Abstract
Objective
Perioperative blood transfusions have been implicated in decreased overall survival (OS) and disease-free survival (DFS) after resection for non–small cell lung cancer (NSCLC). We investigated the effects of single- and multiple-unit blood transfusions on OS, DFS, and recurrence after anatomic pulmonary resection.
Methods
From January 1, 2000, to June 30, 2016, 5709 consecutive patients underwent pulmonary resection for NSCLC at our institution. Exclusion criteria were stage IIIB-IV disease, incomplete resections, ill-defined histological subtypes, and nonanatomic wedge resections. For the 0 versus single-unit analysis, propensity scores were calculated from a logistic regression model that predicted the probability of patients receiving a single-unit transfusion. The resulting matching weights were incorporated into Cox models for OS, DFS, and cumulative incidence of recurrence (CIR), to compare no versus single-unit blood transfusion. We determined whether increasing numbers of blood transfusions influenced survival or recurrence using multivariable Cox models.
Results
Approximately 10% of patients received perioperative blood transfusion (median follow-up, 7.46 years [25th-75th percentile, 3.98–11.8]). There was no difference in OS, DFS, or CIR between patients receiving no transfusion and those receiving single-unit transfusion (P>0.05). However, a dose-response relationship was observed, demonstrating worse OS (overall P<0.001), DFS (overall P<0.001), and recurrence (overall P=0.010) with increasing units of blood transfused.
Conclusions
Although a single-unit blood transfusion did not affect survival in patients undergoing resection for NSCLC, higher unit perioperative blood transfusions were associated with significantly decreased long-term outcomes in a dose-dependent manner, suggesting avoidance or minimization of transfusions could improve long-term survival after lung resection.
INTRODUCTION
Perioperative blood transfusions have been associated with recurrence and decreased survival following surgical resection for non–small cell lung cancer (NSCLC) in some1–5 but not all6, 7 published series. The underlying reasons for this association are unclear, but the immunosuppressive effects of allogeneic blood transfusions have been suggested as one plausible mechanism.8 Historically, 7% to 55% of patients undergoing resection for primary lung cancer receive perioperative blood transfusions,1, 8, 9 with the majority receiving a single unit of packed red blood cells (RBCs). Unsurprisingly, patients receiving transfusions tend to have more medical comorbidities, higher-stage disease, and more postoperative complications. All these patient-level factors have been linked to poorer long-term outcomes, which can lead to confounding by indication. Confounding by indication occurs when the treatment is linked to a clinical condition that prompts the use of the treatment and, simultaneously, increases the risk of the outcome under investigation.10 Previous retrospective studies have attempted to address these challenges through various statistical means, but the extent to which blood transfusions affect long-term outcomes following curative-intent resection for NSCLC remains largely unanswered. A randomized trial to evaluate the effects of blood transfusion on long-term outcomes after resection for NSCLC would be neither feasible nor ethical, limiting us to observational data to further study this question.
Single-unit RBC transfusions are often discretionary and, therefore, arguably avoidable. We sought to determine (1) whether a single-unit perioperative allogeneic blood transfusion is associated with worse overall survival (OS), disease-free survival (DFS), and cumulative incidence of recurrence (CIR) in patients following anatomic pulmonary resection for NSCLC and (2) whether a dose-response relationship exists between increasing volume of blood transfusion and OS, DFS, and recurrence.
METHODS
Patient Selection
This study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center (MSK). Using the MSK Thoracic Service Database, we retrospectively identified patients who underwent curative-intent resection for NSCLC between January 1, 2000, and June 30, 2016. Clinical and demographic variables were obtained from our prospectively maintained database. Given the study time frame, determination of pathologic stage was based on the 7th edition of the American Joint Committee on Cancer Staging Manual.11 In an effort to minimize confounding by indication, we chose to perform the primary analysis by restricting the study population to patients who received either no transfusion or a single-unit blood transfusion. Restriction is an accepted technique that can be used to control for confounding by indication.10, 12 To determine whether there was a dose-response relationship between blood transfusion and long-term outcomes, we expanded our analysis to the whole cohort, including those with >1 RBC units administered, in order to quantify the association between increasing units of transfusion and long-term outcomes. Patients were excluded if their vital status was unknown, they underwent nonanatomic (wedge) resection, they had pathologic stage IIIB or IV disease, they underwent incomplete (R1 or R2) resection, or they had an ill-defined histologic subtype (e.g., NSCLC not otherwise specified). Decisions to transfuse were made at the discretion of the treating physician. There were no specific criteria in place to trigger transfusion.
Recurrence and Follow-Up
Recurrence was identified on the basis of clinical and/or radiologic suspicion and was confirmed with cytologic or histologic evaluation, as previously outlined by our group.13 Postoperative lung cancer surveillance was performed in accordance with National Comprehensive Cancer Network guidelines.14 In general, during the first two years after resection, patients received a physical examination, interval history, and chest/upper abdominal CT scan with or without contrast every 6 months. PET scans were obtained selectively, when surveillance CT scan findings were suspicious for recurrent disease or a new primary malignancy. After two years, follow-up visits and surveillance CT scans were performed annually. At all follow-up visits, the thoracic surgeon or a nurse practitioner trained in thoracic survivorship care reviewed the results of any new studies.
Statistical Analysis
The primary comparison was between no transfusion and a single-unit transfusion (0 vs 1 unit), whereas the secondary analysis was performed to assess the dose-response relationship with respect to the total units of RBCs administered. Two different approaches were used for each objective, to reflect the different focuses of interest. In the first analysis (i.e., 0 vs single-unit transfusion), we applied a matching-weights procedure to identify a group of patients with similar probability of receiving 0 or single-unit transfusion. In the second analysis, to assess the dose-response relationship, we did not apply the matching-weights procedure and instead implemented multivariable regression, adjusting for all clinically relevant variables considered in the matching-weights approach. The use of multivariable models reflected our intention in this analysis to estimate the effect of increasing transfusions on survival and disease recurrence after adjusting for all measurable clinical factors.
