Abstract
The regeneration of brain tissue remains one of the greatest unsolved challenges in medicine and by many is considered unfeasible. Indeed, the adult mammalian brain does not regenerate tissue, but there is ongoing endogenous neurogenesis, which is upregulated after injury and contributes to tissue repair. This endogenous repair response is a conditio sine que non for tissue regeneration. However, scarring around the lesion core and cavitation provide unfavorable conditions for tissue regeneration in the brain. Based on the success of using extracellular matrix (ECM)-based bioscaffolds in peripheral soft tissue regeneration, it is plausible that the provision of an inductive ECM-based hydrogel inside the volumetric tissue loss can attract neural cells and create a de novo viable tissue. Following perturbation theory of these successes in peripheral tissues, we here propose 9 perturbation parts (i.e. requirements) that can be solved independently to create an integrated series to build a functional and integrated de novo neural tissue. Necessities for tissue formation, anatomical and functional connectivity are further discussed to provide a new substrate to support the improvement of behavioral impairments after acute brain injury. We also consider potential parallel developments of this tissue engineering effort that can support therapeutic benefits in the absence of de novo tissue formation (e.g. structural support to veterate brain tissue). It is envisaged that eventually top-down inductive “natural” bioscaffolds composed of decellularized tissues (i.e. ECM) will be replaced by bottom-up synthetic designer hydrogels that will provide very defined structural and signaling properties, potentially even opening up opportunities we currently do not envisage using natural materials.
Keywords: Bioscaffold, Hydrogel, Magnetic resonance imaging, Stroke, Regeneration, Tissue Repair, Biodegradation, Brain
1. Introduction
The persistence of behavioral and cognitive impairments after acute brain injury are a reflection of insufficient tissue repair and a failure of the human brain to regenerate lost tissue [86]. Although there is a lack of spontaneous regeneration of brain tissue after a volumetric tissue loss due to an acute brain injury [55, 76, 144], neurogenesis occurs in the adult brain of all vertebrates [52]. Adult neurogenesis is a precondition for tissue repair and new neurons continually invade damaged tissue surrounding a tissue cavity [83, 97]. It is important to distinguish tissue repair (i.e. replacing lost cells in damaged tissue with new site-appropriate cells) that occurs in all mammalian tissues from tissue regeneration (i.e. replacing injured tissue with homologous tissue) that occurs in some organs, such as the liver, but not the brain. Transplantation of neural stem cells (NSCs) after acute brain injuries, such as a stroke or traumatic brain injury, supplements the endogenous repair process by adding exogenous cells and associated paracrine factors to the damaged tissue without creating de novo tissue in the lesion cavity [136]. A large volumetric tissue loss therefore remains following cell therapy and recovery of function is limited. The development of in situ brain tissue engineering holds the promise to overcome this issue and replace tissue lost due to an acute brain injury, such as stroke or traumatic brain injury.
2. Clinical indications for in situ brain tissue engineering
Acute brain injuries typically constitute a single event that occurs suddenly and causes regionalized damage to the brain [21]. These injuries can be caused by a blockage (i.e. ischemic stroke) or rupture of blood vessels (i.e. intracerebral hemorrhage, ICH) or through exogenous events, such as blunt trauma (i.e. closed head traumatic brain injury, TBI), or a penetrating event, such as a gunshot or knife (i.e. penetrating TBI). Acute brain injuries are common, with 795,000 individuals affected by an ischemic stroke each year in the US [14], 63,000 with an ICH [130] and more than 1.7 million will suffer a traumatic brain injury [48]. Although the molecular mechanisms of cell damage (e.g. excitotoxicity, apoptosis, oxidative stress, free radical production) are common between these injuries and amenable to neuroprotective strategies [21], these mediators have largely failed as therapeutic targets in clinical settings due to their very narrow therapeutic time window [27, 116] (Figure 1). However, with better acute care management in specialized hospital units, more patients survive with severe deficits [91]. Considering the human and economic cost, there is a dire need to develop efficacious strategies to improve outcome [66].
Figure 1. Pathophysiology of acute brain injury and therapeutic interventions.
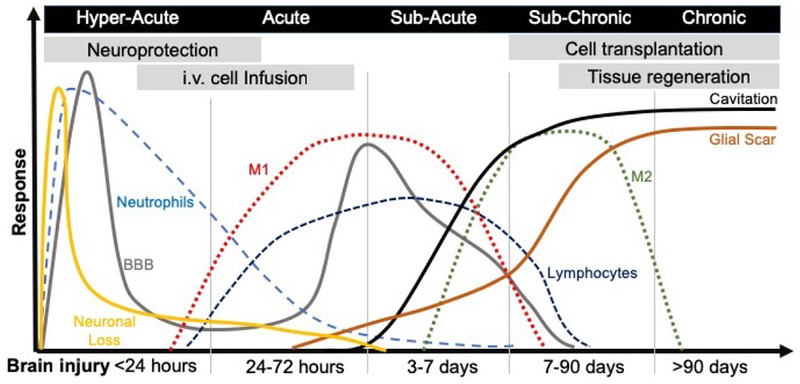
An acute insult to the brain through brute force or ischemia will lead to the rapid loss of neurons at the core of the infarct, this area will eventually cavitate and be sealed off by a glial scar. Apoptosis will further lead to neuronal death, as well as other cells, such as astrocytes, oligodendrocytes and endothelial cells (not illustrated here). Cell stress and cell death will provoke microglia to invoke an immune response that recruits neutrophils into brain tissue and produce a disruption in the blood brain barrier (BBB). The BBB will close again, but a second disruption will occur transitioning between the acute and subacute phase, again characterized by a major influx of peripheral immune cells, such as macrophages and lymphocytes. Most infiltrating macrophages are of a M1-type (i.e. pro-inflammatory) during the cell death and ECM clearance phase. However, as tissue is cleared these macrophages tend to polarize towards an M2-like (i.e. pro-repair) phenotype. It currently remains unclear if these are newly infiltrating cells or if ECM clearance in the brain promotes a shift towards a pro-repair phenotype. The implantation of inductive bioscaffolds needs to be considered against the backdrop of these pathophysiological events, but also in the context of other therapeutics and their time window. Endogenous in situ tissue engineering at present is best considered for treatment after a cavity formed in the sub-chronic phase. However, the use of bioscaffolds to change inflammatory events, promote neuronal survival, or neovascularization might be suitable for earlier interventions.
Behavioral and cognitive impairments reflect the location and extent of neuronal loss. The regional nature of cellular loss after an acute brain injury can lead to volumetric tissue changes that shrink, but preserve, brain tissue (i.e. atrophy) or that lead to a complete destruction of tissue, typically the core, that includes the removal of the extracellular matrix (i.e. cavitation). By 90 days post-stroke, up to 94% of patients will have tissue cavitation, dependent on the location and extent of stroke [110]. The tissue cavity is typically fully formed by 2 weeks post-injury and surrounded by glial scarring that delineates the lesion core from surrounding damaged tissue [138]. The average size of a stroke cavity in patients is approximately 45 ml, but a malignant case of middle cerebral artery occlusion (MCAo) produces tissue cavities of 150 ml [68]. Mild to moderate TBI typically produces tissue atrophy rather than cavitation, but volumetric tissue loss can occur in severe closed and penetrating head injuries. A brain tissue cavity also occurs after glioma resections in 26,000 patients annually [39]. Volumetric brain tissue loss therefore presents a significant clinical challenge that currently does not have any effective treatment options. Although endogenous and exogenous cellular replacement can be harnessed to repopulate damaged tissue [136], a structural support using a bioscaffold is required to retain implanted NSCs in the tissue cavity [16–18, 120].
3. Rationale for implantation of bioscaffolds in acute brain injuries
Bioscaffolds are widely used to treat volumetric tissue defects in peripheral soft tissues [160]. The material for scaffolding is either naturally-derived or composed of a synthetic polymer (Figure 2). Major advantages of synthetic biomaterials include the precision of physicochemical properties and geometric conformation. A robust industrial scale-up production is also achievable. The absence of biological material in these materials reduces the risk of contamination with microbiota or prions [23, 129], but conversely these materials have currently a limited capacity to induce a regenerative response. The use of synthetic polymers to create microcarriers for cells [15, 16] or a sustained and controlled drug delivery after implantation into the brain [18] provide unique opportunities for their use. These properties contrast with naturally-derived materials, which are composed of naturally occurring biological substances, wherein physicochemical properties are more difficult to control. Naturally-derived materials possess bioactive molecules that can exert an inductive host response to support constructive remodeling of the bioscaffold required for the formation of de novo tissue [75, 105]. Synthetic materials provide an opportunity for a bottom-up approach (i.e. reverse engineering), where the addition and combination of particular peptides can drive specific host responses. In contrast, naturally-derived materials deliver a top-down approach (i.e. forward engineering) that is inherent in its composition [93, 140].
Figure 2. Overview of materials used for bioscaffolds.
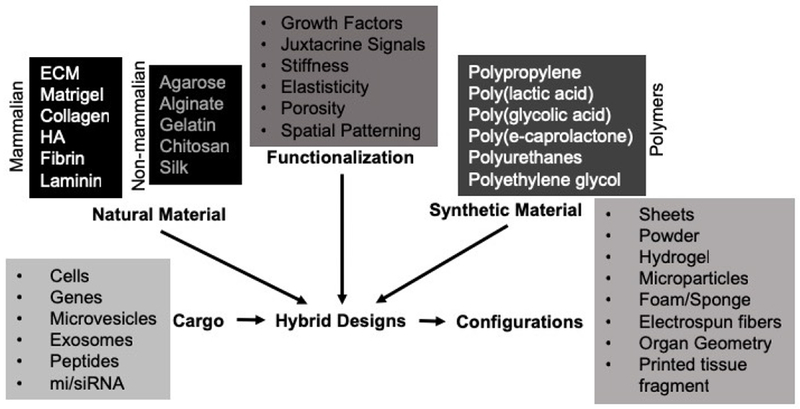
A variety of natural and synthetic materials have been used to create bioscaffolds. Natural material can typically be distinguished based on their provenance from mammalian or non-mammalian species. Of the mammalian sources, extracellular matrix (ECM) produces an inductive response, i.e. it invokes a host cells response that leads to its degradation and replacement with new tissue. Other materials, such as hyaluronic acid (HA) do not by themselves produces these types of response, but do influence cell behavior based on their affinity to its juxtacrine signaling. Combinations of the materials in hybrid designs is possible and further functionalization by altering composition or structure are also undertaken based on specific design criteria (e.g. anisotropic structure for axonal growth requiring spatial patterning). Bioscaffolds can also carry cargo (e.g. cells, growth factors) that is thought to promote specific therapeutic effects. Most commonly cells are integrated into bioscaffolds with an eye towards cell/tissue replacement. Bioscaffolds/biomaterials can improve the survival of delivered cells, but also provide a structural support to promote de novo tissue formation.
Naturally-derived biomaterials, such as gelatin, chitosan, silk and alginate, are commonly used to deliver cells to tissue defects, including the brain [71, 104, 118], but these non-mammalian products do not by themselves induce a constructive remodeling response that promotes endogenous stem cell invasion. Alginate-encapsulated or microcarrier-attached cells have found clinical translation [19, 143], but to date no widespread adoption of this approach has occurred. Cell migration is dependent upon both juxtacrine substrates found in the extracellular matrix (ECM), such as laminin or fibronectin and chemokines, and structural molecules, such as collagens. ECM and purified molecular components of ECM have also been used to improve the implantation and retention of cells after brain damage [17, 139, 145, 146, 158]. In the interstitial space, the structural support for individual cells and the tissue as a whole is provided by ECM molecules, such as hyaluronic acid (HA) and the fibrillar collagens. HA is very abundant in the brain and provides tissue resistance to compression through a counteracting turgor force (i.e. swelling due to water absorption), conveying some of the brain’s elasticity. Collagens are the most abundant structural proteins in ECM and are essential to maintain tissue structure. Collagens by themselves can form hydrogels that provide structural support for cells that is a function of concentration and temperature [5]. In contrast, HA typically requires cross-linking molecules to sustain a 3-dimensional structure. Bioscaffolds composed of these glycosaminoglycans and proteins provide a structural support to cells and provide a tissue-specific spatial organization of resident cells [77, 155].
