene was found in most of the linezolid-resistant enterococci. Further studies should be done to clarify the linezolid resistance mechanism of optrA gene in Enterococcus species.
Acknowledgments
The authors would like to thank Ji Zeng (Department of Clinical Laboratory, Wuhan Fourth Hospital), Qing Yang (Department of Clinical Laboratory, 1st Affiliated Hospital of Zhejiang University, Zhejiang University, Zhejiang, 310003, People’s Republic of China), Rong Zhang (Department of Clinical Laboratory, 2nd Affiliated Hospital of Zhejiang University, Zhejiang University, Zhejiang, 310009, People’s Republic of China), Xiaobo Ma (Department of Clinical Laboratory, 1st Affiliated Hospital of Xiamen University, Xiamen University, Fujian, 361003, People’s Republic of China), Yingmei Liu (Department of Clinical Laboratory, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China), Weiyuan Wu (Department of Clinical Laboratory, Shenzhen People’s Hospital, Shenzhen, 518020, People’s Republic of China) and Dakang Hu (Department of Clinical Laboratory, Taizhou Hospital of Zhejiang Province, Zhejiang, 317000, People’s Republic of China) for collecting the Linezolid-resistant strains.
Abbreviations
- ABC
ATP-binding cassette
- cfr
chloramphenicol-florfenicol resistance
- IS
Insertion sequences
- MATE
Multidrug and toxic compound extrusion
- MDR
Multi-drug resistant
- MGE
Mobile genetic element
- MICs
Minimal inhibitory concentrations
- MRSA
Methicillin-resistant Staphylococcus aureus
- PhLOPSA
Phenicols, lincosamide, oxazolidinones, pleuromutilin, and streptogramin A
- VRE
Vancomycin-resistant enterococci
- VRSA
Vancomycin-resistant Staphylococcus aureus
- WGS
Whole-genome sequencing
Authors’ contributions
HW conceived and designed the study. HC, XW, YY, SL, YZ and QW performed experiments described in this study. HC wrote the draft, and HW revised it. All authors approved the final version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81471990).
Availability of data and materials
The sequences of the optrA-containing regions of 13 enterococci strains have been deposited at GenBank under the following accession numbers MH225413 (1202_13E004), MH225414 (1202_21W014), MH225415 (1203_10W003), MH225416 (1207_26W003), MH225417 (19506), MH225418 (19677), MH225419 (29462), MH225420 (SZ21494), MH225421 (TZ2), MH225422 (WHXH), MH225423 (XM2013_42321), MH225424 (XM2013_71028) and MH225425 (ZJ11066).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongbin Chen and Xiaojuan Wang contributed equally to this work.
Contributor Information
Hongbin Chen, Email: swimmingfish01@163.com.
Xiaojuan Wang, Email: curliele@163.com.
Yuyao Yin, Email: yuyao1213@126.com.
Shuguang Li, Email: sdu_lsg@126.com.
Yawei Zhang, Email: z_yw1990@163.com.
Qi Wang, Email: wangqi99887@sina.com.
Hui Wang, Email: whuibj@163.com.
References
- 1.Brickner SJ, Barbachyn MR, Hutchinson DK, Manninen PR. Linezolid (ZYVOX), the first member of a completely new class of antibacterial agents for treatment of serious gram-positive infections. J Med Chem. 2008;51(7):1981–1990. doi: 10.1021/jm800038g. [DOI] [PubMed] [Google Scholar]
- 2.Meka VG, Pillai SK, Sakoulas G, Wennersten C, Venkataraman L, DeGirolami PC, Eliopoulos GM, Moellering RC, Jr, Gold HS. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J Infect Dis. 2004;190(2):311–317. doi: 10.1086/421471. [DOI] [PubMed] [Google Scholar]
- 3.Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet. 2001;358(9277):207–208. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 4.Fan R, Li D, Fessler AT, Wu C, Schwarz S, Wang Y. Distribution of optrA and cfr in florfenicol-resistant Staphylococcus sciuri of pig origin. Vet Microbiol. 2017;210:43–48. doi: 10.1016/j.vetmic.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 5.RE M, LM D, RN J. Linezolid update: stable in vitro activity following more than a decade of. Drug Resist Updat. 2014;17(1–2):1–12. doi: 10.1016/j.drup.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 6.AM G, SS J, A R, LH H, A G, K L, B V, F K. Identification of 8-methyladenosine as the modification catalyzed by the radical. Rna. 2009;15(2):327–336. doi: 10.1261/rna.1371409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du XD, Dai L, Zhang W, Zhang Q, Shen J. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother. 2012;56(3):1650–4. 10.1128/AAC.06091-11 Epub 02011 Dec 06027. [DOI] [PMC free article] [PubMed]
