Abstract
Sensory neuron nicotinic acetylcholine receptors (nAChRs) contribute to pain associated with tissue injury. However, there are marked differences between rats and mice with respect to both the properties and distribution of nAChR currents in sensory neurons. Since both species are used to understand pain signaling in humans, we sought to determine whether the currents present in either species was reflective of those present in human sensory neurons. Neurons from lumbar 4/5 dorsal root ganglia were obtained from adult male and female organ donors. Nicotine-evoked currents were detected in 40 of 47 neurons (85%). In contrast to the naïve mouse, in which almost all nAChR currents are transient, or the rat, in which both mouse-like transient and more slowly activating and inactivating currents are detected, all the currents in human DRG neurons were slow, but slower than those in the rat. Currents were blocked by the nAChR antagonists mecamylamine (30 μM), but not by the TRPA1 selective antagonist HC-030031 (10μM). Single cell PCR analysis of nicotinic receptor subunit expression in human DRG neurons are consistent with functional data indicating that receptor expression is detected 85 ± 2.1% of neurons assessed (n = 48, from 4 donors). The most prevalent co-expression pattern was α3/β2 (95 ± 4% of neurons with subunits), but α7 subunits were detected in 70 ± 3.4% of neurons. These results suggest that there are not only species differences in the sensory neuron distribution of nAChR currents between rodent and human, but that the subunit composition of the channel underlying human nAChR currents may be different from those in the mouse or rat.
Keywords: Patch clamp, nociceptor, chemosensitive, ionotropic, in vitro
Introduction
Data from rodents and humans indicate that activation of peripheral nicotinic acetylcholine receptors (nAChRs) can be either pro- or antinociceptive. Application of nicotine at a variety of superficial and deep tissue sites evokes the sensation of pain in humans 7, 17 and nociceptive responses in the rat 4, 29. Persistent inflammation is associated with increased α3β4 subunit expression 3 and an increase in nAChR current density in putative nociceptive DRG neurons 35. Furthermore, inflammatory hypersensitivity appears to be due, at least in part, to nAChR activation 3. Several lines of evidence suggest that peripheral nAChR activation may also be anti-nociceptive 25, likely secondary to a nAChR mediated suppression of nociceptor excitability 35. Thus, pre-clinical data support the suggestion that peripheral neuronal nAChR’s may be a viable target for the treatment of inflammatory pain.
nAChRs are pentameric ion channels composed of subunit combinations that contain at least one of 10 α (1–10) and between zero and three β (2–4) subunits. The subunit combination influences biophysical and pharmacological properties of the functional nAChR. Expression data and immunohistochemical studies suggest that at least nine of the 10 α (1–7, 9–10) and all four β (1–4) of the nAChR subunits are detectable in mouse 14, 31 and rat 12, 15, 16 DRG neurons. However, there appears to be a far narrower pattern of nAChR subunit expression in sensory neurons based on the biophysical and pharmacological properties of the evoked currents. These evoked currents primarily fall into one of two groups: (i) a rapidly activating and rapidly inactivating, or transient current, evoked in response to nicotine that is blocked by nM concentrations of methyllycaconitine (MLA), and (ii) a more slowing activating and slowly inactivating, or sustained current, blocked by the non-selective antagonists mecamylamine and hexamethonium, but resistant to MLA 12, 21, 35. The former is consistent with a channel mediated by receptors composed of the α7 subunit, while the latter is consistent with channels containing an α3β4 subunit combination. Based on the relative potency of agonists, Rau and colleagues suggested that there might be a third type of sustained current in DRG neurons 21.
Interestingly, the transient current is the primary type detected in mouse DRG neurons, where any sustained currents appear to be mediated by TRPA1 30. In contrast, both transient and sustained current types are detected in rat DRG neurons 12, 21, 35. Even more interestingly, nAChR currents 3, 30 or evoked Ca2+ transients 27 are only detected in 10–30% of mouse DRG neurons, while currents 21, 35 and evoked Ca2+ transients 26 are detected in 70–80% of rat DRG neurons. These dramatic species differences are a concern given that both the mouse and the rat are used to further our understanding of pain signaling in humans and as model organisms in drug discovery. Furthermore, this concern is amplified by recent results indicating that the pharmacological and biophysical properties of ion channels in human sensory neurons may be quite different from those in rodent sensory neurons 34, 36. Given the potential of the peripheral neuronal nAChR as a therapeutic target, in the present study we sought to determine the extent to which the currents present in either rat or mouse are reflective of those present in human sensory neurons.
Conventional whole cell patch clamp techniques were used with a fast drug application system to record the nicotine evoked current in human DRG neurons obtained from organ donors. Our results suggest that while there are many similarities between the nAChRs currents present in rodent and human sensory neurons, there are marked differences.
Methods
Human Subjects:
L4 and L5 DRGs were collected from organ donors with the consent of family members for the use of their loved one’s tissue for research purposes. Tissue was collected from October 1, 2012 through March 29, 2013, and then in November of 2018. The inclusion and exclusion criteria were based on those around organ donation the donor’s eligibility for organ donation (exclusion criteria would include the presence of communicable disease such as HIV or hepatitis Creutzfeldt-Jakob disease, or cancer that has spread in the last 12 months), and that the next of kin approved the use of tissue for research purposes. All procedures were approved by the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents.
