Abstract
Background and Objective:
Both chronic and aggressive periodontal disease are associated with vitamin D deficiency. The active form of vitamin D, 1,25(OH)2D3, induces the expression of the antimicrobial peptide LL-37 and innate immune mediators in cultured human gingival epithelial cells (GEC). The aim of this study was to further delineate the mechanism by which vitamin D enhances the innate defense against the development of periodontal disease (PD).
Materials and Methods:
Wild-type C57Bl/6 mice were made deficient in vitamin D by dietary restriction. Cultured primary and immortalized GEC were stimulated with 1,25(OH)2D3, followed by infection with Porphyromonas gingivalis, and viable intracellular bacteria were quantified. Conversion of vitamin D3 to 25(OH)D3 and 1,25(OH)2D3 was quantified by ELISA. Effect of vitamin D on basal IL-1α expression in mice was determined by topical administration to the gingiva of wild-type mice, followed by QRT-PCR.
Results:
Dietary restriction of vitamin D led to alveolar bone loss and increased inflammation in the gingiva in the mouse model. In primary human GEC and established human cell lines, treatment of GEC with 1,25(OH)2D3 inhibited the intracellular growth of P. gingivalis. Cultured GEC expressed two 25-hydroxylases (CYP27A1 and CYP2R1), as well as 1-α hydroxylase, enabling conversion of vitamin D to both 25(OH)D3 and 1,25(OH)2D3. Topical application of both vitamin D3 and 1,25(OH)2D3 to the gingiva of mice led to rapid inhibition of IL-1α expression, a prominent proinflammatory cytokine associated with inflammation, which also exhibited more than a two-fold decrease from basal levels in OKF6/TERT1 cells upon 1,25(OH)2D3 treatment, as determined by RNA-seq.
Conclusion:
Vitamin D deficiency in mice contributes to PD, recapitulating the association seen in humans, and provides a unique model to study the development of PD. Vitamin D increases the activity of GEC against the invasion of periodontal pathogens, and inhibits the inflammatory response, both in vitro and in vivo. GEC can convert inactive vitamin D to the active form in situ, supporting the hypothesis that vitamin D can be applied directly to the gingiva to prevent or treat periodontal disease.
Keywords: periodontal disease, inflammation, vitamin D, antimicrobial peptide
1. Introduction
Periodontal disease is an inflammatory disease of the gums and supporting tissues that, if untreated, can lead to tooth loss, and may also affect systemic health. It is associated with a dysbiosis of the commensal microbiota in the subgingival crevice, leading to an increase in specific pathogenic bacteria, including Porphyromonas gingivalis, and a subsequent inflammatory response. This inflammation then leads to bone resorption, and ultimately tooth loss (reviewed in1).
Vitamin D is best known as a principal factor that maintains calcium homeostasis and is required for bone development and maintenance. However, it is becoming clear that Vitamin D has profound effects on immunity and inflammation as well (reviewed in2). The active form of vitamin D, 1,25(OH)2D3, can induce the expression of antimicrobial peptides and other innate immune mediators in a variety of cell types (reviewed in3). Furthermore, 1,25(OH)2D3 exhibits anti-inflammatory activity through the inhibition of pro-inflammatory cytokine gene expression4. Experimental deficiency in a mouse model leads to an increase in bacterial infection in the bladder5.
Periodontal disease has been associated with vitamin D deficiency in numerous populations6–9, although the mechanism by which this occurs is not known. This may be due to the effect of vitamin D on both the innate immune activity of the gingival epithelium against periodontal pathogens to maintain microbial homeostasis10, as well as the inhibition of pro-inflammatory cytokines. We have shown 1,25(OH)2D3, induces the expression of the antimicrobial peptide LL-37 in cultured gingival epithelial cells (GEC), and that this treatment leads to a reduction in the viability of the periodontal pathogen Aggregatibacter actinomycetemcomitans on the surface of the cells11. It was recently demonstrated that injection of 25-hydroxyvitamin D3, or 25(OH)D3 into mice prevented the bone loss in a diabetic periodontitis model with infection of P. gingivalis, through the inhibition of the JAK/STAT3 pathway12. Deletion of the CYP27B1 gene in mice, which encodes the enzyme responsible for the final activation step of vitamin D, leads to accelerated bone loss and an increase in pro-inflammatory cytokines13. Together, this suggests that physiologically sufficient levels of vitamin D, maintained by oral supplementation, can support overall periodontal health.
Indeed, the vast majority of research into the activity and effects of vitamin D in human health has focused on systemic introduction, usually by oral supplementation, as vitamin D is very safe, even at high doses, and easily absorbed and stored14. This has led to mixed results, most likely because oral supplementation can only increase serum 25(OH)D3 levels (and tissue levels of 1,25(OH)2D3) to a limited degree. Similarly, Gui et al.15 showed that while systemic administration of 1,25(OH)2D3 (by daily intraperitoneal injection over two weeks) initially led to reduced inflammation, there were negative effects in the long term. Therefore, we wished to examine the potential for topical administration of vitamin D to the oral cavity. However, the active, 1,25(OH)2D3 form is very labile, and not very suitable for direct application. This form is produced by two sequential hydroxylations of vitamin D. It has been generally accepted that this occurs initially by one of a number of 25-hydroxylases in the liver (leading to 25(OH)D3), and then by 25-hydroxyvitamin D1α-hydroxylase (1α-(OH)ase) in the kidney16. Recently, however, other cell types, including epithelial, breast, prostate and immune system cells (monocytes, macrophages and dendritic cells) have been shown to produce the vitamin D activating 1α-hydroxylase17,18. Therefore, we hypothesized that gingival epithelial cells can activate inactive vitamin D, and allow the topical application of vitamin D to the oral cavity to induce an innate immune response. Here we examine the potential of a novel local mechanism of vitamin D activation in GEC, demonstrate a mouse model of periodontal disease due to vitamin D insufficiency, and examine the feasibility of delivering inactive vitamin D to the oral cavity to regulate innate immune gene expression to maintain periodontal health.
