Abstract
Recently,carbon-based nanomaterials have been attracted much interest among the scientific community due to its extraordinary properties and applications. Mostly the fluorescent carbon nanomaterials are prepared from commercially available precursors. In this work, develop a new strategy for producing carbon nanoparticles (carbon dots) using phosphoric acid as an activating agent from water hyacinth present in Assam, India. These carbon nanoparticles show green fluorescence under UV light, and the sizes are found below 10 nm. These carbon dots are applied as a fluorescence sensor for detecting the herbicide (pretilachlor). The developed PL sensor is exclusively selective and sensitive for detection of this herbicide, and the limit of detection is found to be 2.9 μM.This sensor is also tested for real samples like soil contaminated with pretilachlor.
Keywords: Materials chemistry, Materials science, Carbon dots, Pretilachlor, Herbicide, Sensor, Photoluminescence
1. Introduction
Carbon dots with extraordinary applications and properties have become a rising star as a new nanocarbon member for the scientific community. These consist of mainly sp3 bonded carbon backbone with sizes below 10nm [1, 2]. They are also referred to as carbon nano lights because of their good solubility and strong luminescence. That's why photo-luminescent carbon-based quantum dots are preferred than traditional semiconductor quantum dots and organic dyes due to their high (aqueous) solubility, robust chemical inertness, facile modification and high resistance to photobleaching [3, 4]. The carbon-based quantum dots are entrusted with potential applications in bio-imaging [5], biosensor, biomolecule/drug delivery [6]because of their superior biological properties, and also optoelectronics [7, 8], catalysis,etc.There are numbers of precursors (commercial and natural) reported for the synthesis of carbon dots [9, 10, 11]. Here, wastewater hyacinth leaves are used asa precursor for carbon dots. It is a waste bio-material occurring naturally and hence, prepared CDs are cheap and also biocompatible.
Pesticides are substances that are employed mostly to control agricultural pests, weeds, etc. The term pesticide contains herbicide, insecticides, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, etc. [12, 13, 14]. However, they have increased crop yield as well as reduced post-harvest losses intensification of agricultural practices. Butthis causes an accumulation of pesticide residues that can possess a serious threat to the environment as well as human health worldwide. As most of the pesticides are toxic, the use of these chemicalscan often result in a loss of biodiversity to plants, birds, and animals. These compounds have beenfound widelyin water, soil, sewage sludge, sediment, and the aquatic biota [15, 16]. In human beings, they may increase the risk of different diseases like psychiatric disorders, renal, hepatic, neurological problems, etc. For these reasons, there is an urgent need to monitor and detect such compounds in the environment [17]. The herbicide is the most popular of all of them, which accounts for approximately 80% of all pesticide useare mainly used to control unwanted plants. There are only a fewreports in the literature to detect pesticide using carbon dot based systems, and this area of research is evolving. Long Wang and his co-workers have developed a carbon dot-based fluorescence sensor for glyphosate in environmental water samples [18]. They used thiourea as the carbon source and diethylene glycol asthe reaction medium for the preparation of carbon dots. Fluorescence probe for selective detection of paraoxon-ethyl pesticide is also developed using carbon dots prepared from carbonization of sucrose by M. Chang and his co-workers [19]. A new Fluorescence Detection technique for detection of glyphosate-based on an immune reaction method using carbon dot is also established by Duo Wang et al. [20]. Wool-based microwaved assisted carbon dot as a sensor for glyphosate based on the inner filter effect is also demonstrated [21]. On the other hand,fluorescence-based sensing has become popular in the present times due to the benefits such as excellent sensitivity, short response time and low cost [22]. There is a need to develop cheaper sensing material so as to make it economically viable for commercialization.
PLsensing of the pesticide (herbicide) using carbon dot can be considered as a cheaper, easier method. A herbicide named (pretilachlor) has been used as the analyte for sensing in the present work undertaken. It is used for the control of grasses, sedges and broad-leaved weed like Echinocloa, Cyperus, Panicumin the transplanted rice. This herbicide has a specific working phenomenon where it surpasses the growth of weeds by reducing the cell division [23, 24]. It does not harm crops by leaving yellowing or stunting effects thus, rendering a greening effect on the crops. But this herbicide is poisonous and toxic to humans if inhaled or consumed accidentally or when used in excess. The harmful effects of this herbicide poisoning are as follows-the early symptoms may be a headache, giddiness, vertigo, sweating and excessive lacrimation and salivation. It is also harmful to the skin and eyes as well.
Usually, water hyacinth wastesare used as a natural source for the synthesis of activated carbon. Hereis the first time, carbon dots (CDs) is synthesized from water hyacinth. The prepared CDs have been employed to be used as a PL sensor for sensing the herbicide (namely pretilachlor). This is the first report of detection of any herbicide (pretilachlor) by carbon dot (prepared from water hyacinth).