The extracellular matrix serves both a structural and a signaling function [20]. Decellularization of tissue allows for the isolation of ECM and subsequent fabrication into bioscaffolds that can be configured as layered sheets, sponges, powder, hydrogel or maintain organ geometry [9]. In peripheral tissues, these acellular ECM constructs induce a constructive remodeling response that leads to an endogenous recruitment of host cells with subsequent restoration of functional host tissue in a variety of organs, including bladder [122, 132], skin [78, 147], muscle [133, 170], heart [10, 87], gastrointestinal [74] and breast tissue [46, 112]. A variety of FDA approved, ECM-based products are available for reconstructive surgery [22]. There are differences in the molecular composition of ECM sourced from different tissues [45], including the cargo contained within matrix bound nanovesicles, which represent lipid membrane-bound reservoirs of miRNA, proteins, and other signaling molecules [72, 73]. Differences in ECM content affect cell behavior, as revealed by in vitro studies that compared ECM from different sources. For instance, migration, neuronal differentiation and neurite outgrowth in neural progenitors was greater with UBM-ECM than ECM derived from the CNS [32, 33, 49, 89], although umbilical cord ECM promoted a greater migration of mesenchymal stem cells [89]. Heterologous ECM can therefore potentially provide a greater inductive capability than homologous tissue sources, as well as overcome certain limitations imposed by signaling sources in homologous tissues, such as inhibition of axon sprouting. Age also affects the composition of ECM and its regenerative properties, with younger tissue thought to be more favorable compared to aged tissues [98, 150]. Although ECM can be sourced from the brain [32, 103], its yield is rather sparse in comparison to other tissues/organs, such as the urinary bladder, requiring about 10 times the starting volume [49]. UBM-ECM is hence an attractive source of an inductive bioscaffold that can produce tissue restoration appropriate for the damaged organ. UBM-ECM is here primarily used as an illustrative example for tissue restoration in the brain, but the principles discuss here apply to all inductive scaffolds. Nevertheless, there is currently a lack of comparative in vivo studies to establish which tissue source is the most effective in tissue restoration [49, 151].
From a bio-engineering perspective, regeneration of peripheral tissues/organs with volumetric tissue loss indicates that an insufficient endogenous regenerative response can be overcome by providing the appropriate inductive signaling and structural support for cell invasion. As approximate solutions can be found by identifying the solutions for a related problem, overcoming the lack of regeneration in the brain can potentially be addressed by using an acellular ECM bioscaffold, akin to their use in peripheral soft tissue volumetric defects. To construe a solution for brain tissue restoration, we identified a series of solvable smaller “perturbation parts” to assemble an integrated solution (i.e. a perturbation series). We identified 9 key perturbation parts (i.e. solvable requirements) to achieve endogenous in situ tissue restoration after acute brain injury (Figure 3): 1.) Intracerebral delivery of bioscaffolds; 2.) Structural support for host cell invasion; 3.) Inductive signaling for cell invasion; 4.) Scaffold biodegradation; 5.) Neovascularization; 6.) Site-appropriate phenotypic differentiation; 7.) Dissolution of glial scar; 8.) Axonogenesis and connectivity; and 9.) Synapse formation for functional connectivity.
Figure 3. Perturbation series defined requirements for endogenous in situ brain tissue engineering.
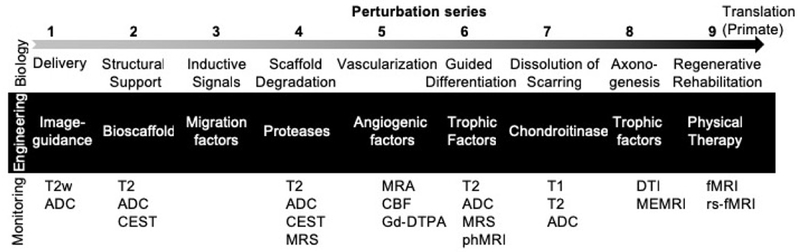
Based on endogenous tissue regeneration is peripheral soft tissue defects, 9 perturbation parts can be defined and arranged in a series to solve the same problem in the brain. For each biological challenge, an engineering solution can be envisaged. To ensure that these concerted parts unfold in vivo, ideally non-invasive monitoring is used to visualize and check that an appropriate biology continues to develop. Magnetic resonance imaging techniques, such as T2-weighted (T2w) images, T2 maps, Apparent diffusion coefficient (ADC) maps, diffusion tensor imaging (DTI), chemical exchange saturation transfer (CEST), magnetic resonance spectroscopy (MRS), as well as functional brain imaging techniques, such as manganese-enhance MRI (MEMRI), functional MRI (fMRI), resting-state fMRI (rs-fMRI) and pharmacological MRI (phMRI) can be employed. To define the integrity of the blood brain barrier (BBB), gadoterate (Gd-DOTA) can be used to visualize leakage of molecules from the vascular compartment into the neuropil. A leaky BBB would occur during angiogenesis, when the barrier has not sufficiently matured and could hence indicate neovascularization.
4. Requirement 1: Intracerebral delivery of bioscaffolds
Unlike many peripheral organs, the brain is encased in the skull, which limits accessibility. Some cavities are also seated deep in the brain with overlaying functional tissue that ideally is not disturbed. In these cases, it is advantageous to have hydrogel that is fluid at room temperature and can be administered through a thin needle to the lesion site before it polymerizes to provide structural support. In cases of open head injuries or decompressive surgery, direct access to the brain is available and other formulations, such as sheets or decellularized tissue pieces, could potentially be implanted. However, care must be taken that no deleterious pressure is exerted on existing brain tissue. Due to the varied topology of cavities, the interface between bioscaffold and brain tissue also needs to be considered to ensure a structural access for invading cells, as well as preventing host tissue deformations due to brain compression. Hydrogels or microbeads can potentially adapt to a varied topology and allow filling of the cavity, while creating a consistent interface with the host brain [16, 17]. Sheets and decellularized tissue pieces are unlikely to provide this geometric conformation.
Intracerebral delivery of the soluble (fluid) phase of an ECM hydrogel through small diameter needles or catheters will produce shear stress [2]. The required ejection pressure will depend on the rheologic properties of the material, as well as the internal diameter of the barrel or tubing. More viscous material and smaller diameters may result in higher shear stress and pressure [156]. Differences in internal diameter between the catheter, the needle and the fluid reservoir (e.g., syringe vessel) will result in different shear stress and pressure within each of these compartments. Ejection pressure in needles can be 20× higher than in a syringe [156]. Transition from one compartment to another may further create pressure points that can affect material properties. Speed of administration (i.e. flow rate) will also affect the applied force for ejection and contribute to the pressure and shear stress in the barrel. Slower speed will produce a higher ejection pressure [3, 156], potentially affecting the material properties of ejected bioscaffolds. These factors should be considered in the context of the injection site. Injection into tissue will produce a resistance that needs to be overcome, but a high pressure will lead to tissue tearing and cause additional damage. Normal intracranial pressure is considered to range between 0.93-1.99 kPa with measurements >3.33 kPa requiring intervention [141]. Injection into a tissue void is less concerning and a higher ejection pressure, as well as injection speed, is tolerable. Speed of injection is also dependent on the time to gelation of the pre-gel fluid. Rapid gelation within the syringe or catheter will not produce an evenly distributed bioscaffold through the tissue void.
Administration of a hydrogel that contains suspended microparticles creates a more complex biophysical situation. A discrepancy in material properties between the fluid and the microparticles can produce a buoyancy or sedimentation effect on microparticles. Size of microparticles will also affect its drag force inside the vehicle for administration and its exposure to shear stress from barrel walls. Larger microparticles require a sufficient needle size to afford passage without damaging the payload. For instance, a 32G needle with an internal diameter of 0.108 mm, is only suitable for very small microparticles, even if these are arranged one on top of the other. Larger microparticles 0.250-0.3 mm in size would require an internal clearance of at least 0.4 mm (i.e. 22G needle at 0.413 mm). However, the larger the needle diameter, the greater the damage caused by the injection tract. A balance therefore needs to be found between reducing damage to the brain due to the injection versus ensuring adequate product delivery [131]. Volume fraction of microparticles in the vehicle will also influence these biomechanical measures. According to the random closed packing paradigm, the highest achievable packing density of spheres of the same size is a 63.6% volume fraction [149]. The higher the volume fraction, the less sedimentation will occur [131], but the effect of shear stress will increase [156]. Microparticle size distribution, density, total payload, as well as needle/catheter and syringe/tubing sizes should be noted to afford a comparison of biophysical aspects between studies.
A targeted delivery of bioscaffolds in hydrogel form requires surgical planning based on non-invasive imaging identifying the site and extent of damage [113]. The site of the lesion is crucial to identify an injection pathway that avoids damaging crucial brain structures, but also avoids passage through enlarged ventricles or rupturing of major blood vessels [15, 101]. In some cases intrathecal delivery is the desired target and image-guidance can assure a targeted delivery [117]. Magnetic resonance imaging (MRI) of brain damage affords a placement of injection sites adjacent to the lesion core [136], but also placement into the tissue cavity [16, 101]. Diffusion MRI is especially useful to identify a lesion core that is the target for implantation of ECM bioscaffolds in hydrogel form [17]. A complete filling of the lesion core with a bioscaffold can be achieved based on administering a volume equivalent to the lesion volume (Figure 4A) [101]. Using a drill hole in the cranium for injection of the biomaterial and a second drill hole to drain superfluous extracellular fluid from the cavity affords a very efficient and homogenous filling without the risk of tissue compression due to an excessive material deposition [58–60, 101]. Although intra-arterial delivery into a stroke lesion is less invasive for the delivery of cell therapy [157], it currently remains unclear if this approach can be adapted to administer large volumes of biomaterials to the lesion core. Diffusion MRI can also verify that the biomaterial was injected into the lesion cavity and if an appropriate tissue is forming over time [17]. Deformation of host brain tissue can also be evaluated using serial MRI [58]. Dense microparticles, such as poly-l-lactic (PLGA) can produce signal changes on T2-weighted images to verify delivery, but this might complicate the evaluation of tissue damage [16], “Doping” of bioscaffolds with MR detectable labels can further assist to verify delivery, as well as biomaterial resorption. For instance, GelinS added to a HA hydrogel [95] or specific molecules in an ECM hydrogel [80] can afford its non-invasive localization using chemical exchange saturation transfer (CEST) MRI. This technique is especially useful to ensure that full coverage of the lesion is achieved and that equivalent concentrations of hydrogel are present throughout the bioscaffold. Image-guided delivery and verification of implantation are essential aspects to ensure a bioscaffold is providing the appropriate support and facilitates host cell invasion. Without imaging-guidance, it is difficult to understand how an appropriate volume could be delivered to the intended site to enable tissue regeneration.
Figure 4. ECM hydrogel implantation for endogenous in situ brain tissue engineering.
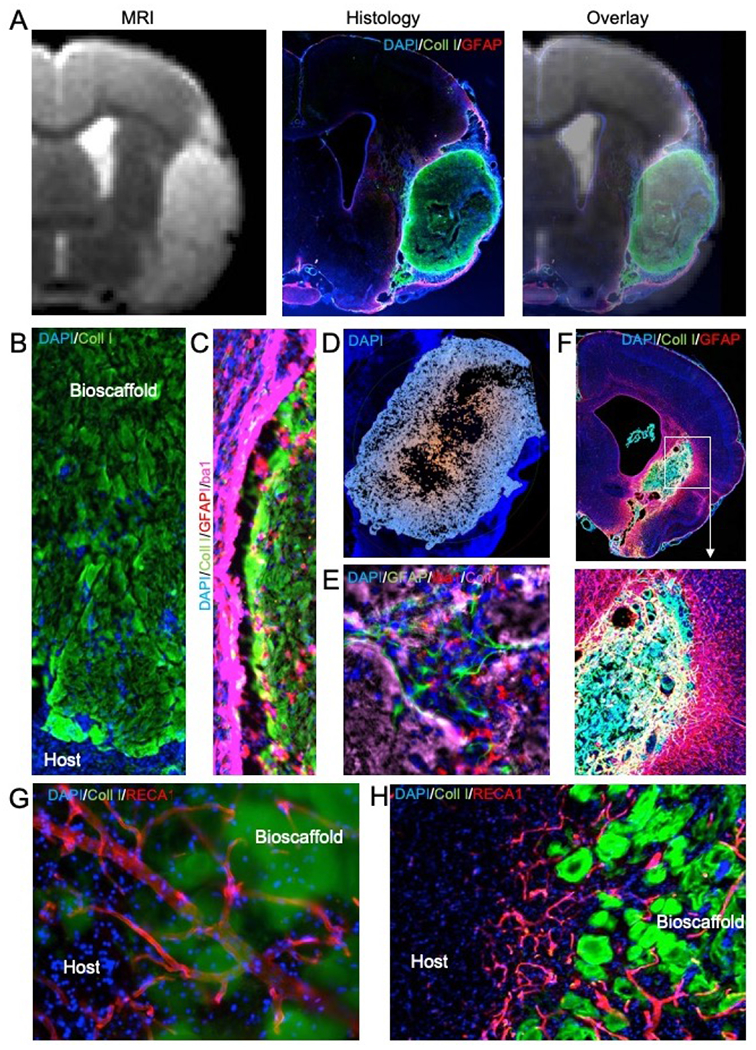
A. Magnetic resonance imaging (MRI)-based image guidance afforded an injection-drainage delivery of ECM hydrogel into a tissue cavity caused by a stroke. This afforded a complete coverage of the cavity with ECM bioscaffold, as evidence by the immunohistochemistry. An overlay of the pre-implantation MR image with 1-day post-mortem histology verifies the accuracy and efficiency of ECM bioscaffold delivery. B. Implantation of an ECM bioscaffold produced host cell invasion following the pattern of chain cell migration. C. The interface between the biomaterial and host tissue is essential to ensure an efficient invasion. D. A poor tissue-biomaterial interface or limited permeation of ECM derived signals into host tissue create invasion blind spots where no cell invasion occurs. Typically, invasion follows an outside-in migration pattern from all sides of the scaffold. E. Invading cells participate in constructive remodeling of the ECM hydrogel. In many cases, small pockets of tissue are forming inside the hydrogel, which gradually is being degraded by phagocytes. F. De novo tissue formed inside the cavity caused by a stroke after implantation of an ECM hydrogel (4 mg/mL). G. Blood vessels are being formed in the de novo tissue, with some vessels presented in the newly formed tissue and penetrating/passing through remnants of the ECM bioscaffold. H. In some cases, neovascularization passed in between ECM patches. The patches reflect a common pattern observed in degrading ECM bioscaffolds. Blood vessels supported newly forming tissue in between these patches that were remodeling the cavity and creating novel tissue.
