Abstract
Allergic eosinophilic esophagitis (EoE) is a chronic, allergen-mediated inflammatory disease of the esophagus, and the most common cause of prolonged dysphagia in children and young adults in the developed world. While initially undistinguished from gastroesophageal reflux disease-associated esophageal eosinophilia, EoE is now recognized as a clinically distinct entity that shares fundamental inflammatory features of other allergic conditions, and is similarly increasing in incidence and prevalence. The clinical and epidemiologic associations between EoE and other allergic manifestations are well established. In addition to exaggerated rates of atopic dermatitis, IgE-mediated food allergy, asthma, and allergic rhinitis in EoE patients, each of these allergic manifestations imparts individual and cumulative risk for subsequent EoE diagnosis. As such, EoE may be a member of the “allergic march”—the natural history of allergic manifestations during childhood. Several determinants likely contribute to the relationship between these conditions, including shared genetic, environmental, and immunologic factors. Herein, we present a comprehensive review of allergic comorbidity in EoE. We discuss areas of the genome associated with both EoE and other allergic diseases, including the well-studied variants encoding thymic stromal lymphopoietin and calpain 14, among other “atopic” regions. We summarize ways that environment factors (such as microbiome-altering pressures and aeroallergen exposure) may predispose to multiple allergic conditions including EoE. Finally, we discuss some fundamental features of type 2 inflammation, and the resulting implications for the development of multiple allergic manifestations. We conclude with an analysis of the “type 2” biologics, and how mechanistic similarities between EoE and the other allergic manifestations have important implications for screening and treatment of the allergic patient.
Keywords: Eosinophilic esophagitis, Atopic dermatitis, Food allergy, Allergic rhinitis, Asthma
Introduction
Allergic eosinophilic esophagitis (EoE) is a chronic inflammatory disease of the esophagus that is thought to be caused by an allergen-specific immune response, and results in progressive esophageal dysfunction[1,2]. EoE is characterized clinically by abdominal pain, reflux, failure to thrive, odynophagia, and food impaction, and is associated histopathologically with progressive esophageal remodeling, stricture formation, and esophageal narrowing. In the 1970s-80s, early case descriptions began to distinguish the discrete characteristics of EoE from gastroesophageal reflux disease-associated esophageal eosinophilia; thought to be the predominant cause of esophagitis at the time [3–5]. In 1993, Attwood and colleagues described EoE as a clinically distinct syndrome [6], and soon thereafter EoE was found to share inflammatory features with other allergic conditions, including descriptions in early case reports [3,4,7]. EoE is now recognized as the most common cause of chronic dysphagia in children and young adults across the developed world[8–10]. Since its first description, rates of EoE diagnosis have steadily increased with current incidence estimates ranging from 5 to 10 cases per 100,000 with a prevalence of 0.5-1 cases per 1000 individuals[10,11].
Though a specific allergen is not able to be identified in every patient, it is generally thought that allergen exposure is a central feature of EoE pathogenesis. Evidence for this comes from early studies that revealed excellent response rates of esophageal inflammation to elemental diets[12,13], as well newer evidence from animal models[14]. Cohort studies from major referral centers have revealed that the most common foods associated with EoE are milk, egg, soy, and wheat—notably overlapping with foods most commonly implicated in IgE-mediated food allergy (IgE-FA). Like IgE-FA, identification and avoidance of the causative food is the primary treatment goal in EoE, when possible [15,16]. In cases where a trigger remains elusive, topical corticosteroids in various formations are highly effective therapies[17,18]. Importantly, and similar to the effects of chronic allergic inflammation seen in asthma, failure to diagnose and treat EoE can result in permanent tissue remodeling of the esophagus leading to stricture formation, odynophagia, and food impaction[19,20].
Epidemiologic associations between EoE and the other allergic manifestations have now been well established, with atopic dermatitis (AD)[1,21,22], IgE-FA, asthma[1,21–23], allergic rhinitis (AR)[1,21,22,24], and pollen food allergy syndrome[25,26] all shown to be common comorbidities in EoE patients. Conversely, patients with AD, IgE-FA, asthma, or AR are at increased risk of subsequently being diagnosed with EoE [27]. The relationship between IgE-FA and EoE seems to be particularly strong, with IgE-FA patients developing EoE almost nine-times faster than healthy peers[28]. Further, the risk relationship between EoE and AR seems to be bidirectional, with each condition imparting risk for the subsequent diagnosis of the other[27]. This final observation is consistent with data suggesting that EoE symptoms can be triggered by specific environmental allergens[7,29,30]. As a result of these various associations, EoE has been proposed as a fifth member of the allergic march[27,31]. Despite this, the recognition that EoE is associated with non-atopic diseases, such as inflammatory bowel disease and connective tissue disorders [32], speaks to the complexity of this disease and the possibility that multiple EoE endotypes exist[33].
Given the considerable allergic burden in EoE, and the relevance of shared allergic features to emerging therapeutics, we discuss the primary literature focusing on allergic comorbidity and type 2 inflammation in EoE. We focus on the shared genetic, environmental, and immunologic features that likely underlie the associations among these various allergic manifestations. We conclude with a discussion of the “type 2” biologics, and how mechanistic similarities between EoE and the other allergic manifestations have important implications for screening and treatment of the allergic patient.
Epidemiologic associations between EoE and the other allergic manifestations
In parallel with the rise in allergic diseases over the past several decades, the prevalence of EoE so too has increased both in pediatric and adult populations[34,35]. In a large pediatric cohort study at Children’s Hospital of Philadelphia, a 70-fold increase in the rate of EoE was demonstrated from 1994-2011 [36]. Similarly, Straumann and Simon found an increase in EoE prevalence from 2/100,000 to 23/100,000 between 1989 and 2004 in an adult population in Switzerland[37]. While these findings may represent a true increase in the incidence of EoE over time, improved recognition and diagnostic capabilities likely also contribute to the increased diagnostic rates.
Additionally, a number of studies have associated EoE clinically with other atopic conditions, noting higher rates of comorbid asthma, AR, AD, asthma and IgE-FA in individuals with EoE compared to the general population[21,38,39]. In review of these early reports, approximately 25-50% of individuals with EoE have concurrent asthma, 30-90% have AR, and 10-25% have AD[24,40–43], with variation in these rates likely influenced by individual study populations and definition of conditions. Further, approximately 10-25% of EoE patients have concurrent or prior history of IgE-FA, compared to a rate of 8% in the general pediatric population[40–42,44]. Interestingly, patients with established IgE-FA have been found to have higher rates of subsequent EoE development compared to the general population, often secondary to the same culprit food responsible for the IgE-mediated allergy [28]. More recent studies in both children and adults within the past 5 years continue to support the association between EoE and atopy (Table 1).
Table 1.
Summary of studies showing the frequency (and odds) of allergic diseases in patients with eosinophilic esophagitis between 2015-2019.
| Author/Year[Ref] | No. EoE Patients |
Population | AR | Asthma | AD | IgE-FA |
|---|---|---|---|---|---|---|
| Dellon et al. 2015 [45] | 81 | Adults | 62% | 27% | 6% | 43% |
| Leung et al. 2015[46] | 23 | Children | 57% | 57% | 30% | 30% |
| Peterson et al. 2015[47] | 4,423 | Both | - | OR: 4.0 | OR: 3.0 | - |
| Duffeyet et al. 2016 [48] | 4,009 | Both | - | OR: 3.95 | - | - |
| Gonzalez-Cervera et al. 2017[21] | 53,542a | Both | OR: 5.09 | OR: 3.01 | OR: 2.85 | - |
| Hill et al. 2017[28] | 1,795 | Children | - | - | - | 68% |
| Capucilli et al. 2018[49] | 428 | Children | 60% OR 7.1 | 60% OR: 5.2 | 18% OR: 3.1 | - |
| Chehade et al. 2018[50] | 705 | Both | 60% | 45% | 46% | 67%, 27%b |
| Leigh et al. 2019 [51] | 950 | Adults | 70% | 36% | 14% | 24% |
Data from meta-analysis report
Rate of FA-anaphylaxis, specified
Abbreviations: EoE, eosinophilic esophagitis; AR, allergic rhinitis; AD, atopic dermatitis; IgE-FA, IgE-mediated food allergy; OR, odds ratio.
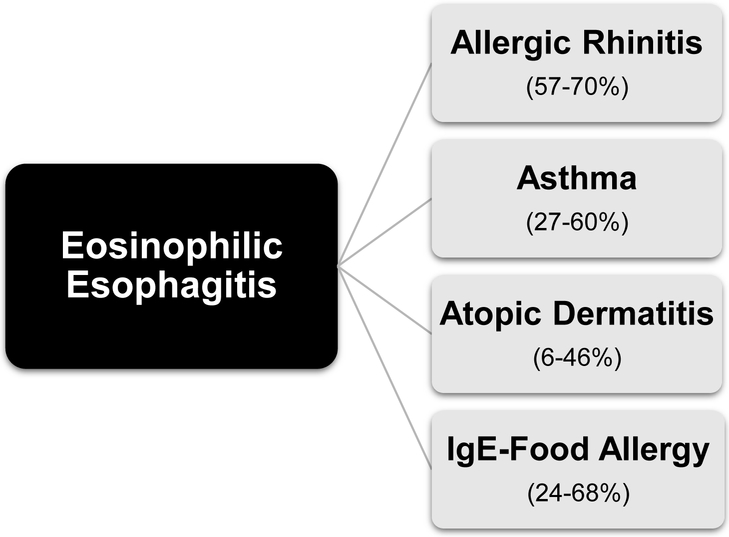
To date, one large meta-analysis has also delineated the atopic burden in patients with EoE, showing significantly increased odds of AD (OR 2.85, 95% CI, 1.87-4.34), asthma (OR 3.01, 95% CI 1.96-4.62), and AR (OR 5.09, 95% CI 2.91-8.90) in those with EoE compared to healthy individuals[21]. We also performed a large analysis assessing children with and without EoE in a single large pediatric primary care network population[49]. In this study, the rates of atopic conditions were found to be significantly higher in subjects with EoE when compared to healthy children, and included AR (60% of EoE vs 17% without EoE; OR 7.1, 95% CI 5.8-8.6), asthma (60% of EoE vs 21% without EoE; OR 5.2, 95% CI 4.3-6.3) and AD (18% of EoE vs 7% without EoE; OR 3.1, 95% CI 2.4-4.0). Finally, in a large multicenter assessment of patients with EoE from the Consortium for Food Allergy Research, concomitant allergic disease was observed in 91% of the study population[50]. The overall prevalence of specific allergic diseases in EoE is depicted in Figure 1.