For both objectives, there were three outcomes of interest: (1) OS, (2) DFS, and (3) recurrence. Survival endpoints were defined from the date of surgery until the date of death for OS or until the date of recurrence or death for DFS. Patients were otherwise censored on the date of the last follow-up. When assessing recurrence, death without recurrence was considered a competing risk. Recurrence is expressed as the cumulative incidence of recurrence (CIR). CIR is based on the cumulative incidence function (or the probability of experiencing an event by a given time). In this case, the event of interest is recurrence. Conventionally, the cumulative incidence can be obtained using the Kaplan-Meier method (1 minus Kaplan-Meier estimate), where patients who do not experience recurrence are considered censored. However, if patients die without recurrence, such events are known as competing risk events, instead of noninformative censoring. Competing risks can alter the probability of experiencing recurrence, and, hence, Kaplan-Meier estimation may not be appropriate. In this case, the cumulative incidence function for recurrence must account for the presence of competing risk events.15
In the primary comparison, between no transfusion and a single-unit blood transfusion, the matching-weights procedure (which is analogous to 1:1 pairwise propensity score matching16) was used to create a pseudopopulation in which two groups of patients (no transfusion vs single-unit blood transfusion) have similar patient characteristics. Unlike 1:1 propensity-score matching, which excludes any unmatched patients, the matching-weights approach does not discard any patients; instead, it down-weighs the contributions of unmatched patients to the analyses. A logistic model was used to calculate the odds of receiving a single-unit blood transfusion. The resulting patient-level matching weights were based on the smaller of the predicted probabilities of receiving a single-unit blood transfusion, divided by the predicted probability of being assigned to the group the patient was actually in. Each patient therefore contributed a fraction toward the overall analysis of the outcome on the basis of matching weights.
The variables included in the logistic model were selected a priori on the basis of all relevant clinical factors associated with the likelihood of receiving a blood transfusion and are listed in Table 1. Performance of the matching-weights method was assessed by the absolute standardized mean difference, before and after applying the matching-weights procedure, between patients who received no transfusion and patients who received a single-unit transfusion, for the variables included in the matching procedure. An absolute standardized mean difference (ASMD) ≤0.1 indicates adequate covariate balance.17 The ASMD for each factor, before and after application of the matching weights, is reported in Table 1. Before applying the matching weights, ASMD values were >0.1 across all factors, confirming differences between the two groups (0 vs single-unit). After applying the matching-weights procedure, the ASMD values were ≤0.1 across every factor considered, indicating successful balance across all clinically relevant factors between the two groups (Table 1, Supplementary Figure 1). In addition, we present the ASMD derived from conventional propensity-score matching procedures (Supplementary Figure 1, either 1-to-1 matching or 5-to-1 matching) to show the superior balancing performance of the matching-weights procedure used in this study. The distribution of the propensity scores before and after application of the matching weights is presented as a mirror histogram to visually assess the success of the matching-weights approach; the mirror histogram shows good overlap in propensity scores between 0 and single-unit transfusion after application of matching weights (Supplementary Figure 2).
Table 1.
Patient demographic and clinical characteristics, before and after matching-weights procedure
| Before matching-weights procedure | After matching-weights procedurea | |||||
|---|---|---|---|---|---|---|
| Variable | No RBC transfusion (0 unit) | Single-unit transfusion (1 unit) | ASMD | No RBC transfusion (0 unit) | Single-unit transfusion (1 unit) | ASMD |
| N | 4352 | 197 | 191.8 | 193.1 | ||
| Age | 68.0 (61.0–75.0) | 69.0 (61.0–76.0) | 0.168 | 70.0 (64.0–76.0) | 69.0 (63.0–76.0) | 0.010 |
| Male | 1862 (42.8) | 86 (43.7) | 0.018 | 83 (43.3) | 83 (42.9) | 0.008 |
| Pack-years | 32.0 (10.0–53.5) | 40.0 (20.0–61.2) | 0.260 | 40.8 (20.0–60.0) | 40.0 (20.0–62.1) | 0.023 |
| Smoking status | 0.203 | 0.023 | ||||
| Never | 714 (16.4) | 19 (9.6) | 17.8 (9.3) | 19.0 (9.8) | ||
| Former | 3039 (69.8) | 147 (74.6) | 144.7 (75.4) | 143.9 (74.5) | ||
| Current | 599 (13.8) | 31 (15.7) | 29.3 (15.3) | 30.3 (15.7) | ||
| Comorbidities | ||||||
| Pulmonary | 1277 (29.3) | 71 (36.0) | 0.143 | 68.1 (35.5) | 68.1 (35.2) | 0.006 |
| Cardiac | 2335 (53.7) | 125 (63.5) | 0.200 | 120.6 (62.9) | 121.9 (63.1) | 0.005 |
| Endocrine | 524 (12.0) | 33 (16.8) | 0.134 | 31.1 (16.2) | 32.0 (16.6) | 0.009 |
| Renal | 81 (1.9) | 10 (5.1) | 0.176 | 10.0 (5.2) | 9.0 (4.8) | 0.018 |
| Preop-Hemoglobin | 13.2 (12.1–14.1) | 11.3 (10.1–12.4) | 1.096 | 11.3 (10.5–12.5) | 11.3 (10.1–12.5) | 0.021 |
| SUV | 5.8 (2.7–10.7) | 10.8 (5.9–17.6) | 0.654 | 11.1 (6.2–16.0) | 10.4 (5.8–16.8) | 0.009 |
| FEV1 | 90.0 (77.0–103.0) | 84.0 (74.0–94.0) | 0.351 | 83.0 (70.0–96.0) | 84.0 (74.0–94.0) | 0.017 |
| DLCO | 80.0 (66.0–95.0) | 69.0 (59.0–80.0) | 0.565 | 68.0 (56.0–82.0) | 69.0 (59.0–88.0) | 0.011 |
| Neoadjuvant chemotherapy | 712 (16.4) | 93 (47.2) | 0.702 | 86.4 (45.1) | 89.4 (46.3) | 0.024 |
| Neoadjuvant radiation | 90 (2.1) | 17 (8.6) | 0.295 | 13.8 (7.2) | 15.6 (8.1) | 0.032 |
| ASA classification | 0.295 | 0.021 | ||||
| I | 12 (0.3) | 0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | ||
| II | 1200 (27.7) | 32 (16.3) | 30.0 (15.6) | 31.7 (16.4) | ||
| III | 2986 (68.8) | 154 (78.6) | 152.0 (79.3) | 151.7 (78.5) | ||
| IV | 140 (3.2) | 10 (5.1) | 9.8 (5.1) | 9.8 (5.1) | ||