2. Experimental
2.1. Chemicals used
Water hyacinth which was collected from Deepor Bil (water body) is located to the south-west of Guwahati, Assam, India. The phosphoric acid and ethanol were purchased from Merck, India and Sigma-Aldrich, India respectively. All the chemicals were used as obtained without further purification. The water used throughout the experiments was from a Milli-Q water purification system.
2.2. Synthesis of CDs from water hyacinth leaves
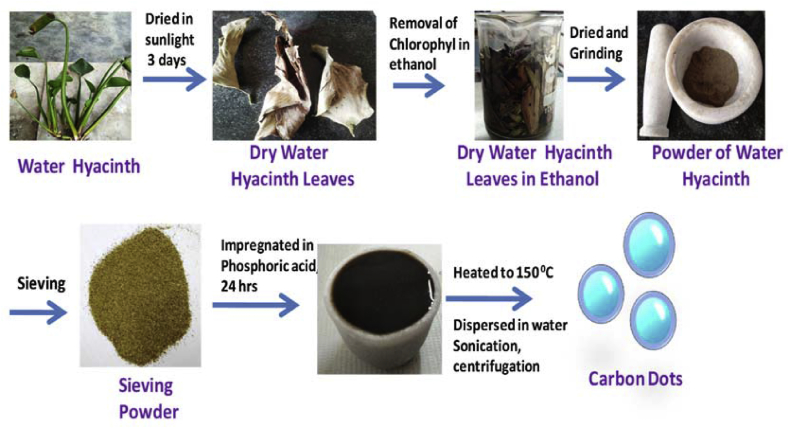
We developed a new strategy to prepare CDs from water hyacinth waste. The collected water hyacinth was washed with water and then dried it in the presence ofsunlight. The dried leaves of water hyacinth were taken was immersed in ethanol overnight for pigmentation. It was then again washed with distilled water and kept it in the oven for 48 h. The material was ground and is impregnated with 50% phosphoric acid and kept for 24 h (oven dried). The material is carbonized using a furnace from room temperature upto the desired temperature at 160 °C with heating at the rate of 10 °C min−1. After heating in the furnace, the carbonized material obtained is a slippery solution which is yellowish black in color. The carbonized material is then dispersed in water and sonication is performed for around 30 min twice. After sonication, the carbonized material is centrifuged several times. The carbon nanomaterial obtained is highly acidic in nature. The acidic carbon nanomaterial is neutralized by adding sodium hydroxide solution, and a pH value of 7 is obtained.
2.3. Characterization
The characterization of water hyacinth based CDs was carried out byDynamic Light Scattering (Malvern Zetasizer Nano series, Nano ZS90), Fourier Transform Infrared Spectrophotometer (Nicolet 6700) UV-Visible absorption spectrophotometer (Shimadzu, UV-2600), Scanning Electron Microscopy (Carl Zeiss ∑igma-VP). To check the fluorescence intensity of CDs Optics Technology UV lamp (365nm) was used. Fluorescence spectra were recorded on a Jascospectrofluorometer FP-8300, between 300 nm to the 400nm excitation wavelength.
3. Results & discussion
In this work,a novel protocol for the synthesis of carbon dots from a natural source (water hyacinth leaves) as a precursoris demonstrated. In this process, phosphoric acid is used as an activating agent to prepare CDs from waste water hyacinth leaves. Schematic representation of the protocol followed for the preparation of CDs is shown in Fig. 1.
Fig. 1.
Schematic illustration of the preparation of carbon dots (CDs) from wastewater hyacinth leaves through hydrothermal strategy.
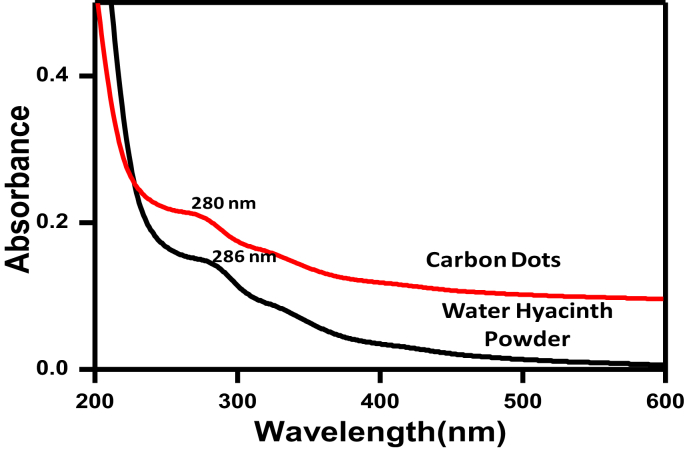
The water hyacinth and synthesized carbon dots were characterized using UV-Visible spectroscopy. It is evident from Fig. 2 it is seen that all CDs show the prominent peak between 280-290 nm. These peaks are due to the n-π* transition of OH, and Carboxylic acid (COOH) groups present on the surfaces of the CDs.Thus, the surface of the CDs is passivated with different oxygenated functional groups.