5. Requirement 2: Structural support for cell invasion
To guarantee a structural support for cell invasion, the bioscaffolds need to be retained within the lesion cavity [101]. The gelation of solubilized ECM hydrogels is within the cavity to provide a substrate that is sufficient to facilitate cell invasion/migration (Figure 4B). For urinary bladder matrix (UBM)-derived ECM hydrogel, a concentration of 4 mg/mL sufficiently gels to be retained within the cavity [101]. Time to 50% gelation is approximately 3 min and therefore requires a reasonably rapid administration of 10 μL/min to fill cavities of >40 μL (>4 min. delivery time). Lower concentrations (<3 mg/mL) do not readily gel to provide a structural support that can support cell invasion into the tissue cavity. However, some benefits of small quantities (5 μL) of low concentration ECM hydrogels have proven efficacious when administered directly into damaged brain tissue that lacks a cavity [162, 168]. A robust gelation occurs at 4 mg/mL UBM-ECM with a storage modulus (G’) of 76.6 Pa and loss modulus (G”) of 11.0 Pa. A denser (8 mg/mL) UBM-ECM hydrogel is stiffer at 460.4 Pa (G’) and 66.4 Pa (G”). Solid microspheres are less desirable on their own as these create an empty space upon their degradation that will not facilitate invasion of cells to create a homogenous tissue. As the rheological properties of hydrogel bioscaffolds widely vary based on their composition and preparation, a key question is whether implanted materials should emulate the rheological properties of mature or developing brain tissue, or reflect the stiffness and elasticity of granulation tissue that supports peripheral soft tissue wound healing.
Estimates of adult brain tissue stiffness vary between 500-1000 Pa, which is consistent with the 8 mg/mL UBM-ECM hydrogel. However, rheological techniques that depend upon instruments, such as rheometers and atomic force microscopes, and that are used in material sciences, produce inconsistent results for storage and loss moduli. The results are dramatically influenced by sample preparation [26]. These techniques typically are not compatible with in vivo measurements and hence provide unreliable estimates of the brain’s rheological properties. In contrast, magnetic resonance elastography (MRE) provides a reliable in vivo measurement of the brain’s rheological properties to indicate a G’ of 3.1 kPA and a G” of 2.5 kPa for grey matter and G’ of 2.7 kPa and G” of 2.5 kPa for white matter in the adult human brain [63]. During aging, global brain viscoelasticity is reduced from 2.5 kPA at 20 years of age to below 1.5 kPA >60 years of age [111]. Softening of tissues is also associated with disease progression, often reflecting neuronal loss or demyelination [81].
Granulation tissue is thought to be much weaker than the mature tissue it is forming due to a predominance of collagen III fibers, rather than collagen type I that is prevalent in mature tissue [67]. Early forming granulation tissue (7 days) in a skin wound healing paradigm yielded an initial stiffness of 18.5 kPa, which increased with greater collagen I deposition to 49.3 kPa for late granulation tissue (12 days) [62]. However, the rheological properties of this tissue will evolve based on the stage of the regenerative process, which is influenced by cellular composition and ECM deposition [65]. Constructive remodeling of bioscaffolds in the brain are likely to produce a similar evolution in rheological properties to influence cell invasion, as well as differentiation. Matching of bioscaffold stiffness to that of adult brain has been suggested as a favorable design characteristic to promote neural regeneration [57]. In vivo measurements using non-invasive imaging techniques, such as MRE, will likely be the gold standard to evaluate rheological properties of live tissues due to rapid changes in brain tissue stiffness post-mortem [64]. This approach can also be applied to characterize tissue constructs in the context of regenerative medicine [119] and potentially provide a non-invasive means to evaluate serially the biomechanical properties of bioscaffolds.
Extensive in vitro studies indicate that softer materials afford a faster migration of cells [166], which is desirable for a more rapid re-population of the tissue cavity. However, there is a preference of cells to remain within stiffer gels, rather than invade softer materials. The in vivo cell invasion into softer ECM hydrogels (3-4 mg/mL) at the cusp of gelation exhibited less invasion than a stiffer bioscaffold within 24 hours in a study by Ghuman et al [59]. The average cell invasion speed was 62.5 μm/hr. The density of cells within the very weak 3 mg/mL hydrogel, nevertheless, increased over 90 days from 1151 to 3095 cells/μL, whereas a linear decrease to 1000 cells/μL was evident for the stiffer material [60]. The 4 mg/mL concentration presented a fairly stable cell density over 90 days between 3000-4000 cells/μL. As in vitro studies mostly used non-inductive hydrogels to investigate stiffness as a single factor, in vivo studies using different concentrations of ECM as ECM hydrogel bioscaffold not only differ with respect to stiffness, but also protein density, which can influence host cell invasion, as well as cellular phenotypes [128, 135]. For instance, during the acute invasion phase (24 hrs), hydrogel concentrations produced a fairly equivalent invasion of neural cells, but by 90 days a robust difference was evident between the stiffer (8mg/mL) and softer (3 and 4 mg/mL) bioscaffold, with the latter ones showing a more robust presence of neurons, astrocytes and oligodendrocytes, as well as endothelial cells [60].
6. Requirement 3: Inductive signaling for cell invasion
A robust interface between bioscaffold and host tissue is required to support the physical migration of cells to invade (Figure 4C), but inductive signals to initiate migration and invasion are also required. If inductive signals cannot be released from the bioscaffold or if the host tissue does not afford a permeation/diffusion of these signaling molecules to reach the appropriate cells, a poor cellular invasion ensues. These issues commonly underlie the case of invasion “blind spots”, where a robust invasion is seen in other parts of the scaffold (Figure 4D), but certain areas at the interface lack presence of cells [101]. Diffusion of inductive signals into host tissue is therefore required to promote cell invasion, although mechanical stimulation of host tissue through ECM bioscaffold implantation is also likely to contribute to the initiation of an immune response. The relative contributions of soluble, juxtacrine and biomechanical signaling to the inductive host response remain poorly understood.
Fractionation of ECM hydrogels into structural and soluble components indicates separate roles for these signaling molecules. Soluble components induced a phagocytic response through Notch and PI3K/Akt signaling that might be required to recruit immune cells into the hydrogel from surrounding host tissue [135]. Other factors, such as monocyte chemoattractant protein-1 (MCP-1/CCL2) are also key signals driving the migration and invasion of macrophages [41]. These signals might be comparative between a regenerative and a destructive response, with M1-like (i.e. destructive pro-inflammatory) macrophages invading through the host tissue [165]. However, neutrophils are also likely to participate in this acute response [38]. The time course and the cellular interplay leading to immune cell recruitment remain poorly understood and represent a main focus of understanding the initial regenerative response [154, 163]. Structural components of ECM bioscaffolds are thought to decrease phagocytosis by juxtacrine MEK/ERK and integrin signaling [135]. Upon invasion into the ECM bioscaffold, phagocytic cells are being polarized towards an M2-like (i.e. pro-repair) phenotype that is involved in a constructive remodeling response [100]. Although the importance of the macrophage response to tissue regeneration has been demonstrated by ablation experiments [153], it remains unclear how the inflammasome impacts the recruitment and differentiation of other host cells required to form a new tissue. Release of soluble factors, such as vascular endothelial growth factor-A (VEGF-A) or brain derived neurotrophic factor (BDNF), from implanted biomaterials will also influence a regenerative response in the brain [25, 30, 115]. Recruitment of neural cells is thought to rely on CXCR4/SDF1 signaling [34], whereas the invasion of endothelial cells is likely driven by a combination of pro-angiogenic soluble (e.g. VEGF-A, PDGF) and juxtacrine signals (e.g. αvβ3). However, detailed mechanistic studies investigating the role of these molecules’ role in inductive signaling through ECM bioscaffolds are lacking.
It is important to distinguish inductive signals which produce a regenerative response from those that provoke a destructive response. Typically a “destructive response” is due to a residual antigenicity that is caused by a poor decellularization or an insufficient removal (>92%) of lipophilic antigens [35, 85]. To avoid a destructive response, 3 key decellularization criteria have been proposed as quality control measures for inductive bioscaffolds: 1) no visible nuclei present (DAPI or H&E stains); 2) denatured DNA strands <200 base pairs; 3) double stranded DNA content <50 ng/mg [31]. Although a poor outcome has been associated with excessive cellular elements, specific roles of these elements in negating a regenerative effect remain unclear. A major advantage of synthetic materials will be that these can designed without these “determinetal” cellular remnants. In the context of allogenic versus xenogeneic tissue sources, there is further concern that some antigen can be retained within the bioscaffold. A slow and delayed adaptive immune sensitization can be invoked through these antigens to trigger a rejection response, calcification or destruction of newly forming tissues [90, 161]. Interestingly, little focus has been on HLA and MHC antigens [53], but the focus has been mostly on the αGal epitope [84] present in, for instance, porcine-sourced tissues, but not present in humans and apes [99]. However, only small quantities of the αGal epitope are present within ECM scaffolds [102], without evidence that these produce adverse effects when compared with knock-out tissues [37]. There are also no reports of zoonotic transmission from porcine whole organ transplants. Sourcing of CNS tissue from porcine sources is advantageous in terms of tissue quantities, as well as a defined age of the source. Human post-mortem CNS tissue is likely of poor quality, as brain death is a requirement for organ harvesting, and these tissues can also harbor the risk of transmitting prions and other transformed extracellular proteins (e.g. amyloid β, α-synuclein) involved in neurodegeneration. Prions and neurodegenerative proteins are unlikely to be eliminated by current decellularization protocols. Xenogenic sources are hence suitable for clinical applications and induce an immune response comparable to allogenic material that is thought to be crucial for ECM scaffold degradation [84].
7. Requirement 4: Scaffold biodegradation
A key role of immune cell invasion is to facilitate and effectuate scaffold degradation to allow structural remodeling by host cells with deposition of a new tissue matrix (Figure 4E). Macrophages degrade the bioscaffold, while secreting soluble factors (e.g. PDGF) that influence the behavior of other cells, including the recruitment of host cells, such as endothelial cells. Deposition of ECM molecules (e.g. fibronectin) by macrophages produces juxtacrine signaling that can guide cell migration and establish a structural support [163]. It remains currently unclear if there is a sequence of ECM molecule degradation or if macrophages trail a particular molecule. There is evidence of chain cell migration during the early phase of bioscaffold degradation, suggesting that a highly regulated process is taking place (Figure 4B). Macrophages are currently thought to be the main effectors of ECM biodegradation, but the specific mechanism(s) by which ECM degradation products interact with other cells and molecules remains under investigation. A distinction between scaffold degradation and remodeling might be required to further distinguish different processes involved in an induced versus a spontaneous regenerative response. ECM hydrogel biodegradation could be defined as the breakdown and loss of molecules integrating within the scaffold, whereas remodeling is a secondary event during which endothelial and neural cells invade to create de novo blood vessels around which tissue fragments emerge within or in between biomaterial patches (Figure 4E). Ideally, non-invasive imaging tools are employed to visualize and distinguish these processes. For instance, CEST-MRI can detect the selective loss of particular ECM molecules, such as fibronectin and chondroitin sulfate, contained within the bioscaffold [80], whereas the addition of specific markers, such as GelinS, can also be used to monitor more generally the degradation profile of hydrogels in vivo [95]. Juxtaposition of bioscaffold degradation and the ensuing tissue restoration by cell infiltration are interdependent, but it remains unclear how these two processes influence each other. At present, there is little evidence that endothelial and neural cells themselves degrade the scaffold material, although tissue elements can be seen forming in or between hydrogel patches indicating their participation in a remodeling response.
