Abstract
CD4 T-cell count is a priority for staging HIV disease and guiding clinical management as part of HIV care. Conventional CD4 T-cell enumeration methods based on flow cytometry are expensive, require well-trained personnel, and are challenging to use in rural, resource-scarce areas. A simple CD4 T-cell count test that can be used at point-of care, the Flow-Through cell Counting Assay (FTCA1), is described in this article. The FTCA is based on the use of: 1) a special membrane that selectively retains white blood cells (WBCs); 2) a sample delivery system; and 3) optical signal detection. To show the feasibility of the FTCA, a proof-of-concept prototype of the FTCA cassette and digital camera or handheld reflectance meter were used for obtaining quantitative assay results within 30 minutes. The results show that the FTCA allows for quantitative enumeration of CD4 T-cells in the clinically relevant range of CD4 T-cell concentrations. The advantages of the FTCA technology, including simplicity, short analysis time, and portability, suggest that FTCA has great potential for use in clinical practice and wide applicability for other cell-based diagnostic tests.
Keywords: HIV, CD4 count, Point-of-care, Flow-through assay, Leukoreduction filter
1. Introduction
HIV infection is a global public health concern. In 2017, approximately 36.9 million people worldwide were living with HIV, including an estimated 1.8 million new infections (UNAIDS, 2018). An estimated 1.1 million people are currently infected with HIV in the United States and nearly 39,000 were diagnosed with HIV in 2017, representing over an 8% decline in annual infections since 2010 (CDC, 2019). AIDS-related deaths worldwide decreased from 1.9 million in 2005 to 940,000 in 2017 (UNAIDS, 2018). During this period, the number of people accessing antiretroviral therapy (ART) increased 10-fold from 2.1 million to 21.7 million. In recent years, much emphasis is being placed on wider HIV screening, and the identification of infected individuals allows implementation of intervention strategies. Individuals with HIV infection and AIDS exhibit abnormalities of the immune system, reflected primarily in their CD4 T-lymphocytes, which are targeted by the virus (Fahey et al., 1990; Lange et al., 1989; Phillips et al., 1991; Stein et al., 1992). The evaluation of CD4 T-cell concentration provides an assessment of immunologic competency and has proven to be an important parameter for disease progression (Di Biagio et al., 2017; Kestens and Mandy, 2017).
The current WHO guidelines recommend ART initiation in all individuals upon HIV diagnosis, irrespective of CD4 T-lymphocyte level (WHO, 2016), as early initiation of ART improves quality of life and considerably increases life expectancy (Katz and Maughan-Brown, 2017; The Antiretroviral Therapy Cohort Collaboration, 2017). The guidelines, however, prioritize ART initiation for individuals (including children) with severe or advanced HIV disease. Therefore, adults with a CD4 count ≤ 350 cells/μL and children < 5 years of age with WHO clinical stage 3 or 4 HIV disease or a CD4 count ≤ 750 cells/μL are considered a priority.
The 2017 update of WHO guidelines strongly recommend the use of CD4 count for disease monitoring where viral load testing is unavailable. The CD4 count provides an important criterion for assessing disease progression, making decisions about ART regime changes, and treating HIV infected individuals with opportunistic infections. The CDC and NIH guidelines provide critical recommendations regarding prophylaxis of potentially life-threatening, recurring, and debilitating opportunistic infections in HIV positive patients whose CD4 counts are ~ 100-200 cells/μL and monitored at regular intervals (every 3-6 months), in some cases regardless of HIV plasma viral load (CDC NIH, 2018). Thus, the CD4 testing will remain an extremely valuable tool at all stages of HIV disease monitoring (Kestens and Mandy, 2017).
Flow cytometry (or FACS) is the current gold standard for CD4 T-lymphocyte counting. Several types of flow cytometers are available, with the BD FACSCount, FACSCalibur (BD Biosciences), and EPICS XL (Beckman Coulter) among the most popular instruments. Besides using the traditional flow cytometers (open platforms that can employ dual or single platform technology), simplified, dedicated single platform CD4 systems have been developed (Coetzee and Glencross, 2017; Peeling et al., 2015). The commercially available dedicated platforms include FACSCount (BD Biosciences), CyFlow Counter (Sysmex Partec), Muse/Guava Auto CD4/CD4% system (EMD Millipore Sigma), and Apogee Auto40 (Apogee Flow Systems). These dedicated platforms allow CD4 T-cell counting with moderate technical complexity, producing absolute CD4 counts and a CD4/CD8 ratio without requiring an external computer. Flow cytometry, despite being the gold standard method for determining lymphocyte subpopulations, has several disadvantages: it requires an expensive instrument (tens of thousands of dollars), an expensive service contract, and well-trained personnel to operate it, effectively limiting its use to well funded laboratories or central facilities.
There are two commercially available, CD4 counting instruments based on digital image analysis - Alere PIMA™ CD4 analyzer (Abbott Laboratories)(“Alere PIMA™ Analyser,” n.d.) and BD FACSPresto™ near-patient CD4 counter (BD Biosciences) (“BD FACSPRESTO - A near-patient complete CD4 testing solution,” n.d.). Both instruments utilize fluorescence imaging cytometry and all reagents are dried in a sealed disposable cartridge. The FACSPresto also measures total hemoglobin (Hb) concentration in addition to the absolute and percentage of CD4 T lymphocytes. Several recent studies demonstrated a good agreement between the PIMA analyzer and FACS-based CD4 enumeration (Diaw et al., 2011; Faye et al., 2016; Jani et al., 2011b, 2011a; Manabe et al., 2012; Mnyani et al., 2012; Mtapuri-Zinyowera et al., 2011; Sukapirom et al., 2011). However, the instrument is rather expensive (> $5,000 US) and sample throughput is very low (3 samples per hour) (Boyle et al., 2012).
Available manual alternatives to FACS for CD4 T-cell quantitation are: VISITECT® CD4 point-of-care test (developed by the Burnet Institute, Melbourne, Australia) (“VISITECT® CD4 POC test,” n.d.) and Dynal T4 Quant Kit (Thermo Fisher Scientific) (“Dynal® T4 Quant Kit,” 2003; Peeling et al., 2015). The VISITECT CD4 is an instrument-free, semi-quantitative test based on lateral-flow technology, and provides test results in 40 minutes. The Dynal T4 Quant Kit uses magnetic Dynabeads (the cell isolation process takes only 30 minutes) and requires the use of an epifluorescent microscope (although it can be performed with only a light microscope), a hemacytometer, a vortex, a tube rocker, a timer, and a magnet (Lutwama et al., 2008). Manual assays cost ~ $5-6 per test and are designed to operate with low sample throughput in resource-limited laboratories. However, manual methods are labor-intensive, require many manual steps and in case of Dynal test an experienced microscopist, making them challenging to implement in many settings (Peter et al., 2008).
In this paper, we show the feasibility of a novel FTCA method. The method is based on an immuno-concentration type assay, with several key characteristics: 1) a special membrane that selectively retains WBCs, preferentially lymphocytes including CD4 T-cells; 2) a sample delivery system which controls the flow of liquids through the filter system; 3) optical assessment of results by automated and quantitative means. The FTCA partially described in our patent (US 9097712B2, EP 2972345B1) (Bystryak and Santockyte) has several advantages over other manual tests, including simplicity and short time of analysis.