Fig 1.
Frequency of allergic diseases in patients with eosinophilic esophagitis from 2015-2019 studies. Prevalence rates shown in parentheses. [21,28,42-48]
Given the clinical associations between EoE and the other allergic manifestations, we hypothesized that EoE might be an intrinsic member of the allergic (or atopic) march[27,31]. The allergic march refers to the natural history of allergic disease manifestations, and time course progression of these conditions throughout infancy and childhood[52]. Initial work by our group determined the peak age of diagnosis of EoE to be approximately 3 years following onset of AD, IgE-FA, and asthma (though actual EoE incidence may be earlier given the known barriers to diagnosis including presentation and histologic evaluation)[31]. Subsequently, in an analysis of over 130,000 children within a large primary care cohort, presence of AD, IgE-FA, asthma and AR were found to be both independently and cumulatively associated with subsequent diagnosis of EoE[27]. Despite these observations, it should be noted that there are varying degrees of EoE presentation, and an allergic trigger is not always identified. This fact speaks to the complexity of this disease process, and to the possible existence of multiple disease endotypes with varying degrees of allergic pathophysiology[33]. In the sections ahead, we discuss the shared genetic, environmental, and immunologic factors that may be responsible for the clinical and epidemiologic associations between EoE and the other allergic manifestations.
Genetic determinants of allergy susceptibility
Several studies, both involving human subjects and murine models, have contributed to our understanding of the immunopathogenesis of EoE[2]. Eosinophilic inflammation is generally thought to be a late-stage effector response to T helper (TH) 2 cell-mediated inflammation, critical to other atopic diseases, and affecting select individuals with specific genetic predispositions. Many of these associations have been comprehensively reviewed previously with discrimination by genetic (disease risk variants, transcriptome) and epigenetic (histones, DNA, microRNA) disease-associated modifications[53]. Here we focus on genetic associations that may predispose to multiple allergic manifestations, including EoE.
Two widely studied genetic components involved in EoE and atopy include thymic stromal lymphopoietin (TSLP) and calpain 14 (CAPN14). Variants in the 5q22 locus encoding the gene for TSLP have been associated with EoE as well as the most common atopic diseases including, AD, asthma, and AR[54–58]. TSLP is an epithelial cell-derived cytokine that is secreted at barrier surfaces in response to allergen exposure, and is involved in the initiation and propagation of type 2 inflammation. In the esophagus, TSLP has been found to be overexpressed in biopsies from subjects with EoE, as compared to healthy individuals[59,60]. Furthermore, TSLP mediates inflammatory basophil responses in the context of experimental EoE[14]. These observations are reminiscent of the overexpression of TSLP that is observed in the skin of in patients with AD [61,62]. Notably, targeting TSLP for neutralization in murine models leads to elimination of esophageal eosinophilia, supporting a central role for TSLP in this disease process[14].
Similarly, variants in the 2p33 locus encoding the CAPN14 gene have been directly associated with EoE and atopy, with upregulation of CAPN14 in the esophagus of those with active esophagitis and following exposure to type 2 cytokines [55,63]. Previous reports have also associated CAPN14 dysregulation with epithelial barrier dysfunction[64,65]. Further, CAPN14 encodes a member of the calcium-dependent, non-lysosomal cysteine protease family and therefore may contribute to the protease hypothesis of EoE described below. Relevant to discussion of TSLP and CAPN14, a recent study investigated the relationship between atopy, EoE, and genetic risk[66]. In this study, single nucleotide polymorphisms in 63 atopy genes were evaluated for EoE associations both in atopic and nonatopic individuals. The results suggested a mechanistic gene-gene interaction whereby atopy-related genes and EoE-specific loci (notably those for IL-4 and TSLP) synergistically cooperate to increase EoE risk. Thus, genetic susceptibility of EoE development is mediated by both EoE-specific and common atopic loci that, when present together, act synergistically to increase susceptibility to allergic disease development.
Other “atopic” regions of the genome previously identified include the 11q13 loci encoding EMSY and LRRC32 which are associated with EoE[55], AD[67,68], asthma[56,69], and AR[56]. Interestingly, this locus has also been associated with inflammatory bowel disease [70], which was recently found to be a significant comorbidity seen in children with EoE[49]. Genome-wide association studies have also identified regions contributing to susceptibility of IgE-FA onset, including the SERPINB gene[71]. While a direct association between SERPINB and EoE has not yet been established, the family of SERPINs genes are highly expressed in the esophagus, and so may be an important target for future exploration. Finally, 16p13 has been identified as an additional risk locus for EoE, associated with various disease including AD and asthma, and AR[56,72], among other immune and autoimmune conditions[73]. Together, these studies indicate that a shared set of genetic changes may predispose an individual to develop multiple allergic manifestations, including EoE.
Environmental factors common to the allergic manifestations
Multiple environmental factors have been associated with development of allergy [74], and the environment in which an individual is raised is a strong contributor to EoE development[54]. Common environmental factors that predispose to multiple allergic manifestations therefore likely contribute to the observed associations between EoE and its comorbidities. Here we review the major environmental considerations thought to contribute to allergy in general, and EoE specifically.
Microbiome-altering environmental pressures
Billions of microbes colonize the mucosal surfaces of mammals in a symbiotic relationship that supports normal physiology. The term microbiome describes the DNA material of these microbial communities which consists of bacteria, viruses, fungi, and protozoa. The human microbiome is both immense, and complex, with 100 trillion microbes representing multiple genera colonizing the gastrointestinal (GI) tract alone [75]. Over the past several decades, the GI microbiome has been a subject of particular focus, and research has added to our appreciation of dysbiosis (microbial imbalance or maladaptation) as an important factor in multiple human disease states [76], including allergy. Previous efforts have sought to understand the role of the microbiome in the context of allergic inflammation[77,78], and microbiome-altering environmental pressures are now thought to be important contributors to allergy risk[79].
Investigators have also focused specifically on the relationship between the esophageal microbiome and EoE. The esophagus itself is colonized by at least hundreds of bacterial species, with members of the Firmicutes and Bacteroides phyla being among the most highly represented in both children and adults [80–83]. Initial studies of the esophageal microbiome in children with EoE have noted a few key associations. Most notably, when examining biopsy samples in children with active EoE, there is shift away from the genera Streptococcus and Atopobium in favor of Neisseria and Corynebacterium when compared with samples from patients with inactive EoE or healthy controls [83]. In another study that examined 16S rRNA from esophageal string tests from children and adults, it was observed that the total bacterial load, as well as members of the Haemophilus genus specifically, were enriched in patients with EoE as compared with controls[82]. In addition, approximately half of subjects with active EoE on proton-pump-inhibitor treatment had an enrichment of members of the Proteobacteria phylum when compared with controls, an enrichment that was also observed upon examination of esophageal biopsies from patients with active EoE[83]. Though preliminary, these studies have started to elucidate the microbiome of the EoE esophagus.
It is particularly useful to note that the esophageal bacterial load seems to be increased in patients with EoE irrespective of treatment status or the degree of mucosal eosinophilia, and that the microbiomes of the inflamed esophagus in patients with EoE or gastrointestinal reflux disease are distinct[82]. Together, these observations suggest that the dysbiosis observed in EoE is may be at least in part the result of a primary mucosal defect in the regulation of microbial communities, as opposed to solely the consequence of mucosal inflammation. That being said, mechanistic studies in animal models (germ-free, gnotobiotic, or otherwise) are required to establish the extent to which microbial changes are a secondary consequence of, or contributing factor to, the esophageal inflammation observed in EoE.
It is challenging to integrate microbiome data across biologic niches, as the commensals that colonize different mucosal and epithelial surfaces are distinct. However, there are a number of common environmental pressures that can impact commensal communities across the body, thereby creating dysbiosis that could contribute to allergy development or progression. Animal models have taught us that normal microbiota help control allergic responses to foods and other allergens [84,85], and antibiotic exposure, birth mode, preterm birth, and diet each dynamically restructure commensal populations [86,87], while increasing risk for allergy[79,88–90]. Similar associations exist with early-life environmental pressures and the development of EoE. For example, antibiotic exposure is associated with EoE in the majority of studies[91–95]. Similarly, cesarean delivery also increases risk of EoE[92,93]. Finally, while diet is known to influence the esophageal microbiome[83], like other allergic manifestations the association between early life breast vs. formula feeding and EoE is less clear[91,96]. Together, these studies support a role for environmental pressures that alter the microbiome as common factors that contribute to the development of EoE, and other allergic manifestations. However, future research is necessary to expand upon this work and define the most relevant microbes and immunologic mechanisms involved.
Exposure to aeroallergens as a trigger of EoE
As noted previously, there is a very high degree of comorbidity between EoE and AR[27]. However, evidence linking these two conditions extends beyond epidemiologic associations. Over the past decade, a body of literature has emerged that describes how aeroallergens can exacerbate EoE in some individuals[97,98]. For example, a case series of 1,180 EoE patients found that 14% had a history of exacerbation by aeroallergens, and 1/5th of those had biopsy-confirmed seasonal variation of esophageal eosinophilia[30]. Several other reports have further supported an intrinsic, seasonal dependence of EoE with a decrease in the diagnosis of EoE during winter months, and increases in diagnosis during times of high pollen as the spring, summer, and fall[99–104]. One study even noted a significantly higher incidence of food bolus impactions in those with EoE during the summer and fall, compared with the winter[105].
