Abstract
Primary gastric squamous cell carcinoma (SCC) is rare, and the simultaneous Helicobacter pylori infection has not been reported in the literature. Here, we presented a patient with concurrent H. pylori gastritis and primary gastric SCC. A 54-year-old Hispanic man presented with diarrhoea, chills, night sweats and weight loss of 16 lbs for the previous 6 weeks. Abdominal CT revealed large exophytic mass from the stomach infiltrating multiple organs. Biopsy was performed and histology showed squamoid features. Immunohistochemistry stain was positive for p40, CK5/6, CK7 and Helicobacter type organisms. Patient was diagnosed with primary gastric SCC and has been receiving chemotherapy. We also reviewed the diagnosis, prognosis and treatment of primary gastric SCC.
Keywords: gastrointestinal system, GI bleeding, gastric cancer
Background
Stomach cancer is the fifth most common cancer in all newly diagnosed cancer cases (after exclusion of non-melanoma skin cancer), yet it is the second most common cause of cancer death worldwide.1 Over 70% of gastric cancers occur in developing countries in Eastern Asia, Eastern Europe and South America.2 Although stomach cancer is less common in developed countries like the USA, in 2019, 27 510 Americans (17 230 in men and 10 280 in women) will be diagnosed with stomach cancer and 11 140 people (6800 men and 4340 women) will die from it.3 Approximately 90%–95% of gastric tumours are adenocarcinomas; 3% are mucosa-associated lymphoid tissue (MALT) lymphomas. Others include gastrointestinal stromal tumour (GIST) and carcinoid tumour. Primary gastric squamous cell carcinomas (SCCs) are very rare tumours of the stomach with a reported prevalence of 0.04%–0.07% of all gastric cancers.4
Association of stomach cancer and Helicobacter pylori has been widely reported in the literature5; however, none reported the association of gastric SCC and H. pylori. H. pylori colonisation and infection affects 80% of the population by age 20 in developing Eastern Asian and part of Latin American countries and approximately 40% by 30–40 years of age in developed countries like the USA.6 H. pylori infection is estimated to be responsible for almost 90% of all non-cardia gastric cancers worldwide and approximately 5% of the total burden from all cancers globally.7 Besides its known risk for MALT lymphoma, H. pylori infection increases the risk of gastric adenocarcinoma in non-cardia gastric cancers by nearly six times.8
Primary gastric SCC has been reported in case and case series studies with a total number of around 100 reported cases9; however, none of the previous publications described H. pylori infection in these patients. Here, we presented a patient with concurrent H. pylori gastritis and primary gastric SCC. We also reviewed the diagnosis, prognosis and treatment of primary gastric SCC.
Case presentation
Patient is a 54-year-old Hispanic man with a medical history of type 2 diabetes, hypertension, obesity, hypothyroidism, one episode of pancreatitis from hypertriglyceridaemia, B12 deficiency and history of neck surgery for cervical spondylosis and occasional smoking. He presented with diarrhoea, chills, night sweats and weight loss of 16 lbs for the previous 6 weeks. He reported a few loose maroon-coloured, malodorous, mucous diarrhoeas per day. Additionally, he also had dull localised left upper quadrant pain (8 to 10 out of 10 on Visual Analogue Scale), worse with sitting and deep respiration. The patient had no nausea, vomiting, haemoptysis, haematemesis or haematochezia.