| Operation type | 0.400 | 0.023 | ||||
| Lobectomy | 3681 (84.6) | 153 (77.7) | 147.5 (76.9) | 150.0 (77.6) | ||
| Bilobectomy | 122 (2.8) | 10 (5.1) | 9.4 (4.9) | 9.6 (5.0) | ||
| Pneumonectomy | 152 (3.5) | 25 (12.7) | 25.2 (13.1) | 24.6 (12.7) | ||
| Segmentectomy | 397 (9.1) | 9 (4.6) | 9.7 (5.0) | 9.0 (4.7) | ||
| VATS | 1555 (35.7) | 19 (9.6) | 0.655 | 18.5 (9.6) | 19.0 (9.8) | 0.007 |
| Robot-assisted | 458 (10.5) | 11 (5.6) | 0.182 | 11.3 (5.9) | 11.0 (5.7) | 0.009 |
| Intrapericardial resection | 60 (1.4) | 10 (5.1) | 0.210 | 9.6 (5.0) | 9.7 (5.0) | 0.001 |
| Chest wall resection | 75 (1.7) | 21 (10.7) | 0.377 | 19.1 (9.9) | 19.0 (9.9) | 0.003 |
| Vascular resection | 24 (0.6) | 5 (2.5) | 0.162 | 4.2 (2.2) | 4.2 (2.2) | 0.001 |
| Pathologic stage | 0.540 | 0.017 | ||||
| IA | 1927 (44.3) | 48 (24.4) | 46.5 (24.2) | 47.2 (24.4) | ||
| IB | 871 (20.0) | 28 (14.2) | 27.3 (14.3) | 27.9 (14.4) | ||
| IIA | 565 (13.0) | 45 (22.8) | 43.8 (22.8) | 44.2 (22.9) | ||
| IIB | 288 (6.6) | 24 (12.2) | 24.3 (12.7) | 23.4 (12.1) | ||
| IIIA | 701 (16.1) | 52 (26.4) | 49.9 (26.0) | 50.5 (26.1) | ||
| Pathologic subtype | 0.403 | 0.044 | ||||
| Adenocarcinoma | 3272 (75.2) | 113 (57.4) | 107.2 (55.9) | 111.6 (57.4) | ||
| Adenosquamous | 66 (1.5) | 7 (3.6) | 7.0 (3.6) | 6.3 (3.3) | ||
| Invasive mucinous adenocarcinoma | 44 (1.0) | 1 (0.5) | 1.1 (0.6) | 1.0 (0.5) | ||
| Large cell carcinoma | 130 (3.0) | 11 (5.6) | 11.6 (6.1) | 10.9 (5.6) | ||
| Squamous cell carcinoma | 747 (17.2) | 59 (29.9) | 58.2 (30.4) | 57.4 (29.7) | ||
| Other NSCLC histologic subtype | 93 (2.1) | 6 (3.0) | 6.6 (3.5) | 6.0 (3.1) | ||
Data are no. (%) or median (interquartile range). ASA, American Society of Anesthesiologists physical status classification; ASMD, absolute standardized mean difference; DLCO, lung diffusion capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; NSCLC, non–small cell lung cancer; SUV, standardized uptake value; VATS, video-assisted thoracoscopic surgery.
The effective sample sizes may be nonintegers, as patients contribute a fraction of themselves to the analysis cohort on the basis of patient-level matching weights.
For the primary comparison of 0 versus 1 unit of blood transfused, survival was estimated using the Kaplan-Meier approach for OS and DFS and the cumulative incidence approach for recurrence (CIR), measured from the time of surgery, incorporating matching weights. For each survival curve, we present unweighted (without matching weights) and weighted (with matching weights) numbers at risk. For the matching-weights analyses, each patient may contribute a fraction of outcomes reflecting the patient-level weights; therefore, the effective sample size may not be an integer. The effect of having a single-unit blood transfusion on prognosis was quantified using the Cox proportional hazards model for OS and DFS and the cause-specific Cox model for recurrence. Patient-level matching weights were incorporated in all analyses.
In the dose-response relationship analyses, we considered the total number of RBC units administered. OS and DFS were estimated using the Kaplan-Meier approach and compared between groups using the log-rank test, whereas CIR was estimated using the cumulative incidence approach and compared between groups using Gray’s test, where death without recurrence was considered a competing event. There was marginal evidence of nonlinearity of the number of RBCs, using restricted cubic splines on a univariable basis (p=0.061). To address potential nonlinearity and to facilitate ease of clinical use, we present the results of the dose-response analyses using a categorical version of RBC “dose” (0 or single-unit, 2 units, 3 to 7 units, and ≥8 units) on the basis of the results from the primary analysis of 0 versus single-unit and the distribution of RBCs administered. The association between total RBC units (as a categorical variable) and OS or DFS was estimated using multivariable Cox proportional hazards model. The association between total RBC units and recurrence was estimated using a cause-specific Cox model. Variables included in the multivariable models were determined a priori on the basis of clinical relevance to the outcome from the same factors used in the matching-weights procedure.
We assessed the patterns of missing data and observed that the assumption of missing completely at random was not violated. To handle missing data, multiple imputation by chained equation was conducted using the mice package in R 3.5.1 (R Core Team, Vienna, Austria) to derive 10 imputed data sets with a maximum of 50 iterations each.18 This method is based on fully conditional specification, in which each incomplete variable is imputed by a separate model. All variables considered in subsequent analyses were included in the imputation models, including survival outcomes.19 The imputation method for pack-years (<1% missing), primary tumor standardized uptake value (18% missing), forced expiratory volume in 1 second (2% missing), diffusion (6% missing), and preoperative hemoglobin level (1% missing) was based on predictive mean modeling to ensure that imputed values were plausible. We used a polytomous logistic regression method to impute missing data for American Society of Anesthesiologists physical status classification (<1% missing). For each long-term outcome, the analysis model was fit on each imputed data set for the outcome of interest, and estimates from each of the 10 imputed data sets were pooled into a single set of estimates and standard errors using Rubin’s rules.20
All statistical tests were 2-sided, and P<0.05 was considered statistically significant. Statistical analyses were conducted using Stata 13.1 (StataCorp, College Station, TX) and R 3.5.1 (R Development Core Team, Vienna, Austria), including the survival, rms, and riskRegression R packages. The matching-weights procedure was performed with the survey and tableone R packages. Multiple imputation was conducted with the mice R package with user-written function to pool estimates according to Rubin’s rule and Barnard-Rubin adjusted degrees of freedom.