Direct evidence for the role of aeroallergens in EoE exacerbations comes from both animal models and clinical observations. Researchers have developed models of experimental EoE in mice through exposure to perennial allergens such as cockroaches, dust mites, or molds[106,107]. Furthermore, EoE development has been seen in patients exposed to large volumes of allergens such as mold, dust, and grass following acute, large-volume accidental exposure[108] or sublingual pollen immunotherapy to trees[109] or grasses[109,110]. Interestingly, associations between aeroallergens and EoE seem to be primarily related to pollens, in contrast to the common “indoor” allergens. There is actually a small protective effect of owning a furred pet when it comes to subsequent EoE development[93,111]. It is not entirely known why pollens are particularly relevant, though it may be due to immunologic cross-reactivity with food allergen components[112].
Despite these associations, the correlation between season and rate of EoE diagnosis is not consistent across studies[113,114]. For example, a large report of over 700 patients found that EoE symptoms did not vary by season suggesting that the causal allergens are present year-round[115], and a meta-analysis of 18 studies and 16,846 patients found no statistical difference in the annual seasonal distribution of newly diagnosed EoE [116]. It is also worth pointing out that the endpoint for this meta-analysis was food bolus impaction requiring medical care—an outcome that favors specificity over sensitivity. None the less, these studies indicate that the role of aeroallergens in EoE may be nuanced, with aeroallergens being relevant in a subset of patients (for example older children where allergic rhinitis is more prevalent), or due to sensitivity to allergens characteristic of distinct seasons, geographic regions, or diets.
Oral or sublingual immunotherapy for IgE-mediated food allergy
Recently, the recognition of EoE in patients undergoing oral immunotherapy (OIT) for IgE-mediated food allergy has raised concern that OIT could trigger EoE in susceptible individuals [117–119]. This association was further explored in a meta-analysis which reported a positive correlation between OIT and EoE, with new onset of EoE occurring in 2.7% of patients undergoing OIT[119]. However, it should be noted that in this meta-analysis EoE diagnosis was defined by biopsy, excluding patients with clinical symptoms of EoE (such as abdominal pain or vomiting) alone [120]. In our previous review of studies that documented discontinuation of OIT due to EoE symptoms and/or consistent biopsy findings, we found that symptoms of EoE occur at a rate between 8 and 14%[28]. Thus, while abdominal pain and vomiting are not diagnostic for EoE, actual rate of EoE during OIT may be higher than previously appreciated.
It is unclear at this time whether OIT causes EoE, or whether OIT exacerbates mild EoE in otherwise sub-clinical individuals. We found that the prevalence of EoE in patients with IgE-mediated food allergy is higher than the rate of EoE in the general population[28], and that a history of IgE-FA to the three of the most common food allergens (milk, egg, and shellfish) was highly associated with the subsequent diagnosis of EoE [28]. In a follow-up study, we observed a hazard ratio of 9.1 for children with IgE-FA going on to develop EoE, the strongest association detected among the various allergic march relationships [27]. Finally, it has been reported that individuals who outgrow IgE-FA can go on to develop EoE to the same food[121–123]. Together, these findings suggest that a common allergen-specific TH2 response could give rise to both IgE-FA and EoE. This body of literature also suggests that the concern for iatrogenic EoE in patients undergoing OIT is warranted, and patients undergoing OIT should be closely monitored for development of EoE symptoms.
Role of infections in EoE predisposition
In investigations into environmental factors that might influence EoE development, an interesting association was made with Helicobacter pylori (H. pylori). H. pylori infects about half of the global population[124], with a wide variation between regions and countries. Since it’s identification as a cause of peptic ulcer disease, gastritis, and several cancers, the prevalence of H. pylori has dramatically decreased in westernized countries[125,126]. This occurred at the same time as an increasing recognition and diagnosis of EoE, resulting in an inverse association between EoE rates and H. pylori infections[127–132].
The inverse association between EoE and H. pylori resulted in the development of a hypothesis that H. pylori, by inducing both a TH1 and TH17 response[133], polarizes the immune system away from allergic inflammation. This hypothesis was supported by animal studies that show a protective effect of H. pylori infection in a model of allergic asthma[134]. However, a recent large, multicenter, case-control study directly examined the relationship between H. pylori and EoE in a prospective manner. A total of 808 individuals were studied, including 170 children. The study found that there was no difference in H. pylori prevalence between cases and controls in either children or adults [135]. Thus, despite the potential for relevant immunologic mechanisms, it is unlikely that there is a clinically-relevant protective effect of H. pylori infection in the setting of EoE.
Shared features of “type 2” inflammation among the allergic manifestations
The observation that most EoE patients have comorbid allergic conditions suggests that EoE is immunopathologically related to the other allergic manifestations. Additional evidence of this relationship comes from the fact that EoE shares many clinical similarities to other allergic conditions including exhibiting a “type 2” or allergic form of inflammation[136,137], and being responsive to allergen avoidance and/or topical steroid applications. For example, elemental diets have been shown to be highly effective in inducing histologic and clinical remission in children and adolescents with EoE[138–140], as have empiric elimination diets based on the most commonly identified etiologic foods[139]. Alternatively, orally administered topical steroids are an effective therapy for EoE, with resolution rates up to 80%[17,18,141–147]. A notable exception to the clinical similarities between EoE and the other allergic manifestations is that allergen testing (skin prick or specific serum IgE) is of limited utility when attempting to determine causal foods [15,138], likely because IgE is not required for EoE pathogenesis (in animal models) [14]. There have been considerable advances over the past decade to our understanding of the immunologic mechanisms underlying EoE pathophysiology, the details of which have been reviewed previously [2,148]. We therefore focus on fundamental features of allergy development, and how they may contribute to the relationship between EoE and its comorbid conditions (Fig. 2).
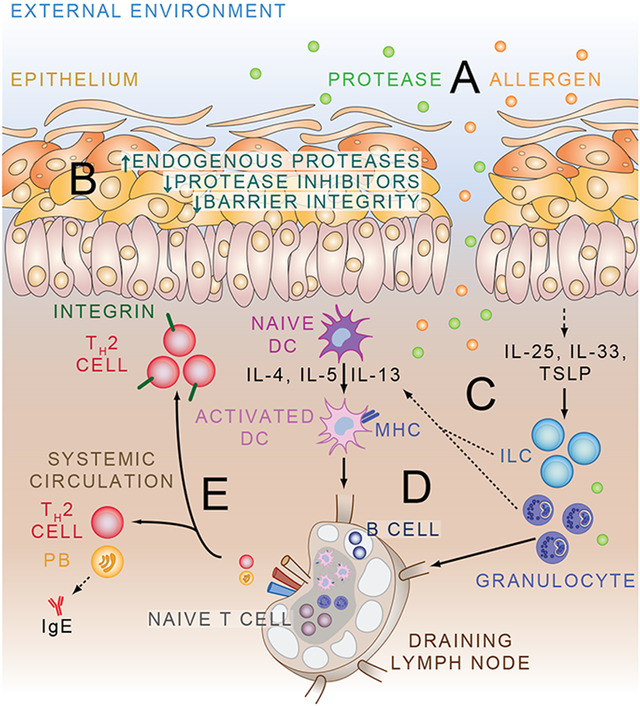
Fig 2.
Model of allergy development. The epithelium (whether it be the airway, skin, or GI tract) is constantly exposed to protease and non-protease allergens (A). Exogenous protease exposure, up-regulation of endogenous proteases, and down-regulation of protease inhibitors is associated with impaired barrier integrity (B). Breakdown of the epithelial barrier allows allergen entry, and causes secretion of alarmin cytokines including IL-25, IL-33, and TSLP that recruit and activate innate lymphoid cells (ILC) and granulocytes (C). Innate cells are potent sources of type 2 cytokines that can help to activate naive dendritic cells (DC) to process antigen, and promote development of type 2 helper T (TH2) cell responses (D). TH2 cells can home back to the site of inflammation via up-regulation of specific chemokine receptors and integrins, or enter the circulation along with allergen-specific IgE-producing plasma blasts to exert effects at distant tissue sites (E).
The protease hypothesis
The protease hypothesis supposes that protease activity is one upstream trigger of type 2 inflammatory responses; the mammalian immune system’s reaction to both parasitic and allergic stimuli. There are evolutionary advantages to mammals evolving sensors of protease activity, as many parasites utilize proteases to both aid host invasion and establish chronic infection[149]. Protease sensors of the immune system include the interleukin (IL)-1 family of cytokines, and in particular the potent TH2 stimulus IL-33, which are directly activated by microbial and allergen-associated proteases[150,151]; direct activation of innate immune cells including basophils, eosinophils, and mast cells[152–155]; and polarization of naive T cells towards a TH2 activation state such as that mediated upon cleavage of the protease-activated receptor 2 [156]. These innate protease sensors may be unintended targets of many of the most clinically-relevant allergens including insect venoms[157], some food allergens[158], and respiratory allergens[151,159,160], all of which can exhibit inherent protease activity. Given the fundamental role of proteases in inducing type 2 inflammatory responses, it is perhaps not surprising that the protease activity of allergens has been shown to be important for generating multiple models of allergic disease[161].
There is also building evidence to support a role for protease dysregulation in EoE pathogenesis. Clinically, a high proportion of patients with EoE display sensitivity to indoor protease allergens derived from insects[107,159,162]. Experimentally, exposure to these same indoor allergens including house dust mite (HDM), cockroach, or mold allergens, can induce EoE-like inflammation in mice[106,107,163]. For example, the major HDM allergen (Derp1) has prominent cysteine protease activity that mediates immune cell activation via cleavage of the low-affinity IgE receptor CD23, and the alpha subunit of the interleukin IL-2 receptor CD25[164,165]. In mice, exposure to HDM allergen results in significant infiltration of eosinophils and mast cells into the esophagus, which is independently dependent on eotaxin-1/2, CCR3, and IL-5.