Animal Subjects:
For the purpose of making side-by-side comparisons, data from seven male Sprague Dawley rats have been included in Figures 1 and 2, and a response typical of that observed in neurons obtained from six male and female mice has been included in Figure 1. We have reanalyzed data originally published 3, 35. The use of animals in these previous studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and in accordance with recommendations from the National Institutes of Health and the International Association for the Study of Pain for the humane use of animals in research. L4 and L5 DRG neurons were harvested and plated as previously described.
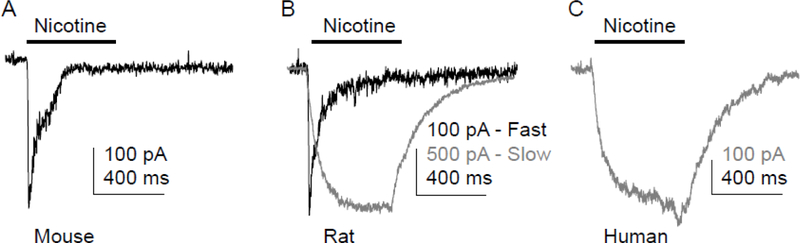
Figure 1.
Only slowly activating and slowly inactivating currents (slow current) were present in human DRG neurons. Representative current evoked with 300 μM nicotine applied for 500 ms to mouse (A), rat (B), and human (C) DRG neurons. The fast and slow currents observed in rat DRG neurons were recorded in different neurons.
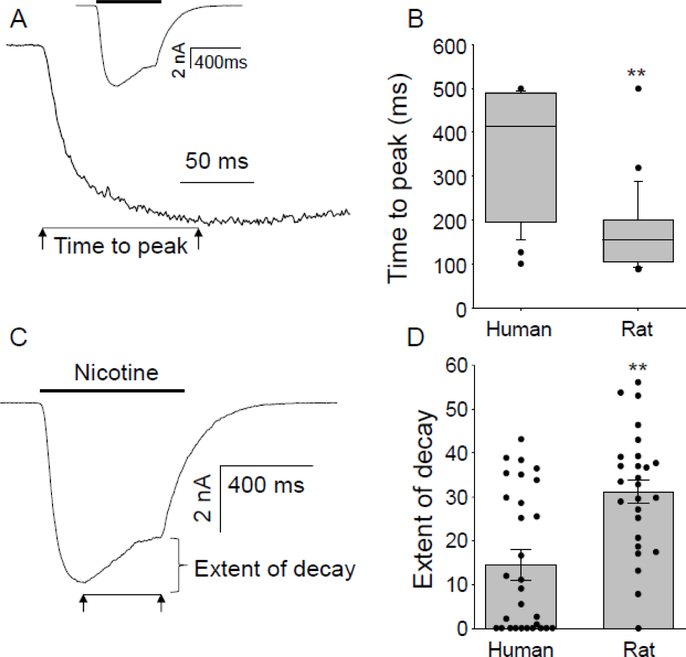
Figure 2.
Biophysical properties of slow currents in human DRG neurons were different from those previously described in the rat. (A). Current activation rate as estimated from the time to peak current was assessed on an expanded time scale than that used to record the current evoked in response to 1000 μM nicotine (inset). (B). Pooled data from human sensory neurons (n = 29) and from a previously published data set of slow current in rat DRG neurons (35, n = 26) that were reanalyzed by the method used for human sensory neurons, are plotted. Slow currents in rat sensory neurons activated more quickly than those in human DRG neurons (** p < 0.01, Mann-Whitney Rank Sum Test). (C) The extent of current inactivation was quantified by measuring the difference between peak evoked current and the peak current at the end of the 500 ms nicotine application. (D). Pooled extent of decay data from human and rat DRG neurons. Dots are data points from individual neurons. The extent of current decay was significantly larger in rat DRG neurons (** p < 0.01, Student’s t-test).
Collection of human DRGs and isolation of DRG neurons:
The detailed methods for collection of L4 and L5 human DRGs has been described in our previous reports 36. Neurons were studied 12 to 36 hrs after culture. To minimize the impact of any single donor or preparation of neurons, neurons from six donors were studied with patch-clamp and four donors with single cell PCR. Any given experiment was performed on neurons from at least two donors. The sample size was based on our assumption that currents would be as prevalent as those observed in rat, as well as our previous experience with neuron yields from organ donors.
Patch clamp recording:
Conventional whole-cell patch-clamp recordings were used to study nicotine-evoked currents in isolated sensory neurons. Recording was performed with an Axopatch 200B controlled with pClamp (v 10.2) software (Molecular Devices, Carlsbad, CA) in combination with a Digidata 1320A A/D converter (Molecular Devices). Data were acquired at 10 kHz and filtered at 2 kHz. Borosilicate glass (WPI, Sarasota, FL) electrodes were 1–2 MΩ when filled with the electrode solution containing (in mM): K-methanesulfonate 110, KCl 30, NaCl 5, CaCl2 1, MgCl2 2, HEPES 10, EGTA 11, Mg-ATP 2, Li-GTP 1, pH 7.2 (adjusted with Tris-base), 310 mOsm (adjusted with sucrose). Currents were recorded in a bath solution containing (in mM): NaCl 130, KCl 3, CaCl2 2.5, MgCl2 0.6, HEPES 10, glucose 10; pH 7.4 (adjusted with Tris-base), 325 mOsm (adjusted with sucrose).