2. Materials and Methods
2.1. Bacteria:
P. gingivalis ATCC 33277 were maintained as frozen stock cultures and grown anaerobically at 37°C in trypticase soy broth supplemented with 1g of yeast extract per liter, 5mg of hemin per liter, and 1mg of menadione per liter.
2.2. Cell cultures:
The human oral keratinocyte cell line OKF6/TERT1 was grown in keratinocyte serum free medium (KSFM) supplemented with L-glutamine and penicillin-streptomycin-fungizone (Sigma-Aldrich) in the presence of 0.03 M calcium chloride and bovine pituitary extract as described previously11. Primary cultures of normal human gingival epithelial cells were grown as previously described19,20.
2.3. Vitamin D:
Vitamin D3, 25(OH)D3 and 1,25(OH)2D3 (Sigma) were dissolved in 100% ethanol at 10−5M, and kept in the dark at −20°C. Stocks were diluted to 10−8M fresh prior to each use in sterile medium or PBS. Control vehicle was 0.1% ethanol in sterile medium or PBS. We observed no toxic effect of either the vitamin D metabolites at this concentration, nor of the vehicle control (data not shown).
2.4. Intracellular P. gingivalis assays:
P. gingivalis ATCC 33277 was cultured anaerobically at 37°C for 24 h in trypticase soy broth supplemented with yeast extract (1 mg/ml), haemin (5 μg/ml) and menadione (1 μg/ml) and harvested by centrifugation at 600 g and 4°C for 10 min. The bacterial pellet was resuspended in Dulbecco’s Phosphate-buffered saline (Sigma) pH 7.3 and the number of bacteria was determined using a Klett-Summerson photometer.
Primary GECs were obtained after oral surgery from healthy gingival tissue as previously described21,22. Cells were cultured as monolayers in serum-free keratinocyte growth medium (KGM) (Lonza) at 37°C in 5% CO2. When GECs were at ~75–80% confluence, they were treated with 1,25(OH)2D3 or vehicle control (0.1% ethanol) for three hours. The medium was removed, and the 1α,25(OH)2D3-treated cells were co-incubated with P. gingivalis at a multiplicity of infection of 100 (MOI=100) for 24 hours. After the incubation, cells were fixed with 4% paraformaldehyde, permeabilized by 0.1% Triton X-100 and stained for one hour at room temperature with a rabbit polyclonal antibody against P. gingivalis ATCC 33277 (1: 500)23. The stained cells were washed with PBS containing Tween-20 and incubated for one hour at room temperature with Alexa Fluor 568 conjugated secondary goat anti-rabbit polyclonal antibody (1:1000; Invitrogen). For labeling of actin-cytoskeleton, phalloidin-tetramethylrhodamine B isothiocyanate was used at a dilution of 1:2000 (Sigma-Aldrich). The stained cells were mounted with VectaShield mounting medium containing DAPI (Vector Laboratories). Images were acquired using Zeiss AxioImager A1 epifluorescence microscope with QImaging MicroPublisher 3.3 cooled microscope camera and QCapture software. Measurement of relative fluorescence intensity was performed using NIH ImageJ software. Cell boundaries were determined from the actin cytoskeleton staining (phalloidin-TRITC, staining red). Mean fluorescence, cell area and the integrated density for each cell were measured by the software. The Corrected Total Cell Fluorescence (CTCF) was calculated as follows: CTCF = Integrated density − (Area of selected cell × Mean fluorescence of background readings)24. A minimum of 20 cells, originating from at least 3 separate experiments, were evaluated for each experimental condition25. A 40X objective was used to obtain the images.
The effect of vitamin D on immortalized gingival cells was determined by treating cultures of OKF6/TERT1 cells for 24 hours with 10nm 1,25(OH)2D3 or 0.1% ethanol. Cultures were washed 4x with PBS and were co-incubated with P. gingivalis (MOI = 100) for 90 minutes. Cells were then washed 4x again in PBS and treated with metronidazole/gentamicin in media for another 60 minute incubation to kill externally adherent P. gingivalis. Cells were washed 4x more in PBS, scraped on ice with ice-cold PBS, and lysed by freeze-thaw at −80°C. Serial dilutions of lysates were plated to quantify viable colonies of P. gingivalis.
