Abstract
Objective:
To investigate the separate and combined associations of obesity and metabolic syndrome (MetS) with depression and the role of inflammation.
Methods:
Depression was assessed with the Patient Health Questionnaire-9 (PHQ-9) and was defined with a cut-point of ≥ 10. Obesity was defined as BMI ≥ 30 kg/m2 from measured height and weight. MetS was defined based on the American Heart Association consensus definition. Participants were divided into four groups: healthy normal weight (MHN), metabolically healthy obese (MHO), metabolically unhealthy normal weight (MUN), and metabolic unhealthy obese (MUO). C-reactive protein was assessed in a subsample.
Results:
A total of 18,025 subjects were included in the analysis. Participants with MUO had the highest prevalence of depression compared to the MHN group (14.8% vs 6.8, P<0.001). While both obesity and MetS were independently associated with depression, there was a significant interaction between the two (P<0.001), indicating that the associations of obesity and metabolic syndrome with depression were synergistic. After adjusting for baseline characteristics, compared to the MHN group, the MUO group had the highest odds of depression (OR 2.30, 95%CI 2.03–2.61), followed by MHO group (OR 1.51, 95% CI 1.30–1.74) and the MUN group (OR 1.39, 95% CI 1.18–1.64).. The MUO group also showed the highest level of C-reactive protein, and the latter partially mediated the effect between MUO and depressive symptoms (20.5% of the total effect).
Conclusion:
Both obesity and MetS are associated with depression independent of each other, but when present together, these conditions have a synergistic association with depression.
Keywords: Depression, Metabolic syndrome, Obesity, Inflammation
Introduction:
Depression is among the highest global public health priorities.(H. Whiteford, Ferrari, & Degenhardt, 2016) The worldwide prevalence of depression is estimated to be 350 million, and an estimated 8% of Americans 20 years of age and older have depression in a given 2-week period.(Brody, Pratt, & Hughes, 2018; Ferrari et al., 2013) Furthermore, depression contributes significantly to the global burden of non-communicable diseases, as the comorbidity of depression with chronic conditions is increasingly being recognized.(H. A. Whiteford et al., 2013)
Both obesity and the metabolic syndrome (MetS) are growing public health problems. The prevalence of both conditions has increased in the United States over the past 30 years, and globally it has more than doubled between 1980 and 2014.(Liu et al., 2007; Martinez-Larrad et al., 2016; Williams, Mesidor, Winters, Dubbert, & Wyatt, 2015) MetS is defined as a combination of abdominal obesity and metabolic abnormalities, including elevated glucose, elevated blood pressure, and altered lipid metabolism, and is a major risk factor for cardiovascular disease.(Lakka et al., 2002; McNeill et al., 2005) However, although obesity and metabolic disturbances are related, they do not always occur together. There has been an increasing interest in discriminating between obesity and metabolic abnormalities related to MetS, since the risk of cardiovascular disease associated with metabolic disturbances varies among individuals with similar body mass index (BMI).(Ferrannini et al., 1997) This has led to the concept of metabolically healthy obesity (MHO), which is defined as obesity in the absence of metabolic syndrome.(Primeau et al., 2011) Several studies have shown that people with MHO have a risk of cardiovascular disease and mortality that is intermediate between those with neither obesity nor metabolic disturbances (metabolically healthy normal weight, or MHN) and those with obesity and metabolic disturbances (metabolically unhealthy obesity (MUO).(Caleyachetty et al., 2017; Eckel, Meidtner, Kalle-Uhlmann, Stefan, & Schulze, 2016; Fan, Song, Chen, Hui, & Zhang, 2013; Kramer, Zinman, & Retnakaran, 2013; Mongraw-Chaffin et al., 2018)
A growing body of evidence has linked obesity measures and other MetS risk factors with depression.(Chang et al., 2013; Gibson-Smith et al., 2018; Hidese, Asano, Saito, Sasayama, & Kunugi, 2018; Vaccarino et al., 2008; Vancampfort et al., 2014) However, whether the association with depression varies across different obesity and metabolic phenotypes is unknown. Furthermore, both obesity and MetS are associated with a chronic, subacute systemic inflammatory state, as shown by elevations in circulating levels of pro-inflammatory cytokines and C-reactive protein (CRP).(Brooks, Blaha, & Blumenthal, 2010) An association between inflammatory markers and depression has also been reported in several studies.(Danner, Kasl, Abramson, & Vaccarino, 2003; Dowlati et al., 2010; Howren, Lamkin, & Suls, 2009; Vaccarino et al., 2007) Based on these findings, inflammation has been proposed as the underlying link between depression and both obesity and MetS.(Capuron et al., 2008) However, few studies have distinguished between obesity and metabolic status when examining these associations.
Using a large nationally representative sample of United States adults, we sought to investigate the separate and combined associations of obesity and MetS with depression. We hypothesized that obesity and MetS would be both associated with depression and inflammation independent of each other, but their joint presence (the MUO group) would have a synergistic effect and be associated with the highest levels of both depression and inflammation. We also hypothesized that elevated inflammation mediates the association between metabolic derangements and depression.
Subjects and Methods
Data source and study sample
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional population-based evaluation of the US non-institutionalized civilian population conducted by the National Center for Health Statistics. For the present study, all adults aged 18 and older were included from the NHANES cycles 2009–2010, 2011–2012, and 2013–2014. Data regarding sociodemographic characteristics, health behaviors, medical conditions, and depressive symptoms were collected through self-administered questionnaires. Standardized measurements of anthropometric data and other cardiovascular risk factors were obtained through an in-person evaluation. All participants provided written informed consent and the protocol was approved by the National Center for Health Statistics Ethics Review Board. Since the NHANES data were anonymized and publically available, no institutional IRB approval was required for the present study.