Fig. 2.
Stacked UV-Visible spectra of water hyacinth powder and carbon dots.
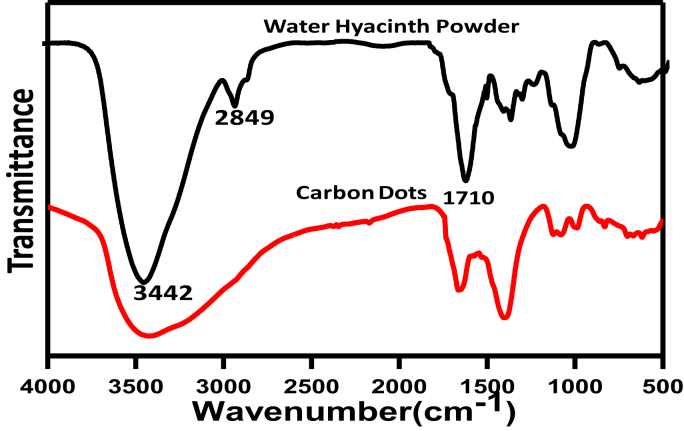
Chemical structure of prepared fluorescence CDs and water hyacinth powder are also investigated by FT-IR and shown in Fig. 3. Both the samples show the wide band at about (3373-3442 cm−1) due to O-H stretching vibration. The shoulders observed at (2849-2932 cm−1) due to aliphatic (C–H) stretching vibration. The bands near 1600 cm−1are due to C=C stretching vibration in the aromatic ring, and the bands at (1711-1713 cm−1) for stretching vibration of carboxyl groups (COO−) on the edges prepared CDs [25]. Compared to water hyacinth powder, the intensity of some peaks in CDs is decreased due to our hydrothermal protocol for preparation of carbon dots.
Fig. 3.
FT-IR spectra of water hyacinth powder and carbon dots (CDs).
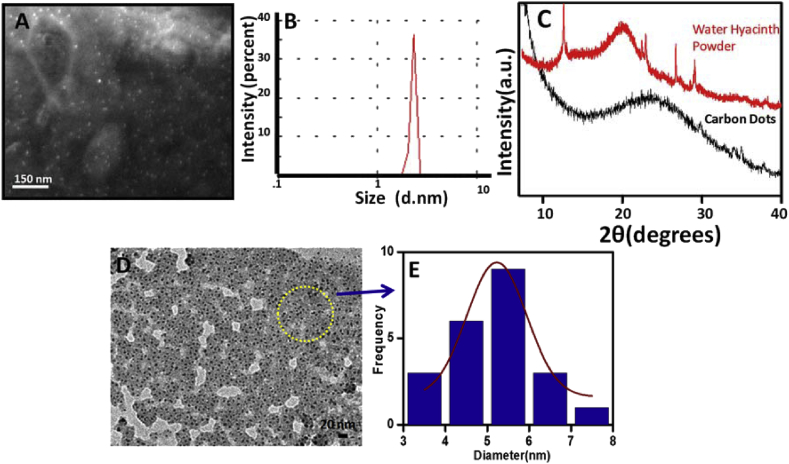
Scanning Electron Microscope Image of CDs is depicted in Fig. 4A. From the figure, it is observed that the sizes of CDs are below 10 nm and also uniformly distributed. Again DLS size (Fig. 4B) of the CDs is also measured and found that the size of the CDs is below 10 nm. SEM images and DLS size results supported each other. The XRD spectra of CDs and water hyacinth powder are also investigated and shown in Fig. 4C. The XRD spectra of water hyacinth powder show some prominent peaks which suggest crystalline nature.
Fig. 4.
A)Scanning Electron Microscope (SEM) image of CDs,B)DLS size of CDs and C) XRD spectra of CDs and water hyacinth powder,D)TEM images of CDs and E) Corresponding size distribution curve of CDs.
On the other hand broad, XRD peaks near 240are observedwhich suggest the amorphous nature of the prepared CDs [26]. Thus the crystallinity of the water hyacinth is diminished after the formation of CDs.The TEM images and corresponding size distribution curve of the CDs are also investigated and shown in Fig.4D, and E. The sizes of the CDs are found to below 10 nm and shows homogeneous size distribution. The average sizes of the prepared CDs are found to be 5.22 nm.