2.5. Quantification of mRNA:
Total nucleic acids were extracted from tissue culture GECs or homogenized gingival tissue with QiaShredder spin columns and the RNAeasy Plus kit (Qiagen) according to the manufacturers guidelines. BioRad’s iScript cDNA library kit (1708891) was used according to the supplied directions. Relative mRNA levels were measured with a SsoAdvanced Universal SYBR Green Supermix (BioRad 1725274) on a BioRad CFX96 Touch Real-Time PCR Detection System thermal cycler (1855195) in 96-well plates (BioRad HASP9601), and quantified according to the 2−ΔΔCq method, relative to β-actin as a housekeeping gene, and control-treated cultures or tissue.
2.6. Mice:
C57Bl/6 mice (Charles River), 8 weeks old, were housed in a barrier facility at the University of Florida. All experimental protocols were approved by the University of Florida Intitutional Animal Care and Use Committee (IACUC) (protocol number 07970). All methods were carried out in accordance with relevant guidelines and regulations. Animals were housed in groups of five. Each study group incorporated 5 mice.
2.7. Vitamin D diets:
After acclimating the mice to their new environment for 2 weeks their diets were replaced with custom-mixed, irradiated diets purchased from Harlan Teklad. To induce vitamin D-deficiency we utilized a diet free of Vitamin D3, containing 0.02% calcium (Harlan Teklad 00562). The control diet (Harlan Teklad 140510) contained standard levels of Vitamin D3 and calcium (1,000 IU and 0.8% respectively). All animals were maintained on their respective diets and Vitamin D levels for 6 weeks. At the end of the 6 weeks, mice were humanely sacrificed by CO2, and gingivae were excised with a scalpel, and placed in RNAlater prior to RNA extraction (Qiagen). Blood was sampled for quantification of serum 25OHD3 (see below). Subsequently, skulls and mandibles were removed for analysis.
2.8. Serum Vitamin D Levels:
Whole blood was collected by cardiac exsanguination, allowed to clot for 30 minutes at room temperature before centrifugation, and the resulting serum was stored at −80°C before being shipped to Heartland Assays, Inc. for the analysis of Vitamin D3, 25(OH)D3, and 1,25(OH)2D3 levels by radioimmunoassay.
2.9. Alveolar bone analysis:
Harvested murine heads were fixed in formalin for 72 hours, washed with PBS, and stored in 70% ethanol for μCT analysis. Maxillae were scanned at 55kVp, 145μA, 16μm voxel resolution using Scanco Medical 40 μCT scanner (Scanco Medical, Brüttisellen, Switzerland). 3-dimensional images were generated and reconstructed for each specimen. These images were rotated with a standard orientation and threshold to discern mineralized and non-mineralized tissue. The region of interest (ROI) was indicated by the contour height of molars (M1–M3) at the cementoenamel junction as the width and the molar cusp tips to root apices as the height as recently reported26. Depth was equal to the buccolingual size of the teeth plus 1.0mm3. Bone volume fraction was calculated as the percentage of bone within the ROI using AnalyzePro software (Seattle, WA). Data are reported in accordance with standardized nomenclature27.
2.10. Histomorphometry:
Following μCT, maxillae were decalcified as previously described28. Specimens were paraffin-embedded and serial sagittal sections were cut through the distal maxillae for alveolar bone analyses. 7μm sections were stained with hematoxylin and eosin (H&E) to assess tissue inflammation. Inflammation was scored in the maxillary periodontal tissues between M1 and M2 using the following scoring system: 0=0–5% inflammatory cell (IC) infiltration, 1=5–25% ICs, 2=25–50% ICs, and 3= >50% ICs as recently reported26. 7μm sections were stained with tartrate resistant acid phosphate (TRAP) and aniline blue (counter stain) to quantify osteoclast cellular endpoints in the interradicular area as recently reported26. TRAP positive area (red-color staining) and eroded bone perimeters were quantified using Visiopharm software (Hoersholm, Denmark). Data are reported in accordance with standardized nomenclature29.
2.11. In vivo administration of vitamin D:
Gingiva of mice were treated by topically applying 50μl 1μM 1,25(OH)2D3 in mineral oil or vehicle control. After 6 hours, mice were sacrificed, and gingivae were excised and placed in RNAlater. Total mRNA was isolated by RNeasy Plus (Qiagen), and relative IL-1α mRNA levels were quantified by qPCR as above.
2.12. RNA-seq:
Triplicate cultures of OKF6/TERT1 cells were treated with 10nM 1,25(OH)2D3 in 0.1% ethanol or vehicle control for 24 hours. Total mRNA was isolated using the RNEasy Plus Mini kit (Qiagen, 74136). cDNA was then prepared using TruSeq Library Prep Kit (Illumina) according to the manufacturer’s protocol, and were sequenced on the NextSeq 500 sequencer (Illumina). Sequences were analyzed via Patterned Alignments for Splicing and Transcriptome Analysis (PASTA) software. Relative expression between sample treatments was calculated for each transcript. Transcripts with greater than a two-fold log2 change that was statistically significant (padj < 0.05 for two-tailed unpaired Student’s t-test) were then further analyzed. The resulting list of over 23,000 genes was trimmed to those which were significantly (adjusted p-value <0.05), and substantially (absolute value log2 fold change >2) changed in expression (3,173 genes). The list was then cross-referenced with the innate immunity gene database (innatedb.com) to identify innate immune genes regulated by vitamin D.
