Abstract
Aims:
To compare long-term criminal justice outcomes among opioid dependent individuals randomized to receive buprenorphine or methadone.
Design, setting and participants:
5-year follow-up was conducted in 2011-14 of 303 opioid-dependent participants entering three opioid treatment programs in California, USA in 2006-09 and randomized to receive either buprenorphine/naloxone or methadone.
Intervention and comparator:
Participants received buprenorphine/naloxone (BUP; n=179) or methadone (MET; n=124) for 24 weeks and then were tapered off their treatment over ≤8 weeks or referred for ongoing clinical treatment. Midway through the study, the randomization scheme was switched from 1:1 BUP:MET to 2:1 because of higher drop out in the BUP arm.
Measurements:
Study outcomes included arrests and self-reported incarceration. Predictors included randomization condition (buprenorphine vs. methadone), age, gender, race/ethnicity, use of cocaine, drug injection in the 30 days prior to baseline, and study site. Treatment status (buprenorphine, methadone, none) during follow-up was included as a time-varying covariate.
Findings:
There was no significant difference by randomization condition in the proportion arrested (buprenorphine: 55.3%, methadone: 54.0%) or incarcerated (40.9%, 47.3%) during follow-up. Among methadone-randomized individuals, arrest was less likely with methadone treatment (0.50, 0.35-0.72) during follow-up (relative to no treatment) and switching to buprenorphine had a lower likelihood of arrest than those receiving no treatment (0.39, 0.18-0.87). Among buprenorphine-randomized individuals, arrest was less likely with receipt of buprenorphine (0.49, 0.33-0.75) during follow-up and switching to methadone had a similar likelihood of arrest as methadone-randomized individuals receiving no treatment. Likelihood of arrest was also negatively associated with older age (0.98, 0.96-1.00); it was positively associated with Hispanic ethnicity (1.63, 1.04-2.56), cocaine use (2.00, 1.33-3.03), injection drug use (2.19, 1.26-3.83), and study site.
Conclusions:
In a US sample of people treated for opioid use disorder, continued treatment with either buprenorphine or methadone was associated with a reduction in arrests relative to no treatment. Cocaine use, injection drug use, Hispanic ethnicity and younger age were associated with higher likelihood of arrest.
Keywords: buprenorphine treatment, methadone treatment, pharmacotherapy, arrests, incarcerations, criminal justice outcomes, opioid use disorder, longitudinal
Introduction
A critical strategy to address the opioid epidemic is increasing access to pharmacotherapy, particularly buprenorphine/naloxone (hereafter referred to as buprenorphine, or BUP). (1, 2) Approved by the U.S. Food and Drug Administration in 2002, BUP is a partial agonist that has a superior safety profile than methadone (MET), a Schedule II full agonist, in terms of overdose risk. (1) Few studies have compared the long-term outcomes of participants randomized to BUP or MET treatment for opioid use disorder (OUD). In our own prior work, we found no differences between the two medications in 5-year mortality and opioid use. (3) However, BUP was inferior to MET in retaining people in treatment over time, (3,4) a finding that is consistent with reports by other studies. (5–8)
What is known about the long-term course of opioid use disorder (9) suggests that differences in treatment retention by medication type may translate into variation in criminal justice outcomes. Specifically, criminal activity by opioid-dependent individuals most often consists of specific drug law violations (e.g., drug possession or sales) or acquisitive crime (e.g., theft, forgery, fraud, handling stolen goods, prostitution) that provide a means to acquire money to purchase opioids and other necessities. (9) Therefore, as individuals are stabilized with medications to treat OUD, opioid use generally decreases as does the need to commit crimes to support opioid use. (10, 11)
Most studies of criminal justice outcomes have focused on individuals receiving methadone treatment; few studies have examined the criminal justice outcomes of individuals started on BUP treatment, particularly relative to those receiving MET treatment, (9, 12) and these report mixed results. Findings include, for example, no differences between BUP and MET in arrests, crime, or incarceration, (13) lower odds of incarceration for BUP compared to MET, (14) and smaller pre-post treatment reductions in criminal charges for BUP than MET. (15) Knowledge is limited by observational study designs, a focus on mostly male and especially vulnerable populations (incarcerated men, individuals being treated for serious co-occurring mental illnesses), reliance on self-reported criminal justice data, and examination of relatively short-term outcomes (3 to 24 months post-baseline).
The present study takes advantage of a recently completed long-term follow-up study of opioid-dependent individuals (3, 16) who participated in a large U.S. trial in which they had been randomized to MET vs. BUP for 24 weeks to compare liver health outcomes. (17) The long-term follow-up study assessed health and social outcomes over approximately 5 to 8 years after randomization. In the present study, we examine criminal justice outcomes among participants who had been treated in California, comparing 5-year arrest and incarceration outcomes for the BUP and MET groups, and we identify participant and treatment factors that influenced arrest.