2. Materials and methods
2.1. Materials
Biotinylated anti-human CD4 antibody, clone SK3, was purchased from BD Biosciences (San Jose, CA). Leukosorb (B) membrane, WBF-2 and LRF10S filters were obtained from Pall Corporation (Port Washington, New York). Streptavidin alkaline phosphatase conjugate (Strep-AP) was purchased from Sigma-Aldrich (Saint Louis, MO). Nitro Blue Tetrazolium (NBT) was purchased from Thermo Fisher Scientific. Alkaline Phosphatase (AP) substrate solution and absorbent pads were purchased from Bio-Rad Laboratories (Hercules, CA). Dynal T4 Quant kit and Dynabeads CD3 were purchased from Invitrogen (Thermo Fisher Scientific).
2.1.1. Clinical samples
Freshly drawn K3EDTA blood samples from different donors were purchased from Biological Specialty Corporation (Colmar, PA). Ten clinical K3EDTA blood samples from HIV-positive patients and two samples from HIV-negative donors were obtained from and processed at the Infectious Disease Laboratory at Ann & Robert H. Lurie Children’s Hospital of Chicago. All de-identified blood samples were processed within 24 hours of collection.
2.1.2. CD3 depleted blood samples
CD3+ T-lymphocytes were depleted using Dynabeads® CD3 (Invitrogen, Thermo Fisher Scientific) as described in the manufacturer’s instructions, with some modifications. Briefly, 50 μL of Dynabeads were added to 1 mL of undiluted whole blood from healthy donors. Samples were incubated with tilting and rotation for 30 min at 4 °C. After 30 min incubation, samples were placed on a magnet for 1 min, after which the supernatant was transferred to a new tube. The new tube with supernatant was placed on a magnet for additional 1 min to ensure complete separation of CD3 depleted blood from Dynabeads. The supernatant was transferred to a new tube once again. The remaining CD4 T-lymphocytes were counted using Dynal® T4 Quant Kit.
2.2. Determination of CD4 counts with Dynal T4 Quant Kit and FACS
The concentration of CD4 T-lymphocytes in whole blood and CD3 T-lymphocyte depleted blood (as described above) was determined using the Dynal® T4 Quant Kit according to the manufacturer’s instructions, with one modification. Briefly, the package insert recommends counting cells in two hemacytometer chambers and averaging the results when the initial count is 100 cells/μL or less. We counted CD4 T-cells in two chambers for all the samples, regardless of total number, calculated the average count, and then calculated the final CD4 count using the formula provided by the manufacturer. If the difference in cell count between two chambers was more than 3 cells, we counted the cells in three chambers and obtained the average before calculating the final CD4 count. CD4 T-cells in HIV positive whole blood were counted at CMH FACS laboratory using Beckman Coulter Epics XL Flow Cytometer.
2.3. FTCA
2.3.1. Principle of the FTCA
The FTCA method is based on measurement of absolute counts of CD4 T-cells. The FTCA employs a special filter that selectively captures WBCs (Bruil et al., 1995), mostly lymphocytes, and a special mechanism for sample delivery. The general scheme for performing the FTCA is shown in Figure 1, and is comprised of the following steps for the determination of CD4 T-cells in whole blood: 1) in Step 1, anti-CD4 antibody enzyme conjugate (or Biotin anti-CD4 – Streptavidin-enzyme complex) is added to the whole blood; 2) after several minutes of incubation at room temperature (RT), in Step 2, the mixture is applied to the FTCA cassette (cartridge) which is comprised of a) a delivery tube or opening; b) a retainer filter and c) an absorbent pad. In this step, the sample applied through the delivery tube or opening travels to the retainer filter (membrane), on which WBCs, including T-cell lymphocytes bound to anti-CD4 antibody labeled with enzyme, are adsorbed; 3) in Step 3, the delivery tube is removed and a washing buffer solution is poured onto the membrane in order to remove weakly bound substances such as red blood cells, enzymes, enzyme inhibitors, proteins, and lipids from the retainer; 4) In Step 4, an enzyme substrate is added, and the result of a color-forming reaction of the enzyme generating an insoluble chromogenic product (colorimetric assay) is read by simple optical means. A description of the components of the FTCA cassette and reagents is provided below in more detail.
Figure 1: General scheme for performing of the flow-through cell counting assay.
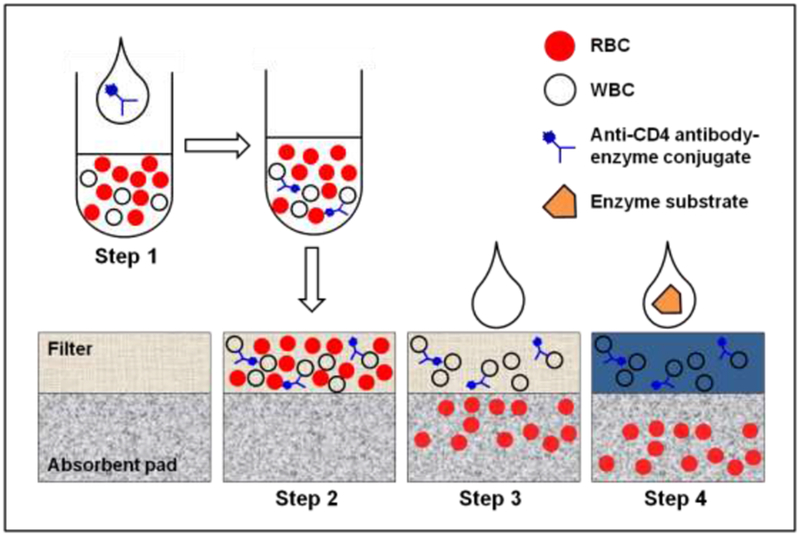
A detailed description is provided in section 2.3.1.
2.3.2. Prototype of the Flow-Through Cassette
The laboratory setup (prototype of the FTCA cassette) which was used in this study is shown in Figure 2B. It is comprised of three major components: a sample delivery tube/cylinder (1); WBC retainer filter (membrane) (2); and an absorbent pad (3). The retainer filter selectivity retains WBCs (mostly lymphocytes), including anti-CD4 antibody-cell complexes. Unlike conventional flow-through immunoassay systems, in which the sample is generally applied directly over a fdter (as in Figure 2A), the sample delivery in the FTCA cassette is controlled using a disposable glass or plastic tube/cylinder (4 mm inner diameter), gently pressed on the membrane placed over an absorbent pad. In this study, various WBC retention membranes of 0.18 mm thickness and 7 mm diameter (cut circles) were used.
Figure 2: Laboratory setups of the FTCA cassette.