2.13. Quantification of vitamin D metabolites:
OKF6/TERT1 cells (n=3) were treated with either 10nM vitamin D3, or 25(OH)D3 in 0.1% ethanol vehicle, which was used as a negative control. Supernatants were collected and 25OHD3 and 1,25(OH)2D3 were measured by enzyme immunoassay (EIA) (Eagle Biosciences) according to the manufacturer’s instructions. Media with vitamin D3 or 25(OH)D3 alone was also assessed for cross-reactivity, and these values were subtracted from the total levels reported.
2.14. Statistical analysis:
Power calculations (alpha=0.05; power=80%) to determine group size in the mouse experiments were carried out using means and standard deviations from preliminary experiments, to provide a significant difference between the groups. Differences between treated and untreated groups were analyzed by two-tailed, unpaired t-test with commercially available software. Significance was set at p<0.05.
3. Results
3.1. Effect of vitamin D deficiency on gingival inflammation and alveolar bone loss
While epidemiological associations between serum vitamin D levels and periodontal disease in humans have been observed, we wished to recapitulate this in a mouse model for further studies. Wild-type (C57Bl/6) mice were fed either a normal diet, including vitamin D, or a diet absent vitamin D for 6 weeks. Serum samples from the mice at the end of 6 weeks showed that the mice fed a vitamin D-absent diet exhibited very low levels of 25(OH)D3 (Figure 1A). While this diet includes reduced calcium as well, this has been shown to lead to no reduction in serum calcium levels, even up to 8 weeks30. Longer depletion of vitamin D would compromise the general health of the mice, potentially confounding the model31. To determine if this vitamin D-deficiency can lead to symptoms associated with periodontal disease, we quantified the alveolar bone in mice fed the vitamin D-deficient diet compared with the vitamin D-replete diet. The results show a clear reduction in alveolar bone in the vitamin D-deficient group compared with the control as measured by microCT analysis of bone volume (Figure 1B) and an increase in osteoclasts, as measured by TRAP staining (Figure 1C). In addition, there was a significant amount of inflammation in the gingival epithelium of the vitamin D-deficient group (Figure 1D).
Figure 1. Effect of vitamin D deficiency on alveolar bone loss and inflammation.

Mice (n=5 per group) fed a normal diet or vitamin D/calcium-deficient diet were sacrificed at the end of the 6-week diet. A. Serum 25(OH)D3 levels after 6 weeks. Maxillae were de-calcified and processed for micro-Ct and histology. B. MicroCT analysis of bone loss. Below the image is the quantification of bone area/tissue area (arrow indicated region of interest). C. Histological analysis of TRAP positive cells. Quantification of osteoclasts above a selected histological image of TRAP staining. Arrow indicates TRAP positive osteoclasts. Scale bar = 200μm. D. Inflammatory score of gingivae. Data in graphs are presented as mean +/− SEM. **P<0.01, ***P<0.001 using 2-tailed, unpaired t-tests.
3.2. Effect of vitamin D on intracellular P. gingivalis in GEC
We examined the effect of vitamin D treatment of GEC on the presence of intracellular P. gingivalis in cultured GEC32. When primary cultures of GEC are pre-treated with 10nM 1,25(OH)2D3 for 24 hours this leads to a concomitant decrease in intracellular P. gingivalis as observed by fluorescence microscopy (Figure 2A, and quantified in Figure 2B). A similar reduction of observed intracellular P. gingivalis in cultured OKF6/TERT1 cells is seen without pre-treatment, when the 1,25(OH)2D3 is added at the same time as the P. gingivalis (2C). Together, the results indicate that the active form of vitamin D can elicit an innate immune defense against periodontal pathogens in GEC.
Figure 2. Effect of vitamin D treatment on antibacterial activity.
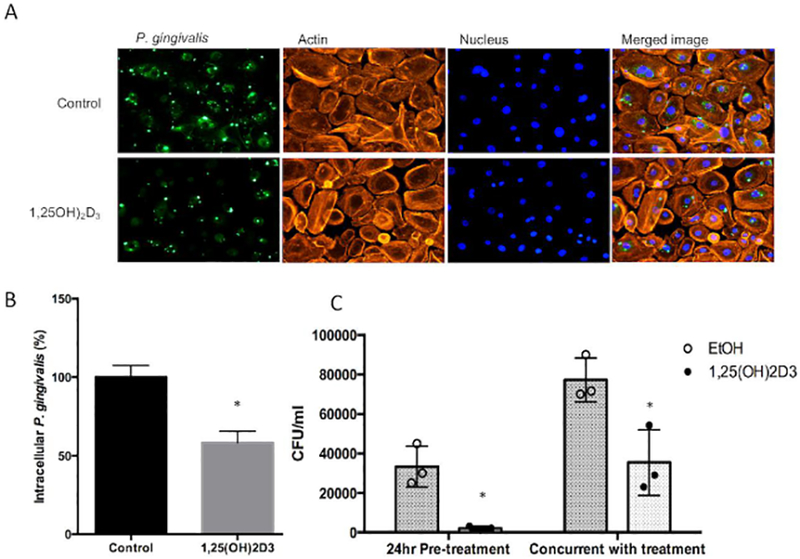
A. Reduction in intracellular P. gingivalis in cultured primary gingival epithelial cells. Primary cultures were pre-treated with 10nM 1,25(OH)2D3 or 0.1% ethanol (vehicle control) for 3 hours, followed by infection for 24 hours. Intracellular bacteria were visualized by detection with a specific anti-P. gingivalis antibody followed by fluorescence microscopy (left panels). B. Fluorescence was quantified by Image J (right panel). c. Reduction in intracellular P. gingivalis in cultured OKF6/TERT1 cells. Cells were treated with 10nM 1,25(OH)2D3, or 0.1% ethanol (vehicle control), either for 24 hours prior to infection, or at the same time as infection (n=3 replicates per condition). After 1 hour, cells were lysed and total bacteria were plated to quantify viable bacteria. Data in graphs are presented as mean +/− SEM. *Differences are significant by two-tailed t-test, p<0.05. Results from both primary and immortalized cultures are from 3 independent experiments, as described in Materials and Methods.