RESULTS
Patient Characteristics
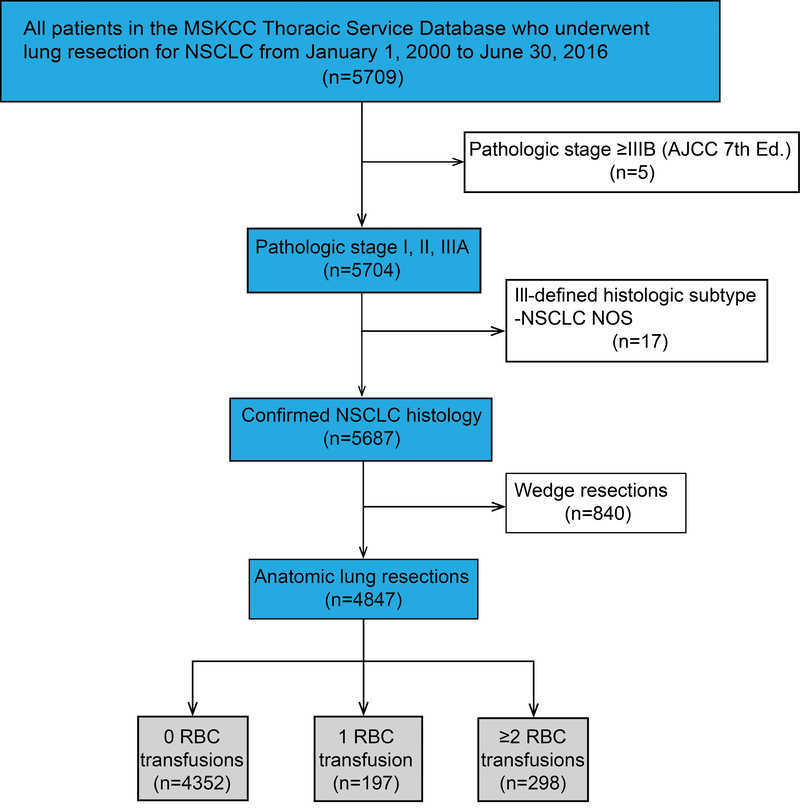
A total of 4847 patients met the inclusion criteria (Figure 1). Of these, 4352 (90%) received no blood transfusions during the postoperative period, 197 (4%) received a single-unit blood transfusion, and 298 (6%) received a blood transfusion with ≥2 units. Four hundred ninety-five patients (10.2%) received ≥1 perioperative blood transfusion. Median duration of follow-up was 7.46 years (25th–75th percentile, 3.98–11.38 years). Patient demographic and clinical characteristics are listed in Table 1. The 5-year OS, DFS and CIR for the entire cohort were 65% (95% CI, 64%−67%), 56% (95% CI, 54%−57%), and 29.3% (95% CI, 28%−30.7%), respectively. The 5-year OS, DFS, and CIR for the no-transfusion group were 68% (95% CI, 66%−69%), 58% (95% CI, 57%−60%), and 28.5% (95% CI, 27.1%−29.9%), respectively.
Figure 1.
CONSORT diagram. The study cohort included all consecutive patients who underwent anatomic R0 resection for pathologic stage I, II, or IIIA non–small cell lung cancer (NSCLC). R0, microscopically margin-negative resection.
Effect of a Single-Unit Blood Transfusion on Outcomes
We first limited our analysis to compare OS, DFS, and CIR between patients who received no transfusion and those who received a single-unit blood transfusion among the subcohort of 4549 patients (4352 with 0 units of blood and 197 with 1 unit of blood). After applying the matching-weights procedure, the effective sample sizes were 192 in the 0-unit group and 193 in the single-unit group (Table 1).
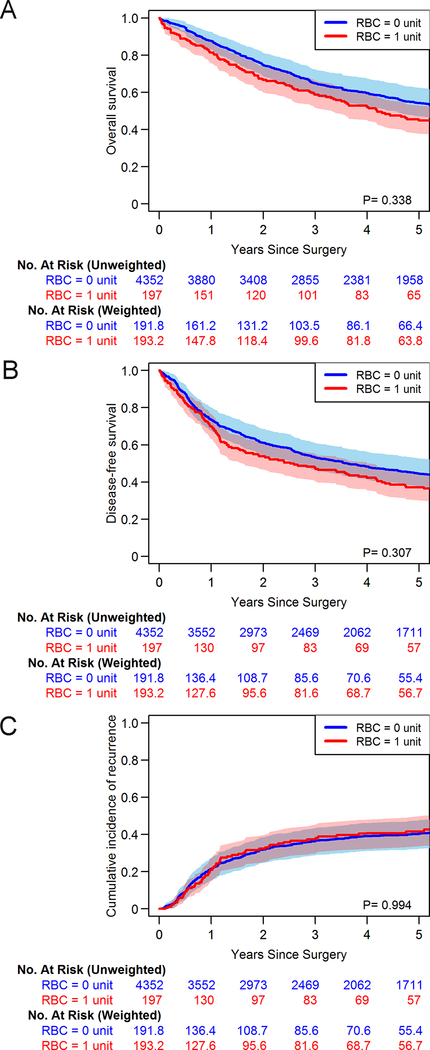
Estimated OS, DFS, and CIR curves are presented in Figure 2A–C, along with model-based P values, both incorporating matching weights. In univariable Cox proportional hazards models, there were no significant associations between receiving 1 and 0 units of blood and OS (HR 1.14; 95% CI, 0.88–1.47; P=0.338), DFS (HR 1.14; 95% CI, 0.89–1.45; P=0.308), or recurrence (cause-specific HR 1.00; 95% CI, 0.72–1.40; P=0.994) (Table 2). We repeated the 0 versus single-unit transfusion comparison among patients with pathologic stage I disease and obtained similar results (Supplementary Figure 3A–C).
