Abstract
Pseudoxanthoma elasticum (PXE) is a rare genetic condition primarily caused by hepatic ABCC6 transporter dysfunction. Most clinical manifestations of PXE are due to premature calcification of elastic fibers. However, the vascular impact of PXE is pleiotropic and remains ill defined. ABCC6 expression has recently been associated with cellular nucleotide export. We studied the impact of ABCC6 deficiency on blood levels of aden-osine triphosphate and related metabolites and on soluble nucleotidase activities in PXE patients and Abcc6−/− mice. In addition, we investigated the expression of genes encoding ectocellular purinergic signaling proteins in mouse liver and aorta. Plasma adenosine triphosphate and pyrophosphate levels were significantly reduced in PXE patients and in Abcc6−/− mice, whereas adenosine concentration was not modified. Moreover, 5′-nucleotidase/CD73 activity was increased in the serum of PXE patients and Abcc6−/− mice. Consistent with alterations of purinergic signaling, the expression of genes involved in purine and phosphate transport/metabolism was dramatically modified in Abcc6−/− mouse aorta, with much less impact on the liver. ABCC6 deficiency causes impaired vascular homeostasis and tissue perfusion. Our findings suggest that these alterations are linked to changes in extracellular nucleotide metabolism that are remote from the liver. This opens new perspectives for the understanding of PXE pathophysiology.
INTRODUCTION
Pseudoxanthoma elasticum (PXE) (OMIM 264800) is an inherited multisystem disorder characterized by fragmentation (elastorrhexis) and progressive calcification of the elastic fibers in the skin, Bruch’s membrane of the retina, and the media layer of the arteries (Li et al., 2009; Neldner, 1988). Mutations causing PXE have been identified in ABCC6, which encodes an adenosine triphosphate (ATP)-binding cassette transporter that is primarily expressed in the liver and kidneys (Le Saux et al., 2000). These findings suggested that PXE is a metabolic disease displaying a silent “central” (liver) causative defect and “distal” (connective tissue) manifestations. It was then assumed that ABCC6 transports anti-calcifying metabolite(s) from hepatocytes into the systemic circulation. In addition to this so-called metabolic hypothesis, a cellular pathophysiological hypothesis of PXE proposes that ABCC6 is expressed at low levels in affected tissues, including dermal fibroblasts and vascular, immune, and nerve cells (Beck et al., 2003; Bergen et al., 2000; Matsuzaki et al., 2005).
Our understanding of the pathophysiology of inherited ectopic calcification evolved in the light of three rare diseases, namely, PXE, general arterial calcification of infancy due to NPP1 deficiency (OMIM 208000), and arterial calcification due to CD73 deficiency (ACDC, OMIM 211800). A recent breakthrough proposed that the ABCC6 transporter contributes to cellular ATP export from hepatocytes. ATP serves as a substrate for ENPP1 to generate pyrophosphate (PPi), a physiological anticalcifying molecule (Jansen et al., 2013) that opposes the effect of inorganic phosphate (Pi) on hydroxyapatite (Ca/Pi) crystal growth. Corroborating this hypothesis, deficiency in ABCC6 or ENPP1 results in ectopic calcifications and reduced levels of plasma PPi (Jansen et al., 2013)), and PPi treatment prevents mineralization in mouse models (Dedinszki et al., 2017; Pomozi et al., 2017). ABCC6-dependent general arterial calcification of infancy and ENPP1-dependent PXE phenotypes have been reported (Nitschke et al., 2012)), indicating (i) the central role of a PPi deficit in the pathophysiology of mineralization and (ii) the overlap of ABCC6 and ENPP1 functions. Finally, mutations in NT5e, which encodes the ecto 5′-nucleotidase adenosine (ADO)-generating enzyme, were associated with mineralization in ACDC, a phenotype with features overlapping with PXE and general arterial calcification of infancy. In ACDC, the calcification is due to insufficient ADO levels, which exert tonic repression of pro-calcifying/Pi-generating alkaline phosphatase (AP).
The redundancy in the clinical presentations of these diseases together sheds light on the central role of extracellular nucleotide phosphohydrolysis in the Pi/PPi ratio. However, little information on the exact molecular function of ABCC6 has been identified.
Beside calcifications, other cardiovascular manifestations are frequent in PXE, including peripheral arterial disease, which represents a major determinant of morbidity associated with PXE; ischemic stroke (Pavlovic et al., 2005; van den Berg et al., 2000); premature arterial occlusion (Pingel et al., 2017)); and carotid malformations (Vasseur et al., 2011), Omarjee et al., submitted). Studies performed on the Abcc6−/− mouse model showed that ABCC6 participates in cardiac protection in the context of dystrophic cardiac calcification (Meng et al., 2007), ischemia reperfusion injury (Mungrue et al., 2011), and cardiac fibrosis (Rau et al., 2015). Moreover, several studies proposed that ABCC6 deficiency is associated with other genetic diseases, such as β-thalassemia (Aessopos et al., 1992; Martin et al., 2011) or general arterial calcification of infancy, as well as with acquired diseases such as chronic kidney disease (Lau et al., 2014). Hence, ABCC6 mutations cause a broad spectrum of cardiovascular manifestations that are not necessarily related to calcification.
Although ectopic calcifications seem to clearly be due to a PPi deficit, structural, ischemic, thrombotic, and other nonmineralizing manifestations associated with PXE may indeed be independent of the mineralization process and extracellular PPi levels. Therefore, we evaluated the global changes in extracellular purine metabolism by measuring the expression of genes implicated in nucleotide and phosphate metabolism; enzymatic activities; and circulating nucleotide, nucleoside, and PPi levels in both PXE patients and the Abcc6−/− mouse model. The changes we observed may cause alterations of purinergic signaling and showed molecular aspects of the pathomechanisms behind PXE that to our knowledge are unreported.