3.3. Conversion of inactive vitamin D to the active form by cultured GEC
To determine whether cultured GEC can respond to the inactive vitamin D form, we treated OKF6/TERT1 cells with 10nM vitamin D, 25OHD3 or 1,25(OH)2D3, for 24 and 48 hours. The results in Figure 3A show an induction of LL-37 mRNA levels in response to all three metabolites, suggesting that in addition to being able to respond to the active form, these cells can convert vitamin D to 25(OH)D3, and from there to 1,25(OH)2D3. To confirm this, and to identify the mechanism, we performed RT-PCR to determine the expression of vitamin D hydroxylases. Visible bands in gel electrophoresis of the RT-PCR products (not shown) demonstrated the expression of two 25-hydroxylases (CYP2R1 and CYP27A1), and the sole 1-α-hydroxylase, CYP27B1. Expression of these genes was not regulated by 1,25(OH)2D3 as measured by QRT-PCR, as shown in Figure 3B. To confirm the activity of these enzymes, we added 10μM vitamin D or 25(OH)D3 to the cultures and quantified the activation products over time. The results in Figure 3C show that 25(OH)D3 is converted to 1,25(OH)2D3 over 24 hours, with a significant increase above background observed by 6 hours. Figure 3D similarly shows the conversion of vitamin D to 25(OH)D3 within 2 hours, although at a much lower level. No significant 25(OH)D3 was observed at longer incubations (data not shown). Thus, inactive vitamin D can be applied to GEC, which is rapidly converted to 25(OH)D3, and from there to 1,25(OH)2D3, which activates a transcriptional response.
Figure 3. Activity and conversion of inactive vitamin D to the active form by gingival epithelial cells.
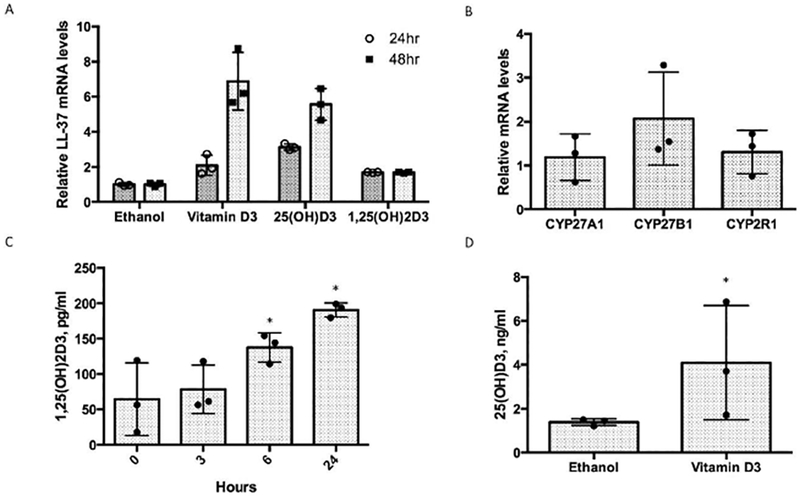
A. Induction of LL-37 gene expression by inactive vitamin D. OKF6/TERT1 cells (n=3) were treated with 0.1% ethanol, 10nM vitamin D3 or 25(OH)D3 in vehicle for 24 or 48 hours. Total mRNA was isolated and LL-37 mRNA levels were quantified by QRT-PCR. B. Expression of vitamin D activating enzymes. OKF6/TERT1 cells were treated as above with either 10nM 1,25(OH)2D3 or ethanol control for 6 hours. Total mRNA was isolated and levels of CYP24A1 (not shown, as its induction was over 200-fold), CYP27A1, CYP27B1 and CYP2R1 were quantified by QRT-PCR. C. Conversion of 25OHD3 to 1,25(OH)2D3. 10μM 25OHD3 was added to triplicate cultures of OKF6/TERT1 cells. Samples of the growth medium were taken at the times indicated, and levels of 1,25(OH)2D3 were quantified by EIA. 10nM 1,25(OH)2D3 in medium was included as a positive control. 10nM 25OHD3 in growth medium was quantified as a negative control for cross-reactivity. D. Conversion of vitamin D3 to 25(OH)D3. 10μM vitamin D3 was added to triplicate cutlures of OKF6/TERT1 cells, and growth medium was sampled at 2 hours. Levels of 1,25(OH)2D3 were quantified by EIA. Data are presented as mean +/− SD. *Differences are significant by two-tailed t-test, p<0.05.