Figure 2.
Kaplan-Meier curves after applying matching weights for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) for patients receiving no red blood cell (RBC) transfusion versus patients receiving a single-unit RBC transfusion. Numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size.
Table 2.
Long-term outcomes for 1 unit of red blood cells transfused versus no transfusion, after applying the matching-weights procedurea
| Outcome, RBC units | N | No. with events | HR (95% CI) | P |
|---|---|---|---|---|
| Overall survivalb | 0.338 | |||
| 0 | 4352 | 1923 | Reference | |
| 1 | 197 | 121 | 1.14 (0.88–1.47) | |
| Disease-free survivalb | 0.308 | |||
| 0 | 4352 | 2240 | Reference | |
| 1 | 197 | 137 | 1.14 (0.89–1.45) | |
| Recurrencec | 0.994 | |||
| 0 | 4352 | 1218 | Reference | |
| 1 | 197 | 68 | 1.00 (0.72–1.40) |
CI, confidence interval; HR, hazard ratio; RBC, red blood cell.
See the Methods section for a list of variables included in the matching-weights procedure.
Cox proportional hazards model
Cause-specific Cox model
Dose-Response Relationship Between Increasing Blood Transfusions and Outcomes
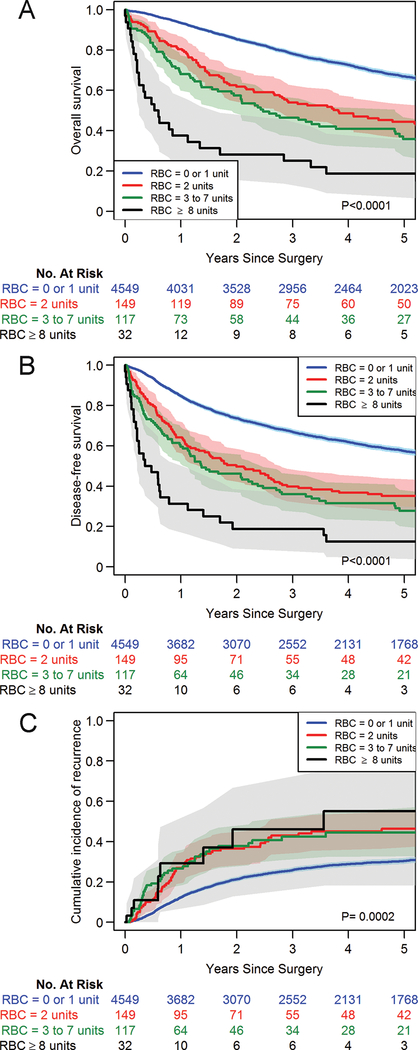
To examine the dose-response relationship between blood transfusion and long-term outcomes, we expanded our analysis to all patients, including those with no transfusion as well as with 1 or more units transfused. On the basis of the lack of significant differences across all three long-term outcomes in the primary analysis of 0 versus single-unit transfusion, we present the dose-response analyses using a categorical version of RBC where the reference group includes patients who received 0 or a single-unit RBC transfusion. Other categories were formed on the basis of the distribution of non-zero units of RBC (25th, 50th, 75th percentile): 2 units, 3 to 7 units, and ≥8 units (top 10th percentile) to represent very high-levels of transfusion (Supplementary Table 1). Estimated OS, DFS, and CIR curves by RBC units are presented in Figures 3A–C. A dose-dependent relationship between units of blood transfused and both OS and DFS was observed with the multivariable Cox proportional hazards analysis (Table 3). Compared with patients transfused with 0 or 1 unit of RBCs, patients administered increasing units of RBCs had worse OS (2-units: HR 1.55 [95% CI, 1.26–1.91; P<0.001]; 3 to 7 units: HR 2.02 [95% CI, 1.61–2.53; P<0.001]; ≥8 units: 4.29 [95% CI, 2.91–6.33; P<0.001]) (Supplementary Table 2). A similar dose-response relationship was observed for DFS, when comparing with patients who received 0 or 1-unit transfusions (2-units: HR 1.44 [95% CI, 1.19–1.76; P<0.0001]; 3 to 7 units: HR 1.85 [95% CI, 1.49–2.30; P<0.0001]); ≥8 units: HR 3.57 (95% CI, 2.45–5.21; P<0.0001]) (Supplementary Table 3). Results from the multivariable cause-specific Cox model also indicated worse hazard of recurrence with increasing units of blood transfused, compared with 0 or single-unit transfusion (2-units: HR 1.35 [95% CI, 1.04–1.75; P=0.023]; 3 to 7 units: HR 1.50 (95% CI, 1.09–2.07; P=0.013]; ≥8 units: HR 1.58 [95% CI, 0.80–3.10; P=0.187]) (Supplementary Table 4). Similar results were observed when the analyses were limited to patients with pathologic stage I disease (Supplementary Figure 4). To further address the potentially confounding effects of complications, we performed post hoc sensitivity analyses for the 0 versus 1-unit transfusion comparison as well as for escalating units of RBC transfusion, which demonstrated no differences on the basis of the presence or absence of postoperative complications (Supplementary Figures 5 and 6). We conducted another post hoc exploratory analysis to determine the effects of intraoperative versus postoperative transfusions on OS, DFS, and CIR. Although there was no significant difference in OS observed in either the 0 versus single-unit or the escalating transfusion analyses in terms of intraoperative versus postoperative transfusions, there was a trend toward significance for DFS with intraoperative transfusions in the dose-escalation analysis (P=0.063) and a trend toward significance for CIR in the 0 versus single-unit transfusion group (P=0.054; Supplementary Figure 7). CIR was significantly higher among patients who received intraoperative transfusion in the dose-escalation analysis (P<0.0001; Supplementary Figure 7).
Figure 3.