Methods
Study design
Data were provided by a large multisite prospective study that examined the long-term outcomes of individuals who had been randomized to buprenorphine (as buprenorphine/naloxone) (BUP) or methadone (MET). The parent Clinical Trials Network (CTN) study “Starting Treatment with Agonist Replacement Therapy” (START) was a phase IV, post-marketing study designed to examine the comparative effects of buprenorphine and methadone on indices of liver health in opioid-dependent participants. (17) START involved nine federally licensed opioid dependence treatment programs located in five states (California, Oregon, Washington, Pennsylvania, and Connecticut) that together randomized 1,269 individuals to receive either BUP (n=740) or MET (n=529) during 2006-2009. Participants received medication for 24 weeks and then were tapered off it over ≤8 weeks or referred for ongoing clinical treatment. Midway through the study, the randomization scheme was switched from 1:1 BUP:MET to 2:1 because of higher drop out in the BUP arm. This change accounted for more participants in the follow-up sample having been randomized to BUP.
Using an intent-to-treat design, a follow-up study of all randomized participants was conducted during 2011-2014, approximately 2 to 8 years (mean 4.5 years) post-randomization.(3) Two sites (189 participants) were dropped during the conduct of the follow-up interviews due to logistical difficulties (i.e., one site recruited only two participants, and the other had difficulty conducting follow-ups). Of the 1,080 participants who were targeted for follow-up, 89.4% were located (n=965) with 797 interviewed - 73.7% of the BUP participants; 73.6% of the MET participants. Of the remainder located, 49 were deceased, 54 refused, 29 were incarcerated, and 36 were too mentally ill or otherwise unable to be interviewed. During their participation in the 24-week trial, participants were compensated in accordance with local site policies for completing tests and assessments.
The findings reported here utilize data on the 179 BUP and 124 MET participants who were recruited from the three California clinics and completed a follow-up interview (total n=303). The present study was restricted to the California participants because it was the only state for which administrative data were available on criminal justice outcomes. The follow-up rate of the originally targeted California sample was 58.7% (303/516). When sites were dropped from the sample targeted for the follow-up interview (described above), the number of California participants targeted for follow-up was reduced to 399. Therefore, the follow-up rate for the present analytic sample is 75.9% (303/399). Compared to California participants who were omitted from analysis, participants who were included were younger (mean [sd] age 42.4 [11.1] vs. 37.9 [11.3]; p<.001) and more were Hispanic (22.4% vs. 17.8%) or Black (13.9% vs. 7.5%) and fewer were White (56.1% vs. 65.7%) (p <.05). About one-third of the analytic sample was female (33.3%) and mean age at baseline was 42.4.
Interview procedures
Research staff at the clinics where participants were originally recruited conducted face-to-face follow-up interviews that lasted approximately 1.5 to 2 hours along with obtaining a urine sample for drug testing. Participants were compensated according to local policies, and generally consisted of a $50 gift card for the assessment and $10 for a urine sample. All study procedures were approved by the Institutional Review Board (IRB) at UCLA and by the local site IRB. A federal Certificate of Confidentiality was obtained to protect against disclosure of sensitive information.
Measures
Measurements included self-reported data and administrative records on involvement with the criminal justice system.
The primary dependent variable was any arrests per month over 60 months from baseline to follow-up as obtained for all participants from the Automated Criminal History System of the California Department of Justice (DOJ).
Criminal justice status in 30 days prior to follow-up was measured with individual self-reported items from the Addiction Severity Index. (18)
Treatment participation over the follow-up period was measured by self-reported receipt of prescribed buprenorphine-naloxone or methadone over 60 months from baseline to follow-up using timeline follow back (TLFB) methodology aided by a calendar and other memory prompts. (3,4, 19–21) It included periods during the original START trial.(3,4) TLFB data were also used to measure incarceration over 60 months from baseline to follow-up.
Participant characteristics were collected at baseline by the parent study and were selected based on prior findings from this cohort. (3, 16) These included age, gender, race/ethnicity, injection drug use, a cocaine positive urine test, and clinic site.
Death occurring during the 5-year follow-up period was determined for all 303 participants using the National Death Index obtained from the Centers for Disease Control and Prevention.
Statistical analysis
We calculated the group difference between randomization conditions by using Chi-square tests for categorical variables and two-tailed independent t-tests for continuous variables. We plotted the proportion arrested and mean arrests per year by randomization group to show the trajectory of arrest over 5-year follow-up. The primary outcome was any arrest per month over 5-years of follow-up (0=no arrest, 1=arrest). To examine associations between arrest and demographic characteristics, randomization condition, and treatment status over time, we used PROC GLIMMIX in SAS to fit a series of generalized linear mixed effects models (GLMMs) with a logit function and Gaussian quadrature. We used BIC and AIC values to select mean and covariance structures. Time-invariant covariates were assessed in Model 1 and 2, with Model 1 including randomization condition (0=MET, 1=BUP), time (coded 0-59 for 60 time-points), and Model 2 adding age at randomization (in years), gender, race/ethnicity, cocaine use, injection drug use, and study site. In Model 3, we included pharmacotherapy during the follow-up time-period as a time-varying covariate (i.e., MET vs. no treatment during follow-up, BUP vs. no treatment during follow-up). In Model 4, we incorporated interaction terms of randomization condition with pharmacotherapy over time to test whether the effects of pharmacotherapy on the odds of arrest differs depending on randomization condition. Given that individuals could not be arrested when incarcerated, we conducted sensitivity analyses in which we included incarceration during the follow-up time-period as a time-varying covariate; results were the same as when incarceration was omitted. Also, in supplemental analysis (Supplement Table 1), we repeated all analyses to examine predictors of incarceration during follow-up. Finally, we attempted to re-run the final model with all covariates using an autoregressive covariance structure (i.e., AR(1), ARMA(1,1), CSH); the models did not converge.