3.4. Effect of topical vitamin D treatment on inflammatory gene expression in vitro and in vivo
To examine the effect of vitamin D treatment on the inflammatory response in vivo, we first identified an appropriate, unstimulated pro-inflammatory cytokine. RNA-seq analysis of OKF6/TERT1 cells treated with 1,25(OH)2D3 yielded 25 transcripts that were associated with innate immunity or inflammation (supplementary table 1). Based on our results, we chose to quantify the expression of the pro-inflammatory cytokine, IL-1α, since it plays an important role in periodontal inflammation and bone loss33. When oral keratinocytes were treated with 10nM 1,25(OH)2D3, we observed an inhibition of basal IL-1α mRNA levels (Figure 4A).
Figure 4. Effect of vitamin D on IL-1α mRNA levels in vitro (A) and in vivo (B).

A. Cultured OKF6/TERT1 cells were treated with either 10nM 1,25(OH)2D3 or 0.1% ethanol (vehicle control) for 24 hours. Total mRNA was isolated and levels of IL-1α mRNA were quantified by QRT-PCR. B. Wild-type C57Bl/6 mice (n=5 per group) were treated with 50μl mineral oil containing either 1μM vitamin D3, 1,25(OH)2D3 or vehicle control. After 6 hours, gingivae were excised, total mRNA was isolated and IL-1α mRNA levels were quantified by QRT-PCR relative to β-actin. Data are shown as mean expression relative to control +/− SEM. Differences are significant, *p<0.0001, by t-test.
We then examined the effect of topical administration of vitamin D in vivo, using mice fed a normal, vitamin D-replete diet. The results in Figure 4B show that topical vitamin D administration of both inactive vitamin D3 and 1,25(OH)2D3 leads to a rapid reduction in levels of IL-1α mRNA in the vitamin D-treated gingivae, compared with vehicle control.
4. Discussion
Vitamin D has long been known to regulate calcium absorption in the body, which can subsequently affect numerous systems, especially bone resorption. As early as 1968, calcium deficiency was observed to lead to alveolar bone loss in a dog model34, and was also observed in a rat model35. Furthermore, supplementation with both calcium and vitamin D have positive effects on periodontal health36. Several studies have shown a correlation between low Vitamin D levels and periodontal disease6–9, however the mechanism is not understood. Our results show that this association between low serum vitamin D levels and periodontal health can be recapitulated in a wild-type mouse model, thus allowing future studies into the specific mechanism that underlies this association. Our results further demonstrate that vitamin D insufficiency leads to increased gingival inflammation in the mouse under specific pathogen-free conditions. As a result of the inflammatory response, alveolar bone loss occurs. It is long-understood that vitamin D maintains bone health, due to its role in the maintenance of calcium homeostasis. Furthermore, in order to maintain a complete deficiency of vitamin D, we used a diet low in calcium37, which has been shown not to affect serum calcium levels30. While mice with experimental periodontitis that were injected with 25(OH)D3 showed decreased bone loss38, as do CYP27B1−/− mice13, our experiments are the first to demonstrate that a dietary deficiency in vitamin D leads to inflammation and alveolar bone loss.
In addition, we have devised a novel mouse model for the development of periodontal disease which does not rely on either mechanical stimulation (i.e., ligatures) or upon the introduction of human pathogens such as P. gingivalis. Furthermore, this model allows for the longitudinal analysis of components of the oral cavity during the development of periodontal disease, as well as the repeated delivery of potential anti-inflammatory drugs, and could thus be useful in pre-clinical testing of new therapies.
In susceptible hosts, colonization by pathobionts such as P. gingivalis into the oral cavity can ultimately lead to inflammation of the gingival tissues, and bone loss that are the hallmarks of periodontal disease39. Based on our earlier results showing that vitamin D stimulated the expression of antimicrobial peptide gene expression and activity in GEC, we hypothesized that physiologically sufficient levels of vitamin D could lead to an increased antimicrobial activity against keystone pathogens, and thus affect overall oral health. Our results showing the reduction in intracellular P. gingivalis in both cultured immortalized GEC and primary GEC support this hypothesis. While early in vitro studies suggested that LL-37, which is induced by vitamin D, exhibits low antimicrobial activity against P. gingivalis and other pathogens associated with periodontal disease40, more recent studies have suggested that conditions found in vivo, both extracellular and intracellular, are more conducive to the antibacterial activity of LL-37 against these species41,42.
While oral supplementation of vitamin D can lead to vitamin D sufficiency in vitamin D-deficient individuals, including those with chronic periodontitis43, the natural feedback mechanism based on the induction of the vitamin D 24-hydroxylase enzyme guarantees that high concentrations of 1,25(OH)2D3 will not occur in the gingival epithelium. Because of this, hypervitaminosis D, and other side effects of high levels of supplementation are rare44. However, to both avoid systemic feedback regulation, and any potential systemic effects of supplementation, we propose that a topical administration of vitamin D could be useful. However, 1,25(OH)2D3 is highly labile, and does not provide a strong foundation for a therapeutic agent. Thus, we would propose to use the inactive vitamin D3 (cholecalciferol) form. In order for this inactive form of vitamin D3 to lead to both antibacterial peptide gene induction and pro-inflammatory cytokine inhibition, it must be converted to the active form in situ. We have previously demonstrated that this two-step activation occurs in airway epithelial cells45, and here we show that the same hydroxylase enzymes are expressed by gingival epithelial cells, allowing the use of cholecalciferol to be applied topically.
