Abstract
Epidemics of neurodegenerative diseases putatively caused by food toxins have been reported in the tropics with no clear understanding of their pathogenetic mechanisms. These diseases include the disease named Konzo that has been well documented in sub-Sahara Africa, mostly among children and women of childbearing age. Outbreaks of Konzo have occurred in the Democratic Republic of Congo, Mozambique, Tanzania, Central African Republic, Angola, Cameroun, and most recently in Zambia. The main clinical picture consists of a symmetrical, permanent and irreversible spastic paraparesis (motor neuron disease) with no signs of sensory or genitourinary impairments. Recently, cognitive impairments and neurodevelopmental delays have been reported among school-aged and very young children. The exact pathogenetic mechanisms of the disease remain unknown. Epidemiological studies consistently show an association between outbreaks of the disease and chronic dietary reliance on insufficiently processed cyanogenic cassava (manioc or tapioca). Biochemical and toxicological studies suggest that the metabolites of linamarin (α-Hydroxyisobutyronitrile β-D-glucopyranoside, the main cassava cyanogen), notably cyanide (mitochondrial toxin), thiocyanate (AMPA chaotropic agent), and cyanate (protein carbamoylating agent) may play an important role in the pathogenesis of Konzo. Experimental data suggest that thiol-redox and protein- folding mechanisms may also be perturbed. Factors of susceptibility including genetics, poor nutrition, poverty and dietary cyanogen exposure, or their interactions have been suggested. Serological studies have ruled out the role of retroviruses such as the human lymphotropic viruses HIV-I/ II or HTLV-I/II. Because there is no cure for Konzo, prevention of the disease remains of paramount importance. Prospects for cognitive rehabilitation still need to be explored and tested.
Keywords: Cassava, Cognition, Cyanogen, Konzo, Motor neuron disease
1. Epidemiology
Cassava toxicity has been incriminated in the pathogenesis of tropical myeloneuropathies, such as the tropical ataxic neuropathy (TAN) and Konzo. TAN is a progressive myeloneuropathy that was first described in Nigeria and is characterized by a progressive onset of ataxia. On the other hand, Konzo is a permanent and clinically distinct upper motor neurone disease of abrupt onset firstly described in the Democratic Republic of Congo (DRC). The word “Konzo” means tied legs and originated from the Yaka tribe in DRC to designate a fetish used by hunters to weaken legs and catch wild animals. The literature suggests that Konzo was known from the Yaka indigenous population of the Bandundu province in Zaïre, presently known as the DRC, since the end of the 19Th century. However, it is only decades later that the syndrome was first documented in the medical literature (Trolli, 1938; Van der Beken, 1993). Since then, outbreaks of Konzo have been reported in many other sub-Sahara African countries notably in Mozambique (where it is called mantakassa), Tanzania, Central African Republic (CAR), Cameroon, Angola, DRC (Chabwine et al., 2011; Ciglenecki et al., 2011; Cliff et al., 2011; Mlingi et al., 2011; Banea et al., 1992; Mbelesso et al., 2009; Bettencourt et al., 2011), and more recently in Zambia (http://www.parliament.gov.zm/node/4637, April 2018). While isolated cases of the disease may be found, the disease mostly occurs as an outbreak suggesting to be triggered by crisis situations such as drought, civil wars or famine. Most epidemiological studies have shown a link between outbreaks of Konzo, agro-ecological collapse, and consumption of insufficiently processed bitter cassava (Manihot esculenta Crantz), a staple for millions of people in sub-Saharan Africa.
Although Konzo affects both men and women, adult males are less frequently affected, and no studies have reported Konzo in children younger than 2 years of age. The disease primarily affects children above the age of three and women in the fertile age group for reasons that are yet to be elucidated (Cliff et al., 2011; Mlingi et al., 2011; Tylleskar et al., 1995, 1992; Okitundu Luwa et al., 2014; Nzwalo and Cliff, 2011). The total number of persons affected by Konzo has been estimated to hundreds of thousands with majority of cases occurring in the DRC. Accurate prevalence estimates (as high as 5% in certain rural areas) have been difficult to obtain because of unreliable demographic data and poor surveillance systems (Tshala-Katumbay et al., 2013).