Neurons were held at −60 mV. Currents were evoked by 500 ms of focal (Fast-Step, Warner Instruments) application of test agents. A neuron was considered responsive to a test agent if there was an increase in current associated with the application of a drug > 3 standard deviations above the baseline fluctuations in holding current.
Single Cell PCR:
Individual neurons cultured between 24–36 hrs, were collected in large bore (~30 μm) pipettes. The contents were expelled into tubes containing 3 μL of lysis buffer (Epicentre, MessageBOOSTER kit), and stored at −80 °C until processed further. Transcripts from single cells were reverse transcribed and linearly preamplified using the MessageBOOSTER kit for cell lysate (Epicentre). After preamplification, the products were cleaned with RNA Cleaner & Concentrator-5 columns (Zymo Research) and transcript levels were quantified using qPCR with optimized primers and SsoAdvanced SYBR Green Master Mix (BioRad). Cycle-time (Ct) values were determined using regression. Quantification threshold was determined to be inter-replicate average of 35 Ct, the point where replicates have a 95% chance of reoccurring, and the GAPDH threshold for cell inclusion was set to 25 Ct to ensure we could detect transcripts a thousand-times less prevalent than GAPDH. The primer sequences employed are listed in table 1.
Table 1:
PCR primers used for the amplification of nicotinic receptor subunits
| Subunit | Gene | Sequence |
|---|---|---|
| α2 | Chrna2 | F - CATCGGGTTCTCGGGACAGC |
| R - GGAGAGAAGTGAGGCATGGCA | ||
| α3 | Chrna3 | F - TCACCACTGACAGATGATTCACA |
| R - GGGCTGCAATTCTGTCCAII 1 1 | ||
| α4 | Chrna4 | F - GGACACTCTAGGGCACGCAG |
| R - CTGCGCCTGAT CCAG CATTT C | ||
| α5 | Chrna5 | F - AAGGCGGAGGAGACCCTATCT |
| R- TAAGGTAGGTAGCTGGCAGGC | ||
| α6 | Chrna6 | F - CAG CACCCACG G CTACAGTAT |
| R - CCACTGGAGGTACAGCAGAAC | ||
| α7 | Chrna7 | F - ACCTCTCCTGTTCCATTGTGTCAT |
| R - CCACCTCAGGGATTAGAAGCAA | ||
| α10 | ChrnalO | F - TGCAGGGCTTTGGCTGTTAC |
| R - ATGTCTGTAGGGCCTCCTGC | ||
| β2 | Chrnb2 | F - TGCTGGCCAGACTCCCATTC |
| R - CATCGAGGGTGGCTGAGTGA | ||
| β3 | Chrnb3 | F - CAAGTTGCCTGGCCTACCGA |
| R - CAGTGGGCTCCAGTTTCACCA | ||
| β4 | Chrnb4 | F - CTTCTTCCTCTTCCCCCAGG |
| R - GGTGGTTAGGGAAGGCCTC |
Test agents:
Drugs were diluted to final concentrations in bath solution from stock solutions at least 1000 times greater than the highest concentration employed. Capsaicin, cytisine, HC-030031(HC3), nicotine, mecamylamine, methyllycaconitine (MLA) were purchased from Sigma-Aldrich (St. Louis, MO). Stock solution of capsaicin (10 mM) and mecamylamine (10 mM) were made in 100% ethanol, stock solution of cytisine (100 mM) and HC3 (100 mM) were made in DMSO. Stock solution of MLA (1mM) was made in water. Nicotine (30 mM or 100 mM) was diluted in bath solution just prior to use to concentrations between 1 μM and 1 mM. The α7 nAChR subunit selective antagonist MLA was used at a concentration of 20 nM 12. The TRPA1 selective antagonist HC3 was used a final concentration of 10 μM based on results from our previous study 35. The α3β4 nAChR subunit selective agonist cytisine was applied at a concentration of 100 μM 12. The TRPV1 selective agonist capsaicin was used at a final concentration of 500 nM.
Data analysis:
All pooled data are presented as mean ± standard error of the mean. Student’s t-test or Mann-Whitney Rank Sum test was used for two-group comparisons. Chi square tests were used when % expression was compared. p < 0.05 was considered statistically significant.
Results
Nicotine evoked currents in different cell sized DRG neurons
A total of 47 DRG neurons from six human organ donors were studied. Four of the six donors were male, all were Caucasian. Their ages were 40, 56, 46, 59, 59, and 60 years old. All neurons were studied within 36 hrs of plating. Neurons were classified as small (cell capacitance below 100 pF, n = 6), medium (capacitance between 100–200 pF, n = 31) and large cells (capacitance above 200 pF, n = 10). Since our mouse and rat data as well as the initial experiments on human DRG suggested 300 μM nicotine was a saturating concentration, all of the neurons were challenged with 300 μM nicotine for 500 ms from a holding potential of −60 mV, 31 were challenged with 1000 μM 3 min after 300 μM. Nicotine (300 μM) evoked currents were observed in 85% (40 of 47) of neurons; no detectable currents were observed in the remaining seven neurons. Nicotine evoked currents are present in three of seven small, 26 of 30 medium, and 9 of 10 large neurons. While the Chi-square test is not accurate when over 20% of expected values in a contingency table of less than five, this difference in the distribution of currents among subpopulations of neurons defined by cell body size, appears to be significant (p = 0.02, Chi-Square). Capsaicin (500 nM) sensitivity was assessed at the end of the recording session on 14 nicotine responsive neurons (12 medium and 2 large). All 14 were responsive to capsaicin, with a peak capsaicin-evoked inward current at −60 mV of 11.2 ± 3.1 nA (range 1.0 to 36 nA).
