Abstract
Adverse birth outcomes can lead to problematic long-term outcomes for children, and are also known to transmit socioeconomic disadvantage across generations, thereby amplifying the importance of identifying their social determinants. However, the full set of factors causing adverse birth outcomes remains unknown. Drawing together theory describing intragenerational (life course) processes linking early life adversity to adult health, and intergenerational transmissions of inequality via birthweight, this study tests a chain of risk that originates within early adolescence, impacts young women’s risky health behaviors in late adolescence/early adulthood and risky health behaviors during pregnancy, and ultimately decreases offspring’s birthweight. We do so using structural equation models and prospective, population-level data on a racially and socioeconomically diverse cohort of young adults (National Longitudinal Study of Adolescent to Adult Health). Results (a) reveal four pathways that fully mediate the association between a young woman’s family-of-origin socioeconomic status in adolescence and her offspring’s birthweight, and (b) identify a trigger effect—a place in the chain of risk where prevention efforts could be targeted, thereby breaking the chain of risk leading to poor offspring health at birth for vulnerable individuals.
Keywords: Intergenerational, birthweight, preconception, life course, prenatal smoking, adolescent smoking, population health, U.S.
INTRODUCTION
Adverse birth outcomes, such as lower birthweight (measured as a continuous variable, in grams) or low birthweight (<2500g), are one mechanism through which socioeconomic disadvantage can be transmitted across generations (Aizer and Currie 2014, Case and Paxson 2006, Conley, Strully, and Bennett 2003, Palloni 2006). This classification holds based on the stark social patterning of adverse birth outcomes observed in the U.S. (meaning, poor mothers tend to exhibit higher risk of adverse birth outcomes), in combination with the generally increased risk of problematic longer-term outcomes (e.g., neurodevelopmental problems, learning disabilities, behavioral problems, lower educational attainment, poorer cardiovascular health) observed among children born with an adverse birth outcome (Behrman and Butler 2007, Conley, Strully, and Bennett 2003, Goldenberg and Culhane 2007). It follows that identifying the etiology of adverse birth outcomes could not only create new opportunities to develop programs that effectively prevented such outcomes, but also potentially disrupt intergenerational transmissions of disadvantage via birthweight.
Despite the considerable attention placed on identifying the determinants of adverse birth outcomes however [for reviews, see (Kramer 1987, Paneth 1995, Goldenberg et al. 2008, Goldenberg and Culhane 2007)], we still do not understand what causes adverse birth outcomes (Savitz and Murnane 2010). Studies in this area have been hampered by a lack of appropriate data and/or methods available to test causal claims at the population-level (Kane and Margerison-Zilko 2017). But, another key contributor is that this literature has historically focused on risk factors operative within the prenatal or immediate preconception period (Johnson et al. 2006, van Dyck 2010), to the exclusion of earlier life course events and risk factors that could set into motion a chain of events that ultimately lead to poor health at birth among offspring (Richardson, Hussey, and Strutz 2012, Atrash et al. 2006). This stands in contrast to a wide body of theory, spanning multiple disciplines from the social sciences to public health, that emphasizes the prominence of the early life environment in shaping health outcomes that unfold within individuals over time (Center on the Developing Child at Harvard University 2010, Hertzman and Boyce 2010, Kuh and Shlomo 2004, Halfon and Hochstein 2002, Elder 1977). Additionally, birth outcomes are a unique case in which both intra- and intergenerational mechanisms are at play (Kane 2015, Palloni 2006). Therefore, fully understanding the complex set of pathways that influence an offspring’s birth outcomes requires drawing from theoretical perspectives spanning both intra- and intergenerational processes.
The current study identifies a set of theoretically-informed mechanisms that operate across women’s early life and impact offspring birthweight, using data from a contemporary, population-based cohort. To do so, this study first draws together theory from sociology and epidemiology describing life course processes linking early life adversity to adult health. From this foundation, we formulate a set of hypotheses relating a mother’s early life environment (in adolescence) to her risk and protective behaviors in late adolescence/early adulthood, and subsequently, her prenatal health behaviors. To describe the significant contributions these pathways may have on the life chances of the next generation (via offspring birthweight), we draw upon theory relating to intergenerational transmissions of inequality. We then test these hypotheses using a nationally representative, prospective dataset containing substantial racial and economic diversity that includes extensive information on individuals spanning multiple domains of health and wellbeing (biological, genetic, social, psychological, behavioral, environmental, and economic), measured at multiple stages in early life. We conclude with a discussion that integrates study findings related to intragenerational pathways with those related to intergenerational transmission of inequality, thereby providing a richer description of the sets of processes shaping adverse birth outcomes.
BACKGROUND
Social conditions are a fundamental cause of disease (Link and Phelan 1995). Early life adversity, in the form of material hardship or socioeconomic disadvantage, is one type of social condition that can adversely impact adult morbidity and mortality as an intragenerational process (Montez and Hayward 2014, Hayward and Gorman 2004, Haas 2008, Almond and Currie 2011), and can also play a key role in the intergenerational transmission of inequality (Kuh and Shlomo 2004, Elder 1977, Elder Jr 1998, Elder Jr, Johnson, and Crosnoe 2003, Palloni 2006). These intra- and inter-generational literatures tend to parallel one another, but need not be mutually exclusive. Indeed, more rigorous study of the intersection of intra- and intergenerational pathways could reveal new insights into disrupting intergenerational transmissions of inequality (Kane 2015, Palloni 2006).
Theoretical Framework
The prominence of early life social conditions for understanding patterns of adult health and wellbeing is perhaps most clearly articulated by life course theory. Within this framework, social conditions and exposures within each life stage are intricately linked across the life course; macro- and micro-level factors interact to shape the exposures, constraints, and opportunities individuals encounter as they move from one life stage to the next. Importantly, individuals enact human agency and personal control over situations they encounter, acting planfully (Elder Jr and Giele 2009, Elder 1977, Elder Jr 1998).