2. Neurological and clinical features
The onset and clinical features of Konzo are so distinct that the disease is easily recognizable by lay people within the affected regions. The disease has a sudden onset often preceded by physical exertion such as a long walk. During the initial phase, affected subjects experience trembling, a sensation of weakness, heaviness, or stiffness in their legs; and muscle cramps usually confined to the calf musculature. Acute reversible somatosensory symptoms are often reported and may include paraesthesia, numbness, muscle ache, and a sensation of electrical discharge in the back of the legs. Blurred vision and swallowing difficulties have been occasionally reported. Clinical deficits are usually greater at the onset of the disease, confining the affected subjects to bed. Within few days, the course of the disease stabilizes and the deficits are mostly confined to the motor system. However, a “second attack” remains possible. The most visible feature is the cross-legged (scissoring) gait of affected subjects who may still be able to walk and/ or run. Once stabilized, the most prominent sign is a symmetrical postural abnormality with a spastic (cross-legged or scissoring) gait during ambulation (Fig. 1).
Fig. 1.

Spastic stance and fixed joint (ankle ankylosis) in a young boy moderately affected by Konzo. Subjects moderately affected by the disease use sticks to walk while those mildly affected may walk with no support. Severely affected subjects may not be able to walk without support.
The spasticity of legs is revealed only when the subject is asked to run among subjects who are mildly affected by the disease, (Tylleskar et al., 1992; Howlett et al., 1990; Tshala-Katumbay et al., 2002a, 2001; Cliff and Nicala, 1997).
The world health organization (WHO) has adopted the following definition and epidemiological criteria for the disease (Trolli, 1938): a heavy reliance on cassava as staple food (Van der Beken, 1993), abrupt onset (< 1 week) of leg weakness and a non-progressive course of the disease in a formerly healthy person (Chabwine et al., 2011), a symmetric spastic abnormality when walking and/or running (Ciglenecki et al., 2011), bilaterally exaggerated knee and/or ankle jerks without signs of disease of the spine. Based on the ability to walk, the following WHO classification of severity has been proposed (Trolli, 1938): mild form = subject is able to walk without support (Van der Beken, 1993), moderate form = subject has to use one or two sticks, and (Chabwine et al., 2011) severe form = subject is unable to walk (Konzo, 1996).
At the neurological examination, the main clinical picture of Konzo consists of an isolated symmetric spastic paraparesis. Deep tendon reflexes of the lower limbs are exaggerated and extensor plantar responses can be seen in most cases when tested in the recumbent position. The ankle clonus is frequently found, as well as pathological reflexes of the upper extremities in severely affected subjects, with a clearly noticeable palmomental reflex. Severely affected subjects may present with tetraparesis associated with weakness of the trunk and pseudobulbar signs in the form of speech and swallowing difficulties (Tylleskar et al., 1992; Howlett et al., 1990; Tshala-Katumbay et al., 2002a, 2001; Cliff and Nicala, 1997). A bilateral optic neuropathy may also be seen in subjects affected by Konzo. This condition encompasses visual impairment, temporal pallor of the optic discs, and defect of visual fields. A pendular nystagmus has been reported in few cases (Mwanza et al., 2003a, b; Mwanza et al., 2005). The presence of visual symptoms at the disease onset and/or optic neuropathy during subsequent examinations do not seem to be correlated with the severity of Konzo. Hearing and sensory function, as well as urinary, bowel and sexual functions appear to be clinically normal (Tshala-Katumbay et al., 2001).
Our recent neuropsychological findings suggested impaired neurocognition in children from Konzo-affected areas compared to those from nearby non-affected areas, raising concerns as to whether the overall human burden of the disease has been underestimated (Boivin et al., 2013). The domains assessed were motor proficiency (fine motor, manual coordination, body coordination, strength and agility), and cognitive performances (sequential processing as measure of visual and auditory working memory, simultaneous processing, learning, planning and delayed recall). More recently, we showed that subjects affected by the disease also have poor neurodevelopmental trajectories including decline in motor and cognitive performances while still relying on improperly processed cassava as the main source of food (Boivin et al., 2017). Our most recent work has extended these findings to children as young as one year of age, suggesting that cassava-associated neurotoxicity may begin when children are weaned from breast milk to cassava porridge (Kashala-Abotnes et al., 2018). In documenting neurocognitive impairments in children with Konzo, Boivin and colleagues also noted sub-clinical symptoms even in Konzo-free children living in Konzo-affected households. These subtler symptoms may constitute a pre-Konzo condition, providing a warning that a child is approaching the disease’s threshold (Boivin et al., 2013). Thus, the neurocognitive effects documented for children without Konzo in Konzo-affected households and communities make it all the more important to ensure food safety in regions dependent on bitter varieties of cassava with high levels of cyanogenic compounds. In these regions, stunting and goitre are commonly found.
3. Neuropathology – nerve conduction and imaging
Peripheral and central nerve conduction studies have shown a prominent dysfunction of the pyramidal system with evidence of subclinical involvement of sensory pathways (Table 1). Non-epileptic electroencephalographic abnormalities are found while magnetic resonance imaging from two subjects has remained unremarkable (Tshala-Katumbay et al., 2002a, b; Tshala Katumbay et al., 2000; Tylleskar et al., 1993). To date, the only observation that was done on an autopsied brain in the 1930′s was not conclusive (Trolli, 1938).