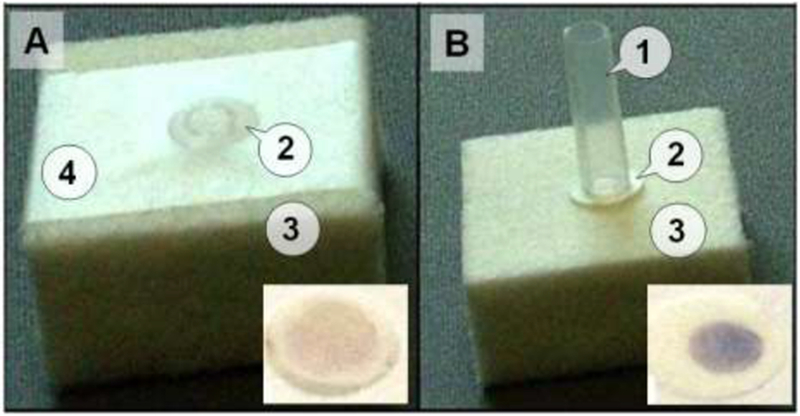
Panel A: A conventional system with retainer filter (membrane) attached with tape (4) on the absorbent pad. The sample is applied directly on part of the membrane through an opening in the tape. Panel B: The FTCA cassette prototype comprised of a sample delivery tube/cylinder (1) held over the membrane either manuall y or by mechanical means; WBC retainer filter (membrane, 2); and an absorbent pad (3). The insets show images of the membranes with final results obtained from a whole blood sample after the color development step.
2.3.3. FTCA procedure
Two immunochemical reaction protocols were used in this study.
Reaction protocol 1
In this protocol, 70 μl of whole blood sample was mixed with 20 μl of 3 μg/ml Biotin anti-CD4 antibody, and the mixture was incubated for 15 min at RT. After incubation, 10 μl of Streptavidin-AP (30 μg/ml) was added and the sample was incubated for an additional 15 min at RT. 50 μL of the sample was applied through sample delivery tube of the FTCA laboratory setup (Figure 2B) and allowed to absorb completely. The delivery tube was removed and the membrane was washed with 2.5 mL of 10 mM Tris, pH 7.4 containing 100 μg/mL NBT. AP substrate (100 μL) containing 3 mM Levamisole was applied to the center of the spot and allowed to react for 2 min at RT in the dark. After 2 min, 200 μL of stop solution (0.1 N HC1) was added to the membrane to stop the blue product development reaction by enzyme.
Reaction protocol 2
In this protocol, Streptavidin-AP (24 μg/mL) was premixed with undiluted Biotin anti-CD4 antibody (3 μg/mL) in a 1:2 ratio. 30 μL of the Streptavidin-AP Biotin-antibody mixture was added to 70 μL of whole blood or CD3 depleted blood and the mixture was incubated with tilting and rotation for 15 min at RT. After incubation, 30 μL of the sample was delivered through the tube. Membrane washing, color development, and stop solution steps were the same as described in Protocol 1 above, except that the development step time was 5 min.
2.3.4. FTCA signal measurement
The intensity of the blue signal at the end of color development step was measured using one of the optical methods described below.
Image analysis
Digital images of the membranes were taken using a Fuji S7000 FinePix digital camera under ambient lighting conditions at a fixed distance from the membrane. Quantitative image analysis of digital photographs was performed using the QuantityOne software (Bio-Rad). The software calculates signal intensity by integrating all the pixel values (0-255) within the defined spot and subtracting background signal intensity.
Reflectance measurements
Colorimetric analysis was done using a handheld reflectance meter commonly used for glucose measurements (Cobas® AccutrendPlus, Roche), following the manufacturer’s instructions with some minor modifications. Briefly, after the color development step, the wet membrane circle was removed from the FTCA laboratory setup and mounted on the test area of the strip provided with the glucose meter. The test strip was carefully positioned such that the color spot on the membrane faced the optical window of the instrument. The displayed numerical result (in glucose concentration units) was used as an arbitrary signal for measurement of the color intensity.
2.3.5. CD4 T-lymphocyte calibration curve using FTCA method
For the preparation of the FTCA calibration curve, we used fresh whole blood samples from healthy donors. Since all these samples contained normal levels of CD4 T-cells, samples containing decreased levels of CD4 T-cells were prepared by depletion of CD3 T-cells or CD4 T-cells. Because CD4 depletion leads to reduction of both CD4 lymphocytes and monocytes, we chose CD3 depleted blood samples to keep monocyte concentration in blood samples unchanged. CD3 T-lymphocytes were depleted using Dynabeads® CD3 as described above (see CD3 depleted blood samples). The final CD4 count in these samples was measured using the manual Dynal® T4 Quant Kit. The FTCA experiments were performed using reaction protocol 1 (section 2.3.3.) and quantitative results were obtained using image analysis (section 2.3.4).
3. Results
3.1. FTCA cassette prototype
Unlike conventional flow-through immunoassays, in which the sample, reagent, and wash solutions are added to the filter directly, FTCA samples are added through a plastic or glass delivery tube/cylinder, which is gently pressed on the retention filter membrane over the absorbent pad. This method provides a more controlled delivery of the solutions within a confined area on the retention membrane. Figure 2 illustrates the effect of using a small tube for sample delivery in our flow-through system. The conventional and FTCA prototypes of cassettes developed for experimental studies are shown in Figure 2. Both prototypes consist of an absorbent pad (Bio-Rad Labs) and a WBC retainer filter. The difference between these two prototypes is that in the conventional scheme, the filter is attached to the absorbent pad using a tape strip with a hole in the center for sample application (Figure 2A), whereas in the second variant, a plastic cylinder (4 mm diameter) is gently pressed on the membrane (Figure 2B). The final results obtained from the two methods are shown as insets in Figures 2A and 2B, respectively. As can be seen in Figure 2, the signal from the tube delivery method is more uniform and stronger than that obtained using the conventional cassette.
3.2. Selection of retainer membrane
In order for the membrane (filter) to be suitable for FTCA, it must exhibit three main capabilities: 1) to selectively capture as many lymphocytes as possible, 2) to allow cells other than white blood cells to be washed out; and 3) to allow the ratio of captured (adsorbed) lymphocytes to monocytes to be as high as possible, in order to avoid monocyte interference. Indeed, one of the main problems associated with all manual tests for CD4 T-cells is the interference of monocytes. Like CD4 T-cells, monocytes carry CD4 antigens, and the anti-CD4 antibodies used for enumeration of CD4 antigens may bind to monocytes and result in increased CD4 count. Therefore, in most CD4 manual tests, monocytes are first removed from blood and then CD4 cells are counted. The additional step of monocyte removal obviously complicates and lengthens the test procedure. Unlike other manual methods, FTCA allows one to count CD4 T-cells without removing monocytes from whole blood. However, in order for FTCA to be accurate for determination of CD4+ T-cells at low CD4 concentrations, the range in which monocyte interference is the greatest, the membrane used in FTCA must adsorb lymphocytes much more efficiently than monocytes.