Only slow current was present in human DRG neurons
In contrast to our previous data from mouse DRG neurons, in which a slow current was detected in one of 13 nicotine responsive neurons (the rest had transient currents) 3, as well as our previous data from the rat, in which both transient and sustained currents were detected in distinct subpopulations of neurons 35, only slow currents were detected in human DRG neurons (Figure 1). Because the only appreciable inactivation of nicotine evoked currents was observed in response to 1000 μM nicotine, the response to this concentration was used for a more detailed analysis of activation and inactivation parameters. The time to peak current was 351 ± 27 ms (Figure 2A, n=29). This was slower than the time to peak for sustained currents in rat DRG neurons (169 ± 17 ms, n=26) described previously 35, reanalyzed in a manner identical to that used for human DRG neurons. This difference was significant (Figure 2B, p < 0.01, Mann-Whitney Rank Sum test). To estimate the current decay in response to 1000 μM nicotine, we calculated the percentage of peak current remaining at the end of the 500 ms application period. As illustrated in Figure 2C, evoked currents had undergone relatively little inactivation over this period of time. Pooled data indicated that the extent of inactivation was only 15.3 ± 3.0% of peak (n = 29). Analyzing our previous data from rat sensory neurons in the same way indicated that the extent of current decay (31.2 ± 2.7%, n = 26) was significantly greater in the rat (Figure 2D, p < 0.01, student’s t test). Of note, while all of the rat data were from males, there was no evidence of an influence of sex on the proportion of neurons with nicotine evoked currents, or the magnitude or biophysical properties of the nicotine evoked currents in human DRG neurons.
Potency of the nicotine evoked current
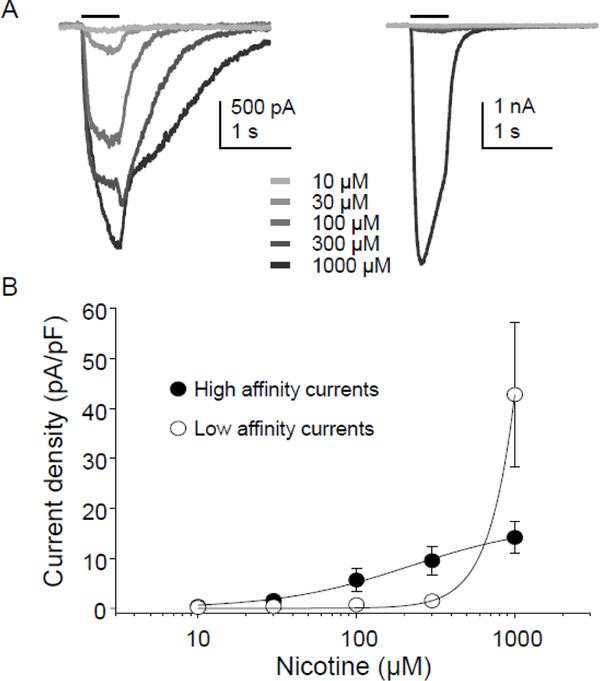
To determine the potency of nicotine evoked currents, a subpopulation of neurons (n = 10) were challenged with increasing concentrations of nicotine ranging from 10 to 1000 μM at an inter-stimulus interval of 3 minutes (Figure 3A). Concentration-response data for each neuron were fitted with the modified Hill equation in order to obtain EC50 and Hill coefficients (n). Based on these stimulus response curves, there appeared to be two populations of neurons: one in which peak evoked current saturated between 300 and 1000 μM nicotine. The EC50 in this population was 156 ± 40 μM (n = 4, Figure 3B). In the second group, the EC50 appeared to be well over 1000 μM nicotine (n = 6). The peak amplitude of current evoked with 1000 μM nicotine in all neurons tested was 29.1 ± 4.5 pA/pF (n=29, range 4.3–108.3 pA/pF). We previously observed a similar slow current density in response to 1000 μM nicotine in DRG neurons from rats (27.1 ± 5.7 pA/pF n=29, p > 0.05)35.
Figure 3.
Concentration response relationship for nicotine evoked currents in human DRG neurons. (A). Current traces evoked with increasing concentrations of nicotine in two types of human DRG neurons: one with an apparently high affinity nicotine-evoked current (Left) and one with an apparently low affinity nicotine-evoked current (Right). (B) Pooled data from the two groups of neurons illustrated in A: neurons with high affinity current (n = 4), and low affinity current (n = 6).
In addition to the lower prevalence of nicotine evoked current in small neurons, there was also a tendency for a smaller current density in this subpopulation compared with that in medium and large neurons. Of the 29 neurons tested with 1000 μM nicotine, current density was 15.8 pA/pF for small n=2 (with a range from 14.9 to 16.2), 24.1 ± 5.8 pA/pF for medium n=19 and 25.6 ± 6.6 pA/pF n=8 for large cells.