Table 1.
Neuro-epidemiology and clinical electrophysiology of Konzo.
| Explorations | Abnormalities |
|---|---|
| Epidemiology (putative causal factors) | Heavy and chronic dietary reliance on insufficiently processed bitter (toxic) cassava |
| Neurology | Spastic para/tetraparesis. |
| Pseudobulbar signs and optic neuropathy | |
| Motor evoked potentials (MEP) | Frequent inability to elicit MEPa. When present, central motor conduction time is often increasedb |
| Peripheral nerve conduction studies | Normal motor and sensory nerve conduction. Increased amplitude of F-waves |
| Somatosensory evoked potentials (SEP) | Cortical responses following tibial stimulation frequently absent. If present, the latency is prolonged. Median SEP often normal |
| Visual evoked potentials (VEP) | Frequent delay and decreased amplitude of P100 |
| Electroencephalography (EEG) | Frequent generalized slowing of background activity and non-specific paroxysmal activities |
Consistent with reduction of the upper motor neuron pool.
Consistent with loss of pyramidal conductivity from spinal tract (axonal) damage.
4. On the biomarkers and mechanisms of food (cassava) cyanide-associated neurological disease
4.1. Biomarkers of exposure
In most studies, the exposure to cyanogenic compounds is ascertained by measuring the concentrations of thiocyanate (SCN), the main cyanide metabolite in urine (U-SCN) or plasma (P-SCN) (Kassa et al., 2011). Levels of U-SCN or P-SCN may be as high as 1720 and 426 μmol/ l, respectively, in Konzo-affected populations. A recent study found a mean ± SD concentration of 520.4 ± 355.7 μmol/l of U-SCN in children with Konzo, which was significantly higher than 382.5 ± 226.3 μmol/l in those with no Konzo (p < 0.05) (Boivin et al., 2017). Samples of cassava flour from 18 consenting households were collected and found to have cyanide concentrations from 30 to 200 ppm with a mean (SD) of 92.2 ( ± 562) ppm, well-above the 10 ppm safe limit proposed by WHO (Boivin et al., 2013; Organization FaAOWH, 2018). Samples of cassava flour and urines in a study of very young children had similar concentrations. The cyanogen content in cassava flour was above the safe limit of 10 ppm (Organization FaAOWH, 2018) and the level of SCN urinary excretion was above 350 μmole/litre in most households (Kashala-Abotnes et al., 2018). The introduction of a new food-processing method has helped reduce the cyanogenic content of cassava and hence, the levels of exposure to its cyanogenic compounds, and a subsequent decrease in the number of incident cases of Konzo was noted in select areas of DRC and Tanzania (Mlingi et al., 2011; Banea et al., 2013, 2014; Banea et al., 2012). The only study that measured the concentration of cyanide in blood of subjects with Konzo found concentrations that can reach ∼ 20X the “accumulation level” of 4 μmol/l in 3 subjects within the first week of Konzo onset (Tylleskar et al., 1992).
4.2. Biomarkers of susceptibility
Current knowledge of cassava toxicity clearly indicates that Konzo is associated with chronic reliance on improperly processed cyanogenic (bitter) cassava as the main source of food. It is possible that outbreaks of the disease and/or it sudden onset are triggered by a peak in the exposure to the cassava cyanogens and hence, to their toxic metabolites (vide infra). While most subjects from Konzo-affected areas rely on cyanogenic cassava as staple food, only a fraction (i.e. ∼ up to 10%) suffers from overt gait abnormalities suggesting that there may be individual factors that dictate susceptibility to the disease. The latter include malnutrition and younger age or female gender for reasons that are not clearly understood, but also poor cyanide detoxification capability and, possibly, genetics. Impaired cyanide detoxification may be seen as a result of poor nutrition (insufficient protein intake and/or availability of sulfur donors) and/or, possibly, genetic polymorphisms (Tshala-Katumbay et al., 2013).