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) from the models. OR>1 indicated an increasing odds of arrest per month, and OR<1 indicated a decreasing odds of arrest per month. Significance was defined as p< 0.05. SAS 9.4 was used for all analyses. (22)
Results
Participant characteristics at baseline
At baseline, more of the participants randomized to BUP tested positive for cocaine than those randomized to MET (28.5% vs. 40.3%, p=0.03) (Table 1). Otherwise, there were no baseline differences in participant characteristics by randomization group. Specifically, for both groups, one-third was female, mean age was about 42.4, more than half was White, 95% tested positive for opiates, about 80% had injected drugs in the prior 30 days, and, according to DOJ data, prior to baseline about 66% had been arrested and 39% had been incarcerated.
Table 1.
Participant characteristics at baseline (n=303)
| BUP (N=179) | MET (N=124) | Total (N=303) | |
|---|---|---|---|
| Female (%) | 33.5 | 33.1 | 33.3 |
| Age (%) | |||
| 18-24 | 9.5 | 10.5 | 9.9 |
| 25-34 | 18.4 | 16.1 | 17.5 |
| 35-44 | 22.4 | 21.8 | 22.1 |
| 45-54 | 36.3 | 43.6 | 39.3 |
| 55+ | 13.4 | 8.1 | 11.2 |
| Age (years), Mean (SD) | 42.2 (11.4) | 42.8 (10.7) | 42.4 (11.1) |
| Race/ethnicity (%) | |||
| White | 54.8 | 58.1 | 56.1 |
| Black | 11.2 | 17.7 | 13.9 |
| Hispanic | 25.1 | 18.6 | 22.4 |
| Other | 8.9 | 5.7 | 7.6 |
| Urine tested positive for use of…(%) | |||
| Opiates/heroin | 96.1 | 93.6 | 95.1 |
| Cocaine* | 28.5 | 40.3 | 33.3 |
| Methamphetamine/amphetamine | 17.7 | 16.8 | 17.2 |
| Cannabis | 21.8 | 25.8 | 23.4 |
| Alcohol (positive breathalyzer) (%) | 22.9 | 26.6 | 24.4 |
| In past 30 days, injected drugs (%) | 83.2 | 80.7 | 82.2 |
| Ever arrested prior to baseline, DOJ data (%) | 65.9 | 66.9 | 66.3 |
| Ever incarcerated prior to baseline, DOJ data (%) | 36.9 | 42.7 | 39.3 |
| Site (%) | |||
| Site 1 | 46.4 | 49.2 | 47.5 |
| Site 2 | 22.4 | 24.2 | 23.1 |
| Site 3 | 31.3 | 26.6 | 29.4 |
p<.05
p<.01
p<.001
Arrests, incarcerations, and treatment over 5 years of follow-up
Over the 5-year period from baseline to follow-up, there was no difference by randomization group in arrests overall (Table 2) or at each time-point examined (Figure 1a and 1b). Overall, 55.3% of participants randomized to BUP and 54.0% randomized to MET were arrested (Table 2). Treatment with each medication was associated with a reduction in arrests over time (Figure 1a and 1b). Also, there was no difference by randomization group in the type of arrests (Table 2). For both groups, the mean number of arrests over 5 years was about 2.2, most arrests were drug-related (followed by property offenses), and there were more felony arrests than misdemeanor arrests. In the 30 days prior to follow-up, there was no difference by randomization group in self-reported criminal justice status -- few participants in both groups self-reported an arrest (about 2%) or criminal involvement (about 6-7%), and about 14-18% were on probation or parole (data not shown).
Table 2.
Involvement with the criminal justice system and treatment experiences over 5 years from baseline to follow-up (n=303)
| BUP (n=179) | MET (n=124) | Total (n=303) | |
|---|---|---|---|
| Involvement with the criminal justice system | |||
| Arrested (%) | 55.3 | 54.0 | 54.8 |
| Number of arrests (%) | |||
| 0 | 44.7 | 46.0 | 45.2 |
| 1 | 14.5 | 8.9 | 12.2 |
| 2 | 10.1 | 14.5 | 11.9 |
| 3 | 10.1 | 8.1 | 9.2 |
| 4+ | 20.6 | 22.5 | 21.5 |
| Number of arrests, Mean (SD) | 2.2 (3.3) | 2.3 (3.3) | 2.2 (3.3) |
| Number of arrests by type, Mean (SD) | |||
| Drug-related | 0.82 (2.1) | 0.75 (1.9) | 0.79 (2.0) |
| Property | 0.46 (2.0) | 0.81 (2.6) | 0.60 (2.2) |
| Violent | 0.05 (0.4) | 0.12 (1.3) | 0.08 (0.9) |
| Sex-related | 0.01 (0.1) | 0.00 (0) | 0.01 (0.1) |
| Other# | 0.74 (2.5) | 0.35 (1.2) | 0.58 (2.0) |
| Number of arrests by offense level, Mean (SD) | |||
| Misdemeanor | 0.55 (1.9) | 0.60 (2.0) | 0.57 (1.9) |
| Felony | 1.18 (2.5) | 1.14 (2.7) | 1.16 (2.6) |
| Moderate | 0.51 (2.2) | 0.56 (1.8) | 0.53 (2.1) |
| Incarcerated (%) | 40.9 | 47.3 | 43.5 |
| Treatment experiences | |||
| % of months in any pharmacotherapy* | 48.7 | 57.1 | 52.2 |
| % of months in BUP*** | 16.1 | 7.2 | 12.4 |
| % of months in MET*** | 30.2 | 48.0 | 37.6 |
| % of months not in any pharmacotherapy (no treatmenta)* | 51.3 | 42.9 | 47.8 |
p<.05
p<.01
p<.001
Pharmacotherapy = medication assisted treatment (e.g., methadone, buprenorphine) for opioid use disorders.