- 8.Antonelli A, D'Andrea MM, Brenciani A, Galeotti CL, Morroni G, Pollini S, Varaldo PE, Rossolini GM. Characterization of poxtA, a novel phenicol-oxazolidinone-tetracycline resistance. J Antimicrob Chemother. 2018;73(7):1763–9. [DOI] [PubMed]
- 9.Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol. 2005;57(4):1064–1073. doi: 10.1111/j.1365-2958.2005.04754.x. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz S, Werckenthin C, Kehrenberg C. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob Agents Chemother. 2000;44(9):2530–2533. doi: 10.1128/AAC.44.9.2530-2533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai L, Wu CM, Wang MG, Wang Y, Huang SY, Xia LN, Li BB, Shen JZ. First report of the multidrug resistance gene cfr and the phenicol resistance gene fexA in a Bacillus strain from swine feces. Antimicrob Agents Chemother. 2010;54(9):3953–3955. doi: 10.1128/AAC.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Wang Y, Schwarz S, Li Y, Shen Z, Zhang Q, Wu C, Shen J. Transferable multiresistance plasmids carrying cfr in Enterococcus spp. from swine and farm environment. Antimicrob Agents Chemother. 2013;57(1):42–48. doi: 10.1128/AAC.01605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Schwarz S, Shen Z, Zhou N, Lin J, Wu C, Shen J. Detection of the staphylococcal multiresistance gene cfr in macrococcus caseolyticus and Jeotgalicoccus pinnipedialis. J Antimicrob Chemother. 2012;67(8):1824–1827. doi: 10.1093/jac/dks163. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li D, Song L, Liu Y, He T, Liu H, Wu C, Schwarz S, Shen J. First report of the multiresistance gene cfr in Streptococcus suis. Antimicrob Agents Chemother. 2013;57(8):4061–4063. doi: 10.1128/AAC.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wu CM, Schwarz S, Shen Z, Zhang W, Zhang Q, Shen JZ. Detection of the staphylococcal multiresistance gene cfr in Proteus vulgaris of food animal origin. J Antimicrob Chemother. 2011;66(11):2521–2526. doi: 10.1093/jac/dkr322. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, He T, Schwarz S, Zhou D, Shen Z, Wu C, Ma L, Zhang Q, Shen J. Detection of the staphylococcal multiresistance gene cfr in Escherichia coli of domestic-animal origin. J Antimicrob Chemother. 2012;67(5):1094–1098. doi: 10.1093/jac/dks020. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Yang Q, Zhang R, He W, Ma X, Zhang J, Xia F, Zhao F, Cao J, Liu Y, et al. In vitro antimicrobial activity of the novel oxazolidinone tedizolid and comparator agents against Staphylococcus aureus and linezolid-resistant gram-positive pathogens: a multicentre study in China. Int J Antimicrob Agents. 2014;44(3):276–277. doi: 10.1016/j.ijantimicag.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Wang Y, Schwarz S. Presence and dissemination of the multiresistance gene cfr in gram-positive and gram-negative bacteria. J Antimicrob Chemother. 2013;68(8):1697–1706. doi: 10.1093/jac/dkt092. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, Mendes RE. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2015;59(10):6256–6261. doi: 10.1128/AAC.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen LH, Vester B. A cfr-like gene from Clostridium difficile confers multiple antibiotic resistance by the same mechanism as the cfr gene. Antimicrob Agents Chemother. 2015;59(9):5841–5843. doi: 10.1128/AAC.01274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Dai L, Sahin O, Wu Z, Liu M, Zhang Q. Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen campylobacter. J Antimicrob Chemother. 2017;72(6):1581–1588. doi: 10.1093/jac/dkx023. [DOI] [PubMed] [Google Scholar]