In most peripheral tissues, approximately 50% of ECM scaffold material in solid form (e.g. sheet form) is degraded within 30 days post-implantation, with a complete resorption evident between 75-90 days [24, 40, 61, 127, 153]. During this time frame, the scaffold is replaced with new tissue (Figure 4F). Little is known about ECM scaffold degradation in the brain or spinal cord and how the immune-privilege of the CNS impacts this process. The blood-brain barrier (BBB) typically does not afford invasion of immune cells to patrol the CNS, although acutely after a brain injury (12-24 hours), this barrier is leaky and immune cells (i.e. neutrophils) are invading. However, this early disruption of the barrier is short-lived and dissipates after invasion of immune cells. A second more prolonged sub-acute opening of the barrier occurs between 3-7 days post-injury [123]. Matrix metalloproteinase-9 (MMP-9) is a key molecule in this opening of the BBB, as well as degradation of native brain ECM in damaged tissues [6]. Microglia, the brain-resident macrophages, are pivotal in this process and regulate the immune response to damage [13]. Opening of the BBB facilitates infiltration of peripheral phagocytic cells (e.g. macrophages) to clear cellular and tissue debris. However, the sub-chronic phase closes when cavitation of tissue completed. Implantation of ECM bioscaffolds in hydrogel form with the associated host cellular response in brain cavities differs from the acute or subacute infiltration of immune cells into damaged tissue. Cellular infiltration occurs through adjacent damaged tissue, as initially there are no blood vessels remaining within the tissue cavity for a peripheral immune cell infiltration to occur directly into the scaffold (i.e. inside-out invasion). Although implantation of an 8 mg/mL ECM hydrogel, compliant with adult brain rheological properties, produced the strongest cell infiltration acutely [59], only 32% of the scaffold was degraded by 90 days [58]. This relatively slow rate of degradation is in stark contrast to the rate of degradation in peripheral tissues. However, in peripheral tissues, ECM-based scaffolds are typically implanted as solid multi-laminate sheets, rather than as a hydrogel, potentially explaining differences in the biodegradation rate. The porosity and stiffness of ECM sheets is different from those in hydrogels and could account for biodegradation characteristics. These studies might therefore not provide a valid comparison or guidance for a time course of biodegradation and cell infiltration into ECM hydrogels in the brain. Less concentrated ECM hydrogels (3 and 4 mg/mL) exhibited a very efficient degradation response, with 95% of the scaffold being degraded by 90 days [60], indicating that appropriate dosing can tailor the biodegradation to favor tissue restoration.
Unlike in peripheral soft tissue wound healing, the CNS does not form granulation tissue. ECM hydrogel implantation within the CNS might therefore act as a ground substance, i.e. an amorphous gel that contains inductive ECM proteins that invoke a host regenerative response [54, 159]. The presence of some fibrous materials, such as collagen, might merely be required to provide a weak structural support and cues for invading cells, but by themselves are not required to induce a regenerative response. As such a weak ECM hydrogel would serve as a granulation tissue in the CNS to induce and support cell invasion into a primitive tissue arrangement. The structural weakness of the hydrogel might therefore be a key characteristic of this intervention, as “brain-compliant” ECM hydrogel did not produce a successful tissue restoration in the cavity. As with granulation tissue, a maturation of cells and tissue characteristics (e.g. collagen I deposition) might be evident that affords a staging of the regenerative process. A mixture of ECM scaffold biodegradation and tissue remodeling (i.e. invasion and organization of host brain cells) might coincide. Although there is a fairly homogenous invasion of cells acutely, the formation of de novo brain tissue and its maturation might be an outside-in progression rather than an inside- out process involved in brain development. Akin to granulation tissue, neovascularization is likely driving the maturation process as oxygen supply to new tissue will be a key factor in its survival.
8. Requirement 5: Neovascularization
Implantation of NSCs on PLGA particles [16] or in ECM hydrogel [17] failed to produce neovascularization of the stroke cavity, although NSCs secrete vascular endothelial growth factor-A [28]. Degradation of PLGA particles afforded a flow of ECF through the cavity that maintained the web-like tissue short-term (7 days), but the development of de novo tissue formed purely by implanted neural cells was not feasible due to a lack of vascularization that compromise long-term tissue survival. In contrast, secretion of VEGF-A from PLGA particles led to an infiltration of endothelial cells into the lesion cavity seeded with human NSCs and afforded the assembly of vascular structures inside the de novo forming tissue [18]. However, endothelial cell invasion did not follow the classical angiogenesis model, where tip cells deviate from the stalk to invade a developing tumor. A widespread invasion of endothelial cells was evident that subsequently organized into vascular structures, which is consistent with vasculogenesis [109]. A heterogenous mix of vasculature developed within this de novo tissue. Some areas developed fairly normal vasculature with a surrounding neuropil, others were hypervascularized, but there were also patches devoid of neovascularization [18]. A differential biodegradation of PLGA microparticles through hydrolysis can explain some of these observations. The lack of revascularization is therefore likely due to a lack of particle degradation due to a restricted availability of water, hence producing an insufficient release of VEGF-A. A limited diffusion of VEGF-A into the surrounding damaged tissue could also compromise endothelial cell invasion. Indeed, scarring around the implant could hinder the diffusion of molecules from the lesion cavity into surrounding tissue, but also prevent cells from invading the scaffold. Co-implantation of NSCs and ECs might circumvent this issue and provide a more rapid re-construction of the neurovascular environment in the cavity [29, 114]. However, even allogenic endothelial cells will require immunosuppression, akin to fetal tissue transplants, due to their high immunogenicity and direct exposure to circulating peripheral immune cells, which will mount an acute rejection response.
Attracting host vasculature is therefore preferable, as it circumvents the need for long-term immunosuppression, but will also ensure that developing arteries and veins are connected into existing vascular systems. Implantation of a brain-compliant acellular 8 mg/mL ECM hydrogel produced a marked invasion of endothelial cells 24 hours post-implantation [59], but long-term failed to produce a vasculature [58]. No tissue developed in these bioscaffolds. In contrast, softer (3 and 4 mg/mL) ECM concentrations produced a robust neovascularization throughout the scaffold area [60]. Neuro-vascular environments formed that in some instances were still engulfed by the bioscaffold (Figure 4G), whereas in others it formed in between patches of hydrogel (Figure 4H). In some cases, a tortuous vasculature was evident, akin to angiogenic areas in peri-infarct stroke, producing a dense mesh of small capillary vessels. In peripheral artery disease, ECM hydrogel promoted revascularization through arteriogenesis [152], potentially indicating multiple ways to promote tissue neovascularization. Acellular ECM hydrogel can therefore attract host endothelial cells to develop a neovasculature in de novo forming tissue. Interestingly, matrix stiffness can affect how endothelial cells respond to the same soluble factors [94], such as VEGF-A, potentially explaining the differential results between soft and brain-compliant ECM hydrogel. Producing a neovasculature from host cells will circumvent the need for immunosuppression and provide the physiological basis for a metabolically active neuro-vascular environment. However, it presently remains unclear if this vascular supply provides sufficient oxygen to support metabolism and allow newly formed tissue to mature long-term.
Vascularization remains a major issue in tissue engineering, especially in cases of large tissue defects [88]. In smaller tissue defects, small blood vessels can form a vascular bed (i.e. plexus) to supply the neuropil with oxygen and nutrients, as well as infiltrating peripheral immune cells. However, it remains unclear to what degree these newly forming blood vessels are leaky and at what stage a basement membrane is formed that would participate in barrier functions. Neovascularization in tissue regeneration is likely different from tumor angiogenesis. In tumor angiogenesis, the neoplastic growth is steadily increasing and requiring a gradual augmentation of blood supply, eventually producing a necrotic core as the metabolic demand can no longer be met. In contrast, induced tissue regeneration produces a large cell invasion that requires a vast area to be rapidly vascularized. Hypoxia can ensue and lead to apoptosis or necrosis in newly forming tissue. A certain level of hypoxia is advantageous, as it will lead to the production of hypoxia-induced factor 1-α (HIF-1α), which is the inducer of VEGF-A and hence can enhance vascularization. Angiogenesis, as seen in tumor growth, and vasculogenesis/arteriogenesis, as observed in ECM hydrogel-mediated tissue regeneration, might share similarities, but there will also be fundamental differences in how this neovascularization occurs and proceeds. The matrigel assay of angiogenesis using ECM from Engelbreth-Holm-Swarm mouse sarcoma cells is unlikely to provide appropriate answers to vessel formation in the context of tissue regeneration as it predominantly relies on angiogenesis with tip cells invading the hydrgoel. Alternative in vitro assays might need to be developed to improve our understanding of vessel formation in regenerating tissues. These assays might also need to distinguish small and large tissue defects, as the microvasculature is insufficient to produce an adequate blood supply in large tissue defects. Large vessels, such as the middle cerebral artery, need to maintain intra-arterial pressure and perfuse newly forming tissues through branching of the microvasculature. Little is known about re-growing these types of vessels in situ and how different brain regions might influence the source and pattern of revascularization.
9. Requirement 6: Site-appropriate phenotypic differentiation
Unlike most other organs, the brain has a distinctive regional specialization. Cytoarchitecturally different phenotypes define brain regions and their functions. It is assumed that tissue regeneration therefore needs to produce site-appropriate phenotypes that characterize the restored area from the endogenous NSC pool [51]. Hydrogel formulation is known to influence cell differentiation [8]. However, the challenges associated with decellularizing brain tissue to form hydrogels [103], as well as the consideration that CNS tissues typically do not support regeneration [76, 144], might limit the therapeutic potential of decellularized brain tissue. A further question is whether a homologous ECM tissue source is required to instruct cells to form a site-appropriate tissue or if the host tissue response induced by these bioscaffolds is not tissue specific, but driven by the invasion of host organ cells (i.e. the brain). In vitro studies indicate that UBM-ECM produced the greatest degree of neuronal differentiation and neurite outgrowth, rather than brain-derived ECM [33, 49, 103]. However, it remains unclear what neuronal sub-types were produced. It is also uncertain if a high level of neuron differentiation is the key factor to select bioscaffolds derived from a particular tissue to drive site-appropriate tissue differentiation. Specific positional information needs to be encoded in neurons to define a brain region. At present, it remains unknown if ECM hydrogel conveys this positional specification or if this is inherent in the invading cells. In the spinal cord, UBM-ECM performed equivalently to spinal cord-derived ECM hydrogel to produce a regenerative response, but provided more favorable degradation characteristics [151]. This also suggests that ECM bioscaffolds do not regulate the final differentiation of neurons. Although direct in vivo comparisons of different ECM sources in acute brain injuries are mostly lacking, UBM-ECM may provide favorable characteristics in terms of hydrogel degradation, more complete neuronal differentiation and neurite outgrowth, as well as being economical and safe.
Positional specification of cells (e.g. cortical versus striatal patterning) might also be influenced by the damaged tissue through which cells must migrate to invade the bioscaffold. The precise mechanism of this cell invasion from the peri-infarct area remains unclear, but it can be surmised that neural cells rely on the soluble SDF-1/CXCR-4 signaling axis [124], as well as juxtacrine integrin (i.e. fibronectin, laminin) signals [92], for migration and ECM invasion. However, detailed mechanistic studies will be required to determine the specific mechanism(s) and if there are differences between migration through damaged tissue versus invasion and migration through an ECM hydrogel. Implantation of a 3 or 4 mg/mL ECM hydrogel recruited approximately 70,000 host neural cells (20% of total cells at 24 hours in the 8 mg/mL ECM hydrogel) to restore tissue in the stroke-damaged striatum [60]. Although the stiffer and denser 8 mg/mL ECM hydrogel produced the highest acute 24 hours invasion from damaged tissue [59], the better long-term invasion of neural cells was found in less concentrated 3 and 4 mg/mL hydrogels [60]. It is conceivable that stiffness of the material in the initial invasion/attraction phase is not the main determining factor, but the concentration of inductive protein could govern this initial response. However, the mechanism by which protein density or its concentration affect cell differentiation remains also poorly understood and will require further in vitro and in vivo studies. Moreover, changing the rheological properties and degradation response might differentially affect cell invasion and differentiation, further highlighting the complex interplay of different variables involved in tissue regeneration.
The rheological properties of hydrogels are known to affect the phenotype of neural cells, with brain-compliant (183 Pa) encapsulating materials achieving the highest neuronal differentiation [12]. However, neural stem cells seeded onto bioscaffolds of different stiffness indicate that softer materials (0.1-1 kPa) are favorable for a neuronal differentiation, whereas stiffer (0.5- 10 kPa) materials produce more astrocytes [7]. This degree of scaffold stiffeness is in contrast to the very stiff surface of cell culture vessels at ~100,000 kPA typically used for in vitro studies [134]. Interestingly, an appropriate differentiation of cells into site-appropriate striatal neuron phenotypes still occurs in culture vessels and on glass slides [47]. Studies in tissue culture flasks therefore suggest that the rheological properties of substrates are merely a contributing, rather than the determining factor that influences the proportion of phenotypes towards one or the other direction. In vitro studies afford the control of very specific variables, but typically fail to replicate the complex in vivo environment that cells encounter. Upon implantation of softer (3 and 4 mg/mL) or stiffer (8 mg/mL) ECM hydrogels into a stroke cavity, the stiffer substrate invoked a significantly higher initial invasion of neural progenitors (8%) at 24 hours compared to the softer scaffolds [60]. In contrast, the softer gels produced twice as many neurons with mature phenotypes (226 neurons/μL, ~5% of total cells), whereas more oligodendrocytes were present in stiffer (~30%) than softer (20%) bioscaffolds at 90 days post-implantation. The most dramatic difference was observed with astrocytes. There was a gradual increase in the soft 3 and 4 mg/mL hydrogels up to 15%, but the stiffer 8 mg/mL concentration was essentially lacking astrocytes. These in vivo results contradict in vitro observations, where stiffer materials produced more astrocytes. However, it is conceivable that the stiffer and denser 8 mg/mL prevents the invasion of astrocyte progenitors and these participate in a tissue response to the material, such as a glial scar, around the implants that seals these off from the host brain [96, 171]. This tissue barrier could further explain the low cell numbers in the stiffer material at later time points. Indeed, the glial scar forming in volumetric tissue loss could affect in situ tissue engineering by limiting cell invasion and integration of newly forming tissues.