4.3. Putative biomarkers of neuropathology
The only available data on neuropathology was not conclusive (Trolli, 1938). Most insight on the site of the lesion in Konzo was provided by electrophysiological studies, which suggest that both motor and somatosensory pathways as well as visual pathways are affected (Table 1) (Tshala-Katumbay et al., 2002a, b; Tshala Katumbay et al., 2000). Additional insights are gained from experimental studies, which suggest that the neuropathology of Konzo may be mediated through mechanisms of oxidative damage or protein carbamoylation induced by cyanide (pro-oxidant) or cyanate (cyanide metabolite, motor system toxicant, and protein-carbamoylating agent) (Kassa et al., 2011; Makila-Mabe et al., 2014; Kimani et al., 2013). We recently confirmed the association between the serum levels of 8,12-iso-iPF2α-VI F2-isoprostane isomer, a marker of lipid peroxidation and thus oxidative damage, and the extent of neurocognitive deficits found in children with Konzo (Makila-Mabe et al., 2014). However, these peripheral markers of oxidative stress may well not reflect the exact neuropathogenic mechanisms taking place in the central nervous system in response to the toxicity of cyanogenic cassava.
5. Differential diagnosis of Konzo
The diagnosis of Konzo is relatively straightforward when the disease occurs in its epidemic form as several families within a community are affected within a common timeframe. The association with poor nutrition and overconsumption of insufficiently processed bitter cassava is required for the diagnosis of Konzo. The disease must be differentiated from lathyrism, another spastic paraparesis associated with poor nutrition and overconsumption of the grass pea lathyrus sativus (Bradbury and Lambein, 2011); and tropical spastic paraparesis (TSP), a neurological entity endemic to tropical Africa, or Latin America, or the Seychelles and Japan islands (Gessain and Mahieux, 2012). In certain parts of the world, for example the Bandundu province of the DRC, clusters of TSP coexists with Konzo (Goubau et al., 1990; Kayembe et al., 1990). While Konzo appears to be a toxico-nutritional disease, the aetiology of TSP is linked to the infection by the human T-cell lymphotropic virus type I (HTLV-I) (Gessain and Mahieux, 2012; Tylleskar et al., 1996). Because of this association, TSP has been named HTLV-I Associated Myelopathy (HAM). The differential diagnosis of Konzo, lathyrism and TSP/HAM may be difficult when (a) either Konzo or lathyrism coexist with TSP/HAM or (b) a TSP/HAM subject tests negative to HTLV-I while residing in a Konzo- or lathyrism-affected area. In these cases, the differential diagnosis is made by carefully taking the history of the disease through a structured interview, the dietary habits, and the findings from the physical examination. TSP/HAM is a slowly progressive spastic paraparesis whereas lathyrism and Konzo are non-progressive conditions usually of acute or subacute onset. In addition, clinical signs of sensory and sphincter involvement may be evident in the extremities of subjects with TSP/HAM. In the absence of co-morbidity, subjects with Konzo or lathyrism should test negative for HTLV-I antibodies or protein immunoblots.
The process of identifying the cause a spastic paraparesis in the tropics may be challenging for the physician in presence of an isolated case. In this situation, the differential diagnosis should be made against other causes of non-compressive myelopathy. These include but are not limited to the subacute myelo-optic neuropathy (SMON) due to clioquinol (5-chloro-7-iodo-8-quinolinol; iodochloroxyquin) intoxication, infections or liver failure, hereditary spastic paraplegia (HSP), primary lateral sclerosis (PLS), or amyotrophic lateral sclerosis (ALS) (Konagaya et al., 2004; Berger and Sabet, 2002; McArthur et al., 2005; Utku et al., 2005). In most cases, the history of the illness, the presence of signs of systemic disease, laboratory analyses, and neuroimaging findings may help to confirm and differentiate the diagnosis. Genetic and serum and/ or cerebrospinal fluid laboratory analyses, virology testing of viruses such as the human immunodeficiency viruses type I (HIV-I) and II (HIV-I-II) are also helpful to differentiate diagnosis. An earlier detection of treatable causes of myelopathy and spastic paraparesis such as tuberculosis remains of paramount importance (Table 2).
Table 2.
Non-compressive spastic/ tetraparaparesis in the tropics.
| Aetiology/Lesion | Toxiconutrional |
Infectious |
Neurodegenerative | |||||
|---|---|---|---|---|---|---|---|---|
| Konzo | Lathyrism | Combined degeneration of the spinal cord | SMON | TSP/HAM | HIV | Tuberculosis/Cysticercosis/Syphilis | ALS/PLS/HSP | |
| Dietary/toxic factors | Cassava (manihot esculenta) | Grass pea (lathyrus sativus) Acute/subacute | B 12 deficiency | Clioquinol intoxication | No | No | No | Controversial in sporadic ALS cases |
| Onset | Acute | Subacute | Subacute | Subacute | Subacute | Subacute | Subacute | |
| Upper motor neuron disorder | Yes | Yes | No | No | No | No | No | Yes (PLS, HSP) No (ALS) |
| Lower motor neuron involvement | No | No | Possible | No | Possible | Possible | Possible | Yes (ALS) No (PLS, HSP) |
| Sensory/sphincter involvement | No | No | Yes | Yes | Yes | Yes | Possible | No |
| Cognition deficits | Yes | Unknown | Possible | Possible | Yes | Yes | Possible | Possible |
| Cranial nerve involvement | Optic neuropathya | No | No | Optic neuropathy | Possible | Possible | Possible | Bulbar palsy but rare in HSP |
| Clinical course | Non-progressive | Non-progressive | Progressive | Progressive | Progressive | Progressive | Progressive | Progressive |
| Virology testing | Negative | Negative | Negative | Negative | Positive | Positive | Negative | Negative |
| Bacterial or parasitic | No | No | No | No | No | No | Yes | No |
| Genetic susceptibility | Postulated | No | No | No | No | No | No | Documented |
More common in cassava-associated ataxic myeloneuropathy.
