Abstract
Background:
High opioid dosage has been associated with overdose, and clinical guidelines have cautioned against escalating dosages above 100 morphine-equivalent mg (MEM) based on the potential harm and the absence of evidence of benefit from high dosages. However, this 100 MEM threshold was chosen somewhat arbitrarily.
Objective:
To examine the association of prescribed opioid dosage as a continuous measure in relation to risk of unintentional opioid overdose to identify the range of dosages associated with risk of overdose at a detailed level.
Methods:
In this nested case-control study with risk-set sampling of controls, cases (opioid overdose decedents) and controls were identified from a population of patients of the Veterans Health Administration who were prescribed opioids and who have a chronic pain diagnosis. Unintentional fatal opioid analgesic overdose was measured from National Death Index records and prescribed opioid dosage from pharmacy records.
Results:
The average prescribed opioid dosage was higher (P < 0.001) for cases (mean = 98.1 MEM, SD = 112.7; median = 60, interquartile range, 30–120), than controls (mean = 47.7 MEM, SD = 65.2; median = 25, interquartile range, 15–45). In a ROC analysis, dosage was a moderately good “predictor” of opioid overdose death, indicating that, on average, overdose cases had a prescribed opioid dosage higher than 71% of controls.
Conclusions:
A clear cut-point in opioid dosage to distinguish between overdose cases and controls was not found. However, lowering the recommended dosage threshold below the 100 MEM used in many recent guidelines would affect proportionately few patients not at risk for overdose while potentially benefitting many of those at risk for overdose.
Keywords: pain, opioid analgesics, patient safety
In recent years, the public health problem of prescription opioid overdose (also called “poisoning”) emerged when opioid prescribing and usage increased in response to changes in treatment practices for chronic, noncancer pain.1 Evidence indicated that many opioid overdose victims were prescribed opioids before their death.2 Given the concurrent trends in overdose deaths and increased prescribing for chronic pain, the safety of long-term opioid prescribing for chronic, noncancer pain in particular has been increasingly scrutinized.3,4
In 2010–2011, 3 observational studies reported an association between higher prescribed opioid dosage and risk of opioid overdose.5–7 These studies found increases in risk starting at dosages above 20 morphine-equivalent milligrams (MEM) per day, with dramatic increases in overdose rates for patients prescribed 100 MEM per day or greater. In the absence of evidence that patients gain benefit from higher dosage prescribing,8 these findings have led to a reconsideration of whether there is a threshold beyond which patients’ dosages should not be escalated. As summarized by Nuckols et al,9 guidelines written after these studies suggested thresholds of 80–100 MEM,10–12 compared with the 200 MEM suggested threshold of earlier guidelines.13–15 A recent evidence review16 and guideline17 also suggested that prescribers reevaluate treatment when opioid dosage reaches 50–100 MEM per day, which is a fairly large range within which overdose risk may vary.
The prior studies of opioid dosage and overdose have used generally similar categories in analysis, which were 1– < 20, 20– < 50, 50– < 100, and 100+5,6 or 100– < 200 and 200+ MEM.7 These categories were driven more by the base 10 number system (ie, round numbers used by convention) than common opioid dosages or the pharmacokinetics of opioids. In light of how these studies influenced guidelines, a refined understanding of the association between opioid dosage and overdose is needed. We analyzed prescribed opioid dosage as a continuous, rather than categorical, measure in relation to risk of opioid overdose death among patients with chronic pain in a large national dataset originating from the Veterans Health Administration (VHA) system. The objective of this analysis was to identify the range of dosages associated with overdose at a detailed level.
METHODS
Design
This study used a nested case-control design based on analysis of treatment and mortality records. In this design, both cases and controls are selected from an underlying population. The study used a risk-set sampling approach, in which the controls are randomly sampled from among patients “at risk” for the outcome on the case’s date of death (ie, alive and still under observation). This sampling strategy results in controls that are matched to the specific cases, and exposure is measured for both a case and his or her matched control in the period leading up to the date of the case’s death18; this date is called the “index date” here to apply equally to cases and controls.
We sought to also match cases and controls on several diagnosis and treatment characteristics to minimize differences between the 2 groups and thus to have greater confidence in any observed differences in prescribed opioid dosage. This was prioritized over full representativeness of all cases; patients who died of an overdose who were unable to be matched to a control because of unique characteristics were not included. Study procedures received approval from the Ann Arbor VA human studies committee, which waived the requirement for informed consent.