These principles are also central tenets of life course epidemiology—a growing subfield of public health that builds on life course theory by bringing together social and biological origins of disease from across the life course (Ben-Shlomo and Kuh 2002, Kuh et al. 2003). Situated within this framework, adverse exposures—whether environmental, socioeconomic, or behavioral—are thought to accumulate over time, ultimately degrading health by wearing down the body’s ability to continually repair damage. Oftentimes, this accumulation involves multiple risk factors that independently influence a health outcome, but are also clustered, meaning they stem from a common source—such as family socioeconomic status; this is known as an accumulation model with risk clustering. A special case of this model is an additive chains of risk model, wherein one adverse exposure increases the risk of a subsequent adverse exposure and each exposure maintains both an indirect and direct effect on the health outcome. Another type of chain of risk model involves a trigger effect, whereby a chain of risk may stem from a trigger event, such as a health event, a move, the death of a loved one, experiencing abuse or trauma, etc., that increases one’s risk of engaging in unhealthy behaviors and ultimately increases the risk of a poor health outcome (Kuh et al. 2003). Trigger events hold particular importance because these are places where chains of risk could be averted or broken for vulnerable individuals (Kuh and Shlomo 2004).
Another aspect of life course epidemiology that relates to the present study is the notion that the timing of the exposure matters. Sensitive periods are stages in the life course in which the effect of a stressor on health is unique, such that, the same effect on health would not be observed if the stressor were encountered in a different stage of life. A prime example is adversity encountered in the sensitive period of childhood which has distinct and critical implications for adult health and wellbeing (Umberson et al. 2014, Ben-Shlomo and Kuh 2002, Miller, Chen, and Parker 2011, Shonkoff, Boyce, and McEwen 2009, Hayward and Gorman 2004, Haas 2008).
Applied to the case of adverse birth outcomes, an accumulation model with risk clustering would suggest that adverse exposures across childhood and adulthood accumulate, each maintaining a direct effect on adverse birth outcomes while also sharing a common source, such as family socioeconomic status. The special case of this model, an additive chain of risk, links these adverse exposures through a chain, or pathway. Each adverse exposure may retain a direct effect on the birth outcome (an additive chain of risk model), or not (a trigger effect chain of risk model). The logic underlying these claims is that early life stressors induce physiologic dysregulation that ultimately impact adverse birth outcomes, either directly or indirectly through social and biological risk factors. Next we seek to identify adverse exposures that may comprise such a pathway.
Maternal Early Life Adversity and Offspring Adverse Birth Outcomes
Using data from the 1958 birth cohort in Great Britain, Harville and colleagues (2010) demonstrated a link between early life adversity, indicated by material hardship and childhood neglect/abuse, and an increased risk of low birth weight and preterm birth; some of this association was mediated by prenatal smoking. Using more recent data from the U.S. [Waves I and III of The National Longitudinal Study of Adolescent to Adult Health (Add Health)] Gavin and colleagues (2012) found that childhood SES and childhood maltreatment lowered offspring birthweight; some of this association was mediated by adolescent substance abuse (measured as a latent variable indicated by adolescent smoking, heavy drinking, and drug use) and prenatal smoking. The U.S. study was limited however by (a) including a select group of young (largely teen) mothers, (b) using data (from Wave III) that systematically undercounted births, and (c) not examining adolescent smoking, drinking, and drug use as independent risk factors. The sample issue described in (a) is problematic because the salience of adolescent substance abuse for normative, later-timed births is unknown. As a result of the measurement issue described in (b) it is impossible to know if the study’s results are generalizable to the U.S. population. Additionally, without being able to distinguish the unique effects of each form of substance abuse [implied by (c)], each of which may well imply a distinct prevention strategy, scholars have yet to clearly identify modifiable risk factors on the pathway between mother’s early life environment and offspring’s birth outcomes in the U.S.
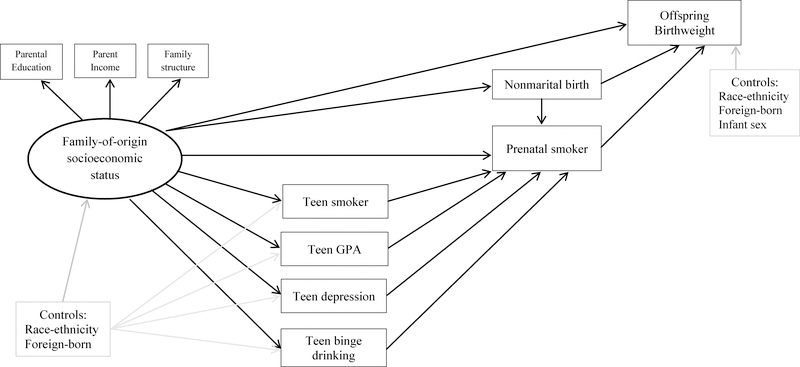
Figure 1 presents the measurement model guiding the present study. We begin by drawing a path linking a young woman’s family-of-origin socioeconomic status (SES) in adolescence to her offspring’s birthweight. Next we identify risk factors that potentially mediate this association. Based on past research (Harville et al. 2010, Gavin et al. 2012), we consider prenatal smoking to be a probable mediator. Given that the association between prenatal smoking and offspring birthweight is well-supported by evidence emerging from economics suggesting a causal association (Lien and Evans 2005, Wehby et al. 2011, Dietz et al. 2010), the present study considers whether prenatal smoking continues to perform mediation after accounting for the endogeneity of prenatal smoking; this has never before been assessed. This is an important knowledge gap given that prenatal smoking is widely considered to be the leading preventable cause of perinatal morbidity and mortality in the U.S. (Cnattingius 2004). Population-level prevalence of prenatal smoking declined from roughly 20% in 1989 to 10–12% in 2012 (Child Trends Databank 2014). However, these estimates are likely to be conservative. Deception rates of prenatal smoking tend to range from 5% to 23% (Pickett et al. 2005) with rates as high as 73% being detected in some locations (Webb et al. 2003), leading researchers to avoid using clinician’s reports of prenatal smoking whenever possible. In contrast, prenatal smoking rates gathered using computer-assisted telephone interviewing techniques are of higher quality and show less misclassification relative to administrative data (Srisukhumbowornchai, Krikov, and Feldkamp 2012). Questions with multiple response options are also preferable, as they can reduce non-disclosure by as much as 40% (Mullen et al. 1991).
Figure 1.
Measurement Model of Family-of-Origin Socioeconomic Status, Adolescent Risk/Protective Factors, Prenatal Smoking, and Birthweight
Next we consider factors that may mediate the association between family-of-origin SES and prenatal smoking. The first of which we assess is adolescent-onset of smoking (see Figure 1). This is plausible on both empirical [see (Gavin et al. 2011) for indirect evidence] and conceptual grounds. Most adult smokers initiate tobacco use in adolescence (Apelberg et al. 2014); those who do so display higher levels of addiction and are at increased risk of developing a longer-term smoking habit (Centers for Disease Control and Prevention 2014). Heavier smoking behavior increases women’s risk of persistently smoking throughout pregnancy, relative to spontaneously quitting upon learning of a pregnancy (Quinn, Mullen, and Ershoff 1991). More broadly, adolescent substance abuse predicts risky health and social behaviors in adulthood (Wodarski and Smyth 1994). Thus, we anticipate adolescent smokers to be at increased risk of prenatal smoking. Adolescent smoking is also linked with low childhood SES (Hanson and Chen 2007), establishing it as a potential mediator of the association between family-of-origin SES and prenatal smoking.