Measures
The Patient Health Questionnaire (PHQ-9) was used to assess depression and suicidal ideation (Kroenke, Spitzer, & Williams, 2001). This 9-item instrument assesses the frequency of depressive symptoms over the past 2 weeks and has shown high reliability and validity in the general population.(Martin, Rief, Klaiberg, & Braehler, 2006) We used a cut-off of ≥ 10 to identify significant depressive symptoms. This cut-off has shown a sensitivity and specificity of 88% for major depressive disorder, (Kroenke, Spitzer, Williams, & Lowe, 2010) and has been commonly used in research studies. (Kroenke et al., 2010; Moriarty, Gilbody, McMillan, & Manea, 2015)
Participants underwent a physical exam to ascertain height, body weight and waist circumference. Obesity was defined as BMI ≥30 kg/m2. Blood pressure was measured following 5 minutes of quiet rest, and the average of 3 consecutive blood pressure readings was calculated.
Fasting plasma glucose was assayed by a hexokinase method. Triglyceride and high-density lipoprotein (HDL) levels were assessed enzymatically using a two-reagent, endpoint reaction that is specific for triglycerides. CRP levels were only available for the 2009–2010 cycle and were measured via a high sensitivity assay with latex-enhanced nephelometry. Five participants who had a CRP level higher than 10 mg/dl were excluded as these values may be indicative of active infection.
We classified MetS based on the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) definition.(Grundy et al., 2005) Participants were considered to have MetS if at least three of the following five cardiovascular risk factors were present: (1) impaired fasting glucose ≥ 100 milligrams per deciliter (mg/dL) or pharmacological treatment for diabetes; (2) low HDL cholesterol (<40 mg/dL in men or <50 mg/dL in women) or pharmacological treatment for an abnormal HDL cholesterol level; (3) triglycerides ≥ 150 mg/dL or pharmacological treatment for hypertriglyceridemia; (4) a waist circumference ≥ 102 cm in men or ≥ 88 cm in women; and (5) blood pressure ≥ 130/85 mm Hg or pharmacological treatment for hypertension. Participants were classified into 4 groups, based on their obesity and metabolic status consistent with previous literature,(Mongraw-Chaffin et al., 2018) as shown in Table 1.
Table 1.
Baseline characteristics for the NHANES participants by Obesity and MetS Status
| MHN BMI < 30 kg/m2, without MetS (N= 9,386) | MUN BMI < 30 kg/m2, with MetS (N= 2,294) | MHO BMI ≥ 30 kg/m2, without MetS (N=2,951) | MUO BMI ≥ 30 kg/m2, with MetS (N=3,394) | P value | |
|---|---|---|---|---|---|
| Mean age ± SD (years) | |||||
| Male sex, N (%) | 44 ± 18 | 62 ± 14 | 42 ± 15 | 55 ± 15 | <0.001 |
| Race, N (%) | 4,832 (51.5) | 1,154 (50.3) | 1,230 (41.7) | 1,531 (45.1) | <0.001 |
| White | <0.001 | ||||
| African-American | 3,994 (42.6) | 1,053 (45.9) | 1,083 (36.7) | 1,490 (43.9) | |
| Hispanic | 1,754 (18.7) | 394 (17.2) | 873 (29.6) | 873 (25.7) | |
| Other | 2,113 (22.5) | 532 (23.2) | 802 (27.2) | 874 (25.8) | |
| Education, N (%) | 1,525 (16.2) | 315 (13.7) | 193 (6.5) | 157 (4.6) | |
| Less than high school | <0.001 | ||||
| High school graduate | 2,001 (22.2) | 729 (31.9) | 667 (23.2) | 957 (28.4) | |
| Some college | 1,881 (20.9) | 534 (23.4) | 668 (23.3) | 815 (24.2) | |
| College graduate | 2,553 (28.4) | 612 (26.8) | 947 (33.0) | 1,057 (31.4) | |
| Marital status, N (%) | 2,565 (28.5) | 410 (17.9) | 588 (20.5) | 536 (15.9) | |
| Single | <0.001 | ||||
| Married | 2,094 (24.8) | 172 (9.0) | 692 (25.3) | 441 (14.8) | |
| Divorced/separated | 5,199 (61.7) | 1,381 (72.0) | 1,634 (59.7) | 1,960 (65.6) | |
| History of current smoking, N (%) | 1,135 (13.5) | 366 (19.1) | 409 (15.0) | 587 (19.6) | |
| Number of people in household, mean ± SD | 1,433 (15.2) | 416 (18.1) | 414 (14.0) | 603 (17.7) | <0.001 |
| Household income, N (%) | 3 ± 1 | 4 ± 1 | 3 ± 1 | 3 ± 1 | 0.82 |
| < $35,000 | 0.002 | ||||
| $35,000–$65,000 | 3,438 (38.9) | 1005 (46.4) | 1207 (42.5) | 1522 (46.8) | |
| > $65,000 | 1953 (22.1) | 514 (23.8) | 655 (23.1) | 764 (23.5) | |
| Physical activity, N (%) | 3,447 (39.0) | 645 (29.8) | 979 (34.5) | 969 (29.8) | |
| Sedentary | <0.001 | ||||
| Low | 5,835 (62.2) | 1,601 (69.8) | 1,760 (59.6) | 2,187 (64.4) | |
| Moderate | 588 (6.3) | 146 (6.4) | 163 (5.5) | 219 (6.5) | |
| Vigorous | 1,359 (14.5) | 290 (12.7) | 477 (16.2) | 465 (13.7) | |
| Average number of drinks per day, mean (SD) | 1,604 (17.1) | 257 (11.2) | 551 (18.7) | 523 (15.4) | |
| Comorbidities, N (%) | 3.6 (2.5) | 3.09 (2.7) | 2.86 (1.4) | 3.8 (1.4) | 0.84 |
| Hypertension | |||||
| Diabetes mellitus | 1816 (19.3) | 1550 (67.6) | 702 (23.8) | 2298 (67.7) | <0.001 |
| Coronary artery disease | 307 (3.3) | 667 (29.1) | 107 (3.6) | 1075 (31.7) | <0.001 |
| Congestive heart failure | 186 (2.0) | 213 (9.3) | 27 (0.9) | 256 (7.5) | <0.001 |
| Stroke | 125 (1.3) | 148 (6.5) | 40 (1.4) | 230 (6.8) | <0.001 |
| Pulmonary disease | 231 (2.5) | 183 (8.0) | 60 (2.0) | 184 (5.4) | <0.001 |
| Chronic kidney disease | 379 (4.0) | 192 (8.4) | 150 (5.1) | 322 (9.5) | <0.001 |
| Liver disease | 232 (2.5) | 146 (6.4) | 82 (2.8) | 190 (5.6) | <0.001 |
| Malignancy | 253 (2.7) | 131 (5.7) | 75 (2.5) | 183 (5.4) | <0.001 |
| Metabolic risk factors, N (%)a | 709 (7.6) | 369 (16.1) | 157 (5.3) | 420 (12.4) | <0.001 |
| Impaired fasting glucose | |||||
| Low HDL level | 1,120 (11.9) | 1,515 (66.0) | 284 (9.6) | 2,181 (64.3) | <0.001 |
| Elevated triglycerides | 1,404 (15.0) | 1,111 (48.4) | 760 (25.8) | 1,950 (57.5) | <0.001 |
| Elevated blood pressure | 1,230 (13.1) | 1,797 (78.3) | 205 (6.9) | 2,242 (66.1) | <0.001 |