RESULTS
ABCC6 deficiency is associated with reduced plasma concentrations of adenine nucleotides and PPi
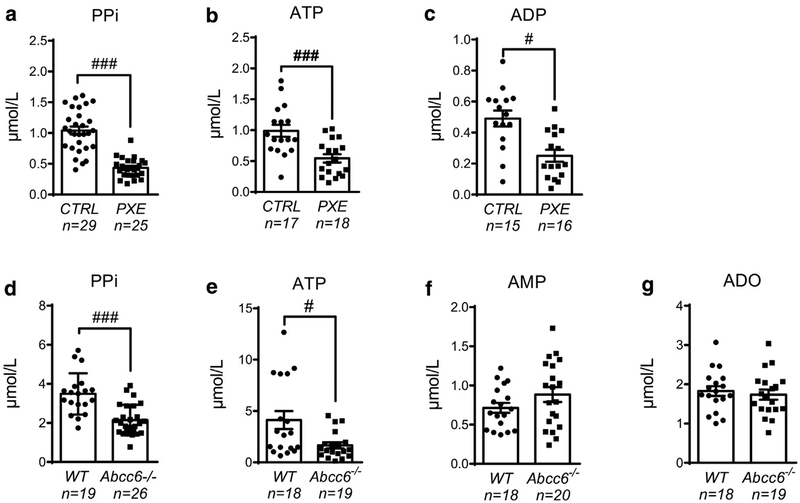
To evaluate circulating nucleotide and PPi concentrations in ABCC6 deficiency, we recruited PXE patients and age-and sex-matched healthy control individuals (Table 1). The characteristics of the PXE population, including the pathology severity (Phenodex) score (Legrand et al., 2017) and genetic mutations, as well as the sample used for the different assays, are presented in Supplementary Table S1 online. Together with a highly significant reduction in PPi concentrations in PXE patients (P = 0.0001) (Figure 1a), corroborating the previous findings of Jansen et al. (2014), plasma ATP and adenosine diphosphate (ADP) concentrations were significantly reduced within the same range (2-fold) compared with those of healthy control individuals (P = 0.0005 and P = 0.015, respectively) (Figure 1b and c). The deficit in PPi concentrations was corroborated in Abcc6−/− plasma mouse samples (Figure 1d), as previously described (Jansen et al., 2013), again with an equivalent 2-fold decrease in plasma ATP concentration (P = 0.008) (Figure 1e). We did not observe a correlation between age and any of the quantified metabolites in the plasma of Abcc6−/− or wild-type (WT) mice (data not shown). ADO quantification requires a specific procedure to avoid active cellular uptake and catabolism (Saadjian et al., 2002). Because of the specificity of the procedure, the limited number of patients, and their low frequency of consultation, we were not able to evaluate ADO in PXE patients. We therefore measured adenosine monophosphate (AMP) and ADO in WT and Abcc6−/− mice. Both AMP and ADO were maintained in Abcc6−/− mice (Figure 1f and g), indicating that the extracellular ATP deficit does not affect ADO concentration.
Table 1.
Patient Characteristics
| Subgroup | n | Age in Years, Mean ± SD |
|---|---|---|
| Patient characteristics for plasma ATP and ADP determination | ||
| Control | ||
| All | 17 | 45 ± 11 |
| Female | 12 | 44 ± 11 |
| Male | 5 | 47 ± 9 |
| PXE | ||
| All | 18 | 46 ± 13 |
| Female | 13 | 45 ± 14 |
| Male | 5 | 47 ± 9 |
| Patient characteristics for plasma PPi determination | ||
| Control | ||
| All | 29 | 38 ± 12 |
| Female | 20 | 38 ± 12 |
| Male | 9 | 42 ± 12 |
| PXE | ||
| All | 25 | 43 ± 19 |
| Female | 15 | 46 ± 16 |
| Male | 10 | 39 ± 22 |
| Patient characteristics for serum enzymatic activities determination | ||
| Control | ||
| All | 20 | 46 ± 3 |
| Female | 15 | 45 ± 14 |
| Male | 5 | 47 ± 9 |
| PXE | ||
| All | 26 | 44 ± 12 |
| Female | 19 | 43 ± 13 |
| Male | 7 | 46 ± 9 |
Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; PPi, pyrophosphate; PXE, pseudoxanthoma elasticum; SD, standard deviation.
Figure 1. Quantification of plasma adenine nucleotide, ADO, and PPi levels during ABCC6 deficiency.
Concentrations of (a) PPi, (b) ATP, and (c) ADP were determined in blood plasma from healthy control (CTRL) and PXE individuals. (d) PlasmaPPi, (e) ATP, (f) AMP, and (g) adenosine were measured in WT and Abcc6−/− mice. Data represent the mean ± standard error of the mean of the indicated number of plasma samples. #P < 0.05, ###P < 0.005. ADO, adenosine; AMP, adenosine monophosphate; ATP, adenosine triphosphate; CTRL, control; PPi, pyrophosphate; PXE, pseudoxanthoma elasticum; WT, wild type.
Alteration of soluble nucleotide metabolizing activities in ABCC6 deficiency
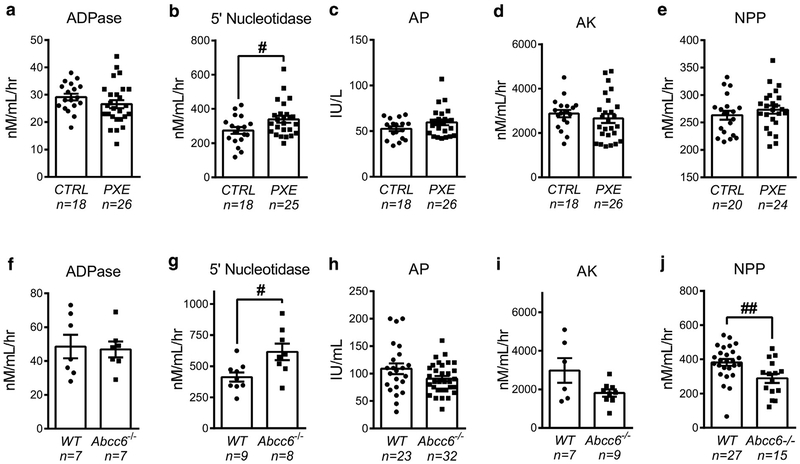
Soluble purine-converting enzymes are present in the bloodstream where they contribute, along with membrane-bound ecto-nucleotidases, to the metabolism of circulating nucleotides in the vasculature (Yegutkin et al., 2012). Here, we quantified the activities of several key enzymes—NTPDase1/CD39, 5′-nucleotidase/CD73, AP, adenylate kinase (AK), and NPP1—in the sera of PXE patients and Abcc6−/− mice. Although PXE patients displayed a slight up-regulation in serum AP activity, it did not reach statistical significance (P = 0.10). PXE patients also showed increased 5′-nucleotidase/CD73 activity (P = 0.015, Figure 2b), but no change was found for the other soluble enzymes: NTPDase1/ADPase, AP, AK, and NPP1 (Figure 2a–e). The activity of 5′-nucleotidase/CD73 was also elevated in serum from Abcc6−/− mice (P = 0.030), paralleling decreased NPP1 (P = 0.006) activity, but not those of ADPase, AP, and AK (Figure 2f–j).
