Abstract
An estimated 466 million people suffer from hearing loss worldwide. Sensorineural hearing loss is characterized by degeneration of key structures of the sensory pathway in the cochlea such as the sensory hair cells, the primary auditory neurons and their synaptic connection to the hair cells – the ribbon synapse. Various strategies to protect or regenerate these sensory cells and structures are the subject of intensive research. Yet despite recent advances in our understandings of the capacity of the cochlea for repair and regeneration there are currently no pharmacological or biological interventions for hearing loss. Current research focusses on localized cochlear drug, gene and cell-based therapies. One of the more promising drug-based therapies is based on neurotrophic factors for the repair of the ribbon synapse after noise exposure, as well as preventing loss of primary auditory neurons and regrowth of the auditory neuron fibers after severe hearing loss. Drug therapy delivery technologies are being employed to address the specific needs of neurotrophin and other therapies for hearing loss that include the need for high doses, long-term delivery, localised or cell-specific targeting and techniques for their safe and efficacious delivery to the cochlea. Novel biomaterials are enabling high payloads of drugs to be administered to the cochlea with subsequent slow-release properties that are proving to be beneficial for treating hearing loss. In parallel, new gene therapy technologies are addressing the need for cell specificity and high efficacy for the treatment of both genetic and acquired hearing loss with promising reports of hearing recovery. Some biomaterials and cell therapies are being used in conjunction with the cochlear implant ensuring therapeutic benefit to the primary neurons during electrical stimulation. This review will introduce the auditory system, hearing loss and the potential for re pair and regeneration in the cochlea. Drug delivery to the cochlea will then be reviewed, with a focus on new biomaterials, gene therapy technologies, cell therapy and the use of the cochlear implant as a vehicle for drug delivery. With the current pre-clinical research effort into therapies for hearing loss, including clinical trials for gene therapy, the future for the treatment for hearing loss is looking bright.
Keywords: Sensorineural hearing loss, biomaterials, gene therapy, cell-based therapy, drug delivery, cochlea, cochlear implant
1. Introduction
Over 5% of the world’s population is living with a disabling hearing loss impacting on the individual’s ability to communicate with others (WHO 2018). Despite being a very important part of our lives, hearing is often taken for granted until it begins to deteriorate. The severity of the pathology ranges from loss of the synapse between the hair cells that convert sound input into electrical signals and the spiral ganglion neurons (SGNs) that transmit the electrical signals to the brain, to more severe cases with loss of hair cells and loss of SGNs. There are currently very few treatment options for people with hearing loss. Hearing aids may be used to amplify sounds for people with a mild or moderate hearing loss whereas cochlear implants are suitable for people with more significant hearing loss. While hearing aids and cochlear implants partially reverse the symptoms of hearing loss they do not restore the underlying pathology: cellular degeneration in the cochlea and loss of connection between the cochlea and the central auditory processing areas of the brain. At the pre-clinical level there is mounting evidence of therapeutic protection and/or regeneration of hair cells, SGNs and other affected cell types with numerous examples of hearing protection and hearing recovery. Therapeutic strategies focus on overcoming some of the obstacles of providing safe and efficacious localised drug delivery to the cochlea. This review will provide an overview of the auditory system, hearing loss, capacity for repair and regeneration and the current status of targeted therapies for drug delivery to the cochlea.
2. Normal cochlear anatomy and function
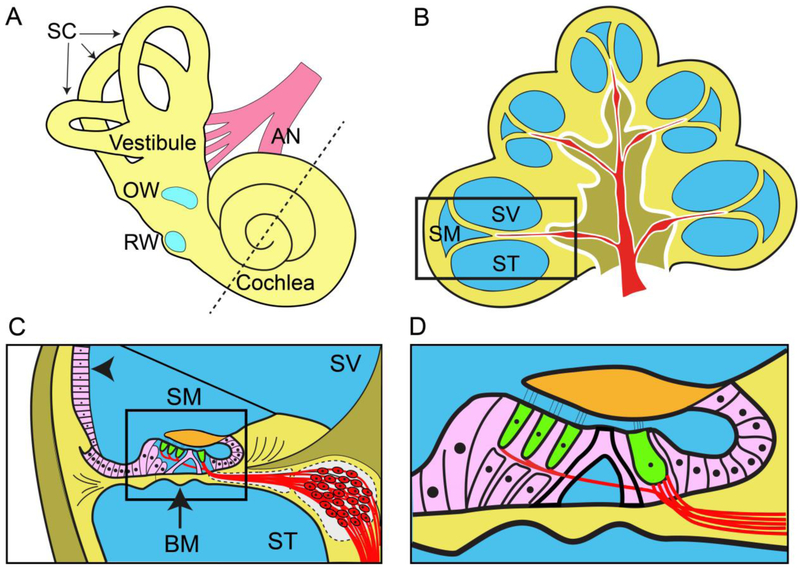
The peripheral auditory system consists of the external, middle and inner ear - or cochlea. The external auditory canal provides efficient transmission of the acoustic stimulus to the tympanic membrane or ear drum. The middle ear cavity, bound by the tympanic membrane laterally and the cochlea medially, efficiently couples sound energy from the air-filled external auditory canal to the fluid filled cochlea via the ossicular chain consisting of the malleus, incus and stapes (Peake and Rosowski 1991). Fluid in the cochlea is displaced by movement of the stapes onto the oval window located in the base of the cochlea (Figure 1A). The round window membrane, also located near the base of the cochlea, vibrates in opposite phase to the oval window as the fluid in the cochlea is displaced in response to acoustic stimulation. The round window provides a site for surgical access to the cochlea for both cochlear implants and drug delivery, as discussed later in the review, but the potential risks of additional hearing loss from targeting this membrane must be carefully considered when developing safe and effective drug delivery therapies (Figure 1A).
Figure 1.
(A) Schematic image of the inner ear depicting the three semi-circular canals (SC), the oval window (OW), the round window (RW) and the auditory nerve (AN). Drugs can be delivered to the inner ear via the semi-permeable round and oval windows, or directly into the semi-circular canals or cochlea via a small hole drilled through the bony wall or by piercing the round window. The dotted line bisects the cochlea in half which is depicted schematically in (B). (B) Transverse section of the human cochlea illustrating the three fluid-filled cochlear ducts shown in blue - scala vestibuli (SV), scala media (SM), and scala tympani (ST) spiralling around the modiolus containing the auditory nerve (red). The boxed region is shown in (C) in greater detail. (C) The organ of Corti (boxed) is located on the basilar membrane (BM, arrow) that separates the scala tympani from the scala media. The lateral wall of the scala media contains the stria vascularis (arrow head) which is responsible for the generation of the endocochlear potential. The SGN soma (red) are located in a small bony canal (Rosenthal’s canal) that also spirals from the cochlear base to its apex. (D) The organ of Corti contains the two types of sensory hair cells - a single row of inner hair cells and three rows of outer hair cells (green) - together with supporting cells (pink). Peripheral fibres from spiral ganglion neurons (red) form synapses with the inner and outer hair cells.
The cochlea is a spiral shaped labyrinth embedded within the temporal bone. Three fluid-filled scalae form the membranous labyrinth (Figure 1B). The basilar membrane separates the scala tympani from the scala media while Reissner’s membrane separates the scala media from the scala vestibuli (Figure 1C). Both the scala tympani and scala vestibuli contain perilymph, an extracellular fluid similar to cerebrospinal fluid (Salt and Hirose 2018). In contrast, the scala media contains endolymph, a unique extracellular fluid which has a high concentration of potassium and a low concentration of sodium, a composition that is important for the normal process of transduction in the hair cells (Smith et al. 1954). The cells lining the perimeter of the scala media are joined by interce11ular tight junctions that maintain the unique electrolyte composition of endolymph (Nadol 1979). In the majority of cases, cochlear therapy techniques focus on delivery of a drug to the perilymph. In humans, the total perilymph volume is approximately 158 mm3 which includes the cochlea and semi-circular canals as there is free passage of fluids between these organs. The endolymph is sometimes targeted for therapies, with its volume being just 34 mm3 (Buckingham and Valvassori 2001, Glueckert et al. 2018).
The sensory cells of the cochlea are located within the organ of Corti, a complex structure attached to the basilar membrane (Figure 1D). In the human, the basilar membrane is approximately 32 mm in length from the cochlea base to the apex. The mechanical characteristics of the basilar membrane allow the cochlea to resolve different frequencies at different sites along its length; high frequencies in the base of the cochlea close to the middle ear, and lower frequencies at progressively more distal sites within more apical regions of the cochlea (Liberman 1982).
The sensory hair cells consist of a single row of inner hair cells and three rows of outer hair cells (Figure 1D). The human cochlea contains approximately 3200 inner hair cells and 10,000 outer hair cells (Schuknecht 1974a). Both inner and outer hair cells contain up to 150 stereocilia protruding from their apical surface. On displacement of the basilar membrane, mechanically sensitive ion channels located in the stereocilia initiate depolarisation of the hair cell. This in turn results in the release of the excitatory neurotransmitter glutamate, depolarizing primary afferent neurons innervating the hair cell - the SGNs (Guinan et al. 2012, Patuzzi and Robertson 1988). Both the hair cells and the SGNs are the most common target cell types for therapies to prevent or reverse hearing loss.
Inner and outer hair cells are innervated by specific SGN populations; the difference in their afferent innervation reflects the different roles these two hair cell types play in the transduction process. Type I SGNs, which represent ~90% of the total SGN population, exclusively innervate inner hair cells while Type II SGNs exclusively innervate outer hair cells (Lorente de Nó 1933, Ryugo et al. 1991, Spoendlin 1978). The two hair cell populations play different and unique roles in auditory perception; inner hair cells convey auditory stimuli to the central auditory pathway via their innervation with the primary SGN population while outer hair cells provide biological amplification within the cochlea that increases hearing sensitivity to low intensity stimuli (Patuzzi and Robertson 1988). The central fibres of the SGNs form the auditory nerve and projects to the cochlear nucleus - the first relay centre within the central auditory pathway (Lorente de Nó 1933). There are approximately 30,000 SGNs in a normal human cochlea (Spoendlin 1984).
The peripheral fibres of both Type I and II SGNs project from their soma in Rosenthal’s canal, through a small opening in the bony wall called the habenula perforata, to the organ of Corti. Each Type I peripheral fibre makes a single synaptic connection with the closest inner hair cell. This synapse, known as a ribbon synapse, promotes the rapid release of neurotransmitter and can maintain high firing rates important for the temporal fidelity of sound processing (Griesinger et al. 2005). In contrast, Type II peripheral fibres cross the floor of the organ of Corti and synapse with multiple outer hair cells (Spoendlin 1984). The ribbon synapses in the inner hair cells have recently been identified as sensitive to even moderate noise exposure, preferentially affecting high-threshold neurons and is therefore also a specific target for drug therapies in addition to hair cells and SGNs more generally (Kujawa and Liberman 2009, Lin et al. 2011c, Liu et al. 2012a, Shi et al. 2013), as outlined later in the review.
3. Deafness
3.1. Impact on people.
Over 432 million adults and 34 million children have a disabling hearing loss, making it a major health and economic burden on society (WHO 2018). Hearing impairment can result in significant communication disorders including poor development of spoken and written language in children, leading to significant educational, social and vocational ramifications that can adversely affect quality of life. In adults, hearing loss affects employment, income and social interaction and selfesteem (Theunissen et al. 2014, Wilson et al. 2017). Moreover, hearing loss is also a potential risk factor for accelerated cognitive decline and cognitive impairment in the elderly associated with increased social isolation (Curhan et al. 2019, Jayakody et al. 2018b, Lin et al. 2011a, Lin et al. 2013, Tay et al. 2006). There are also links to the development of depression and its impact on the individual (Jayakody etal. 2018a, Jayakody etal. 2018b, Livingston etal. 2017, Thomson etal. 2017).
3.2. Rehabilitation
There are two forms of deafness; conductive and sensorineural. Conductive deafness is a mechanically based hearing loss associated with the external or middle ear. It can occur as a result of middle ear infections or ossification of middle ear bones that affect the capacity of the auditory structures to transmit sound information to the inner ear. It is typically a partial hearing loss and surgery and/or amplification via a hearing aid are the standard treatments. Sensorineural hearing loss (SNHL) is the most common form of deafness and typically occurs following damage to, or dysfunction of, the sensory hair cells within the inner ear (Schuknecht 1974b), their synapse with primary auditory neurons (termed synaptopathy) (Kujawa and Liberman 2015) or the stria vascularis (Wangemann 2006). Extensive hair cell damage can result in a hearing loss that encompasses the important frequency range for human speech (~300 Hz-8 kHz). While a mild SNHL can be clinically managed using hearing aids, people with severe-to-profound SNHL may benefit from a cochlear implant which uses electrical stimulation to activate the auditory nerve. Cochlear implants enable recipients to understand speech in good listening conditions but results can vary considerably and performance in challenging or noisy environments is typically poor (Roche and Hansen 2015, Wilson and Dorman 2008).
Auditory rehabilitation, including the use of hearing aids and cochlear implants, results in positive improvements in terms of educational outcomes in children (Leigh et al. 2013, Nikolopoulos and Vlastarakos 2010, Verhaert et al. 2008) as well as a decline in social isolation, depression and cognitive deterioration in the elderly (Castiglione et al. 2016). However, a perceived stigma of hearing loss and their limited effectiveness in challenging environments often prevents people from taking up these technologies (Wilson et al. 2017). The implementation of drug therapies for hearing loss has the potential to not only reach a greater portion of affected individuals but also to improve the outcomes when used in conjunction with devices.
3.3. Causes of SNHL
SNHL may have hereditary causes or may be acquired as a result of infection or injury, long-term exposure to loud noise or drugs that are toxic to hair cells. There is also a strong correlation between ageing and hearing loss suggesting that many factors increase the risk or susceptibility to SNHL later in life (Gates and Mills 2005). As this review will discuss, the aetiology of hearing loss has a major impact on the potential for repair and regeneration and shapes the development of technologies for the treatment of hearing loss.
3.3.1. Genetic factors
Over 60% of cases of SNHL can be attributed to a genetic cause (Marazita et al. 1993), classified as non-syndromic (most common) or syndromic conditions affecting auditory function at different sites along the auditory transduction pathway (for review see (Angeli et al. 2012)). For instance, mutations to cochlear gap junctions is a common cause of genetic hearing loss, in particular connexin 26 that enables the passage of potassium ions during normal cochlear function (Estivill et al. 1998). Indeed, connexin 26 mutations are responsible for ~50% of cases of non-syndromic hearing loss (Wingard and Zhao 2015). Other causes of genetic hearing loss include mutations to genes important for hair cell function, for example the structural protein Myo7A (Weil et al. 1995), or to genes that encode for transmembrane channels essential for mechanotransduction in the hair cell stereocillia such as Transmembrane channel-like protein 1 (TMC1) (Pan et al. 2013).
3.3.2. Infections and injury
Hearing loss can result from infections contracted or injuries sustained at any stage of life. Viral infections such as cytomegalovirus are a major cause of congenital hearing loss (Goderis et al. 2014) and traumatic injuries to the temporal bone may cause significant hearing loss. Diseases such as auditory nerve neuromas may require surgical intervention that can have significant impact on hearing.
3.3.3. Ageing
Hearing loss associated with ageing, termed presbycusis, can have significant implications to heathy ageing. Disentangling the specific effects of ageing on cochlear health can be problematic with life-long environmental factors such as exposure to noise in combination with genetic predisposition to hearing loss making the task difficult (Kujawa and Liberman 2006). Nevertheless, there is a clear link between progression of hearing loss and ageing with nearly two thirds of people over 70 having significant hearing impairment as assessed by standard audiometry testing (Lin et al. 2011b). Typically hearing loss is symmetrical with a progressive loss of hearing sensitivity over time. The characteristics of hearing loss are variable between people. Similar to the effects of excitotoxic injury from noise, it is likely that presbycusis manifests as a combination of loss of hearing sensitivity brought about by loss of ribbon synapses, hair cells and SGNs. Animal models of presbycusis can exhibit significant SGN loss without appreciable hair cell loss (Sergeyenko et al. 2013, Viana et al. 2015) suggesting that auditory neuropathy is a likely contributor to the loss of hearing function in humans (Makary et al. 2011). Dysfunction to cochlear homeostasis, brought about by metabolic changes that affect the stria vascularis lining the outer wall of the scala media, are also likely to contribute to age-related hearing loss (Shi 2016).