| Increased waist circumference | 2,562 (27.3) | 1,937 (84.4) | 862 (29.2) | 2,778 (81.9) | <0.001 |
| BMI, Mean (SD) | 1,765 (18.8) | 1,531 (66.7) | 2,510 (85.1) | 3,246 (95.6) | <0.001 |
| Mean PHQ-9 score, (SD) | 24.3 (3.1) | 26.6 (2.4) | 35.4 (5.6) | 36.4 (6.2) | <0.001 |
| Antidepressant use, N (%) | 2.48 (0.4) | 3.08 (0.9) | 3.37 (0.9) | 4.1 (1) | <0.001 |
| Statin use, N (%) | 329 (3.5) | 108 (4.7) | 201 (6.8) | 336 (9.9) | <0.001 |
| Mean age ± SD (years) | 741 (7.9) | 858 (37.4) | 378 (4.4) | 1,635 (48.2) | <0.001 |
MHN = metabolically healthy normal weight; MHO = metabolically healthy obesity; MUN = metabolically unhealthy normal weight; MUO = metabolically unhealthy obesity; BMI= body mass index, MetS= Metabolic syndrome, PHQ = Patient Health Questionnaire, SD= Standard deviation
Impaired fasting glucose defined as fasting glucose ≥ 100 milligrams per deciliter (mg/dL), low HDL cholesterol defined as values <40 mg/dL in men or <50 mg/dL in women, Elevated triglycerides defined as values ≥ 150 mg/dL; elevated blood pressure defined as values ≥ 130/85 mm Hg: increased waist circumference defined as measurements ≥ 102 cm in men or ≥ 88 cm in women: Obesity defined as BMI ≥30.
Other study measures
Data regarding subject’s sociodemographic factors including age, sex, race/ethnicity (White, African American, Hispanic, other), education level (less than high school diploma, high school diploma, some college without degree, college degree or higher), marital status (never married, married, divorced/separated, widowed) and household income were collected through self-administered questionnaires. Medical history data were also obtained through questionnaires and included history of smoking, coronary artery disease, congestive heart disease, stroke, hypertension, diabetes mellitus, pulmonary disease (chronic bronchitis, emphysema, or asthma), kidney disease, liver disease, and cancer. Use of antidepressants and statins was also obtained through self-administered questionnaires.
Physical activity was assessed with the Global Physical Activity Questionnaire, and levels of moderate and vigorous activity calculated by multiplying the minutes of activity per day times the number of days per week of activity.(Bull, Maslin, & Armstrong, 2009; Cleland et al., 2014) To get an estimate of metabolic equivalent (MET) of activity per week, the physical activity score (per minutes per week) was then multiplied by 4 for moderate and 8 for vigorous activity).(Belcher et al., 2015) Moderate or vigorous physical activity was defined as MET-minutes per week ≥ 500, and low physical activity as MET-minutes between 1 and 500.(Garber et al., 2011) Participants who did not engage in any physical activity were considered sedentary. Alcohol intake was assessed by measuring the estimated number of alcoholic drinks per day over the past 12 months.
Statistical analysis
Differences in demographic and clinical variables between different metabolic groups were assessed using ANOVA for continuous variables and Chi-square tests for categorical variables. Depression was analyzed both as a categorical variable (PHQ-9 ≥ 10) and as a continuous variable (PHQ-9 score). CRP levels were log transformed to achieve normality and were examined as a continuous variable, and also dichotomized using a cutoff of 3 mg/dl. Prior studies have shown that a cutoff of ≥3 mg/dl signifies a higher risk of cardiovascular disease.(Pearson et al., 2003). Geometric levels of CRP were compared according to depression as a categorical variable using Student’s T tests. Logistic regression models were used to estimate the associations between depression as a categorical outcome variable and metabolic status groups with MHN as the reference, as well as log-transformed CRP levels. Linear regression analysis was also used to determine the association between the PHQ-9 score as the outcome variable and metabolic groups and log-transformed CRP levels. All models were adjusted for potential confounding factors, including demographic characteristics (age, sex, race/ethnicity, education, marital status, and household income), smoking status, physical activity, comorbid conditions that may influence both body weight and depression (coronary artery disease, heart failure, stroke, pulmonary disease, chronic kidney disease, liver disease, and malignancy) and medication use (antidepressants and statins). As hypertension and diabetes mellitus are part of the definition of MetS, we did not to adjust for these conditions. Using the Bonferroni correction, P values for pairwise comparisons between different metabolic subgroups were considered significant at an α level of 0.05/3=0.01. The interaction of obesity with MetS was also formally tested by entering the interaction term in the regression models.
Sensitivity analyses were conducted for depression as the outcome variable by stratifying patients based on sex, age (< 50 and ≥ 50 years, based on the median age of the entire sample) and race/ethnicity, adjusting for the same characteristics as above, and interaction effects were tested. ANOVA was used to compare the geometric means of CRP values between men and women for all participants as well as by metabolic subgroups. A sensitivity analysis was also performed with exclusion of patients with comorbidities including hypertension, diabetes mellitus, coronary artery disease, heart failure, stroke, pulmonary disease, chronic kidney disease, liver disease and malignancy. We also examined whether results were similar when using the harmonized definition of MetS by the International Diabetes Federation, which includes increased waist circumference plus two of the above criteria.(Alberti, Zimmet, & Shaw, 2006).