Figure 2. Changes in soluble nucleotide-converting activities in PXE patients and Abcc6−/− mice.
The activities of key soluble enzymes, (a, f) NTPDase1/CD39 (ADPase), (b, g) ecto-5′-nucleotidase/CD73, (c, h) alkaline phosphatase (AP), (d, i) adenylate kinase (AK), and (e, j) nucleotide pyrophosphatase phosphodiesterase (NPP) were determined in (a–e) the serum of healthy control individuals and PXE patients and in (f–j) Abcc6−/− and WT mice. Data represent the mean ± standard error of the mean of the indicated number of serum samples. #P < 0.05; ##P < 0.01. CTRL, control; IU, international unit; nM/mL/hr, nano molar/milliliter/hour; PXE, pseudoxanthoma elasticum.
Altered arterial expression of purinergic signaling partners in Abcc6−/− mice
To evaluate the impact of the PXE condition on hepatic (central) and arterial (distal) gene expression, we used a quantitative real-time PCR approach on liver and thoracic aorta from WT and Abcc6−/− mice. Because PXE symptoms develop with age, we compared 5-month-old (adult) and 24-month-old (senescent) animals. The mineralizing phenotype, a marker of PXE disease progression, was verified in old animals by measuring calcium accumulation in whiskers (Le Corre et al., 2012) and osteopontin (Spp1) arterial expression (see Supplementary Figure S1 online).
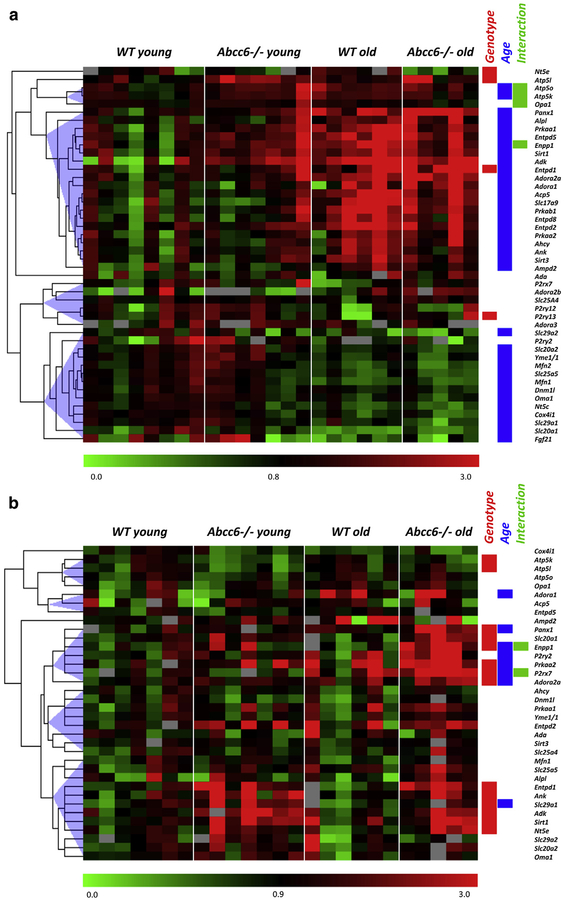
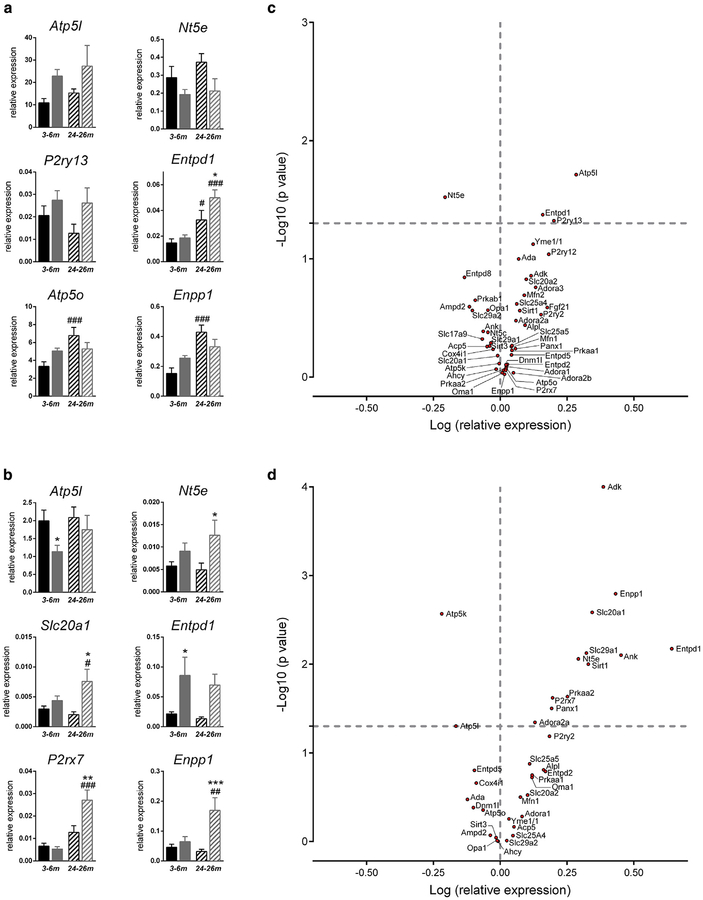
Gene expression levels showed important changes according to aging in the liver (76% of investigated genes, Figure 3a), but less in the aorta (22%, Figure 3b). By contrast, focusing on the impact of the Abcc6−/− genotype, we found a much more profound effect in aorta compared with liver. Bar graphs representative of the different gene expression patterns are shown in Figure 4a and b. Detailed statistical analysis according to the Abcc6 genotype and aging relative to appropriate controls are shown in Supplementary Tables S3 (liver) and S4 (aorta) online. In the liver, a significant age/genotype interaction was found for four genes (Atp5o, Atp5k, Opa1, and Enpp1), showing their increased expression with aging in WT but not in Abcc6−/− mice (Figure 4a). In aorta, two genes displayed interaction (Enpp1 and P2rx7). In this case, their overexpression occurred with aging only in Abcc6−/− mice, suggesting a consequence of the pathogenic process (Figure 4b).