3.3.4. Noise exposure
Noise exposure can result in a temporary loss of hearing sensitivity that can resolve over days and weeks following the noise exposure. This temporary threshold shift was previously thought to reflect temporary changes to the structure of the outer hair cell stereocilia, the tectorial membrane and other changes in the organ of Corti (Nordmann et al. 2000). More recently, evidence has begun to emerge that more permanent changes occur in the cochlea despite a return to normal hearing thresholds (Ryan et al. 2016). As noted above, this phenomenon has been called synaptopathy or hidden hearing loss (Schaette and McAlpine 2011) as the deficit in hearing cannot be detected in common audiometry testing where hearing thresholds are monitored at specific frequencies using a pure tone audiogram. The mechanism for hidden hearing loss is thought to be the loss of synaptic connections between the SGN peripheral fibres and the inner hair cells (Kujawa and Liberman 2009, 2015, Lin et al. 2011c, Valero et al. 2017). In particular, SGNs with higher activation thresholds are more susceptible to damage while the low threshold neurons are more resilient to this noise induced damage (Furman et al. 2013). The resulting outcome is that hearing sensitivity is maintained but the capacity to process auditory information in more challenging environments is impacted due to the loss of information provided by the higher threshold neurons. There is degradation in the capacity to process temporal information of suprathreshold input. This type of deficit can manifest itself as difficulty in understanding speech in challenging sound environments such as a noisy restaurant. In terms of the impact on the SGNs, significant loss of synaptic contact can result in ongoing and progressive degeneration of the nerve fibres and ultimately death of the SGN (Kujawa and Liberman 2006), irrespective to the survival of the inner hair cells (Liberman 2017).
Longer noise exposure or exposure to louder noise can lead to excitotoxic hair cell damage and loss due to excessive glutamate release at ribbon synapses (Pujol and Puel 1999). There is a release of reactive oxygen species, increases in calcium levels in cochlear hair cells and a cascade of intracellular events within the hair cell leading to functional damage and death via apoptotic or necrotic mechanisms in the days to weeks following damaging noise exposure (Ping Yang et al. 2004). Local cochlear inflammation and the upregulation of pro-inflammatory cytokines also occur following noise exposure (Tan et al. 2016). Excessive noise exposure can also result in mechanical damage to the organ of Corti such as rupture of the reticular lamina that can allow the endolymph and perilymph to mix leading to further damage of the hair cells, supporting cells and nerve fibres (Bohne et al. 2017). The outer hair cells are typically damaged and lost prior to the loss of the inner hair cells (Chen and Fechter 2003).
3.3.5. Ototoxic drugs
Cochlear hair cells are sensitive to exposure to ototoxic drugs such as platinum-based chemotherapeutics (e.g. cisplatin) and aminoglycoside antibiotics (e.g. neomycin and kanamycin). Cisplatin is a chemotherapeutic agent widely used in the treatment of several forms of cancer. Ototoxicity is a common adverse side effect associated with the administration of this drug, particularly affecting the higher frequencies (Anniko and Sobin 1986). Cisplatin enters the hair cell cytoplasm mainly via mammalian copper transport 1 and organic cationic transporter, where it Increases the oxidative stress of the cell promoting apoptotic and necrotic cell death (Sheth et al. 2017). It is anticipated that targeting these transporters and/or the application of antioxidants locally within the cochlea would reduce the incidence of hearing loss while maintaining the efficacy of cisplatin as a chemotherapy agent (Sheth et al. 2017).
The broad-spectrum antibacterial activity of aminoglycoside drugs are used to treat Gram-negative bacterial infections and are widely used throughout the world, often as a last resort option. The ototoxic compounds enter the cochlea via the stria vascularis and then enter into the apical surface of hair cells via the mechanotransduction channels located in the stereocilia. The compounds affect metabolic function of the hair cells by damaging mitochondrial ribosomes leading to the production of oxygen free radicals and initiating cell death mechanisms (Forge and Schacht 2000). In animal models ototoxic drugs can result in profound deafness and elimination of all cochlear hair cells. The ototoxic drugs can directly affect the cochlear synapses, causing rapid loss of the ribbon synapse and subsequent retraction of the peripheral nerve fibres (Liu et al. 2013). The peripheral nerve fibres degenerate and retract within their myelin sheath followed by the breakdown and loss of myelin sheath (Wise et al. 2017).
3.3.6. Strial/metabolic hearing loss
The stria vascularis, located in the scala media (Figure 1C), maintains the high potassium concentration (150 nM K+) of the endolymph via the functioning of ion pumps, producing the highly positive endocochlear potential (+80 to +100 mV). There are three main layers to the stria vascularis composed of marginal, intermediate and basal cells. Any dysfunction of the endocochlear potential could impact on hair cell depolarisation in response to acoustic input with the overall effect of reduction in hearing sensitivity. For example, mutations in the potassium channel subunit KCNQ1 in marginal cells disrupt the endocochlear potential and cause severe congenital deafness; Jervell and Lange-Nielsen syndrome (Jervell and Lange-Nielsen 1957). Strial dysfunction is also thought to contribute to age related hearing loss (Kujawa and Liberman 2019) with the cochlear apex (low frequency) less susceptible to changes in the endocochlear potential compared to the basal (high frequency) region. Consequently the loss in hearing sensitivity due to strial dysfunction is likely to result in a gradual reduction in hearing sensitivity in the hearing frequency range (Dubno et al. 2013, Heeringa and Koppl 2019).
3.3.7. Loss of residual hearing with cochlear implantation
The indication criteria for cochlear implantation have expanded over the last decade to include patients with useable low frequency hearing. Typically, patients that combine the residual low frequency acoustic hearing with the high frequency electric hearing from the cochlear implant have superior performance compared to profoundly deaf patients (Gantz et al. 2005). A significant concern and risk for these patients is the loss of residual hearing that can occur following cochlear implant surgery. The loss can occur immediately post-surgery (Adunka et al. 2006), presumably due to factors such as altered cochlear mechanics, acute trauma or immediate inflammatory effects of implantation (Fayad and Linthicum 2006, Fayad et al. 2009, Lee et al. 2010, Nadol and Eddington 2006, Nadol et al. 2001). However, some patients can lose residual hearing months following implantation via mechanisms that are not well understood. Additional research is required to elucidate the causes of delayed loss of residual hearing. Other research has shown correlations between the extent of fibrous tissue in the cochlea (especially new bone growth), the population of remaining auditory neurons and cochlear implant performance (Kamakura and Nadol 2016).
3.4. Potential for repair or regeneration
Given the diversity of causes of hearing loss, the pathology in the cochlea will also be extremely varied in terms of cells affected and the severity of damage. Damaged cells and debris are eliminated by resident and migrating macrophages (Hirose et al. 2017). If damage is severe and widespread, a complete loss of the sensory epithelium can occur resulting in a so-called flattened sensory epithelium. Such profoundly damaged cochleae represent a potentially insurmountable obstacle for regenerative therapies; essentially trying to recapitulate the highly structured and complex sensory epithelium from scratch (Taylor et al. 2012). Consequently, research efforts have been focused towards the development of protective therapies and therapies designed to return hearing function in cochleae that have not sustained profound sensory cell loss. The main targets for these therapies are the hair cells, SGNs and the ribbon synapse.
3.4.1. Hair cells
It has long been known that hair cells in birds and non-mammalian vertebrates have the capacity to regenerate following damage (Balak et al. 1990, Corwin and Cotanche 1988, Ryals and Rubel 1988). Regeneration occurs via either direct transdifferentiation, whereby neighbouring supporting cells are converted to hair cells, or by mitotic regeneration in which supporting cells divide and are subsequently converted to a hair cell. Conversely, in the mature mammalian auditory system, spontaneous regeneration of auditory hair cells following damage to the sensory epithelium is extremely limited and short lived (Cox et al. 2014). Attempts to induce hair cell regeneration in the mature mammalian cochlea by cellular reprogramming have had varied success. Some studies have reported conversion of supporting cells to hair cells by increasing the expression of the Atoh1 gene, a basic helix-loop-helix transcription factor, in supporting cells via gene therapy or molecular techniques with associated recovery of hearing thresholds (Izumikawa et al. 2005, Kraft et al. 2013). Manipulation of the Notch signalling pathway is another method of increasing Atoh1 gene expression and this has also successfully regenerated hair cells in the cochlea with recovery of hearing after noise injury (Du et al. 2018, Mizutari et al. 2013, Yeh et al. 2018). Others have reported hair cell conversion that was either too limited, short-lived or incomplete to result in recovery of hearing (Atkinson et al. 2014, Izumikawa et al. 2008, Kawamoto et al. 2003, Kelly et al. 2012, Kuo et al. 2015, Liu et al. 2014, Liu et al. 2012b, Yang et al. 2013, Yang et al. 2012). Factors that may affect the efficacy of hair cell regeneration and hearing recovery include the degree of pathology in the cochlea, maturity of the organ of Corti or age-related epigenetic modifications, timing of the therapy and the expression dynamics of the transfected gene (Zhang etal. 2018). The sub-type of supporting cell being converted may also impact on outcomes with pillar and Deiters cells being more resistant to conversion (Walters etal. 2017). Newly regenerated hair cells have been noted in some cases to attract neuronal fibres and express synaptic markers suggesting that the hair cells can be reconnected (Gubbels et al. 2008, Kawamoto et al. 2003, Kuo et al. 2015, Walters et al. 2017). It is also noteworthy that recovery of vestibular function has also been observed for Atoh1 gene therapy following vestibular hair cell ablation as a result of vestibular hair cell regeneration (Schlecker et al. 2011, Staecker et al. 2007).
A clinical trial is underway to investigate the potential for Atoh1 gene therapy to restore hearing in patients with profound hearing loss ( NCT02132130). An adenovirus carrying the human Atohl transcription factor gene is being assessed for safety, tolerability and efficacy in patients with severe-to-profound hearing loss. An experimental dose of 20-60 μL is delivered via the intra-labyrinthine route in order to access the cochlear supporting cells in the sensory epithelium.
A single-factor approach to reprogramming of supporting cells may too simplistic (Jahan et al. 2015) with some reports of increased conversion and improved survival and function of converted hair cells with a combinatorial approach. Co-factors to the Atoh1 gene have included beta-catenin; a component of the Wnt signalling pathway (Kuo et al. 2015), transcription factors Gfi1 and Pou4f3 (Costa et al. 2015, Walters et al. 2017), deletion of p27KIP1; a factor associated with quiescence of hair cells and supporting cells (Walters etal. 2017) and the GATA3 transcription factor (Walters et al. 2017).
Direct transdifferentiation of supporting cells to hair cells depletes the supporting cell population that normally plays a critical mechanical role in auditory transduction and homeostasis. To overcome this, the supporting cell population may first require proliferation priorto trans-differentiation to hair cells (mitotic regeneration). Recent research has shown that the inner ear contains a distinct population of progenitor cells that can form new hair cells and neurons in culture (Oshima et al. 2007). These resident cells serve as hair cell precursors during development (Chai et al. 2012, Shi et al. 2012). By altering intracellular signalling pathways such as Wnt and Notch activation the progenitor cells can be ‘switched on’ and stimulated to divide and differentiate into cochlear hair cells (McLean et al. 2017). In explanted cochlear tissue depleted of hair cells by aminoglycosides, the addition of small molecules that activate Wnt and Notch signalling pathways resulted in an increase in the number of hair cells by stimulating the division and differentiation of Lgr5-positive cells without the loss of supporting cells that occurs with Atoh1 gene therapy (McLean et al. 2017).
3.4.2. SGNs
Following loss of hair cells, there is retraction of the SGN peripheral fibres. The SGN cell bodies within Rosenthal’s canal shrink before the loss of the neuronal cell body via apoptotic or necrotic processes (Wise et al. 2017). Interestingly, once the neuronal cell body has degenerated the cochlear Schwann cell that provided myelin to the SGN appears to alter its phenotype towards a non-myelinating form and interacts with remaining neurons (Wise et al. 2017). This dynamic behaviour of the remaining Schwann cells is likely to facilitate the capability of remaining neurons to resprout their peripheral fibres, which often occurs spontaneously after a cochlear insult such as aminoglycoside damage, but can be enhanced by the delivery of neurotrophic growth factors. In terms of functional changes in the deafened cochlea as a consequence of neural and myelin degeneration, changes in auditory nerve functioning in response to electrical stimulation are more subtle. Auditory neurons maintain the ability to respond to electrical stimuli, however, reductions in refractory periods and increases in electrical thresholds have been reported in long-term deafened animals (Shepherd et al. 2004) and myelin-deficient mice (Zhou et al. 1995).
The relative slow decline of SGNs (compared to hair cell loss) makes them an amenable target for drug therapies in the cochlea. Given that the number of SGNs is an important factor influencing post-implant hearing performance clinically (Kamakura and Nadol 2016, Seyyedi et al. 2014), it is important to develop effective strategies aimed at their rescue. There is a large body of work on the use of neurotrophins in the cochlea for the protection of SGNs, regrowth of the peripheral fibres and repair of the hair cell ribbon synapse (see Section 3.4.3. and review (Ramekers et al. 2012)).
3.4.3. Neurotrophins and their role in the treatment of hearing loss
Neurotrophins are a family of proteins that play a major role in both the development and maintenance of neurons in the central and peripheral nervous systems (Kashyap et al. 2018). During cochlear development, SGNs receive neurotrophic support, including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), from hair cells, supporting cells of the organ of Corti and second order cochlear nucleus neurons (Fritzsch et al. 1999, Schecterson and Bothwell 1994, Stankovic et al. 2004, Ylikoski et al. 1993). The high-affinity receptors for these neurotrophins (TrkB and TrkC, respectively) are expressed on SGNs (Schecterson and Bothwell 1994, Ylikoski et al. 1993). Importantly, the recent identification of BDNF and its high affinity receptor in developing human cochleae suggests a similar role in human SGNs (Johnson Chacko et al. 2017).
Both BDNF and NT-3 also play a role in the maintenance of SGNs in the adult inner ear (Qun et al. 1999, Ylikoski et al. 1993). Removal of this support leads to the gradual degeneration of SGNs via apoptotic cell death (Alam et al. 2007, Fritzsch et al. 1999). Significantly, the neuroprotective effects of exogenous neurotrophins on SGNs in vivo have been demonstrated (Gillespie et al. 2004, Leake et al. 2011, McGuinness and Shepherd 2005, Miller et al. 1997, Shinohara et al. 2002, Staecker et al. 1996) (Figure 2). Associated with this rescue effect is regrowth of peripheral SGN peripheral fibres compared with deafened controls (Budenz et al. 2015, Leake et al. 2011, Richardson et al. 2007, Wise et al. 2005), with implications in reducing excitation thresholds when electrically stimulated with a cochlear implant (Landry et al. 2013). Finally, exogenous neurotrophins have been shown to promote synaptic regeneration of the SGN peripheral fibres to the hair cell (i.e. the ribbon synapse) and rescue of hearing function in adult animals following acoustic trauma (Sly et al. 2016, Suzuki et al. 2016, Wan et al. 2014). While protective effects of neurotrophin administration have been observed for at least 2 weeks post-therapy (Agterberg et al. 2009, Sly et al. 2016), it appears that long-term exogenous neurotrophin delivery to the cochlea may be required for ongoing SGN protection (Gillespie et al. 2003). In contrast, promoting SGN peripheral fibres to re-synapse with sensory hair cells via exogenous neurotrophin delivery would probably not require long durations of therapy as the connection would presumably be maintained by the endogenous supply via the hair cell and supporting cells of the organ of Corti (Sly et al. 2016, Suzuki et al. 2016).
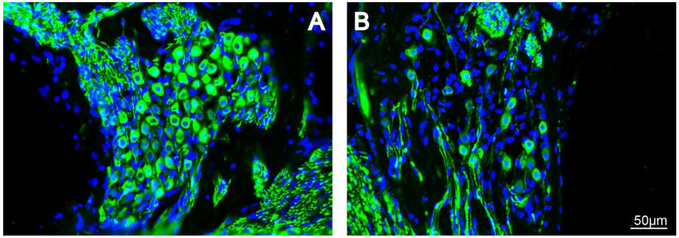
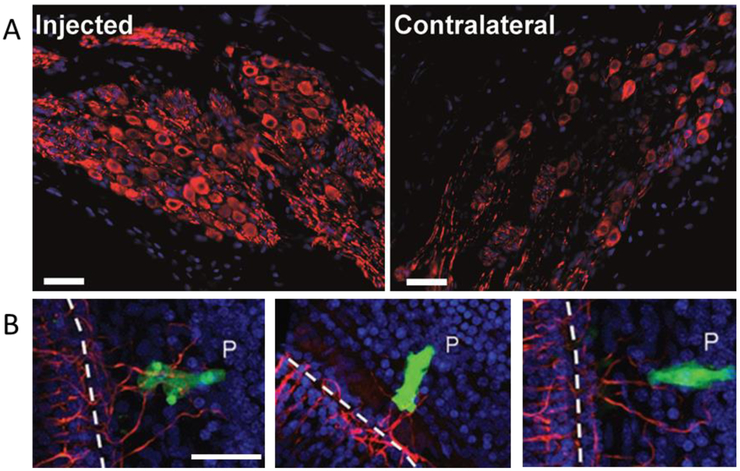
Figure 2.