10. Requirement 7: Dissolution of the glial scar
At the central core of the acute brain injury, liquefactive/colliquative necrosis occurs to form the tissue cavitation. Akin to hemostasis in wound healing, the local tissue response is to stabilize potentially viable from necrotic tissue by compartmentalizing this area using the formation of a scar to create a physical and endocytic barrier. This liquid viscous mass within the cavity is known to be toxic to brain cells for at least 7 weeks. This material diffuses into surrounding brain tissue despite the formation of a glial scar [167]. Reactive astrocytes, microglia, fibroblast and ependymal cells derived from the peri-vasculature, as well as circulating macrophages, participate in the development of the scar [1]. Activated astrocytes are thought to have the potential to undergo one round of cell division and give rise to NSCs, potentially participating in local tissue repair. Astrogliosis, the presence of individual activated astrocytes in areas of damage, also occurs and potentially plays a key role in the establishment of the glial scar and tissue repair [137]. Akin to macrophages, an emergent literature suggests differential roles of activated A1 (pro-inflammatory/neurotoxic) and A2 astrocytes (pro-repair) in peri-lesional tissues [11], which could be influenced by biomaterials [148]. Typically, established scar tissue is considered to contain astrocytes, NG2 glia (also known as oligodendrocyte progenitor cells, OPC), and microglia [1]. The glial scar also sees a dramatic upregulation of chondroitin sulfate proteoglycans (CSPG), which might further limit the invasion of neuronal progenitors and axonal re-growth [50]. Digestion of CSPG can, for instance, promote a pathway to reconstruction by transplanted dopamine progenitors in Parkinsonian mice [82]. Recently, it has been reported that glial scarring weakened the mechanical properties of CNS tissue [108]. Still, it currently remains unclear how changes in rheological properties of damaged tissue, as well as migration through the scar affect progenitor cells. The glial scar and associated changes in tissue microenvironment might hence pose a major obstacle to tissue regeneration by limiting cell invasion, influencing phenotypes of invading cells, preventing neovascularization, and ultimately precluding connectivity between veterate (i.e. pre-existing) and de novo tissue.
Implantation of ECM hydrogel into the tissue cavity using the injection-drainage approach, cleared the liquefactive material and provided a biocompatible substrate for cell invasion [101]. These results indicate that scarring by itself is not a limitation to attract host cells into the cavity [59, 60]. Although some reports indicate a change in glial scarring and astrocytes, UBM-ECM hydrogel did not alter the scarring or peri-lesional astrogliosis characteristics after a sub-chronic implantation time [60]. However, in the stiffer 8 mg/mL ECM bioscaffold no astrocytes invaded, suggesting that the peri-lesion scar is altered and potentially encapsulates stiffer material to prevent long-term astrocyte invasion. Still, it is unclear why this would not also affect other neuronal progenitors or oligodendrocytes. Lower concentrations of ECM that produced softer hydrogel revealed a significant presence of astrocytes in the scaffold. The 3 and 4 mg/mL ECM scaffolds also revealed axons passing through the hydrogels, but it remains unclear how extensive this connectivity is and if indeed functional synapses are being formed by new tissue.
11. Requirement 8: Axonogenesis and connectivity
The regional specialization of the brain requires interconnectivity to support diverse functions [142]. This interconnectivity is achieved by 1) local intra-regional and 2) multi-regional long-distance axons. Axons link different neurons with each other to conduct information processing and produce an integrated function. Although each neuron will have a single axon, it can be connected with more than 1000 other neurons. Restoration of complex behavioral or cognitive functions are therefore thought to require not only a reconstitution of neuronal cells in a de novo tissue, but also will need to establish axonal connections between these cells in local cell assemblies, as well as connect these with existing neuronal circuitry [107]. Based on our current understanding of the developmental neurobiology underpinning axonal connectivity and the limited number of studies investigating tissue regeneration in the brain, this will likely be the most challenging requirement.
After ECM implantation into a stroke cavity, axons are present within and passing through the scaffold [60]. However, it is unknown how many axons are newly formed, if these mostly support local connectivity, or if these produce long-range connectivity. It is also unclear if host axons project into the newly formed tissue and if synapses are being formed that could support functional connectivity. Hyaluronic acid (HA) hydrogels with VEGF-A induce angiogenesis, and an axonal network akin to the contralateral hemisphere in the infarct region [115]. It has further been suggested that axonogenesis necessitates angiogenesis, as blocking of angiogenesis did not afford axonogenesis in the infarct area [115]. This would suggest that a spontaneous arrangement of fibers could occur in cortical tissue after promoting a regenerative environment using bioscaffolds. However, it remains undetermined to what degree alignment needs to be predetermined to generate appropriate grey matter tissue (e.g. entorhinal cortex) in the brain and if cells other than endothelial cells, such as astrocytes or microglia, could support axonogenesis [121]. For instance, in the spinal cord axonogenesis is observed after implantation of inductive bioscaffolds [70, 89], but an extensive effort is dedicated to patterning anisotropic channels that would guide axonal growth [44, 169]. The delivery of specific molecules driving axonogenesis might further improve the connectivity between veterate and de novo tissues [4, 126]. The interdependence of biological processes involved in tissue regeneration is evident and shows the complex and concerted interplay that is required to restore a physiological tissue. Blocking or enhancing of a single signaling pathway can dramatically change the entire process. However, creating a physiologically active tissue does not necessarily produce a “functional brain tissue”. Functional tissue will also require synapse formation of axons and dendrites that are responsible for brain activity and define participation in neuronal circuitry.
12. Requirement 9: Synapse formation for a functional integration of de novo tissue
Axonal pathways provide the structural basis of connectivity, but synaptogenesis determines functional connectivity [142]. Synapses are promiscuously formed during development (i.e. exuberant synaptogenesis). Pruning of unused synapses and strengthening of synaptic connections occurs to refine neuronal circuitry based on activity i.e. Hebbian synapses). Maturation of tissue is hence also reflected in synaptic density. Any behavioral changes due to the formation of new synaptic connections are likely to undergo a similar process of exuberant synaptogenesis due to both new neurons being formed in de novo tissue and projections into damaged or previously denervated regions. Conversely, re-innervation of the lesion cavity from veterate tissue would also be required. The formation of Hebbian synapses, i.e. two synapses that fire together wire together, are thought to be a key component of restoring behavioral functions [36]. We are currently not aware of any studies that have directly investigated synapse formation in de novo brain tissue, although DREADD (designer receptor exclusively activated by designer drugs) ligands have been used to demonstrate that areas injected with HA hydrogel and VEGF nanoparticles underpinned behavioral functions associated with recovery after a cortical stroke [115].
Promoting synaptogenesis and the formation of novel functional connections is fundamental to physical and cognitive therapy after stroke. In cell therapy, it remains a matter of investigation if physical therapy is a requirement or merely facilitates novel neural progenitors to form functional connections that underpin behavioral recovery [106]. This regenerative rehabilitation [125] has been found to be an essential component to regrow functional skeletal muscle after ECM implantation [56, 133]. However, a distinction is probably required between individual progenitor cells being injected into damaged tissues, where neuronal circuitry has been disrupted, versus forming an entirely new circuitry. In the brain, this would be exemplified by studies that implanted fetal tissue to promote a recovery of function. These materials did not result in the seamless integration of individual cells into existing brain tissue, but formed a “mass” within brain tissue or cavities. In these cases, “training” of grafts implanted into the striatum, for instance, showed task specificity and a clear benefit from functional inputs [42, 43]. However, restitution of behavioral functions in a tissue regeneration paradigm might occur without the addition of a rehabilitation component [115]. This again raises the question of rehabilitation as a requirement for behavioral improvements [107]. In cases of ECM hydrogels implanted into a stroke cavity, the lack of an efficient degradation of the bioscaffold and regeneration of tissue failed to produce any behavioral improvements [58]. However, it currently remains unclear if softer gels that produce tissue regeneration could produce behavioral recovery. Interestingly, small volumes (5 μL) of ECM hydrogels injected into damaged tissue were sufficient to produce improvements in behavioral functions [162, 168]. To establish the rules governing the restoration of behavioral functions through tissue regeneration will require both successes and failures to define aspects important to efficacy and guide the translation of these novel therapies into the clinic.
13. Special considerations for clinical translation
The two main considerations for clinical translation are safety and efficacy. Inductive biomaterials promoting tissue regeneration in peripheral soft tissue have been widely used clinically and have proven to be safe. It is unlikely that implantation into the brain would involve a different safety profile of the materials themselves, although specific product specifications (i.e. hydrogels versus material sheets) need to be considered, especially since rheological properties of the material affect their regenerative potential. Safety considerations in the brain also need to consider potential adverse effects that could occur due to implantation of the material. A selective targeted delivery to tissue cavities, while avoiding displacement of fragile damaged tissue, remains a major challenge and is difficult to evaluate in small rodent brains. Changes in intracranial pressure due to implantation of too much material, or swelling of the gels, could, for instance, produce iatrogenic complications that would raise safety concerns [101]. Moreover, inductive materials produce a proliferative response of progenitor cells. Consequently, it is important to ensure that no neoplastic growth develops in the cavity. No reports of this possibility have occurred in peripheral tissue and it is unlikely that brain cells would differ in this respect. Restitution of tissue and its connectivity with the host brain also harbors a risk to develop uncontrolled spontaneous brain activity (i.e. generation of an epileptic focus). Although there are no reports so far of any of these potential side-effects, the safety of this approach for clinical translation will need to consider these potential additional complications.
Efficacy is defined as a significant improvement over clinical equipoise. However, efficacy can encompass many measures. For instance, stabilizing a patient and preventing further damage to occur assumes a certain trajectory of the condition. In the case of a malignant MCA stroke, this involves the removal of the cytotoxic edema to save the patient’s life [68]. Reducing the midline shift that commonly follows the decompressive surgery significantly improves survival of patients at 6 months [79]. Implantation of a stiff ECM bioscaffold that degrades very slowly could hence stabilize the veterate tissue in patients and provide a means to improve the survival rate, even without promoting tissue regeneration [58]. Stabilizing the veterate tissue in patients with smaller strokes that do not undergo decompressive surgery could also provide a benefit by reducing secondary Wallerian degeneration of axonal tracts due to morphological shifts in brain tissue. These changes are difficult to measure and might not be readily detectable in rodent models of stroke, including a lack of dramatic worsening commensurate with slow and subtle anatomical changes.
Removal of cytotoxic fluid from the tissue cavity can also reduce a secondary loss of neurons in the peri-lesion tissue [167]. Halting or slowing the progression of neurodegeneration in TBI could provide a sparing of function, rather than a reversal of behavioral deficits. Inductive biomaterials in damaged tissue will promote a shift of immune cells towards a repair phenotype, as well as promote angiogenesis, which can lead to tissue repair [162, 168]. Implantation of ECM hydrogel into the tissue cavity is also likely to increase the presence of neural progenitor cells in damaged tissue, which could exert beneficial effects, even in the absence of a functional tissue regeneration. However, it is unclear if these events are sufficient to drive a significant behavioral change that can be measured in rodent models of acute brain injury. Manifestation of these benefits might take a protracted lesion evolution. Rodent models’ life-span might be limiting in this regard [107]. Developments of large animal models might also be key to refine the delivery of large volumes of bioscaffolds into complex lesion environments and to define appropriate measures to monitor the repair/regeneration process, as well as to better define outcome measures that go beyond standard behavioral measures currently used to assess efficacy in small animal models [107]. Subtle effects (~10% change) might require a large cohort of animals (>15) to establish efficacy and robustly demonstrate the potential of these therapeutic effects for patients.