Figure 3. Gene expression pattern in liver and aorta of Abcc6−/− mice.
Heat map of gene expression in the (a) liver and (b) aorta of young (5 months, n = 7) and old (24 months, n = 6, except for Abcc6−/−aorta, for which n = 5) Abcc6+/+ and Abcc6−/− mice. Results represent color-coded expression relative to the control group (WT young). On the right are noted genes that are differently expressed (P < 0.05, two-way analysis of variance) according to genotype (red box) and to age (blue box) and the significance of the interaction between the two factors (green box). WT, wild type.
Figure 4. Gene expression pattern in liver and aorta of Abcc6−/− mice.
Bar graphs illustrating representative gene expression pattern in young andold WT and Abcc6−/− mice in the (a) liver and (b) aorta. Genes represented were selected according to (i) the significant effect of Abcc6 genotype and(ii) the significance of aging/genotype interaction. Black bars indicate WT mice, and gray bars indicate Abcc6−/− mice; solid fill indicatesadult mice, and hatched fill indicates old mice. Significant differences according to age (#) or genotype (*) (P < 0.05). Volcano plots showing the distribution of relative gene expression (log of fold changes) and P-values (log10) in (c) Abcc6−/− mice liver (n = 13) and (d) aorta (n = 13 WT and n = 12 Abcc6−/−). Data are the mean of five to seven values obtained from different animals. Values of log P above 1.3 (P < 0.05) are statistically significant. #P < 0.05; ###P < 0.001; *P < 0.05; **P < 0.01; ***P < 0.001. WT, wild type.
Volcano plots illustrate the global impact of Abcc6 deficiency on gene expressions independently of aging. It evidences both the importance (log expression relative to WT) and significance (−log10 P-value, two way analysis of variance) of the results (Figure 4c and d). Among 46 genes investigated in the liver, three (Atp5l, Entpd1, and P2ry13) were significantly overexpressed, whereas Nt5e was down-regulated in Abcc6−/− mice. Strikingly, in the thoracic aorta, 12 genes were overexpressed (Adk, Enpp1, Slc20a1, Entpd1, Slc29a1, Ank, Nt5e, Sirt1, Prkaa2, P2rx7, Panx1, and Adora2a) and two were down-regulated (Atp5k and Atp5l) in Abcc6−/− mice out of 36 genes investigated.
DISCUSSION
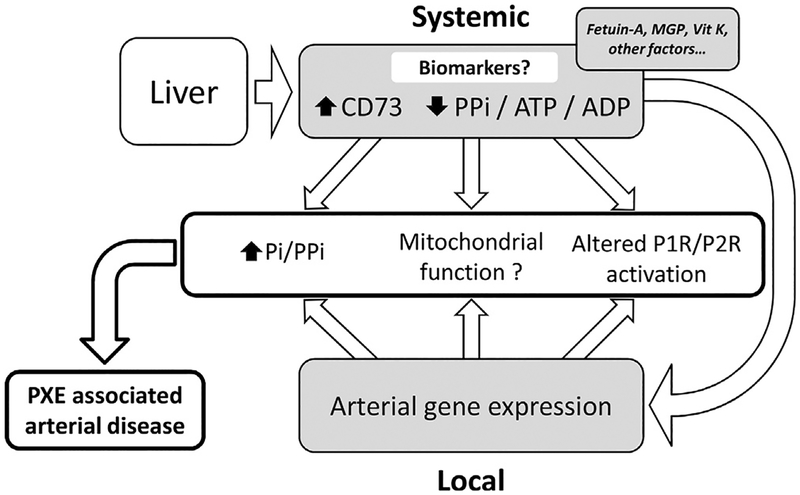
Calcifications mostly caused by a lack of PPi (Zhao et al., 2017) represent the tip of the iceberg of extensive pathological processes affecting vascular homeostasis in PXE. We show in this study that PXE broadly affects intravascular nucleotide metabolism, thus representing a molecular pathway for nonmineralizing manifestations of PXE such as ischemia, thrombosis, carotid malformation, or systemic oxidative stress (Figure 5).
Figure 5. Proposal of a summary diagram for the pathophysiology of PXE.
In the context of liver (central) ABCC6 deficiency, plasma levels of PPi and adenine nucleotides (ATP, ADP) are decreased and 5′-nucleotidase activity is increased each of these, representing potential disease biomarkers. ABCC6 deficiency is also associated with modifications of purine and phosphate gene expression in (remote) affected tissues like arteries. Both systemic and local alterations in purine and phosphate metabolism contribute to PXE-associated peripheral arterial disease. ADP, adenosine diphosphate; ATP, adenosine triphosphate; MGP, matrix gla protein; Pi, inorganic phosphate; PPi, pyrophosphate; PXE, pseudoxanthoma elasticum; Vit, vitamin.