Next we assess low educational performance, as indicated by grade point average (GPA) in high school (see Figure 1); no studies we are aware of have yet considered this as a mediator of the maternal early life SES—prenatal smoking association. We believe this is a potentially important mediator. The association between family-of-origin SES and high school academic performance is well established [see for example, Sandel (2012)]. High school academic performance is an important predictor of high school completion (Rumberger 1987) and a developmentally-appropriate measure of educational success in this life stage. In terms of potential linkages between high school GPA and prenatal smoking, it has been widely shown that women who smoke during pregnancy are disproportionately low-educated (high school degree or less) (Child Trends Databank 2014). Furthermore, among women who reported smoking three months prior to conception, the odds of quitting during pregnancy were lower among those who did not complete high school, relative to those who did (Colman and Joyce 2003). From a broader theoretical perspective, academic performance may play a key role in this pathway because it reflects both cognitive and non-cognitive skills (Heckman 2000), each of which can influence health behaviors such as smoking (Chiteji 2010, Mendolia and Walker 2014). Education may also increase problem-solving capacity and tends to promotes individual agency and self-efficacy; these are key resources that cultivate health more generally (Mirowsky and Ross 2003).
Adolescent depressive symptomology and binge drinking may also mediate the association between family-of-origin SES and prenatal smoking. This assertion is based partly on the observed comorbidities of teen smoking [adolescent smokers (versus non-smokers) report higher levels of serious psychological distress and higher rates of binge drinking (Curry et al. 2007)], but also on evidence linking both risk factors to family-of-origin SES and prenatal smoking. Adolescent depression has been shown to mediate the association between SES and adolescent substance use (Goodman and Huang 2002). The literature linking varying indicators of family SES with adolescent drinking is mixed, although many studies show an inverse association (Hanson and Chen 2007). Higher levels of maternal depressive symptomology are associated with prenatal smoking (Orr et al. 2005). On the basis that some forms of major depressive symptomology are characterized by episodes that recur over the life course (National Institute of Mental Health, 2014), we hypothesize adolescent psychological distress will be a precursor to prenatal smoking behavior. We anticipate the same holds for binge drinking [see (Gavin et al. 2011) for indirect evidence of this pathway]. This line of reasoning is further supported by the notion of education as learned effectiveness (Mirowsky and Ross 2003) and is consistent with Healthy People 2020 goals identifying adolescent mental health and substance abuse as key preconception factors influencing birth outcomes.
As a final risk factor, we assess being unmarried at the time of birth (see Figure 1). Besides being intricately linked to family-of-origin SES (Duncan et al. 1998), and being a key risk factor for adverse birth outcomes [above and beyond conditions that select women into being married at the time of birth (Buckles and Price 2013, Kane 2016)], women who are unmarried three months prior to conception are more likely to smoke than their married counterparts (Colman and Joyce 2003). Marriage is an important social tie that influences health. Spouses can monitor and promote healthy behaviors (Umberson, Crosnoe, and Reczek 2010, Waite 1995), or broker information about social norms that can instill a sense of responsibility in their partners to adopt protective health behaviors (Umberson and Montez 2010). Social norms around prenatal smoking appear to be particularly salient, likely due to wide-reaching media messaging campaigns since the 1970s conveying risks to the fetus. Therefore, we hypothesize being unmarried is associated with an increased risk of prenatal smoking and is a potential mediator of the family-of-origin SES—prenatal smoking association.
METHODS
Study Design and Sample
This study uses data from the National Longitudinal Study of Adolescent to Adult Health (Add Health), a school-based, nationally representative sample of 20,745 7th-12th graders in1994–1995. Respondents were re-interviewed in 1996 (Wave II), 2001–02 (Wave III), and 2008–09 (Wave IV) (Harris 2010, Harris et al. 2009). At Wave IV, respondents were 24–32 years old (mean age = 28.5). The analytic sample includes female respondents (n = 10,480) participating in the Wave IV interview (8,352), that: had a live birth between the Wave I and Wave IV interview (3,852); were black, Hispanic, or white (3,640); had a valid sampling weight (3,404); and provided a non-missing, plausible response for birthweight and gestational length (n = 3,328). As some women in the sample had more than one birth between Waves I and IV, we selected only firstborns for inclusion in all analyses.
Variables
Offspring birth outcome and prenatal smoking data were collected by the Add Health team using computer-assisted self-interviewing (CASI) at Wave IV. Respondents were asked to report all births, and then were asked a series of questions about each birth. This study analyzes birth outcomes and prenatal smoking of the respondent’s first birth. As part of this sequence, respondents reported each infant’s birthweight in pounds and ounces; we converted birthweight to grams (range: 992–5,330g). Prenatal smoking during each pregnancy was assessed using multiple response options (“During this pregnancy with [initials of partner], how many cigarettes did you smoke? None; a few cigarettes, but not every week; a few cigarettes a week, but not every day; 10 or fewer a day; 11 to 20 a day; 21 to 30 a day; 31 or more a day; refused; don’t know”) which was collapsed into a binary variable (1 = any smoking during the pregnancy).
Family-of-origin SES was indicated using three variables from the Wave I interview: parental education (years completed), parental income, and family structure (1 = respondent reported living with two biological parents). [Supplementary analyses omitted parental income to explore if the relatively high level of missingness (compared to other Add Health variables) impacted results. Findings were extremely similar to those presented here.] Adolescent risk/protective factors (gathered in Wave I) included: if the respondent currently smoked, current grade point average, level of depressive symptomology in the last week [based on an abbreviated CES-D scale, validated in past research (Perreira et al. 2005)], and level of binge drinking in the last year [based on a scale used in past research; (Amato and Kane 2011)]. Marital status at birth was gathered from Wave IV (1 = unmarried at birth). Controls for respondents’ race-ethnicity (black, Hispanic; reference = white), native-born status (1 = born outside the U.S.), and infant sex (1 = male) were included.