14. Conclusions
The treatment of acute brain injuries to date is mostly focused on stabilizing patients and providing neuroprotection to avoid a further loss of brain tissue. Although limiting brain tissue loss is expected to have the biggest clinical impact, little focus has been devoted to repair or regenerate brain tissue as secondary therapeutic options. Especially the regeneration of lost brain tissue offers novel therapeutic opportunities that can be realized using biomaterials to provide scaffolding in tissue cavities and to introduce cell signaling using juxtacrine and soluble factors that can influence the repair process. Akin to peripheral soft tissue regeneration, tissue regeneration can be broken down into individual perturbation parts that can be solved, as outlined here. Therapeutic benefits are not merely restricted to a complete restoration to a pre-insult tissue architecture, but many smaller effects can provide worthwhile interventions. Indeed, it is currently unclear if regenerating tissue needs to mirror the developmental architecture of veterate tissue or if other configurations of cell assemblies can achieve the same function. The ultimate creation of a de novo functional brain tissue might require all these perturbation parts to integrate into a series to provide a new substrate to support behavioral improvements. Akin to the workings of a Swiss watch, all elements might be required to work together to make this ultimate aim feasible, with the lack of each one of these preventing the formation of a de novo functional brain tissue. Conceptually and technically novel directions result from developing in situ brain tissue engineering that will lead to novel therapeutic opportunities parallel to tissue regeneration, such as the local and sustained delivery of therapeutic drugs or cells [69, 164].
However, there remains much to learn to make in situ tissue engineering in the brain a viable treatment option for patients with acute brain injury. Placing the sole emphasis on improving behavioral functions might produce false positives and limit discovery of other opportunities. Developing a roadmap that defines specific requirements will help to identify individual perturbation parts that need to be solved as part of the bigger endeavor. Still, the interdependence of many of these processes (e.g. angiogenesis and axonogenesis) requires an integrated view to inform the design of new synthetic scaffolds. Importantly, a greater emphasis needs to be put on in vivo experiments and understanding how bioscaffolds interact with existing veterate tissue. Understanding the process of tissue restoration using inductive bioscaffolds will form the foundation to develop bottom-up designer hydrogels that produce specific activities appropriate to repair or regenerate a particular brain tissue. These intriguing opportunities will undoubtedly spur new innovations to improve the care of patients with acute brain injuries.
Highlights.
Conceptualization of brain tissue restoration in perturbation parts
Defined the importance of importance of intracerebral delivery of bioscaffolds
Discussion of the immune systems role in scaffold degradation and initiating a restoration response
Reviewed the role of juxtacrine and paracrine factors in tissue formation
Acknowledgments
The authors acknowledge the generous funding of NINDS (R01NS08226), NIBIB (R01EB016629), Pennsylvania Department of Health, ACell, Vertex Pharmaceuticals, and C.R. Bard that support our effort in developing in situ tissue engineering to replace tissue lost after a stroke.
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article. None of the funders had a role in the design of the studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Adams KL, Gallo V, The diversity and disparity of the glial scar, Nat Neurosci, 21 (2018) 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amer MH, Rose F, Shakesheff KM, Modo M, White LJ, Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges, NPJ Regen Med, 2 (2017) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amer MH, Rose FR, White LJ, Shakesheff KM, A Detailed Assessment of Varying Ejection Rate on Delivery Efficiency of Mesenchymal Stem Cells Using Narrow-Bore Needles, Stem Cells Transl Med, 5 (2016) 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV, Required growth facilitators propel axon regeneration across complete spinal cord injury, Nature, 561 (2018) 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Antoine EE, Vlachos PP, Rylander MN, Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport, Tissue Eng Part B Rev, 20 (2014) 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aoki T, Sumii T, Mori T, Wang X, Lo EH, Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats, Stroke, 33 (2002) 2711–2717. [DOI] [PubMed] [Google Scholar]
- [7].Aurand ER, Lampe KJ, Bjugstad KB, Defining and designing polymers and hydrogels for neural tissue engineering, Neurosci Res, 72 (2012) 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aurand ER, Wagner JL, Shandas R, Bjugstad KB, Hydrogel formulation determines cell fate of fetal and adult neural progenitor cells, Stem Cell Res, 12 (2014) 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Badylak SF, Freytes DO, Gilbert TW, Extracellular matrix as a biological scaffold material: Structure and function, Acta Biomater, 5 (2009) 1–13. [DOI] [PubMed] [Google Scholar]
- [10].Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, Kelly DJ, Ignotz RA, Gaudette GR, The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium, Cell Transplant, 15 Suppl 1 (2006) S29–40. [DOI] [PubMed] [Google Scholar]
- [11].Baldwin KT, Eroglu C, Molecular mechanisms of astrocyte-induced synaptogenesis, Curr Opin Neurobiol, 45 (2017) 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS, The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells, Biomaterials, 30 (2009) 4695–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J, The role of microglia and myeloid immune cells in acute cerebral ischemia, Front Cell Neurosci, 8 (2014) 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American E Heart Association Council on, C. Prevention Statistics, S. Stroke Statistics, Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association, Circulation, 137 (2018) e67–e492. [DOI] [PubMed] [Google Scholar]
- [15].Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M, Attachment of stem cells to scaffold particles for intra-cerebral transplantation, Nat Protoc, 4 (2009) 1440–1453. [DOI] [PubMed] [Google Scholar]
- [16].Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M, The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles, Biomaterials, 30 (2009) 2985–2994.19278723 [Google Scholar]
- [17].Bible E, Dell’Acqua F, Solanky B, Balducci A, Crapo PM, Badylak SF, Ahrens ET, Modo M, Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI, Biomaterials, 33 (2012) 2858–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bible E, Qutachi O, Chau DY, Alexander MR, Shakesheff KM, Modo M, Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles, Biomaterials, 33 (2012) 7435–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bloch J, Bachoud-Levi AC, Deglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, Bourdet C, Gaura V, Remy P, Brugieres P, Boisse MF, Baudic S, Cesaro P, Hantraye P, Aebischer P, Peschanski M, Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study, Hum Gene Ther, 15 (2004) 968–975. [DOI] [PubMed] [Google Scholar]
- [20].Bonnans C, Chou J, Werb Z, Remodelling the extracellular matrix in development and disease, Nat Rev Mol Cell Biol, 15 (2014) 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bramlett HM, Dietrich WD, Pathophysiology of cerebral ischemia and brain trauma: similarities and differences, J Cereb Blood Flow Metab, 24 (2004) 133–150. [DOI] [PubMed] [Google Scholar]
- [22].Brown BN, Badylak SF, Extracellular matrix as an inductive scaffold for functional tissue reconstruction, Transl Res, 163 (2014) 268–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJ, Zaat SA, Schultz MJ, Grainger DW, Biomaterial-associated infection: locating the finish line in the race for the surface, Sci Transl Med, 4 (2012) 153rv110. [DOI] [PubMed] [Google Scholar]
- [24].Carey LE, Dearth CL, Johnson SA, Londono R, Medberry CJ, Daly KA, Badylak SF, In vivo degradation of 14C-labeled porcine dermis biologic scaffold, Biomaterials, 35 (2014) 8297–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chan SJ, Love C, Spector M, Cool SM, Nurcombe V, Lo EH, Endogenous regeneration: Engineering growth factors for stroke, Neurochem Int, 107 (2017) 57–65. [DOI] [PubMed] [Google Scholar]
- [26].Cheng S, Clarke EC, Bilston LE, Rheological properties of the tissues of the central nervous system: a review, Med Eng Phys, 30 (2008) 1318–1337. [DOI] [PubMed] [Google Scholar]
- [27].Cheng YD, Al-Khoury L, Zivin JA, Neuroprotection for ischemic stroke: two decades of success and failure, NeuroRx, 1 (2004) 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chou CH, Modo M, Human neural stem cell-induced endothelial morphogenesis requires autocrine/paracrine and juxtacrine signaling, Sci Rep, 6 (2016) 29029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chou CH, Sinden JD, Couraud PO, Modo M, In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells, PLoS One, 9 (2014) e106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cook DJ, Nguyen C, Chun HN, I LL, Chiu AS, Machnicki M, Zarembinski TI, Carmichael ST, Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke, J Cereb Blood Flow Metab, 37 (2017) 1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Crapo PM, Gilbert TW, Badylak SF, An overview of tissue and whole organ decellularization processes, Biomaterials, 32 (2011) 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crapo PM, Medberry CJ, Reing JE, Tottey S, van der Merwe Y, Jones KE, Badylak SF, Biologic scaffolds composed of central nervous system extracellular matrix, Biomaterials, 33 (2012) 3539–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Crapo PM, Tottey S, Slivka PF, Badylak SF, Effects of biologic scaffolds on human stem cells and implications for CNS tissue engineering, Tissue engineering. Part A, 20 (2014) 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cui L, Qu H, Xiao T, Zhao M, Jolkkonen J, Zhao C, Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia, Restor Neurol Neurosci, 31 (2013) 239–251. [DOI] [PubMed] [Google Scholar]
- [35].Dalgliesh AJ, Parvizi M, Lopera-Higuita M, Shklover J, Griffiths LG, Graft-specific immune tolerance is determined by residual antigenicity of xenogeneic extracellular matrix scaffolds, Acta Biomater, 79 (2018) 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dalise S, Ambrosio F, Modo M, Brain plasticity and recovery in preclinical models of stroke, Arch Ital Biol, 152 (2014) 190–215. [DOI] [PubMed] [Google Scholar]
- [37].Daly KA, Stewart-Akers AM, Hara H, Ezzelarab M, Long C, Cordero K, Johnson SA, Ayares D, Cooper DK, Badylak SF, Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model, Tissue engineering. Part A, 15 (2009) 3877–3888. [DOI] [PubMed] [Google Scholar]
- [38].de Oliveira S, Rosowski EE, Huttenlocher A, Neutrophil migration in infection and wound repair: going forward in reverse, Nat Rev Immunol, 16 (2016) 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, Day L, Lam D, Jette N, The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis, Neuro Oncol, 17 (2015) 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dearth CL, Slivka PF, Stewart SA, Keane TJ, Tay JK, Londono R, Goh Q, Pizza FX, Badylak SF, Inhibition of COX1/2 alters the host response and reduces ECM scaffold mediated constructive tissue remodeling in a rodent model of skeletal muscle injury, Acta Biomater, 31 (2016) 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Deshmane SL, Kremlev S, Amini S, Sawaya BE, Monocyte chemoattractant protein-1 (MCP-1): an overview, J Interferon Cytokine Res, 29 (2009) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dobrossy MD, Dunnett SB, The effects of lateralized training on spontaneous forelimb preference, lesion deficits, and graft-mediated functional recovery after unilateral striatal lesions in rats, Experimental neurology, 199 (2006) 373–383. [DOI] [PubMed] [Google Scholar]
- [43].Dobrossy MD, Nikkhah G, Role of experience, training, and plasticity in the functional efficacy of striatal transplants, Prog Brain Res, 200 (2012) 303–328. [DOI] [PubMed] [Google Scholar]
- [44].Dumont CM, Carlson MA, Munsell MK, Ciciriello AJ, Strnadova K, Park J, Cummings BJ, Anderson AJ, Shea LD, Aligned hydrogel tubes guide regeneration following spinal cord injury, Acta Biomater, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dziki JL, Wang DS, Pineda C, Sicari BM, Rausch T, Badylak SF, Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype, J Biomed Mater Res A, 105 (2017) 138–147. [DOI] [PubMed] [Google Scholar]
- [46].Eichler C, Schulz C, Vogt N, Warm M, The Use of Acellular Dermal Matrices (ADM) in Breast Reconstruction: A Review, Surg Technol Int, 31 (2017) 53–60. [PubMed] [Google Scholar]
- [47].El-Akabawy G, Medina LM, Jeffries A, Price J, Modo M, Purmorphamine increases DARPP-32 differentiation in human striatal neural stem cells through the Hedgehog pathway, Stem Cells Dev, 20 (2011) 1873–1887. [DOI] [PubMed] [Google Scholar]