“Other” offenses include obstruction of justice (e.g. disobey court order, fail to appear), local ordinance violations, driving under influence, family offenses (e.g. child neglect), and weapons offenses.
No treatment is defined as not having received either BUP or MET treatment. It includes opioid medications other than BUP or MET (e.g., levo-α-acetylmethadol or LAAM). It also includes treatment without opioid medications such as outpatient, residential, detoxification, or other treatments with no receipt of opioid medications.
Figure 1a. Proportion arrested per year over 5 years by randomization group (n=303).
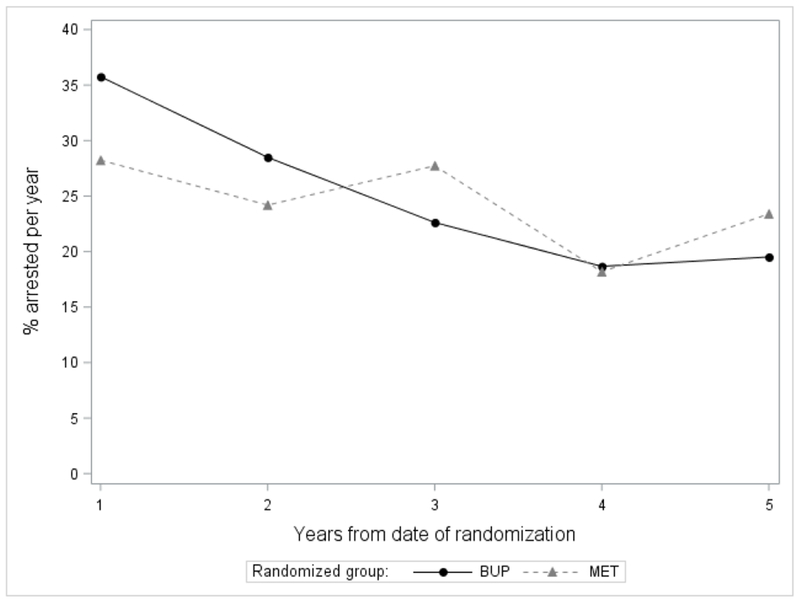
At each time-point, the difference in arrests by randomization group is not statistically significant.
Figure 1b. Number of arrests per year over 5 years by randomization group (n=303).
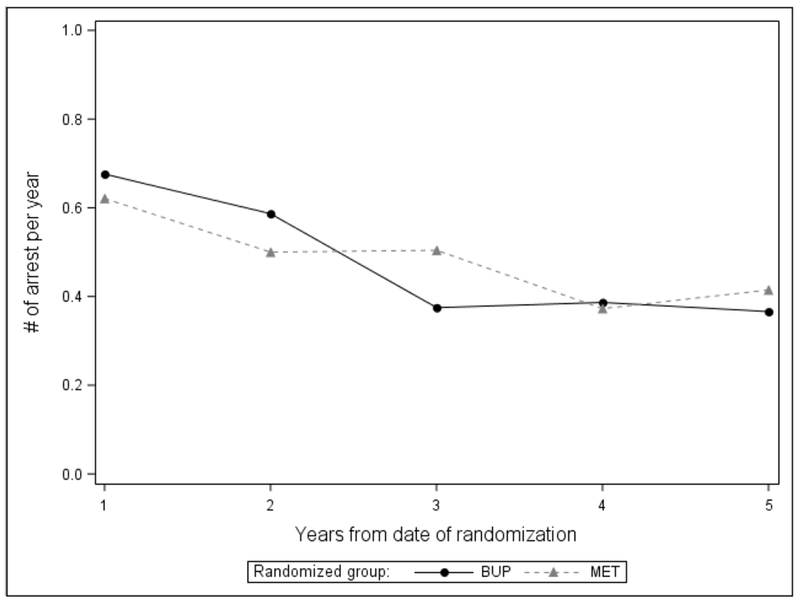
At each time-point, the difference in arrests by randomization group is not statistically significant.
There was no difference by randomization group in self-reported incarceration experiences over the 5-year period from baseline to follow-up. Overall, 40.9% of participants randomized to BUP and 47.3% randomized to MET were incarcerated. The mean number of months incarcerated during following was 4.6 for the BUP group and 4.8 for the MET group (no difference) (data not shown).
Over the 5-year follow-up period, individuals randomized to BUP spent less time in any pharmacotherapy than individuals randomized to MET. Specifically, individuals randomized to BUP spent about 48.7% of the months from baseline to follow-up receiving pharmacotherapy for opioid use disorder, whereas those randomized to MET spent about 57.1% of those months receiving pharmacotherapy (p=0.02). When time in pharmacotherapy during follow-up was calculated in mean months, individuals randomized to BUP received pharmacotherapy for about 29.2 months and those randomized to MET received pharmacotherapy for about 34.2 months (data not shown). Results are consistent with those from the parent study.(3,4)
There was one death in each randomization group over the 5-year follow-up (data not shown).