Extracellular nucleotides and ADO (a nucleoside) act as autocrine/paracrine messengers through P2 and P1 membrane receptor activation, respectively (Eltzschig et al., 2013). We show here that PXE patients display a highly significant decrease in ATP and ADP concentrations, and we confirm a major drop in the plasmatic PPi concentration. Important questions emerge from these observations. Although there are multiple sources of extracellular nucleotides (Praetorius and Leipziger, 2009), our results suggest that systemic concentrations of purines and PPi largely depend on liver production and/or release through an ABCC6-dependent mechanism. Furthermore, reduced intravascular concentrations of ATP and ADP may alter inflammatory, thrombotic, and other cardiovascular manifestations linked to P2 purinergic receptor activation (Eltzschig et al., 2013). Hence, altered P2 receptor activation likely takes part in the pathophysiology of PXE and could underlie unexplained manifestations previously observed in Abcc6−/− mice, such as cardiomyocyte hypertrophy (Prunier et al., 2013), increased tone of resistant arteries (Kauffenstein et al., 2014), increased inflammatory infiltrate after ischemia reperfusion (Mungrue et al., 2011), and fibrosis (Rau et al., 2015). However, these changes appear to have no major influence on the life expectancy of Abcc6−/− animals (see Supplementary Figure S2 online). Finally, the data we present also suggest a potential link between ABCC6 function, extracellular purines, and mitochondrial ATP production. Pasquali-Ronquetti et al. (2006) previously showed that the mitochondrial membrane potential was higher in the fibroblasts of PXE patients, likely causing oxidative stress with no impact on intracellular adenine nucleotides. More recently, based on altered mitochondrial respiration in Abcc6−/− mouse tissues, it was proposed that the ABCC6 transporter was localized in mitochondria-associated membranes (Martin et al., 2012). The localization of ABCC6 in mitochondria-associated membranes was later disputed (Pomozi et al., 2013). However, the exploration of a possible link between ABCC6 and mitochondrial function and/or adenylate energy charge undoubtedly deserves further attention.
ADO, through activation of specific P1 receptors, exerts anti-inflammatory, anti-thrombotic, and vasculoprotective action. A link between the ABCC6 transporter and ADO has indeed been evoked, because a 5′nucleotidase (ADO-generating enzyme) gene mutation in ACDC causes ectopic calcifications similar to those of PXE (St Hilaire et al., 2011). In this condition, inhibition of the P1 receptor is responsible for AP overexpression and calcification. Because of the similarity between PXE and ACDC manifestations, it has been proposed that ABCC6 could transport ADO (Markello et al., 2011), which would consequently be reduced in PXE patients. However, in vitro studies did not confirm ADO transport through ABCC6 (Szabo et al., 2011). Because most extracellular ADO originates from adenine nucleotide phosphohydrolysis, one would expect its reduction in Abcc6−/− plasma, together with ATP and ADP concentrations. However, we found no significant reduction in ADO or its precursor AMP in Abcc6−/− mice plasma. These data corroborate a recent metabolomics study that reported a similar amount of AMP and xanthine, an ADO degradation product, in Abcc6−/− mice compared with WT animals (Rasmussen et al., 2016). The levels of uric acid, the end product of the purinergic catabolic chain, were no different in PXE patients than in healthy control individuals (data not shown), suggesting again that the adenylic nucleotide (ATP/ADP) deficit does not affect downstream hydrolysis products(i.e., AMP, ADO, xanthine, uric acid). These data notably suggest that PXE pathogenesis does not involve an ADO deficit.
Along with membrane-bound nucleotidases and other ectoenzymes, soluble purine-converting activities are constitutively present in the bloodstream, thereby representing an important auxiliary effector system for local regulation of purine levels in the vasculature (Yegutkin, 2008, 2014). Although the physiological relevance of coupling these activities to intravascular nucleotide homeostasis remains questionable, the activities of several key soluble nucleotidases may represent valuable biomarkers of disease, such as liver dysfunction, chronic hypoxia, atherosclerosis, and other cardiovascular diseases. Serum ATPDase activity depends on ecto-NTPDase1/CD39, whereas AMPase activity is carried out by ecto-NT5E/CD73. Together, these enzymes sequentially hydrolyze ATP to ADO. Whereas CD39 ADPase activity was unchanged, 5′-nucleotidase was significantly increased in PXE patients and Abcc6−/− mice. Other soluble enzymes (AK and NPP) were down-regulated in these animals compared with WT littermates. The Abcc6−/− mouse recapitulates the phenotype of PXE patients (calcifications, ocular involvement, biology, etc.) and is considered as a good model. However, the latter changes, although showing modifications in extracellular purine metabolism, were not observed in humans and likely reflect the existence of species-specific differences.
An increase in CD73 AMPase activity could be responsible for enhanced AMP hydrolysis and normalized ADO plasma concentration despite reduced ATP and ADP levels in Abcc6−/− mice. Circulating 5′-nucleotidase activity is used as a biomarker for hepatic diseases (cholestasis), being complementary to but more specific than AP, which displays a broader expression in bone, placenta, and intestine. Of interest, soluble CD73 activity was recently shown to be positively correlated to hypoxemic conditions (Liu et al., 2016) and atherosclerosis progression in patients with peripheral arterial disease (Jalkanen et al., 2015). Hence, 5′-nucleotidase could be a useful biomarker in PXE. Although the possibility cannot be fully excluded, these changes are unlikely to be associated with liver dysfunction, which is absent in PXE patients. The potential of CD73 activity as a biomarker, in particular the correlation with vascular manifestations of PXE, warrants further testing.
Several in vitro studies using skin fibroblasts with inactivating ABCC6 mutations (Boraldi et al., 2014b; Dabisch-Ruthe et al., 2014; Ziegler et al., 2017), or in cultured HepG2 cell lines with ABCC6 knockdown (Miglionico et al., 2014), showed that ABCC6 deficiency has an impact on the expression and activity of proteins that contribute to extra-cellular nucleotide hydrolysis and Pi and PPi homeostasis. These data indicated overall a ratio between inducers and inhibitors of PPi metabolism that is shifted toward the procalcifying state (up-regulation of TNAP, down-regulation of NPP1 and NT5E) and, of note, suggested a transcriptomic impact of ABCC6.