Neurotrophin therapy results in SGN survival after hearing loss in guinea pigs. (A) An intracochlear BDNF therapy applied 1 week after ototoxic hearing loss maintains the survival of SGN cell bodies (green) in Rosenthal’s canal as well as the peripheral fibres over a 4 week period. (B) The SGN population deteriorates over 5 weeks in deafened guinea pigs that receive a control therapy (Wise et al. 2016).
These pre-clinical studies have shown that there are a number of opportunities for drug therapies for hearing loss that each presents a set of unique requirements, such as specific cellular targeting or slow-release delivery, as well as universal requirements such as the need to protect residual cochlear function and for reliable dosing. The next sections will focus on current and new technologies being developed to meet the demand for a drug therapy that can be applied to the cochlea for preservation and regeneration of hair cells, SGNs, ribbon synapses or other affected cell types.
4. Delivery of drugs to the inner ear
Drug based therapies targeting inner ear disease have been used clinically for over 60 years, initially using systemic administration to deliver aminoglycosides for the treatment of severe bilateral Meniere’s disease, and more recently the application of steroids for sudden SNHL. Although still in clinical practise, these therapies exhibit significant limitations including highly variable pharmacokinetics due to the blood-cochlear barrier and clinical variability (e.g. patient age; renal function; aetiology; previous inner ear pathology; genetic disposition), and potential undesirable side-effects associated with systemic drug administration (Shepherd 2011).
In an attempt to improve clinical outcomes, investigators developed drug delivery techniques specifically targeting the inner ear by delivering drugs directly to the round window membrane at the base of the cochlea. Intra-tympanic clinical delivery techniques include direct trans-tympanic injection into the middle ear or the use of Gelfoam™ as a means to maintain drug contact with the round window membrane. Similarly, the Silverstein Microwick™(Summit Medical Inc.) is a device that is placed onto the round window via a small incision in the tympanic membrane allowing drugs to be transported from the external auditory meatus to the round window. These techniques have been used to provide a more targeted approach in the treatment of Meniere’s disease and sudden SNHL after systemic treatment has failed (McCall et al. 2010, Swan et al. 2008). This route offers a short-term delivery strategy that is suitable for some clinical applications but also has a number of limitations including loss of drug through the eustachian tube, variability in pharmacokinetics due to variability and thickening of the round window membrane (Goycoolea 2001), and the very slow diffusion of the drug through the cochlea (Salt and Plontke 2009, Swan et al. 2008).
The most efficient route is delivery of the drug directly into the inner ear. The anatomy of the cochlea makes it relatively isolated and local therapy can be applied with few off-site effects. The scala tympani provides a small, relatively contained fluid-filled compartments into which drugs can be delivered at high doses to reach all target cells including hair cells, supporting cells, SGNs and the stria vascularis (Richardson et al. 2019, Richardson et al. 2005, Richardson et al. 2004); only the scala media is not accessible via this route due to the presence of tight junctions (Swan et al. 2008). In addition to the clear advantage of delivering predictable amounts of a drug into the inner ear, this technique offers the potential for safe delivery of drugs that have a history of adverse side effects when administered systemically. While efficient, direct administration of drugs to the cochlea brings with it increased risks of both short and long-term damage to inner ear structures and therefore requires careful preclinical investigation to develop safe surgical access and delivery techniques prior to clinical application. This is particularly important in mild forms of SNHL where further loss of sensory hair cells or their peripheral fibres could result in a profound hearing loss. In cases involving cochlear implantation, where surgical access to the inner ear is part of the procedure and there is little or no residual hearing, the risks of “doing harm” are significantly reduced. It is for this reason that a major clinical application of therapeutic drugs into the cochlea, at least in the short term, will be performed in association with cochlear implantation with a focus on preserving residual hearing and/or promoting SGN survival and peripheral fibre regeneration (Plontke et al. 2017, Wise et al.2011a).
5. Novel cochlear therapy technologies
The key features of a clinically relevant drug delivery strategy are that the system must be capable of delivering a therapeutic dose of the drug safely to the inner ear over durations that are required for a clinically meaningful outcome. The active agent is typically delivered to the round window membrane or to the intracochlear environment depending upon the clinical indication that is being targeted. While there are sophisticated microfluidic based drug delivery pumps under development with application to the cochlea (Peppi et al. 2018) the present review concentrates on non-pump based delivery techniques. Some of the considerations for these systems are described in the following section.
5.1. Biomaterials
The observations in preclinical studies that neurotrophin delivery (often using mini-pump systems) can protect and promote re-sprouting of SGNs in deafened models have been the motivation for the development of new strategies to deliver neurotrophins that are suitable for clinical application. As neurotrophins have a short biological half-life, poor pharmacokinetics and poor permeability across biological barriers (Bartus 2012, Khalin et al. 2015) it is difficult and expensive to systemically administer them for therapy in the cochlea. Thus localized biocompatible nanotechnology-based neurotrophin delivery systems are being developed such as nanogels, hydrogel, micelles, microspheres, electrospun nanofibers, nanoparticles and supraparticles. Neurotrophins can be immobilized in nanotechnology-based systems via physical entrapment, adsorption or electrostatic interaction. The sustained release of neurotrophins from the drug carriers can then be achieved via the diffusion of neurotrophins and/or the degradation of the carriers. Controlled and sustained release systems can increase the residence time of neurotrophins in the middle ear/cochlea. In this section, we will focus on the most commonly used delivery strategies under investigation for neurotrophin therapy (or other therapeutic molecules) for hearing impairment: hydrogels, nanoparticles and supraparticles.
5.1.1. Hydrogels
Hydrogels are comprised of a porous network of lightly hydrophilic cross-linked polymers, which enables them to easily swell or dehydrate in aqueous environments based on triggering mechanisms such as pressure, temperature, electrical potential or pH, thus achieving drug loading and release (Aurand et al. 2012). Hydrogels can be synthesized by various polymerization approaches (Aurand et al. 2012, Khademhosseini and Langer 2007) and have been widely used as drug delivery reservoirs in tissue engineering and neuroscience applications. For the treatment of hearing loss, hydrogels loaded with neurotrophins are typically applied onto the round window membrane (Havenith et al. 2011, Noushi et al. 2005). Because of their high viscosity and viscoelastic properties, they prevent the neurotrophins from rapidly flowing through the eustachian tube in the middle ear, thus increasing the residence time to achieve more uniform delivery to the inner ear. Release of the drug is often rapid and occurs by diffusion (Burdick et al. 2006, Noushi et al. 2005). The injection of hydrogels onto the round window membrane is less invasive compared to the intracochlear administration route and is therefore a potential platform to translate this approach into clinical application.
Collagen, gelatin and agarose are natural biomaterials that can easily form a hydrogel or be simply modified into a synthetic injectable hydrogel (Madduri and Gander 2012). Pre-clinical studies using hydrogels infiltrated with neurotrophins have evaluated the technique for the protection or rescue of SGNs post-hearing loss. For example, encapsulation of BDNF in a biodegradable hydrogel, and its implantation onto the round window membrane of deafened guinea pigs resulted in BDNF delivery to the cochlea over a 7 day period, protecting SGNs and significantly lowering electrically evoked auditory brain-stem response thresholds compared to controls (Endo et al. 2005). Similarly, Gelfoam™, a biodegradable gelatin matrix, loaded with BDNF and positioned onto round window membrane of a deafened guinea pig, promoted survival of SGNs in the basal region of the cochlea after 2 weeks treatment (Havenith et al. 2011) and after 4 weeks of treatment with NT-3 via alginate (Noushi et al. 2005).
For more sustained drug release, researchers have looked towards layering technology. Sustained release of lysozyme, a similar protein in size, molecular weight and isoelectric point to BDNF, for up to a month was reported from an agarose hydrogel where the protein was encapsulated in poly(ethylene glycol)/poly(acrylic acid) which was assembled layer-by-layer (Mehrotra et al. 2010). The ease of application of hydrogels delivered transtympanically for treatment of hearing loss is offset by some potential challenges, such as the relatively quick release profile of most hydrogels which might not be applicable for long term drug delivery and the variations in the thickness of the round window membrane observed in the clinical setting.
5.1.2. Nanoparticles
Since neurotrophins are rapidly released from most hydrogels, it is essential to develop other drug delivery carriers that form strong bonds with neurotrophins such as electrostatic interaction for longer term release. Nanoparticle/nanocarrier systems are promising drug delivery technologies that have the ability to encapsulate and transport neurotrophins to the inner ear (Figure 3A, B). Encapsulation of neurotrophins results in stabilization of the protein and controlled and sustained drug release, avoiding neurotoxicity of highly concentrated neurotrophins (Scharfman et al. 2002, Suzuki et al. 2016, Swan et al. 2008).
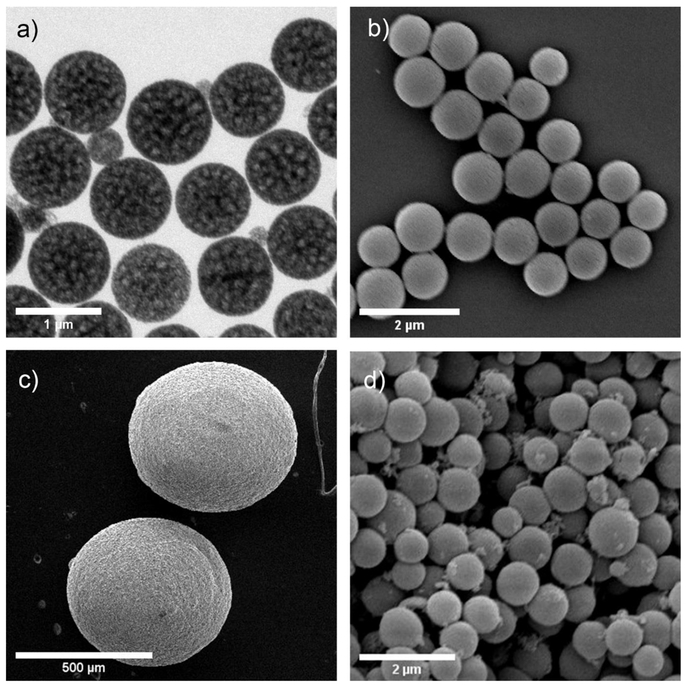
Figure 3.
Characterization of silica nanoparticles and silica supraparticles. (a) Transmission electron microscope image of silica nanoparticles (b) Scanning electron microscope (SEM) image of the silica nanoparticles. (c) SEM image of silica supraparticles. (d) SEM image of the surface structure of a silica supraparticle where individual nanoparticles can be seen clustered together. Modified with permission (Ma et al. 2018).
Specific targeting of nanoparticles to auditory hair cells and SGNs has been achieved via surface modifications. For instance, poly (d,l)-lactide-co-glycolide acid (PLGA) nanoparticles functionalized with hydrophilic molecules like poloxamer 407, showed greater cellular uptake in outer hair cells compared to unmodified PLGA nanoparticles and PLGA nanoparticles with chitosan and methoxy poly(ethylene glycol) modifications (Wen et al. 2016). Moreover, poly(ethylene glycol)-b-poly (ε-caprolactone) nanoparticles with surface modifications of nerve growth factor derived peptides demonstrated specificity for SGNs (Roy et al. 2010). Recently,a peptide (termed A666) with specific binding affinity for prestin, an abundant outer hair cell protein, was conjugated to poly(lactide) nanoparticles in order to specifically target outer hair cells. The peptide was coupled via the SH-terminal to maleimide groups of poly(ethylene glycol) on the surface of the nanoparticles. The aim was to use the nanoparticles to deliver a protective payload of dexamethasone to outer hair cells for protection of hearing during cisplatin-based cancer treatment, where ototoxicity is a common side-effect (Anniko and Sobin 1986). As a result, dexamethasone loaded A666-nanoparticles enhanced outer hair cell viability and afforded some hearing protection following cisplatin therapy in guinea pigs, with a slight advantage over dexamethasone or non-coated dexamethasone loaded nanoparticles. The nanoparticles were confirmed to cross the intact round window membrane and targeted to outer hair cells, with internalisation of the nanoparticles shown in an inner ear cell line HEI-OC1 (Wang et al. 2018).
The stabilization and sustained drug release properties of nanoparticles have made them an attractive means for the delivery of neurotrophins to the cochlea as a hearing loss therapy. For example, Tan et al. demonstrated poly (L-glutamic acid) nanoparticles loaded with BDNF could achieve sustained delivery for 70 days although nearly 90 percent of the BDNF was released in the first 30 days. The biological activity of BDNF was not affected by interaction with the nanoparticles and when applied to the cochlea of deafened guinea pigs prevented the degeneration of SGNs. However, the long-term stability of the BDNF in the nanoparticles was not measured (Tan et al. 2012). Similarly, phytantriollipid-based crystalline nanoparticles were shown to load a large amount of nerve growth factor and improve the availability of nerve growth factor in the cochlea following placement on the round window membrane of the cochlea as compared to application of nerve growth factor in the absence of nanoparticles (Bu et al. 2015). Recently, Schmidt et al. has used silica nanoparticles with an amino group surface modification to encapsulate BDNF and demonstrated sustained release for a period of 80 days. The BDNF released from the silica nanoparticle-based drug delivery system was shown to retain bioactivity for at least 39 days that was equivalent to or greater than the addition of 50 ng/ml BDNF to cultured SGNs (Schmidt et al. 2018). Technologies that maintain the activity of neurotrophic factors is an important consideration in the development of successful slow-release delivery systems.
Exosomes are cell-derived membrane lipid nanoparticles secreted by many cells to mediate cell to cell communication by delivering proteins, nucleic acids or metabolites. They are now being considered as a drug delivery tool due to their intrinsic biocompatibility, stability, targeting properties and ability to overcome natural barriers (Valadi et al. 2007). Exosomes can be used to package and deliver genetic material such as RNA, DNA, gene editing tools and viral gene therapy as well as small molecules and peptides. In the auditory system, exosomes have been shown to enable delivery of adeno-associated virus to otherwise hard-to-transfect outer hair cells of the cochlea, outperforming conventional viral vectors (Gyorgy et al. 2017).
Small nanoparticles have the potential to migrate from the cochlea to the brain via the cochlear aqueduct-a small canal connecting the basal turn of the scala tympani to the subarachnoid space of the cranium (Salt and Hirose 2018), presenting a safety concern that is not unique to nanoparticles (Kho et al. 2000, Zhang et al. 2013). Moreover, some nanoparticle technologies exhibit a rapid release profile over the first few days followed by prolonged release of only very small amounts of neurotrophin (Tan et al. 2012, Wise and Gillespie 2012). Many therapeutic applications in the auditory system have a need for a more regulated release of neurotrophins over a longer period of time.
5.1.3. Supraparticles
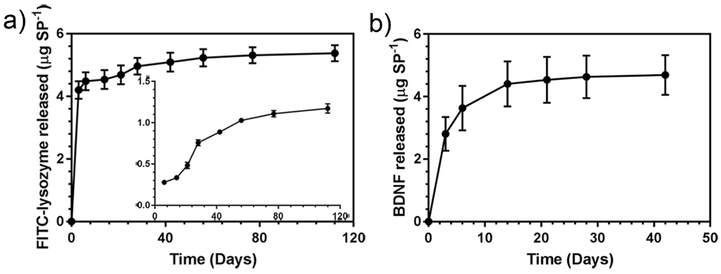
Supraparticles consist of thousands of colloidal nanoparticles with a total size range of 10 μm to 1000 μm. The overall larger size of supraparticles together with the large surface area provided by the individual porous nanoparticles and the pores between the nanoparticles provide an opportunity for a large drug loading capacity compared to individual nanoparticles (Sperling and Gradzielski 2017) (Figure 3C, D). The larger size of supraparticles also addresses the safety concerns of nanoparticle delivery systems where nanoparticles may unintentionally flow into cerebral spinal fluid through the cochlear aqueduct (Zhang et al. 2013). Furthermore, BDNF-loaded supraparticles synthesized by an evaporation-induced self-assembly method also showed a reduction in the initial burst of release commonly seen with other biomaterials and demonstrated sustained BDNF release over at least 70 days in vitro, presumably due to the highly convoluted path that the neurotrophin must take to elute from the particle (Wang et al. 2014). After 70 days there was still about 89% of the initial amount remaining in the particle and with the release rate still steady. When applied to deafened guinea pig cochleae, the BDNF-loaded particles maintained SGN numbers at near-normal levels after 28 days treatment (Wang et al. 2014, Wise et al. 2016). Co-delivery of two drugs important for hearing loss therapies, BDNF and dexamethasone, was demonstrated over 30 days using gelatin hybrid mesoporous silica supraparticles synthesized via a polydimethylsiloxane mould approach (Maina et al. 2014). In an important step towards clinical translation and commercialization, large porous silica supraparticles shown in Figure 3 were prepared by an automatable electrospray technique prior to lysozyme or BDNF encapsulation as an approach for long-term drug delivery to treat inner ear disease (Ma et al. 2018). The large porous silica supraparticles (~550 μm) are biocompatible, they can be loaded with large amounts of lysozyme (10 μg per supraparticle) or BDNF (7 μg per supraparticle), and showed sustained release profiles (lysozyme > 110 days; BDNF > 40 days; Figure 4). The cumulative BDNF release of the supraparticles was 4000-fold larger than the reported silica nanoparticles after 40 days (Schmidt et al. 2018).