Predictors of arrest over 5 years of follow-up
Results of four models of arrest over the follow-up period are presented in Table 3. The pattern of results is consistent across models (with various covariates). For parsimony, we describe the results of Models 3 and 4 only.
Table 3.
Modeling results predicting any arrest per month over the 60-month follow-up period (n=303)
| OR (95% CI) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Intercept | 0.03 (0.02, 0.04)*** | 0.01 (0.00, 0.04)*** | 0.02 (0.01, 0.06)*** | 0.02 (0.01, 0.07)*** |
| Slope (month) | 0.97 (0.96, 0.98)*** | 0.97 (0.96, 0.98)*** | 0.96 (0.95, 0.97)*** | 0.96 (0.95, 0.97)*** |
| Randomized condition | ||||
| BUP (vs. MET) | 1.04 (0.69, 1.58) | 1.13 (0.76, 1.66) | 1.15 (0.78, 1.69) | 0.93 (0.60, 1.44) |
| Age at randomization | 0.98 (0.96, 0.99)** | 0.98 (0.96, 1.00)* | 0.98 (0.96, 1.00)* | |
| Male (vs. Women) | 1.26 (0.83, 1.89) | 1.25 (0.84, 1.86) | 1.26 (0.85, 1.88) | |
| Race (ref: White) | ||||
| Black | 1.07 (0.56, 2.04) | 1.02 (0.55, 1.92) | 1.01 (0.54, 1.88) | |
| Hispanic | 1.78 (1.12, 2.83)* | 1.63 (1.04, 2.56)* | 1.62 (1.03, 2.54)* | |
| Other race | 1.28 (0.61, 2.70) | 1.26 (0.61, 2.60) | 1.27 (0.62, 2.63) | |
| Cocaine use | 2.05 (1.34, 3.14)** | 2.00 (1.33, 3.03)** | 2.00 (1.33, 3.03)** | |
| Injection use | 2.44 (1.38, 4.32)** | 2.19 (1.26, 3.83)** | 2.16 (1.24, 3.75)** | |
| Site (ref: Site 1) | ||||
| Site 2 | 1.91 (1.19, 3.06)** | 1.85 (1.17, 2.93)** | 1.81 (1.15, 2.86)* | |
| Site 3 | 2.25 (1.30, 3.88)** | 2.46 (1.45, 4.19)*** | 2.41 (1.41, 4.09)** | |
| Time-varying covariates | ||||
| MET (vs. no treatmenta) | 0.65 (0.50, 0.83)*** | 0.50 (0.35, 0.72)*** | ||
| BUP (vs. no treatmenta) | 0.46 (0.32, 0.67)*** | 0.39 (0.18, 0.87)* | ||
| BUP (vs. MET treatment)b | 0.72 (0.48, 1.07) | 0.59 (0.36, 0.96)* | ||
| Interaction of randomized condition and treatment status | ||||
| BUP group*MET treatment | 1.68 (1.01, 2.81)* | |||
| BUP group*BUP treatment | 1.26 (0.51, 3.09) | |||
| AIC | 4656.97 | 4627.77 | 4501.67 | 4501.66 |
| BIC | 4675.54 | 4679.76 | 4561.09 | 4568.5 |
| −2 Log Likelihood | 4646.97 | 4599.77 | 4469.67 | 4465.66 |
OR 95% CI=Odds Ratio with 95% Confidence Intervals MET=methadone; BUP=buprenorphine
p<0.05
p<0.01
p<0.001
No treatment is defined as not having received either BUP or MET treatment. It includes opioid medications other than BUP or MET (e.g., levo-α-acetylmethadol or LAAM). It also includes treatment without opioid medications such as outpatient, residential, detoxification, or other treatments with no receipt of opioid medications.
The odds ratios were indirectly calculated by exponentiating the coefficients in the models.
There was no difference by randomization condition in the likelihood of arrest (Table 3, Model 3). Likelihood of arrest was negatively associated with each additional month of time (Odds Ratio [OR] 0.96, 95% Confidence Interval [CI] 0.95-0.97, p<.001) and older age at baseline (0.98, 0.96-1.00, p=.01); it was positively associated with Hispanic ethnicity (1.63, 1.04-2.56, p=.03), cocaine use (2.00, 1.33-3.03, p=.001), injection drug use (2.19, 1.26-3.83, p=.006), and study site.
There were three types of pharmacotherapy status during follow-up: MET treatment, BUP treatment, and no treatment. Therefore, we reparameterized the odds ratios using MET with no treatment and BUP with no treatment as the reference, respectively, to interpret the results. Individuals who received during follow-up either buprenorphine (0.46, 0.32-0.67, p<.001) or methadone treatment (0.65, 0.50-0.83, p<.001) were less likely to be arrested relative to no treatment during follow-up (Table 3, Model 3). The interaction between randomization condition and pharmacotherapy (MET treatment vs. no treatment) is significant (p=0.046). Furthermore, moderation effects (Table 4) revealed that among methadone-randomized individuals, arrest was less likely with receipt of methadone (0.50, 0.35-0.72, p<.001) during follow-up (relative to no treatment during follow-up) and switching to buprenorphine had a lower odds of arrest compared to no treatment during follow-up (0.39, 0.18-0.87, p=.02). Among buprenorphine-randomized individuals, arrest was less likely with receipt of buprenorphine (0.49, 0.33-0.75, p<.001) during follow-up whereas switching to methadone had a similar odds of arrest as receiving no treatment during follow-up.