Sample
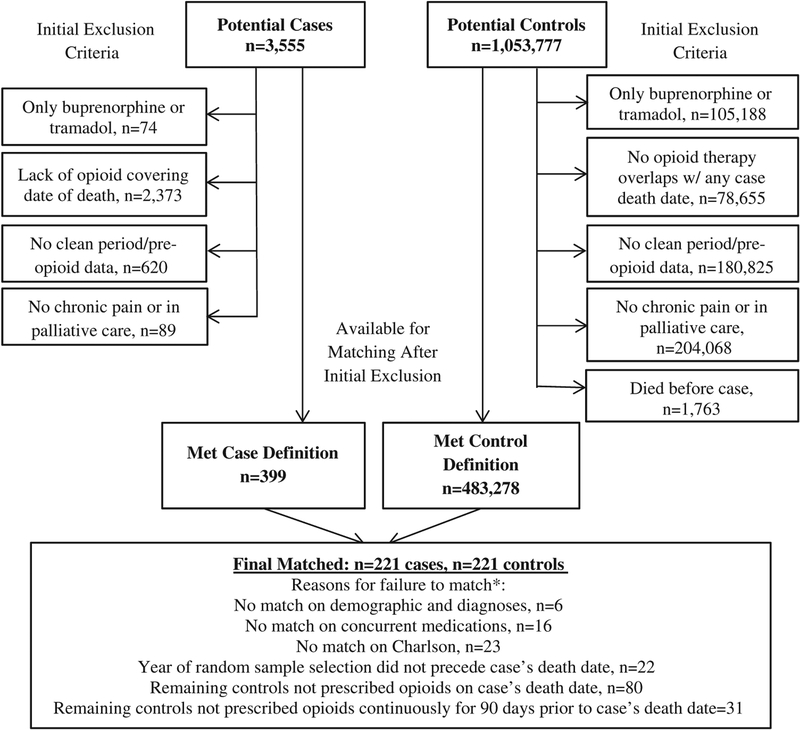
The sample was selected from patients in the VHA system during fiscal years (October 1 of the prior calendar year to September 30) 2004–2009. Inclusion criteria were: (1) filled an opioid prescription at a VHA facility during the study timeframe; (2) had a chronic pain diagnosis in the medical record [based on ICD-9-CM19 codes 307.81, 337.0, 337.1, 338.0, 338.2, 338.4, 339.x, 346.x, 350.2, 354.0, 354.4, 355.x-357.x, 377.x, 710.x-729.x (except 729.1), 730.x-739.x, and 784.0]; (3) were prescribed to be taking an opioid on the index date (see the Design section above); and (4) started a “new” opioid treatment episode at some point during fiscal years 2002–2009, defined as having an opioid prescription fill after a 2-year (or greater) period with use of VHA treatment services and without an opioid prescription fill. The last criterion was intended to allow sufficient opportunity to measure patient and treatment characteristics from the medical records preopioid treatment to generate matching variables. Patients were excluded if they had either of the following: (1) hospice or palliative care; or (2) only opioids prescribed were tramadol (due to the unique mechanism of action and evidence suggesting lower risk for overdose specifically,20,21 although potentially high risk for other adverse outcomes22) or buprenorphine (because it is only on formulary for opioid use disorder treatment in VA23). Figure 1 displays the sample flowchart.
FIGURE 1.
Sample selection and matching.
*Reasons for failure to match are hierarchical as listed; n given is for number of cases not matched.
Data Sources
This study used linked administrative and surveillance data sources. Data from the VHA’s National Patient Care Database were used to identify VHA clinical encounters. From these encounters, information on patient demographic characteristics and diagnoses was obtained. Outpatient prescription medication data came from the VHA’s Pharmacy Benefits Management service.
Cause of death data from the National Death Index24 were obtained by the VHA Office of Mental Health Operations and their use was approved by the VA/DoD Suicide Data Repository Board of Governance. The National Death Index (NDI) is a compilation of death certificates from all state vital statistics offices and has the greatest sensitivity among population-level sources of mortality data.25 The National Death Index searches for the VHA population have been described elsewhere.26 Matching27 included 2 definitions: (1) full match on Social Security Number (SSN) and sex and match on at least 2 of the 3 parts (day, month, year) of date of birth; or (2) match on at least 7 digits of the SSN plus full match on date of birth, sex, first name, and last name and middle initial when present. More than 99% of deaths had a full match on SSN.