Additional evidence of a role for proteases in EoE pathogenesis comes from genome-wide studies that have identified polymorphisms in protease inhibitors that associate with the disease. For example, EoE has been linked to polymorphisms in the CAPN14 gene[63], a calcium-activated cysteine protease that is induced by IL-13, and contributes to the esophageal epithelial barrier impairment observed in EoE [64]. Conversely, polymorphisms that interfere with potentially protective, anti-peptidases expressed by the esophageal epithelium are also associated with EoE. The SERPIN gene family, which encode serine and other protease inhibitors, are highly expressed in the esophagus and associated with FA on a genome-wide level[71]. Intriguingly, the SERPINs are among the most dysregulated esophagus-specific protein families in EoE[166]. Similarly, the serine protease inhibitor Kazal-type 7 (SPINK7) is important for normal epithelial differentiation. SPINK7 is lost in the epithelium of EoE patients, and experimental manipulations of SPINK7 have shown that it plays a homeostatic role in maintaining barrier function and allergic inflammatory responses[167]. Together, these results identify mammalian-encoded proteases, and protease inhibitors, and integral components of normal esophageal epithelial barrier function and immune responses, and emphasize the role of both mechanisms in EoE pathogenesis (Fig. 2AB)[168].
A failure of barrier integrity
An additional shared feature of type 2 inflammatory diseases, whether they be of the skin, gastrointestinal (GI) tract, or airway, is an impairment of epithelial barrier integrity [169]. When this important barrier is disrupted, immune activating microorganisms and antigens can gain access to body’s immune system, with resulting inflammatory responses. The GI tract is one of the best studied of the mucosal sites, and is unique in that it is one of two organ systems (along with the lungs) that has evolved to facilitate biologic interactions with the external world. Consequently, the large surface area of the GI tract is charged with absorbing essential minerals and nutrients while being simultaneously exposed to inert compounds, allergens, toxins, commensal organisms, and pathogens.
As a result of this complicated task, the GI tract has evolved physical, biochemical, and immunologic barriers between the internal and external environment[76]. Epithelial cells of the GI tract express various intercellular junctions (apical tight junctions, subjacent adherens junctions, and desmosomes) all of which control the absorption of fluid and solutes while impeding access of pathogens and other harmful molecules[170]. In addition, biochemical adaptations (such as mucus, and antimicrobial peptides) confer broad-spectrum antimicrobial properties to the epithelium[170]. Finally, the human GI tract is home to one of the highest densities of immune cells in the entire body which must identify potentially harmful stimuli and mount a protective response [171].
When these adaptations fail, the results can be catastrophic. One of the best examples of this comes from our understanding of inflammatory bowel disease where an overwhelming immune response to normally commensal microbes results in significant morbidity and mortality [172]. However, defects in barrier function are characteristic of the allergic manifestations, where microbial exposure activates the innate immune system, and subsequent TH2 responses can develop against otherwise inert antigens. For example, AD is characterized by defects in filaggrin (a filament-associated epidermal differentiation complex protein essential for regulation of epidermal homeostasis) [173], desmosomal proteins[174,175], and tight junction proteins[176], while disruption of both epithelial tight and adherens junctions is characteristic of asthma[177].
Consistent with epithelial barrier dysfunction being a common feature of allergy, genes and molecules important for epithelial integrity (including CRISP3, CLDN10, DSG1, and FLG) have been linked to EoE in patients and disease models [167,178,179]. For example, it has been noted that patients with EoE have reduced levels of DSG1, an intercellular adhesion molecule that is important for maintaining suprabasal epithelial integrity, as compared with controls[180]—a defect which is also observed in AD[174,175]. Treatment of EoE with topical steroids results in normalization of DSG1 expression, suggesting a central role in the barrier dysfunction observed in EoE[181]. Patients have also been identified with homozygous mutations in DSG1, and the resulting severe allergic phenotype includes eosinophilic esophageal inflammation[174,175]. Mechanistically, deletion of DSG1 in cultured epithelial cells results in a transcriptional phenotype similar of EoE biopsies [180]. While IL-13 treatment or overexpression of calpain 14 (a protease discussed above) reduces DSG1 expression in epithelial cells and results in impaired barrier function[64,180,182]. Together, these observations identify epithelial barrier dysfunction as a central feature of EoE pathology, that is common to the other allergic manifestations (Fig. 2C).
Promiscuity of type 2 inflammatory responses
An additional mechanistic question related to the clinical associations between EoE and the other allergic manifestations is whether there is an additive effect of existing type 2 inflammation on the subsequent development of food-specific TH2 cell responses. This effect could contribute to the progression of the allergic march in a manner that is complementary too genetic and environmental factors. Perhaps the best understood example of this phenomenon is the increased likelihood of allergic sensitization that occurs when the immune system is exposed to an antigen via inflamed skin, such as that seen in atopic dermatitis. In this case, the resulting inflammatory milieu of a primary skin defect promotes the development of specific T- and B-cell responses, and subsequent allergic disease. This mode of sensitization is certainly relevant to IgE-FA and respiratory allergy, as well as EoE (Fig. 2D)[14]
However, there is also evidence to suggest that systemic allergic inflammation could act as an adjuvant for the development of TH2 cell responses. Basophils are one potential mechanism for this effect as they are potent innate sources of IL-4, and are recruited to lymph nodes quickly after exposure to infectious or allergic stimuli where they cooperate with DCs to promote TH2 cell development[183]. What is particularly notable about basophils however is that their number in the blood in the steady-state, and their recruitment to lymph nodes after allergen exposure, are both directly related to serum IgE levels in an antigen-independent manner [84,184]. Thus, elevated IgE levels as a result of multiple factors could potentiate the subsequent development of basophil-facilitated TH2 responses at other tissue sites, including the esophagus [185]. While these observations offer preliminary insights into immunologic mechanisms by which the presence of allergic inflammation at one site may potentiate the development of additional allergic manifestations, additional basic and translational research is necessary to establish its relevance.
The role of “Type 2” targeted biologics in EoE management
Given the shared features of EoE and the other allergic manifestations, it is reasonable to consider the role of new, type 2-targeted biologics in EoE management. Indeed, biologic therapies have proved to be innovative therapeutics for various allergic diseases, and several of these agents have also been evaluated for use in EoE. The possibility to treat allergic patients with a single medication that is effective against multiple allergic manifestations may represent a reality for the future of allergy care. Here we review biologics that target immunologic pathways that are common to multiple allergic manifestations including EoE.
IL-5-targeted therapies
IL-5, integral for multiple facets of eosinophil biology, and has perhaps been the most widely studied target for treatment of EoE. Studies have primarily focused on mepolizumab, a humanized monoclonal antibody currently approved for the treatment of asthma and for other hypereosinophilic syndromes including eosinophilic granulomatosis withpolyangiitis[186–188]. Initial murine studies using IL-5 targeted therapy demonstrated promising results with significant reductions in airway inflammation/hyperresponsiveness, airway eosinophilia and reduced response to methacholine[189–191]. Use of mepolizumab for EoE was initially openly studied in a small phase 1-2 study of adults that found significant reductions in esophageal eosinophilia and clinical symptoms in the treatment group[192]. These results were not replicated, however, in prospective, randomized, double-blind placebo-controlled trials (RDBPCT) including one adult study in which both the histologic and symptom-based end points were not reached[193]. In the pediatric RDBPCT trial, only 8.8% of patients reached the pathologic primary end point, with no significant improvement in clinical symptoms [194].
Other IL-5 inhibitors studied in the treatment of EoE include Benralizumab and Reslizumab, both currently approved for use in eosinophilic asthma in adults, with additional approval for use of Benralizumab in adolescents [188]. While no specific RDBPCTs using Benralizumab (a IL-5 receptor antagonist) for EoE exist to date, significant histologic and symptomatic improvement was demonstrated in patients with hypereosinophilic syndrome involving the gastrointestinal tract, whereby depletion of gut tissue eosinophilia was demonstrated[195]. In the randomized placebo-controlled trial of Reslizumab, involving a large cohort (n=226) of children with EoE, a significant reduction in peak esophageal eosinophils was positively achieved[196]. Specifically, compared to the 24% reduction demonstrated in the placebo group, those treated with Reslizumab demonstrated a 59-67% reduction in peak eosinophil counts, however, no significant differences in patient symptoms or physician global assessment was found between groups. Given the overall mixed nature of these data, further studies using targeted IL-5 therapy are needed.
IgE-targeted therapies
Omalizumab, a humanized mouse anti-IgE monoclonal antibody currently used in the management of asthma and chronic urticaria[184,197,198], has also been studied for use in EoE with variable outcomes. In a 12-week open label study, 33% of patients who underwent omalizumab therapy achieved the study’s pathologic end point in pathologic reduction of esophageal eosinophils, with nearly half of these patients experiencing symptomatic improvement[199]. Similar to the anti-IL-5 studies, results were unable to be replicated in an RDBPCT of mostly adults with EoE, where no significant difference in EoE-related symptoms or esophageal eosinophils was observed between those in the treatment vs placebo groups[200]. These negative results could indicate that IgE is not required for EoE pathogenesis. However, in the open label study, omalizumab-induced remission of EoE was limited to subjects with low peripheral blood absolute eosinophil counts, which may suggest specific utility in a subset of patients, though further studies are needed.
IL-13 and IL-4-targeted therapies
Three additional RDBPCTs involving biologic therapies targeted towards the treatment of EoE should be mentioned. These include two studies targeting IL-13, an integral TH2 cytokine secreted by active eosinophils and necessary for eotaxin-mediated eosinophil recruitment, among other functions[7,201]. The first study involved 23 adult patients with EoE treated with the monoclonal IgG1 anti-IL-13 antibody, QAX576[202]. Although the primary endpoint of reduced peak eosinophils was not reached, mean eosinophil count was significant reduced in the treatment group compared to controls. Additionally, in the QAX576 group, several EoE-relevant transcription markers (eotaxin-3, periostin and markers of mast cells) were improved. In the second study, adult patients who underwent 16-week treatment with RPC4046, a humanized IgG1 kappa anti-IL13 antibody showed improvement in EoE features, including improvement in clinical symptoms, clinician’s global assessment of disease severity, and histology, with a significant decrease in esophageal eosinophils compared to those receiving placebo[203]. Finally, a single study evaluating the utility of Dupilumab, a human anti-IL-4 receptor alpha monoclonal antibody that inhibits signaling both of IL-4 and IL-13, and currently approved in the treatment of adult severe atopic dermatitis, was also conducted in patients with EoE. In this phase 2 RDBPCT involving multiple clinical centers, adults with EoE in the treatment group were found to have significant improvement in comprehensive histologic evaluation, including significant reduction in peak esophageal eosinophils and in symptoms, compared to the placebo group[204]. Together, these studies suggest IL-13 (and potentially IL-4) as targets for EoE therapeutics, with the potential that future studies could evaluate IL-13 as a target for atopic diseases such as asthma and AD, specifically in patients with comorbid EoE diagnosis.