Figure 4.
In vitro cumulative release profiles of (a) FITC-lysozyme and (b) BDNF release from silica supraparticles. Insert in (a) shows the in vitro FITC-lysozyme release profile starting from day 6. Adapted with permission (Ma et al. 2018).
Overall, the studies on the silica supraparticles have played an important role in the development of controlled and sustained neurotrophins delivery. However, the pharmacokinetics of neurotrophins in the cochlea released from supraparticles has not been well studied. In addition, the therapeutic dose of neurotrophins and the distribution of the degradation components from supraparticles in different organs have not been investigated. These studies are required in order to translate this drug delivery system into clinical applications.
5.2. Gene therapy
Recent developments in the field of cochlear molecular therapies are showing exciting trends towards clinical therapies for hearing loss, particularly for the repair of genetic defects that result in loss of hair cells. Molecular therapies include gene replacement, gene augmentation, gene editing, post-translational modifications and alternative splicing. The transfer of genetic material into cells can be achieved via viral or non-viral vectors, including adenovirus, adeno-associated virus (AAV), herpes simplex virus I, vaccinia virus and non-viral technologies such as lipid gene transfer systems. The challenges for the field of gene therapy have been to prove that the technique applied to the cochlea is safe, highly efficacious and targeted. Recent advances in the field are stepping up to meet these goals through improvements in the mode of delivery to the cochlea and advances in viral vector technology.
5.2.1. Viral vectors
AAVs are generally considered to have the best profile of safety and efficacy for clinical translation (Colella et al. 2018). AAVs are non-pathogenic, have low immunogenicity and provide long-term gene expression in non-dividing cells. The safety profile of AAVs has improved with all viral genes removed from the current generation of viruses. The multitude of serotypes gives diverse targeting capability which means that specific AAV serotypes can be matched to different indications. The main limitations of AAVs are a restriction on the size of the gene of interest and pre-existing immunity to AAV which may render the treatment ineffective.
The limited cargo capacity of AAVs was previously an obstacle for genes such as otoferlin, also known as DFNB9. Mutations in the large otoferlin gene cause profound hearing loss due to defects in vesicle exocytosis (Roux et al. 2006). Researchers have overcome this limitation through a dual AAV approach where large genes have been split between two AAV vectors. The gene is then reassembled in the transfected cells. Partial restoration of hearing thresholds was observed in otoferlin knockout mice following delivery of dual AAV vectors into the cochlea (Al-Moyed et al. 2019). Full length otoferlin was observed in an average of 19 or 30% inner hair cells (based on two different dual AAV strategies) with restoration of fast vesicle exocytosis. Weaker expression was observed in outer hair cells. With further improvements to the dual AAV vectors to achieve higher full-length protein expression, this study demonstrates the potential for this technique for transferring large genes into cells within the auditory system.
Work is also underway to address the problem of pre-existing immunity to AAVs. The synthetic vector, AAV2/Anc80L65, first described by Zinn, is comprised of ancestral sequences of AAV capsids (Zinn et al. 2015). Cross reactivity to AAV2/Anc80L65 was partially reduced in AAV8 pre-immunized animals whereas AAV8 was completely neutralized (Zinn et al. 2015). The AAV2/Anc80L65 vector also heralded an important breakthrough in terms of cellular targeting in the cochlea. AAV2/Anc80L65 targeted both inner and outer hair cells of the cochlea with high efficiency, while other viral vectors had previously shown poor uptake in outer hair cells. Furthermore, AAV2/Anc80L65 transfected SGNs more efficiently than had been previously achieved following gene therapy into the fluids of the cochlea (Landegger et al. 2017). The vector also had faster transfection kinetics compared to other AAVs with expression visible as early as 1 day post-therapy (Carvalho et al. 2018).
AAVs are the subject of numerous clinical safety and efficacy studies for various diseases including inherited blindness, Parkinson’s disease and cystic fibrosis to name a few. Many studies are showing promising results such as improvements in visual acuity in blind patients with choroideremia (MacLaren et al. 2014). However, an adenovirus is the subject of the only current clinical trial ( NCT02132130) for the treatment of hearing loss, as discussed previously.
5.2.2. Application and distribution
Typically, gene therapy viral vectors are delivered directly to the cochlear fluid compartments: the perilymph or the endolymph. The perilymphatic compartment can be accessed via the round window or oval window membranes of the cochlea or by drilling a cochleostomy through the otic capsule of the cochlea to directly access the scala tympani (Figure 1A). The round window approach is reported to be the least damaging to residual hearing (Chien et al. 2015), sometimes with no reported loss of hearing thresholds following surgical access and injection of viral vectors (Gyorgy et al. 2019). While early studies on gene therapy to the cochlea were often hampered by low transfection efficiency, largely a result of the method of delivery to the cochlea or the cell specificity of the viral serotype, recent developments in mode of delivery and viral vector technology in cochlear gene therapy research are eliminating this problem (Lustig and Akil 2018).
Enormous inter-subject and inter-study variation in transfection levels following direct injection into the cochlear perilymph fluid highlight the difficulty of this approach experimentally. Reported transfection rates to inner hair cells following round window membrane or cochleostomy injection of viral vectors varied from 15% to 100% at the base of the cochlea, with declining transfection rates towards the apex (Akil et al. 2012, Askew et al. 2015, Chien et al. 2016, Chien et al. 2015). Up to 50% inter-subject variation was also reported (Askew et al. 2015). A major cause of variation is the rapid efflux of the perilymphatic fluid that occurs once the otic capsule or the round window membrane has been opened whilst gaining access to the cochlea for application of gene therapy. During efflux of perilymph from the cochlea, cerebrospinal fluid flows into the cochlea via the cochlear aqueduct to maintain fluid homeostasis (Salt and Hirose 2018). Injection of a viral vector into the cochlea is therefore against the perilymph/CSF flow with inevitable loss of the injected material, unknown dosing and difficultly in transfecting the apical turns of the cochlea. Techniques designed to carefully seal potential fluid leaks from the cochlea during the injection such as with gels or glues have shown increased efficacy (Plontke et al. 2016). Another group addressed low transfection rates by creating a flow-system during injection in which the cochlea was inoculated with AAV2/9 via the round window membrane while perilymph was drawn out from a distal location in the semi-circular canals (Yoshimura et al. 2018). The resulting near complete transfection in inner hair cells was consistent throughout the cochlea in both young (P15-16) and adult (P56-60) mice, while the effect on hearing was minimal. In direct comparison, injection through the round window membrane without distal venting resulted in 68% inner hair cell transfection in the base, only 21% in the middle turn and as little as 2% in the apex with high variability between animals (Yoshimura et al. 2018). Finally, other causes of variation in transfection may come from the use of different AAV serotypes, different doses, animal age/strain differences and variations in surgical experience.
Outer hair cells of the cochlea are typically more difficult to transfect with viral vectors compared to inner hair cells. As mentioned previously, the AAV2/Anc80L65 synthetic vector drastically improved targeting to outer hair cells compared to other AAV serotypes (Landegger et al. 2017, Suzuki et al. 2017). Another group employed an exosome delivery technique to improve targeting to both inner and outer hair cells with AAV1 or AAV9. The in vitro data demonstrated that exosome-AAV1 vectors could improve inner hair cell transfection (65% transfected) and outer hair cells (50% transfected) compared to non-exosome based AAV vectors in cochlear explant cultures (as low as 20% for AAV1 for both inner and outer hair cells). Up to 95% outer hair cells were targeted with exosome-AAV9, demonstrating significantly enhanced efficiency of gene delivery with the exosome packaging (Gyorgy et al. 2017). Similar enhanced transfection of hair cells was demonstrated by injecting exosome-AAVl vectors into the cochlear perilymph of neonatal mice with 88% inner hair cell transfection (compared to 65% for conventional AAV1) and 28% outer hair cell transfection (compared to 14% for conventional AAV1). The gene delivery technique was tested in Lhfpl5−/− mice, a strain of mice with profound hearing loss and vestibular dysfunction due to deficiency in a protein integral to mechanotransduction in inner and outer cells (Xiong et al. 2012). Presence of the correctly localised Lhfpl5 protein was demonstrated in inner and outer hair cells, with overall improved hair cell survival, functional mechanotransduction, partial improved hearing thresholds in 9 out of 12 animals (thresholds at ~70 dB SPL), a detectable startle response and rescue of balance in 5 animals (Gyorgy et al. 2017).
A few studies have investigated delivery to the endolymph within the scala media (Figure 1B) which is much smaller and more difficult to access surgically. Access is achieved by injection through the basilar membrane but causes more hearing damage compared to perilymphatic injection (Shibata et al. 2009, Wise et al. 2010); or via a cochleostomy through the stria vascularis, also with the potential to damage the strial cells responsible for cochlear fluid dynamics and homeostasis (Wangemann 2006). In the guinea pig, injection of an adenovirus carrying neurotrophin genes directly into the endolymph resulted in greater transfection in the hair cells and supporting cells of the organ of Corti compared to perilymphatic injection, attributable to the more contained nature of the endolymphatic space compared to the perilymph (Shibata et al. 2009, Wise et al. 2010). Endolymphatic injection was also successfully applied to mice for the correction of a genetic defect resulting in hearing loss. KCNQ1 is a potassium channel subunit essential for the generation of the endocochlear potential and the high potassium content of endolymph which are crucial for the transduction of sound by hair cells (Casimiro et al. 2001, Lee et al. 2000). Mutations in KCNQ1 are responsible for Jervell and Lange-Nielsen syndrome resulting in severe congenital deafness (Jervell and Lange-Nielsen 1957). Neonatal mice lacking the KCNQ1 gene were injected with an AAV carrying the KCNQ1 gene via the scala media with resulting expression in 61-75% of cochlear marginal cells of the stria vascularis in all cochlear turns. KCNQ1 gene therapy to the endolymph, but not the perilymph, corrected the severe morphological defects caused by the mutation, restored the endocochlear potential and improved hearing thresholds (Chang et al. 2015).
The risk of loss of residual cochlear function is obviously a significant issue when it comes to therapies that aim to protect or restore hearing function. One method employed to minimise the impact on hearing function was gene therapy via the posterior semi-circular canal, first trialled with adenovirus carrying a reporter gene with a significant advantage in hearing preservation (Kawamoto et al. 2001). AAV2/Anc80L65 containing a reporter gene was injected into the posterior semi-circular canal of 6 week old mice, with resulting transfection of nearly 100% of cochlear inner hair cells and high transfection of outer hair cells throughout the cochlea without affecting hearing thresholds (Suzuki et al. 2017). Furthermore, up to 10% of SGNs in the cochlea were also transfected, with greater efficiency in the apical turn. Vestibular hair cells, supporting cells and ganglion cells were also transfected without any obvious vestibular dysfunction or changes in vestibular evoked thresholds. This technique therefore has merit for drug therapies targeting both cochlear and balance disorders (Suzuki et al. 2017). A similar finding was reported for neonatal mice injected with AAV8 via the posterior semi-circular canal that showed moderately high cochlear inner hair cell and vestibular hair cell transfection without balance or hearing dysfunction (Guo et al. 2017, Isgrig and Chien 2018).
5.2.3. Gene replacement
In animal models of genetic loss-of-function hearing loss, gene therapy has demonstrated successful repair of genetic defects in hair cells and partial or near complete restoration of hearing, although often the therapy is required at early stages of hearing development with obvious translational challenges.
The Whirlin gene is required for actin polymerisation in hair cell stereocilia. As the name suggests Whirler mice exhibit circling and head tossing behaviour indicative of vestibular dysfunction as well as a profound hearing loss due to a frameshift mutation in the Whirlin gene (Lane 1963). Delivery of a viral vector containing the Whirlin gene to the perilymph of the cochlea via the round window of neonatal Whirler mice restored the morphology of the stereocilia in transfected cells (Chien et al. 2016). However, there was no improvement in overall hearing sensitivity as a result of the low transfection efficiency in this study (15% hair cells at the base of the cochlea) (Chien et al. 2016).
TMC1 is another gene required for hair cell transduction channel function (Pan et al. 2018). Delivery of an AAV2/1 through the round window membrane of neonatal mice that were bred for recessive TMC1 deafness resulted in nearly 60% inner hair cell transfection and the return of some hearing function (Askew et al. 2015). To target outer hair cells more efficiently, the group then used the synthetic AAV2/Anc80L65 serotype in the TMC1 model of hearing loss and found dramatically improved transfection in both inner (91%) and outer hair cells (50%). Restoration of hearing thresholds of up to 50 dB was observed in TMC1 mutant mice (Nist-Lund et al. 2019). Since the cochlear injection approach also resulted in expression in vestibular hair cells, the researchers tested whether gene therapy could restore balance and vestibular function in TMC1/TMC2 mutant mice, as vestibular hair cells express both TMC1 and TMC2 (Kawashima et al. 2011). Following neonatal injection of AAV2/Anc80L65 containing the TMC1 or TMC2 gene into the cochlea via the round window the mice showed vestibular function similar to wild type mice (Nist-Lund et al. 2019).
Vesicular glutamate transporter type 3 (VGLUT3) is required for synaptic transmission in hair cells. Mice lacking the VGLUT3 gene are profoundly deaf due to lack of glutamate release from hair cells (Seal et al. 2008). Restoration of auditory brainstem responses to within 10 dB of wild-type mice as well as a partial restoration of behavioural responses to auditory stimuli were demonstrated following injection of an AAV1 containing VGLUT3 into VGLUT3 knockout neonatal mice via the round window membrane. In this case, nearly every inner hair cell was transfected, demonstrating the enormous potential of gene therapy when efficient and timely targeting of cells is achieved (Akil et al. 2012). However, variable loss of hearing was then observed approximately 7 weeks after gene therapy suggesting transgene inactivation or a slow progressive hearing loss resulting from the surgical delivery of the transgene (Akil et al. 2012).
Ushers syndrome is responsible for 3-6% of childhood deafness (Boughman et al. 1983). Patients with the most severe form experience profound hearing loss, balance issues and progressive blindness. A disrupted harmonin protein is responsible for this phenotype, causing disorganised hair cell stereocilia bundles and loss of mechanotransduction. Using the synthetic AAV serotype AAV2/Anc80L65, harmonin-a1 or harmonin-b1 was introduced into the cochlear and vestibular hair cells in a mouse model of Usher syndrome. Following neonatal cochlear injection, when there are still surviving hair cells, inner hair cell transfection rates were 80-90% with correctly localised harmonin protein at the tips of the stereocilia. Partial recovery of inner and outer hair cell function, auditory thresholds and behaviourally relevant auditory function were observed in the majority of mice injected with harmonin-b1 6 weeks after injection, particularly in the low frequencies (Pan et al. 2017). Out of five mice examined 6 months after injection, three showed continued low frequency hearing attributable to the survival of outer hair cells (Pan et al. 2017).
To summarise, gene therapy to the cochlea via AAV viral vectors has resulted in partial or sometimes near-complete correction of genetic defects in the auditory pathway. Several challenges are still remaining such as the timing of treatment with respect to the degenerative changes of the disease. In many cases experimentally, the gene therapy is administered to neonatal mice in order to repair the gene in the hair cell prior to hair cell degeneration. Given that the development of human hearing occurs prior to birth (Hall 2000), the translation of these approaches would potentially require gene therapy in utero. Characterisation of long-term expression and outcomes will also be required for each type of gene therapy as long-term outcomes are variable.