To evaluate in vivo the local and distal impacts of Abcc6 deficiency on purine and phosphate homeostasis-related gene expression, we used a transcriptomic approach that compared liver, an unaffected tissue expressing Abcc6, versus arteries, which are affected by the pathology but do not express Abcc6. We report that the expressions of 4 of the 46 investigated genes was modified in Abcc6−/− liver, whereas 14 of 36 genes were modified in arteries. These results point to a remote transcriptional impact of ABCC6 deficiency, in agreement with the metabolic hypothesis of PXE (Li et al., 2009). In Abcc6−/− liver, the expressions of the mitochondrial complex Atp5l, the vascular ATPDase Entpd1 (CD39), and the P2ry13 ADP receptor were increased, and Nt5e was reduced, corroborating the effect of ABCC6 silencing in HepG2 cells (Miglionico et al., 2014). To some extent, these limited changes are consistent with the absence of reported liver disease in PXE. However, altered lipid metabolism with a plausible liver origin has been reported in PXE patients and would be responsible for their increased tendency to develop atherosclerosis (Gorgels et al., 2005; Kuzaj et al., 2014; Pomozi et al., unpublished data; Wang et al., 2001. In this context, changes of P2ry13 expression were significant. Because this receptor contributes to reverse cholesterol transport and biliary lipid secretions (Fabre et al., 2010), we hypothesize that alteration in its expression or function (because of insufficient ligand ADP) may contribute to reducing high-density lipoprotein and other lipid metabolism alterations in the PXE condition. Moreover, four genes (Atp5o, Atp5k, Opa1, and Enpp1) showed significant increased expression with aging in WT but not in Abcc6−/− mice. This may suggest that Abcc6 loss of function abrogates the spontaneous induction in these genes, the pathophysiological impact remaining to be determined.
In the aorta of Abcc6−/− mice, 12 of 36 genes were significantly overexpressed, and 2 of 36 were down-regulated compared with those in WT animals. With our quantitative real-time PCR approach, we could not detect significant Abcc6 expression in mouse aorta (see Supplementary Figure S3 online), in contrast to previous data (Beck et al., 2003; Bergen et al., 2000) and supporting the remote reprogramming of arterial gene expression rather than a local effect. Several inhibitors of arterial calcifications were over-expressed in Abcc6−/− aorta, including the intracellular-toextracellular PPi transporter Ank and the PPi-generating enzyme NPP1, which is consistent with the findings of studies conducted on PXE fibroblasts (Boraldi et al., 2014a) but contradicts the results of another study (Dabisch-Ruthe et al., 2014). We measured the overexpression of genes involved in nucleotide phosphohydrolysis (Entpd1, Enpp1) and in ADO local homeostasis. These genes encompassed molecules implicated in the generation (Entpd1 and Nt5e), uptake (Slc29a1/Ent1), phosphorylation (Adk), and cellular effects (Adora2A) of ADO. These changes may reflect an adaptive process that preserves the local concentrations of PPi and ADO and facilitates purine salvage. Conversely, the procalcifying gene encoding the Pi transporter, Slc20a1/Pit1, was also overexpressed in Abcc6−/− arteries, likely modifying the pro-versus anti-calcifying ratio. We and other researchers previously reported that osteochondrogenic marker expression is increased in Abcc6−/− arteries with aging (Kauffenstein et al., 2014) or after dystrophic cardiac calcifications (Sowa et al., 2013). In this respect, the contribution of P1 and P2 receptors in these phenotypic changes is likely. Although it is difficult to say to what extent these changes contribute to PXE manifestations, these results reflect the existence of transcriptional feedback linking circulating levels of nucleotides/ADO and the vascular expression of the enzymatic machinery involved in their regulation. Expression patterns of two genes, P2rx7 and Enpp1, displayed a marked overexpression associated with aging evidenced only in Abcc6−/− mice. Again, this may suggest that these changes are consequences of the pathological process rather than being a cause of it. Here, we show in vivo that Enpp1 expression is tightly linked to Abcc6 (in liver and arteries) as previously proposed in vitro (Boraldi et al., 2014a; Dabisch-Ruthe et al., 2014; Ziegler et al., 2017).
Finally, two genes involved in mitochondrial biogenesis (Sirt1) and energy sensing (Prkaa2/protein kinase AMP-activated) were up-regulated, whereas the ATP synthase subunits Atp5l and Atp5k were down-regulated. These results might indirectly suggest a link to mitochondrial function with potential alteration of energy production.
Overall, these molecular data suggest an alteration of specific gene expressions in Abcc6−/− mice, which nevertheless should be confirmed in human PXE.
In conclusion, we show here that ABCC6 loss of function has a profound impact on vascular purine metabolism through modified arterial gene expression, soluble nucleotidase activities, and, finally, circulating nucleotide levels. These results may bring some reconciliation between metabolic versus local influence in PXE etiology, because they associate modifications of both circulating factors and altered gene expression specifically in the affected tissue. Purinergic signaling alterations most likely contribute to thrombotic and low-grade inflammation associated with PXE, and the real impact of this disease on mitochondrial function and energy metabolism remains to be investigated. Moreover, the diagnostic and prognostic potential of 5′-nucleotidase in PXE warrants specific investigations. A deeper characterization of purinergic signaling alterations will open new perspectives in our understanding of the pathogenesis of PXE and might help identify new therapeutic targets.
MATERIALS AND METHODS
Detailed Materials and Methods are available in the Supplementary Materials online.
Patients
Human participants gave written informed consent that was approved by the institutional ethics committee. Phenodex score (Legrand et al., 2017) and carried mutations are presented in Supplementary Table S1.
Animals
Animals were manipulated in accordance with the European Community standards on the care and use of laboratory animals. Abcc6−/− mice were generated as described (Gorgels et al., 2005).
Quantification of ATP in plasma
Human blood was collected on EDTA anticoagulated tubes. ATP and ADP concentrations were determined by enzyme-coupled assay (Yegutkin et al., 2012).
Quantification of PPi in plasma
PPi in human plasma was determined enzymatically as previously described (Jansen et al., 2013).