6. Treatment, prognosis and prevention
Up to date, there is no effective treatment for Konzo that has been documented. The neurological damages are debilitating, and permanent. Severely affected subjects may use walking aids, but once the neurodamaging process has stabilized, the disability remains unchanged and irreversible. There has been attempts with physical therapy to reduce muscle spasms and contractures, and with reconstructive surgery. A proximal tendon release of the calf has proven successful in a handful of patients in Central African Republic where the patients have regained the ability to stand on their whole feet, thus improving both standing and gait (Fig. 2). A possible option is to trial centrally acting spasmolytics, dorsal rhizotomy or intramuscular injection of botulinum toxin used with success to reduce adductor spasticity in patients with cerebral palsy. However, no such therapeutic trials have been tested in patients with Konzo so far.
Fig. 2.
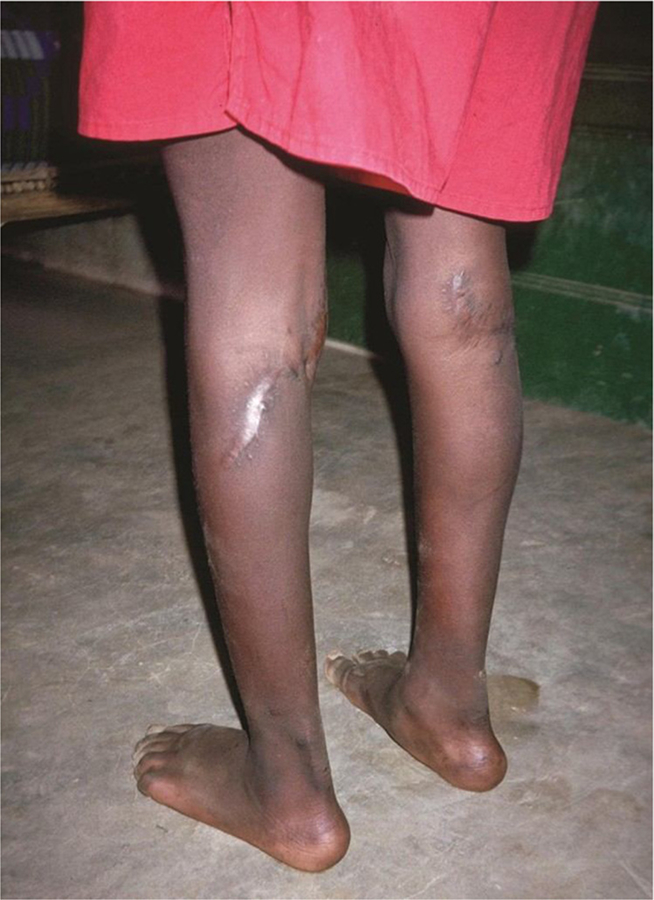
A patient from Central African Republic (CAR) after a successful reconstructive surgery by an Italian missionary surgeon who has done proximal tendon release which has restored the patient’s stance and gait to a large extent, he is able to stand on the whole foot. Photo T. Tylleskär.
Konzo-affected regions face the challenges of the agricultural, educational, and public health capacity and infrastructure needed to implement the necessary dietary changes. For the same reasons, these regions fail to diversify their food staples. Presently, the key to eradicating Konzo is prevention. Because there is no cure for the neurological damage that Konzo causes, the battle against the disease must focus on prevention. In 2005, an Australian scientist discovered an efficient cassava processing method to reduce cyanogen content in cassava. The method called wetting method has been used successfully in the DRC and has helped prevent outbreaks of Konzo (Mlingi et al., 2011; Banea et al., 2013, 2014; Banea et al., 2012).
Konzo victims are able to survive for decades with their handicap, depending on how supportive the environment is. It will therefore be possible to document a ‘post-Konzo’ syndrome with increased pain and weakness due to contractures and joint malalignment in old patients.