Case and Control Definitions
Unintentional overdose case status was based on NDI data. Cases were those with an underlying cause of death corresponding to unintentional (n = 195) or undetermined intent (n = 26) drug poisoning (X40-X45 or Y10–15 in WHO International Classification of Diseases-Tenth Revision) and a T-code indicating that the drug involved was a prescription opioid (T 40.2, 40.3, or 40.4).5 NDI records include only 1 underlying cause of death but are able to accommodate multiple T-codes when the medical examiner or coroner records that the overdose was caused by >1 drug or medication; in this study, cases were eligible even if their overdose was caused by other substances in combination with prescription opioids. Of note, although the term “overdose” implies taking a dose larger than a specific threshold that demarcates toxicity, prior research has found that changes in tolerance and interactions with alcohol and other medications also influence the risk of overdose.28,29
Controls were selected from a random sample registry maintained by the VA Serious Mental Illness Treatment Resource and Evaluation Center. To be selected as a match to a specific case, a control had to be alive and prescribed to be taking opioids on the case’s date of death. The random sample registry was expanded on an annual basis, and all available treatment records for all years prior and subsequent to that year are included in the registry for each selected patient. Consequently, in the present study, we further required that the year for which the control was sampled for registry inclusion was before the case’s date of death to be matched to that case. This was because controls added to the registry in a year subsequent to the case’s death could not be considered “at risk” on that case’s date of death under the study methods because they had had to survive longer than the case to be included in the sample. This criterion helps to avoid disproportionately selecting controls who are healthier than other patients.
Prescribed Opioid Dosage
All opioids used in VHA for pain during the study years were included aside from the exceptions described above; these were codeine, morphine, oxycodone, hydrocodone, oxymorphone, hydromorphone, fentanyl, meperidine, pentazocine, propoxyphene, and methadone. The ratios for morphine-equivalent doses across formulations used in these analyses were compiled by the CDC National Center for Injury Prevention and Control.30 Dosage calculations also accounted for the difference between routes of administration. Supplemental Digital Content 1 (http://links.lww.com/MLR/B112) reports the dosage conversions for this study. Methods to calculate the maximum prescribed daily opioid dosage (henceforth called “prescribed opioid dosage”) on the index date followed prior research in this population.5 This calculation uses an “as-prescribed” approach,31 which assumes that patients take all prescribed opioids at the dosage and on the schedule described in their prescriptions; for prescriptions written for “as needed”/PRN use, the pills are assumed to be consumed at the maximum frequency permitted by the prescription. When patients filled a prescription for the same formulation at the same dosage and schedule as a prior fill and the new fill occurred during the days covered by the prior fill, it was assumed that the new fill was a continuation of the same treatment. Thus, it was assumed that the patient started taking the new fill after the end of the days covered by the prior fill. However, patients taking opioid medications who filled prescriptions for a different dosage, schedule, or opioid medication were assumed to have had their opioid therapy augmented and to have begun taking the new prescription on the date that it was filled. Each patient’s prescribed opioid dosage on their index date was calculated by adding the daily doses of all fills that covered that particular day. This measurement of dosage reflects the opioid dosage prescribed and not necessarily the actual dosage consumed.
Variables Used in Matching of Cases and Controls
Cases and controls were matched on a number of measures obtained from medical records. These included demographic information recorded in the medical chart, diagnoses recorded as part of medical visits, and use of specific concurrent medications from pharmacy records. All matching variables were measured in the year before the most recent new opioid treatment episode (see above). This was done to avoid matching on variables measured after treatment started and thus could be on the causal pathway between prescribed opioid dosage and risk of overdose.