5.2.4. Modulating gene translation
Not all genetic mutations require gene replacement for repair of the hearing defect. Some point mutations result in aberrant gene splicing such that the translated protein is truncated or frame-shifted and inactive. In such cases, manipulating splicing could potentially correct the defect by blocking the offending splice site and directing splice site selection to normal. As mentioned above, harmonin is a protein encoded by the USH1C gene and is located in stereocilia and ribbon synapses of hair cells in the cochlea and vestibular system where it has a role in stereocilia structure and mechanotransduction (Verpy et al. 2000). About 6-8% of mutations in Usher syndrome are in the USH1C gene, attributed to a USH1Cc.216G>A mutation which causes mis-splicing and ultimately a truncated non-functional harmonin protein (Lentz et al. 2005, Verpy et al. 2000). Antisense oligonucleotide therapy was developed to base-pair at the position of the mutation, blocking access of splicing factors to the aberrant splice site and redirecting the splicing to the correct location in the gene. Deviating from many of the other gene therapy strategies described above where the gene therapy is localised to the cochlea, antisense oligonucleotide therapy in a mouse model of this particular Usher mutation (Lentz 2007) was administered via systemic administration in neonates (Donaldson et al. 2018, Lentz et al. 2013, Vijayakumar et al. 2017) or embryonically via the amniotic fluids (Depreux et al. 2016) as there is a critical time period during the development of hearing in which therapy must be administered. Antisense oligonucleotide therapy afforded long-term significant rescue of hearing and correction of vestibular function associated with restoration of hair cell stereocilia morphology (Donaldson et al. 2018, Lentz et al. 2013, Vijayakumar et al. 2017). Earlier treatment had a greater impact on hearing restoration consistent with the developmental pattern of expression of harmonin that occurs between P4 and P16 (Lentz 2013).
The ease of delivery, specificity and efficacy of antisense oligonucleotides make this form of therapy an exciting option for altering gene expression in diseases with a known genetic component. Spliceswitching oligonucleotide therapy is currently in clinical trials for the treatment of Duchenne muscular dystrophy and spinal muscular atrophy (Havens and Hastings 2016) showing relatively low toxicity and good tolerability.
RNA interference is a technique that can be used to silence specific genes linked to a disease, particularly for gain-of-function mutations. Gain-of-function mutations occur when a mutation results in an altered gene product which then interferes with the normal functioning of a biological pathway. An example in the auditory system is connexin-26, encoded by the Gap Junction Protein Beta 2 gene (GJB) which is a part of a larger complex of proteins that form a connexon, the pore-forming unit of gap junctions (Kumar 1999). A point mutation in connexin-26 results in a protein that still complexes with the other five connexins but interferes with the function of gap junctions, preventing hearing transduction in the cochlea through deficient potassium recycling (Cohen-Salmon et al. 2002, Denoyelle et al. 1998, Richard et al. 1998, Rouan et al. 2001). To suppress the mutant protein, a short-interfering RNA was complexed to a liposome delivery system and applied to the intact round window membrane of a mouse model of the connexin point mutation. The RNA interference treatment significantly and specifically suppressed the mutant GJB mRNA, preventing hearing loss in the mouse model (Maeda et al. 2005). Similarly, in a mouse model of a gain-of-function TMC1 mutation known as Beethoven (Vreugde et al. 2002), a single intracochlear injection of a microRNA delivered by an AAV slowed the progression of hearing loss through suppression of the mutated gene. At the 35-week time point, mice in the RNA interference group had a 40 dB improvement in hearing thresholds over non-treated controls (Shibata et al. 2016). Other gain-of-function mutations in the auditory system that may benefit from RNA interference include missense mutations of the KCNQ4 gene that affect potassium channels (Coucke et al. 1999, Kubisch et al. 1999) and the DFNA5 gene which produces a protein of currently unknown function (Van Laer et al. 1998, Van Laer et al. 2004).
As discussed in Section 3.4.1, new hair cells can be generated in mammals by increasing expression of the Atoh1 transcription factor, decreasing Notch signalling or regulating other pathways following hearing loss (Samarajeewa et al. 2019). Silencing of hairy and enhancer of split-1 (Hes1), a downstream effector of Notch signalling and inhibitor of Atoh1 (Lanford et al. 1999, Zine et al. 2000), is also sufficient to promote transdifferentiation of supporting cells to hair cells in the mouse cochlear tissue (Du et al. 2013, Korrapati et al. 2013, Slowik and Bermingham-McDonogh 2013). To test these results in vivo, a Hes1 small interfering RNA was delivered to adult guinea pig cochleae via PLGA nanoparticles 72 hours after noise-induced hearing loss by mini-osmotic pump infusion into the perilymph over 24 hours. As a result of the treatment there were fewer missing inner and outer hair cells across the entire length of the cochlear spiral, some correctly positioned and some ectopic. There was also progressive recovery of hearing of more than 10 dB, particularly in the epicentre of the noise-induced damage and near the infusion site (Du et al. 2018).
In an exciting development for gene modulation therapies, the US Food and Drug Administration has approved the first gene-silencing technology based on RNA interference: the drug, patisiran, which has improved outcomes in patients with a rare heart and nerve function condition (Adams et al. 2018).
5.2.5. Cell targeting
The majority of experimental cochlear gene therapies to date aim to target cells in the sensory epithelium such as the hair cells or supporting cells. SGNs have proven to be relatively resistant to transfection. There is recent interest in transducing SGNs, particularly for optogenetics research which requires expression of a light-sensitive ion channel in SGNs. The aim is to develop an optical cochlear implant for improved spectral resolution during SGN stimulation in the cochlea (Jeschke and Moser 2015, Richardson et al. 2017). At first embryonic gene therapy was performed in order to achieve efficient SGN transfection (Hernandez et al. 2014). When E11.5-12.5 embryos were transfected, there was 40% SGN transfection which was sufficient to evoke activity within the central auditory pathway to optical stimulation of the transfected SGN. Clinical translation of this technique requires a solution in which post-natal, preferably adult SGNs, are transfected. One solution was the use of a novel AAV serotype which improved SGN transfection in post-natal mice. In 5-7 day old early post-natal mice, strong SGN expression of the opsin was achieved across all turns (about 55% in base and 75% in apex) when using a novel serotype with improved neural transfection, AAV-PHP.B (Deverman et al. 2016) via a round window membrane injection (Keppeler et al. 2018) or 74% transfection when using the AAV2/Anc80L65 viral vector in P4 mice (Duarte et al. 2018). Interestingly, another solution came in the form of a novel mode of delivery to the cochlea. In adult gerbils, AAV2/6 was injected directly into the modiolus of the cochlea. This technique resulted in up to 30% transfection in SGNs in all turns of the cochlea in about half of the injected animals. The technique was damaging to SGNs though, with approximately 25% loss of SGNs (Wrobel et al. 2018). Subsequent optical stimulation of SGNs expressing the light-sensitive ion channels resulted in auditory percepts and behavioural responses in transfected gerbils (Wrobel et al. 2018).
5.2.6. Gene editing
Usher syndrome is an inherited disorder causing deafness and blindness with up to 13 associated genes. More than 50% of Usher syndrome cases harbour a mutation in the USH2A gene. The large size of the USH2A gene makes it difficult to develop a conventional gene therapy for these patients, but the CRISPR/Cas9 gene editing technology now offers an alternative strategy. The Cas9 nuclease is guided with precision by small RNAs to the target sequence for editing. Successful targeting and repair of the USH2A mutation was demonstrated in fibroblasts in vitro (Fuster-Garcia et al. 2017). More recently CRISPR/Cas9 technology has been used to restore hearing in animals with a genetic form of deafness that normally results in loss of hair cells. The faulty TMC1 gene present in cochlear hair cells was targeted by the gene-editing tool to repair a single base-pair mutation. In a novel take on the CRISPR/Cas9 technology, the authors used a special DNA-free, virus-free delivery technique in which the Cas9 complex was encapsulated in lipid packaging to directly deliver the Cas9 complex inside the hair cell rather than having the cell make the Cas9 from DNA (Zuris et al. 2015). This reduced the off-target editing thereby improving the precision of the editing. The outcome in these mice was significantly greater hair cell survival and prevention of loss of hearing (Gao et al. 2018).
Genome editing with nucleases such as Cas9 can be subject to extraneous insertions and deletions (indels) and is largely restricted to mitotic cells (Cox et al. 2015). To overcome these limitations, researchers have used a nuclease-free base editing technique which increased the ratio of editing compared to indels (Komor et al. 2016). Lipid-mediated delivery of the beta-catenin base editor to the mouse inner ear resulted in the replacement of Ser 33 to Tyr in the beta-catenin gene. This resulted in altered post-translational protein modification (prevention of phosphorylation) and therefore, up-regulation of Wnt signalling. This in turn promoted mitosis in supporting cells of the cochlea and reprogramming of the cells towards the hair cell lineage (Yeh et al. 2018).
5.2.7. Gene augmentation
Viral gene therapy is not just limited to the correction of hereditary disorders. Gene therapy can be utilised to augment gene expression, for example to restore neurotrophin levels in the cochlea following the loss of hair cells and supporting cells that occurs with SNHL (Atkinson et al. 2012, Budenz et al. 2015, Fukui et al. 2012, Wise et al. 2010, Wise et al. 2011b). When neurotrophin gene therapy was administered to the scala media of guinea pigs that were deafened by aminoglycosides there was a preservation of the SGN population along with outgrowth of the peripheral fibres towards neurotrophin-expressing cells in the sensory epithelium (Wise et al. 2010, Wise et al. 2011b) (Figure 5). It is yet to be determined whether neurotrophin gene therapy results in improvements in cochlear implant function as has been shown with long-term recombinant BDNF delivery to the cochlea (Shepherd et al. 2005). Guinea pig cochleae that were inoculated with AAV containing the NT-3 gene and then implanted with an electrode array showed measurable and long-lasting SGN survival, but variability in transfection made electrophysiological assessment difficult (Pfingst et al. 2017).
Figure 5.
Neurotrophin gene therapy provides cellular sources of neurotrophins for SGN survival after hearing loss (A) Comparison of spiral ganglion neuron cell bodies (red) in a cochlea treated with neurotrophin gene therapy and the contralateral non-injected cochlea. Guinea pigs were deafened with aminoglycosides 1 week prior to gene therapy with adenoviral vectors containing BDNF and NT3 genes into the scala tympani. After three weeks there was a dramatic difference in the number of surviving spiral ganglion neurons between the treated and non-treated cochleae. Scale bars are 50 μm. (B) Surface view of the organ of Corti with peripheral fibres of spiral ganglion neurons (red) attracted towards pillar cells (P) that have been transfected with neurotrophin genes (as labelled by a green-fluorescent protein reporter gene). A DAPI counterstain is in blue. Scale bars are 50 μm. The dotted line indicates the position of the habenula perforata. Adapted from (Wise etal. 2011b)
Finally, the potential to use the cochlear implant to drive transfection of DNA into resident cells lining the scala tympani via brief but intense pulses from the electrode array provides a unique transfection mechanism for gene therapy. Using this electroporation technique Housley and colleagues were able to transfect mesenchymal cells with a complementary DNA (cDNA) gene construct resulting in the expression of BDNF (Pinyon et al. 2014b). Using a deafened guinea pig model, they were able to show reduced electrical thresholds and improved SGN survival with significant regrowth of the nerve fibres within the scala tympani. The resultant improvement in the electrode-neural interface may lead to clinical improvements for cochlear implant recipients following completion of an upcoming Phase 1 clinical trial.
5.3. Cochlear implant combination therapies
Cochlear implant electrode arrays are implanted into the scala tympani of severe-to-profoundly deaf patients. There is therefore the potential to use the device to deliver therapeutics to the cochlea. The validity of this approach was first investigated by manufacturing a cannula internally to the electrodes which was connected to a pump for the continuous delivery of neurotrophins to the guinea pig cochlea following hearing loss (Shepherd and Xu 2002). SGN survival was enhanced compared to deaf controls and further improvement in the SGN density was observed in animals that were simultaneously given electrical stimulation (Shepherd et al. 2008, Shepherd et al. 2005). However, the presence of a cannula or any cavity in the electrode array is problematic as it increases the infection risk and significantly alters the mechanical characteristics of the electrode array. For this reason some studies examined drug release from a cochlear implant coating that has been modified to be loaded with drugs for subsequent release into the cochlea. These have included a coating of cells that have been modified to express and release neurotrophins and degradable coatings.
In one study cochlear implant electrodes were coated with the electro-active polymer polypyrrole. The polypyrrole could be doped with NT-3 or BDNF that was only released during electrical stimulation (Richardson et al. 2007, Thompson et al. 2006, Thompson et al. 2011, Thompson et al. 2009). Arrays that were coated with polypyrrole containing NT-3 were implanted in guinea pigs that had been ototoxically deafened and electrical stimulation was provided for 4 weeks. It was found that there was greater nerve survival in guinea pigs implanted with coated compared to control arrays (Richardson et al. 2009) although the technique is limited to a relatively small and finite payload of neurotrophin.
Inflammation and fibrosis following cochlear implantation is believed to be the cause of some post implantation-related hearing loss that can occur in recipients with residual hearing (Chang et al. 2009, Eastwood et al. 2010). Pharmacological interventions to reduce the immune response are being investigated with a particular emphasis on the anti-inflammatory drug dexamethasone. Local delivery of dexamethasone to the cochlea can prevent some of the loss of residual hearing and reduces the tissue response around the implanted array (Lee et al. 2013). Some researchers have been looking to the cochlear implant itself as a means to elute drugs like dexamethasone over the time frame of inflammation and fibrosis which occurs in stages during the days and weeks following cochlear implantation. The dexamethasone was added during the polymerisation of silicone elastomer such that the drug is an integral part of the implant (Farahmand Ghavi et al. 2010). When tested in guinea pigs the dexamethasone-eluting cochlear implant attenuated some inflammatory cell infiltration in the cochlea between 3 and 13 days post-implantation (Farhadi et al. 2013). This approach has also demonstrated a trend towards hearing protection, however the results to date have not been statistically significant and may depend on the level of damage during insertion (Astolfi et al. 2016, Stathopoulos et al. 2014).
5.4. Cell-based therapies
Another potential solution to the damage or loss of hair cells or SGNs is cell-based therapies for the replacement or restoration of these cells. Stem cells are one of the most commonly used in the field of cell-based therapies due to their potential to differentiate into different cell types including hair cells and SGNs. To date, there are quite a few studies examining the differentiation of murine embryonic stem cells (Coleman et al. 2007b, Lin et al. 2009, Reyes et al. 2008) or human embryonic stem cells (Buckiova et al. 2012, Chen et al. 2009, Shi et al. 2007) into functional hair cells or auditory neurons.
When transplanted into the cochlear environment, differentiated stem cells have been shown to survive and integrate into the tissue (Coleman et al. 2006, Tateya et al. 2003). Strikingly, transplanted neural progenitor cells appeared to respond to natural differentiation and guidance cues in the tissue environment with engrafted embryonic stem cells in the cochlear nerve trunk projecting fibres towards the sensory epithelium (Corrales et al. 2006). Human otic progenitor cells that were differentiated towards cells with morphological and electrophysiological features of hair cells and SGNs in vitro promoted recovery of auditory brainstem response thresholds when transplanted into SGN-depleted gerbils (Chen et al. 2012). Induced pluripotent stem cells (IPSCs), differentiated cells that have been reprogrammed to an embryonic state, also received much attention as they can be transplanted without immune response since they are generated from the patients’ own cells (Takahashi and Yamanaka 2006). In one study, mouse IPSC-derived neural progenitors could be differentiated into a neural lineage. After transplantation into the mouse cochlea in vivo, a small percentage of the transplanted IPSC-derived neural progenitors expressed VGLUT1, a marker for glutamatergic neurons (Nishimura et al. 2009).
In addition to the transplantation of differentiated neural stem cells to replace degenerated sensory cells or neurons in deafened cochlea, a second cell-based therapy approach involves the delivery of cells designed to rescue sensory cells or neurons from ongoing degeneration. For example, in vitro experiments indicated that genetically modified Schwann cells could maintain the survival of SGNs (Pettingill et al. 2008). To avoid recognition by the immune system and cellular migration from the implantation site, BDNF-over-expressing Schwann cells were encapsulated by a semi-permeable alginate membrane where oxygen and nutrients could diffuse into the cells and the BDNF and waste could diffuse away from the cells (Pettingill et al. 2011). The results showed that alginate encapsulated BDNF-over-expressed Schwann cells could promote the survival of SGNs in deafened guinea pigs. Further, porcine choroid plexus cells, which naturally secrete a cocktail of neurotrophins (Skinner et al. 2009), encapsulated in an alginate membrane promoted survival of SGNs and their peripheral fibers for several months in deafened guinea pigs with a combination of chronic electrical stimulation via cochlear implantation with minimal tissue response to the grafted cells (Wise et al. 2011a).
There are still a number of challenges for clinical application of cell based therapies. It is essential that the transplanted cells survive for long periods of time and not interfere with normal cochlear function or affect residual hearing (Coleman et al. 2007a, Hildebrand et al. 2008). Potential immunological responses to embryonic stem cells must be considered (Beisel et al. 2008) alongside the risk of tumorigenesis if they are not fully differentiated (Erdo et al. 2003, Gunewardene et al. 2012). An additional uncertainty is whether the generated cells have the same functional response as normal SGNs or hair cells (Mittal et al. 2017, Takeda et al. 2018). It is also challenging to precisely inject the cells into the target without damage to existing structures and for the cell to integrate into the existing tissue and to form functional connections (Altschuler et al. 2008, Backhouse et al. 2008, Coleman et al. 2007a, Takeda et al. 2018).