We performed a mediation analysis with bootstrapping (1000 bootstrap samples and a 95 percent confidence interval) to test the hypothesis that CRP levels mediate the relationship between metabolic status and depression using the method by Preacher and Hayes.(Preacher & Hayes, 2008). Each metabolic subgroup was separately included in the mediation analysis and compared with the MHN group. Since the mediation analysis requires a continuous variable as the outcome variable, we used the PHQ-9 score as the outcome variable for this analysis.
All analyses were conducted using Stata 14 (StataCorp., College Station, Texas). A P-value of < 0.05 was considered statistically significant.
Results
Study Sample
Of a total of 30,468participants from NHANES cycles 2009–2010, 2011–2012, and 2013–2014, 18,025 had data on the PHQ-9 and metabolic status and were included in the final analysis. The baseline characteristics were similar between participants who were included and those who were excluded from the study (Supplemental Table 1). Table 1 describes the baseline characteristics of the study population according to the four obesity/MetS groups. Metabolic unhealthy groups (MUN and MUO) tended to be older and have more comorbidities than the other two groups, while the obese groups (MHO and MUO) were more likely to be female and African American. Compared to the MUN group, subjects with MUO had higher frequency of increased waist circumference (P<0.001), and reduced HDL (P<0.001), but were less likely to have elevated blood pressure (P=0.006), and elevated triglyceride levels (P<0.001, Table 1). The frequency of impaired glucose tolerance was similar between the MUN and MUO groups (P=0.09).
Obesity-Metabolic Phenotypes and Depression
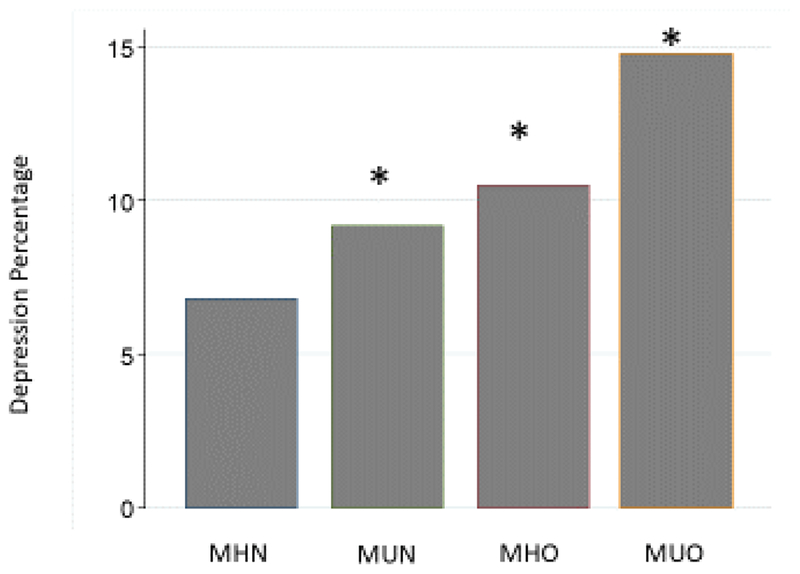
Among all participants, 1,658 (9.2%) had PHQ-9 levels ≥ 10, consistent with clinically significant depressive symptoms. As shown in Figure 1, patients in the MUO group had the highest prevalence of a PHQ-9 ≥ 10, followed by the MHO and MUN groups. Similar results were observed for the mean PHQ-9 scores, with the MUO group having the highest scores (4.16 ± 0.09), followed by the MHO group (3.37 ± 0.08), the MUN group (3.08 ± 0.09), and the MHN group (2.48 ± 0.04) (P<0.001).
Figure 1. Frequency of depression, defined as a Patient Health Questionnaire (PHQ)-9 score ≥10 in participants classified by obesity and metabolic status.
MHN: Metabolically healthy normal weight, MHO: Metabolically healthy obesity, MUN: Metabolically unhealthy normal weight, MUO: Metabolically unhealthy obesity; *: P< 0.001 compared to MHN.
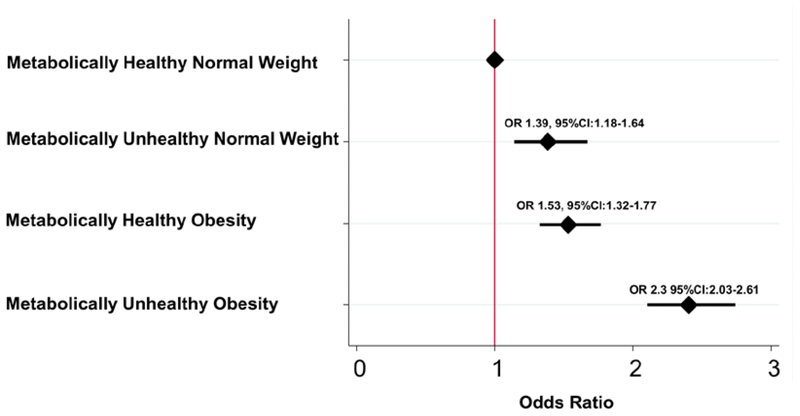
After adjusting for demographic factors, co-morbidities, and medication use, all three subgroups of obesity and/or MetS had significantly higher odds of having PHQ ≥ 9 compared to the MHN group (Figure 2). Patients in the MUO group had the highest odds ratio (OR), with more than twice the odds of having a PHQ-9 ≥10 compared to the MHN group. In addition, the MUO group had higher odds of PHQ-9 ≥10 compared to the MUN group (OR 1.04, 95% CI 1.03 – 1.05) and the MHO group (OR 1.03, 95% CI 1.02 – 1.04). While both obesity and MetS were independently associated with a PHQ-9 ≥10, there was a significant interaction between the two (P<0.001), indicating that the coexistence of obesity and MetS was associated with a higher likelihood of depression than the two conditions alone. Similarly, compared to the MHN as the reference group, PHQ-9 scores as a continuous variable were found to be independently associated with MUO (β 1.00, 95% CI 0.79 – 1.21), MHO (β 0.63, 95% CI 0.44 – 0.82), and MUN (β 0.25, 95% CI 0.12 – 0.39) groups.