It has been proposed to switch from bitter to sweet cassava (low in toxin), but it is not the solution because only bitter cassava can grow in the region due to the acidity of the soil. According to the FAO/ UNESCO Soil map of the world (1977), the soil in the Southern Bandundu Province in DRC, which is one of the most Konzo-affected areas in the world, is not suitable for modern agriculture and livestock due to mineral deficiencies and low ferralic arenosols.
7. Future perspectives
The human model of food (cassava) cyanide poisoning offers invaluable opportunities to explore treatment options, e.g., antidotes for cyanide poisoning and elucidate the neuropathogenic mechanisms underlying the long-term impact of cyanide exposure on the brain. Other opportunities to be explored include testing and validation of point-of-care diagnostic tools to measure and monitor levels of cyanide exposure and metabolites in relation to risks for neurological diseases and child neurodevelopment. Further studies should explore the differential roles of cyanide (mitochondrial toxicant), thiocyanate (AMPA-receptor chaotropic metabolite), cyanate (protein-carbamoylating metabolite), and 2-iminothiazolidine-4-carboxylic acid (seizure inducer) in the pathogenesis of cassava-associated neurological damage (Spencer, 1999). Whole genome or exome sequencing, metagenomics and epigenetics may, possibly, unveil other factors of individual susceptibility to cyanide related neurological disease (Tshala-Katumbay et al., 2013). Further studies should focus on the burden of social challenges such as poverty, maternal mental health, and malnutrition (stunting) that have been reported in households affected by Konzo. Therapeutic trials with substances that can reduce adductor spasticity, such as in patients with cerebral palsy could be tested to alleviate symptoms. Prospects on cognitive rehabilitation have yet to be explored.
Acknowledgments
Recent advances on the understanding of Konzo have been made possible thank to a grant from the Research Council of Norway through the Global Health and Vaccination Program grant (GLOBVAC, project number 234469, PI: Espérance Kashala-Abotnes) and the NIH GRANT NIEHS/FIC R01ES019841 (PI: Désiré Tshala-Katumbay).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- Banea JPM, Bikangi N, Nahimana G, Nunga M, Tylleskar T, Rosling H, 1992. High prevalence of Konzo associated with a food shortage crisis in the Bandundu region of zaire. Ann. Soc. Belg. Med. Trop 72 (4), 295–309. [PubMed] [Google Scholar]
- Banea JP, Nahimana G, Mandombi C, Bradbury JH, Denton IC, Kuwa N, 2012. Control of in DRC using the wetting method on cassava flour. Food Chem. Toxicol 50 (5), 1517–1523. [DOI] [PubMed] [Google Scholar]
- Banea JP, Bradbury JH, Mandombi C, Nahimana D, Denton IC, Kuwa N, et al. , 2013Control of Konzo by detoxification of cassava flour in three villages in the Democratic Republic of Congo. Food Chem. Toxicol 60, 506–513. [DOI] [PubMed] [Google Scholar]
- Banea JP, Bradbury JH, Mandombi C, Nahimana D, Denton IC, Kuwa N, et al. , 2014. Effectiveness of wetting method for control of Konzo and reduction of cyanide poisoning by removal of cyanogens from cassava flour. Food Nutr. Bull 35 (1), 28. [DOI] [PubMed] [Google Scholar]
- Berger JR, Sabet A, 2002. Infectious myelopathies. Semin. Neurol 22 (2), 133–142. [DOI] [PubMed] [Google Scholar]
- Konzo in Angola In: Bettencourt M, Paquisse M, Zangulo A, Resende I (Eds.), World Congress of Neurology; Morocco. [Google Scholar]
- Boivin MJ, Okitundu D, Makila-Mabe Bumoko G, Sombo MT, Mumba D, Tylleskar T, et al. , 2013. Neuropsychological effects of Konzo: a neuromotor disease associated with poorly processed cassava. Pediatrics 131 (4), e1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Okitundu D, Makila-Mabe B, Sombo MT, Mumba D, Sikorskii A, et al. , 2017. Cognitive and motor performance in Congolese children with Konzo during 4 years of follow-up: a longitudinal analysis. Lancet Glob. Health 5 (9), e936–e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JH, Lambein F, 2011. Konzo and neurolathyrism: similarities and dissimilarities between these crippling neurodegenerative diseases of the poor. Food Chem. Toxicol 49 (3), 537–538. [DOI] [PubMed] [Google Scholar]