Pharmacological properties of the nicotine evoked current
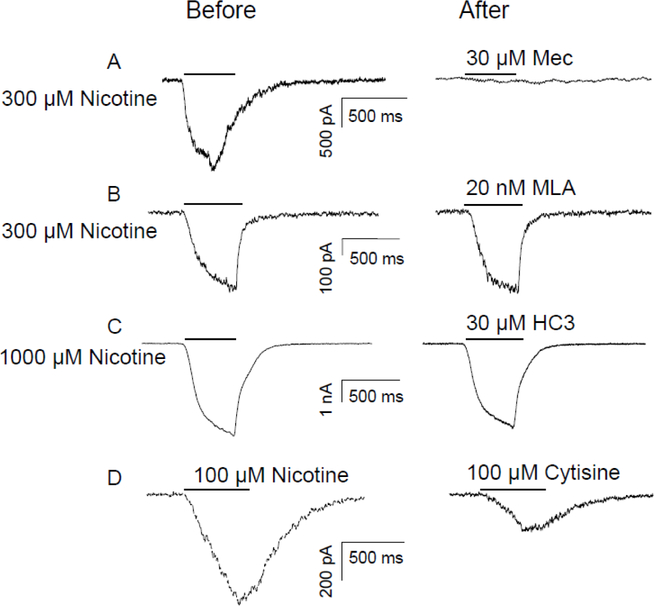
We performed a series of pharmacological experiments to further characterize the nicotine evoked currents in human DRG neurons. Nicotine (300 or 1000 μM) was applied before and then three minutes after antagonist application. Current evoked with 300 μM nicotine was blocked by the non-selective nAChR antagonists mecamylamine (Mec, 30 μM) (Figure 4A, n =6). In contrast, the α−7 subunit specific antagonist, methyllycaconitine citrate (MLA, 20 nM) had no impact on 300 μM nicotine-evoked current (Figure 4B, n = 4). Based on previous results from mouse trigeminal ganglion neurons and heterologous expression of human TRPA1 suggesting that nicotine can activate TRPA1 channels 30, we also determined whether TRPA1 may contribute to the nicotine evoked current in human DRG neurons. Consistent with results from our studies in rat and mouse 3, 35, the putative TRPA1 selective antagonist HC-030031 (HC3, 10 μM) had no detectable influence on the current evoked by nicotine (1000 μM) in human DRG neurons (Figure 4C, n =7). Finally, to implicate the contribution of β4 subunit containing nAChR to the current evoked in human DRG neurons, the relatively β4-subunit selective agonist cytisine (100 μM) was applied to neurons previously challenged with 100 μM nicotine. In 4 of 4 neurons, the cytisine evoked current was smaller than the nicotine evoked current (Figure 4D, mean = 48 ± 8 % of nicotine evoked current).
Figure 4.
Pharmacological properties of the nicotine-evoked current in human DRG neurons. Slow currents were evoked with nicotine for 500 ms (indicated by the bar above each trace) before and after the application of mecamylamine (MEC, 30 μM), a nAChRs antagonist (A), methyllycaconitine citrate (MLA, 20nM), an α−7 subunit selective antagonist (B), HC-030031(HC-03, 30μM), a TRPA1 receptor antagonist (C). (D) Neurons were also challenged with cytisine (100 μM) for 500 ms (indicated by the bar above the figure). The concentration of nicotine applied was indicated at left side of the traces.
Nicotinic receptor subunit expression in single DRG neurons
Twelve neurons in which successful cDNA synthesis was confirmed via GAPDH levels, were analyzed for nicotinic receptor subunit expression, from each of four donors (two male, of ages 22, 29, 36, and 70, all of whom were Caucasian). An α subunit (α2, 3, 4, 5, 6, 7, and 10) was detected in at least 10 out of 12 (85 ± 2.1%) neurons from each donor. Of the neurons with at least one α subunit, the most common co-expression pattern was α3/β2, which was present in 96 ± 4% of neurons with any α subunit. Very few neurons were detected with α2 (2.1 ± 2.1%), α4 (6.3 ± 2.1%) or α5 (6.3 ± 2.1%). However, the α6 (45.8 ± 8.0), α7 (72.9 ± 4.0%), and α10 (45.8 ± 4.2%) subunits were more common. Of the β subunits, β2 was present in most neurons (81.3 ± 4.0%), followed by β4 (47.9 ± 9.2%) and then β3 (41.7 ± 11.3%).
Discussion
The purpose of the present study was to characterize the distribution and properties of nicotine evoked currents in human sensory neurons. Robust currents were detected in the majority of neurons (85%) irrespective of cell body diameter and all neurons tested were also responsive to capsaicin. In all neurons tested, currents were slowly activating and slowly inactivating (persistent current), demonstrating a concentration-dependent increase in inactivation that was only readily detectable when currents were evoked with 1 mM nicotine. The current density (at −60mV) was, on average, larger than that of GABAA currents in human DRG neurons 36, but smaller than that of voltage-gated Na+ currents 34, with an EC50 in a subpopulation of neurons between that previously reported for human α3β4 nAChR expressed in HEK293 cells (~40 μM, 28) and Xenopus oocytes (~330 μM, 24). Nicotine-evoked currents were completely blocked by the non-selective nAChR antagonist mecamylamine, but not by the α−7 subunit specific antagonist, MLA (at 20 nM), nor by the TRPA1 antagonist, HC-030031. Consistent with the distribution of functional nicotinic receptors in human DRG neurons, nicotinic receptor subunit mRNA was detected in the majority (83%) of neurons assessed. The α3/β2 subunits were present in virtually all neurons in which an α subunit was detected. However, the α7 subunit was also detected in the majority of neurons assessed. Taken together, these data indicate that under the appropriate conditions nicotine evoked currents may play a significant role in the activation of human sensory neurons.