Kaplan-Meier curves depicting a dose-response relationship for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) between patients receiving no or single-unit red blood cell (RBC) transfusion and those receiving transfusion with increasing units of blood. No matching weights were applied in this set of analyses.
Table 3.
Long-term outcomes for red blood cells transfused, compared with 0 or 1 units, after multivariable analysesa
| Outcome, RBC units | N | No. with events | HR (95% CI) | P | Overall P |
|---|---|---|---|---|---|
| Overall survivalb | <0.0001 | ||||
| 0 or 1 | 4549 | 2044 | Reference | ||
| 2 | 149 | 102 | 1.55 (1.26–1.91) | <0.0001 | |
| 3 to 7 | 117 | 85 | 2.02 (1.61–2.53) | <0.0001 | |
| ≥8 | 32 | 29 | 4.29 (2.91–6.33) | <0.0001 | |
| Disease-free survivalb | <0.0001 | ||||
| 0 or 1 | 4549 | 2377 | Reference | ||
| 2 | 149 | 113 | 1.44 (1.19–1.76) | <0.0001 | |
| 3 to 7 | 117 | 90 | 1.85 (1.49–2.30) | <0.0001 | |
| ≥8 | 32 | 30 | 3.57 (2.45–5.21) | ><0.0001 | |
| Recurrencec | 0.0099 | ||||
| 0 or 1 | 4549 | 1286 | Reference | ||
| 2 | 149 | 64 | 1.35 (1.04–1.75) | 0.023 | |
| 3 to 7 | 117 | 41 | 1.50 (1.09–2.07) | 0.013 | |
| ≥8 | 32 | 9 | 1.58 (0.80–3.10) | 0.187 |
CI, confidence interval; HR, hazard ratio; RBC, red blood cell.
See Supplementary Tables 1, 2 and 3 for full multivariable models results.
Cox proportional hazards model
Cause-specific Cox model
DISCUSSION
A single-unit perioperative blood transfusion was not associated with worse OS, DFS, or recurrence following anatomic lung resection for NSCLC. However, for escalating blood transfusions above 1 unit, a significant dose-response relationship was observed for OS, DFS, and recurrence. These findings are consistent with those of some studies1–5, 21 and contrary to the findings of others.6, 22, 23
Wang and colleagues performed a meta-analysis, including 23 eligible studies, to evaluate the effects of perioperative blood transfusion on clinical outcomes in patients with resected stage I-IV NSCLC.5 The results of this pooled analysis, which included all patients regardless of stage, mirrored those of the present study in that OS, DFS, and recurrence rates were worse in the transfused group. However, when the authors limited their analysis to stage I patients, in contrast to our results for this subgroup, Wang et al. observed significantly higher rates of disease recurrence in transfused patients. One possible explanation for this difference is that we used the cumulative incidence function to analyze recurrence, which accounts for competing risk events (i.e., death before recurrence), and censoring, neither of which is addressed by the relative risk approach used in the Wang et al study. Of importance, our group recently showed that noncancer-specific death in patients with stage I NSCLC is a significant competing event and that it increases with advancing patient age.24 To minimize heterogeneity and reduce confounding, Nosotti et al. and Ng et al. limited inclusion in their respective studies to patients with stage I NSCLC who underwent lobectomy.1, 2 Both studies demonstrated worse OS and DFS in patients who received perioperative blood transfusions—similar to the results of the present study. In our prespecified subset analysis of stage I patients, we observed similar findings with respect to OS and DFS.
Several published studies demonstrate findings at least partially inconsistent with those in the present study. Interestingly, in 1988 Keller et al. reported on a retrospective cohort of 352 pathologic stage I and II NSCLC patients from our institution, demonstrating no difference in DFS or recurrence.6 The authors did not report on OS. More recently, Cata and colleagues at the MD Anderson Cancer Center reported on a total of 636 patients who underwent resection for NSCLC. After propensity matching, blood transfusion had a significant negative effect on OS but not on recurrence-free survival.22 A recent review paper by Churchhouse and colleagues highlights the lack of consensus regarding the role blood transfusion plays in long-term postoperative outcomes for patients with NSCLC.23
The present study can contribute to the current evidence in several ways. First, it demonstrates a clear dose-dependent effect of increasing volume of blood transfusion on OS, DFS, and recurrence. Although causality cannot be assigned to transfusions, given the retrospective nature of this study, the survival curves for the no-transfusion and the ≥2-unit transfusion groups continue to diverge somewhat over time, lending further support to this relationship. Second, to our knowledge, the finding that a single-unit blood transfusion is not sufficient to have an effect on recurrence or survival in patients with NSCLC has not been previously reported in the literature. Third, we limited our patient sample to those who underwent complete anatomic resection; wedge resections were excluded because they have been associated with poorer OS, DFS, and recurrence rates. Finally, this is the largest single-center series to evaluate the effects of blood transfusion on postoperative recurrence and survival in patients with NSCLC, permitting a more robust statistical analysis using propensity-matched weighting. Unlike the more commonly used 1:1 propensity matching, this technique allows for the inclusion of all patients in the data set, rather than a smaller subset of matched pairs—thus, it maintains the full benefit of the large cohort size.
This study has several limitations. First, this is a retrospective study that is subject to confounding by factors not available in our data set. Despite this, the results of our study could have still been influenced by the clinical indication for blood transfusion, which was unavailable in our database. In terms of the 0 versus 1-unit analysis, a single-unit transfusion may be insufficient to affect long-term outcomes, or the lack of an association may simply be an issue of inadequate sample size in the single-unit transfusion group. Second, we had information on perioperative blood transfusions only during the index hospitalization (i.e., admission for surgery). Blood transfusions given before or after this admission were not included in the analysis. Third, we included a heterogeneous patient population with stage I through IIIA NSCLC undergoing a variety of procedure types and approaches; however, to address this, we performed a subgroup analysis of only patients with stage I disease, which demonstrated similar results in terms of OS and DFS but not CIR. The lack of an observed difference in recurrence between the no-transfusion and transfusion groups may also be related to small sample size and the limited number of recurrence events in the stage I population.