Conclusions and clinical implications
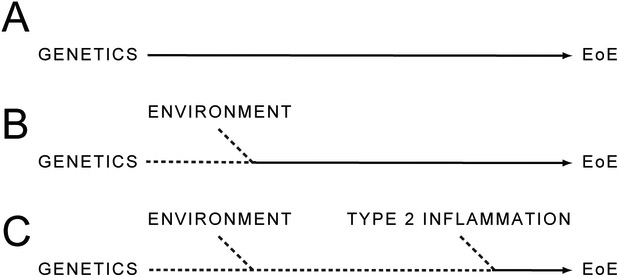
In sum, evidence suggests that EoE is a primarily allergic disease, that is closely associated with AD, IgE-FA, asthma, and AR. While the relationship between these conditions is likely the result of shared genetic, environmental, and immunologic features, the details of these relationships continue to be elucidated. For example, there is likely a subset of patients who are significantly predisposed to develop EoE as a result of their genetics alone (Fig. 3A). However, others may require contributions from their environment and/or concurrent type 2 inflammation at sites other than the esophagus to potentiate EoE development (Fig. 3B,C). Future studies must focus on establishing the relative contributions of these “allergic determinants” to EoE development, with particular focus on the immunologic mechanisms that link one allergic manifestation to the development of another.
Fig 3.
Factors underlying the development of EoE. Cumulative influence of an individual’s genetics (A), environment (B), and existing type 2 inflammation (C) on risk of EoE development.
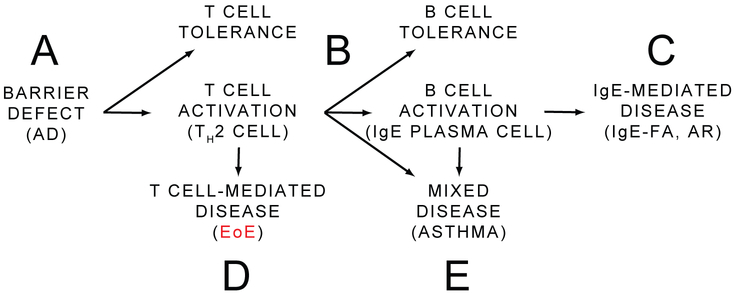
When considering the pathophysiology of EoE and the other allergic manifestations, a model emerges that can place EoE in immunologic and clinical context. In this simplified model, a foreign protein is exposed to the immune system as a result of a barrier defect such as AD (Fig. 4A). Upon exposure, there are two broad opportunities for tolerance—one at the point of the T cell, and one at the point of the B cell (Fig 4B). Should both of these checks fail, the individual develops an IgE-mediated disease such as AR, or IgE-FA (Fig. 4C). Individuals where allergen-specific TH2 cell response form, but B cell tolerance is maintained (or develops over time such as the case with children who “outgrow” IgE-FA), may still be at risk for developing a primarily T cell-mediated disease such as EoE (Fig. 4D). There is some precedent for such discordance between allergen-specific T and B cell responses, as food allergen-specific IgE and basophil activation correlate with clinical IgE-FA, while presence of allergen-specific TH2 cells do not[205]. This model also provides a potential explanation for the limited utility of skin prick testing in EoE[15,138], as food-specific T cell responses can exist in the absence of circulating food-specific IgE. Further, a natural extension of this logic suggests that the ability to measure food-specific T cell responses may aid in the identification of EoE-causal foods[206]. Finally, there are mixed IgE/T cell-mediated conditions such as asthma (Fig. 4E). While this model is useful to broadly categorize the relationship between the allergic manifestations, the authors are the first to acknowledge that EoE subtypes may exist that result from a mixed, IgE/T cell-mediated mechanism. None the less, the prevailing view at this time is that IgE may contribute, but is not required for EoE pathogenesis in most cases[207].
Fig 4.
Simplified model of the pathologic relationship between EoE and the other allergic manifestations. Allergy often is initiated at a site of barrier defect such as is the case in atopic dermatitis (AD) (A). There are two broad checkpoints at which antigen-specific activation or tolerance can occur: the T cell and the B cell (B). Should T and B cell tolerance fail, an IgE-mediated disease such as IgE-mediated food allergy (IgE-FA) or allergic rhinitis (AR) ensues (C). In the presence of B cell tolerance, allergen-specific T cells can cause eosinophilic esophagitis (EoE) (D). Mixed IgE/T cell-mediated disease can also occur, such as is the case with asthma (E).
From a clinical stand point, early diagnosis and institution of an effective treatment regimen is paramount in preventing long-term sequelae of EoE [208]. In 2018, the Updated International Consensus Criteria for EoE were published, establishing important changes in screening methods for patients suspected to have EoE [209]. Given the clinical associations between EoE and the other allergic manifestations, the use of allergic history in evaluating a patients risk for EoE is an attractive consideration[210]. It is reasonable that family or personal history of a preexisting allergic disease should increase the suspicion for EoE, as it has been reproducibly demonstrated across multiple studies that individuals with EoE have a high prevalence of allergic comorbidity. The relationship between IgE-FA and EoE seems to be particularly strong. Thus, physicians should suspect EoE in patients with chronic symptoms of esophageal dysfunction, especially in those with personal history of a significant atopic burden, and with particular emphasis on current or prior IgE-mediated food allergy. Finally, physicians should be vigilant about screening for symptoms of EoE in patients undergoing immunotherapy for IgE-FA. By continuing to elucidate the clinical and pathophysiologic relationship between these conditions, we will have the best chance of identifying and developing new preventative and therapeutic approaches that are common among EoE, and the other allergic manifestations.
Acknowledgements:
We thank Jonathan Spergel and Neil Romberg for their helpful suggestions.
Sources of Funding: DAH is supported by the NIH (K08 DK116668) and a Children’s Hospital of Philadelphia Junior Faculty grant.
Abbreviations:
- EoE
eosinophilic esophagitis
- AD
atopic dermatitis
- IgE-FA
IgE-mediated food allergy
- AR
allergic rhinitis
- GI
gastrointestinal
- TSLP
thymic stromal lymphopoietin
- CAPN14
calpain 14
- OIT
oral immunotherapy
- RDBPCT
randomized, double-blind placebo-controlled trial
- H. pylori
Helicobacter pylori
- TH
T helper
- IL
interleukin
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Cianferoni A, Spergel J. Eosinophilic esophagitis: A comprehensive review. Clin Rev Allergy Immunol. 2016;50(2): 159–74. [DOI] [PubMed] [Google Scholar]
- 2.Hill DA, Spergel JM. The Immunologic Mechanisms of Eosinophilic Esophagitis. Curr Allergy Asthma Rep. 2016;16(2):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breier-Mackie S Cultural competence and patient advocacy: The new challenge for nurses. Gastroenterol Nurs. 2007;30(2): 120–2. [DOI] [PubMed] [Google Scholar]
- 4.Picus D FP. Eosinophilic esophagitis. AJR Am J Roentgenol. 1981;136(5): 1001–3. [DOI] [PubMed] [Google Scholar]
- 5.Walsh S V, Antonioli DA, Goldman H, Fox VL, Bousvaros A, Leichtner AM, et al. Allergic esophagitis in children: A clinicopathological entity. Am J Surg Pathol. 1999;23(4):390–6. [DOI] [PubMed] [Google Scholar]
- 6.Attwood SEA, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia - A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1): 109–16. [DOI] [PubMed] [Google Scholar]
- 7.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a TH2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108(6):954–61. [DOI] [PubMed] [Google Scholar]
- 8.Arias A, Pérez-Martinez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: The incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43(1):3–15. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):319–332.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merves J, Muir A, Modayur Chandramouleeswaran P, Cianferoni A, Wang M-L, Spergel JM. Eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2014;112(5):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina-Infante J, Lucendo AJ. Dietary therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):41–7. [DOI] [PubMed] [Google Scholar]
- 13.Arias Á, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology. 2014;146(7): 1639–48. [DOI] [PubMed] [Google Scholar]
- 14.Noti M, Wojno EDT, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8): 1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130(2). [DOI] [PubMed] [Google Scholar]
- 16.Kagalwalla AF, Shah A, Li BUK, Sentongo TA, Ritz S, Manuel-Rubio M, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53(2):145–9. [DOI] [PubMed] [Google Scholar]
- 17.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010; 139(2). [DOI] [PubMed] [Google Scholar]
- 18.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010; 139(5): 1526–37. [DOI] [PubMed] [Google Scholar]
- 19.Debrosse CW, Franciosi JP, King EC, Buckmeier Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011; 128(1): 132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Cervera J, Arias Á, Redondo-González O, Cano-Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic esophagitis: A systematic review and meta-analysis. Ann Allergy, Asthma Immunol. 2017;118(5):582–590.e2. [DOI] [PubMed] [Google Scholar]
- 22.Spergel JM. An allergist’s perspective to the evaluation of Eosinophilic Esophagitis. Best Pract Res Clin Gastroenterol. 2015;29(5):771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon D, Marti H, Heer P, Simon HU, Braathen LR, Straumann A. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases [3], J Allergy Clin Immunol. 2005;115(5):1090–2. [DOI] [PubMed] [Google Scholar]
- 24.Sugnanam KKN, Collins JT, Smith PK, Connor F, Lewindon P, Cleghorn G, et al. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy Em J Allergy Clin Immunol. 2007;62(11): 1257–60. [DOI] [PubMed] [Google Scholar]
- 25.Mahdavinia M, Bishehsari F, Hayat W, Elhassan A, Tobin MC, Ditto AM. Association of eosinophilic esophagitis and food pollen allergy syndrome. Ann Allergy, Asthma Immunol. 2017;118(1): 116–7. [DOI] [PubMed] [Google Scholar]
- 26.Letner D, Farris A, Khalili H, Garber J. Pollen-food allergy syndrome is a common allergic comorbidity in adults with eosinophilic esophagitis. Dis Esophagus. 2018;31(2):1–8. [DOI] [PubMed] [Google Scholar]
- 27.Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic Esophagitis Is a Late Manifestation of the Allergic March. J Allergy Clin Immunol Pract. 2018;6(5):1528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract. 2017;5(2):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SK, Sabharwal G, Ghaffari G. A review of the evidence linking eosinophilic esophagitis and food allergy. Allergy Asthma Proc. 2015;36(1):26–33. [DOI] [PubMed] [Google Scholar]
- 30.Ram G, Lee J, Ott M, Brown-Whitehom TF, Cianferoni A, Shuker M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy, Asthma Immunol. 2015;115(3):224–8. [DOI] [PubMed] [Google Scholar]