Table 4.
The adjusted odds ratios of any arrest over 60-months of follow-up by randomization status
| Randomization condition | Treatment | Odds ratios |
|---|---|---|
| MET | MET vs. No treatment | 0.50 (0.35, 0.72) |
| BUP vs. No treatment | 0.39 (0.18, 0.87) | |
| BUP | MET vs. No treatment | 0.84 (0.62, 1.20) |
| BUP vs. No treatment | 0.49 (0.33-0.75) |
MET=methadone; BUP=buprenorphine
Based on results from Table 3, Model 4.
In supplemental analysis (Supplement Table 1) we repeated all analyses to examine predictors of incarceration during follow-up; results were mostly the same as when the outcome was arrest. A notable exception, individuals who received buprenorphine during follow-up were less likely to be incarcerated than those who received methadone (0.47, 0.25-0.86, p=.02).
Discussion
Summary of primary findings and implications
Among adults in California who had been randomized to be treated with either buprenorphine or methadone for opioid use disorder, there was no difference by randomization condition in the proportion arrested (about 55%) or incarcerated (about 41-47%) over 5 years of follow-up. Also, individuals who received either buprenorphine or methadone treatment during follow-up were less likely to be arrested or incarcerated relative to no treatment during follow-up. Findings suggest that in terms of criminal justice outcomes, treatment of opioid use disorder with either medication is superior to treatment with no pharmacotherapy. The finding that individuals who received buprenorphine during follow-up were less likely to be incarcerated than those who received methadone warrants replication.
We also found that among methadone-randomized individuals, arrest was less likely with receipt of methadone during follow-up (relative to no treatment during follow-up). Among buprenorphine-randomized individuals, arrest was less likely with receipt of buprenorphine during follow-up whereas switching medication type during follow-up had a similar likelihood of arrest as receiving no treatment. Findings contrast with other emerging evidence indicating, for example, that switching non-responding buprenorphine patients to methadone can yield reductions in criminal offences and incarceration rates. (23) Clearly, more studies are needed to understand the characteristics of individuals who change medication types, predictors of medication changes, and why such changes are associated with differential criminal justice outcomes.
Finally, we found that Hispanics were more likely than Whites to be arrested, and arrests were associated both with clinic site and with factors that are often considered to be proxy indicators for greater addiction severity or involvement with the criminal justice system (younger age, cocaine use, injection drug use). Findings underscore the need for public health efforts to prevent or mitigate criminal justice consequences that may disproportionately impact certain groups with opioid use disorder over others.
More broadly, arrest and incarceration places individuals in a setting in which opioids are less readily available, which in the short-term reduces overdose risks and increases public safety, however growing evidence suggests that experiences of repeated incarceration do not yield beneficial health effects. Instead, a return to opioid use after release from incarceration is a common occurrence and the period immediately following release is a high-risk one for overdose-related mortality. (24, 25) Additionally, exposure to incarcerated settings increases the likelihood of severe health limitations (26) and it is associated with greater disparities in health conditions. (27) Unlike in several European countries and elsewhere, where pharmacotherapy to treat opioid use disorder is offered to prisoners during and after incarceration, (28–31) incarceration in the U.S. has historically been associated with an interruption of such pharmacotherapy, (32) a practice that reduces the likelihood of prisoners re-engaging with pharmacotherapy after their release and increases risks for other poor health and social outcomes. (33, 34) In light of this context, our findings support current efforts (35–37) to improve within U.S. criminal justice settings the delivery and outcomes of pharmacotherapy for opioid use disorder.
Limitations
Study findings must be considered within the context of several limitations. Findings are generated from a sample of 303 treated individuals who participated in a randomized clinical trial implemented by three community addiction treatment clinics in California. The trial was designed to evaluate effects of buprenorphine versus methadone on liver function, it lasted for only 6 months, and across study sites there was variability in post-trial treatment availability. For these reasons, we included site as a covariate. However, findings may not be representative of participants treated in office-based settings, and warrant replication with a large and diverse sample of treated people. Also, arrest outcomes are measured with administrative records. These administrative data provide a means to measure outcomes on all participants, including those who did not complete a follow-up interview, a key reason why these data are useful for assessing addiction treatment outcomes,(38, 39) but they provide information only on those crimes that resulted in an arrest and occurred in California. Experiences of treatment and incarceration during follow-up were provided by self-reported TLFB data, which have been determined to provide adequate reliability and validity,(20, 40, 41) but nevertheless may be subject to recall bias. ASI data were self-reported and excluded people who were incarcerated at follow-up and thus could not complete the interview. The extent to which individuals may have participated in intensive psychosocial treatment was not captured and therefore any potential effects were not examined. There were differences in the demographic characteristics of participants included and omitted from analysis, which may have resulted in attrition bias. Finally, since treatment was provided by the 24-week trial for a limited period, and to remain in treatment after the trial participants had to make additional arrangements, the rates of follow-up treatment engagement may not reflect what would occur in routine clinical care.