In conclusion, a single-unit perioperative blood transfusion did not affect OS, DFS, or recurrence in patients undergoing anatomic pulmonary resection for NSCLC. However, a dose-response relationship was observed, demonstrating increasing recurrence rates and decreasing survival with escalating RBC transfusions. These results suggest that improved perioperative blood conservation efforts and the avoidance of discretionary transfusions may result in lower recurrence and improved long-term survival after lung resection for NSCLC.
Supplementary Material
Supplementary Figure 1. Exploratory analyses to justify the choice of the matching-weights approach, compared with the propensity-score matching approach. PSMatch refers to the two cases where we applied the propensity-score matching procedure, with either 5:1 (PSMatch: 5 to 1) or 1:1 (PSMatch: 1 to 1) nearest neighbor greedy matching algorithm with caliper width, as suggested by Austin17 (i.e., 0.2 of the standard deviation of the logit of the propensity scores obtained using the same set of variables to calculate the matching weights). Propensity-score matching was conducted using the “matchit” package in R 3.1.1 (R Development Core Team, Vienna, Austria). Optimal performance of an approach can be determined by the balance of covariates between the groups after application of the approach. Here, the balance of covariates between 0 and 1 unit of blood was assessed by the absolute standardized mean difference (ASMD). ASMD ≤0.1 indicates balance in the covariate between the two groups. In the original data (Unweighted), all but 1 variable was well-balanced between the two groups. Application of the propensity-score matching procedure, whether 5 to 1 or 1 to 1 only managed to balance a subset of the covariates. The matching-weights approach performed the best in terms of balancing across all covariates between the two groups. The matching-weights procedure does not require the specification of a caliper width.
Supplementary Figure 2. A mirror histogram plotting the distribution of the propensity scores before and after the matching-weights procedure. Above the horizontal line is the histogram of the propensity scores in the 0-unit group, and below is that of the 1-unit group. The white bars represent the distribution of the propensity score of the original sample. The green shaded bars depict the effective sample after application of the matching-weights procedure. The height of the shaded bar is the summation of the weights of the (treated or control) subjects within the corresponding stratum. It may not be an integer. Analogous to the case of 1:1 pair matching, the shaded areas are always within the white histogram bars.
Supplementary Figure 3. Kaplan-Meier curves for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) among patients with pathologic stage I disease receiving no red blood cell (RBC) transfusion versus those receiving a single-unit RBC transfusion. Numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size.
Supplementary Figure 4. Kaplan-Meier curves depicting a dose-response relationship for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) between patients with pathologic stage I disease receiving no or single-unit red blood cell (RBC) transfusion versus those receiving transfusion with increasing units of blood. No matching weights were applied in this set of analyses.
Supplementary Figure 5. Kaplan-Meier curves for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) by complication status (No or Yes) among patients receiving no red blood cell (RBC) transfusion versus those receiving a single-unit RBC transfusion. Numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size.
Supplementary Figure 6. Kaplan-Meier curves depicting a dose-response relationship for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) by complication status (No or Yes) among patients receiving no or single-unit red blood cell (RBC) transfusion versus those receiving transfusion with increasing units of blood. No matching weights were applied in this set of analyses.
Supplementary Figure 7. Exploratory analyses to examine the relationship between timing of transfusion (intraoperative or postoperative) and outcomes. Kaplan-Meier curves for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) by complication status (No or Yes) among patients receiving no red blood cell (RBC) transfusion versus those receiving a single-unit RBC transfusion (left panel) or in increasing units of blood (right panel). In the left panel, numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size. In the right panel, no matching weights were applied in this set of analyses.
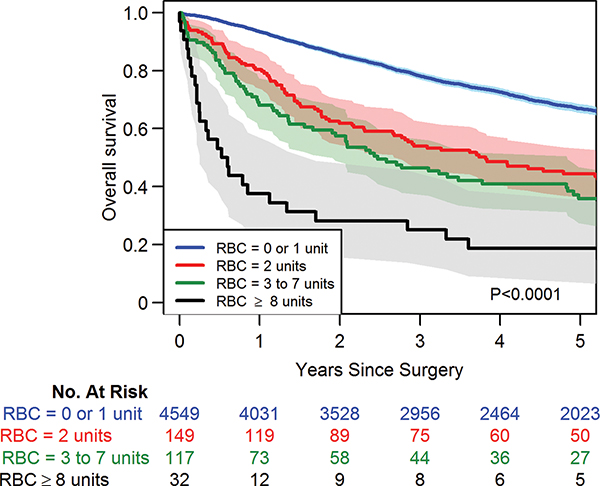
Central Picture
Relationship between increasing number of blood transfusions and overall survival.
Central Message
The avoidance or minimization of blood transfusions may decrease recurrence and improve long-term survival in patients undergoing anatomic lung resection for non-small cell lung cancer.
Perspective Statement.
Perioperative allogeneic blood transfusions are known to be immunosuppressive; however, their effect on long-term survival following surgical resection for non–small cell lung cancer is not well understood. The findings of this study demonstrate a dose-dependent association of blood transfusion on survival and recurrence following anatomic resection for non-small cell lung cancer.
ACKNOWLEDGMENTS
We are grateful to Joe Dycoco for his assistance with data management and David Sewell and Alex Torres for their editorial assistance.
Funding Support: This work was supported, in part, by the National Institutes of Health Comprehensive Cancer Center Core Grant (P30 CA008748).