- 31.Hill DA, Spergel JM. Is eosinophilic esophagitis a member of the atopic march? Ann Allergy, Asthma Immunol. 2018;120(2):113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkins D, Furuta GT, Liacouras CA, Spergel JM. Eosinophilic esophagitis phenotypes: Ready for prime time? Pediatr Allergy Immunol. 2017;28(4):312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoda T, Wen T, Aceves SS, Abonia JP, Atkins D, Bonis PA, et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. lancet Gastroenterol Hepatol. 2018;3(7):477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sicherer SH, Sampson HA. Peanut allergy: Emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120(3):491–503. [DOI] [PubMed] [Google Scholar]
- 35.Sicherer SH, Muñoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999;103(4):559–62. [DOI] [PubMed] [Google Scholar]
- 36.Spergel JM, Brown-Whitehom TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: Clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48(1):30–6. [DOI] [PubMed] [Google Scholar]
- 37.Straumann A, Simon HU. Eosinophilic esophagitis: Escalating epidemiology? [1]. J Allergy Clin Immunol. 2005;115(2):418–9. [DOI] [PubMed] [Google Scholar]
- 38.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. [DOI] [PubMed] [Google Scholar]
- 39.Benninger MS, Strohl M, Holy CE, Hanick AL, Bryson PC. Prevalence of atopic disease in patients with eosinophilic esophagitis. Int Forum Allergy Rhinol. 2017;7(8):757–62. [DOI] [PubMed] [Google Scholar]
- 40.Guajardo JR, Plotnick LM, Fende JM, Collins MH, Putnam PE, Rothenberg ME. Eosinophil-associated gastrointestinal disorders: A world-wide-web based registry. J Pediatr. 2002;141(4):576–81. [DOI] [PubMed] [Google Scholar]
- 41.Jyonouchi S, Brown-Whitehorn TA, Spergel JM. Association of Eosinophilic Gastrointestinal Disorders with Other Atopic Disorders. Immunol Allergy Clin North Am. 2009;29(1):85–97. [DOI] [PubMed] [Google Scholar]
- 42.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198–206. [DOI] [PubMed] [Google Scholar]
- 43.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. J Allergy Clin Immunol. 2007; 119(3):731–8. [DOI] [PubMed] [Google Scholar]
- 44.Baumert JL. Detecting and Measuring Allergens in Food. Risk Manag Food Allergy. 2013;215–26. [Google Scholar]
- 45.Dellon E S, Rusin S, Gebhart JH, Covey S, Speck O, Woodward K, et al. A Clinical Prediction Tool Identifies Cases of Eosinophilic Esophagitis Without Endoscopic Biopsy: A Prospective Study. Am J Gastroenterol. 2015;110(9): 1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung AJT, Persad S, Slae M, Abdelradi A, Kluthe C, Shirton L, et al. Intestinal and gastric permeability in children with eosinophilic esophagitis and reflux esophagitis. J Pediatr Gastroenterol Nutr. 2015;60(2):236–9. [DOI] [PubMed] [Google Scholar]
- 47.Peterson K, Fang JC FR. Familial risk of eosinophilic gastrointestinal disorders (EGID) and atopy in eosinophilic esophagitis (EoE). Gastroenterology. 2015;148(Se414). [Google Scholar]
- 48.Duffey H, Peterson K FR. Population-based study suggests strong genetic association between eosinophilic esophagitis and asthma. J Allergy Clin Immunol. 2016;137(AB400). [Google Scholar]
- 49.Capucilli P, Cianferoni A, Grundmeier RW, Spergel JM. Comparison of comorbid diagnoses in children with and without eosinophilic esophagitis in a large population. Ann Allergy, Asthma Immunol. 2018;121(6):711–6. [DOI] [PubMed] [Google Scholar]
- 50.Chehade M, Jones SM, Pesek RD, Burks AW, Vickery BP, Wood RA, et al. Phenotypic Characterization of Eosinophilic Esophagitis in a Large Multicenter Patient Population from the Consortium for Food Allergy Research. J Allergy Clin Immunol Pract. 2018;6(5):1534–1544.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leigh LY, Spergel JM. An in-depth characterization of a large cohort of adult patients with eosinophilic esophagitis. Ann Allergy, Asthma Immunol. 2019;122(1):65–72.e.l. [DOI] [PubMed] [Google Scholar]
- 52.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy, Asthma Immunol. 2018;120(2):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherrill JD, Rothenberg ME. Genetic and epigenetic underpinnings of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134(5):1084–1092.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sleiman PMA, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira MAR, Matheson MC, Tang CS, Granell R, Ang W, Hui J, et al. Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014; 133(6): 1564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weidinger S, Willis-Owen SAG, Kamatani Y, Baurecht H, Morar N, Liang L, et al. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum Mol Genet. 2013;22(23):4841–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinds DA, McMahon G, Kiefer AK, Do CB, Eriksson N, Evans DM, et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet. 2013;45(8):907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42(4):289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–80. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Hener P, Zhang Z, Ganti KP, Metzger D CP. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J Invest Dermatol. 2009;129(2):498–502. [DOI] [PubMed] [Google Scholar]
- 63.Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet. 2014;46(8):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitis- linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Litosh VA, Rochman M, Rymer JK, Porollo A, Kottyan LC, Rothenberg ME. Calpain-14 and its association with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;139(6):1762–1771.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin LJ, He H, Collins MH, Abonia JP, Biagini Myers JM, Eby M, et al. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J Allergy Clin Immunol. 2018;141(5):1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greisenegger EK, Zimprich F, Zimprich A, Gleiss A, Kopp T. Association of the chromosome 11q13.5 variant with atopic dermatitis in Austrian patients. Eur J Dermatology. 2013;23(2):142–5. [DOI] [PubMed] [Google Scholar]
- 68.O’Regan GM, Campbell LE, Cordell HJ, Irvine AD, McLean WHI, Brown SJ. Chromosome 11q13.5 variant associated with childhood eczema: An effect supplementary to filaggrin mutations. J Allergy Clin Immunol. 2010;125(l-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferreira MAR, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souëf P, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378(9795):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang SK, Hong M, Zhao W, Jung Y, Baek J, Tayebi N, et al. Genome-wide association study of Crohn’s disease in Koreans revealed three new susceptibility loci and common attributes of genetic susceptibility across ethnic populations. Gut. 2014;63(1):80–7. [DOI] [PubMed] [Google Scholar]
- 71.Marenholz I, Grosche S, Kalb B, Rüschendorf F, Blümchen K, Schlags R, et al. Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat Commun. 2017;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellinghaus D, Baurecht H, Esparza-Gordillo J, Rodríguez E, Matanovic A, Marenholz I, et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet. 2013;45(7):808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kottyan LC, Maddox A, Braxton JR, Stucke EM, Mukkada V, Putnam PE et al. (2018) Genetic variants at the 16p13 locus confer risk for eosinophilic esophagitis. Genes Immun:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jensen ET, Dellon ES. Environmental factors and eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Consortium THMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill DA, Artis D. Intestinal Bacteria and the Regulation of Immune Cell Homeostasis. Annu Rev Immunol. 2010;28(1):623–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch S V, Boushey HA The microbiome and development of allergic disease. Curr Opin Allergy Clin Immunol. 2016;16(2):165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L (2018) Recent developments and highlights in mechanisms of allergic diseases: microbiome. Allergy 73(12):2314–2327 [DOI] [PubMed] [Google Scholar]
- 79.Mitselou N, Hallberg J, Stephansson O, Almqvist C, Melén E, Ludvigsson JF. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J Allergy Clin Immunol. 2018;142(5):151–04. [DOI] [PubMed] [Google Scholar]
- 80.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci. 2004;101(12):4250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Norder Grusell E, Dahlén G, Ruth M, Ny L, Quiding-Järbrink M, Bergquist H, et al. Bacterial flora of the human oral cavity, and the upper and lower esophagus. Dis Esophagus. 2013;26(1):84–90. [DOI] [PubMed] [Google Scholar]
- 82.Dellon ES. The Esophageal Microbiome in Eosinophilic Esophagitis. Gastroenterology. 2016;151(2):364–5. [DOI] [PubMed] [Google Scholar]
- 83.Benitez AJ, Hoffmann C, Muir AB, Dods KK, Spergel JM, Bushman FD, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. 2015;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012:18(4):538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci. 2014; 111(36): 13145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3(2):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kummeling I, Stelma FF, Dagnelie PC, Snijders BEP, Penders J, Huber M, et al. Early Life Exposure to Antibiotics and the Subsequent Development of Eczema, Wheeze, and Allergic Sensitization in the First 2 Years of Life: The KOALA Birth Cohort Study. Pediatrics. 2007;119(l):e225–31. [DOI] [PubMed] [Google Scholar]
- 89.Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Lang JE, Garssen J, et al. Early-life antibiotic exposure increases the risk of developing allergic symptoms later in life: A meta-analysis. Allergy Eur J Allergy Clin Immunol. 2018;73(5):971–86. [DOI] [PubMed] [Google Scholar]
- 90.Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med. 2018; 15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;57(1):67–71. [DOI] [PubMed] [Google Scholar]
- 92.Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2014;2(4). [DOI] [PubMed] [Google Scholar]