Investigations
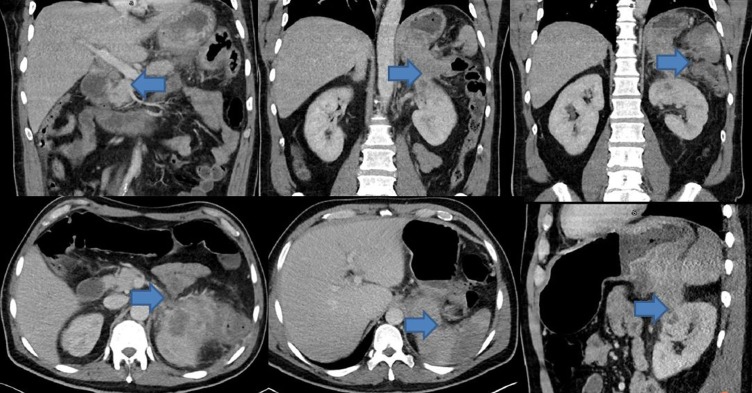
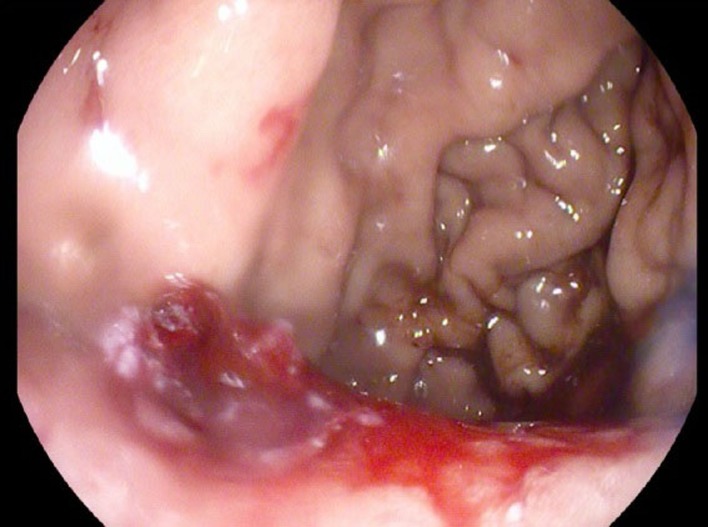
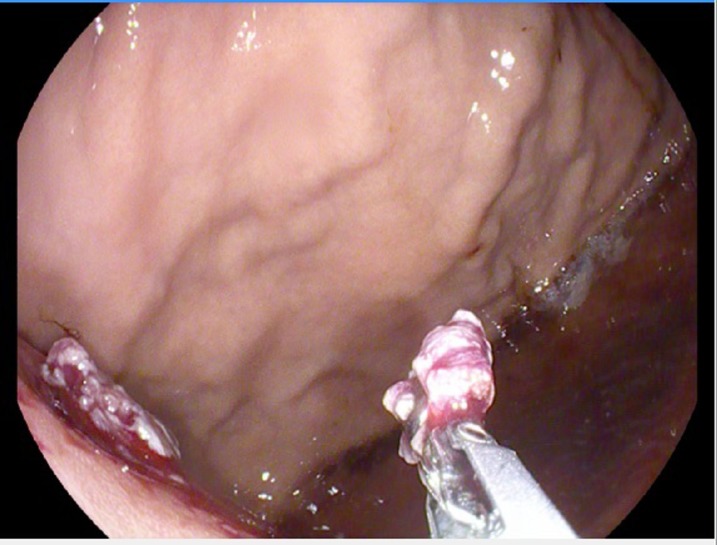
Significant lab workup included haemoglibin 10.3 g/L, and white blood cell count 14.9×109/L, carbohydrate antigen (CA) 19–9 elevation at 144.2, normal alpha-fetoprotein (AFP) at 1.2 and borderline elevation of carcinoembryonic antigen (CEA) at 5.4. Abdominal CT with intravenous contrast revealed large infiltrative heterogeneous mass in the left upper quadrant extending throughout the pancreatic tail, into the stomach, splenic flexure of the colon, left kidney, left adrenal gland and occlusion of the splenic artery with extensive splenic infarction (figure 1). Oesophageaogastric endoscopy revealed normal oesophageal mucosa in its entirety with no mucosal abnormalities or ulcerations and well demarcated squamocolumnar junction, extrinsic compression of the posterior wall of the fundus of the stomach immediately distal to the gastric cardia by a retrogastric exophytic mass in the lesser curvature that displayed central ulceration (figures 2 and 3). Biopsy of the mass was performed and histopathology evaluation was completed.
Figure 1.
Large mixed density mass involving the tail of pancreas, stomach, spleen, left adrenal gland and the superior pole of the left kidney. The blue arrows indicate cancer-affected organs.
Figure 2.
Gastric ulceration.
Figure 3.
Gastric intrusion from the retrogastric exophytic mass.