In this study, we used leukocytes reduction filters which are widely employed in blood transfusion industry. These filters adsorb cells, mainly leukocytes, based on size and charge of the cells (Bruil et al., 1995). The exact mechanism of adsorption of different subsets of leukocytes including lymphocytes depends on the particular type of the filter and its manufacturing process, and usually unknown. We examined the suitability of three commercially available filters for FTCA with the highest reported ratio of adsorbed lymphocytes to monocytes: LRF10S, Leukosorb (B), and WBF-2 (Sowemimo-Coker SO, Zinn F, Kim A et al., 2002). In order to compare these filters, we tested samples with low or depleted CD4 counts using these filters in FTCA system. The LRF10S filter membrane was found to be more efficient than the Leukosorb (B) and WBF-2 membranes (data not shown). These results are in general agreement with those reported by Pall (the filter manufacturer) (Sowemimo-Coker SO, Zinn F, Kim A et al., 2002) and presented in Figure 3, which shows the three differential WBC fractions (lymphocytes, granulocytes, and monocytes) for unfiltered whole blood vs. the WBC fractions recovered from leukoreduction filters WBF-2 and LRF10S after whole blood filtration. As can be seen in Figure 3, the ratio of lymphocytes to monocytes recovered from WBF-2 and LRF10S filters relative to the ratio in unfiltered whole blood is 2.2- and 3.3-fold, respectively. Thus, a significant relative enrichment of lymphocytes is observed on the LRF10S filter membrane. We found that the LRF10S filter provided the best absolute lymphocyte adsorption efficiency, a maximal signal to noise ratio for samples containing high and low CD4 counts, and minimal error due to CD4 monocytes adsorbed on the filter (see below). Therefore, the LRF10S filter membrane was chosen for subsequent studies using the FTCA system.
Figure 3. Differential WBC retention/recovery from WBF-2 and LRF10S leukoreduction filters.
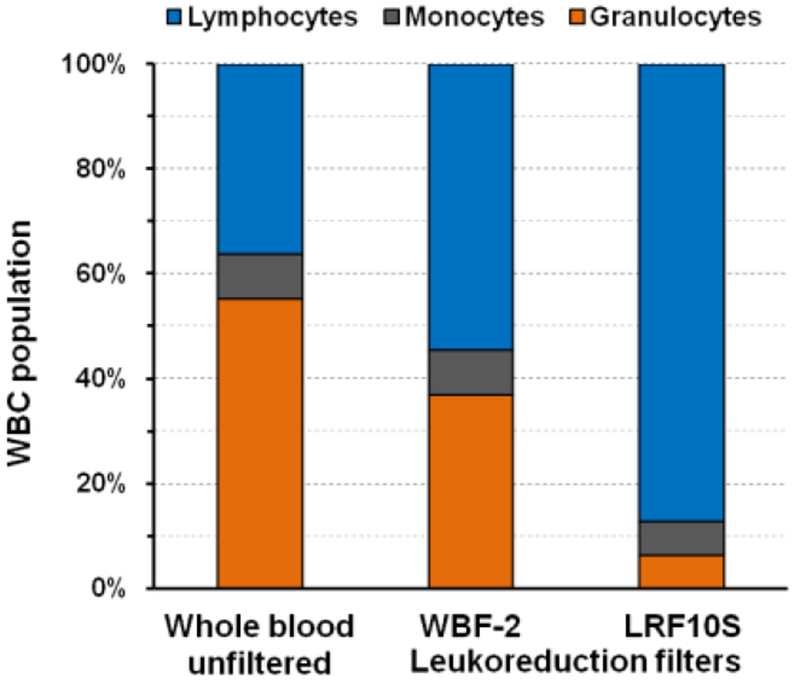
Distribution of three WBC fractions (lymphocytes, monocytes, and granulocytes) in unfiltered whole blood and after retention and recovery from two leukoreduction filters of whole blood (Sowemimo-Coker SO, Zinn F, Kim A et al., 2002).
3.3. CD4 calibration curve using FTCA
The CD4 calibration curve obtained by reaction protocol 1 (section 2.3.3) and image analysis (section 2.3.4) is shown in Figure 4 as a plot of signal intensity vs. CD4 count (normal whole blood or CD3 depleted blood) from triplicate samples. Since CD4 depletion leads to reduction of CD4 lymphocytes and CD4 monocytes, we chose to deplete CD3 cells to keep monocyte concentrations unchanged while making artificial blood samples with reduced CD4 T-lymphocyte counts. It is apparent from Figure 4 that the calibration curve is linear in the entire clinically significant range of CD4 T-cell concentrations (200 – 1,200 CD4 cells/μL). The most important feature of the FTCA calibration curve is that it allows one to accurately quantify the signal in the range of CD4 T-cell counts used for clinical HIV management (including opportunistic infections), ~100 - 350 cells/μL. Figure 4 also shows that the error (coefficient of variation, CV) of the measurements is < 20 % which is acceptable for this kind of assay (Stevens et al., 2008).
Figure 4. CD4 calibration curve using FTCA with image analysis.
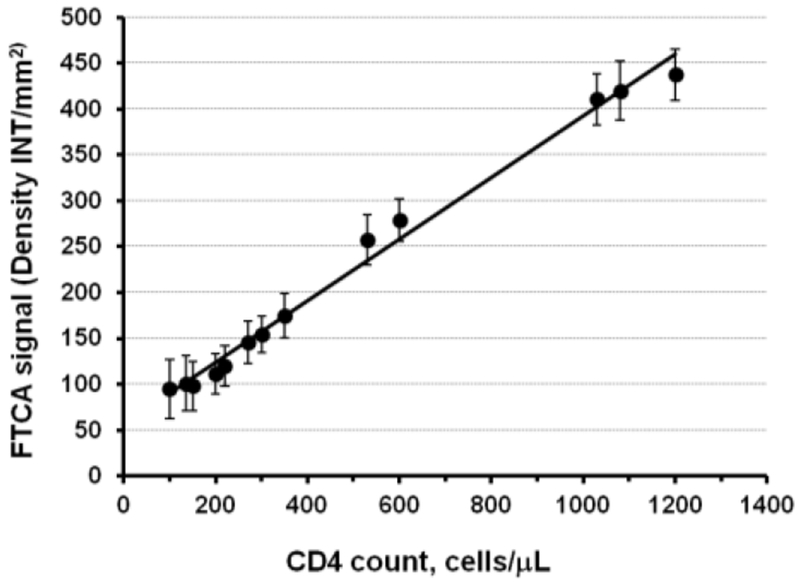
Data obtained using FTCA reaction protocol 1 and image analysis (sections 2.3.3 and 2.3.4). Sample CD4 counts were measured using Dynal® T4 Quant Kit. Samples with CD4 count < 1000 cells/μL were prepared by depletion of CD3 T-cells (section 2.1.2). The calibration curve fits well to a linear regression analysis with an equation, y = 0.336 x + 56.2 and R2 = 0.99.
3.4. Possible interference of monocytes on CD4 T-cell count
To test monocyte interference in the FTCA method, we compared signals obtained for CD3 depleted samples (high monocyte concentration, low CD4 T-cell concentration) with signals obtained for diluted whole blood samples (low monocyte concentration, low CD4 T-cell concentration). Table 1 shows that the difference between the signals for blood samples containing approximately the same low CD4 T-cell concentration (~200 cells/μL) and high monocyte concentration (800 cells/μL) or low monocyte concentration (160 cells/μL) did not exceed the error of our method. It is worth noting that in our experiments, the CD3 depleted samples contained a practically maximal normal clinical concentration of monocytes, which is approximately 800 cells/μL. Similar results were obtained for undiluted clinical samples containing different concentrations of monocytes (see Table 1).
Table 1.