Vitamin D inhibits the expression of pro-inflammatory cytokines46, most often demonstrated by the activity of 1,25(OH)2D3 in cultured cells that are stimulated with LPS or IL-1β to induce the expression of these cytokines47,48. To demonstrate the activity of topical, inactive vitamin D in vivo in periodontally healthy mice, we identified a pro-inflammatory cytokine, IL-1α, whose basal expression was inhibited by vitamin D treatment in vitro. IL-1α is an important pro-inflammatory cytokine in the development of bone loss in periodontal disease33, and thus we examined the effect of vitamin D on its expression in vitro and in vivo. Our results demonstrate for the first time that topical administration of vitamin D in vivo can lead to a localized inhibition of the inflammatory response. This not only supports the hypothesis that normal levels of vitamin D maintain an anti-inflammatory state in the oral cavity, but that it may be possible to use topical administration of vitamin D to prevent or treat the inflammation associated with periodontal disease, in addition to enhancing the natural antimicrobial activity of the tissue.
Supplementary Material
Acknowledgements
This study was supported by grants from the US Public Health Service: NIH 1R01DE22723 to GD; R21DE027017 and R01DE028258 (KLK), P30GM103331 (KLK); R01DE016593 (ÖY); and 5T90DE021990–07 (DCB). EP was a recipient of the University of Florida University Scholars award. We thank Dr. Susmita Datta, Department of Biostatistics, College of Public Health and Health Professions, University of Florida, for assistance with the statistical analysis.
Footnotes
Competing financial interests
The authors have no competing financial interests.
References
- 1.Hajishengallis G Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christakos S, Hewison M, Gardner DG, et al. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin R Crosstalk between Vitamin D Metabolism, VDR Signalling, and Innate Immunity. Biomed Res Int. 2016;2016:1375858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hertting O, Luthje P, Sullivan D, Aspenstrom P, Brauner A. Vitamin D-deficient mice have more invasive urinary tract infection. PLoS One. 2017;12(7):e0180810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonoglou GN, Knuuttila M, Niemela O, et al. Low serum level of 1,25(OH)2 D is associated with chronic periodontitis. J Periodontal Res. 2015;50(2):274–280. [DOI] [PubMed] [Google Scholar]
- 7.Antonoglou GN, Suominen AL, Knuuttila M, et al. Associations between serum 25-hydroxyvitamin d and periodontal pocketing and gingival bleeding: results of a study in a non-smoking population in Finland. J Periodontol. 2015;86(6):755–765. [DOI] [PubMed] [Google Scholar]
- 8.Laky M, Bertl K, Haririan H, et al. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin Oral Investig. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Je DI, Won SJ, Paik DI, Bae KH. Association between vitamin D deficiency and periodontal status in current smokers. Community Dent Oral Epidemiol. 2015;43(5):471–478. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JS, Atanasova KR, Lee J, et al. Opportunistic Pathogen Porphyromonas gingivalis Modulates Danger Signal ATP-Mediated Antibacterial NOX2 Pathways in Primary Epithelial Cells. Front Cell Infect Microbiol. 2017;7:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun. 2011;79(6):2250–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Li H, Xie H, et al. 25-Hydroxyvitamin D3 attenuates experimental periodontitis through downregulation of TLR4 and JAK1/STAT3 signaling in diabetic mice. J Steroid Biochem Mol Biol. 2013;135:43–50. [DOI] [PubMed] [Google Scholar]
- 13.Gong A, Chen J, Wu J, et al. 1,25-Dihydroxyvitamin D deficiency accelerates alveolar bone loss independent of aging and extracellular calcium and phosphorus. J Periodontol. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Kearns MD, Alvarez JA, Seidel N, Tangpricha V. Impact of vitamin D on infectious disease. Am J Med Sci. 2015;349(3):245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gui B, Chen Q, Hu C, Zhu C, He G. Effects of calcitriol (1, 25-dihydroxy-vitamin D3) on the inflammatory response induced by H9N2 influenza virus infection in human lung A549 epithelial cells and in mice. Virol J. 2017;14(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikle DD, Adams JS, Christakos S. Vitamin D: production, metabolism, mechanism of action and clinical requirements In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Wiley Blackwell; 2013:235–248. [Google Scholar]
- 17.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab.21(6):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26(1):21–28. [DOI] [PubMed] [Google Scholar]
- 19.Laube D, Dongari-Bagtzoglou A, Kashleva H, Eskdale J, Gallagher G, Diamond G. Differential regulation of innate immune response genes in gingival epithelial cells stimulated with Aggregatibacter actinomycetemcomitans J Periodontal Res. 2008;43:116–123. [DOI] [PubMed] [Google Scholar]