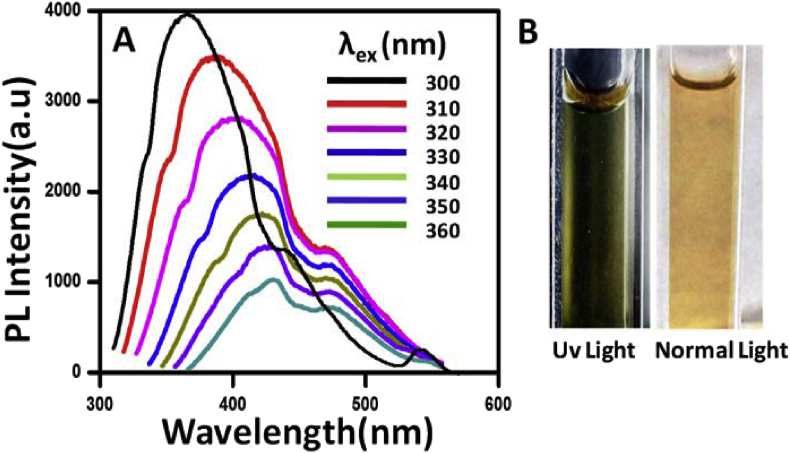
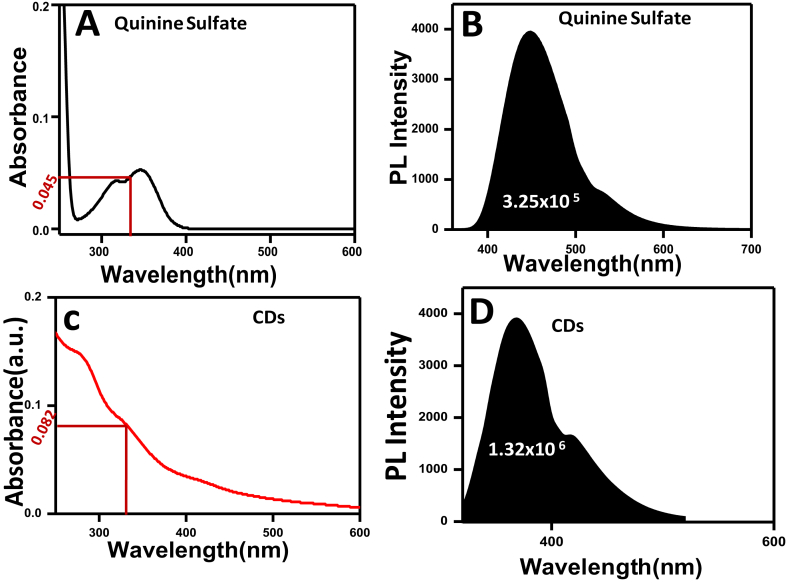
PL property of CDs on the water was also investigated and is shown in Fig. 5A. The PL spectra obtained after excitation of carbon dots at different wavelengths ranging from 300 nm to 360 nm is investigated. The highest emission intensity at 370 nm was found at an excitation wavelength of 300 nm. But after that excitation wavelength (300 nm) the PL spectra of CDs shows shifting to higher wavelength accompanied by a decrease in fluorescence intensity. It is interesting to note that the waste assisted CDs also show excitation-dependent emission behavior shown by other carbon dots prepared from different precursors. The main reason for dependent PL properties of CDs has led many assumptions like solvent relaxation around CDs, the presence of different emissive states due to small particle size distribution, discrete multiple electronic states of CDs which participated in and so forth [27, 28, 29, 30]. Thus, the PL of our synthesis CDs istunable by changing the excitation wavelength. The CDs show beautiful green PL under Uv light, and the images (snapshots) are shown in Fig. 5B. The quantum yield of the CDs is also calculated taking quinine sulfate as a reference dye [29]. The quantum yield of the CDs is found to be 17.02%.The detailed procedure is discussed in supporting information section (Fig. 6).
Fig. 5.
A) Stacked PL spectra of carbon dots excited at different wavelengths. B) Images of CDs dots in normal light (right) and under UV-lamp (left).
Fig. 6.
A) UV-Vis spectra of quinine sulfate B) integrated photoluminescence intensity of quinine sulfate C) UV-Vis spectra of CDs and D) integrated photoluminescence intensity of CDs.
The quantum yield (φ) of calculated using equation (1)
| (1) |
Where Iis the measured integrated emission intensity, Ais the optical density, η is the refractive index of the solvent, and the subscript Rrefers to the reference standard. Here we use quinine sulfate (φR= 0.54)in 0.1M H2 SO4 (η = 1.33) as standard while the CDs are dispersed in water (η = 1.33). The absorbance of all the samples is maintained less than 0.1 to minimize the inner-filter effects.
The quantum yield (φ) of CDs from Fig. 6 is found to be 17.02 %.
3.1. CDs act as a sensor for herbicide
As our oxygenated synthesis CDs shows beautiful green PL and water-soluble thus, it can be used as apotential candidate tosense pesticide. On the other hand, PL sensing using CDs can be considered as an easier and cheaper technique over others. We have used the fluorescence property of waste assisted CDs to detect pretilachlor inwater becauseherbicide becomes toxic and hazardous tothe environment if when used in excess or consumed accidentally. Thus, PL detection of herbicide through CDs isa novel approach.