CD4 count bias between FTCA and reference methods for some clinical samples with high and low monocyte concentrations.
| Sample No. | Sample type | Monocytes count, cells/μL | CD4 count, cells/μL | Bias (%) | |
|---|---|---|---|---|---|
| Reference methods | FTCA* | ||||
| 1 | CD3 depleted | 800 | 220 | 205 | −15 (−6.8 %) |
| 2 | CD3 depleted | 800 | 204 | 188 | −16 (−7.8 %) |
| 3 | Diluted blood | 160 | 210 | 194 | −16 (−7.6 %) |
| 4 | Whole blood | 730 | 451 | 477 | +26 (+5.8 %) |
| 5 | Whole blood | 440 | 409 | 391 | −18 (−4.4 %) |
Calculated from formula: y = 0.336 x + 56.2 (from calibration plot Trendline)
The above experiments show that the presence of monocytes carrying CD4 antigen in whole blood does not affect the performance of the FTCA. This could be driven by following: 1) compared to T-cells, monocytes express about an order of magnitude less surface CD4 antigens (Davis et al., 1998), which reduces the number of AP conjugate molecules bound to the surface of the monocytes; 2) monocytes adsorbed onto the LRF10S membrane are depleted significantly relative to lymphocytes as compared to whole blood samples (see also Figure 3).
3.5. Correlation between FTCA and the FACS and Dynal T4 Quant methods
In order to perform preliminary correlation analysis between our FTCA method and two reference methods, FACS and Dynal T4 Quant, CD4 T-cell count was measured by FTCA in all samples (n = 54), by Dynal T4 Quant Kit in 15 HIV-negative whole blood samples and 27 CD3 depleted HIV-negative samples, and by FACS in 2 HIV-negative samples and 10 HIV-positive samples. The correlation between CD4 T-cell counts assessed by FTCA vs. FACS and Dynal T4 Quant Kit for different sets of samples is shown in Figure 5 and Table 2
Figure 5. Correlation analysis of CD4 cell counts measured by FTCA vs reference methods.
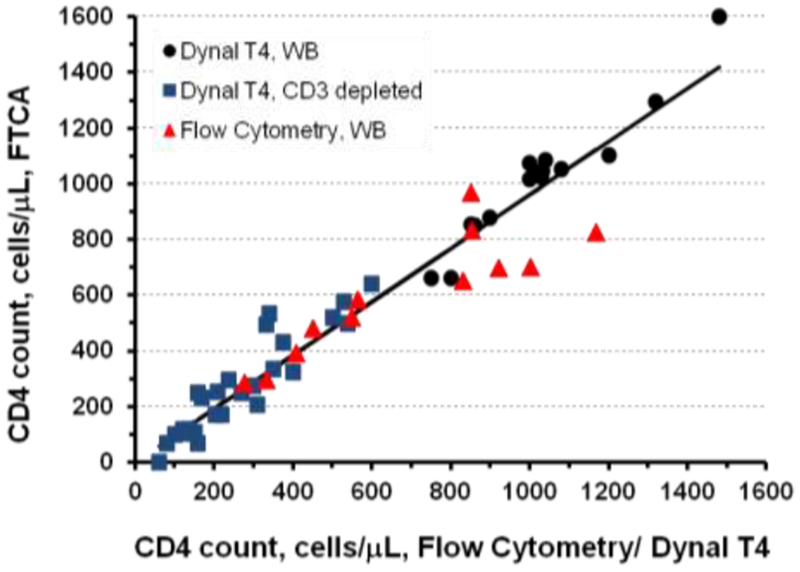
CD4 cell counts of all samples (all data points, n = 54) were determined by FTCA calibration curve. HIV negative whole blood (WB) samples (black circles, n = 15) and CD3 depleted blood samples (blue squares, n = 27) were analyzed by Dynal® T4 Quant Kit. Flow cytometry (red triangles) was used to analyze HIV positive samples (n = 10) and HIV negative samples (n = 2, CD4 ≥ 1000 cells/μL). Statistical parameters of the correlation analyses are listed in Table 2.
Table 2.
Statistical parameters from the correlation analysis of FTCA vs FACS and Dynal CD4 counting methods.
| Sample set (counting method, sample type, number of samples) | Linear fit parameters | Pearson correlation coefficient | ||
|---|---|---|---|---|
| Slope | Intercept | R2 | ||
| HIV negative (Dynal T4, whole blood, n = 15) | 1.16 | −177.3 | 0.94 | 0.97 |
| HIV negative (Dynal T4, CD3 depleted, n = 27) | 1.12 | −33.9 | 0.84 | 0.92 |
| HIV positive (Flow Cytometry, whole blood, n = 10) | 0.85 | 57.4 | 0.83 | 0.91 |
| HIV positive + negative (Flow Cytometry, whole blood, n = 10 + 2) | 0.66 | 150.7 | 0.77 | 0.88 |
| All samples (all methods, all sample types, n = 54) | 0.96 | 1.1 | 0.94 | 0.97 |
The regression slope of the correlation, R2 value, and Pearson correlation coefficient are close to 1 for all but one (FTCA vs. FACS, 10 HIV positive + 2 negative samples) sets of samples, indicating a strong correlation and very good agreement with reference methods. Indeed, of the 27 whole blood samples tested, there were 4 outlier results that differed by more than 100 CD4 T-cells: The instances of poor correlation between FTCA and FACS were observed with two HIV-positive samples with CD4 count > 600 cells/μL and two HIV-negative samples with CD4 counts > 1000 cells/μL. In these samples, FTCA results are approximately 100-150 cells/μL less than FACS results. As can be seen in Figure 5, the FTCA allows one to determine CD4 counts in the total range of all analyzed samples, 60 - 1480 cells/μL. which effectively covers the entire range of clinical CD4 counts (200 – 1,200 cells/μL). Moreover, in the most clinically significant range of CD4 counts (~100 – 350 cells/μL), there is a very good correlation between FTCA vs Dynal T4 Quant and FACS methods.
3.6. FTCA signal measurement using a portable reflectivity meter
All quantitative FTCA results described above were obtained using a digital camera and a commercially available image analysis software (QuantityOne from Bio-Rad). Though this approach can be justified as a proof-of-concept for the FTCA, a small, portable, easy-to-use, and inexpensive device is needed to make the assay more suitable for use in the field. For this purpose, we also demonstrated that colorimetric analysis of the FTCA test can be done using reflectivity measurements. In optical reflectometry, changes in color are used to determine analyte concentration. A simple glucose meter (Cobas® AccutrendPlus, Roche) that uses reflectance photometry to quantify color intensity was employed in this study. For CD4 count measurement, a test strip with a mounted LRF10S membrane was inserted into the Accutrend device, and the result was displayed in less than 15 seconds. In order to prepare a CD4 count calibration curve using a glucose meter, the FTCA tests were performed utilizing the simplified reaction protocol 2, which uses Biotin anti-CD4 antibody -Streptavidin-AP conjugate prepared in advance (see Materials and Methods section 2.3.3) and whole and CD3 depleted blood samples containing varying CD4 counts. The calibration curve, which is a function of the reflected light intensity expressed as glucose concentration units (mg/dL) versus CD4 counts, is presented in Figure 6.
Figure 6. Preliminary CD4 calibration curve using FTCA with reflectance measurements.