- 93.Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2018;141(l):214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jenson E, Shaheen O, Koutlas N, Chang Ao, Martin L, Rothenberg M, et al. Early life factors are associated with risk for eosinophilic esophagitis diagnosed in adulthood. Gastroenterology. 2017;152(5):S861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Witmer CP, Susi A, Min SB, Nylund CM. Early Infant Risk Factors for Pediatric Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2018;67(5):610–5. [DOI] [PubMed] [Google Scholar]
- 96.Munblit D, Peroni DG, Boix-Amorós A, Hsu PS, Van’t Land B, Gay MCL, et al. Human milk and allergic diseases: An unsolved puzzle. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis [1]. J Allergy Clin Immunol. 2003;112(4):796–7. [DOI] [PubMed] [Google Scholar]
- 98.Onbasi K, Sin AZ, Doganavsargil B, Onder GF, Bor S, Sebik F. Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy. 2005;35(11): 1423–31. [DOI] [PubMed] [Google Scholar]
- 99.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41(5):451–3. [DOI] [PubMed] [Google Scholar]
- 100.Moawad FJ, Veerappan GR, Lake JM, Maydonovitch CL, Haymore BR, Kosisky SE, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31(4):509–15. [DOI] [PubMed] [Google Scholar]
- 101.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of Eosinophilic Esophagitis Over Three Decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10): 1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104(4):828–33. [DOI] [PubMed] [Google Scholar]
- 103.Iwanczak B, Janczyk W, Ryzko J, Banaszkiewicz A, Radzikowski A, Jarocka-Cyrta E, et al. Eosinophilic esophagitis in children: Frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci. 2011;56(2):151–7. [DOI] [PubMed] [Google Scholar]
- 104.Fahey L, Robinson G, Weinberger K, Giambrone AE, Solomon AB. Correlation between aeroallergen levels and new diagnosis of eosinophilic esophagitis in New York City. J Pediatr Gastroenterol Nutr. 2017;64(l):22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larsson H, Bergquist H, Bove M. The incidence of esophageal bolus impaction: Is there a seasonal variation? Otolaryngol - Head Neck Surg. 2011; 144(2): 186–90. [DOI] [PubMed] [Google Scholar]
- 106.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107(l):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rayapudi M, Mavi P, Zhu X, Pandey AK, Abonia JP, Rothenberg ME, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2010;88(2):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. J Gastrointest Liver Dis. 2013;22(2):205–8. [PMC free article] [PubMed] [Google Scholar]
- 109.Miehlke S, Alpan O, Schröoder S, Straumann A. Induction of eosinophilic esophagitis by sublingual pollen immunotherapy. Case Rep Gastroenterol. 2013;7(3):363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Antico A, Fante R. Esophageal hypereosinophilia induced by grass sublingual immunotherapy. J Allergy Clin Immunol. 2014;133(5):1482–4. [DOI] [PubMed] [Google Scholar]
- 111.Jensen ET, Kuhl JT, Martin LJ, Langefeld CD, Dellon ES, Rothenberg ME. Early-life environmental exposures interact with genetic susceptibility variants in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2018;141(2):632–637.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Rhijn BD, Van Ree R, Versteeg SA, Vlieg-Boerstra BJ, Sprikkelman AB, Terreehorst I, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy Eur J Allergy Clin Immunol. 2013;68(11): 1475–81. [DOI] [PubMed] [Google Scholar]
- 113.Frederickson NW, Bayman L, Valestin J, Redd M, Lee YJ, Soubra M, et al. Lack of seasonal variation in the incidence of eosinophilic oesophagitis in adolescent and adult non-PPI-responsive oesophageal eosinophilia midwestern US populations. United Eur Gastroenterol J. 2014;2(2):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: The experience in children living in rural communities. J Clin Gastroenterol. 2013;47(3):287–8. [DOI] [PubMed] [Google Scholar]
- 115.Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: Epidemiology, clinical presentation and seasonal variation. J Gastroenterol. 2013;48(l):81–5. [DOI] [PubMed] [Google Scholar]
- 116.Lucendo AJ, Arias A, Redondo-González O, González-Cervera J. Seasonal distribution of initial diagnosis and clinical recrudescence of eosinophilic esophagitis: A systematic review and meta-analysis. Allergy Eur J Allergy Clin Immunol. 2015;70(12):1640–50. [DOI] [PubMed] [Google Scholar]
- 117.Fuentes-Aparicio V, Alvarez-Perea A, Infante S, Zapatero L, D’Oleo A, Alonso-Lebrero E. Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergol Immunopathol (Madr). 2013;41(3):143–50. [DOI] [PubMed] [Google Scholar]
- 118.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009; 124(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucendo AJ, Arias Á, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: A systematic review with meta-analysis. Ann Allergy, Asthma Immunol. 2014;113(6):624–9. [DOI] [PubMed] [Google Scholar]
- 120.Hsieh FH. Oral food immunotherapy and iatrogenic eosinophilic esophagitis: An acceptable level of risk? Ann Allergy, Asthma Immunol. 2014;113(6):581–2. [DOI] [PubMed] [Google Scholar]
- 121.Hill DA, Shuker M, Cianferoni A, Wong T, Ruchelli E, Spergel JM, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. J allergy Clin Immunol Pract. 3(1): 123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soller L, Mill C, Avinashi V, Teoh T, Chan ES. Development of anaphylactic cow’s milk allergy following cow’s milk elimination for eosinophilic esophagitis in a teenager. J allergy Clin Immunol Pract. 5(5): 1413–4. [DOI] [PubMed] [Google Scholar]
- 123.Ho H-E, Chehade M. Development of IgE-mediated immediate hypersensitivity to a previously tolerated food following its avoidance for eosinophilic gastrointestinal diseases. J allergy Clin Immunol Pract. 6(2):649–50. [DOI] [PubMed] [Google Scholar]
- 124.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420–9. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen T, Ramsey D, Graham D, Shaib Y, Shiota S, Velez M, et al. The Prevalence of Helicobacter pylori Remains High in African American and Hispanic Veterans. Helicobacter. 2015;20(4):305–15. [DOI] [PubMed] [Google Scholar]
- 126.Roberts SE, Morrison-Rees S, Samuel DG, Thorne K, Akbari A, Williams JG. Review article: The prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol Ther. 2016;43(3):334–45. [DOI] [PubMed] [Google Scholar]
- 127.Ronkainen J, Talley NJ, Aro P, Storskrubb T, Johansson SE, Lind T, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: The population-based Kalixanda study. Gut. 2007;56(5):615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elitsur Y, Alrazzak BA, Preston D, Demetieva Y. Does Helicobacter pylori Protect against Eosinophilic Esophagitis in Children? Helicobacter. 2014;19(5):367–71. [DOI] [PubMed] [Google Scholar]
- 129.Von Amim U, Wex T, Link A, Messerschmidt M, Venerito M, Miehlke S, et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43(7):825–30. [DOI] [PubMed] [Google Scholar]
- 130.Sonnenberg A, Dellon ES, Turner KO, Genta RM. The influence of Helicobacter pylori on the ethnic distribution of esophageal eosinophilia. Helicobacter. 2017;22(3). [DOI] [PubMed] [Google Scholar]
- 131.Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH GR. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011; 141(5): 1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furuta K, Adachi K, Aimi M, Ishimura N, Sato S, Ishihara S, et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr. 2013;53(1):60–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blosse A, Lehours P, Wilson KT, Gobert AP. Helicobacter: Inflammation, immunology, and vaccines. Helicobacter [Internet]. 2018;23:e12517 Available from: http://doi.wiley.com/10.1111/hel.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011; 121(8):3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Molina-Infante J, Gutierrez-Junquera C, Savarino E, Penagini R, Modolell I, Bartolo O, et al. Helicobacter pylori infection does not protect against eosinophilic esophagitis: results from a large multicenter case-control study. Am J Gastroenterol. 2018;113(7):972–9. [DOI] [PubMed] [Google Scholar]
- 136.Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local esophageal cytokine expression profde in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sherrill JD, Kiran KC, Blanchard C, Stucke EM, Kemme KA, Collins MH, Abonia JP, Putnam PE, Mukkada VA, Kaul A, Kocoshis SA, Kushner JP, Plassard AJ, Karns RA, Dexheimer PJ, Aronow BJ RM. Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes Immun. 2014; 15(6):361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012; 129(6): 1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lucendo AJ. Meta-Analysis-Based Guidance for Dietary Management in Eosinophilic Esophagitis. Curr Gastroenterol Rep. 2015;17(10). [DOI] [PubMed] [Google Scholar]
- 140.Markowitz JE, Spergel JM, Ruchelli E LC. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98(4):777–82. [DOI] [PubMed] [Google Scholar]
- 141.Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy Eur J Allergy Clin Immunol. 2010;65(1): 109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajan J, Newbury RO, Anilkumar A, Dohil R, Broide DH, Aceves SS. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol. 2016;137(1):147–156.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Fluticasone Propionate for Pediatric Eosinophilic Esophagitis. Gastroenterology. 2006; 131 (5): 1381–91. [DOI] [PubMed] [Google Scholar]
- 144.Noel RJ, Putnam PE, Collins MH, Assa’ad AH, Guajardo JR, Jameson SC, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2(7):568–75. [DOI] [PubMed] [Google Scholar]
- 145.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone inpatients with eosinophilic esophagitis. Gastroenterology. 2014; 147(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, Schoepfer A SH. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9(5):400–9.e1. [DOI] [PubMed] [Google Scholar]
- 147.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: A potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102(10):2271–9. [DOI] [PubMed] [Google Scholar]
- 148.Caldwell JM, Paul M, Rothenberg ME. Novel immunologic mechanisms in eosinophilic esophagitis. Curr Opin Immunol. 2017;48:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zarowiecki M, Berriman M. What helminth genomes have taught us about parasite evolution. Parasitology. 2015;142:S85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Scott IC, Rees DG, Cohen ES. New perspectives on IL-33 and IL-1 family cytokines as innate environmental sensors. Biochem Soc Trans. 2018;46(5): 1345–53. [DOI] [PubMed] [Google Scholar]