Conclusions
This study is the first to examine criminal justice outcomes of opioid dependent individuals randomized to two types of pharmacotherapy for opioid use disorder and followed prospectively for 5 years. This study shows that continued treatment for opioid use disorder with either buprenorphine or methadone is associated with a reduction in arrests (relative to no treatment), with changes to methadone yielding similar outcomes as no pharmacotherapy among buprenorphine-randomized individuals. In addition to ongoing pharmacotherapy, addressing cocaine use and injection drug use, and attending to risk factors that are unique to Hispanic ethnicity, younger age, and setting are likely to reduce arrests individuals with opioid use disorder.
Supplementary Material
ACKNOWLEDGEMENT
Sincere appreciation to our participating networks: the Pacific Northwest Node and Evergreen Treatment Services; the Western States Node and CODA Inc. and Bi-Valley Medical Clinic; the New England Node and Connecticut Counseling Centers and Yale and Hartford Dispensary; the Delaware Valley Node and NET Steps; the Pacific Region Node and Matrix Institute; Emmes Corporation; the CCTN and NIDA.
Funding source:
Main study funding was provided by the National Institute on Drug Abuse (NIDA) through the Clinical Trials Network (CTN) through a series of grants provided to each participating node:
The Pacific Northwest Node (U10 DA01714)
The Western States Node (U10 DA 015815)
The New England Node (U10 DA13038)
The Delaware Valley Node (U10 DA13043)
The Pacific Region Node (U10 DA13045)
The Greater New York Node (UG1 DA013035)
Dr. Evans is supported by The Greenwall Foundation, NIDA UG3 DA0044830-02S1, and Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Substance Abuse Treatment (CSAT) Grant No. 1H79T1081387-01. Dr. Hser is supported by NIDA R33DA045844 and UG1 DA013035.
Footnotes
Declaration of Interest: Authors disclosing relevant financial interests, activities, relationships, and affiliations are:
All other authors report no financial or other possible conflicts of interest.
Trial registration: The START Follow-up Study on ClinicalTrials.gov (NCT01592461).
Contributor Information
Elizabeth A. Evans, Email: eaevans@umass.edu.
Yuhui Zhu, Email: yhzhu@ucla.edu.
Caroline Yoo, Email: cyoo5@ucla.edu.
David Huang, Email: yhuang@ucla.edu.
Yih-Ing Hser, Email: yhser@ucla.edu.
References
- 1.Bruneau J, Ahamad K, Goyer MÈ, Poulin G, Selby P, Fischer B, et al. Wood E. Management of opioid use disorders: a national clinical practice guideline. CMAJ. 2018. March; 190(9): E247–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services (HHS), Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: HHS, November 2016. [PubMed] [Google Scholar]
- 3.Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, et al. , Ling W Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016. April; 111(4): 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. , Ling W Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014. January; 109(1): 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns L, Gisev N, Larney S, Dobbins T, Gibson A, Kimber J, et al. Degenhardt L A longitudinal comparison of retention in buprenorphine and methadone treatment for opioid dependence in New South Wales, Australia. Addiction. 2015; 110(4): 646–655. [DOI] [PubMed] [Google Scholar]
- 6.Hickman M, Steer C, Tilling K, Lim AG, Marsden J, Millar T, et al. Macleod J The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction. 2018. August; 113(8):1461–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014. February;(2): CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proctor S, Copeland A, Kopak A, Herschman P, Polukhina N. A naturalistic comparison of the effectiveness of methadone and two sublingual formulations of buprenorphine on maintenance treatment outcomes: Findings from a retrospective multisite study. Exp Clin Psychopharm 2014; 22(5): 424–433. [DOI] [PubMed] [Google Scholar]
- 9.Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015. Mar-Apr; 23(2): 76–89. [DOI] [PubMed] [Google Scholar]
- 10.Russolillo A, Moniruzzaman A, McCandless LC, Patterson M, Somers JM. Associations between methadone maintenance treatment and crime: a 17-year longitudinal cohort study of Canadian provincial offenders. Addiction. 2018. April; 113(4):656–667. [DOI] [PubMed] [Google Scholar]
- 11.Teesson M, Mills K, Ross J, Darke S, Williamson A, Havard A. The impact of treatment on 3 years’ outcome for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS). Addiction. 2008. January; 103(1): 80–8. [DOI] [PubMed] [Google Scholar]
- 12.Perry AE, Neilson M, Martyn-St James M, Glanville JM, McCool R, Duffy S, et al. , Hewitt C Pharmacological interventions for drug-using offenders. Cochrane Database Syst Rev 2013. December 19; (12): CD010862. [DOI] [PubMed] [Google Scholar]
- 13.Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, Rosenblum A. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009. January 1; 99(1-3): 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson AG, Easter MM, Lin HJ, Frisman LK, Swanson JW, Swartz MS. Associations between pharmacotherapy for opioid dependence and clinical and criminal justice outcomes among adults with co-occurring serious mental illness. J Subst Abuse Treat. 2018. March; 86: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastegar DA, Sharfstein Kawasaki S, King VL, Harris EE, Brooner RK. Criminal charges prior to and after enrollment in opioid agonist treatment: a comparison of methadone maintenance and office-based buprenorphine. Subst Use Misuse. 2016. June 6;51(7):803–11. [DOI] [PubMed] [Google Scholar]