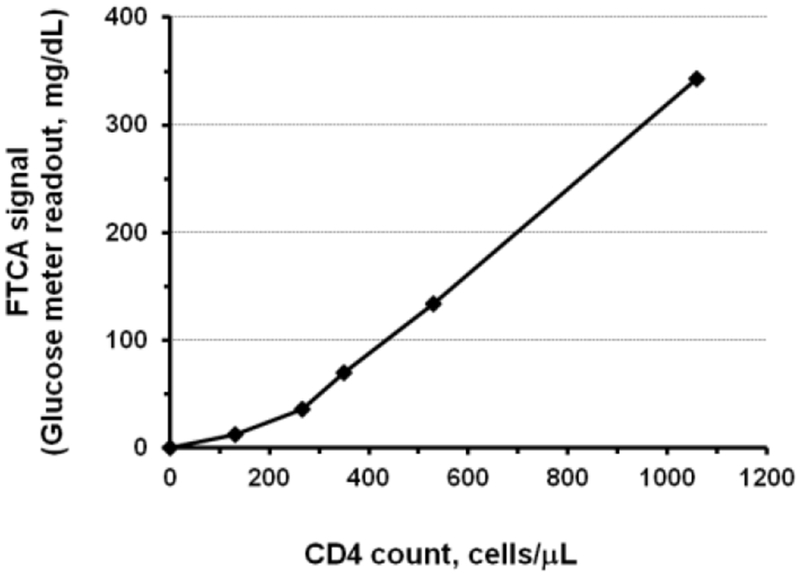
Data obtained using FTCA reaction protocol 2 and reflectance measurements (sections 2.3.3 and 2.3.4). The reflectance meter readout is reported in glucose concentration units of mg/dL. CD4 counts of samples were measured using Dynal® T4 Quant Kit. Samples with CD4 count < 600 cells/μL were prepared by depletion of CD3 T-cells (section 2.1.2).
4. Discussion
In this paper, we describe the development of a novel FTCA system and show its application for the enumeration of CD4 count in whole blood samples. A prototype of the FTCA cassette with a controlled application of sample through a delivery tube/cylinder was found to give more uniform and stronger signal compared to a conventional system with open/direct sample application on the membrane. For the CD4 count measurement application, a leukoreduction fdter membrane was employed. In order for the fdter membrane to be suitable for this application, it must possess three main properties: 1) ability to capture WBCs with high capacity, 2) ability to selectively retain lymphocytes upon wash and allow other cells and interfering substances (e.g. enzyme inhibitors, etc.) to be washed out, and 3) ability to minimize interference from remaining monocytes. Unlike other manual methods, FTCA allows one to count CD4 T-cells without removing monocytes from whole blood. However, in order for FTCA to accurately quantify CD4 T-cells at low CD4 concentrations, the range in which monocyte interference could be significant, the membrane used in FTCA must retain lymphocytes much more efficiently than monocytes. The present proof-of-concept experiments show that the presence of monocytes at highest normal level (~ 800 cells/μL) has no significant effect on the performance of the FTCA for CD4 T-cell measurement at the clinically relevant concentration of ~200 CD4 cells/μL, an important threshold level for making decision about failing ART. It is however worth noting that in our experiments, the CD3 depleted samples contained a practically maximal clinical concentration of monocytes of normal range (200 - 800 cells/μL), which is 800 cells/μL. Monocytosis, the condition with greater than 800 monocytes/μL is usually observed in patients with chronic infections, autoimmune disorders, and certain cancers (Chara et al., 2015, 2012). The prevalence of monocytosis among HIV-positive patients is rather low, around 3% (Rahman et al., 2014). Therefore, the probability of monocyte interference for the overall HIV positive population should be low. Regardless, a more detailed study including samples containing greater than 800 monocytes/μL is required in the future to find out if any possible interference towards an accurate CD4 T-cell enumeration by the FTCA method occurs.
Our results demonstrate that the FTCA works well on whole blood HIV negative samples, CD3 depleted samples (samples with decreased lymphocyte concentrations), and HIV positive samples. Based on the current proof-of-concept studies, the FTCA calibration curve allows measurement of CD4 counts in the studied range of 60 - 1480 cells/μL. The linearity of the FTCA calibration curve is very good in the range of CD4 T-cell counts (200-1000 cells/μL) that is clinically important for monitoring HIV progression. Testing clinical samples in parallel with commercially available CD4 tests showed a high degree of correlation between the FTCA vs Dynal T4 Quant and FACS (Table 2). The correlation-coefficient between Dynal T4 and flow cytometry is 0.97 (“Dynal® T4 Quant Kit,” 2003), approximately the same as between FTCA and reference methods (Dynal T4 + flow cytometry). Minor discrepancies were observed in the range of CD4 counts exceeding 600 cells/μL, which is considered in the normal range and is, therefore, not relevant for HIV disease monitoring. Although the cause of this discrepancy is unknown, a much larger number of clinical samples must be tested in order to draw a compelling conclusion about the correlation between FTCA and FACS.
Current WHO guidelines emphasize HIV viral load testing for ART initiation and HIV disease management. However, CD4 count remains an important criteria at various stages of HIV disease management and treatment against opportunistic infections (CDC NIH, 2018; Ford et al., 2017; WHO, 2016). Developments in point-of-care (POC) CD4 counting technologies have focused on methods that could be used in resource-limited countries, in any health care setting or in the field. Time to result for the tests designated to be used in the resource-limited settings varies from 6 to 40 min, with sample throughput of 3-10 samples per hour. Commercially available PIMA™ CD4 test from Abbott Laboratories is based on static image analysis and counting principles (Sukapirom et al., 2011). In general, the development of a POC diagnostic test from laboratory proof-of-concept to meeting regulatory requirements is a long and challenging path. Several CD4 enumeration technologies have been developed (Givens et al., 2017; Glynn et al., 2013; Kanakasabapathy et al., 2017; Logan et al., 2013; Peeling et al., 2015; Rodriguez et al., 2005), and although these tests are promising, they require complex and relatively expensive equipment such as microfluidic devices and signal detection and analysis systems. These tests also need to be evaluated in clinical trials and may find it challenging to progress to late development stages (e.g. Zyomyx CD4) (Price, 2016). Published data is available for the PIMA CD4 test (Mtapuri-Zinyowera et al., 2011; Sukapirom et al., 2011; Zeh et al., 2017), and has shown that the test is prone to erroneous outcomes as a result of instrument rejections due to failed internal control criteria (Fajardo et al., 2015).
The FTCA is based on a modified immuno-concentration method and colorimetric analysis by means of image analysis of digital photographs or by measuring reflectance with a portable glucose-type reflectivity meter. In the near future, we intend to develop the next generation of FTCA cartridge in conjunction with an inexpensive signal analysis system. We envisage developing the FTCA POC test with the imaging system as a standalone, portable, battery operated device, with an integrated image analysis software, and an LCD panel to display CD4 counts directly. Alternatively, an affordable handheld device like a reflectance meter can be used to measure signal intensity and provide output as CD4 count. The test cartridge will consist of a liquid delivery port for incubated sample, the retention membrane, and the absorbent pad. The design of the FTCA cassette will allow all steps, from sample application to signal detection, to occur with minimal manipulation and without requiring a skilled technician. The time to CD4 count results from FTCA will be 30-40 minutes, and multiple tests (≤ 10) can be performed in parallel by a single operator in one hour.