Statistical Analysis
We estimated a structural equation model (SEM) in Mplus v7, employing survey weights and adjustments for clustering to account for the complex sample design. We included correlations terms for the residuals of all (endogenous) adolescent risk/protective factors, an approach that essentially controls for commonalities between unobserved variables affecting these risk/protective factors (Heckman 1979). We calculated total, direct, and indirect effects of key pathways (Bollen 1989). We report three goodness-of-fit statistics: the Confirmatory Fit Index (CFI), the Tucker-Lewis Index (TLI) [values for both must be above .90 to accept the model and above .95 to deem the model as a good fit], and the Root Means Square Error of Approximation (RMSEA) [values less than .05 indicate adequate model fit]. The TLI performs well for large sample sizes while adjusting for model complexity; the RMSEA adjusts for error in the population, making it ideal for use with large population-level samples (Bollen and Long 1993).
RESULTS
Table 1 describes the characteristics of our analytic sample. The average birthweight is 3,285g. Roughly one third of women transitioned to motherhood before age 20 (32.42%). The mean age at first birth in the sample was 21.86 years (median age = 21.42; not shown). Low birth weight (<2,500 grams) is observed among 7.8% of births, a proportion similar to that reported on birth certificate records among similarly-aged mothers nationwide in 2001—the median year in which women in our sample gave birth—9.4% among 15–19 year olds and 7.5% among 20–24 year olds (Martin et al. 2002). Using audio-CASI, one fifth (21%) of mothers reported prenatal smoking; as anticipated, this is slightly higher than nationwide estimates based on birth certificate records [in 2001, 19% among 18–19 year olds and 17% among 20–24 year olds; (Martin et al. 2002)]. More than half of births in the study sample were to unmarried mothers (60%), which is comparable to nonmarital childbearing rates nationwide in 2001: 79% of births to 15–19 year olds and 50% of births to 20–24 year olds (Martin et al. 2002).
Table 1.
Characteristics of Study Sample (N = 3,328), National Longitudinal Study of Adolescent Health
| Mean or Percent | Standard Deviation | |
|---|---|---|
| Birth weight (grams, range = 992 – 5,330) | 3282.50 | 569.17 |
| Prenatal Smoking | 21.66% | |
| Family-of-origin socioeconomic status | ||
| Level of parent’s education (range = 0 – 9) | 5.02 | 2.15 |
| (Log) Parental income (range = 0 – 6.8) | 3.40 | .82 |
| Living with two biological parents | 49.69% | |
| Current smoker, Wave I | 33.50% | |
| Grade point average, Wave I (range = 1 – 4) | 2.79 | .74 |
| Depressive symptomology, Wave I (range = 0 – 2.9) | .78 | .54 |
| Binge drinking, Wave I (range = 0 – 6) | .78 | 1.14 |
| Race-Ethnicity | ||
| non-Hispanic White (reference) | 72.31% | |
| non-Hispanic Black | 16.86% | |
| Hispanic | 10.83% | |
| Foreign-born | 2.76% | |
| Unmarried at birth | 58.70% | |
| Teen birth (1 = 19 or less, 0 = 20+) | 32.42% | |
| Infant is male | 51.80% | |
Notes: Statistics are weighted and adjusted for clustering.
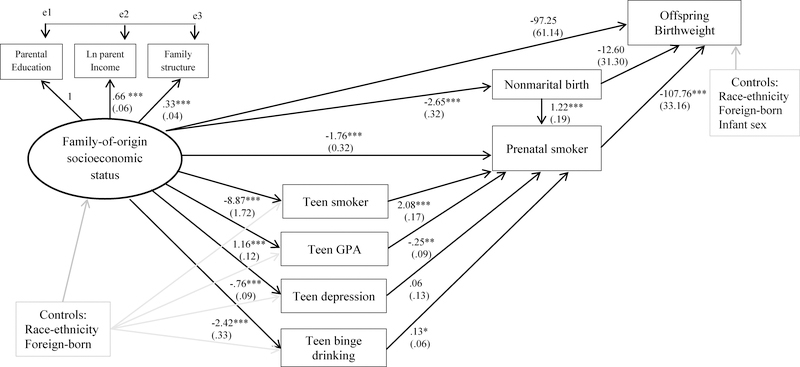
Figure 2 presents path coefficients and standard errors from the SEM. The total effect of family-of-origin SES on offspring birthweight is statistically significant (b = 60.68, SE = 20.83, p < .01), but the direct effect is not. Four indirect pathways fully mediate this association. First, higher levels of family-of-origin SES are associated with a decreased risk of prenatal smoking (b = −1.77, SE = .32; p < .001); in turn, prenatal smoking is associated with a decrease of 108g in birthweight (SE = 33.16; p < .001; indirect effect, p < .01). The next two pathways operate through adolescent risk/protective factors: higher levels of family-of-origin SES are associated with (a) decreased risk of teen smoking and (b) higher GPA in adolescence; teen smoking increases, and higher GPA reduces, the risk of prenatal smoking; in turn, prenatal smoking reduces birthweight (p < .05 and p < .10 for the indirect effects, respectively). Notably, adolescent smoking is associated with a substantial increase in the odds of prenatal smoking (b = 2.08, OR = eb = 8.00). The fourth indirect path shows higher levels of family-of-origin SES are associated with a lower risk of nonmarital birth; nonmarital birth is positively associated with prenatal smoking which is a risk factor for lower birthweight (indirect effect: p < .01).
Figure 2.
Parameter Estimates from the Structural Equation Model of Family-of-Origin Socioeconomic Status, Adolescent Risk/Protective Factors, Prenatal Smoking, and Birthweight
Notes: ***p < .001, **p < .01, *p < .05 (two-tailed). Analyses are weighted. N=3,328. RMSEA = 0.01, CFI = 0.99, TLI = 0.98. Correlations between all exogenous variables were included in the model but are not shown.
Results also suggest higher levels of family-of-origin SES reduce the risk of teen depression and teen binge drinking, although only the latter is associated with prenatal smoking (b = .13, SE = .06, p < .05). Neither pathway is a statistically significant mediator of the family-of-origin SES—birthweight association. Direct effects of adolescent risk factors (teen smoking, low GPA, depressive symptomology, and binge drinking) on offspring birthweight were assessed; none were statistically significant and therefore were omitted from the final model.
The R-squared of prenatal smoking provides a sense of how well the model explains prenatal smoking behavior: pathways included here account for 50% of the variance, a relatively large proportion given the parsimonious set of variables included. Model fit indices indicate excellent fit: the CLI and TLI are above 0.95, 0.99 and 0.98, respectively, and the RMSEA is well below 0.05 (0.01).