Outcome and follow-up
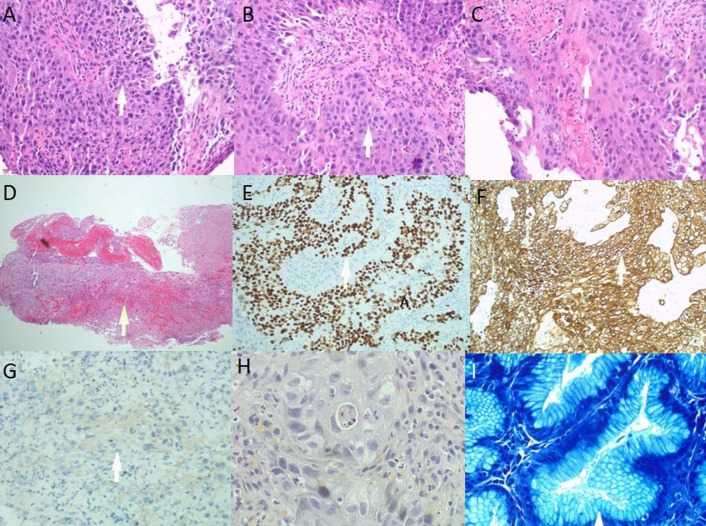
Histology showed involvement of the gastric mucosa by nests and sheets of large atypical epithelial cells with enlarged oval to pleomorphic nuclei with prominent single to multiple nucleoli and moderate amounts of eosinophilic cytoplasm (figure 4A,B). Some cell groups showed intercellular bridges and evidence of keratinisation including some individual cell keratinisation (figure 4C). Several mitotic figures and some apoptotic bodies were noted. Extensive areas of ulceration and granulation tissue were seen (figure 4D). Focal areas showed some residual gastric mucosa with chronic active gastritis. No intestinal metaplasia was appreciated while scant residual areas showed intact gastric epithelium/mucosa. Immunohistochemical stains were performed with adequate controls. Neoplastic cells were positive for p40 (figure 4E), CK5/6 (figure 4F) and CK7 while negative for TTF-1 (figure 4G), CK20 and CDX2. To rule out primary lung SCC, mucicarmine special stain block B2 was performed and did not show any intracytoplasmic mucin and neoplastic cells (figure 4H). Giemsa stain showed residual gastric mucosa with numerous Helicobacter type organisms, consistent with H. pylori gastritis (figure 4I). The morphology and immunophenotype features of immunohistochemistry were consistent with SCC, moderately differentiated.
Figure 4.
(A, B) Squamoid features; (C) individual cell keratinisation; (D) surface ulceration; (E) p40 stain; (F) CK5/6 stain; (G) TTF-1 stain; (H) mucicarmine special stain; (I) Helicobacter pylori stain.
Whole body positron emission tomography (PET) scan did not reveal any suspicious metastatic disease. Patient was diagnosed with primary gastric SCC and has been receiving chemotherapy. He had a follow-up abdominal CT half month later and revealed increased cancer mass size.
Discussion
The following diagnostic criteria for primary gastric SCC were proposed by Parks in196710: (1) the tumour must not be located in the cardia; (2) the tumour must not extend into the oesophagus and (3) there should be no evidence of SCC in any other part of the body. In 2011, the Japanese Gastric Cancer Association proposed the following diagnostic criteria: (1) SCC cells without any glandular cancer cells and (2) sufficient evidence supporting cancer from the gastric mucosa.11 Histopathological diagnostic criteria for SCC include at least one of the following: keratin pearls, mosaic cell arrangement, intercellular bridges and high concentration of sulfhydryl and/or disulfide groups indicating the presence of keratin or prekeratin.12 In the present case, the invasive nature of the disease ruled out background squamous epithelial heterotopia in the stomach which is a benign condition. The immediately distal to the gastric cardia location, normal oesophageal mucosa in its entirety, immunohistochemistry findings and PET scan of no suspicious cancerous changes outside stomach confirm the diagnosis of primary gastric SCC.
Because of its rarity, understandings of primary gastric SCC are mainly based on case reports and case series studies.13 Gastric SCC predominantly affects male patients with an age of around 60,13 14 and abdominal pain and weight loss are the two most common symptoms. The most common location of the tumour is lesser curvature, followed by greater curvature. Tumour in the fundus of the stomach and posterior gastric wall has also been reported. The present case depicted a 54-year-old man with gastric SCC at retrogastric lesser curvature who presented with left upper quadrant abdominal pain and weight loss together with some acute features.
The present case had exophytic growth compressing and invading multiple extragastric organs and caused splenic thrombosis. These presentations are consistent with the description by Hera et al15; whereas differ from others like the one reported by Segura et al.16 These findings indicate that primary gastric SCC may manifest with either an intragastric (as reported by Segura et al16) or exophytic mass (as described by Hera et al15). Intragastric mass usually can be managed with surgery whereas exophytic mass usually compresses and invades multiple organs and thus these patients are usually poor candidates for surgery.
Surgical excision may improve the prognosis and remains the only potential cure for primary gastric SCC.13 Unfortunately, because of its advanced stage upon diagnosis, the majority of the patients are not surgical candidate; various palliative chemo regimens including fluoropyrimidine-based, platin-based and taxane-based combined chemotherapy have been reported. With these chemotherapies, primary gastric SCC has an overall survival ranging from 2 to 22 months (median 7 months) in the previous reports, usually longer in patients who had surgery with a progression-free survival of 10 months from the date of surgery to recurrence or metastasis.13 In two case reports with concurrent chemoradiotherapy, the overall survival was actually increased to 27 months17 and 60 months.18 It is worth noting that Chen et al9summarised data of 21 patients with primary gastric SCC and found no significant difference in median survival time between patients treated with surgery alone and those with surgery plus adjuvant therapy (46 vs 51 months, p=0.310).