Figure 2. Association of depression with obesity with and without metabolic syndrome.
All models are adjusted for demographic characteristics (age, sex, race/ethnicity, education, marital status, smoking history, physical activity, and household income), co-morbidities (coronary artery disease, congestive heart disease, stroke, pulmonary disease, chronic kidney disease, liver disease, and malignancy), and medication use (antidepressants and statins).
As shown in Table 2, stronger associations between obesity-metabolic phenotypes and a PHQ ≥ 9 were present for women. While all subgroups with obesity or metabolic dysregulation were significantly associated with a PHQ-9 ≥10 among women, the relationship between depression and metabolic status was attenuated among men, with only the MUO group showing significant associations. Among men, the MOU group was associated with 62% higher odds of a PHQ ≥9 compared with the MHN group, while among women, it was associated with 185% higher odds of a PHQ ≥ 9. Results remained similar when excluding waist circumference from the MetS definition (Table 2). No material changes in results were also noted when patients with a history of hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, stroke, pulmonary disease, chronic kidney disease, liver disease and malignancy were excluded from analysis. Finally, results did not change when using the harmonized definition of MetS from the International Diabetes Federation.
Table 2.
Sensitivity analysis for depression by obesity-related metabolic status
| Metabolic Status | N | OR | 95% CI | |
|---|---|---|---|---|
| Entire sample | ||||
| MHN | 9,386 | Ref | ||
| MHO | 2,294 | 1.51 | 1.30–1.74 | |
| MUN | 2,951 | 1.39 | 1.18–1.64 | |
| MUO | 3,394 | 2.31 | 2.02–2.67 | |
| Sex | ||||
| Men | MHN | 4,832 | Ref | |
| MHO | 1,154 | 1.25 | 0.97–1.69 | |
| MUN | 1,230 | 1.07 | 0.82–1.40 | |
| MUO | 1,531 | 1.63 | 1.31–2.10 | |
| Women | MHN | 4,554 | Ref | |
| MHO | 1.140 | 1.71 | 1.42–2.09 | |
| MUN | 1,721 | 1.62 | 1.30–2.04 | |
| MUO | 1,863 | 2.83 | 2.40–3.30 | |
| P value for interaction | <0.001 | |||
| Age | ||||
| <50 yrs | MHN | 5,869 | Ref | |
| MHO | 422 | 1.22 | 0.85–1.77 | |
| MUN | 2,008 | 1.44 | 1.10–1.60 | |
| MUO | 1,183 | 2.09 | 1.65–2.55 | |
| ≥50 yrs | MHN | 3,487 | Ref | |
| MHO | 1,872 | 1.31 | 1.08–1.62 | |
| MUN | 943 | 1.49 | 1.14–1.98 | |
| MUO | 2,211 | 2.16 | 1.78–2.55 | |
| P value for interaction | 0.41 | |||
| Race/ethnicity | ||||
| White | MHN | 3,994 | Ref | |
| MHO | 1,053 | 1.66 | 1.33–2.08 | |
| MUN | 1,083 | 1.19 | 0.92–1.53 | |
| MUO | 1,490 | 2.23 | 1.84–2.69 | |
| African American | MHN | 1,754 | Ref | |
| MHO | 394 | 1.03 | 0.77–1.03 | |
| MUN | 873 | 1.47 | 0.81–2.39 | |
| MUO | 873 | 1.57 | 1.20–2.06 | |
| Hispanic | MHN | 2,113 | Ref | |
| MHO | 532 | 1.61 | 1.22–2.13 | |
| MUN | 802 | 1.76 | 1.28–2.40 | |
| MUO | 874 | 2.60 | 2.04–3.32 | |
| P value for interaction | 0.71 | |||
| MetS defined without waist circumference | ||||
| MHN | 8049 | Ref | ||
| MHO | 3631 | 1.57 | 1.36–1.82 | |
| MUN | 2823 | 1.53 | 1.31–1.80 | |
| MUO | 3522 | 2.57 | 2.24–2.94 |
All models are adjusted for demographic characteristics (age, sex, race/ethnicity, education, marital status, history of smoking, physical activity, and household income) comorbidities (coronary artery disease, congestive heart disease, stroke, pulmonary disease, chronic kidney disease, liver disease, and malignancy) and medication use (antidepressants and statins). Bold indicates estimates that are significantly different from the reference at the p < 0.05 level. OR = odds ratio; CI = confidence interval; Ref = reference; MHN = metabolically healthy normal weight; MUN = metabolically unhealthy normal weight; MHO = metabolically healthy obesity; MUO = metabolically unhealthy obesity.
Obesity-Related Metabolic Phenotypes and C-Reactive Protein
CRP levels were available for the 5,874 participants in the NHANES 2009–2010 cycle. The baseline characteristics of subjects with available CRP values were similar to the overall sample with respect to age (mean age 48.4 vs 48.8 years), male sex (51.3% vs 51.5%) and white race (42.6% vs 42.5%), Supplemental Table 2. The geometric mean levels were highest among the MUO group (0.34 ± 0.03), followed by the MHO (0.32 ± 0.02), MUN (0.19 ± 0.02) and MHN (0.11 ± 0.03) groups (P<0.001). The association between metabolic status and CRP remained significant after adjusting for the above factors, with all metabolic unhealthy and obese groups (MHO, MUN, MUO) having significantly higher adjusted geometric mean levels of CRP compared to the MHN group (P<0.001). Also, a significant interaction existed between MetS and obesity with respect to CRP levels (P<0.001), indicating that the coexistence of obesity and MetS was associated with higher levels of CRP than the two conditions alone. Although the prevalence of an elevated CRP using a cutoff of 3 mg/dl was low in this predominantly young and healthy population, the pattern of results remained similar, with the MUO group showing the highest proportion of individuals with CRP ≥ 3 mg/dl (Supplemental Figure).