Quantification of AMP and ADO in mouse plasma
Mouse blood was collected using a method adapted from (Saadjian et al., 2002). Plasma were deproteinized, extracted, and incubated with chloroacetaldehyde 1 mol/L H2PO4 buffer (pH 4). The resulting etheno-fluorescent nucleotides were separated with a C18 reverse phase for quantification.
Measurement of soluble purinergic enzyme activities in serum
Soluble enzymes were measured in the serum according to the method previously described (Yegutkin et al., 2012). ENPP1 activity was determined by hydrolysis of the chromogenic substrate thymi-dine 5-monophosphate p-nitrophenyl ester (p-Nph-5-TMP; Sigma-Aldrich, Saint Quentin Fallavier, France). Alkaline phosphatase activity was measured using routine hospital procedures.
Real-time quantitative PCR
Tissue RNA was extracted using the RNeasy micro kit and was used to synthesize cDNA using the QuantiTect Reverse Transcription kit (Qiagen, Hilden, Germany). Quantitative real-time PCR was performed with Sybr Select Master Mix (Applied Biosystems, Foster City, CA) using a Light cycler 480 Real-Time PCR System (Roche, Basel, Switzerland). Primer sequences are shown in Supplementary Tables S2. Heat mapping was performed using the Multi-experiment Viewer (MeV) software (http://mev.tm4.org).
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical analysis was performed using GraphPad PRISM (La Jolla, CA). Differences between groups were assessed using unpaired t-test or two-way analysis of variance followed by Fisher least significant difference multiple comparison when indicated. P-values less than0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Frank Rutsch and Yvonne Nitschke for technical advice on NPP1 activity determination; Jennifer Deschamps for maintenance and care of the animals; and Barbara Every, ELS, of BioMedical Editor, for English language editing. MITOVASC was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), the University of Angers, and the Centre Hospitalier Universitaire (CHU) of Angers. GK was supported by PXE France. Financial support to OL and VP came from National Institutes of Health grants HL108249 and P20GM113134.
Abbreviations:
- ACDC
arterial calcification due to CD73 deficiency
- ADO
adenosine
- ADP
adenosine diphosphate
- AK
adenylate kinase
- AMP
adenosine monophosphate
- AP
alkaline phosphatase
- ATP
adenosine triphosphate
- Pi
inorganic phosphate
- PPi
pyrophosphate
- PXE
pseudoxanthoma elasticum
- WT
wild type
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2018.02.023.
REFERENCES
- Aessopos A, Savvides P, Stamatelos G, Rombos I, Tassiopoulos T, Karagiorga M, et al. Pseudoxanthoma elasticum-like skin lesions and angioid streaks in beta-thalassemia. Am J Hematol 1992;41:159e64. [DOI] [PubMed] [Google Scholar]
- Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem 2003;51:887e902. [DOI] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause Pseudoxanthoma elasticum. Nat Genet 2000;25:228e31. [DOI] [PubMed] [Google Scholar]
- Boraldi F, Annovi G, Bartolomeo A, Quaglino D. Fibroblasts from patients affected by Pseudoxanthoma elasticum exhibit an altered PPi metabolism and are more responsive to pro-calcifying stimuli. J Dermatol Sci 2014a;74: 72e80. [DOI] [PubMed] [Google Scholar]
- Boraldi F, Bartolomeo A, Li Q, Uitto J, Quaglino D. Changes in dermal fibroblasts from Abcc6(−/−) mice are present before and after the onset of ectopic tissue mineralization. J Invest Dermatol 2014b;134:1855e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabisch-Ruthe M, Kuzaj P, Gotting C, Knabbe C, Hendig D. Pyrophosphates as a major inhibitor of matrix calcification in Pseudoxanthoma elasticum. J Dermatol Sci 2014;75:109e20. [DOI] [PubMed] [Google Scholar]
- Dedinszki D, Szeri F, Kozak E, Pomozi V, Tokesi N, Mezei TR, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med 2017;9:1463e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 2013;368:1260. [DOI] [PubMed] [Google Scholar]
- Fabre AC, Malaval C, Addi Ben A, Verdier C, Pons V, Serhan N, et al. P2Y13 receptor is critical for reverse cholesterol transport. Hepatology 2010;52: 1477e83. [DOI] [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of Pseudoxanthoma elasticum. Hum Mol Genet 2005;14:1763e73. [DOI] [PubMed] [Google Scholar]
- Jalkanen J, Yegutkin GG, Hollmen M, Aalto K, Kiviniemi T, Salomaa V, et al. Aberrant circulating levels of purinergic signaling markers are associated with several key aspects of peripheral atherosclerosis and thrombosis. Circ Res 2015;116:1206e15. [DOI] [PubMed] [Google Scholar]
- Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, et al. ATP-binding cassette subfamily C member 6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyro-phosphate in the systemic circulation. Arterioscler Thromb Vasc Biol 2014;39:1985e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, et al. ABCC6 prevents ectopic mineralization seen in Pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci USA 2013;110:20206e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Pizard A, Le Corre Y, Vessieres E, Grimaud L, Toutain B, et al. Disseminated arterial calcification and enhanced myogenic response are associated with abcc6 deficiency in a mouse model of Pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol 2014;34:1045e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzaj P, Kuhn J, Dabisch-Ruthe M, Faust I, Gotting C, Knabbe C, et al. ABCC6-a new player in cellular cholesterol and lipoprotein metabolism? Lipids Health Dis 2014;13:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WL, Liu S, Vaziri ND. Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am J Nephrol 2014;40:51e5. [DOI] [PubMed] [Google Scholar]
- Le Corre Y, Le Saux O, Froeliger F, Libouban H, Kauffenstein G, Willoteaux S, et al. Quantification of the calcification phenotype of Abcc6-deficient mice with microcomputed tomography. Am J Pathol 2012;180:2208e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause Pseudoxanthoma elasticum. Nat Genet 2000;25:223e7. [DOI] [PubMed] [Google Scholar]