- [48].Faul M, Xu L, Wald MM, Coronado VG, Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002-2006, in, Centres for Disease Control and Prevention, National Centre for Injury Prevenetion and Control, Atlanta, GA, 2010. [Google Scholar]
- [49].Faust A, Kandakatla A, van der Merwe Y, Ren T, Huleihel L, Hussey G, Naranjo JD, Johnson S, Badylak S, Steketee M, Urinary bladder extracellular matrix hydrogels and matrix-bound vesicles differentially regulate central nervous system neuron viability and axon growth and branching, J Biomater Appl, 31 (2017) 1277–1295. [DOI] [PubMed] [Google Scholar]
- [50].Fawcett JW, The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease, Prog Brain Res, 218 (2015) 213–226. [DOI] [PubMed] [Google Scholar]
- [51].Ferretti P, Neural stem cell plasticity: recruitment of endogenous populations for regeneration, Curr Neurovasc Res, 1 (2004) 215–229. [DOI] [PubMed] [Google Scholar]
- [52].Ferretti P, Is there a relationship between adult neurogenesis and neuron generation following injury across evolution?, Eur J Neurosci, 34 (2011) 951–962. [DOI] [PubMed] [Google Scholar]
- [53].Fishman JM, Lowdell MW, Urbani L, Ansari T, Burns AJ, Turmaine M, North J, Sibbons P, Seifalian AM, Wood KJ, Birchall MA, De Coppi P, Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model, Proc Natl Acad Sci U S A, 110 (2013) 14360–14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Forbes SJ, Rosenthal N, Preparing the ground for tissue regeneration: from mechanism to therapy, Nat Med, 20 (2014) 857–869. [DOI] [PubMed] [Google Scholar]
- [55].Fry EJ, Central nervous system regeneration: mission impossible?, Clin Exp Pharmacol Physiol, 28 (2001) 253–258. [DOI] [PubMed] [Google Scholar]
- [56].Gentile NE, Stearns KM, Brown EH, Rubin JP, Boninger ML, Dearth CL, Ambrosio F, Badylak SF, Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss, Am J Phys Med Rehabil, 93 (2014) S79–87. [DOI] [PubMed] [Google Scholar]
- [57].Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA, Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures, Biophys J, 90 (2006) 3012–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ghuman H, Gerwig M, Nicholls FJ, Liu JR, Donnelly J, Badylak SF, Modo M, Long-term retention of ECM hydrogel after implantation into a sub-acute stroke cavity reduces lesion volume, Acta Biomater, 63 (2017) 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ghuman H, Massensini AR, Donnelly J, Kim SM, Medberry CJ, Badylak SF, Modo M, ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate, Biomaterials, 91 (2016) 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ghuman H, Mauney C, Donnelly J, Massensini AR, Badylak SF, Modo M, Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke, Acta Biomater, 80 (2018) 66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gilbert TW, Stewart-Akers AM, Badylak SF, A quantitative method for evaluating the degradation of biologic scaffold materials, Biomaterials, 28 (2007) 147–150. [DOI] [PubMed] [Google Scholar]
- [62].Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B, Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers, J Cell Biol, 172 (2006) 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Green MA, Bilston LE, Sinkus R, In vivo brain viscoelastic properties measured by magnetic resonance elastography, NMR Biomed, 21 (2008) 755–764. [DOI] [PubMed] [Google Scholar]
- [64].Guertler CA, Okamoto RJ, Schmidt JL, Badachhape AA, Johnson CL, Bayly PV, Mechanical properties of porcine brain tissue in vivo and ex vivo estimated by MR elastography, J Biomech, 69 (2018) 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gurtner GC, Werner S, Barrandon Y, Longaker MT, Wound repair and regeneration, Nature, 453 (2008) 314–321. [DOI] [PubMed] [Google Scholar]
- [66].Hachinski V, Donnan GA, Gorelick PB, Hacke W, Cramer SC, Kaste M, Fisher M, Brainin M, Buchan AM, Lo EH, Skolnick BE, Furie KL, Hankey GJ, Kivipelto M, Morris J, Rothwell PM, Sacco RL, Smith SC Jr., Wang Y, Bryer A, Ford GA, Iadecola C, Martins SC, Saver J, Skvortsova V, Bayley M, Bednar MM, Duncan P, Enney L, Finklestein S, Jones TA, Kalra L, Kleim J, Nitkin R, Teasell R, Weiller C, Desai B, Goldberg MP, Heiss WD, Saarelma O, Schwamm LH, Shinohara Y, Trivedi B, Wahlgren N, Wong LK, Hakim A, Norrving B, Prudhomme S, Bornstein NM, Davis SM, Goldstein LB, Leys D, Tuomilehto J, Stroke: working toward a prioritized world agenda, Stroke; a journal of cerebral circulation, 41 (2010) 1084–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hakkinen L, Larjava H, Koivisto L, Granulation tissue formation and remodeling., Endodontic Topics, 24 (2011) 94–129. [Google Scholar]
- [68].Heiss WD, Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions, Cerebrovasc Dis, 41 (2016) 1–7. [DOI] [PubMed] [Google Scholar]
- [69].Ho MT, Teal CJ, Shoichet MS, A hyaluronan/methylcellulose-based hydrogel for local cell and biomolecule delivery to the central nervous system, Brain Res Bull, 148 (2019) 46–54. [DOI] [PubMed] [Google Scholar]
- [70].Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC, Kim BG, An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling, Nat Commun, 8 (2017) 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hopkins A, De Laporte L, Tortelli F, Spedden E, Staii C, Atherton TJ, Hubbell JA, Kaplan DL, Silk hydrogels as soft substrates for neural tissue engineering., Advanced Functional Materials, 23 (2013) 5140–5149. [Google Scholar]
- [72].Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME, Pineda Molina C, LoPresti ST, Brown BN, Naranjo JD, Badylak SF, Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype, Tissue engineering. Part A, 23 (2017) 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, Stolz DB, Badylak SF, Matrix-bound nanovesicles within ECM bioscaffolds, Sci Adv, 2 (2016) e1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hussey GS, Cramer MC, Badylak SF, Extracellular Matrix Bioscaffolds for Building Gastrointestinal Tissue, Cell Mol Gastroenterol Hepatol, 5 (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hussey GS, Dziki JL, Badylak SF, Extracellular matrix-based materials for regeneative medicine., Nature Reviews Materials, 3 (2018) 159–173. [Google Scholar]
- [76].Illis LS, Central nervous system regeneration does not occur, Spinal Cord, 50 (2012) 259–263. [DOI] [PubMed] [Google Scholar]
- [77].Irons HR, Cullen DK, Shapiro NP, Lambert NA, Lee RH, Laplaca MC, Three-dimensional neural constructs: a novel platform for neurophysiological investigation, J Neural Eng, 5 (2008) 333–341. [DOI] [PubMed] [Google Scholar]
- [78].Jeon M, Kim SY, Application of a paste-type acellular dermal matrix for coverage of chronic ulcerative wounds, Arch Plast Surg, 45 (2018) 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jeon SB, Kwon SU, Park JC, Lee DH, Yun SC, Kim YJ, Ahn JS, Kwun BD, Kang DW, Choi HA, Lee K, Kim JS, Reduction of Midline Shift Following Decompressive Hemicraniectomy for Malignant Middle Cerebral Artery Infarction, J Stroke, 18 (2016) 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jin T, Nicholls FJ, Crum WR, Ghuman H, Badylak SF, Modo M, Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke, Biomaterials, 113 (2017) 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Johnson CL, Telzer EH, Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain, Dev Cogn Neurosci, 33 (2018) 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kauhausen JA, Thompson LH, Parish CL, Chondroitinase improves midbrain pathway reconstruction by transplanted dopamine progenitors in Parkinsonian mice, Mol Cell Neurosci, 69 (2015) 22–29. [DOI] [PubMed] [Google Scholar]
- [83].Kazanis I, Gorenkova N, Zhao JW, Franklin RJ, Modo M, Ffrench-Constant C, The late response of rat subependymal zone stem and progenitor cells to stroke is restricted to directly affected areas of their niche, Experimental neurology, 248 (2013) 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Keane TJ, Badylak SF, The host response to allogeneic and xenogeneic biological scaffold materials, J Tissue Eng Regen Med, 9 (2015) 504–511. [DOI] [PubMed] [Google Scholar]
- [85].Keane TJ, Londono R, Turner NJ, Badylak SF, Consequences of ineffective decellularization of biologic scaffolds on the host response, Biomaterials, 33 (2012) 1771–1781. [DOI] [PubMed] [Google Scholar]
- [86].Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisen J, Human Adult Neurogenesis: Evidence and Remaining Questions, Cell Stem Cell, 23 (2018) 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Khalil HH, Kalkat M, Malahias MN, Rhobaye S, Ashour T, Djearaman MG, Naidu B, Chest Wall Reconstruction with Porcine Acellular Dermal Matrix (Strattice) and Autologous Tissue Transfer for High Risk Patients with Chest Wall Tumors, Plast Reconstr Surg Glob Open, 6 (2018) e1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ko HC, Milthorpe BK, McFarland CD, Engineering thick tissues--the vascularisation problem, Eur Cell Mater, 14 (2007) 1–18; discussion 18-19. [DOI] [PubMed] [Google Scholar]
- [89].Koci Z, Vyborny K, Dubisova J, Vackova I, Jager A, Lunov O, Jirakova K, Kubinova S, Extracellular Matrix Hydrogel Derived from Human Umbilical Cord as a Scaffold for Neural Tissue Repair and Its Comparison with Extracellular Matrix from Porcine Tissues, Tissue Eng Part C Methods, 23 (2017) 333–345. [DOI] [PubMed] [Google Scholar]
- [90].Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M, Klepetko W, Wolner E, Ankersmit HJ, Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery, Eur J Clin Invest, 35 (2005) 17–23. [DOI] [PubMed] [Google Scholar]
- [91].Lakshminarayan K, Berger AK, Fuller CC, Jacobs DR Jr., Anderson DC, Steffen LM, Sillah A, Luepker RV, Trends in 10-year survival of patients with stroke hospitalized between 1980 and 2000: the Minnesota stroke survey, Stroke, 45 (2014) 2575–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lam J, Truong NF, Segura T, Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds, Acta Biomater, 10 (2014) 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lampe KJ, Heilshorn SC, Building stem cell niches from the molecule up through engineered peptide materials, Neurosci Lett, 519 (2012) 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].LaValley DJ, Reinhart-King CA, Matrix stiffening in the formation of blood vessels., Advances in Regenerative Biology, 1 (2014) 25247. [Google Scholar]
- [95].Liang Y, Bar-Shir A, Song X, Gilad AA, Walczak P, Bulte JW, Label-free imaging of gelatin-containing hydrogel scaffolds, Biomaterials, 42 (2015) 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lind G, Linsmeier CE, Schouenborg J, The density difference between tissue and neural probes is a key factor for glial scarring, Sci Rep, 3 (2013) 2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lindvall O, Kokaia Z, Neurogenesis following Stroke Affecting the Adult Brain, Cold Spring Harb Perspect Biol, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].LoPresti ST, Brown BN, Effect of Source Animal Age upon Macrophage Response to Extracellular Matrix Biomaterials, J Immunol Regen Med, 1 (2018) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Macher BA, Galili U, The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance, Biochim Biophys Acta, 1780 (2008) 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, Burgdorf S, Engelholm LH, Behrendt N, Holmbeck K, Weigert R, Bugge TH, M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway, J Cell Biol, 202 (2013) 951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Massensini AR, Ghuman H, Saldin LT, Medberry CJ, Keane TJ, Nicholls FJ, Velankar SS, Badylak SF, Modo M, Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity, Acta Biomater, 27 (2015) 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].McPherson TB, Liang H, Record RD, Badylak SF, Galalpha(1,3)Gal epitope in porcine small intestinal submucosa, Tissue Eng, 6 (2000) 233–239. [DOI] [PubMed] [Google Scholar]
- [103].Medberry CJ, Crapo PM, Siu BF, Carruthers CA, Wolf MT, Nagarkar SP, Agrawal V, Jones KE, Kelly J, Johnson SA, Velankar SS, Watkins SC, Modo M, Badylak SF, Hydrogels derived from central nervous system extracellular matrix, Biomaterials, 34 (2013) 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Medelin M, Porrelli D, Aurand ER, Scaini D, Travan A, Borgogna MA, Cok M, Donati I, Marsich E, Scopa C, Scardigli R, Paoletti S, Ballerini L, Exploiting natural polysaccharides to enhance in vitro bio-constructs of primary neurons and progenitor cells, Acta Biomater, 73 (2018) 285–301. [DOI] [PubMed] [Google Scholar]
- [105].Meng F, Modo M, Badylak SF, Biologic scaffold for CNS repair, Regen Med, 9 (2014) 367–383. [DOI] [PubMed] [Google Scholar]
- [106].Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H, Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion, Journal of neuroscience methods, 104 (2000) 99–109. [DOI] [PubMed] [Google Scholar]
- [107].Modo MM, Jolkkonen J, Zille M, Boltze J, Future of Animal Modeling for Poststroke Tissue Repair, Stroke, 49 (2018) 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Moeendarbary E, Weber IP, Sheridan GK, Koser DE, Soleman S, Haenzi B, Bradbury EJ, Fawcett J, Franze K, The soft mechanical signature of glial scars in the central nervous system, Nat Commun, 8 (2017) 14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Moon JJ, West JL, Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials, Curr Top Med Chem, 8 (2008) 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Moreau F, Patel S, Lauzon ML, McCreary CR, Goyal M, Frayne R, Demchuk AM, Coutts SB, Smith DE, Cavitation after acute symptomatic lacunar stroke depends on time, location, and MRI sequence, Stroke, 43 (2012) 1837–1842. [DOI] [PubMed] [Google Scholar]