Mean geometric CRP levels were also higher among participants with a PHQ ≥ 9 compared to non-depressed subjects (0.48 ± 0.02 vs 0.40 ± 0.01 mg/dl respectively, P=0.014). In multivariable analysis adjusted for the above variables, for every unit increase in LogCRP levels, the odds of a PHQ-9 ≥10 were increased by 36% (OR 1.36, 95% CI 1.16 – 1.60). Additionally, after adjusting for the same factors as above, every unit increase in the CRP levels was associated with a 3.2% increase in PHQ-9 score (P<0.001).
Women had higher geometric means of CRP levels compared to men (0.46 ± 0.74 vs 0.35 ± 0.75, P<0.001). While both metabolic status and sex were independently associated with CRP levels, there was a significant interaction between the two (P<0.001). Specifically, women had significantly higher CRP levels than men in the MUO (0.68 ± 0.69, vs 0.48 ± 0.71, P<0.001) and MHO (0.71 ± 0.87 vs 0.39 ± 0.46, P<0.001) groups, while there was a smaller sex differences in CRP levels in the MUN group (0.39 ± 0.71 in men and 0.46 ± 1.03 in women, P=0.24) and similar CRP levels in the MHN group (0.27 ± 0.73 in men and 0.26 ± 0.66 in women, P=0.64).
Mediation analysis
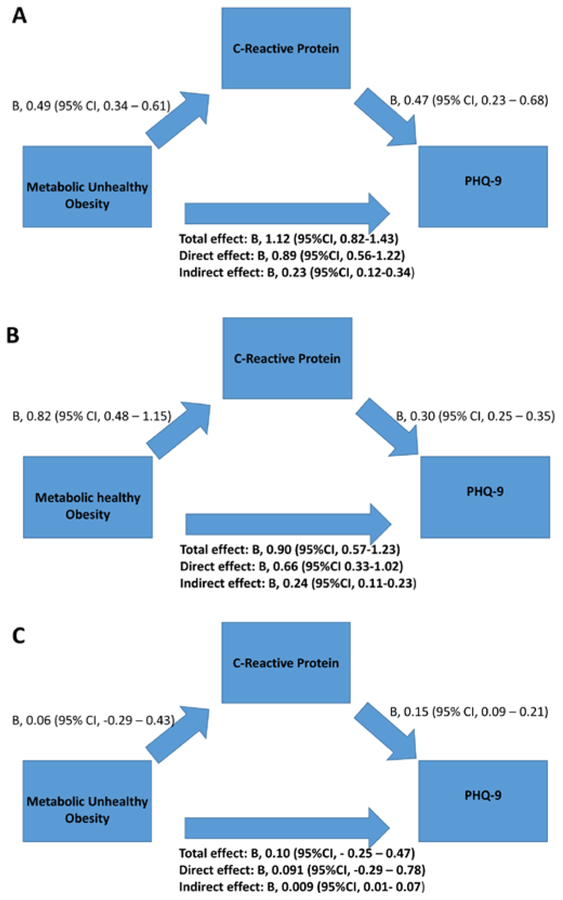
As shown in Figure 3, the MUO group had direct relationships with both PHQ-9 and CRP levels (P<0.001). The total effect of MHO and MUO on PHQ-9 was significant (P<0.001), but not the effect of MUN (Figure 3). After adjusting for the above factors, CRP levels partially mediated the relationship between metabolic status and PHQ-9 by 24.4% and 20.5% for MHO and MUO, respectively.
Figure 3. Mediation analysis for CRP levels as mediator of the relationship between metabolic unhealthy obesity and depressive symptoms.
A. Effect of Metabolic Unhealthy Obesity, compared with Metabolically healthy normal weight, on PHQ-9. B. Effect of Metabolic Healthy Obesity, compared with Metabolically healthy normal weight, on PHQ-9. C. Effect of Metabolic Unhealthy normal weight, compared with Metabolically healthy normal weight, on PHQ-9PHQ-9: Patient Health Questionnaire; Total effect: effect of metabolic subgroup on PHQ-9; Direct effect: effect of metabolic subgroup on PHQ-9 after controlling for CRP levels. Indirect effect: effect of metabolic subgroup on PHQ-9 through the CRP pathway, corresponding to the total effect minus the direct effect.
Model adjusted for demographic characteristics (age, sex, race/ethnicity, education, marital status, smoking history, physical activity, and household income)co-morbidities (coronary artery disease, congestive heart failure, stroke, pulmonary disease, chronic kidney disease, liver disease, and malignancy), and medication use (antidepressants and statins).
Discussion
In a large, nationally representative sample, we found that both obesity and MetS were associated with significant depressive symptoms independent of each other, and that participants with both conditions had the highest rate of depression compared to the other groups. This group also showed the highest levels of systemic inflammation measured by CRP. These associations were independent of baseline characteristics, including demographic factors, behavioral factors, comorbidities, and medication use.
A large body of evidence supports an association between depression and both obesity and MetS.(Chang et al., 2013; Gibson-Smith et al., 2018; Hidese et al., 2018; Vaccarino et al., 2008; Vancampfort et al., 2014) Building on this foundation, our results provide evidence, for the first time, that participants who are both obese and also fulfill criteria for MetS have a particularly high prevalence of depression, which is almost double the rate of depression in the general population (Brody et al., 2018). Also, this group showed a 2.3 times higher odds of depression compared to participants without obesity or metabolic syndrome. Our findings suggest that this subgroup could be most vulnerable to depression and may need closer monitoring and early intervention.