The relationship between H. pylori infection and primary gastric SCC is unclear. The possibilities are: H. pylori increases risk of SCC, SCC increases the risk for H. pylori infection, the interaction between SCC and H. pylori increases the risk for both diseases, or the two has no association in the stomach. Nonetheless, their simultaneous occurrences indicate that H. pylori infection will less likely be beneficial for the development and/or treatment of primary gastric SCC.
Researchers have proposed complete eradication of H. pylori for the prevention of gastric cancers, nearly all referring to gastric adenocarcinoma.5 In a recent randomised controlled trial, patients with early gastric cancer who received H. pylori eradication had a lower rate of metachronous gastric cancer recurrence and more improvement from baseline in the grade of gastric glandular atrophy at the corpus.19 Our case study supports the idea of H. pylori screening and treatments in patients of Asian or Latin ethnicity with gastrointestinal symptoms and population at risk. Eradication of H. pylori may also be beneficial for the prevention of primary gastric SCC.
This case study reported a case with concurrent primary gastric SCC and H. pylori infection. H. pylori screening and treatments in population at risk may not only be beneficial for the prevention of gastric adenocarcinoma, but also primary SCC of the stomach.
Learning points.
Primary gastric squamous cell carcinoma (SCC) can be present together with Helicobacter pylori infection.
Left upper abdominal pain and weight loss with acute symptoms are common.
Surgery remains the cure, but palliative chemotherapy or chemoradiotherapy is usually required for primary gastric SCC.
H. pylori screening and eradication may be necessary for high risk patients for peptic ulcer and gastric cancer.
Footnotes
Contributors: KZ wrote the majority of the article; AF wrote a portion of the manuscript; MM and MT provided constructive feedback during the manuscript preparation and drafting process.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol 2014;20:4483–90. 10.3748/wjg.v20.i16.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Key Statistics About Stomach Cancer [Internet]. https://www.cancer.org/cancer/stomach-cancer/about/key-statistics.html
- 4. Hwang SH, Lee JH, Kim K, et al. Primary squamous cell carcinoma of the stomach: A case report. Oncol Lett 2014;8:2122–4. 10.3892/ol.2014.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:1113–24. 10.1053/j.gastro.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 6. Conteduca V, Sansonno D, Lauletta G, et al. H. pylori infection and gastric cancer: state of the art (review). Int J Oncol 2013;42:5–18. 10.3892/ijo.2012.1701 [DOI] [PubMed] [Google Scholar]
- 7. Moss SF. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol Gastroenterol Hepatol 2017;3:183–91. 10.1016/j.jcmgh.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347–53. 10.1136/gut.49.3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Zhu H, Xu F, et al. Clinicopathological Characteristics, Treatment, and Prognosis of 21 Patients with Primary Gastric Squamous Cell Carcinoma. Gastroenterol Res Pract 2016;2016:1–6. 10.1155/2016/3062547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. PARKS RE. Squamous neoplasms of the stomach. Am J Roengen 1967;101:447–9. 10.2214/ajr.101.2.447 [DOI] [PubMed] [Google Scholar]
- 11. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 12. Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer 1965;18:181–92. [DOI] [PubMed] [Google Scholar]
- 13. Meng Y, Zhang J, Wang H, et al. Poorer prognosis in patients with advanced gastric squamous cell carcinoma compared with adenocarcinoma of the stomach: Case report. Medicine 2017;96:e9224 10.1097/MD.0000000000009224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guzman Rojas P, Parikh J, Vishnubhotla P, et al. Primary Gastric Squamous Cell Carcinoma. Cureus 2018;10:e2389 10.7759/cureus.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hara J, Masuda H, Ishii Y, et al. Exophytic primary squamous cell carcinoma of the stomach. J Gastroenterol 2004;39:299–300. 10.1007/s00535-003-1294-5 [DOI] [PubMed] [Google Scholar]
- 16. Segura S, Pender J, Dodge J, et al. Primary Squamous Cell Carcinoma of the Stomach: A Case Report and Review of the Literature. Conn Med 2016;80:209–12. [PubMed] [Google Scholar]
- 17. von Waagner W, Wang Z, Picon AI. A Rare Case of a Primary Squamous Cell Carcinoma of the Stomach Presenting as a Submucosal Mass. Case Rep Surg 2015;2015:1–5. 10.1155/2015/482342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt C, Schmid A, Lüttges JE, et al. Primary squamous cell carcinoma of the stomach. Report of a case and review of literature. Hepatogastroenterology 2001;48:1033–6. [PubMed] [Google Scholar]
- 19. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med 2018;378:1085–95. 10.1056/NEJMoa1708423 [DOI] [PubMed] [Google Scholar]