6. How close are we and which delivery technique is best?
This review has covered a number of novel drug therapy technologies for the potential treatment of hearing loss. The therapies are varied by necessity to mirror the diverse aetiologies of hearing loss. A number of the therapies discussed have demonstrated partial reversal or slowed progression of hearing loss in relevant animal models. These have included hair cell regeneration following acquired damage to hair cells via the manipulation of transcription factor pathways by gene or drug therapies (Du et al. 2018, Izumikawa et al. 2005, Kraft et al. 2013, Mizutari et al. 2013, Yeh et al. 2018). The most promising hair cell regeneration therapies are those that use a combination of factors (Costa et al. 2015, Kuo et al. 2015, Walters et al. 2017) and those that promote mitotic regeneration such that the supporting cell population is maintained during hair cell regeneration (McLean et al. 2017). For acquired synaptopathy or hidden hearing loss, delivery of neurotrophic factors such as NT-3 to the cochlea has been shown to rescue hearing function via the regeneration of the ribbon synapse following noise trauma (Sly et al. 2016, Suzuki et al. 2016). The simplicity of the trans-tympanic delivery technique will make this therapy readily translatable, despite the short window of opportunity for effective treatment. Neurotrophins are also protective to spiral ganglion neurons that can otherwise degenerate following hearing loss and hence are a promising drug therapy that could be applied to a range of hearing loss conditions. In many cases, extended exposure to neurotrophins is beneficial to the therapy, hence research into drug delivery technologies such as biomaterials (incorporating hydrogels, nanoparticles, supraparticles) or cell-based therapies that can not only stabilise the drug over time but also provide sustained release of the drug. With so many tools available, gene therapy has the potential to treat a diverse range of SNHL such as gene editing of point mutations, replacement of defective genes, silencing of gain-of-function mutations, suppression of aberrant splice variants, alteration of post-translational modifications, manipulation of transcription factor pathways for regeneration and augmentation with the rapeutic genes such as neurotrophins. Using mouse models of known human mutations or acquired damage to hair cells, there have been many reports of long-term correction of genetic defects ranging from partial to near-complete reversal/prevention of hearing loss with early intervention (Akil et al. 2012, Al-Moyed et al. 2019, Askew et al. 2015, Chang et al. 2015, Donaldson et al. 2018, Gao et al. 2018, Gyorgy et al. 2019, Lentz et al. 2013, Maeda et al. 2005, Nist-Lund et al. 2019, Pan et al. 2017, Shibata et al. 2016, Vijayakumar et al. 2017). Furthermore, with a direct readout of transfection efficiency in the form of a fluorescent reporter gene, gene therapy research has led to significant improvements in cochlear delivery techniques that will benefit all forms of therapy to the cochlea. In contrast, there have been relatively few examples of hearing restoration by cell based therapies (Chen et al. 2012) compared to gene therapy techniques highlighting the challenges of differentiation stem cells into functional auditory sensory cells, integrating the cells into the auditory pathway and maintaining long-term survival in the cochlea.
While there are relatively large numbers of clinical trials incorporating trans-tympanic drug delivery into the middle ear (Peppi et al. 2018, Staecker et al. 2017) there are few trials incorporating drug delivery directly into the cochlea due to concerns with potential damage to residual hair cells. As noted above, the only clinical trial registered with ClinicalTrials.gov incorporating direct intra-labyrinthine drug delivery is designed to evaluate the safety, tolerability, and the potential ability of the drug CGF166, an adenovirus containing the human Atoh1 gene, to improve hearing in patients with severe-profound SNHL ( NCT02132130).
The various delivery systems have specific advantages and disadvantages that must be taken into consideration in developing a clinical based delivery system. For example, delivery using biomaterials – particularly nano- and supraparticles – can deliver large payloads including a cocktail of drugs delivered over different time-constants. These systems can deliver both native protein and appropriate synthetic drugs. Biomaterial-based delivery techniques typically have short or moderate release profiles and must be carefully tuned to ensure they do not produce high concentrations of the drug(s) at the target site. In contrast, cell and gene based delivery techniques offer potentially longer-term solutions, whether for gene augmentation, genetic correction or cell regeneration, with a high degree of cell specificity. However, many of the promising therapies for hereditary hearing loss would require early intervention before complete loss of the hair cells or irr eversible morphological changes.
In addition to these technical considerations, it is also important when developing these systems to consider the manufacturing and clinical implications of the technology (Shepherd et al. 2018). For example, in a clinical setting the technology will need to conform to Good Manufacturing Practice guidelines and recognized standards of sterilization for healthcare products and packaging systems, and show a reasonable shelf-life at room temperature. If drug, gene or cell loading is to be performed during surgery it must be undertaken using sterile techniques under appropriate guidelines for handling biological material.
Finally, it is likely that the initial delivery techniques will target hearing preservation (Lyu et al. 2018), SGN peripheral fibre regeneration (Landry et al. 2013, Pinyon et al. 2014a) and a reduction in inflammation (Chambers et al. 2019) in cochlear implant patients. This work, together with novel techniques designed to safely target either the round or oval windows to treat disorders including Meniere’s disease (King et al. 2017) and hidden hearing loss (Sly et al. 2016, Wan et al. 2014) are likely to dominate inner ear drug delivery in the near-term. With the clinical experience gained from these approaches, more trials evaluating direct drug delivery to the cochlea in patients are expected. Disorders including tinnitus, sudden hearing loss and hearing protection following exposure to loud noise or ototoxic drugs would be potential candidates for this route.
7. Conclusions
Hearing loss is a significant problem world-wide. The sensory hair cells and SGNs of the cochlea are often affected and do not spontaneously regenerate in humans. A number of factors have been identified that can preserve, repair or regenerate these cell types with a growing number of studies showing recovery of hearing in animal models of acquired and genetic forms of hearing loss. The anatomy of the cochlea presents some unique challenges for the development of a therapy for hearing loss. The cochlear tissues and membranes are extremely sensitive, difficult to access and consistent dosing is often hard to achieve. To overcome these limitations, researchers are using novel drug delivery techniques and technologies. The cochlear implant itself is also the subject of a range of drug delivery strategies, from electroactive biomaterial coatings to using electrical stimulation to transfect cochlear cells with DNA encoding neurotrophic factors. Hydrogel biomaterials are being considered for the simplicity of application to the round window of the cochlea for non-invasive and safe drug delivery to the cochlea. Nano- or supraparticle biomaterial technologies offer slow-release and high pay-load drug delivery properties that are attractive for neurotrophin delivery to the cochlea to protect SGNs from degeneration. There are now numerous examples of hearing restoration in genetic models of hearing loss following gene therapies due to new viral vector and gene editing technologies and improvements in delivery techniques. The potential to activate progenitor cells in the cochlea have also been explored for hair cell regeneration alongside cell-based therapies for repopulating the SGN population or delivering neurotrophins for SGN protection after hearing loss. As the potential for a drug treatment to restore hearing becomes evident in pre-clinical studies, advancements in drug therapy technologies such as those described here will help ensure that translation of these discoveries can be achieved.
Acknowledgements
This work was supported by the following funding agencies for which we are most grateful: US Department of Defence Translation Research Award (RH170019), the National Institute on Deafness and other Communication Disorders (R01DC015031), Action on Hearing Loss (G30, G39, G89), the Garnett Passe and Rodney Williams Memorial Foundation, Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (project number CE140100036), the Robert Bulley Foundation and the Australian National Health and Medical Research Council (GNT1024350, GNT1064375, GNT1122055, and GNT1142910). The Bionics Institute acknowledges the support of the Victorian Government through its Operational Infrastructure Support Program.
List of abbreviations
- AAV
adeno-associated virus
- AN
auditory nerve
- BM
basilar membrane
- BDNF
brain derived neurotrophic factor
- CRISPR
clustered regularly interspaced short palindromic repeats
- CSF
cerebrospinal fluid
- IPSCs
induced pluripotent stem cells
- NT-3
neurotrophin-3
- OW
oval window
- PLGA
poly (d, l-lactide-co-glycolide acid)
- RNA
RNA interference
- RW
round window
- SC
semi-circular canal
- SM
scala media
- ST
scala tympani
- SV
scala vestibuli
- SEM
scanning electron microscope
- SGNs
spiral ganglion neurons
- SNHL
sensorineural hearing loss
- TMC1
transmembrane channel-like protein 1
- VGLUT1/3
vesicular glutamate transporter type 1/3
Footnotes
Conflict of Interest Statement
The authors A.K.W, Y.M and R.K.S are named inventors on a patent describing drug delivery technique that is owned by the Bionics Institute. There is no financial conflict of interest by these or any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams D, et al. 2018. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 379:11–21. [DOI] [PubMed] [Google Scholar]
- Adunka O, Roush P, Grose J, Macpherson C, Buchman CA. 2006. Monitoring of cochlear function during cochlear implantation. Laryngoscope 116:1017–1020. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. 2009. Enhanced Survival of Spiral Ganglion Cells After Cessation of Treatment with Brain-Derived Neurotrophic Factor in Deafened Guinea Pigs. J Assoc Res Otolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, Lustig LR. 2012. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Moyed H, Cepeda AP, Jung S, Moser T, Kugler S, Reisinger E. 2019. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice. EMBO Mol Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SA, Robinson BK, Huang J, Green SH. 2007. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol 503:832–852. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, O'Shea KS, Miller JM. 2008. Stem cell transplantation for auditory nerve replacement. Hear Res 242:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli S, Lin X, Liu XZ. 2012. Genetics of Hearing and Deafness. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 295:1812–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anniko M, Sobin A. 1986. Cisplatin: evaluation of its ototoxic potential. American journal of otolaryngology 7:276–293. [DOI] [PubMed] [Google Scholar]
- Askew C, Rochat C, Pan B, Asai Y, Ahmed H, Child E, Schneider BL, Aebischer P, Holt JR. 2015. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med 7:295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Simoni E, Giarbini N, Giordano P, Pannella M, Hatzopoulos S, Martini A. 2016. Cochlear implant and inflammation reaction: Safety study of a new steroid-eluting electrode. Hear Res 336:44–52. [DOI] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Hume CR, O'Leary SJ, Shepherd RK, Richardson RT. 2012. Neurotrophin gene therapy for sustained neural preservation after deafness. PLoS One 7:e52338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT. 2014. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One 9:e102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurand ER, Lampe KJ, Bjugstad KB. 2012. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res 72:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhouse S, Coleman B, Shepherd R. 2008. Surgical access to the mammalian cochlea for cell-based therapies. Experimental Neurology 214:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak K, Corwin J, Jones J. 1990. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. The Journal of Neuroscience 10:2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT. 2012. Translating the therapeutic potential of neurotrophic factors to clinical 'proof of concept': a personal saga achieving a career-long quest. Neurobiol Dis 48:153–178. [DOI] [PubMed] [Google Scholar]
- Beisel K, Hansen L, Soukup G, Fritzsch B. 2008. Regenerating cochlear hair cells: quo vadis stem cell. Cell Tissue Res 333:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Kimlinger M, Harding GW. 2017. Time course of organ of Corti degeneration after noise exposure. Hearing Research 344:158–169. [DOI] [PubMed] [Google Scholar]
- Boughman JA, Vernon M, Shaver KA. 1983. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis 36:595–603. [DOI] [PubMed] [Google Scholar]
- Bu M, Tang J, Wei Y, Sun Y, Wang X, Wu L, Liu H. 2015. Enhanced bioavailability of nerve growth factor with phytantriol lipid-based crystalline nanoparticles in cochlea. Int J Nanomedicine 10:6879–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham RA, Valvassori GE. 2001. Inner ear fluid volumes and the resolving power of magnetic resonance imaging: can it differentiate endolymphatic structures? Ann Otol Rhinol Laryngol 110:113–117. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Ranjan S, Newman TA, Johnston AH, Sood R, Kinnunen PK, Popelar J, Chumak T, Syka J. 2012. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine (Lond) 7:1339–1354. [DOI] [PubMed] [Google Scholar]
- Budenz CL, Wong HT, Swiderski DL, Shibata SB, Pfingst BE, Raphael Y. 2015. Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Sci Rep 5:8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick JA, Ward M, Liang E, Young MJ, Langer R. 2006. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 27:452–459. [DOI] [PubMed] [Google Scholar]
- Carvalho LS, et al. 2018. Synthetic Adeno-Associated Viral Vector Efficiently Targets Mouse and Nonhuman Primate Retina In Vivo. Hum Gene Ther 29:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro MC, Knollmann BC, Ebert SN, Vary JC Jr., Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K. 2001. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A 98:2526–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione A, et al. 2016. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiology & neuro-otology 21 Suppl 1:21–28. [DOI] [PubMed] [Google Scholar]
- Chai R, et al. 2012. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A 109:8167–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S, Newbold C, Stathopoulos D, Needham K, Miller C, Risi F, Enke YL, Timbol G, Cowan R. 2019. Protecting against electrode insertion trauma using dexamethasone. Cochlear implants international 20:1–11. [DOI] [PubMed] [Google Scholar]
- Chang A, Eastwood H, Sly D, James D, Richardson R, O'Leary S. 2009. Factors influencing the efficacy of round window dexamethasone protection of residual hearing post-cochlear implant surgery. Hear Res 255:67–72. [DOI] [PubMed] [Google Scholar]
- Chang Q, Wang J, Li Q, Kim Y, Zhou B, Wang Y, Li H, Lin X. 2015. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med 7:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-D, Fechter LD. 2003. The relationship between noise-induced hearing loss and hair cell loss in rats. Hearing Research 177:81–90. [DOI] [PubMed] [Google Scholar]
- Chen W, Johnson SL, Marcotti W, Andrews PW, Moore HD, Rivolta MN. 2009. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells 27:1196–1204. [DOI] [PubMed] [Google Scholar]
- Chen W, et al. 2012. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WW, Isgrig K, Roy S, Belyantseva IA, Drummond MC, May LA, Fitzgerald TS, Friedman TB, Cunningham LL. 2016. Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice. Mol Ther 24:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WW, McDougald DS, Roy S, Fitzgerald TS, Cunningham LL. 2015. Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope 125:2557–2564. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, et al. 2002. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol 12:1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Ronzitti G, Mingozzi F. 2018. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol Ther Methods Clin Dev 8:87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, de Silva MG, Shepherd RK. 2007a. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells 25:2685–2694. [DOI] [PubMed] [Google Scholar]
- Coleman B, Fallon JB, Pettingill LN, de Silva MG, Shepherd RK. 2007b. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp Cell Res 313:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, Hardman J, Coco A, Epp S, de Silva M, Crook J, Shepherd R. 2006. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant 15:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. 2006. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol 66:1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J, Cotanche D. 1988. Regeneration of sensory hair cells after acoustic trauma. Science 240:1772–1774. [DOI] [PubMed] [Google Scholar]
- Costa A, Sanchez-Guardado L, Juniat S, Gale JE, Daudet N, Henrique D. 2015. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development 142:1948–1959. [DOI] [PubMed] [Google Scholar]
- Coucke PJ, et al. 1999. Mutations in the KCNQ4 gene are responsible for autosomal dominant deafness in four DFNA2 families. Hum Mol Genet 8:1321–1328. [DOI] [PubMed] [Google Scholar]
- Cox BC, et al. 2014. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141:816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DB, Platt RJ, Zhang F. 2015. Therapeutic genome editing: prospects and challenges. Nature Medicine 21:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan SG, Willett WC, Grodstein F, Curhan GC. 2019. Longitudinal study of hearing loss and subjective cognitive function decline in men. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle F, Lina-Granade G, Plauchu H, Bruzzone R, Chaib H, Levi-Acobas F, Weil D, Petit C. 1998. Connexin 26 gene linked to a dominant deafness. Nature 393:319–320. [DOI] [PubMed] [Google Scholar]
- Depreux FF, Wang L, Jiang H, Jodelka FM, Rosencrans RF, Rigo F, Lentz JJ, Brigande JV, Hastings ML. 2016. Antisense oligonucleotides delivered to the amniotic cavity in utero modulate gene expression in the postnatal mouse. Nucleic Acids Res 44:9519–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, et al. 2016. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 34:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson TN, Jennings KT, Cherep LA, McNeela AM, Depreux FF, Jodelka FM, Hastings ML, Wallace DG. 2018. Antisense oligonucleotide therapy rescues disruptions in organization of exploratory movements associated with Usher syndrome type 1C in mice. Behav Brain Res 338:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Cai Q, West MB, Youm I, Huang X, Li W, Cheng W, Nakmali D, Ewert DL, Kopke RD. 2018. Regeneration of Cochlear Hair Cells and Hearing Recovery through Hes1 Modulation with siRNA Nanoparticles in Adult Guinea Pigs. Mol Ther 26:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Li W, Gao X, West MB, Saltzman WM, Cheng CJ, Stewart C, Zheng J, Cheng W, Kopke RD. 2013. Regeneration of mammalian cochlear and vestibular hair cells through Hes1/Hes5 modulation with siRNA. Hearing Research 304:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte MJ, et al. 2018. Ancestral Adeno-Associated Virus Vector Delivery of Opsins to Spiral Ganglion Neurons: Implications for Optogenetic Cochlear Implants. Mol Ther 26:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Eckert MA, Lee FS, Matthews LJ, Schmiedt RA. 2013. Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol 14:687–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood H, Chang A, Kel G, Sly D, Richardson R, O'Leary SJ. 2010. Round window delivery of dexamethasone ameliorates local and remote hearing loss produced by cochlear implantation into the second turn of the guinea pig cochlea. Hear Res 265:25–29. [DOI] [PubMed] [Google Scholar]