There is growing evidence to suggest that inflammation is an important biological pathway linking depression to cardiometabolic risk.(Brooks et al., 2010; Dowlati et al., 2010; Howren et al., 2009) Individuals with elevated inflammation are more likely to be obese and meet criteria for MetS.(Rethorst, Bernstein, & Trivedi, 2014) Lipid accumulation causes adipocytes to directly activate the innate immune system by secreting pro-inflammatory cytokines and setting up a chronic inflammatory status.(Shelton & Miller, 2010) The resulting inflammatory response has been associated with insulin resistance, ultimately leading to type 2 diabetes and other manifestations of MetS.(Tilg & Moschen, 2006, 2008) Inflammation has also been implicated in the etiology of depression as higher levels of pro-inflammatory cytokines are seen in depressed patients compared to healthy controls.(Dowlati et al., 2010; Howren et al., 2009) The results of the present study indicate that, compared to healthy controls, individuals who have both obesity and MetS have the highest levels of systemic inflammation in addition to the highest rates of depression, supporting the notion that inflammation linked to metabolic abnormalities may play an etiological role in depression. This idea was further strengthened by our observation that CRP partially mediated the association between MUO and depression, although it only explained 20% of the total effect. It should be noted that the opposite pathway may also play a role, through which hypercortisolemia and autonomic and immune dysregulation in depression may lead to insulin resistance, abdominal obesity and chronic inflammation that may underlie the association between depression and cardiometabolic disease.(McIntyre et al., 2009)
Several prior studies have reported that the relationship between depression and metabolic dysregulation differs for men and women.(Carpenter, Hasin, Allison, & Faith, 2000; Faith, Matz, & Jorge, 2002; Istvan, Zavela, & Weidner, 1992; Kinder, Carnethon, Palaniappan, King, & Fortmann, 2004) Collectively, these studies indicated that, compared to men, women with obesity or MetS are more likely to develop depression.(Carpenter et al., 2000; Faith et al., 2002; Istvan et al., 1992; Kinder et al., 2004) Our results are in line with these previous reports, showing that among women, all obesity/metabolic categories were associated with higher odds of depression compared with the MHN group, and the combination of both conditions was associated with an almost threefold higher odds of depression. Among male individuals, only the MUO group had higher odds of depression compared to the MHN group. In general, women have higher levels of inflammation, as well as higher rates of autoimmune diseases compared to men.(Quintero, Amador-Patarroyo, Montoya-Ortiz, Rojas-Villarraga, & Anaya, 2012; Yang & Kozloski, 2011) There is evidence to suggest that women’s susceptibility to inflammation may contribute to sex differences in depression.(Derry, Padin, Kuo, Hughes, & Kiecolt-Glaser, 2015; Quintero et al., 2012; Yang & Kozloski, 2011) Additionally, transient increases in inflammation have been shown to affect women’s mood to a greater extent than their male counterparts.(Moieni et al., 2015) Our results showed significant interactions also between sex and metabolic status for CRP, with higher levels of CRP among women in the MUO and MHO groups compared to men. These findings further validate the idea that women may be especially susceptible to depression as a consequence of metabolic and inflammatory derangements.
Our study had a number of limitations. First, the cross-sectional design precluded determination of causality between the metabolic status and risk of depression, and limited the interpretability of our mediation analysis. Second, information was not available on psychiatric history, including a clinical history of major depression and psychiatric medications. Finally, CRP levels were only available for a subgroup, and other inflammatory biomarkers were not available.
Despite these limitations, our study is strengthened by the large, representative sample, with in-person measurement of anthropometric data and objective measures of MetS risk factors, as well as detailed data on demographic and lifestyle factors.
In conclusion, we report for the first time that both obesity and MetS are independently related to depression, but the combination of these two conditions is associated with especially higher odds of depression. This finding is especially marked among women. Thus, individuals, especially women, with both obesity and MetS represent a group potentially at highest risk for depression. Our data also suggest that systemic inflammation may be implicated in these results. These findings highlight the magnitude of the comorbidity between metabolic derangements and depression, and the importance of developing strategies to target individuals with MetS and obesity not only for cardiovascular disease prevention, but also for mental health improvement.
Supplementary Material
Sources of Funding:
This work was supported by the National Institutes of Health through the following grants: R01 HL125246, R01 HL136205, K23 HL127251, K12HD085850 and T32 HL130025.
Footnotes
Disclosures
All authors report no conflicts of interest. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- Alberti KG, Zimmet P, & Shaw J (2006). Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med, 23(5), 469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- Belcher BR, Moser RP, Dodd KW, Atienza AA, Ballard-Barbash R, & Berrigan D (2015). Self-Reported Versus Accelerometer-Measured Physical Activity and Biomarkers Among NHANES Youth. J Phys Act Health, 12(5), 708–716. doi: 10.1123/jpah.2013-0193 [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pratt LA, & Hughes JP (2018). Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013–2016. NCHS Data Brief(303), 1–8. [PubMed] [Google Scholar]
- Brooks GC, Blaha MJ, & Blumenthal RS (2010). Relation of C-reactive protein to abdominal adiposity. Am J Cardiol, 106(1), 56–61. doi: 10.1016/j.amjcard.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Bull FC, Maslin TS, & Armstrong T (2009). Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health, 6(6), 790–804. [DOI] [PubMed] [Google Scholar]
- Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, & Nirantharakumar K (2017). Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J Am Coll Cardiol, 70(12), 1429–1437. doi: 10.1016/j.jacc.2017.07.763 [DOI] [PubMed] [Google Scholar]
- Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, … Vaccarino V (2008). Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry, 64(10), 896–900. doi: 10.1016/j.biopsych.2008.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS, Allison DB, & Faith MS (2000). Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health, 90(2), 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Yen AM, Lee CS, Chen SL, Chiu SY, Fann JC, & Chen HH (2013). Metabolic syndrome and the risk of suicide: a community-based integrated screening samples cohort study. Psychosom Med, 75(9), 807–814. doi: 10.1097/PSY.0000000000000014 [DOI] [PubMed] [Google Scholar]
- Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, & Tully MA (2014). Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health, 14, 1255. doi: 10.1186/1471-2458-14-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner M, Kasl SV, Abramson JL, & Vaccarino V (2003). Association between depression and elevated C-reactive protein. Psychosomatic Medicine, 65(3), 347–356. [DOI] [PubMed] [Google Scholar]