- 16.Hser YI, Huang D, Saxon AJ, Woody G, Moskowitz AL, Matthews AG, Ling W. Distinctive trajectories of opioid use over an extended follow-up of patients in a multisite trial on buprenorphine+naloxone and methadone. J Addict Med 2017. Jan-Feb; 11(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxon A, Ling W, Hillhouse M et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug Alcohol Depen 2013; 128(1-2): 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. , Argeriou M The fifth edition of the Addiction Severity Index. J Subst Abuse Treat 1992; 9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 19.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption In: Litten RZ, Allen JP, eds. Measuring alcohol consumption . Totowa, NJ: Humana Press; 1992: 41–72. [Google Scholar]
- 20.Hser Y, Anglin M, Chou C. Reliability of retrospective self-report by narcotics addicts. Psychol Assessment. 1992; 4(2): 207–213. [Google Scholar]
- 21.Murphy D, Hser Y, Huang D, Brecht M, Herbeck D. Self-Report of longitudinal substance use: a comparison of the UCLA natural history interview and the addiction severity index. J Drug Issues. 2010;40(2):495–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SAS Institute Inc. SAS Enterprise Guide 5.1. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 23.Carrieri P, Vilotitch A, Nordmann S, Lions C, Michel L, Mora M, et al. , Roux P; Methaville Study Group. Decrease in self-reported offences and incarceration rates during methadone treatment: A comparison between patients switching from buprenorphine to methadone and maintenance treatment incident users (ANRS-Methaville trial). Int J Drug Policy. 2017. January; 39: 86–91. [DOI] [PubMed] [Google Scholar]
- 24.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013. November; 159(9): 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bukten A, Stavseth MR, Skurtveit S, Tverdal A, Strang J, Clausen T. High risk of overdose death following release from prison: variations in mortality during a 15-year observation period. Addiction. 2017. August; 112(8): 1432–1439. [DOI] [PubMed] [Google Scholar]
- 26.Schnittker J, John A. Enduring stigma: the long-term effects of incarceration on health. J Health Soc Behav 2007; 48: 115–130. [DOI] [PubMed] [Google Scholar]
- 27.Wildeman C, Wang EA. Mass incarceration, public health, and widening inequality in the USA. Lancet. 2017. April; 389(10077): 1464–1474. [DOI] [PubMed] [Google Scholar]
- 28.Bird SM, Fischbacher CM, Graham L, Fraser A. Impact of opioid substitution therapy for Scotland’s prisoners on drug-related deaths soon after prisoner release. Addiction. 2015; 110(10):1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Degenhardt L, Larney S, Kimber J, Gisev N, Farrell M, Dobbins T, et al. , Burns L The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction. 2014. August;109(8):1306–1317. [DOI] [PubMed] [Google Scholar]
- 30.Favrod-Coune T, Baroudi M, Casillas A, Rieder JP, Gétaz L, Barro J, et al. , Wolff H Opioid substitution treatment in pretrial prison detention: a case study from Geneva, Switzerland. Swiss Med Wkly. 2013. November 1;143:w13898. [DOI] [PubMed] [Google Scholar]
- 31.Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, et al. , Hickman M Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction. 2017. August;112(8):1408–1418. [DOI] [PubMed] [Google Scholar]
- 32.Bruce RD, Schleifer RA. Ethical and human rights imperatives to ensure medication-assisted treatment for opioid dependence in prisons and pre-trial detention. Int J Drug Policy. 2008. February;19(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkley-Rubinstein L, McKenzie M, Macmadu A, Larney S, Zaller N, Dauria E, Rich J. A randomized, open label trial of methadone continuation versus forced withdrawal in a combined US prison and jail: Findings at 12 months post-release. Drug Alcohol Depend 2018. March 1;184:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rich JD, McKenzie M, Larney S, Wong JB, Tran L, Clarke J, et al. , Zaller N Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2015. July 25;386(9991):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkley-Rubinstein L, Zaller N, Martino S, Cloud DH, McCauley E, Heise A, Seal D. Criminal justice continuum for opioid users at risk of overdose. Addict Behav 2018. November; 86:104–110. [DOI] [PubMed] [Google Scholar]
- 36.Chandler RK, Finger MS, Farabee D, Schwartz RP, Condon T, Dunlap LJ, et al Lee JD. The SOMATICS collaborative: Introduction to a National Institute on Drug Abuse cooperative study of pharmacotherapy for opioid treatment in criminal justice settings. Contemp Clin Trials. 2016. May; 48: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krawczyk N, Picher CE, Feder KA, Saloner B. Only one in twenty justice-referred adults in specialty treatment for opioid use receive methadone or buprenorphine. Health Aff (Millwood). 2017. December; 36(12): 2046–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans E, Grella CE, Murphy DA, Hser YI. Using administrative data for longitudinal substance abuse research. J Behav Health Serv Res 2010. April; 37(2): 252–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hser YI, Evans E. Cross-system data linkage for treatment outcome evaluation: lessons learned from the California Treatment Outcome Project. Eval Program Plann. 2008. May; 31(2): 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol 2000. February; 68(1):134–144. [DOI] [PubMed] [Google Scholar]
- 41.Sutton JE, Bellair PE, Kowalski BR, Light R, Hutcherson DT. Reliability and validity of prisoner self-reports gathered using the life event calendar method. J Quant Criminol 2011. June; 27(2): 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