5. Conclusion
In this paper we demonstrated the feasibility of a simple, flow-through cell counting assay for enumeration of CD4 cells using the prototype FTCA cassette, digital camera and image analysis software, or simple blood glucose meter for the detection of a test signal. The FTCA allows one to determine CD4 cell count quantitatively in the clinically significant range of CD4 concentrations. Further development of the assay will provide great potential not only for CD4 T-lymphocyte enumeration, but also for other cell-based point-of-care tests in resource-constrained countries.
HIGHLIGHTS.
Development of a colorimetric flow-through cell counting assay (FTCA).
Assembly of a proof of-concept prototype of the FTCA cassette/device.
Rapid and inexpensive point-of-care testing for enumeration of CD4 T-cells.
Acknowledgments
We thank Bill Kabat and staff of Infectious Disease Laboratory at Ann & Robert H. Lurie Children’s Hospital of Chicago for providing HIV positive clinical samples and FACS data.
Funding
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health [1R43HL097933-01A1].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None declared.
Abbreviations: AP, alkaline phosphatase; ART, antiretroviral therapy; FACS, fluorescence-activated cell sorting; FTCA, flow-through cell counting assay; NBT, Nitro Blue Tetrazolium; POC, point-of-care; RT, room temperature.
References:
- Alere PIMA™ Analyser [WWW Document], n.d. URL https://www.alere.com/en/home/product-details/PimaAnalyserOUS.html (accessed 5.2.19) .
- BD FACSPRESTO - A near-patient complete CD4 testing solution [WWW Document], n.d. URL https://www.bdbiosciences.com/eu/instruments/clinical/cell-analyzers/bd-facspresto/m/4595881/overview (accessed 5.2.19).
- Boyle DS, Hawkins KR, Steele MS, Singhal M, Cheng X, 2012. Emerging technologies for point-of-care CD4 T-lymphocyte counting. Trends Biotechnol 30, 45–54. doi:S0167-7799(11)00119-3 [pii] 10.1016/j.tibtech.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruil A, Beugeling T, Feijen J, van Aken WG, 1995. The mechanisms of leukocyte removal by filtration. Transfus. Med. Rev IX, 145–166. doi: 10.1016/S0887-7963(05)80053-7 [DOI] [PubMed] [Google Scholar]
- Bystryak S, Santockyte R, n.d. A flow through cell counting assay. [DOI] [PMC free article] [PubMed]; (A) US Patent No. 9,097,712 (2015);; (B) European Patent No. EP2972345B1 (2018).
- CDC, 2019. HIV in the United States and Dependent Areas. URL https://www.cdc.gov/hiv/pdf/statistics/overview/cdc-hiv-us-ataglance.pdf (accessed 5.2.19).
- CDC NIH, 2018. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents.
- Chara L, Sánchez-Atrio A, Pérez A, Cuende E, Albarrán F, Turrión A, Chevarria J, del Barco AA, Sanchez MA, Monserrat J, Prieto A, de la Hera A, Sanz I, Diaz D, Alvarez-Mon M, 2015. The number of circulating monocytes as biomarkers of the clinical response to methotrexate in untreated patients with rheumatoid arthritis. J. Transl. Med 13, 1–10. doi: 10.1186/sl2967-014-0375-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chara L, Sánchez-Atrio A, Pérez A, Cuende E, Albarrán F, Turrión A, Chevarria J, M. A, Sánchez MA, Monserrat J, de la Hera A, Prieto A, Sanz I, Diaz D, Alvarez-Mon M, 2012. Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res. Ther. 14, 1–11. doi: 10.1186/ar3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee L-M, Glencross DK, 2017. Performance verification of the new fully automated Aquios flow cytometer PanLeucogate (PLG) platform for CD4-T-lymphocyte enumeration in South Africa. PLoS One 12, 1–17. doi: 10.1371/journal.pone.0187456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Abrams B, Iyer SB, Hoffman RA, Bishop JE, 1998. Determination of CD4 antigen density on cells: role of antibody valency, avidity, clones, and conjugation. Cytometry 33, 197–205. doi:10.1002/(SICI)1097-0320(19981001)33:2<197::AID-CYT014>3.0.CO;2-P [pii] [DOI] [PubMed] [Google Scholar]
- Di Biagio A, Ameri M, Sirello D, Cenderello G, Di Bella E, Taramasso L, Giannini B, Giacomini M, Viscoli C, Cassola G, Montefiori M, 2017. Is it still worthwhile to perform quarterly cd4+ t lymphocyte cell counts on hiv-1 infected stable patients? BMC Infect. Dis. 17, 1–6. doi: 10.1186/sl2879-017-2199-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaw PA, Daneau G, Coly AA, Ndiaye BP, Wade D, Camara M, Mboup S, Kestens L, Dieye TN, 2011. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr 58, e103–111. doi: 10.1097/QAI.0b013e318235b378 [DOI] [PubMed] [Google Scholar]
- Dynal® T4 Quant Kit [WWW Document], 2003. URL https://assets.thermofisher.com/TFS-Assets/LSG/brochures/CEL.F.030.01.pdf (accessed 4.25.19).
- Fahey JL, Taylor JM, Detels R , Hofmann B, Melmed R, Nishanian P, Giorgi JV, 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med 322, 166–172. doi: 10.1056/NEJM199001183220305 [DOI] [PubMed] [Google Scholar]
- Fajardo E, Metcalf C, Piriou E, Gueguen M, Maman D, Chaillet P, Cox V , Rumaney MB, Tunggal S, Kosack C, Roberts T, 2015. Errors generated by a point-of-care CD4+ T-lymphocyte analyser: a retrospective observational study in nine countries. Bull World Heal. Organ 93, 623–630. doi: 10.2471/BLT.14.146480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye B, Mbow M, Cheikh Seek M, Mbengue B, Wade D, Camara M, Cisse C, Diouf SG, Ndao B, Djibo A, Sylla Niang MD, Ndiaye T, Grillo MP, Dieye A, 2016. Evaluation of PIMATM CD4 System for Decentralization of Immunological Monitoring of HIV-Infected Patients in Senegal. PLoS One 11, 1–11. doi: 10.1371/journal.pone.0154000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford N, Meintjes G, Vitoria M, Greene G, Chiller T, 2017. The evolving role of CD4 cell counts in HIV care. Curr. Opin. HIV AIDS 12, 123–128. doi: 10.1097/COH.0000000000000348 [DOI] [PubMed] [Google Scholar]
- Givens M, Weaver A, Bickman S, Logan C, Noormahomed EV, Patel S, Schooley RT, Benson CA, Lochhead MJ, 2017. Near patient CD4 count in a hospitalized HIV patient population. Cytom. Part B - Clin. Cytom. 92B, 451–455. doi: 10.1002/cyto.b.21248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn MT, Kinahan DJ, Ducree J, 2013. CD4 counting technologies for HIV therapy monitoring in resource-poor settings--state-of-the-art and emerging microtechnologies. Lab Chip 13, 2731–2748. doi: 10.1039/c31c50213a [DOI] [PubMed] [Google Scholar]
- Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF, 2011a. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet 378, 1572–1579. doi: 10.1016/S0140-6736(11)61052-0 [DOI] [PubMed] [Google Scholar]
- Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa 0, Lehe JD, Peter TF, 2011b. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS 25, 807–812. doi: 10.1097/QAD.0b013e328344f424 [DOI] [PubMed] [Google Scholar]
- Kanakasabapathy MK, Pandya HJ, Draz MS, Chug MK, Sadasivam M, Kumar S, Etemad B, Yogesh V, Safavieh M, Asghar W, Li JZ, Tsibris AM, Kuritzkes DR, Shafiee H, 2017. Rapid, label-free CD4 testing using a smartphone compatible device. Lab Chip 17, 2910–2919. doi: 10.1039/c71c00273d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IT, Maughan-Brown B, 2017. Improved life expectancy of people living with HIV: who is left behind? Lancet HIV 4, e324–e326. doi: 10.1016/S2352-3018(17)30086-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestens L, Mandy F, 2017. Thirty-Five Years of CD4 T-Cell Counting in HIV Infection: From Flow Cytometry in the Lab to Point-of-Care Testing in the Field. Cytometry B. Clin. Cytom. 92, 437–444. doi: 10.1002/cyto.b.21400 [DOI] [PubMed] [Google Scholar]
- Lange JM, de Wolf F, Goudsmit J, 1989. Markers for progression in HIV infection. AIDS 3 Suppl 1, S153–160. [DOI] [PubMed] [Google Scholar]
- Logan C, Givens M, Ives JT, Delaney M, Lochhead MJ, Schooley RT, Benson CA, 2013. Performance evaluation of the MBio Diagnostics point-of-care CD4 counter. J. Immunol. Methods 387, 107–113. doi: 10.1016/j.jim.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutwama F, Serwadda R, Mayanja-Kizza H, Shihab HM, Ronald A, Kamya MR, Thomas D, Johnson E, Quinn TC, Moore RD, Spacek LA, 2008. Evaluation of Dynabeads and Cytospheres compared with flow cytometry to enumerate CD4+ T cells in HIV-infected Ugandans on antiretroviral therapy. J Acquir Immune Defic Syndr 48, 297–303. doi: 10.1097/QAI.0b013e31817bbc3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe YC, Wang Y, Elbireer A, Auerbach B, Castelnuovo B, 2012. Evaluation of Portable Point-of-Care CD4 Counter with High Sensitivity for Detecting Patients Eligible for Antiretroviral Therapy. PLoS One 7, 1–5. doi:10.1371/journal.pone.0034319 PONE-D-11–24422 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyani CN, McIntyre JA, Myer L, 2012. The reliability of point-of-care CD4 testing in identifying HIV-infected pregnant women eligible for antiretroviral therapy. J Acquir Immune Defic Syndr 60, 260–264. doi: 10.1097/QAI.0b013e318256b651 [DOI] [PubMed] [Google Scholar]
- Mtapuri-Zinyowera S, Chideme M, Mangwanya D, Mugurungi 0, Gudukeya S, Hatzold K, Mangwiro A, Bhattacharya G, Lehe J, Peter T, 2011. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. Acquir Immune Defic Syndr 55, 1–7. doi: 10.1097/QAI.0b013e3181e93071 [DOI] [PubMed] [Google Scholar]
- Peeling RW, Sollis KA, Glover S, Crowe SM, Landay AL, Cheng B, Barnett D, Denny TN, Spira TJ, Stevens WS, Crowley S, Essajee S Vitoria M, Ford N, 2015. CD4 enumeration technologies: a systematic review of test performance for determining eligibility for antiretroviral therapy. PLoS One 10, 1–26. doi: 10.1371/journal.pone.0115019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter T, Badrichani A, Wu E, Freeman R, Ncube B, Ariki F, Daily J, Shimada Y, Murtagh M, 2008. Challenges in implementing CD4 testing in resource-limited settings. Cytom. Part B Clin. Cytom. 74B, S123–S130. doi: 10.1002/cyto.b.20416 [DOI] [PubMed] [Google Scholar]
- Phillips AN, Lee CA, Elford J, Janossy G, Timms A, Bofill M , Kernoff PB, 1991. Serial CD4 lymphocyte counts and development of AIDS. Lancet 337, 389–392 . [DOI] [PubMed] [Google Scholar]
- Price D, 2016. Eyes Wide Open: Good reasons for a bad investment in a low-cost HIV test. [WWW Document]. Stanford Soc. Innov. Rev URL https://ssir.org/articles/entry/eyes_wide_open# (accessed 5.2.19).
- Rahman Md Mizanur, Giti S, Islam MS, Md Mostafizur Rahman, 2014. Haematological Changes in Peripheral Blood of HIV - Infected Persons with Correlation to CD4 Cell Count. J. Bangladesh Coll. Physicians Surg. 32, 130–136. doi: 10.3329/jbcps.v32i3.26050 [DOI] [Google Scholar]
- Rodriguez WR, Christodoulides N, Floriano PN, Graham S, Mohanty S, Dixon M, Hsiang M, Peter T, Zavahir S, Thior I, Romanovicz D, Bernard B, Goodey AP, Walker BD, McDevitt JT, 2005. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS Med 2, 0663–0672. doi:05-PLME-RA-0042R2 [pii] 10.1371/journal.pmed.0020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowemimo-Coker SO, Zinn F, Kim A, Sowemimo-Coker AJ, Zinn SO, Kim F, Angelbeck A, J., 2002. A Simple Procedure For Recovery Of Viable Leukocytes from Pall Filters. Transfusion 42, poster 57A. [Google Scholar]
- Stein DS, Korvick JA, Vermund SH, 1992. CD4+ lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J. Infect. Dis 165, 352–363. [DOI] [PubMed] [Google Scholar]
- Stevens W, Gelman R, Glencross DK, Scott LE, Crowe SM, Spira T, Rebecca G, Glencross DK, Scott LE, Crowe SM, Spira T, 2008. Evaluating new CD4 enumeration technologies for resource-constrained countries. Nat Rev Microbiol 6, S29–S38. [DOI] [PubMed] [Google Scholar]
- Sukapirom K, Onlamoon N, Thepthai C, Polsrila K, Tassaneetrithep B, Pattanapanyasat K, 2011. Performance Evaluation of the Alere PIMA CD4 Test for Monitoring HIV-Infected Individuals in Resource-Constrained Settings. J Acquir Immune Defic Syndr 58, 141–147. doi: 10.1097/QAI.0b013e31822866a2 [DOI] [PubMed] [Google Scholar]
- The Antiretroviral Therapy Cohort Collaboration, 2017. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 4, e349–e356. doi: 10.1016/S2352-3018(17)30066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS, 2018. Fact Sheet - World AIDS Day 2018, URL http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed 5.2.19).
- VISITECT® CD4 POC test [WWW Document], n.d. URL http://www.omegadiagnostics.com/Products/Infectious-Diseases/HIV/CD4 (accessed 5.2.19).
- WHO, 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization. [PubMed] [Google Scholar]
- Zeh C, Rose CE, Inzaule S, Desai MA, Otieno F, Humwa F, Akoth B, Omolo P, Chen RT, Kebede Y, Samandari T, 2017. Laboratory-based performance evaluation of PIMA CD4 + T-lymphocyte count point-of-care by lay-counselors in Kenya. J. Immunol. Methods 448, 44–50. doi: 10.1016/j.jim.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]