To assess the extent to which our results were robust to the endogeneity of prenatal smoking, we performed a sensitivity analysis whereby we estimated the same SEM (shown in Figure 2) but included a variable, state cigarette tax, to statistically identify prenatal smoking. State cigarette tax has been shown in past research to be a valid instrument, meaning it only affects birthweight through prenatal smoking and is uncorrelated with other unobserved factors related to birthweight (Evans and Ringel 1999). Results, presented in Appendix Table A, are markedly similar to those presented in Figure 2. This suggests our findings are robust.
DISCUSSION
Adverse birth outcomes are known to transmit inequality across generations, yet we do not yet fully understand their social determinants. Consistent with theory and past empirical work situating early life adversity as a prominent factor shaping adult health that also plays a key role in the intergenerational transmission of inequality (Kuh and Shlomo 2004, Elder 1977, Elder Jr 1998, Elder Jr, Johnson, and Crosnoe 2003, Palloni 2006), this study draws from life course theory and life course epidemiology and uses structural equation modeling to analyze chains of risk that originate within early life adversity, impact later-observed risky health behaviors in adolescence and during pregnancy, and ultimately increase the risk of adverse birth outcomes. Our study is the first to assess these pathways using accurate (and more normatively-timed) birth data among a contemporary, population-based sample in the U.S., and to assess these pathways while accounting for the endogeneity of prenatal smoking.
Consistent with a life course epidemiological model of accumulation with risk clustering (Kuh and Shlomo 2004), results demonstrated that adolescent risk factors (teen smoking, low academic performance, depressive symptomology, and binge drinking) are not only clustered by family-of-origin SES, but are also embedded within a chain of risk linking family-of-origin SES to an increased risk of prenatal smoking; in turn, prenatal smoking was associated with lower offspring birthweight. In particular, four pathways fully mediated the association between maternal family-of-origin SES and offspring birthweight; identifying the full set of pathways explaining this association is the first contribution of our study. The first pathway suggested lower family-of-origin SES was associated with an increased risk of prenatal smoking, which in turn was associated with lower birthweight. The present study is the first to provide evidence of this pathway after accounting for the endogeneity of prenatal smoking, suggesting it is robust to the inclusion of factors that confound (and bias) the estimated effect of prenatal smoking on birthweight. Therefore, this finding considerably strengthens the current evidence base classifying prenatal smoking as a mediator of the childhood poverty—adverse birth outcome association (Harville et al. 2010, Gavin et al. 2011).
The other three pathways documented by our study suggest that women exposed to lower levels of family-of-origin SES were at increased risk of poor educational performance in high school, adolescent-uptake of smoking, and nonmarital childbearing; each factor independently increased the risk of prenatal smoking, which in turn was associated with decreased birthweight. No studies have yet assessed high school academic performance or marital status as potential mediators of the association between family-of-origin SES and prenatal smoking, although the former is consistent with the theoretical notion of education as learned effectiveness that has important benefits for health behaviors (Mirowsky and Ross 2003), and with empirical work suggesting that both cognitive and non-cognitive skills (that are reflected in GPA) influence smoking (Chiteji 2010, Mendolia and Walker 2014).
Moreover, that adolescent smoking, but not binge drinking, significantly mediated the association between family-of-origin SES and prenatal smoking sharpened a finding from past research exploring the effect of a latent variable of adolescent substance abuse (including smoking, drinking, and drug use) on prenatal smoking (Gavin et al. 2012). Results of the present study reveal which form of substance abuse is implicated as a key risk factor of prenatal smoking (namely, teen smoking) and which is not (teen binge drinking). This is a key distinction that can fundamentally inform future prevention efforts aimed at preventing prenatal smoking; this is the second contribution of our study. More specifically, young women who began smoking as teenagers were eight times more likely to engage in prenatal smoking than their non-teen-smoking counterparts. In the language of life course epidemiology (Kuh and Shlomo 2004), adolescent-uptake of smoking appears to be a trigger that increases the risk of engaging in unhealthy behaviors later on, ultimately increasing the risk of poor offspring health at birth. Under this interpretation, adolescent smoking is therefore a place in the chain of risk where prevention efforts could be targeted, thereby potentially averting or breaking the chain of risk leading to poor offspring health at birth for vulnerable individuals.
This contribution, in and of itself, is rather intuitive, but has dramatic implications for prenatal smoking efforts. In contrast to current cessation efforts that largely target women who are already pregnant [and, which demonstrate modest results, such as a 5% reduction in prenatal smoking behavior (Ershoff et al. 1999, Chamberlain et al. 2013)], this finding suggests a more efficacious strategy may be to redouble efforts aimed at preventing adolescent-uptake of smoking. This shift in the target population buttresses past recommendations to focus smoking prevention efforts earlier in the life course and further reduce the number of young women who initiate smoking (Ebrahim et al. 2000).
Taken together, these four pathways add new insights that can directly inform Healthy People 2020 efforts to identify, measure, and track preconception risk factors of adverse birth outcomes at the population-level, thereby buttressing the, as yet, scant evidence base upon which Healthy People 2020 is based (Richardson, Hussey, and Strutz 2012). Future research should build on the current study by exploring racial/ethnic differences in these four pathways. While beyond the scope of the current study, such an examination would enrich this evidence base, given the stark racial patterning observed in early life adversity, prenatal smoking, and birthweight in the U.S. Broadly speaking, study findings also reveal salient social patterning in the chain of risk leading to adverse birth outcomes, a finding that buttresses the wide body of scholarship highlighting the centrality of adversity encountered within the sensitive periods of childhood and adolescence in terms of shaping adult health and wellbeing (Umberson et al. 2014, Ben-Shlomo and Kuh 2002, Miller, Chen, and Parker 2011, Shonkoff, Boyce, and McEwen 2009, Hayward and Gorman 2004, Haas 2008).
A key implication of these documented pathways is that efforts to minimize maternal early life adversity (observed here in the form of low family-of-origin SES) could potentially disrupt the chain of risks leading to poor offspring health at birth. An emphasis on early life interventions is logically consistent with Heckman’s (2008) work showing a higher rate of return on investments in early life. It is also consistent with Link and Phelan’s (1995) foundational argument, proposing that little gains will be made in improving health without first changing the social conditions that are fundamental causes of disease. Given the unique intra- and intergenerational implications of adverse birth outcomes, the stakes are even higher, suggesting a successful intervention along these lines could potentially disrupt intergenerational transmissions of inequality at the population-level.