Glossary of Abbreviations
- ASMD
absolute standardized mean difference
- CIR
cumulative incidence of recurrence
- DFS
disease-free survival
- MSK
Memorial Sloan Kettering Cancer Center
- NSCLC
non–small cell lung cancer
- OS
overall survival
- RBC
red blood cell
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Nosotti M, Rebulla P, Riccardi D, Baisi A, Bellaviti N, Rosso L, et al. Correlation between perioperative blood transfusion and prognosis of patients subjected to surgery for stage I lung cancer. Chest. 2003;124:102–7. [DOI] [PubMed] [Google Scholar]
- 2.Ng T, Ryder BA, Chern H, Sellke FW, Machan JT, Harrington DT, et al. Leukocyte-depleted blood transfusion is associated with decreased survival in resected early-stage lung cancer. J Thorac Cardiovasc Surg. 2012;143:815–9. [DOI] [PubMed] [Google Scholar]
- 3.Moores DW, Piantadosi S, McKneally MF. Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg. 1989;47:346–51. [DOI] [PubMed] [Google Scholar]
- 4.Piantadosi S, Moores DW, McKneally MF. The adverse effect of perioperative blood transfusion in lung cancer. Chest. 1994;106:382S–4S. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Luo L, Huang H, Yu J, Pan C, Cai X, et al. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. 2014;97:1827–37. [DOI] [PubMed] [Google Scholar]
- 6.Keller SM, Groshen S, Martini N, Kaiser LR. Blood transfusion and lung cancer recurrence. Cancer. 1988;62:606–10. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P, Michelet P, Barlesi F, Thirion X, Doddoli C, Giudicelli R, et al. Impact of blood transfusions on outcome after pneumonectomy for thoracic malignancies. Eur Respir J. 2007;29:565–70. [DOI] [PubMed] [Google Scholar]
- 8.Cata JP, Gutierrez C, Mehran RJ, Rice D, Nates J, Feng L, et al. Preoperative anemia, blood transfusion, and neutrophil-to-lymphocyte ratio in patients with stage i non-small cell lung cancer. Cancer Cell Microenviron. 2016;3:e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54. [DOI] [PubMed] [Google Scholar]
- 10.Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–54. [DOI] [PubMed] [Google Scholar]
- 11.Edge SBBD, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 12.McMahon AD. Approaches to combat with confounding by indication in observational studies of intended drug effects. Pharmacoepidemiol Drug Saf. 2003;12:551–8. [DOI] [PubMed] [Google Scholar]
- 13.Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz NM. The evolution of cardiothoracic critical care. J Thorac Cardiovasc Surg. 2011;141:3–6. [DOI] [PubMed] [Google Scholar]
- 15.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9:215–34. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. 2011. 2011;45:67. [Google Scholar]
- 19.Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–101. [DOI] [PubMed] [Google Scholar]
- 20.Rubin D Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 1987. [Google Scholar]
- 21.Little AG, Wu HS, Ferguson MK, Ho CH, Bowers VD, Segalin A, et al. Perioperative blood transfusion adversely affects prognosis of patients with stage I non-small-cell lung cancer. Am J Surg. 1990;160:630–2; discussion 3. [DOI] [PubMed] [Google Scholar]
- 22.Cata JP, Chukka V, Wang H, Feng L, Gottumukkala V, Martinez F, et al. Perioperative blood transfusions and survival in patients with non-small cell lung cancer: a retrospective study. BMC Anesthesiol. 2013;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchhouse AM, Mathews TJ, McBride OM, Dunning J. Does blood transfusion increase the chance of recurrence in patients undergoing surgery for lung cancer? Interact Cardiovasc Thorac Surg. 2012;14:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eguchi T, Bains S, Lee MC, Tan KS, Hristov B, Buitrago DH, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol. 2017;35:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Exploratory analyses to justify the choice of the matching-weights approach, compared with the propensity-score matching approach. PSMatch refers to the two cases where we applied the propensity-score matching procedure, with either 5:1 (PSMatch: 5 to 1) or 1:1 (PSMatch: 1 to 1) nearest neighbor greedy matching algorithm with caliper width, as suggested by Austin17 (i.e., 0.2 of the standard deviation of the logit of the propensity scores obtained using the same set of variables to calculate the matching weights). Propensity-score matching was conducted using the “matchit” package in R 3.1.1 (R Development Core Team, Vienna, Austria). Optimal performance of an approach can be determined by the balance of covariates between the groups after application of the approach. Here, the balance of covariates between 0 and 1 unit of blood was assessed by the absolute standardized mean difference (ASMD). ASMD ≤0.1 indicates balance in the covariate between the two groups. In the original data (Unweighted), all but 1 variable was well-balanced between the two groups. Application of the propensity-score matching procedure, whether 5 to 1 or 1 to 1 only managed to balance a subset of the covariates. The matching-weights approach performed the best in terms of balancing across all covariates between the two groups. The matching-weights procedure does not require the specification of a caliper width.
Supplementary Figure 2. A mirror histogram plotting the distribution of the propensity scores before and after the matching-weights procedure. Above the horizontal line is the histogram of the propensity scores in the 0-unit group, and below is that of the 1-unit group. The white bars represent the distribution of the propensity score of the original sample. The green shaded bars depict the effective sample after application of the matching-weights procedure. The height of the shaded bar is the summation of the weights of the (treated or control) subjects within the corresponding stratum. It may not be an integer. Analogous to the case of 1:1 pair matching, the shaded areas are always within the white histogram bars.
Supplementary Figure 3. Kaplan-Meier curves for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) among patients with pathologic stage I disease receiving no red blood cell (RBC) transfusion versus those receiving a single-unit RBC transfusion. Numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size.
Supplementary Figure 4. Kaplan-Meier curves depicting a dose-response relationship for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) between patients with pathologic stage I disease receiving no or single-unit red blood cell (RBC) transfusion versus those receiving transfusion with increasing units of blood. No matching weights were applied in this set of analyses.
Supplementary Figure 5. Kaplan-Meier curves for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) by complication status (No or Yes) among patients receiving no red blood cell (RBC) transfusion versus those receiving a single-unit RBC transfusion. Numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size.
Supplementary Figure 6. Kaplan-Meier curves depicting a dose-response relationship for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) by complication status (No or Yes) among patients receiving no or single-unit red blood cell (RBC) transfusion versus those receiving transfusion with increasing units of blood. No matching weights were applied in this set of analyses.
Supplementary Figure 7. Exploratory analyses to examine the relationship between timing of transfusion (intraoperative or postoperative) and outcomes. Kaplan-Meier curves for overall survival (A), disease-free survival (B), and cumulative incidence of recurrence (C) by complication status (No or Yes) among patients receiving no red blood cell (RBC) transfusion versus those receiving a single-unit RBC transfusion (left panel) or in increasing units of blood (right panel). In the left panel, numbers at risk refer to the original sample size (“No. at risk [unweighted]”) and the fractional number of patients after applying the matching weights (“No. at risk [weighted]”), reflecting the effective sample size. In the right panel, no matching weights were applied in this set of analyses.