The distribution and properties of nicotine evoked currents in rat sensory neurons appear to be more similar to human than to mouse sensory neurons. That is, in contrast to 10% 30 and 20% 3 of mouse sensory neurons, in which hexamethonium-sensitive nicotine evoked currents are detected, these currents are present in the majority of both rat (64%, 35) and human (85%) sensory neurons. Similarly, the dominant current type (in 13 of 14 neurons) present in mouse sensory neurons is a transient MLA sensitive current 3. And while the transient current is also detected in a subpopulation of rat sensory neurons, a slow current with properties similar to those in human sensory neurons is detected in the majority of rat sensory neurons 21, 35. Interestingly, α7 subunit expression was detected in a majority of human DRG neurons. As this subunit should generate an MLA sensitive transient current, the failure to detect such a current in human DRG neurons suggests that either the mRNA is not efficiently translated, or that receptor is differentially trafficked in human and rodent sensory neurons. Furthermore, while we were unable to replicate the observation 3, there are data suggesting that nicotine activates TRPA1 in mouse sensory neurons 30. In contrast, the sustained currents in both rat and human DRG neurons were resistant to the TRPA1 antagonist HC-030031. Taken together, these results not only highlight the impact of species differences in mechanisms that may be important for pain processing, but suggest that at least in the context of nicotinic receptor signaling in sensory neurons, the rat may be a more appropriate model system than the mouse, despite the vastly wider array of genetic tools available for the study of mice.
Based on the biophysical and pharmacological properties of the transient currents present in mouse and rat sensory neurons, we suggested the currents are mediated by homomeric α7 containing nAChR 3, 35. These α7 containing nAChRs have a larger Ca2+ permeability and appear to be localized presynaptically, enabling them to contribute to the regulation of transmitter release 10, 13. There is also evidence that α7 containing nAChRs mediate the suppressive effects of 1 μM nicotine on experimental colitis-induced hyperexcitability of mouse colonic DRG neurons 1. These inhibitory actions were thought to mediate the improvement in global clinical score of colitis associated with the use of the nicotine 5, 19. Such a possibility would also be consistent with the suggestion that α7 subunits are differentially trafficked in human sensory neurons, where the receptors may be preferentially targeted to peripheral terminals. However, it is also possible that other receptors subtypes mediate the inhibitory actions of nicotine. Consistent with this suggestion, we have demonstrated that 1 μM nicotine can also decrease the excitability of rat cutaneous sensory neurons with slow currents 35. Given that both the inhibitory and excitatory actions of nicotine are concentration dependent, the same receptor subtype could account for both the therapeutic and painful aspects of peripheral nicotine.
Several different nAChR subunit combinations may underlie slow currents. Consequently, differences in agonist potency have been used to implicate the relative involvement of channels with different subunit composition in native tissues. For example, based on potency profiles of dimethyl phenyl piperazinium (DMPP) > cytisine > nicotine and DMPP = cytisine > nicotine, Rau and colleagues suggested there were two slow currents in rat sensory neurons carried by α3β4 and α3β4α5 subunit containing nAChRs, respectively 21. Interestingly, while a greater potency for cytisine over nicotine was considered a characteristic feature of β4 subunit containing nAChRs (α3β4 or α3α5β4 or other) 12, cytisine appeared to have a lower potency than nicotine for the activation of slow current in human DRG neurons. Consistent with the possibility that a β4 subunit containing receptor is not the dominant nAChR subtype in human sensory neurons, the α3/β2 subunits was the most common subunit combination in human DRG neurons. That there may be two slow currents in human sensory neurons distinct from those described in rat sensory neurons is suggested by the two populations of neurons defined by differences in nicotine potency. The presence of both inactivating and non-inactivating current in response to 1000 μM nicotine (see Figure 2 D), and number of different receptor subunits detected in human DRG neurons, are also consistent with this possibility. In this regard, it is worth noting that an α4-α4 interface present in (α4)3(β2)2 accounts for a significant decrease in agonist potency 2. Thus, subunit composition is at least one possible explanation for the apparently distinct nAChR subtypes in human DRG neurons. Regardless of the mechanism, however, these observations raise the possibility that it is not only the pattern of nAChR expression in human DRG neurons that is different from rat and mouse, but the subunit composition and trafficking of the receptors as well. Additional pharmacological, expression, and subunit localization data will be needed to address this possibility.
While gene expression data should always be interpreted with caution and in the context of functional protein analysis, it is informative to consider our results in relation to recently published transcriptomic data from mouse and human DRG neurons. The pattern of nAChR subunit expression we observed in single human DRG neurons was similar to the relative levels of subunit expression recently reported by Ray and colleagues in whole DRG 22. That is, consistent with our results, these authors detected very little expression of the α2, 4 and 5 subunits, and the highest level of expression of the α3 subunit. They also observed a relatively high level of expression of the α7 subunit. The β2 subunit was the most highly expressed of the β subunits. These authors performed a comparative analysis with mouse DRG, and reported a qualitatively similar pattern of nAChR subunit expression as was observed in human DRG except for α6 and α9 subunits which appeared to be expressed in opposite patterns in mouse and human: in the mouse, α6 was highly expressed and α9 was undetectable, while in the human α6 was expressed at a low level and α9 more highly expressed 22. Of note, we originally chose not to include the α9 subunit in our analysis based on evidence that nicotine does not activate these channels 11. And while nicotine also fails to activate nAChRs with the α10 subunit 11, we included this subunit based on evidence that the receptor may have disease modifying properties 6.