Matching variables were selected based on having an association with overdose risk in prior studies of this population.5,32,33 These included the following measures: (1) sex; (2) age ( ± 2 y); (3) race and ethnicity (with an option for none specified); (4) substance use disorder diagnosis; (5) depression diagnosis; (6) other psychiatric diagnosis; (7) acute pain condition; (8) comorbid chronic diseases [COPD, CVD, sleep apnea, cancer (except skin)], each present/absent; (9) Charlson score (0, 1, 2+), a measure of co-morbidity34; (10) use of benzodiazepines; (11) use of antidepressants; (12) use of anticonvulsants; and (13) whether the patient had been prescribed opioids continuously for the 90 days before index date, allowing for a 30-day gap between fills.35
Analysis
Within cases and controls, measures of distribution and central tendency of prescribed opioid dosage were calculated and the distribution was graphed using Kernel density estimation, based on a Gaussian function. A box plot and whiskers diagram was used to supplement the examination of distribution of opioid dosage by group. To examine the degree to which prescribed dosage differentiates cases from controls, we developed a receiver operating characteristic (ROC) curve, from which we measured area under the curve. To account for the matched design in the ROC, we ran a logistic regression model that was adjusted for all variables used in matching, following the recommendations of Pepe et al.36 The ROC was estimated based on this logistic model. We also calculated the sensitivity, specificity, and positive likelihood ratio (LR)37 for specific dosage cutpoints.
We partitioned the sample into 10 groups according to their predicted probabilities for being an overdose case from a conditional logistic regression model. The Hosmer-Lemeshow goodness-of-fit χ2 statistic was used to determine whether the observed number of cases in each group differed from the predicted number of cases. Low χ2 values with high P-values provide evidence for good model fit.
RESULTS
There were 399 decedents meeting the unintentional overdose case definition, and 221 were able to be matched to controls. Table 1 describes the characteristics of the included sample as well as all possible cases and controls from the base population. Notably, this table indicates that the process of finding matches on the many characteristics considered resulted in a final set of cases and controls that had fewer comorbidities than the overall population from which they were sampled.
TABLE 1.
Distribution of Characteristics in the Sample
| Characteristics | Included Sample (%)* | All Possible Cases (%) | All Possible Controls (%) |
|---|---|---|---|
| Demographic | |||
| Age [mean (SD)] | 48.4 (9.1) | 46.9 (10.5) | 60.6 (14.4) |
| Male sex | 97.7 | 89.6 | 93.0 |
| Race | |||
| American Indian | 0.5 | 0.6 | 0.5 |
| African American | 6.3 | 7.3 | 17.2 |
| Asian/Pacific | 0.0 | 0.6 | 1.3 |
| Islander | |||
| White | 86.9 | 69.3 | 72.0 |
| Multiracial | 0.0 | 0.6 | 0.6 |
| Unknown | 6.3 | 21.7 | 8.2 |
| Hispanic ethnicity | 0.5 | 3.1 | 5.3 |
| Diagnoses | |||
| Substance use disorder | 20.8 | 37.7 | 10.2 |
| Depression | 32.1 | 38.8 | 21.1 |
| Other psychiatric | 32.6 | 41.6 | 21.0 |
| Chronic obstructive pulmonary disease | 5.9 | 10.2 | 13.1 |
| Cardiovascular disease | 37.1 | 35.2 | 61.5 |
| Sleep apnea | 2.7 | 3.6 | 6.0 |
| Cancer | 5.0 | 7.8 | 21.8 |
| Acute pain | 8.6 | 22.0 | 18.5 |
| Charlson comorbidity index score | |||
| 0 | 70.1 | 62.7 | 44.1 |
| 1 | 17.7 | 20.7 | 23.5 |
| 2+ | 12.2 | 16.7 | 32.4 |
| Medication use | |||
| 90+ d of opioid use before index date | 66.5 | — | — |
| Benzodiazepine use | 24.9 | 32.4 | 14.3 |
| Antidepressant use | 52.0 | 53.8 | 33.3 |
| Anticonvulsant use | 38.5 | 37.6 | 22.7 |
All variables except sex, race, ethnicity, and 90+ days of opioid use were measured in the year before start of opioid treatment.
Per the design of matching cases and controls, the distribution of these characteristics are the same among cases and controls.
In the sample, the prescribed opioid dosage was higher (P < 0.001) for cases, with a mean of 98.1 MEM (SD = 112.7), than controls, whose prescribed opioid dosage had a mean of 47.7 MEM (SD = 65.2). The median similarly indicated higher prescribed opioid dosages among cases (60 MEM; interquartile range, 30–120 MEM) compared with controls (25 MEM; interquartile range, 15–45 MEM). To account for the potential role of extremely high outliers in creating differences between overdoses and controls, we repeated the analyses excluding pairs when either the case or control were prescribed 300+ MEM. In this subset of 206 pairs, we found a prescribed opioid dosage mean of 74.7 (SD = 57.6) in cases and 40.2 (SD = 41.2) among controls (P < 0.001).