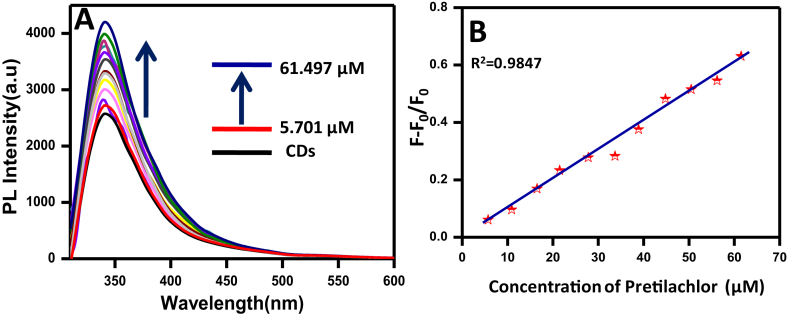
The PL responseof prepared CDswas investigatedin the presence of pretilachlor via fluorescence spectroscopy. The stacked PL emission spectra of CDs and CDs/pretilachlor system was depicted in Fig. 7. From the figure, it was observed that CDs have the highest PL intensity value (λmax) at 360 nm at an excitation wavelength of (λex)300 nm. Enhancement of PL intensity was observed linearly upon addition of pretilachlor from 5.701μM to 61.497μM. The corresponding Stern–Volmer plot of the change in PL intensity versus concentration of pretilachlor added is shown in Fig. 7B. From this curve, the linear regression constant (R2) value was calculated and found to be 0.9847. This shows a very good linear response and the line shows closeness to the fitted regression line. Here the minimum detected limits for the herbicide (pretilachlor) is found to be 2.9 μM which is equivalent to 0.9 mg/L. It is already reported that for living organism the toxicity limit of the pretilachlor is in the range of 1–8 mg/L [31]. Hence, this carbon dot based PL sensor can easily detect this herbicide if the toxicity limit falls in this range.
Fig. 7.
A) Stacked PL emission spectra of CDs and in the presence of pretilachlor at a constant λex (300 nm), B) Stern–Volmer plot of CDs-pretilachlor system showing the linear regression constant value.
3.2. Tested in a real sample(soil sample)
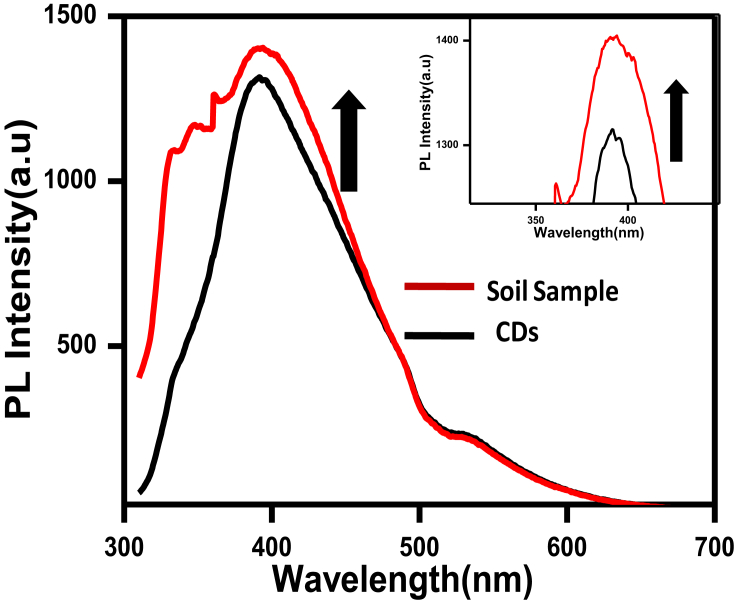
Herbicides (weed killers), e.g.,pretilachlor are chemical substances, sprayed in the soil to control unwanted plants in the crop field. It is already discussed that pretilachlor concentration higher than the permissible limit is very toxic for the environment as well as for living organism. To confirm the excellent specificity of our PL sensor, it is also checkedon contaminated soil samples. For that purpose, one crop field with contaminated soil was collected, transferred to the aqueous medium, and tested with carbon dots system. Fig. 8 shows the stacked PL spectra of CDs, and then after addition of soil sample to CDs. The addition of soil samples to CDs resulted in an increase in PL intensity (turn on) which confirms the system has good selectivity and sensitive for real sample too. This is tough to measure the exact concentration of soil contaminated with the herbicide. To prove our concept, used that collected herbicides and found that there is an increase in PL intensity of CDs in the presence of soil contaminated herbicide. Thus, this detection technique is also applicable to real soil sample contaminated with pretilachlor.