- Endo T, Nakagawa T, Kita T, Iguchi F, Kim TS, Tamura T, Iwai K, Tabata Y, Ito J. 2005. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope 115:2016–2020. [DOI] [PubMed] [Google Scholar]
- Erdo F, et al. 2003. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab 23:780–785. [DOI] [PubMed] [Google Scholar]
- Estivill X, et al. 1998. Connexin-26 mutations in sporadic and inherited sensorineural deafness. The Lancet 351:394–398. [DOI] [PubMed] [Google Scholar]
- Farahmand Ghavi F, Mirzadeh H, Imani M, Jolly C, Farhadi M. 2010. Corticosteroid-releasing cochlear implant: a novel hybrid of biomaterial and drug delivery system. J Biomed Mater Res B Appl Biomater 94:388–398. [DOI] [PubMed] [Google Scholar]
- Farhadi M, Jalessi M, Salehian P, Ghavi FF, Emamjomeh H, Mirzadeh H, Imani M, Jolly C. 2013. Dexamethasone eluting cochlear implant: Histological study in animal model. Cochlear Implants Int 14:45–50. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH Jr., 2006. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope 116:1310–1320. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Makarem AO, Linthicum FH Jr., 2009. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol Head Neck Surg 141:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Schacht J. 2000. Aminoglycoside antibiotics. Audiol Neurootol 5:3–22. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. 1999. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell & Tissue Research 295:369–382. [DOI] [PubMed] [Google Scholar]
- Fukui H, Wong HT, Beyer LA, Case BG, Swiderski DL, Di Polo A, Ryan AF, Raphael Y. 2012. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci Rep 2:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. 2013. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of neurophysiology 110:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Garcia C, Garcia-Garcia G, Gonzalez-Romero E, Jaijo T, Sequedo MD, Ayuso C, Vazquez-Manrique RP, Millan JM, Aller E. 2017. USH2A Gene Editing Using the CRISPR System. Mol Ther Nucleic Acids 8:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. 2005. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope 115:796–802. [DOI] [PubMed] [Google Scholar]
- Gao X, et al. 2018. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 553:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. 2005. Presbycusis. Lancet 366:1111–1120. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. 2003. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. Journal of Neuroscience Research 71:785–790. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Marzella PL. 2004. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport 15:1121–1125. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Johnson Chacko L, Rask-Andersen H, Liu W, Handschuh S, Schrott-Fischer A. 2018. Anatomical basis of drug delivery to the inner ear. Hearing Research 368:10–27. [DOI] [PubMed] [Google Scholar]
- Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. 2014. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 134:972–982. [DOI] [PubMed] [Google Scholar]
- Goycoolea MV. 2001. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol 121:437–447. [DOI] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. 2005. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature 435:212–215. [DOI] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. 2008. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ Jr., Salt A, Cheatham MA. 2012. Progress in cochlear physiology after Bekesy. Hearing research 293:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunewardene N, Dottori M, Nayagam BA. 2012. The convergence of cochlear implantation with induced pluripotent stem cell therapy. Stem Cell Rev 8:741–754. [DOI] [PubMed] [Google Scholar]
- Guo JY, Liu YY, Qu TF, Peng Z, Xie J, Wang GP, Gong SS. 2017. Cochleovestibular gene transfer in neonatal mice by canalostomy. Neuroreport 28:682–688. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, et al. 2019. Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate. Mol Ther Methods Clin Dev 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, et al. 2017. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol Ther 25:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW 3rd. 2000. Development of the ear and hearing. J Perinatol 20:S12–20. [DOI] [PubMed] [Google Scholar]
- Havenith S, Versnel H, Agterberg MJ, de Groot JC, Sedee RJ, Grolman W, Klis SF. 2011. Spiral ganglion cell survival after round window membrane application of brain-derived neurotrophic factor using gelfoam as carrier. Hear Res 272:168–177. [DOI] [PubMed] [Google Scholar]
- Havens MA, Hastings ML. 2016. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res 44:6549–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa AN, Koppl C. 2019. The aging cochlea: Towards unraveling the functional contributions of strial dysfunction and synaptopathy. Hear Res. [DOI] [PubMed] [Google Scholar]
- Hernandez VH, et al. 2014. Optogenetic stimulation of the auditory pathway. Journal of Clinical Investigation 124:1114–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, Dahl HH, Rose SD, Behlke MA, Smith RJ. 2008. Advances in molecular and cellular therapies for hearing loss. Mol Ther 16:224–236. [DOI] [PubMed] [Google Scholar]
- Hirose K, Rutherford MA, Warchol ME. 2017. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hearing Research 352:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgrig K, Chien WW. 2018. Posterior Semicircular Canal Approach for Inner Ear Gene Delivery in Neonatal Mouse. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. 2008. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res 240:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. 2005. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Medicine 11:271–276. [DOI] [PubMed] [Google Scholar]
- Jahan I, Pan N, Fritzsch B. 2015. Opportunities and limits of the one gene approach: the ability of Atoh1 to differentiate and maintain hair cells depends on the molecular context. Front Cell Neurosci 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakody DMP, Almeida OP, Speelman CP, Bennett RJ, Moyle TC, Yiannos JM, Friedland PL. 2018a. Association between speech and high-frequency hearing loss and depression, anxiety and stress in older adults. Maturitas 110:86–91. [DOI] [PubMed] [Google Scholar]
- Jayakody DMP, Friedland PL, Martins RN, Sohrabi HR. 2018b. Impact of Aging on the Auditory System and Related Cognitive Functions: A Narrative Review. Front Neurosci 12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervell A, Lange-Nielsen F. 1957. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J 54:59–68. [DOI] [PubMed] [Google Scholar]
- Jeschke M, Moser T. 2015. Considering optogenetic stimulation for cochlear implants. Hear Res 322:224–234. [DOI] [PubMed] [Google Scholar]
- Johnson Chacko L, Blumer MJF, Pechriggl E, Rask-Andersen H, Dietl W, Haim A, Fritsch H, Glueckert R, Dudas J, Schrott-Fischer A. 2017. Role of BDNF and neurotrophic receptors in human inner ear development. Cell and tissue research 370:347–363. [DOI] [PubMed] [Google Scholar]
- Kamakura T, Nadol JB Jr., 2016. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res 339:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap MP, Roberts C, Waseem M, Tyagi P. 2018. Drug Targets in Neurotrophin Signaling in the Central and Peripheral Nervous System. Molecular neurobiology 55:6939–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. 2003. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 23:4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Oh SH, Kanzaki S, Brown N, Raphael Y. 2001. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther 4:575–585. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, et al. 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121:4796–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. 2012. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:6699–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppeler D, et al. 2018. Ultrafast optogenetic stimulation of the auditory pathway by targeting-optimized Chronos. Embo J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R. 2007. Microengineered hydrogels for tissue engineering. Biomaterials 28:5087–5092. [DOI] [PubMed] [Google Scholar]
- Khalin I, Alyautdin R, Kocherga G, Bakar MA. 2015. Targeted delivery of brain-derived neurotrophic factor for the treatment of blindness and deafness. Int J Nanomedicine 10:3245–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho ST, Pettis RM, Mhatre AN, Lalwani AK. 2000. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Molecular Therapy 2:368–373. [DOI] [PubMed] [Google Scholar]
- King EB, Shepherd RK, Brown DJ, Fallon JB. 2017. Gentamicin Applied to the Oval Window Suppresses Vestibular Function in Guinea Pigs. Journal of the Association for Research in Otolaryngology : JARO 18:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. 2016. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. 2013. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS One 8:e73276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft S, Hsu C, Brough DE, Staecker H. 2013. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. The Laryngoscope 123:992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. 1999. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96:437–446. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. 2006. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26:2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. 2009. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. 2015. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. 2019. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear Res 377:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NM. 1999. Molecular biology of the interactions between connexins. Novartis Found Symp 219:6–16; discussion 16-21, 38-43. [PubMed] [Google Scholar]
- Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. 2015. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of beta-Catenin and Atoh1. J Neurosci 35:10786–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegger LD, et al. 2017. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol 35:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry TG, Fallon JB, Wise AK, Shepherd RK. 2013. Chronic neurotrophin delivery promotes ectopic neurite growth from the spiral ganglion of deafened cochleae without compromising the spatial selectivity ofcochlear implants. J Comp Neurol 521:2818–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PW. 1963. Whirler Mice: A Recessive Behavior Mutation in Linkage Group Viii. J Hered 54:263–266. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. 1999. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet 21:289–292. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O. 2011. Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol 519:1526–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ismail H, Lee JH, Kel G, O'Leary J, Hampson A, Eastwood H, O'Leary SJ. 2013. Effect of both local and systemically administered dexamethasone on long-term hearing and tissue response in a Guinea pig model of cochlear implantation. Audiol Neurootol 18:392–405. [DOI] [PubMed] [Google Scholar]
- Lee J, Nadol JB Jr., Eddington DK. 2010. Depth of electrode insertion and postoperative performance in humans with cochlear implants: a histopathologic study. Audiol Neurootol 15:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MP, et al. 2000. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest 106:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J, Dettman S, Dowell R, Briggs R. 2013. Communication development in children who receive a cochlear implant by 12 months of age. Otology & neurotology 34:443–450. [DOI] [PubMed] [Google Scholar]
- Lentz J, Savas S, Ng SS, Athas G, Deininger P, Keats B. 2005. The USH1C 216G-->A splice-site mutation results in a 35-base-pair deletion. Hum Genet 116:225–227. [DOI] [PubMed] [Google Scholar]
- Lentz JJ, Jodelka FM, Hinrich AJ, McCaffrey KE, Farris HE, Spalitta MJ, Bazan NG, Duelli DM, Rigo F, Hastings ML. 2013. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nature Medicine 19:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. 1982. The cochlear frequency map for the cat: Labeling auditory nerve fibers of known characteristic frequency. The Journal of the Acoustical Society of America 72:1441–1449. [DOI] [PubMed] [Google Scholar]
- Liberman MC. 2017. Noise-induced and age-related hearing loss: new perspectives and potential therapies. F1000Res 6:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. 2011a. Hearing loss and incident dementia. Archives of neurology 68:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. 2011b. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci 66:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM. 2013. Hearing loss and cognitive decline in older adults. JAMA internal medicine 173:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. 2011c. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol 12:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Feng L, Hamajima Y, Komori M, Burns TC, Fukudome S, Anderson J, Wang D, Verfaillie CM, Low WC. 2009. Directed differentiation of mouse cochlear neural progenitors in vitro. Am J Physiol Cell Physiol 296:C441–452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu K, Jiang X, Shi C, Shi L, Yang B, Shi L, Xu Y, Yang W, Yang S. 2013. Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Mol Neurobiol 48:647–654. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang H, Shi L, Almuklass A, He T, Aiken S, Bance M, Yin S, Wang J. 2012a. Silent damage of noise on cochlear afferent innervation in guinea pigs and the impact on temporal processing. PLoS One 7:e49550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Fang J, Dearman J, Zhang L, Zuo J. 2014. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS One 9:e89377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZY, Dearman JA, Cox BC, Walters BJ, Zhang LL, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. 2012b. Age-Dependent In Vivo Conversion of Mouse Cochlear Pillar and Deiters' Cells to Immature Hair Cells by Atoh1 Ectopic Expression. Journal of Neuroscience 32:6600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, et al. 2017. Dementia prevention, intervention, and care. Lancet 390:2673–2734. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R 1933. Anatomy of the eighth nerve. III. General plan of structure of the primary cochlear nuclei. Laryngoscope 43:327–350. [DOI] [PubMed] [Google Scholar]
- Lustig L, Akil O. 2018. Cochlear Gene Therapy. Cold Spring Harb Perspect Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu AR, Kim DH, Lee SH, Shin DS, Shin SA, Park YH. 2018. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: A comparison of administration routes. PloS one 13:e0195230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, et al. 2018. Gel-Mediated Electrospray Assembly of Silica Supraparticles for Sustained Drug Delivery. ACS Appl Mater Interfaces 10:31019–31031. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, et al. 2014. Retinal gene therapy in patients with choroideremia: initial findings from a phase ½ clinical trial. Lancet 383:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madduri S, Gander B. 2012. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J Control Release 161:274–282. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Nishizaki K, Smith RJ. 2005. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet 14:1641–1650. [DOI] [PubMed] [Google Scholar]
- Maina JW, Cui J, Bjornmalm M, Wise AK, Shepherd RK, Caruso F. 2014. Mold-templated inorganic-organic hybrid supraparticles for codelivery of drugs. Biomacromolecules 15:4146–4151. [DOI] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. 2011. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol 12:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. 1993. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet 46:486–491. [DOI] [PubMed] [Google Scholar]
- McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. 2010. Drug del ivery for treatment of inner ear disease: current state of knowledge. Ear Hear 31:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. 2005. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol 26:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, Karp JM, Edge ASB. 2017. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep 18:1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S, Lynam D, Maloney R, Pawelec KM, Tuszynski MH, Lee I, Chan C, Sakamoto J. 2010. Time Controlled Protein Release from Layer-by-Layer Assembled Multilayer Functionalized Agarose Hydrogels. Adv Funct Mater 20:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. 1997. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci 15:631–643. [DOI] [PubMed] [Google Scholar]
- Mittal R, Nguyen D, Patel AP, Debs LH, Mittal J, Yan D, Eshraghi AA, Van De Water TR, Liu XZ. 2017. Recent Advancements in the Regeneration of Auditory Hair Cells and Hearing Restoration. Front Mol Neurosci 10:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. 2013. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB Jr., 1979. Intercellular fluid pathways in the organ of Corti of cat and man. Annals of Otology, Rhinology & Laryngology 88:2–11. [DOI] [PubMed] [Google Scholar]
- Nadol JB Jr., Eddington DK. 2006. Histopathology of the inner ear relevant to cochlear implantation. Adv Otorhinolaryngol 64:31–49. [DOI] [PubMed] [Google Scholar]
- Nadol JB Jr., et al. 2001. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol 110:883–891. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos TP, Vlastarakos PV. 2010. Treating options for deaf children. Early human development 86:669–674. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nakagawa T, Ono K, Ogita H, Sakamoto T, Yamamoto N, Okita K, Yamanaka S, Ito J. 2009. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport 20:1250–1254. [DOI] [PubMed] [Google Scholar]
- Nist-Lund CA, et al. 2019. Next generation gene therapy improves hearing, balance and secondary outcomes in mouse models of genetic inner ear disorder Nature Communications (accepted). [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. 2000. Histopathological differences between temporary and permanent threshold shift. Hear Res 139:13–30. [DOI] [PubMed] [Google Scholar]
- Noushi F, Richardson RT, Hardman J, Clark G, O'Leary S. 2005. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol 26:528–533. [DOI] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. 2007. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol 8:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, et al. 2018. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 99:736–753 e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, et al. 2017. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol 35:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patuzzi R, Robertson D. 1988. Tuning in the mammalian cochlea. Physiological reviews 68:1009–1082. [DOI] [PubMed] [Google Scholar]
- Peake WT, Rosowski JJ. 1991. Impedance matching, optimum velocity, and ideal middle ears. Hearing research 53:1–6. [DOI] [PubMed] [Google Scholar]
- Peppi M, Marie A, Belline C, Borenstein JT. 2018. Intracochlear drug delivery systems: a novel approach whose time has come. Expert Opin Drug Deliv 15:319–324. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Minter RL, Shepherd RK. 2008. Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro. Neuroscience 152:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettingill LN, Wise AK, Geaney MS, Shepherd RK. 2011. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PLoS One 6:e18733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, Hughes AP, Strahl SB, Sinan M, Raphael Y. 2017. Neurotrophin Gene Therapy in Deafened Ears with Cochlear Implants: Long-term Effects on Nerve Survival and Functional Measures. J Assoc Res Otolaryngol 18:731–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Yang W, Henderson D, Hua Hu B, Nicotera TM. 2004. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hearing Research 196:69–76. [DOI] [PubMed] [Google Scholar]