A strength of our study was the use of audio computer assisted interviewing to ascertain prenatal smoking status, an approach shown to reduce misclassification error relative to administrative data (Srisukhumbowornchai, Krikov, and Feldkamp 2012), and to employ data gathered through a question with multiple responses—an approach that likely further reduced nondisclosure (Mullen et al. 1991). We were therefore able to detect a higher prevalence of prenatal smoking (21%) than has been previously documented using birth certificate data, buttressing existing arguments for assessing sensitive health behaviors using modes other than clinician’s report/administrative data.
A limitation of our study was the assumption (imposed by data constraints) that marital status at birth approximated marital status at conception. This assumption would not hold in the case of shotgun marriage, however, nowadays this event is rare (Rackin and Gibson Davis 2012). Our study was also limited to primiparous women, a group of women that may be more likely to spontaneously quit upon learning of a pregnancy (Colman and Joyce 2003). Future research should replicate this study within multiparous women. Due to data constraints, women were also not observed past age 32 (although a fifth wave of interviews is underway, when respondents are between 32 and 42 years old). Future research should explore whether the pathways demonstrated in this study differ among women transitioning to motherhood beyond age 32 as rates of prenatal smoking are lower among mothers aged 25+ years (versus 15–24 years) (Child Trends Databank 2014). Lastly, while the parsimonious set of pathways included herein explained 50% of the variance in prenatal smoking and therefore likely describe a meaningful set of preconception processes contributing to the risk of prenatal smoking, future studies could explore other adolescent risk factors, such as conduct disorder or dietary factors. These limitations are weighed against the fact that the potential for recall bias in our data is likely to be relatively low, given the time elapsed between birth and reporting of birth is relatively short.
In conclusion, study findings point to the importance of unpacking longer-term, intragenerational processes—whereby the deleterious effects of maternal early life adversity spill over into adult health and wellbeing—that appear to be key inputs into the intergenerational transmission of inequality via birthweight. When these intragenerational pathways are integrated within a multigenerational process, as was the case in this study, this approach can reveal insight into the minimal success health interventions have demonstrated, in terms of reducing the substantial and seemingly intransient class- and race-based disparities in infant health in the U.S.
Supplementary Material
Research Highlights.
A chain of risk links maternal family-of-origin SES to offspring birthweight.
Prenatal smoking is a key part of this chain of risk.
Teen smoking, low GPA, and being unmarried link family SES to prenatal smoking.
Preventing teen smoking may break the chain of risks, improving infant birthweight.
Efforts to prevent adolescent-uptake of smoking should be redoubled.
Acknowledgements
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). This research received support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: K99/R00 HD075860 (PI: Kane), T32-HD52468 (PI: Siega-Riz), P01-HD31921 (PI: Harris), and P2C HD050924 (PI: Morgan). Opinions reflect those of the authors and not necessarily those of the granting agencies.
Footnotes
Ethics approval
This research was approved by the Institutional Review Board at University of California, Irvine (9300).
REFERENCES
- Aizer Anna, and Currie Janet. 2014. “The intergenerational transmission of inequality: Maternal disadvantage and health at birth.” Science 344 (6186):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond Douglas, and Currie Janet. 2011. “Killing me softly: The fetal origins hypothesis.” The Journal of Economic Perspectives 25 (3):153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato Paul R, and Kane Jennifer B. 2011. “Life Course Pathways and the Psychosocial Adjustment of Young Adult Women.” Journal of Marriage and Family 73 (1):279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelberg Benjamin J, Corey Catherine G, Hoffman Allison C, Schroeder Megan J, Husten Corinne G, Caraballo Ralph S, and Backinger Cathy L. 2014. “Symptoms of Tobacco Dependence Among Middle and High School Tobacco Users: Results from the 2012 National Youth Tobacco Survey.” American Journal of Preventive Medicine 47 (2):S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrash HK, Johnson K, Adams M, Cordero JF, and Howse J 2006. “Preconception Care for Improving Perinatal Outcomes: The Time to Act.” Maternal and Child Health Journal 10 (1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman Richard E, and Adrienne Stith Butler. 2007. Preterm birth: causes, consequences, and prevention: National Academy Press. [PubMed] [Google Scholar]
- Ben-Shlomo Y, and Kuh D 2002. “A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives.” International Journal of Epidemiology 31 (2):285–293. [PubMed] [Google Scholar]
- Bollen KA 1989. Structural equations with latent variables. Vol. 8. [Google Scholar]
- Bollen Kenneth A, and Long J Scott. 1993. Testing Structural Equation Models. Newbury Park, California: Sage Publications. [Google Scholar]
- Buckles Kasey S, and Price Joseph. 2013. “Selection and the Marriage Premium for Infant Health.” Demography:1–25. [DOI] [PubMed] [Google Scholar]
- Case A, and Paxson Christina H. 2006. “Children’s health and social mobility.” The Future of Children 16 (2):151–173. [DOI] [PubMed] [Google Scholar]
- Center on the Developing Child at Harvard University. 2010. “The Foundations of Lifelong Health Are Built in Early Childhood.” http://developingchild.harvard.edu/.
- Centers for Disease Control and Prevention. 2014. “Tobacco Use Among Middle and High School Students—United States, 2013.” Morbidity and Mortality Weekly Report 63 (45):1021–6. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain C, O’Mara-Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, and Thomas J 2013. “Psychosocial interventions for supporting women to stop smoking in pregnancy.” Cochrane Database of Systematic Reviews (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child Trends Databank. 2014. “Mothers who smoke while pregnant.” Available at http://www.childtrends.org/?indicators=mothers-who-smoke-while-pregnant.