- 22.Shore AC, Brennan OM, Ehricht R, Monecke S, Schwarz S, Slickers P, Coleman DC. Identification and characterization of the multidrug resistance gene cfr in a Panton-valentine leukocidin-positive sequence type 8 methicillin-resistant Staphylococcus aureus IVa (USA300) isolate. Antimicrob Agents Chemother. 2010;54(12):4978–4984. doi: 10.1128/AAC.01113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70(8):2182–2190. doi: 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 24.Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, Yu S, Zhao K, Gu D, Wang X, et al. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010-2014. Clin Microbiol Infect. 2015;21(12):1095.e1091–1095.e1094. doi: 10.1016/j.cmi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Fan R, Li D, Wang Y, He T, Fessler AT, Schwarz S, Wu C. Presence of the optrA gene in methicillin-resistant Staphylococcus sciuri of porcine origin. Antimicrob Agents Chemother. 2016;60(12):7200–7205. doi: 10.1128/AAC.01591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He T, Shen Y, Schwarz S, Cai J, Lv Y, Li J, Fessler AT, Zhang R, Wu C, Shen J, et al. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother. 2016;71(6):1466–1473. doi: 10.1093/jac/dkw016. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Wang Y, Schwarz S, Cai J, Fan R, Li J, Fessler AT, Zhang R, Wu C, Shen J. Co-location of the oxazolidinone resistance genes optrA and cfr on a multiresistance plasmid from Staphylococcus sciuri. J Antimicrob Chemother. 2016;71(6):1474–1478. doi: 10.1093/jac/dkw040. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Wu W, Ni M, Liu Y, Zhang J, Xia F, He W, Wang Q, Wang Z, Cao B, et al. Linezolid-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: molecular epidemiology and resistance mechanisms. Int J Antimicrob Agents. 2013;42(4):317–321. doi: 10.1016/j.ijantimicag.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, Jones RN. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother. 2010;65(11):2329–2335. doi: 10.1093/jac/dkq331. [DOI] [PubMed] [Google Scholar]
- 30.Nurk S, Bankevich A, Antipov D, Gurevich AA, Korobeynikov A, Lapidus A, Prjibelski AD, Pyshkin A, Sirotkin A, Sirotkin Y, et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol. 2013;20(10):714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu2153. [DOI] [Google Scholar]
- 32.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(Database issue):D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 35.Cui L, Wang Y, Lv Y, Wang S, Song Y, Li Y, Liu J, Xue F, Yang W, Zhang J. Nationwide surveillance of novel Oxazolidinone resistance gene optrA in Enterococcus isolates in China from 2004 to 2014. Antimicrob Agents Chemother. 2016;60(12):7490–7493. doi: 10.1128/AAC.01256-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darini AL, Palepou MF, Woodford N. Effects of the movement of insertion sequences on the structure of VanA glycopeptide resistance elements in Enterococcus faecium. Antimicrob Agents Chemother. 2000;44(5):1362–1364. doi: 10.1128/AAC.44.5.1362-1364.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raze D, Dardenne O, Hallut S, Martinez-Bueno M, Coyette J, Ghuysen JM. The gene encoding the low-affinity penicillin-binding protein 3r in Enterococcus hirae S185R is borne on a plasmid carrying other antibiotic resistance determinants. Antimicrob Agents Chemother. 1998;42(3):534–539. doi: 10.1128/AAC.42.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai JC, Hsueh PR, Chen HJ, Tseng SP, Chen PY, Teng LJ. The erm(T) gene is flanked by IS1216V in inducible erythromycin-resistant Streptococcus gallolyticus subsp. pasteurianus. Antimicrob Agents Chemother. 2005;49(10):4347–4350. doi: 10.1128/AAC.49.10.4347-4350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciric L, Brouwer MS, Mullany P, Roberts AP. Minocycline resistance in an oral Streptococcus infantis isolate is encoded by tet(S) on a novel small, low copy number plasmid. FEMS Microbiol Lett. 2014;353(2):106–115. doi: 10.1111/1574-6968.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kehrenberg C, Schwarz S. Florfenicol-chloramphenicol exporter gene fexA is part of the novel transposon Tn558. Antimicrob Agents Chemother. 2005;49(2):813–815. doi: 10.1128/AAC.49.2.813-815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences of the optrA-containing regions of 13 enterococci strains have been deposited at GenBank under the following accession numbers MH225413 (1202_13E004), MH225414 (1202_21W014), MH225415 (1203_10W003), MH225416 (1207_26W003), MH225417 (19506), MH225418 (19677), MH225419 (29462), MH225420 (SZ21494), MH225421 (TZ2), MH225422 (WHXH), MH225423 (XM2013_42321), MH225424 (XM2013_71028) and MH225425 (ZJ11066).