Fig. 8.
Stacked PL emission spectra of CDs and in the presence of soil sample at a constant λex (300 nm). Inset: zoom image of stacked PL emission spectra.
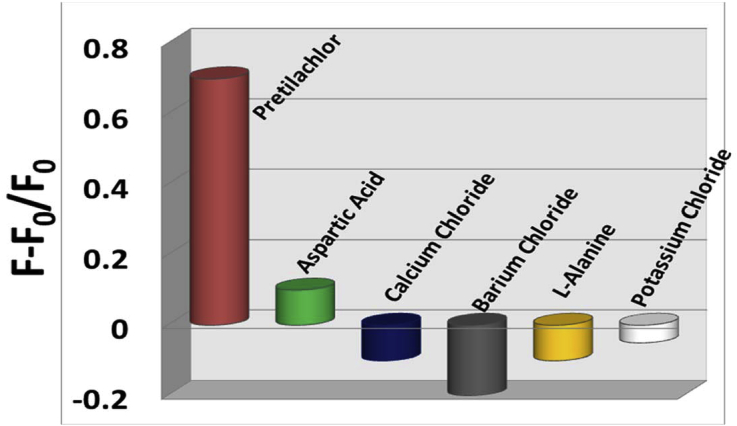
Fig. 9 Shows the comparative histogram plot of the change in PL intensity of CDs system in the presence of herbicide and some others structurally similar compounds in the same concentration range. From the plot, it is very clear that enhancement of PL intensity is observed exclusively for herbicide (pretilachlor) and whereas for other similar compounds, the enhancement of PL intensity is negligible rather some shows negligible PL quenching. Thus, our waste assisted CDs system is exclusively selective for pretilachlor.
Fig. 9.
CDs based system toward various potential interfering structurally similar molecules (aromatic and non-aromatic).
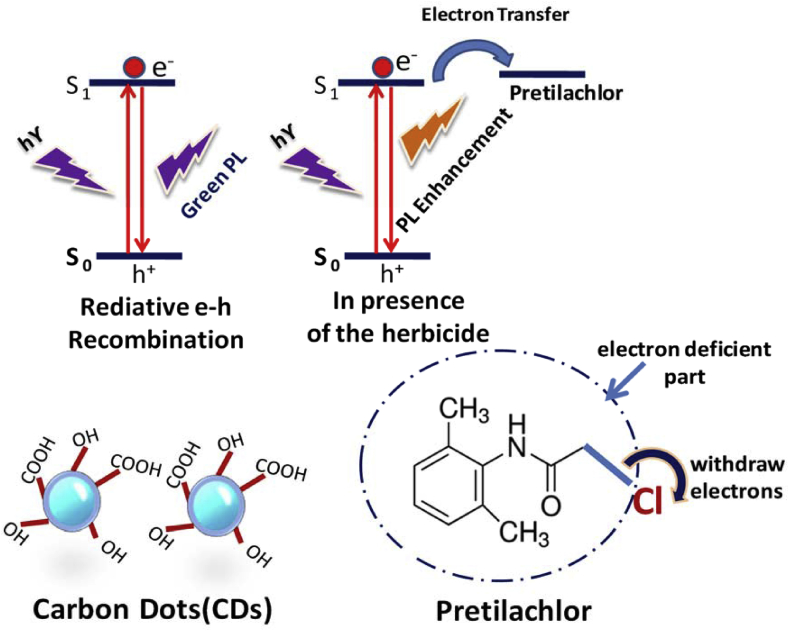
The mechanism of CDs fluorescence properties is still not well established and hence a matter of debate. From literature, it is found that there are several factors responsible for CD luminescence. These are mainly quantum confinement effect, photogenerated electron-hole pairs recombination on the surface of the CDs, the presence of different emissive traps due to various functionalities, excitons of carbon,etc. In our water hyacinth assisted CDs are passivated with oxygenated functional groups, e.g., COOH, OH,etc. Due to which the fluorescence is generated from surface trapped states as well as electron-hole recombination pathways. When excited 300nm-400 nm, the electrons are jumped to a higher energy level and thus shows beautiful green fluorescence. It is interesting to note that in the presence of CDs with pretilachlor, the higher energy electron of CDs can move to the electron deficient part of the pretilachlor (herbicide). As in this case, the chlorine atom withdraws the electron and makes the other parts of pretilachlor electron deficient. Thus, the electron transfer mechanism may be responsible for the enhancement of PL intensity in the presence ofpretilachlor (Fig. 10). We have also compared the minimum detection limit of the pretilachlor by different methods and depicted in Table 1.
Fig. 10.
Proposed mechanism for PL enhancement.
Table 1.
Comparison between different methods for pretilachlor detection.