- Derry HM, Padin AC, Kuo JL, Hughes S, & Kiecolt-Glaser JK (2015). Sex Differences in Depression: Does Inflammation Play a Role? Curr Psychiatry Rep, 17(10), 78. doi: 10.1007/s11920-015-0618-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctot KL (2010). A meta-analysis of cytokines in major depression. Biol Psychiatry, 67(5), 446–457. doi: 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, & Schulze MB (2016). Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur J Prev Cardiol, 23(9), 956–966. doi: 10.1177/2047487315623884 [DOI] [PubMed] [Google Scholar]
- Faith MS, Matz PE, & Jorge MA (2002). Obesity-depression associations in the population. J Psychosom Res, 53(4), 935–942. [DOI] [PubMed] [Google Scholar]
- Fan J, Song Y, Chen Y, Hui R, & Zhang W (2013). Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol, 168(5), 4761–4768. doi: 10.1016/j.ijcard.2013.07.230 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, & Mingrone G (1997). Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest, 100(5), 1166–1173. doi: 10.1172/JCI119628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, … Whiteford HA (2013). Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med, 10(11), e1001547. doi: 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, … American College of Sports, M. (2011). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc, 43(7), 1334–1359. doi: 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- Gibson-Smith D, Bot M, Snijder M, Nicolaou M, Derks EM, Stronks K, … Penninx B (2018). The relation between obesity and depressed mood in a multi-ethnic population. The HELIUS study. Soc Psychiatry Psychiatr Epidemiol. doi: 10.1007/s00127-018-1512-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, … Blood I (2005). Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- Hidese S, Asano S, Saito K, Sasayama D, & Kunugi H (2018). Association of depression with body mass index classification, metabolic disease, and lifestyle: A web-based survey involving 11,876 Japanese people. J Psychiatr Res, 102, 23–28. doi: 10.1016/j.jpsychires.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med, 71(2), 171–186. doi: 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- Istvan J, Zavela K, & Weidner G (1992). Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord, 16(12), 999–1003. [PubMed] [Google Scholar]
- Kinder LS, Carnethon MR, Palaniappan LP, King AC, & Fortmann SP (2004). Depression and the metabolic syndrome in young adults: findings from the Third National Health and Nutrition Examination Survey. Psychosom Med, 66(3), 316–322. [DOI] [PubMed] [Google Scholar]
- Kramer CK, Zinman B, & Retnakaran R (2013). Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med, 159(11), 758–769. doi: 10.7326/0003-4819-159-11-201312030-00008 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. doi:jgi01114 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, & Lowe B (2010). The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry, 32(4), 345–359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, & Salonen JT (2002). The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA, 288(21), 2709–2716. [DOI] [PubMed] [Google Scholar]
- Liu J, Grundy SM, Wang W, Smith SC Jr., Vega GL, Wu Z, … Zhao D (2007). Ten-year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J, 153(4), 552–558. doi: 10.1016/j.ahj.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Martin A, Rief W, Klaiberg A, & Braehler E (2006). Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry, 28(1), 71–77. doi: 10.1016/j.genhosppsych.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Martinez-Larrad MT, Corbaton-Anchuelo A, Fernandez-Perez C, Lazcano-Redondo Y, Escobar-Jimenez F, & Serrano-Rios M (2016). Metabolic syndrome, glucose tolerance categories and the cardiovascular risk in Spanish population. Diabetes Res Clin Pract, 114, 23–31. doi: 10.1016/j.diabres.2016.02.003 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Rasgon NL, Kemp DE, Nguyen HT, Law CW, Taylor VH, … Goldstein BI (2009). Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep, 9(1), 51–59. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, … Heiss G (2005). The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care, 28(2), 385–390. doi:28/2/385 [pii] [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, & Eisenberger NI (2015). Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology, 40(7), 1709–1716. doi: 10.1038/npp.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongraw-Chaffin M, Foster MC, Anderson CAM, Burke GL, Haq N, Kalyani RR, … Vaidya D (2018). Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J Am Coll Cardiol, 71(17), 1857–1865. doi: 10.1016/j.jacc.2018.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty AS, Gilbody S, McMillan D, & Manea L (2015). Screening and case finding for major depressive disorder using the Patient Health Questionnaire (PHQ-9): a meta-analysis. Gen Hosp Psychiatry, 37(6), 567–576. doi: 10.1016/j.genhosppsych.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, … American Heart, A. (2003). Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107(3), 499–511. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, … Rabasa-Lhoret R (2011). Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond), 35(7), 971–981. doi: 10.1038/ijo.2010.216 [DOI] [PubMed] [Google Scholar]
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, & Anaya JM (2012). Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun, 38(2–3), J109–119. doi: 10.1016/j.jaut.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Bernstein I, & Trivedi MH (2014). Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009–2010 National Health and Nutrition Examination Survey (NHANES). J Clin Psychiatry, 75(12), e1428–1432. doi: 10.4088/JCP.14m09009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, & Miller AH (2010). Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol, 91(4), 275–299. doi: 10.1016/j.pneurobio.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, & Moschen AR (2006). Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol, 6(10), 772–783. doi: 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- Tilg H, & Moschen AR (2008). Inflammatory mechanisms in the regulation of insulin resistance. Mol Med, 14(3–4), 222–231. doi: 10.2119/2007-00119.Tilg [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, … Blood I (2007). Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol, 50(21), 2044–2050. doi: 10.1016/j.jacc.2007.07.069 [DOI] [PubMed] [Google Scholar]
- Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, … Merz CN (2008). Depression, the metabolic syndrome and cardiovascular risk. Psychosomatic Medicine, 70(1), 40–48. 10.1097/PSY.0b013e31815c1b85 [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, … De Hert M (2014). Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med, 44(10), 2017–2028. doi: 10.1017/S0033291713002778 [DOI] [PubMed] [Google Scholar]
- Whiteford H, Ferrari A, & Degenhardt L (2016). Global Burden Of Disease Studies: Implications For Mental And Substance Use Disorders. Health Aff (Millwood), 35(6), 1114–1120. doi: 10.1377/hlthaff.2016.0082 [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet, 382(9904), 1575–1586. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Williams EP, Mesidor M, Winters K, Dubbert PM, & Wyatt SB (2015). Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr Obes Rep, 4(3), 363–370. doi: 10.1007/s13679-015-0169-4 [DOI] [PubMed] [Google Scholar]
- Yang Y, & Kozloski M (2011). Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci, 66(5), 493–500. doi: 10.1093/gerona/glr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.