- Chiteji Ngina. 2010. “Time-preference, non-cognitive skills and well-being across the life course: Do non-cognitive skills encourage healthy behavior?” The American Economic Review 100 (2):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius Sven. 2004. “The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes.” Nicotine & Tobacco Research 6 (Supplement 2):S125–S140. [DOI] [PubMed] [Google Scholar]
- Colman Gregory J, and Joyce Ted. 2003. “Trends in Smoking Before, During, and After Pregnancy in Ten States.” American Journal of Preventive Medicine 24 (1):29–35. [DOI] [PubMed] [Google Scholar]
- Conley Dalton, Strully Kate Wetteroth, and Bennett Neil G. 2003. The starting gate: Birth weight and life chances: Univ of California Press. [Google Scholar]
- Curry Susan J, Sporer Amy K, Pugach Oksana, Campbell Richard T, and Emery Sherry. 2007. “Use of tobacco cessation treatments among young adult smokers: 2005 National Health Interview Survey.” American Journal of Public Health 97 (8):1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz Patricia M, England Lucinda J, Shapiro-Mendoza Carrie K, Tong Van T, Farr Sherry L, and Callaghan William M. 2010. “Infant Morbidity and Mortality Attributable to Prenatal Smoking in the U.S.” American Journal of Preventive Medicine 39 (1):45–52. [DOI] [PubMed] [Google Scholar]
- Duncan Greg J, Yeung W Jean, Brooks-Gunn Jeanne, and Smith Judith R. 1998. “How much does childhood poverty affect the life chances of children?” American sociological review:406–423. [Google Scholar]
- Ebrahim Shahul H, Floyd R Louise, Merritt Robert K II, Decoufle Pierre, and Holtzman Deborah. 2000. “Trends in pregnancy-related smoking rates in the United States, 1987–1996.” Journal of the American Medical Association 283 (3):361–366. [DOI] [PubMed] [Google Scholar]
- Elder GH 1977. “Family history and the life course.” Journal of Family History 2 (4):279. [DOI] [PubMed] [Google Scholar]
- Elder GH Jr 1998. “The life course as developmental theory.” Child development 69 (1):1–12. [PubMed] [Google Scholar]
- Jr Elder, Glen H, and Giele Janet Z. 2009. “Life course studies: An evolving field” In The Craft of Life Course Research, edited by Elder GH Jr and Giele Janet Z, 1–24. New York: The Guilford Press. [Google Scholar]
- Jr Elder, Glen H, Johnson Monica Kirkpatrick, and Crosnoe Robert. 2003. The Emergence and Development of Life Course Theory Edited by Mortimer Jeylan T and Shanahan Michael J, Handbook of the Life Course. New York: Springer. [Google Scholar]
- Ershoff Daniel H., Quinn Virginia P., Boyd Neal R., Stern Julie, Gregory Margaret, and Wirtschafter David. 1999. “The Kaiser Permanente prenatal smoking-cessation trial: When more isn’t better, what is enough?” American Journal of Preventive Medicine 17 (3):161–168. doi: 10.1016/S0749-3797(99)00071-9. [DOI] [PubMed] [Google Scholar]
- Evans William N, and Ringel Jeanne S. 1999. “Can higher cigarette taxes improve birth outcomes?” Journal of public Economics 72 (1):135–154. [Google Scholar]
- Gavin Amelia R, Hill Karl G, Hawkins J David, and Maas Carl. 2011. “The role of maternal early-life and later-life risk factors on offspring low birth weight: Findings from a three-generational study.” Journal of Adolescent Health 49 (2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin Amelia R, Thompson Elaine, Rue Tessa, and Guo Yuqing. 2012. “Maternal Early Life Risk Factors for Offspring Birth Weight: Findings from the Add Health Study.” Prevention Science 13 (2):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg Robert L, and Culhane Jennifer F. 2007. “Low birth weight in the United States.” The American Journal of Clinical Nutrition 85 (2):584S–590S. [DOI] [PubMed] [Google Scholar]
- Goldenberg Robert L, Culhane Jennifer, Iams JD, and Romero R 2008. “Epidemiology and causes of preterm birth.” The Lancet 371 (9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Elizabeth, and Huang Bin. 2002. “Socioeconomic status, depressive symptoms, and adolescent substance use.” Archives of pediatrics & adolescent medicine 156 (5):448–453. [DOI] [PubMed] [Google Scholar]
- Haas SA 2008. “Trajectories of functional health: the ‘long arm’of childhood health and socioeconomic factors.” Social Science & Medicine 66 (4):849–861. [DOI] [PubMed] [Google Scholar]
- Halfon N, and Hochstein M 2002. “Life course health development: an integrated framework for developing health, policy, and research.” Milbank Quarterly 80 (3):433–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson Margaret D, and Chen Edith. 2007. “Socioeconomic status and health behaviors in adolescence: a review of the literature.” Journal of behavioral medicine 30 (3):263. [DOI] [PubMed] [Google Scholar]
- Harris KM 2010. “An integrative approach to health.” Demography 47 (1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Halpern CT, Whitsel E, Hussey J, Tabor J, Entzel P, and Udry JR 2009. The National Longitudinal Study of Adolescent Health: Research Design. http://www.cpc.unc.edu/projects/addhealth/design: Carolina Population Center, University of North Carolina at Chapel Hill. [Google Scholar]
- Harville Emily W, Boynton-Jarrett Renée, Power Chris, and Hypponen Elina. 2010. “Childhood hardship, maternal smoking, and birth outcomes: A prospective cohort study.” Archives of Pediatrics & Adolescent Medicine 164 (6):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward Mark D, and Gorman Bridget K. 2004. “The long arm of childhood: The influence of early-life social conditions on men’s mortality.” Demography 41 (1):87–107. [DOI] [PubMed] [Google Scholar]
- Heckman James J. 1979. “Sample selection bias as a specification error.” Econometrica: Journal of the Econometric Society:153–161. [Google Scholar]
- Heckman James J. 2000. “Policies to foster human capital.” Research in Economics 54 (1):3–56. [Google Scholar]
- Heckman James J. 2008. “Schools, skills, and synapses.” Economic inquiry 46 (3):289–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman Clyde, and Boyce Tom. 2010. “How experience gets under the skin to create gradients in developmental health.” Annual Review of Public Health 31:329–347. [DOI] [PubMed] [Google Scholar]
- Johnson Kay, Posner Samuel F., Biermann Janis, Cordero José F., Atrash Hani K., Parker Christopher S., Boulet Sheree, and Curtis Michele G. 2006. “Recommendations to Improve Preconception Health and Health Care: A Report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care.” Morbidity and Mortality Weekly Report 55 (RR06):1–23. [PubMed] [Google Scholar]