4. Conclusion
In summary, successfully synthesizedcarbon dots from a natural source (water hyacinth waste) using phosphoric acid as the activating agent. The synthesized carbon dots show green fluorescence, and the size was found to be less than 10 nm. FT-IR and UV-Visible spectroscopy showed the presence of the oxygenated functional group on the surface of carbon dots. It possesses excitation dependent PL behavior like other carbon nanomaterials and well dispersed in an aqueous medium. The carbon dot is used as a fluorescent sensor to detect the herbicide (pretilachlor), and the PL spectra from the herbicide sensing denote the enhancement of PL intensity linearly on the addition of the herbicide from 5.7 μM to 61.5 μM, and the minimum detectable limit of herbicide was found to be 2.9 μM.This PL sensor also shows excellent selectivity and specificity for real soil sample. Hence the sensing application of carbon dot in the detection of the herbicide can be considered an important step in protecting our environment. The use of a cheap source (water hyacinth waste) as a precursor material for developing the sensor makes the process sustainable.
Declarations
Author contribution statement
Okhil Kumar Medhi, N. C. Talukdar: Conceived and designed the experiments.
Devasish Chowdhury: Conceived and designed the experiments; Wrote the paper.
Parlie Dutta, Sewaljyoti Sarma: Performed the experiments; Analyzed and interpreted the data.
Manash Jyoti Deka: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by DBT, New Delhi (Project No. BT/PR15722/NER/95/24/2015) and DST, New Delhi (No. 85).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Georgakilas V., Perman J.A., Tucek J., Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015;115:4744–4822. doi: 10.1021/cr500304f. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y., H Kwon O., Kim B.S. Carbon dots: bottom-up syntheses, properties, and light-harvesting applications. Chem. Asian J. 2018;13:586–598. doi: 10.1002/asia.201701736. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya D., Mishra M.K., De G. Carbon dots from a single source exhibiting tunable luminescent colors through the modification of surface functional groups in ORMOSIL films. J. Phys. Chem. 2017;C121:28106–28116. [Google Scholar]
- 4.Han M., Zhu S., Lu S., Song Y., Feng T., Tao S., Liu J., Yang B. Recent progress on the photocatalysis of carbon dots: classification, mechanism,andapplication. Nano Today. 2018;19:201–218. [Google Scholar]
- 5.Cao L., Wang X., Meziani M.J., Lu F., Wang H., Luo P.G., Lin Y., Harruff B.A., Veca L.M., Murray D., Xie S.-Y., Sun Y.-P. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007;129:11318–11319. doi: 10.1021/ja073527l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Huang X., Long Y., Wang X., Zhang H., Zhu R., Liang L., Teng P., Zheng H. Hollow luminescent carbon dots for drug delivery. Carbon. 2013;59:192–199. [Google Scholar]
- 7.Yuan F., Li S., Fan Z., Meng X., Fan L., Yang S. Shining carbon dots: synthesis and biomedical and optoelectronic applications. Nano Today. 2016;11:565–586. [Google Scholar]
- 8.Wan J.-Y., Yang Z., Liu Z.-G., Wang H.-X. Ionic liquid-assisted thermal decomposition synthesis of carbon dots and graphene-like carbon sheets for optoelectronic application. RSC Adv. 2016;6:61292–61300. [Google Scholar]
- 9.Wang Y., Hu A. Carbon quantum dots: synthesis, properties and applications. J. Mater. Chem. 2014;C2:6921–6939. [Google Scholar]
- 10.Wu Z.L., Liu Z.X., HYuan Y. Carbon dots: materials, synthesis, properties,and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B. 2017;5:3794–3809. doi: 10.1039/c7tb00363c. [DOI] [PubMed] [Google Scholar]
- 11.Mao L.-H., Tang W.-Q., Deng Z.-Y., Liu S.-S., Wang C.-F., Chen S. Facile access to white fluorescent carbon dots toward light-emitting devices. Ind. Eng. Chem. Res. 2014;53:6417–6425. [Google Scholar]
- 12.Hillocks R.J. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Protect. 2012;31:85–93. [Google Scholar]
- 13.Popp J., Nagy P.K. Pesticide productivity and food security. a review. Agron. Sustain. Dev. 2013;33:243–255. [Google Scholar]
- 14.Albuquerque A.F., Ribeiro J.S., Kummrow F., Nogueira A.J.A., Montagner C.C., Umbuzeiro G.A. Pesticides in Brazilian freshwaters: a critical review. Environ. Sci. Processes Impacts. 2016;18:779–787. doi: 10.1039/c6em00268d. [DOI] [PubMed] [Google Scholar]