- 151.Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. 2018;19(4):375–85. [DOI] [PubMed] [Google Scholar]
- 152.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9(3):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Farhan RK, Vickers MA, Ghaemmaghami AM, Hall AM, Barker RN, Walsh GM. Effective antigen presentation to helper T cells by human eosinophils. Immunology. 2016;149(4):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Svensson L, Rudin A, Wennerås C. Allergen extracts directly mobilize and activate human eosinophils. Eur J Immunol. 2004;34(6):1744–51. [DOI] [PubMed] [Google Scholar]
- 155.Machado DC, Horton D, Harrop R, Peachell PT, Helm BA. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur J Immunol. 1996;26(12):2972–80. [DOI] [PubMed] [Google Scholar]
- 156.Liang G, Barker T, Xie Z, Charles N, Rivera J, Druey KM. Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel T H2 immunity. J Allergy Clin Immunol. 2012;129(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39(5):976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Masilamani M, Commins S, Shreffler W. Determinants of Food Allergy. Immunol Allergy Clin North Am. 2012;32(1): 11–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chapman MD, Wunschmann S, Pomés A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep. 2007;7(5):363–7. [DOI] [PubMed] [Google Scholar]
- 160.Reed CE, Kita H The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114(5):997–1008. [DOI] [PubMed] [Google Scholar]
- 161.Borisov IN, Zashikhin AL. Specific secretory inclusions in the pyloric part of the mouse stomach. Bull Exp Biol Med. 1978;86(6):1672–4. [PubMed] [Google Scholar]
- 162.Pesek RD, Rettiganti M, O’Brien E, Beckwith S, Daniel C, Luo C, et al. Effects of allergen sensitization on response to therapy in children with eosinophilic esophagitis. Ann Allergy, Asthma Immunol. 2017; 119(2): 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129(3):985–94. [DOI] [PubMed] [Google Scholar]
- 164.John RJ, Rusznak C, Ramjee M, Lamont AG, Abrahamson M, Hewitt EL. Functional effects of the inhibition of the cysteine protease activity of the major house dust mite allergen Der p 1 by a novel peptide-based inhibitor. Clin Exp Allergy. 2000;30(6):784–93. [DOI] [PubMed] [Google Scholar]
- 165.Schulz O, Sewell HF, Shakib F. Proteolytic Cleavage of CD25, the α Subunit of the Human T Cell Interleukin 2 Receptor, by Der p 1, a Major Mite Allergen with Cysteine Protease Activity. J Exp Med. 1998; 187(2):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rochman M, Travers J, Miracle CE, Bedard MC, Wen T, Azouz NP, et al. Profound loss of esophageal tissue differentiation in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;140(3):738–749.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Azouz NP, Ynga-Durand MA, Caldwell JM, Jain A, Rochman M, Fischesser DM, et al. The antiprotease SPINK7 serves as an inhibitory checkpoint for esophageal epithelial inflammatory responses. Sci Transl Med. 2018; 10(444). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rochman M, Azouz NP, Rothenberg ME. Epithelial origin of eosinophilic esophagitis. J Allergy Clin Immunol 2018;142(1): 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schleimer RP, Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. 2017;139(6):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann NY Acad Sci. 2017;1397(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–85. [DOI] [PubMed] [Google Scholar]
- 172.Hill DA.; Faubion W. The Intestinal Immune System During Homeostasis and Inflammatory Bowel Disease. Pediatric inflammatory bowel disease: Third Edition 3rd ed. Mamula P, Grossman A, Baldassano R, Kelsen JMJ, editor. Pediatric Inflammatory Bowel Disease: Third Edition. Springer International Publishing; 2017. [Google Scholar]
- 173.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–6. [DOI] [PubMed] [Google Scholar]
- 174.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet. 2013;45(10): 1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McAleer MA, Pohler E, Smith FJD, Wilson NJ, Cole C, Macgowan S, et al. Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin. J Allergy Clin Immunol. 2015;136(5):1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139(1):72–81.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Simon D, Page B, Vogel M, Bussmann C, Blanchard C, Straumann A, et al. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid-treated eosinophilic esophagitis. Allergy Eur J Allergy Clin Immunol. 2018;73(1):239–47. [DOI] [PubMed] [Google Scholar]
- 179.Kc K, Rothenberg ME SJ. In vitro model for studying esophageal epithelial differentiation and allergic inflammatory responses identifies keratin involvement in eosinophilic esophagitis. PLoS One. 2015; 10(6):e0127755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7(3):718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Van Rhijn BD, Verheij J, Van Den Bergh Weerman MA, Verseijden C, Van Den Wijngaard RMJGJ, De Jonge WJ, et al. Histological Response to Fluticasone Propionate in Patients with Eosinophilic Esophagitis is Associated with Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol. 2015; 110(9): 1289–97. [DOI] [PubMed] [Google Scholar]
- 182.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: Transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007; 120(6): 1292–300. [DOI] [PubMed] [Google Scholar]
- 183.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132(4):789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy Eur J Allergy Clin Immunol. 2014;69(5):674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Venturelli N, Lexmond WS, Ohsaki A, Nurko S, Karasuyama H, Fiebiger E, et al. Allergic skin sensitization promotes eosinophilic esophagitis through the IL-33-basophil axis in mice. J Allergy Clin Immunol. 2016; 138(5): 1367–1380.e5. [DOI] [PubMed] [Google Scholar]
- 186.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon H-U, et al. Treatment of Patients with the Hypereosinophilic Syndrome with Mepolizumab. N Engl J Med. 2008;358(12): 1215–28. [DOI] [PubMed] [Google Scholar]
- 187.Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010; 125(6): 1336–43. [DOI] [PubMed] [Google Scholar]
- 188.Busse WW. Biological treatments for severe asthma. Curr Opin Allergy Clin Immunol [Internet]. 2018;18(6):509–18. Available from: http://insights.ovid.com/crossref?an=00130832-201812000-00010 [DOI] [PubMed] [Google Scholar]
- 189.Hamelmann E, Cieslewicz G, Schwarze J, Ishizuka T, Joetham A, Heusser C, et al. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am J Respir Crit Care Med. 1999;160(3):934–41. [DOI] [PubMed] [Google Scholar]
- 190.Lee JJ, Dimina D, Macias MMP, Ochkur SI, McGarry MP, O’Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science (80-). 2004;305(5691):1773–6. [DOI] [PubMed] [Google Scholar]
- 191.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH GC. A critical role for eosinophils in allergic airways remodeling. Science (80-). 2004;305(5691): 1776–9. [DOI] [PubMed] [Google Scholar]
- 192.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006; 118(6): 1312–9. [DOI] [PubMed] [Google Scholar]
- 193.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. [DOI] [PubMed] [Google Scholar]
- 194.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG AS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–604. [DOI] [PubMed] [Google Scholar]
- 195.Kuang FL, Alao H, Kumar S, Powers A, Quezado M, Wang Z et al. Benralizumab (anti-IL5Ra) depletes gut tissue eosinophilia and improves symptoms in hypereosionphilic syndrome with gastrointestinal involvement. J Allergy Clin Immunol. 2018;141(AB):196. [Google Scholar]
- 196.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2). [DOI] [PubMed] [Google Scholar]
- 197.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa G Della, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2): 184–90. [DOI] [PubMed] [Google Scholar]
- 198.Bernstein JA, Kavati A, Tharp MD, Ortiz B, MacDonald K, Denhaerynck K, et al. Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic/spontaneous urticaria: a systematic review of ‘real-world’ evidence. Expert OpinBiol Ther. 2018;18(4):425–48. [DOI] [PubMed] [Google Scholar]
- 199.Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–9. [DOI] [PubMed] [Google Scholar]
- 201.Mishra A, Rothenberg ME. Intratracheal IL-13 Induces Eosinophilic Esophagitis by an IL-5, Eotaxin-1, and STAT6-Dependent Mechanism. Gastroenterology. 2003;125(5):1419–27. [DOI] [PubMed] [Google Scholar]
- 202.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135(2):500–7. [DOI] [PubMed] [Google Scholar]
- 203.Mishra A, Rothenberg ME (2003) Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 125(5):1419–1427 [DOI] [PubMed] [Google Scholar]
- 204.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson KA, Chehade M et al. Dupilumab efficacy and safety in adult patients with active eosinophilic esophagitis: a randomized double-blind placebo-controlled phase 2 trial. Am Coll Gastroenterol Natl Meet Orlando. 2017;October. [Google Scholar]
- 205.Berin MC, Grishin A, Masilamani M, Leung DYM, Sicherer SH, Jones SM, et al. Egg-specific IgE and basophil activation but not egg-specific T-cell counts correlate with phenotypes of clinical egg allergy. J Allergy Clin Immunol. 2018. July;142(1): 149–158.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cianferoni A, Ruffner MA, Guzek R, Guan S, Brown-Whitehorn T, Muir A, et al. Elevated expression of activated TH2 cells and milk-specific TH2 cells in milk-induced eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;120(2):177–183.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71(5):611–20. [DOI] [PubMed] [Google Scholar]
- 208.Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon H-U, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013. December;145(6): 1230-6.e1–2. [DOI] [PubMed] [Google Scholar]
- 209.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155(4):1022–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ruffner MA, Capucilli P, Hill DA, Spergel !M. Screening children for eosinophilic esophagitis: allergic and other risk factors. Expert Rev Clin Immunol. 2019. February 5;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]