- Pinyon JL, Tadros FS, Froud KE, Wong ACW, Tompson IT, Crawford EN, Ko M, Morris R, Klugmann M, Housley GD. 2014a. Close-Field Electroporation Gene Delivery Using the Cochlear Implant Electrode Array Enhances the Bionic Ear. Sci. Transl. Med 6. [DOI] [PubMed] [Google Scholar]
- Pinyon JL, Tadros SF, Froud KE, AC YW, Tompson IT, Crawford EN, Ko M, Morris R, Klugmann M, Housley GD. 2014b. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci Transl Med 6:233ra254. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Gotze G, Rahne T, Liebau A. 2017. Intracochlear drug delivery in combination with cochlear implants : Current aspects. HNO 65:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke SK, Hartsock JJ, Gill RM, Salt AN. 2016. Intracochlear Drug Injections through the Round Window Membrane: Measures to Improve Drug Retention. Audiology & neuro-otology 21:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Puel J-L. 1999. Excitotoxicity, Synaptic Repair, and Functional Recovery in the Mammalian Cochlea: A Review of Recent Findings. Annals of the New York Academy of Sciences 884:249–254. [DOI] [PubMed] [Google Scholar]
- Qun LX, Pirvola U, Saarma M, Ylikoski J. 1999. Neurotrophic factors in the auditory periphery. Ann N Y Acad Sci 884:292–304. [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Grolman W, Klis SF. 2012. Neurotrophins and their role in the cochlea. Hearing Research 288:19–33. [DOI] [PubMed] [Google Scholar]
- Reyes JH, O'Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. 2008. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci 28:12622–12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ. 1998. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet 103:393–399. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Hu QY, Shi F, Nguyen T, Fallon JB, Flynn BO, Wise AK. 2019. Pharmacokinetics and tissue distribution of neurotrophin 3 after intracochlear delivery. J Control Release 299:53–63. [DOI] [PubMed] [Google Scholar]
- Richardson RT, O'Leary S, Wise A, Hardman J, Clark G. 2005. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res 204:37–47. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Thompson AC, Wise AK, Needham K. 2017. Challenges for the application of optical stimulation in the cochlea for the study and treatment of hearing loss. Expert Opin Biol Ther 17:213–223. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Thompson B, Moulton S, Newbold C, Lum MG, Cameron A, Wallace G, Kapsa R, Clark G, O'Leary S. 2007. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials 28:513–523. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Wise A, O'Leary S, Hardman J, Casley D, Clark G. 2004. Tracing neurotrophin-3 diffusion and uptake in the guinea pig cochlea. Hear Res 198:25–35. [DOI] [PubMed] [Google Scholar]
- Richardson RT, et al. 2009. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials 30:2614–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche JP, Hansen MR. 2015. On the Horizon: Cochlear Implant Technology. Otolaryngol Clin North Am 48:1097–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G. 2001. trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci 114:2105–2113. [DOI] [PubMed] [Google Scholar]
- Roux I, et al. 2006. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 127:277–289. [DOI] [PubMed] [Google Scholar]
- Roy S, Johnston AH, Newman TA, Glueckert R, Dudas J, Bitsche M, Corbacella E, Rieger G, Martini A, Schrott-Fischer A. 2010. Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: potential tool for drug delivery. International journal of pharmaceutics 390:214–224. [DOI] [PubMed] [Google Scholar]
- Ryals B, Rubel E. 1988. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240:1774–1776. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Kujawa SG, Hammill T, Le Prell C, Kil J. 2016. Temporary and Permanent Noise-induced Threshold Shifts: A Review of Basic and Clinical Observations. Otol Neurotol 37:e271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Dodds LW, Benson TE, Kiang NY. 1991. Unmyelinated axons of the auditory nerve in cats. Journal of Comparative Neurology 308:209–223. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hirose K. 2018. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res 362:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Plontke SK. 2009. Principles of local drug delivery to the inner ear. Audiol Neurootol 14:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarajeewa A, Jacques BE, Dabdoub A. 2019. Therapeutic potential of Wnt and Notch signalling and epigenetic regulation in mammalian sensory hair cell regeneration. Molecular Therapy (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. 2011. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31:13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL, Croll SD. 2002. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol 174:201–214. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. 1994. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res 73:92–100. [DOI] [PubMed] [Google Scholar]
- Schlecker C, Praetorius M, Brough DE, Presler RG Jr., Hsu C, Plinkert PK, Staecker H. 2011. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther 18:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N, Schulze J, Warwas DP, Ehlert N, Lenarz T, Warnecke A, Behrens P. 2018. Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS One 13:e0194778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF. 1974a. Pathology of the ear. Harvard University Press. [Google Scholar]
- Schuknecht HF. 1974b. Pathology of the ear. Harvard University press. [Google Scholar]
- Seal RP, et al. 2008. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 57:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. 2013. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33:13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyyedi M, Viana LM, Nadol JB Jr., 2014. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol 35:1446–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK. 2011. Rescuing the Cochlea: the challenges. ENT Audiol News 19:49–52. [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. 2008. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res 242:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. 2005. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol 486:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG. 2004. Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. Eur J Neurosci 20:3131–3140. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Villalobos J, Burns O, Nayagam DAX. 2018. The development of neural stimulators: a review of preclinical safety and efficacy studies. J Neural Eng 15:041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Xu J. 2002. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res 172:92–98. [DOI] [PubMed] [Google Scholar]
- Sheth S, Mukherjea D, Rybak LP, Ramkumar V. 2017. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front Cell Neurosci 11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Corrales CE, Liberman MC, Edge AS. 2007. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur J Neurosci 26:3016–3023. [DOI] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. 2012. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:9639–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu L, He T, Guo X, Yu Z, Yin S, Wang J. 2013. Ribbon synapse plasticity in the cochleae of Guinea pigs after noise-induced silent damage. PLoS One 8:e81566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X 2016. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res 338:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Di Pasquale G, Cortez SR, Chiorini JA, Raphael Y. 2009. Gene transfer using bovine adeno-associated virus in the guinea pig cochlea. Gene Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Ranum PT, Moteki H, Pan B, Goodwin AT, Goodman SS, Abbas PJ, Holt JR, Smith RJH. 2016. RNA Interference Prevents Autosomal-Dominant Hearing Loss. Am J Hum Genet 98:1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. 2002. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A 99:1657–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner SJ, Geaney MS, Lin H, Muzina M, Anal AK, Elliott RB, Tan PL. 2009. Encapsulated living choroid plexus cells: potential long-term treatments for central nervous system disease and trauma. J Neural Eng 6:065001. [DOI] [PubMed] [Google Scholar]
- Slowik AD, Bermingham-McDonogh O. 2013. Hair cell generation by notch inhibition in the adult mammalian cristae. J Assoc Res Otolaryngol 14:813–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly DJ, Campbell L, Uschakov A, Saief ST, Lam M, O'Leary SJ. 2016. Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy After Noise-induced Hearing Loss. Otol Neurotol 37:1223–1230. [DOI] [PubMed] [Google Scholar]
- Smith CA, Lowry OH, Wu ML. 1954. The electrolytes of the labyrinthine fluids. The Laryngoscope 64:141–153. [DOI] [PubMed] [Google Scholar]
- Sperling M, Gradzielski M. 2017. Droplets, evaporation and a superhydrophobic surface: Simple tools for guiding colloidal particles into complex materials. Gels 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H 1978. The afferent innervation of the cochlea Pages 21–41 in Naunton RF, Fernandez C, eds. Evoked Electrical Activity in the Auditory Nervous System. New York: Academic Press. [Google Scholar]
- Spoendlin H 1984. Primary neurons and synapses. Butterworths. [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. 1996. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport 7:889–894. [DOI] [PubMed] [Google Scholar]
- Staecker H, Morelock M, Kramer T, Chrbolka P, Ahn JH, Meyer T. 2017. Safety of Repeated-Dose Intratympanic Injections with AM-101 in Acute Inner Ear Tinnitus. Otolaryngol Head Neck Surg 157:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K, Brough DE. 2007. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol 28:223–231. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. 2004. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci 24:8651–8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos D, Chambers S, Enke YL, Timbol G, Risi F, Miller C, Cowan R, Newbold C. 2014. Development of a safe dexamethasone-eluting electrode array for cochlear implantation. Cochlear Implants Int 15:254–263. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Corfas G, Liberman MC. 2016. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep 6:24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Hashimoto K, Xiao R, Vandenberghe LH, Liberman MC. 2017. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep 7:45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. 2008. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev 60:1583–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. [DOI] [PubMed] [Google Scholar]
- Takeda H, Hosoya M, Fujioka M, Saegusa C, Saeki T, Miwa T, Okano H, Minoda R. 2018. Engraftment of Human Pluripotent Stem Cell-derived Progenitors in the Inner Ear of Prenatal Mice. Sci Rep 8:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Wang Y, Yip X, Glynn F, Shepherd RK, Caruso F. 2012. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv Mater 24:3362–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WJ, Thorne PR, Vlajkovic SM. 2016. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem Cell Biol 146:219–230. [DOI] [PubMed] [Google Scholar]
- Tateya I, Nakagawa T, Iguchi F, Kim TS, Endo T, Yamada S, Kageyama R, Naito Y, Ito J. 2003. Fate of neural stem cells grafted into injured inner ears of mice. Neuroreport 14:1677–1681. [DOI] [PubMed] [Google Scholar]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. 2006. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology 52:386–394. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, Forge A. 2012. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One 7:e30577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen SC, Rieffe C, Netten AP, Briaire JJ, Soede W, Schoones JW, Frijns JH. 2014. Psychopathology and its risk and protective factors in hearing-impaired children and adolescents: a systematic review. JAMA Pediatr 168:170–177. [DOI] [PubMed] [Google Scholar]
- Thompson BC, Moulton SE, Ding J, Richardson R, Cameron A, O'Leary S, Wallace GG, Clark GM. 2006. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J Control Release 116:285–294. [DOI] [PubMed] [Google Scholar]
- Thompson BC, Moulton SE, Richardson RT, Wallace GG. 2011. Effect of the dopant anion in polypyrrole on nerve growth and release of a neurotrophic protein. Biomaterials 32:3822–3831. [DOI] [PubMed] [Google Scholar]
- Thompson BC, Richardson RT, Moulton SE, Evans AJ, O'Leary S, Clark GM, Wallace GG. 2009. Conducting polymers, dual neurotrophins and pulsed electrical stimulation - Dramatic effects on neurite outgrowth. J Control Release. [DOI] [PubMed] [Google Scholar]
- Thomson RS, Auduong P, Miller AT, Gurgel RK. 2017. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig Otolaryngol 2:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. [DOI] [PubMed] [Google Scholar]
- Valero MD, Burton JA, Hauser SN, Hackett TA, Ramachandran R, Liberman MC. 2017. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hearing Research 353:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laer L, et al. 1998. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet 20:194–197. [DOI] [PubMed] [Google Scholar]
- Van Laer L, Vrijens K, Thys S, Van Tendeloo VF, Smith RJ, Van Bockstaele DR, Timmermans JP, Van Camp G. 2004. DFNA5: hearing impairment exon instead of hearing impairment gene? J Med Genet 41:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaert N, Willems M, Van Kerschaver E, Desloovere C. 2008. Impact of early hearing screening and treatment on language development and education level: evaluation of 6 years of universal newborn hearing screening (ALGO) in Flanders, Belgium. International journal of pediatric otorhinolaryngology 72:599–608. [DOI] [PubMed] [Google Scholar]
- Verpy E, et al. 2000. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet 26:51–55. [DOI] [PubMed] [Google Scholar]
- Viana LM, O'Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC. 2015. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research 327:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar S, Depreux FF, Jodelka FM, Lentz JJ, Rigo F, Jones TA, Hastings ML. 2017. Rescue of peripheral vestibular function in Usher syndrome mice using a splice-switching antisense oligonucleotide. Hum Mol Genet 26:3482–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugde S, et al. 2002. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet 30:257–258. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, Zuo J. 2017. In Vivo Interplay between p27(Kip1), GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice. Cell Rep 19:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G. 2014. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen Y, Tao Y, Gao Y, Yu D, Wu H. 2018. A666-conjugated nanoparticles target prestin of outer hair cells preventing cisplatin-induced hearing loss. Int J Nanomedicine 13:7517–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wise AK, Tan J, Maina JW, Shepherd RK, Caruso F. 2014. Mesoporous silica supraparticles for sustained inner-ear drug delivery. Small 10:4244–4248. [DOI] [PubMed] [Google Scholar]
- Wangemann P 2006. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, et al. 1995. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374:60–61. [DOI] [PubMed] [Google Scholar]
- Wen X, Ding S, Cai H, Wang J, Wen L, Yang F, Chen G. 2016. Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int J Nanomedicine 11:5959–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2018. World Health Organisation https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
- Wilson BS, Dorman MF. 2008. Cochlear implants: a remarkable past and a brilliant future. Hear Res 242:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Tucci DL, Merson MH, O'Donoghue GM. 2017. Global hearing health care: new findings and perspectives. Lancet 390:2503–2515. [DOI] [PubMed] [Google Scholar]
- Wingard JC, Zhao HB. 2015. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss - A Common Hereditary Deafness. Front Cell Neurosci 9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Fallon JB, Neil AJ, Pettingill LN, Geaney MS, Skinner SJ, Shepherd RK. 2011a. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics 8:774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Gillespie LN. 2012. Drug delivery to the inner ear. J Neural Eng 9:065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O'Leary SJ, Shepherd RK, Richardson RT. 2010. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther 18:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Pujol R, Landry TG, Fallon JB, Shepherd RK. 2017. Structural and Ultrastructural Changes to Type I Spiral Ganglion Neurons and Schwann Cells in the Deafened Guinea Pig Cochlea. J Assoc Res Otolaryngol 18:751–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. 2005. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol 487:147–165. [DOI] [PubMed] [Google Scholar]
- Wise AK, Tan J, Wang Y, Caruso F, Shepherd RK. 2016. Improved Auditory Nerve Survival with Nanoengineered Supraparticles for Neurotrophin Delivery into the Deafened Cochlea. PLoS One 11:e0164867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Tu T, Atkinson PJ, Flynn BO, Sgro BE, Hume C, O'Leary SJ, Shepherd RK, Richardson RT. 2011b. The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection. Hear Res 278:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel C, Dieter A, Huet A, Keppeler D, Duque-Afonso CJ, Vogl C, Hoch G, Jeschke M, Moser T. 2018. Optogenetic stimulation of cochlear neurons activates the auditory pathway and restores auditory-driven behavior in deaf adult gerbils. Sci Transl Med 10:eaao0540. [DOI] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, Kazmierczak P, Muller U. 2012. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151:1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cong N, Han Z, Huang Y, Chi F. 2013. Ectopic hair cell-like cell induction by Math1 mainly involves direct transdifferentiation in neonatal mammalian cochlea. Neurosci Lett 549:7–11. [DOI] [PubMed] [Google Scholar]
- Yang SM, Chen W, Guo WW, Jia S, Sun JH, Liu HZ, Young WY, He DZ. 2012. Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PLoS One 7:e46355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WH, Chiang H, Rees HA, Edge ASB, Liu DR. 2018. In vivo base editing of post-mitotic sensory cells. Nat Commun 9:2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. 1993. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res 65:69–78. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Shibata SB, Ranum PT, Smith RJH. 2018. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci Rep 8:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kim SM, Wang W, Cai C, Feng Y, Kong W, Lin X. 2018. Cochlear Gene Therapy for Sensorineural Hearing Loss: Current Status and Major Remaining Hurdles for Translational Success. Front Mol Neurosci 11:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen G, Wen L, Yang F, Shao AL, Li X, Long W, Mu L. 2013. Novel multiple agents loaded PLGA nanoparticles for brain delivery via inner ear administration: in vitro and in vivo evaluation. Eur J Pharm Sci 48:595–603. [DOI] [PubMed] [Google Scholar]
- Zhou R, Abbas PJ, Assouline JG. 1995. Electrically evoked auditory brainstem response in peripherally myelin- deficient mice. Hear Res 88:98–106. [DOI] [PubMed] [Google Scholar]
- Zine A, Van de Water T, de Ribaupierre F. 2000. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development 127:3373–3383. [DOI] [PubMed] [Google Scholar]
- Zinn E, et al. 2015. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Rep 12:1056–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR. 2015. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol 33:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]