- Chabwine JN, Masheka C, Balol’ebwami Z, Maheshe B, Balegamire S, Rutega B, et al. , 2011. Appearance of Konzo in South-Kivu, a wartorn area in the Democratic Republic of Congo. Food Chem. Toxicol 49 (3), 644–649. [DOI] [PubMed] [Google Scholar]
- Ciglenecki I, Eyema R, Kabanda C, Taafo F, Mekaoui H, Urbaniak V, 2011. Konzo outbreak among refugees from Central African Republic in Eastern region, Cameroon. Food Chem. Toxicol 49 (3), 579–582. [DOI] [PubMed] [Google Scholar]
- Cliff J, Nicala D, 1997. Long-term follow-up of Konzo patients. Trans. R. Soc. Trop. Med. Hyg 91 (4), 447–449. [DOI] [PubMed] [Google Scholar]
- Cliff J, Muquingue H, Nhassico D, Nzwalo H, Bradbury JH, 2011. Konzo and continuing cyanide intoxication from cassava in Mozambique. Food Chem. Toxicol 49 (3), 631–635. [DOI] [PubMed] [Google Scholar]
- Gessain A, Mahieux R, 2012. Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects. Rev. Neurol. (Paris) 168 (3), 257–269. [DOI] [PubMed] [Google Scholar]
- Goubau P, Carton H, Kazadi K, Muya KW, Desmyter J, 1990. HTLV seroepidemiology in a central African population with high incidence of tropical spastic paraparesis. Trans. R. Soc. Trop. Med. Hyg 84 (4), 577–579. [DOI] [PubMed] [Google Scholar]
- Howlett WP, Brubaker GR, Mlingi N, Rosling H, 1990. Konzo, an epidemic upper motor neuron disease studied in Tanzania. Brain 113 (Pt 1), 223–235. [DOI] [PubMed] [Google Scholar]
- Kashala-Abotnes E, Sombo MT, Okitundu DL, Kunyu M, Bumoko Makila-Mabe G, Tylleskar T, et al. , 2018. Dietary cyanogen exposure and early child neurodevelopment: an observational study from the Democratic Republic of Congo. PloS One 13 (4), e0193261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa RM, Kasensa NL, Monterroso VH, Kayton RJ, Klimek JE, David LL, et al. , 2011. On the biomarkers and mechanisms of Konzo, a distinct upper motor neuron disease associated with food (cassava) cyanogenic exposure. Food Chem. Toxicol 49 (3), 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayembe K, Goubau P, Desmyter J, Vlietinck R, Carton H, 1990. A cluster of HTLV-1 associated tropical spastic paraparesis in Equateur (Zaire): ethnic and familial distribution. J. Neurol. Neurosurg. Psychiatry 53 (1), 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani S, Moterroso V, Lasarev M, Kipruto S, Bukachi F, Maitai C, et al. , 2013. Carbamoylation correlates of cyanate neuropathy and cyanide poisoning: relevance to the biomarkers of cassava cyanogenesis and motor system toxicity. Springerplus 2, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konagaya M, Matsumoto A, Takase S, Mizutani T, Sobue G, Konishi T, et al. , 2004. Clinical analysis of longstanding subacute myelo-optico-neuropathy: sequelae of clioquinol at 32 years after its ban. J. Neurol. Sci 218 (1–2), 85–90. [DOI] [PubMed] [Google Scholar]
- Konzo WHO, 1996. A distinct type of upper motor neuron disease. Wkly. Epidemiol. Rec 71, 225–232.8718826 [Google Scholar]
- Makila-Mabe BG, Kikandau KJ, Sombo TM, Okitundu DL, Mwanza JC, Boivin MJ, et al. , 2014. Serum 8,12-iso-iPF2alpha-VI isoprostane marker of oxidative damage and cognition deficits in children with Konzo. PLoS One 9 (9), e107191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbelesso P, Yogo ML, Yangatimbi E, Paul-Senekian V, Nali NM, Preux PM, 2009. Outbreak of Konzo disease in health region No. 2 of the Central African Republic. Rev. Neurol. (Paris) 165 (5), 466–470. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A, 2005. Neurological complications of HIV infection. Lancet Neurol 4 (9), 543–555. [DOI] [PubMed] [Google Scholar]