One caveat with the analysis of whole DRG is that the expression patterns observed are not necessarily neuronal. That this might be the case for some of the transcripts in the Ray et al study is suggested by their observation that the α3 nAChR subunit was relatively highly expressed in the mouse, whereas analysis of DRG neuronal populations such as that performed by Zeisel and colleagues 33 suggest little if any α3 is expressed in DRG neurons. That said, the exceptionally high level of α7 subunit expression detected by Ray and colleagues in whole DRG is consistent with the mouse functional data, which is also consistent with the Zeisel data indicating that this subunit is expressed in only two subpopulations of DRG neurons 33. Unfortunately, comparable data are not available for the rat. Nevertheless, these results are consistent with the suggestion that in addition to species differences in the patterns of nAChR subunit expression, there may also be differences in receptor trafficking.
With increased understanding of the nAChRs signaling in peripheral or central nervous system, there continues to be interest in targeting nAChRs for the treatment of pain 18, 20, 32. For example, epibatidine, a highly potent nAChR agonist was shown to have an analgesic potency greater than morphine in animal models 18. Similarly, another nAChR agonist ABT-594 was shown to produce analgesia when administered systemically or intradermally 8, 18. However, compounds such as these are generally first identified in screens of heterologously expressed human nAChRs with selectivity and behavioral pharmacology subsequently performed in rats and mice. And while epibatidine never made it to clinical trials because of the predicted side effect profile, the more selective ABT-594 made it to a phase 2 trial for neuropathic pain 23. Analgesic efficacy was demonstrated. However, there was only a two-fold separation between the dose needed for analgesic efficacy and that associated with adverse effects 9. Given the widespread distribution of nAChRs throughout the body, it may never be possible to avoid deleterious side effects with even the most selective agonists or antagonists. Nevertheless, the possibility that receptors in human sensory neurons may be distinct from those in rodents suggests that the limited success with these compounds in clinical trials may not be as much a problem with the drugs developed, as it is with the strategy for the identification and validation of the therapeutic targets. Regardless, these data add additional support to the suggestion human sensory neurons maybe an essential screening tool for those considering moving novel therapeutics targeting primary afferents into clinical trials.
Highlights for Nicotine-evoked currents in human primary sensory neurons.
Nicotine-evoked currents were present in the majority (85%) of human DRG neurons
Currents in human DRG neurons were slowly activating with pharmacology of nAChRs
α3β2 subunit containing nAChRs likely carried currents in human DRG neurons
Humans are not just big rodents with respect to nicotine currents in DRG neurons
Perspective:
The properties and distribution of nicotine-evoked currents in human sensory neurons were markedly different from those previously observed in mice and rats. These observations add additional support to the suggestion human sensory neurons maybe an essential screening tool for those considering moving novel therapeutics targeting primary afferents into clinical trials.
Acknowledgements:
The authors would like to thank the staff of the Center for Organ Recovery and Education for the tremendous work they do in enabling the organ donation process, for their diligent collection and de-identification of medical records, and for their technical assistance in the recovery of DRG for this project.
Disclosures:
This study was supported by the National Institutes of Health (R01NS083347 and R01 DK107966), the National Natural Science Funds of China (81670686), Shandong Natural Science Funds (ZR2015HM001), and Eli Lilly Co. None of the authors have any conflicts of interest with respect to the work described in this manuscript. Dr. Belfer contributed to this article in her personal capacity. The views expressed are her own and do not necessarily represent the views of the National Institutes of Health or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Abdrakhmanova GR, AlSharari S, Kang M, Damaj MI, Akbarali HI. {alpha}7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 299:G761–768, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahring PK, Olsen JA, Nielsen EO, Peters D, Pedersen MH, Rohde LA, Kastrup JS, Shahsavar A, Indurthi DC, Chebib M, Gajhede M, Balle T. Engineered alpha4beta2 nicotinic acetylcholine receptors as models for measuring agonist binding and effect at the orthosteric low-affinity alpha4-alpha4 interface. Neuropharmacology. 92:135–145, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Albers KM, Zhang XL, Diges CM, Schwartz ES, Yang CI, Davis BM, Gold MS. Artemin growth factor increases nicotinic cholinergic receptor subunit expression and activity in nociceptive sensory neurons. Mol Pain. 10:31, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardini N, Sauer SK, Haberberger R, Fischer MJ, Reeh PW. Excitatory nicotinic and desensitizing muscarinic (M2) effects on C-nociceptors in isolated rat skin. J Neurosci. 21:3295–3302, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonapace CR, Mays DA. The effect of mesalamine and nicotine in the treatment of inflammatory bowel disease. The Annals of pharmacotherapy. 31:907–913, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Del Bufalo A, Cesario A, Salinaro G, Fini M, Russo P. Alpha9 alpha10 nicotinic acetylcholine receptors as target for the treatment of chronic pain. Curr Pharm Des. 20:6042–6047, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Dessirier JM, O’Mahony M, Carstens E. Oral irritant effects of nicotine. Psychophysical evidence for decreased sensation following repeated application of and lack of cross-desensitization to capsaicin. Ann N Y Acad Sci. 855:828–830, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Donnelly-Roberts DL, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, Piattoni-Kaplan M, McKenna DG, Wasicak JT, Holladay MW, Williams M, Arneric SP. ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: a novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: I. In vitro characterization. J Pharmacol Exp Ther. 285:777–786, 1998 [PubMed] [Google Scholar]