- Kane Jennifer B. 2015. “An Integrative Model of Inter- and Intragenerational Preconception Processes Influencing Birthweight in the United States.” Journal of Health and Social Behavior 56 (2):246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane Jennifer B. 2016. “Marriage Advantages in Perinatal Health: Evidence of Marriage Selection or Marriage Protection?” Journal of Marriage and Family 78 (1):212–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane Jennifer B., and Margerison-Zilko Claire. 2017. “Theoretical Insights into Preconception Social Conditions and Perinatal Health: The Role of Place and Social Relationships.” Population Research and Policy Review 36 (5):639–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS 1987. “Determinants of low birth weight: methodological assessment and meta-analysis.” Bulletin of the World Health Organization 65 (5):663. [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, and Power C 2003. “Life course epidemiology.” Journal of Epidemiology and Community Health 57 (10):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, and Shlomo YB 2004. A life course approach to chronic diseases epidemiology. Vol. 2: Oxford University Press, USA. [Google Scholar]
- Lien DS, and Evans WN 2005. “Estimating the Impact of Large Cigarette Tax Hikes The Case of Maternal Smoking and Infant Birth Weight.” Journal of Human Resources 40 (2):373–392. [Google Scholar]
- Link BG, and Phelan J 1995. “Social conditions as fundamental causes of disease.” Journal of Health and Social Behavior (Extra Issue):80–94. [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM, and Sutton PD 2002. “Births: Final Data for 2001.” National Vital Statistics Reports 51 (2). [PubMed] [Google Scholar]
- Mendolia Silvia, and Walker Ian. 2014. “The effect of noncognitive traits on health behaviours in adolescence.” Health economics 23 (9):1146–1158. [DOI] [PubMed] [Google Scholar]
- Miller Gregory E, Chen Edith, and Parker Karen J. 2011. “Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms.” Psychological bulletin 137 (6):959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky John, and Ross Catherine E. 2003. Education, social status, and health. New York: Aldine de Gruyter. [Google Scholar]
- Montez Jennifer Karas, and Hayward Mark D. 2014. “Cumulative childhood adversity, educational attainment, and active life expectancy among US adults.” Demography 51 (2):413–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen Patricia Dolan, Carbonari Joseph P, Tabak Ellen R, and Glenday Marianna C. 1991. “Improving disclosure of smoking by pregnant women.” American journal of obstetrics and gynecology 165 (2):409–413. [DOI] [PubMed] [Google Scholar]
- Orr Suezanne T, Newton Edward, Tarwater Patrick M, and Weismiller David. 2005. “Factors associated with prenatal smoking among black women in eastern North Carolina.” Maternal and child health journal 9 (3):245–252. [DOI] [PubMed] [Google Scholar]
- Palloni Alberto. 2006. “Reproducing inequalities: Luck, wallets, and the enduring effects of childhood health.” Demography 43 (4):587–615. [DOI] [PubMed] [Google Scholar]
- Paneth NS 1995. “The problem of low birth weight.” The Future of Children 5 (1):19–34. [PubMed] [Google Scholar]
- Perreira Krista M, Deeb-Sossa Natalia, Kathleen Mullan Harris, and Bollen Kenneth. 2005. “What are we measuring? An evaluation of the CES-D across race/ethnicity and immigrant generation.” Social Forces 83 (4):1567–1601. [Google Scholar]
- Pickett Kate E, Rathouz Paul J, Kasza Kristen, Wakschlag Lauren S, and Wright Rosalind. 2005. “Self reported smoking, cotinine levels, and patterns of smoking in pregnancy.” Paediatric and perinatal epidemiology 19 (5):368–376. [DOI] [PubMed] [Google Scholar]
- Quinn Virginia P, Mullen Patricia Dolan, and Ershoff Daniel H. 1991. “Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery.” Addictive behaviors 16 (1–2):29–40. [DOI] [PubMed] [Google Scholar]
- Rackin Heather, and Gibson Christina M Davis. 2012. “The Role of Pre and Postconception Relationships for First Time Parents.” Journal of Marriage and Family 74 (3):526–539. [Google Scholar]
- Richardson Liana J, Hussey Jon M, and Strutz Kelly L. 2012. “A Life Course Perspective in Maternal and Child Health” In Maternal and Child Health: Programs, Problems, and Policy, edited by Kotch Jonathan B., 65–85. Gaithersburg, MD: Aspen Publishers, Inc. [Google Scholar]
- Rumberger Russell W. 1987. “High school dropouts: A review of issues and evidence.” Review of Educational Research 57 (2):101–121. [Google Scholar]
- Sandel Michael J. 2012. What money can’t buy: the moral limits of markets: Macmillan. [Google Scholar]
- Savitz DA, and Murnane P 2010. “Behavioral influences on preterm birth: a review.” Epidemiology 21 (3):291. [DOI] [PubMed] [Google Scholar]
- Shonkoff Jack P, Boyce W Thomas, and McEwen Bruce S. 2009. “Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention.” JAMA 301 (21):2252–2259. [DOI] [PubMed] [Google Scholar]
- Srisukhumbowornchai Sivithee, Krikov Sergey, and Feldkamp Marcia L. 2012. “Self reported maternal smoking during pregnancy by source in Utah, 2003–2007.” Birth Defects [DOI] [PubMed] [Google Scholar]
- Research Part A: Clinical and Molecular Teratology 94 (12):996–1003. [DOI] [PubMed] [Google Scholar]
- Umberson D, Crosnoe Robert, and Reczek Corinne. 2010. “Social relationships and health behavior across life course.” Annual Review of Sociology 36:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, and Montez JK 2010. “Social Relationships and Health A Flashpoint for Health Policy.” Journal of Health and Social Behavior 51 (1 suppl):S54–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson Debra, Williams Kristi, Thomas Patricia A Liu Hui, and Thomeer Mieke Beth. 2014. “Race, gender, and chains of disadvantage childhood adversity, social relationships, and health.” Journal of health and social behavior 55 (1):20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck PC 2010. “Celebrating 75 Years of Title V (Maternal and Child Health) and Re-exploring Our Roots.” Maternal and Child Health Journal 14 (6):817–821. [DOI] [PubMed] [Google Scholar]
- Waite LJ 1995. “Does marriage matter?” Demography 32 (4):483–507. [PubMed] [Google Scholar]
- Webb David A, Boyd Neal R, Messina Darlene, and Windsor Richard A. 2003. “The Discrepancy Between Self Reported Smoking Status and Urine Cotinine Levels Among Women Enrolled in Prenatal Care at Four Publicly Funded Clinical Sites.” Journal of Public Health Management and Practice 9 (4):322–325. [DOI] [PubMed] [Google Scholar]
- Wehby GL, Fletcher JM, Lehrer SF, Moreno LM, Murray JC, Wilcox A, and Lie RT 2011. “A Genetic Instrumental Variables Analysis of the Effects of Prenatal Smoking on Birth Weight: Evidence from Two Samples.” Biodemography and Social Biology 57 (1):3–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarski John S, and Smyth Nancy J. 1994. “Adolescent substance abuse: A comprehensive approach to prevention intervention.” Journal of Child & Adolescent Substance Abuse 3 (3):33–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.