- 15.Mourato S., OzdemirogluI E., Foster V. Evaluating health and environmental impacts of pesticide use:implications for the design of ecolabels and pesticide taxes. Environ. Sci. Technol. 2000;34:1456–1461. [Google Scholar]
- 16.Hayo van der Werf M.G. Assessing the impact of pesticides on the environment Agriculture. Ecosyst. Environ. 1996;60:81–96. [Google Scholar]
- 17.Chang P.-L., M Hsieh M., Chiu T.-C. Recent advances in the determination of pesticides in environmental samples by capillary electrophoresis. Int. J. Environ. Res. Public Health. 2016:409–429. doi: 10.3390/ijerph13040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Bi Y., Gao J., Li Y., Ding H., Ding L. Carbon dots based turn-on fluorescent probes for the sensitive determination ofglyphosate in environmental water samples. RSC Adv. 2016;6:85820–85828. [Google Scholar]
- 19.Chang M.M.F., Ginjom I.R., Ng S.M. Single-shot ‘turn-off’ optical probe for rapid detection ofparaoxon-ethyl pesticide on vegetable utilising fluorescencecarbon dots. Sensor. Actuator. B Chem. 2017:1050–1056. [Google Scholar]
- 20.Wang D., Lin B., Cao Y., Guo M., Yu Y. A highly selective and sensitive fluorescence detection method ofglyphosate-based on an immune reaction strategy of carbon DotLabeled antibody and antigen magnetic beads. J. Agric. Food Chem. 2016;64:6042–6050. doi: 10.1021/acs.jafc.6b01088. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Bi Y., Hou J., Li H., Xu Y., Wang B., Ding H., Ding L. Facile, green and clean one-step synthesis of carbon dots from wool: application as a sensor for glyphosate detection based on the inner filter effect. Talanta. 2016;160:268–275. doi: 10.1016/j.talanta.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Nie H., Minjie H.L., Li Q., Liang S., Tan Y., Sheng L., Shi W., Xio-An Zhang S. Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing. Chem. Mater. 2014:3104–3112. [Google Scholar]
- 23.Sahoo S., Adak T., Bagchi T.B., Kumar U., Munda S., Saha S., Berliner J., Jena M., Mishra B.B. Effect of pretilachlor on soil enzyme activities in tropical RiceSoil. Bull. Environ. Contam. Toxicol. 2017;98:439–445. doi: 10.1007/s00128-016-1943-z. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J., Chen Y., Yu R., Zhao X., Wang Q., Cai L. Pretilachlor has the potential to induce endocrine disruption,oxidative stress, apoptosis and immunotoxicity during zebrafish embryo development. Environ. Toxicol. Pharmacol. 2016;42:42125–42134. doi: 10.1016/j.etap.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim M., Kühnc O., Scheyttd T. Molecular spectroscopic study of water hyacinth dry matter. Open Chem. Phys. J. 2009;2:1–6. [Google Scholar]
- 26.Deka M.J., Chowdhury D. CVD assisted hydrophobic graphene quantum dots: fluorescence sensor for aromatic amino acids. ChemistrySelect. 2017;(2):1999–2005. [Google Scholar]
- 27.Deka M.J., Chowdhury D. Chiral carbon dots and their effect on the optical properties of photosensitizers. RSC Adv. 2017;7:53057–53063. [Google Scholar]
- 28.Baruah U., Gogoi N., Konwar A., Deka M.J., Chowdhury D., Majumdar G. Carbon dot based sensing of dopamine and ascorbic acid. J. Nanoparticles. 2014;2014:8. [Google Scholar]
- 29.Deka M.J., Dutta A., Chowdhury D. Tuning the wettability and photoluminescence of graphene quantum dots via covalentmodification. New J. Chem. 2018;42:355–362. [Google Scholar]
- 30.Baruah U., Deka M.J., Chowdhury D. Reversible on/ofswitching of fluorescence via esterification of carbon dots. RSC Adv. 2014;4:36917–36922. [Google Scholar]
- 31.Maryam P., Mehdi M., Morteza S., Masood F., Abbasali Z., Firouz A. Determination of the acute toxicity of pretilachlor on liver and gill issues as well as glucose and cortisol levels in fingerling grass carps (Ctenopharyngodonidella) J. Fish. Aquat. Sci. 2013;8:721–726. [Google Scholar]
- 32.Kaur P., Kaur P. Optimization of matrix solid phase dispersion method for quantification of pretilachlorin soil and rice. Agric. Res. J. 2015;52:138–142. [Google Scholar]
- 33.Jun-kai L., Xue H., Li-hua Y., Ye-jie L., Dao-xin G. Determination of pretilachlor in paddy field by HPLC. Adm. Techn. Environ. Monit. 2014;01 [Google Scholar]
- 34.Cai C., Cheng H., Wang Y. Determination of pretilachlor in soil and rice using matrix solid-phase dispersion extraction by capillary electrophoresis with field amplified sample injection and electrochemiluminescence detection. Anal. Methods. 2014;6:2767–2773. [Google Scholar]