- [111].Murphy MC, Huston J 3rd, Ehman RL, MR elastography of the brain and its application in neurological diseases, Neuroimage, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Nafisi N, Akbari ME, Mahjoub F, Mohseni MJ, Sabetkish S, Khorramirouz R, Tehrani M, Kajbafzadeh AM, Application of Human Acellular Breast Dermal Matrix (ABDM) in Implant-Based Breast Reconstruction: An Experimental Study, Aesthetic Plast Surg, 41 (2017) 1435–1444. [DOI] [PubMed] [Google Scholar]
- [113].Naumova AV, Modo M, Moore A, Murry CE, Frank JA, Clinical imaging in regenerative medicine, Nat Biotechnol, 32 (2014) 804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nicholls FJ, Ling W, Ferrauto G, Aime S, Modo M, Simultaneous MR imaging for tissue engineering in a rat model of stroke, Sci Rep, 5 (2015) 14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nih LR, Gojgini S, Carmichael ST, Segura T, Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke, Nat Mater, 17 (2018) 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW, 1,026 experimental treatments in acute stroke, Ann Neurol, 59 (2006) 467–477. [DOI] [PubMed] [Google Scholar]
- [117].Oliveira JM, Carvalho L, Silva-Correia J, Vieira S, Majchrzak M, Lukomska B, Stanaszek L, Strymecka P, Malysz-Cymborska I, Golubczyk D, Kalkowski L, Reis RL, Janowski M, Walczak P, Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: clinical needs, biomaterials, and imaging technologies, NPJ Regen Med, 3 (2018) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Osama I, Gorenkova N, McKittrick CM, Wongpinyochit T, Goudie A, Seib FP, Carswell HVO, In vitro studies on space-conforming self-assembling silk hydrogels as a mesenchymal stem cell-support matrix suitable for minimally invasive brain application, Sci Rep, 8 (2018) 13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Othman SF, Curtis ET, Plautz SA, Pannier AK, Butler SD, Xu H, MR elastography monitoring of tissue-engineered constructs, NMR Biomed, 25 (2012) 452–463. [DOI] [PubMed] [Google Scholar]
- [120].Park KI, Teng YD, Snyder EY, The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue, Nat Biotechnol, 20 (2002) 1111–1117. [DOI] [PubMed] [Google Scholar]
- [121].Pawar K, Prang P, Muller R, Caioni M, Bogdahn U, Kunz W, Weidner N, Intrinsic and extrinsic determinants of central nervous system axon outgrowth into alginate-based anisotropic hydrogels, Acta Biomater, 27 (2015) 131–139. [DOI] [PubMed] [Google Scholar]
- [122].Pearlman AM, Mujumdar V, McAbee KE, Terlecki RP, Outcomes of adult urethroplasty with commercially available acellular matrix, Ther Adv Urol, 10 (2018) 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Prakash R, Carmichael ST, Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury, Current opinion in neurology, 28 (2015) 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Prokoph S, Chavakis E, Levental KR, Zieris A, Freudenberg U, Dimmeler S, Werner C, Sustained delivery of SDF-1alpha from heparin-based hydrogels to attract circulating pro-angiogenic cells, Biomaterials, 33 (2012) 4792–4800. [DOI] [PubMed] [Google Scholar]
- [125].Rando TA, Ambrosio F, Regenerative Rehabilitation: Applied Biophysics Meets Stem Cell Therapeutics, Cell Stem Cell, 22 (2018) 608. [DOI] [PubMed] [Google Scholar]
- [126].Rao JS, Zhao C, Zhang A, Duan H, Hao P, Wei RH, Shang J, Zhao W, Liu Z, Yu J, Fan KS, Tian Z, He Q, Song W, Yang Z, Sun YE, Li X, NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury, Proc Natl Acad Sci U S A, 115 (2018) E5595–E5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, Badylak SF, In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair, Biomaterials, 22 (2001) 2653–2659. [DOI] [PubMed] [Google Scholar]
- [128].Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF, Degradation products of extracellular matrix affect cell migration and proliferation, Tissue engineering. Part A, 15 (2009) 605–614. [DOI] [PubMed] [Google Scholar]
- [129].Rimondini L, Fini M, Giardino R, The microbial infection of biomaterials: A challenge for clinicians and researchers. A short review, J Appl Biomater Biomech, 3 (2005) 1–10. [PubMed] [Google Scholar]
- [130].Rincon F, Mayer SA, The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008, Neurocrit Care, 19 (2013) 95–102. [DOI] [PubMed] [Google Scholar]
- [131].Rossetti T, Nicholls F, Modo M, Intra-cerebral cell implantation: Preparation and characterization of cell suspensions, Cell Transplant, (2015). [DOI] [PubMed] [Google Scholar]
- [132].Santucci RA, Barber TD, Resorbable extracellular matrix grafts in urologic reconstruction, Int Braz J Urol, 31 (2005) 192–203. [DOI] [PubMed] [Google Scholar]
- [133].Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EH, Dziki JL, Fisher LE, Brown S, Badylak SF, An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss, Sci Transl Med, 6 (2014) 234ra258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Skardal A, Mack D, Atala A, Soker S, Substrate elasticity controls cell proliferation, surface marker expression and motile phenotype in amniotic fluid-derived stem cells, J Mech Behav Biomed Mater, 17 (2013) 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Slivka PF, Dearth CL, Keane TJ, Meng FW, Medberry CJ, Riggio RT, Reing JE, Badylak SF, Fractionation of an ECM hydrogel into structural and soluble components reveals distinctive roles in regulating macrophage behavior, Biomater Sci, 2 (2014) 1521–1534. [DOI] [PubMed] [Google Scholar]
- [136].Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, Stevanato L, Sinden JD, Modo M, Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke, Stem Cells, 30 (2012) 785–796. [DOI] [PubMed] [Google Scholar]
- [137].Sofroniew MV, Astrogliosis, Cold Spring Harb Perspect Biol, 7 (2014) a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sofroniew MV, Vinters HV, Astrocytes: biology and pathology, Acta Neuropathol, 119 (2010) 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Stabenfeldt SE, Garcia AJ, LaPlaca MC, Thermoreversible laminin-functionalized hydrogel for neural tissue engineering, J Biomed Mater Res A, 77 (2006) 718–725. [DOI] [PubMed] [Google Scholar]
- [140].Stabenfeldt SE, Munglani G, Garcia AJ, LaPlaca MC, Biomimetic microenvironment modulates neural stem cell survival, migration, and differentiation, Tissue engineering. Part A, 16 (2010) 3747–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Steiner LA, Andrews PJ, Monitoring the injured brain: ICP and CBF, Br J Anaesth, 97 (2006) 26–38. [DOI] [PubMed] [Google Scholar]
- [142].Stiles J, Jernigan TL, The basics of brain development, Neuropsychol Rev, 20 (2010) 327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Stover NP, Bakay RA, Subramanian T, Raiser CD, Cornfeldt ML, Schweikert AW, Allen RC, Watts RL, Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease, Arch Neurol, 62 (2005) 1833–1837. [DOI] [PubMed] [Google Scholar]
- [144].Tanaka EM, Ferretti P, Considering the evolution of regeneration in the central nervous system, Nat Rev Neurosci, 10 (2009) 713–723. [DOI] [PubMed] [Google Scholar]
- [145].Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC, Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain, J Tissue Eng Regen Med, 3 (2009) 208–217. [DOI] [PubMed] [Google Scholar]
- [146].Tate MC, Shear DA, Hoffman SW, Stein DG, Archer DR, LaPlaca MC, Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain, Cell Transplant, 11 (2002) 283–295. [PubMed] [Google Scholar]
- [147].Tchanque-Fossuo CN, Dahle SE, Lev-Tov H, Li CS, Isseroff RR, Cellular versus acellular grafts for diabetic foot ulcers: altering the protocol to improve recruitment to a comparative efficacy trial, Cutis, 100 (2017) E18–E21. [PubMed] [Google Scholar]
- [148].Thompson R, Sakiyama-Elbert S, Using biomaterials to promote pro-regenerative glial phenotypes after nervous system injuries, Biomed Mater, 13 (2018) 024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Torquato S, Truskett TM, Debenedetti PG, Is random close packing of spheres well defined?, Phys Rev Lett, 84 (2000) 2064–2067. [DOI] [PubMed] [Google Scholar]
- [150].Tottey S, Johnson SA, Crapo PM, Reing JE, Zhang L, Jiang H, Medberry CJ, Reines B, Badylak SF, The effect of source animal age upon extracellular matrix scaffold properties, Biomaterials, 32 (2011) 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Tukmachev D, Forostyak S, Koci Z, Zaviskova K, Vackova I, Vyborny K, Sandvig I, Sandvig A, Medberry CJ, Badylak SF, Sykova E, Kubinova S, Injectable Extracellular Matrix Hydrogels as Scaffolds for Spinal Cord Injury Repair, Tissue engineering. Part A, 22 (2016) 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ungerleider JL, Johnson TD, Hernandez MJ, Elhag DI, Braden RL, Dzieciatkowska M, Osborn KG, Hansen KC, Mahmud E, Christman KL, Extracellular Matrix Hydrogel Promotes Tissue Remodeling, Arteriogenesis, and Perfusion in a Rat Hindlimb Ischemia Model, JACC Basic Transl Sci, 1 (2016) 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF, Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds, Tissue engineering. Part A, 15 (2009) 1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Vasconcelos DP, Aguas AP, Barbosa MA, Pelegrin P, Barbosa JN, The inflammasome in host response to biomaterials: Bridging inflammation and tissue regeneration, Acta Biomater, 83 (2019) 1–12. [DOI] [PubMed] [Google Scholar]
- [155].Vukasinovic J, Cullen DK, LaPlaca MC, Glezer A, A microperfused incubator for tissue mimetic 3D cultures, Biomed Microdevices, 11 (2009) 1155–1165. [DOI] [PubMed] [Google Scholar]
- [156].Wahlberg B, Ghuman H, Liu JR, Modo M, Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery, Sci Rep, 8 (2018) 9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Walczak P, Wojtkiewicz J, Nowakowski A, Habich A, Holak P, Xu J, Adamiak Z, Chehade M, Pearl MS, Gailloud P, Lukomska B, Maksymowicz W, Bulte JW, Janowski M, Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system, J Cereb Blood Flow Metab, 37 (2017) 2346–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Wang JY, Liou AK, Ren ZH, Zhang L, Brown BN, Cui XT, Badylak SF, Cai YN, Guan YQ, Leak RK, Chen J, Ji X, Chen L, Neurorestorative effect of urinary bladder matrix-mediated neural stem cell transplantation following traumatic brain injury in rats, CNS Neurol Disord Drug Targets, 12 (2013) 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wheatley DN, Diffusion, perfusion and the exclusion principles in the structural and functional organization of the living cell: reappraisal of the properties of the ‘ground substance’, J Exp Biol, 206 (2003) 1955–1961. [DOI] [PubMed] [Google Scholar]
- [160].Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF, Naturally derived and synthetic scaffolds for skeletal muscle reconstruction, Adv Drug Deliv Rev, 84 (2015) 208–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wong ML, Griffiths LG, Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization, Acta Biomater, 10 (2014) 1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wu Y, Wang J, Shi Y, Pu H, Leak RK, Liou AK, Badylak SF, Liu Z, Zhang J, Chen J, Chen L, Implantation of Brain-derived Extracellular Matrix Enhances Neurological Recovery after Traumatic Brain Injury, Cell Transplant, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wynn TA, Vannella KM, Macrophages in Tissue Repair, Regeneration, and Fibrosis, Immunity, 44 (2016) 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Youngblood RL, Truong NF, Segura T, Shea LD, It’s All in the Delivery: Designing Hydrogels for Cell and Non-viral Gene Therapies, Mol Ther, 26 (2018) 2087–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yu T, Wang W, Nassiri S, Kwan T, Dang C, Liu W, Spiller KL, Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels, J Biomater Sci Polym Ed, 27 (2016) 721–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zaari N, Rajagopalan P, Kim SK, Engler AJ, Wong JY, Photopolymerization in microfluidic gradient generators: Microscale control of substrate compliance to manipulate cell response, Adv Mater, 16 (2004) 2133–+. [Google Scholar]
- [167].Zbesko JC, Nguyen TV, Yang T, Frye JB, Hussain O, Hayes M, Chung A, Day WA, Stepanovic K, Krumberger M, Mona J, Longo FM, Doyle KP, Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts, Neurobiol Dis, 112 (2018) 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zhang L, Zhang F, Weng Z, Brown BN, Yan H, Ma XM, Vosler PS, Badylak SF, Dixon CE, Cui XT, Chen J, Effect of an inductive hydrogel composed of urinary bladder matrix upon functional recovery following traumatic brain injury, Tissue engineering. Part A, 19 (2013) 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Zhang Z, Yao S, Xie S, Wang X, Chang F, Luo J, Wang J, Fu J, Effect of hierarchically aligned fibrin hydrogel in regeneration of spinal cord injury demonstrated by tractography: A pilot study, Sci Rep, 7 (2017) 40017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zhao JY, Bass KD, Skeletal muscle regeneration by extracellular matrix biological scaffold: a case report, J Wound Care, 27 (2018) S11–S14. [DOI] [PubMed] [Google Scholar]
- [171].Zuidema JM, Gilbert RJ, Gottipati MK, Biomaterial Approaches to Modulate Reactive Astroglial Response, Cells Tissues Organs, (2018) 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