- Mlingi NL, Nkya S, Tatala SR, Rashid S, Bradbury JH, 2011. Recurrence of Konzo in southern Tanzania: rehabilitation and prevention using the wetting method. Food Chem. Toxicol 49 (3), 673–677. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Lysebo DE, Kayembe DL, Tshala-Katumbay D, Nyamabo LK, Tylleskar T, et al. , 2003a. Visual evoked potentials in Konzo, a spastic paraparesis of acute onset in Africa. Ophthalmologica 217 (6), 381–386. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Tshala-Katumbay D, Kayembe DL, Eeg-Olofsson KE, Tylleskar T, 2003b. Neuro-ophthalmologic findings in Konzo, an upper motor neuron disorder in Africa. Eur. J. Ophthalmol 13 (4), 383–389. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Tshala-Katumbay D, Tylleskar T, 2005. Neuro-ophthalmologic manifestations of Konzo. Environ. Toxicol. Pharmacol 19 (3), 491–496. [DOI] [PubMed] [Google Scholar]
- Nzwalo H, Cliff J, 2011. Konzo: from poverty, cassava, and cyanogen intake to toxico-nutritional neurological disease. PLoS Negl. Trop. Dis 5 (6), e1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okitundu Luwa EAD, Bumoko Makila-Mabe G, Ayanne MT, Kikandau JK, Mashukano N, Kazadi Kayembe T, et al. , 2014. Persistence of Konzo epidemics in Kahemba, Democratic Republic of Congo: phenomenological and socio-economic aspects. Pan Afr. Med. J 18, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization FaAOWH, 1991. Joint FAO/ WHO Food Standars Programme Rome; Contract No.: Supplement 4. [Google Scholar]
- Spencer PS, 1999. Food toxins, ampa receptors, and motor neuron diseases. Drug Metab. Rev 31 (3), 561–587. [DOI] [PubMed] [Google Scholar]
- Trolli G, 1938. Paraplégie spastique épidémique, “Konzo” des indigènes du Kwango. In: Trolli G (Ed.), Résumé des observations réunies, au Kwango, au sujet de deux affections d´órigine indéterminée Fonds Reine Elisabeth, Brussels, pp. 1–36. [Google Scholar]
- Tshala Katumbay D, Lukusa VM, Eeg-Olofsson KE, 2000. EEG findings in Konzo: a spastic para/tetraparesis of acute onset. Clin. Electroencephalogr 31 (4), 196–200. [DOI] [PubMed] [Google Scholar]
- Tshala-Katumbay D, Eeg-Olofsson KE, Tylleskar T, Kazadi-Kayembe T, 2001. Impairments, disabilities and handicap pattern in Konzo–a non-progressive spastic para/tetraparesis of acute onset. Disabil. Rehabil 23 (16), 731–736. [DOI] [PubMed] [Google Scholar]
- Tshala-Katumbay D, Eeg-Olofsson KE, Kazadi-Kayembe T, Tylleskar T, Fallmar P, 2002a. Analysis of motor pathway involvement in Konzo using transcranial electrical and magnetic stimulation. Muscle Nerve 25 (2), 230–235. [DOI] [PubMed] [Google Scholar]
- Tshala-Katumbay D, Edebol Eeg-Olofsson K, Kazadi-Kayembe T, Fallmar P, Tylleskar T, Kayembe-Kalula T, 2002b. Abnormalities of somatosensory evoked potentials in Konzo–an upper motor neuron disorder. Clin. Neurophysiol 113 (1), 10–15. [DOI] [PubMed] [Google Scholar]
- Tshala-Katumbay D, Mumba N, Okitundu L, Kazadi K, Banea M, Tylleskar T, et al. , 2013. Cassava food toxins, Konzo disease, and neurodegeneration in sub-Sahara Africans. Neurology 80 (10), 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylleskar T, Banea M, Bikangi N, Cooke RD, Poulter NH, Rosling H, 1992. Cassava cyanogens and Konzo, an upper motoneuron disease found in Africa. Lancet 339 (8787), 208–211. [DOI] [PubMed] [Google Scholar]
- Tylleskar T, Howlett WP, Rwiza HT, Aquilonius SM, Stalberg E, Linden B, et al. , 1993. Konzo: a distinct disease entity with selective upper motor neuron damage. J. Neurol. Neurosurg. Psychiatry 56 (6), 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylleskar T, Banea M, Bikangi N, Nahimana G, Persson LA, Rosling H, 1995. Dietary determinants of a non-progressive spastic paraparesis (Konzo): a case-referent study in a high incidence area of Zaire. Int. J. Epidemiol 24 (5), 949–956. [DOI] [PubMed] [Google Scholar]
- Tylleskar T, Banea M, Bottiger B, Thorstensson R, Biberfeld G, Rosling H, 1996. Konzo, an epidemic spastic paraparesis in Africa, is not associated with antibodies to HTLV-I, HIV, or HIV gag-encoded proteins. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol 12 (3), 317–318. [DOI] [PubMed] [Google Scholar]
- Utku U, Asil T, Balci K, Uzunca I, Celik Y, 2005. Hepatic myelopathy with spastic paraparesis. Clin. Neurol. Neurosurg 107 (6), 514–516. [DOI] [PubMed] [Google Scholar]
- Van der Beken A, 1993. In: Karthala (Ed.), Proverbes yaka du Zaire Harmattan, Paris. [Google Scholar]