- Legrand A, Cornez L, Samkari W, Mazzella JM, Venisse A, Boccio V, et al. Mutation spectrum in the ABCC6 gene and genotype-phenotype correlations in a French cohort with Pseudoxanthoma elasticum. Genet Med 2017;19:909e17. [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Pfendner E, Varadi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol 2009;18:1e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang Y, Wu H, D’Alessandro A, Yegutkin GG, Song A, et al. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 2016;134: 405e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in Pseudoxanthoma elasticum. Mol Genet Metab 2011;103:44e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Douet V, VanWart CM, Heller MB, Le Saux O. A mouse model of beta-thalassemia shows a liver-specific down-regulation of Abcc6 expression. Am J Pathol 2011;178:774e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Lau E, Singh H, Vergnes L, Tarling EJ, Mehrabian M, et al. ABCC6 localizes to the mitochondria-associated membrane. Circ Res 2012;111: 516e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Nakano A, Jiang QJ, Pulkkinen L, Uitto J. Tissue-specific expression of the ABCC6 gene. J Invest Dermatol 2005;125:900e5. [DOI] [PubMed] [Google Scholar]
- Meng H, Vera I, Che N, Wang X, Wang SS, Ingram-Drake L, et al. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc Natl Acad Sci USA 2007;104:4530e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglionico R, Armentano MF, Carmosino M, Salvia AM, Cuviello F, Bisaccia F, et al. Dysregulation of gene expression in ABCC6 knockdown HepG2 cells. Cell Mol Biol Lett 2014;19:517e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungrue IN, Zhao P, Yao Y, Meng H, Rau C, Havel JV, et al. Abcc6 deficiency causes increased infarct size and apoptosis in a mouse cardiac ischemiareperfusion model. Arterioscler Thromb Vasc Biol 2011;31:2806e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol 1988;6:1e159. [DOI] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and Pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 2012;90:25e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I, Garcia-Fernandez MI, Boraldi F, Quaglino D, Gheduzzi D, De Vincenzi Paolinelli C, et al. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: possible role in the pathogenesis of clinical manifestations. J Pathol 2006;208:54e61. [DOI] [PubMed] [Google Scholar]
- Pavlovic AM, Zidverc-Trajkovic J, Milovic MM, Pavlovic DM, Jovanovic Z, Mijajlovic M, et al. Cerebral small vessel disease in Pseudoxanthoma elasticum: three cases. Can J Neuro Sci 2005;32:115e8. [DOI] [PubMed] [Google Scholar]
- Pingel S, Pausewang KS, Passon SG, Blatzheim AK, Gliem M, Charbel Issa P, et al. Increased vascular occlusion in patients with Pseudoxanthoma elasticum. Vasa 2017;46:47e52. [DOI] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, van de Wetering K, Zoll J, Calio B, Pham K, et al. Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-deficient mice. Am J Pathol 2017;187:1258e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Le Saux O, Brampton C, Apana A, Ilias A, Szeri F, et al. ABCC6 is a basolateral plasma membrane protein. Circ Res 2013;112:e148e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. ATP release from non-excitable cells. PurinergicSignal 2009;5:433e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier F, Terrien G, Le Corre Y, Apana AL, Biere L, Kauffenstein G, et al. Pseudoxanthoma elasticum: cardiac findings in patients and Abcc6-deficient mouse model. PLoS One 2013;8(7):e68700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MR, Nielsen KL, Laursen MR, Nielsen CB, Svendsen P, Dimke H, et al. Untargeted metabolomics analysis of ABCC6-deficient mice discloses an altered metabolic liver profile. J Proteome Res 2016;15:4591e600. [DOI] [PubMed] [Google Scholar]
- Rau CD, Wang J, Avetisyan R, Romay MC, Martin L, Ren S, et al. Mapping genetic contributions to cardiac pathology induced by Beta-adrenergic stimulation in mice. Circ Cardiovasc Genet 2015;8:40e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadjian AY, Levy S, Franceschi F, Zouher I, Paganelli F, Guieu RP. Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation 2002;106:569e74. [DOI] [PubMed] [Google Scholar]
- Sowa AK, Kaiser FJ, Eckhold J, Kessler T, Aherrahrou R, Wrobel S, et al. Functional interaction of osteogenic transcription factors Runx2 and Vdr in transcriptional regulation of Opn during soft tissue calcification. Am J Pathol 2013;183:60e8. [DOI] [PubMed] [Google Scholar]
- St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, et al. NT5E mutations and arterial calcifications. N Engl J Med 2011;364:432e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo Z, Varadi A, Li Q, Uitto J. ABCC6 does not transport adenosine—relevance to pathomechanism of Pseudoxanthoma elasticum. Mol Genet Metab 2011;104:421e2. [DOI] [PubMed] [Google Scholar]
- van den Berg JS, Hennekam RC, Cruysberg JR, Steijlen PM, Swart J, Tijmes N, et al. Prevalence of symptomatic intracranial aneurysm and ischaemic stroke in Pseudoxanthoma elasticum. Cerebrovasc Dis 2000;10:315e9. [DOI] [PubMed] [Google Scholar]
- Vasseur M, Carsin-Nicol B, Ebran JM, Willoteaux S, Martin L, Leftheriotis G. Carotid rete mirabile and Pseudoxanthoma elasticum: an accidental association? Eur J Vasc Endovasc Surg 2011;42:292e4. [DOI] [PubMed] [Google Scholar]
- Wang J, Near S, Young K, Connelly PW, Hegele RA. ABCC6 gene polymorphism associated with variation in plasma lipoproteins. J Hum Genet 2001;46:699e705. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide-and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 2008;1783:673e94. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol 2014;49:473e97. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Wieringa B, Robson SC, Jalkanen S. Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities. FASEB J 2012;26: 3875e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kingman J, Sundberg JP, Uitto J, Li Q. Plasma PPi deficiency is the major, but not the exclusive, cause of ectopic mineralization in an Abcc6−/−mouse model of PXE. J Invest Dermatol 2017;137:2336e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SG, Ferreira CR, MacFarlane EG, Riddle RC, Tomlinson RE, Chew EY, et al. Ectopic calcification in Pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci Transl Med 2017;9(393):eaa1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.