- 20.Yao L, Jermanus C, Barbetta B, et al. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25(2):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park Y, Yilmaz O, Jung IY, Lamont RJ. Identification of Porphyromonas gingivalis genes specifically expressed in human gingival epithelial cells by using differential display reverse transcription-PCR. Infect Immun. 2004;72(7):3752–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö. Human Primary Epithelial Cells Acquire an Epithelial-Mesenchymal-Transition Phenotype during Long-Term Infection by the Oral Opportunistic Pathogen,. Front Cell Infect Microbiol. 2017;7:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Roberts JS, Atanasova KR, Chowdhury N, Yilmaz Ö. A novel kinase function of a nucleoside-diphosphate-kinase homologue in Porphyromonas gingivalis is critical in subversion of host cell apoptosis by targeting heat-shock protein 27. Cell Microbiol. 2018;20(5):e12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atanasova K, Lee J, Roberts J, Lee K, Ojcius DM, Yilmaz Ö. Nucleoside-Diphosphate-Kinase of P. gingivalis is Secreted from Epithelial Cells In the Absence of a Leader Sequence Through a Pannexin-1 Interactome. Sci Rep. 2016;6:37643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K, Roberts JS, Choi CH, Atanasova KR, Yilmaz Ö. Porphyromonas gingivalis traffics into endoplasmic reticulum-rich-autophagosomes for successful survival in human gingival epithelial cells. Virulence. 2018;9(1):845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinkamp HM, Hathaway-Schrader JD, Chavez MB, et al. Tristetraprolin Is Required for Alveolar Bone Homeostasis. J Dent Res. 2018:22034518756889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- 28.Novince CM, Michalski MN, Koh AJ, et al. Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J Bone Miner Res. 2012;27(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quach HP, Noh K, Hoi SY, et al. Alterations in gene expression in vitamin D-deficiency: Down-regulation of liver Cyp7a1 and renal Oat3 in mice. Biopharm Drug Dispos. 2018;39(2):99–115. [DOI] [PubMed] [Google Scholar]
- 31.Belenchia AM, Johnson SA, Kieschnick AC, Rosenfeld CS, Peterson CA. Time Course of Vitamin D Depletion and Repletion in Reproductive-age Female C57BL/6 Mice. Comp Med. 2017;67(6):483–490. [PMC free article] [PubMed] [Google Scholar]
- 32.Lamont RJ, Yilmaz O. In or out: the invasiveness of oral bacteria. Periodontol 2000. 2002;30:61–69. [DOI] [PubMed] [Google Scholar]
- 33.Nishida E, Hara Y, Kaneko T, Ikeda Y, Ukai T, Kato I. Bone resorption and local interleukin-1alpha and interleukin-1beta synthesis induced by Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2001;36(1):1–8. [DOI] [PubMed] [Google Scholar]
- 34.Henrikson PA. Periodontal disease and calcium deficiency. An experimental study in the dog. Acta Odontol Scand. 1968;26:Suppl 50:51–132. [PubMed] [Google Scholar]
- 35.Sones AD, Wolinsky LE, Kratochvil FJ. Osteoporosis and mandibular bone resorption in the Sprague Dawley rat. Calcif Tissue Int. 1986;39(4):267–270. [DOI] [PubMed] [Google Scholar]
- 36.Garcia MN, Hildebolt CF, Miley DD, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol. 2011;82(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutuzova GD, Sundersingh F, Vaughan J, et al. TRPV6 is not required for 1alpha, 25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci U S A. 2008;105(50):19655–19659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang P, Zhang W, Zhang D, et al. 25-Hydroxyvitamin D3 -enhanced PTPN2 positively regulates periodontal inflammation through the JAK/STAT pathway in human oral keratinocytes and a mouse model of type 2 diabetes mellitus. J Periodontal Res. 2018. [DOI] [PubMed] [Google Scholar]
- 39.Hajishengallis G Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007;42(5):410–419. [DOI] [PubMed] [Google Scholar]
- 41.Gutner M, Chaushu S, Balter D, Bachrach G. Saliva enables the antimicrobial activity of LL-37 in the presence of proteases of Porphyromonas gingivalis. Infect Immun. 2009;77(12):5558–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sol A, Skvirsky Y, Nashef R, et al. Actin enables the antimicrobial action of LL-37 peptide in the presence of microbial proteases. J Biol Chem. 2014;289(33):22926–22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khammissa RAG, Ballyram R, Jadwat Y, Fourie J, Lemmer J, Feller L. Vitamin D Deficiency as It Relates to Oral Immunity and Chronic Periodontitis. Int J Dent. 2018;2018:7315797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D Toxicity-A Clinical Perspective. Front Endocrinol (Lausanne). 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiFranco KM, Mulligan JK, Sumal AS, Diamond G. Induction of CFTR gene expression by 1,25(OH)2 vitamin D3, 25OH vitamin D3, and vitamin D3 in cultured human airway epithelial cells and in mouse airways. J Steroid Biochem Mol Biol. 2017;173:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun J Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010;26(6):591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakashyan V, Tipton DA, Karydis A, Livada R, Stein SH. Effect of 1,25(OH)2 D3 and 20(OH)D3 on interleukin-1beta-stimulated interleukin-6 and −8 production by human gingival fibroblasts. J Periodontal Res. 2017. [DOI] [PubMed] [Google Scholar]
- 48.Andrukhov O, Andrukhova O, Hulan U, Tang Y, Bantleon HP, Rausch-Fan X. Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells. PLoS One. 2014;9(2):e90301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