- 9.Dutta S, Hosmane BS, Awni WM. Population analyses of efficacy and safety of ABT-594 in subjects with diabetic peripheral neuropathic pain. The AAPS journal. 14:168–175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fucile S, Sucapane A, Eusebi F. Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J Physiol. 565:219–228, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fucile S, Sucapane A, Eusebi F. Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors. Cell Calcium. 39:349–355, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 86:1773–1782, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Gopalakrishnan M, Buisson B, Touma E, Giordano T, Campbell JE, Hu IC, Donnelly-Roberts D, Arneric SP, Bertrand D, Sullivan JP. Stable expression and pharmacological properties of the human alpha 7 nicotinic acetylcholine receptor. Eur J Pharmacol. 290:237–246, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Goswami SC, Mishra SK, Maric D, Kaszas K, Gonnella GL, Clokie SJ, Kominsky HD, Gross JR, Keller JM, Mannes AJ, Hoon MA, Iadarola MJ. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J Pain. 15:1338–1359, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Autonomic neuroscience : basic & clinical. 113:32–42, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hone AJ, Meyer EL, McIntyre M, McIntosh JM. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4* subtype. FASEB J. 26:917–926, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keele CA, Armstrong D: Substances Producing Pain and Itch, The Williams and Wilkins company, Baltimore, 1964. [Google Scholar]
- 18.Kesingland AC, Gentry CT, Panesar MS, Bowes MA, Vernier JM, Cube R, Walker K, Urban L. Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain. 86:113–118, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Lashner BA, Hanauer SB, Silverstein MD. Testing nicotine gum for ulcerative colitis patients. Experience with single-patient trials. Dig Dis Sci. 35:827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Loram LC, Taylor FR, Strand KA, Maier SF, Speake JD, Jordan KG, James JW, Wene SP, Pritchard RC, Green H, Van Dyke K, Mazarov A, Letchworth SR, Watkins LR. Systemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley rats. J Pain. 13:1162–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rau KK, Johnson RD, Cooper BY. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. J Neurophysiol. 93:1358–1371, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G, Price TJ. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain. 159:1325–1345, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 146:245–252, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Rush R, Kuryatov A, Nelson ME, Lindstrom J. First and second transmembrane segments of alpha3, alpha4, beta2, and beta4 nicotinic acetylcholine receptor subunits influence the efficacy and potency of nicotine. Mol Pharmacol. 61:1416–1422, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Saika F, Kiguchi N, Kobayashi Y, Kishioka S. Peripheral alpha4beta2 nicotinic acetylcholine receptor signalling attenuates tactile allodynia and thermal hyperalgesia after nerve injury in mice. Acta physiologica (Oxford, England). 213:462–471, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Smith NJ, Hone AJ, Memon T, Bossi S, Smith TE, McIntosh JM, Olivera BM, Teichert RW. Comparative functional expression of nAChR subtypes in rodent DRG neurons. Frontiers in cellular neuroscience. 7:225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spies M, Lips KS, Kurzen H, Kummer W, Haberberger RV. Nicotinic acetylcholine receptors containing subunits alpha3 and alpha5 in rat nociceptive dorsal root ganglion neurons. J Mol Neurosci. 30:55–56, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Stauderman KA, Mahaffy LS, Akong M, Velicelebi G, Chavez-Noriega LE, Crona JH, Johnson EC, Elliott KJ, Gillespie A, Reid RT, Adams P, Harpold MM, Corey-Naeve J. Characterization of human recombinant neuronal nicotinic acetylcholine receptor subunit combinations alpha2beta4, alpha3beta4 and alpha4beta4 stably expressed in HEK293 cells. J Pharmacol Exp Ther. 284:777–789, 1998 [PubMed] [Google Scholar]
- 29.Steen KH, Reeh PW. Actions of cholinergic agonists and antagonists on sensory nerve endings in rat skin, in vitro. Journal of Neurophysiology. 70:397–405, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 12:1293–1299, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 18:145–153, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Vincler M Neuronal nicotinic receptors as targets for novel analgesics. Expert opinion on investigational drugs. 14:1191–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular Architecture of the Mouse Nervous System. Cell. 174:999–1014.e1022, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Priest BT, Belfer I, Gold MS. Voltage-gated Na+ currents in human dorsal root ganglion neurons. eLife. 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XL, Albers KM, Gold MS. Inflammation-induced increase in nicotinic acetylcholine receptor current in cutaneous nociceptive DRG neurons from the adult rat. Neuroscience. 284:483–499, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang XL, Lee KY, Priest BT, Belfer I, Gold MS. Inflammatory mediator-induced modulation of GABAA currents in human sensory neurons. Neuroscience. 310:401–409, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]