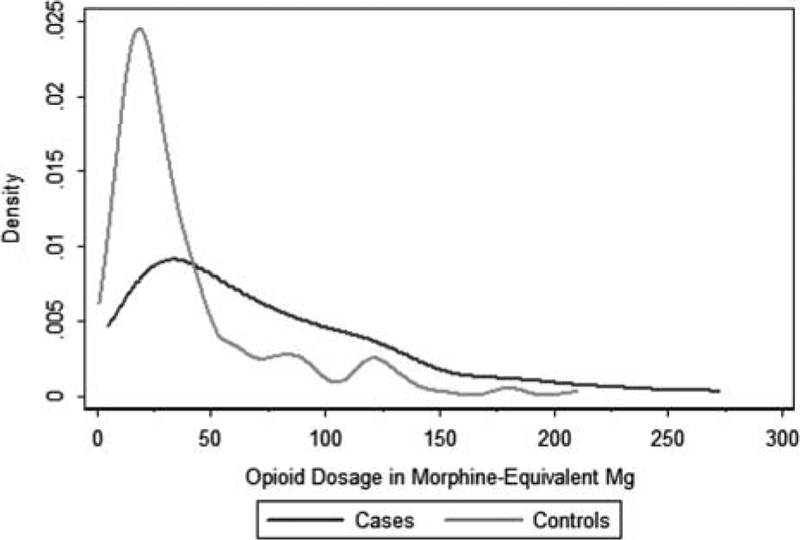
The Kernel density plot in Figure 2 displays the distribution of prescribed opioid dosage among cases and controls, with the outliers above 300 MEM excluded. In addition to further demonstrating that opioid dosages were generally lower among controls than cases, this figure shows that the patients who died of an opioid overdose were prescribed a wide range of opioid dosages at the time of their death, including relatively low dosages. Per the box and whiskers plot reported in Supplemental Digital Content 2 (http://link-s.lww.com/MLR/B113), the 50th percentile in dosage among cases is above the 75% percentile in dosage among controls.
FIGURE 2.
Distribution of opioid dosages by group.
Table 2 reports the sensitivity and specificity for prescribed opioid dosage as a measure to identify patients at risk of overdose. If both sensitivity and specificity were relatively high at a given dosage level, this would indicate a clear dosage threshold for prescribing recommendations, but this was not the case. However, it is notable that <25% of controls had prescribed opioid dosages above 50 MEM, but almost 60% of cases had dosages above this level. The LRs indicated that opioid dosages levels of 40 MEM and above had a small (or, greater than minimal) increase in risk of death, but no specific level reached the LR threshold of 5 that would be considered a moderate increase.37
TABLE 2.
Distribution of Opioid Overdose Cases and Controls at Specific Opioid Dosage Levels
| Dose in MEM | Cases Above (Sensitivity) (%) | Controls at or Below (Specificity) (%) | Likelihood Ratio (+) |
|---|---|---|---|
| 10 | 97 | 14 | 1.12 |
| 20 | 87 | 41 | 1.47 |
| 30 | 71 | 63 | 1.94 |
| 40 | 66 | 71 | 2.27 |
| 50 | 59 | 76 | 2.50 |
| 60 | 48 | 81 | 2.50 |
| 70 | 45 | 82 | 2.50 |
| 80 | 41 | 84 | 2.60 |
| 90 | 33 | 88 | 2.67 |
| 100 | 31 | 89 | 2.83 |
| 110 | 28 | 90 | 2.82 |
| 120 | 21 | 93 | 3.06 |
| 130 | 20 | 95 | 3.67 |
| 140 | 17 | 95 | 3.70 |
| 150 | 15 | 96 | 3.67 |
| 160 | 15 | 96 | 3.67 |
| 170 | 14 | 96 | 3.45 |
| 180 | 12 | 97 | 3.71 |
| 190 | 11 | 97 | 3.43 |
| 200 | 10 | 97 | 3.28 |
MEM indicates morphine-equivalent mg.
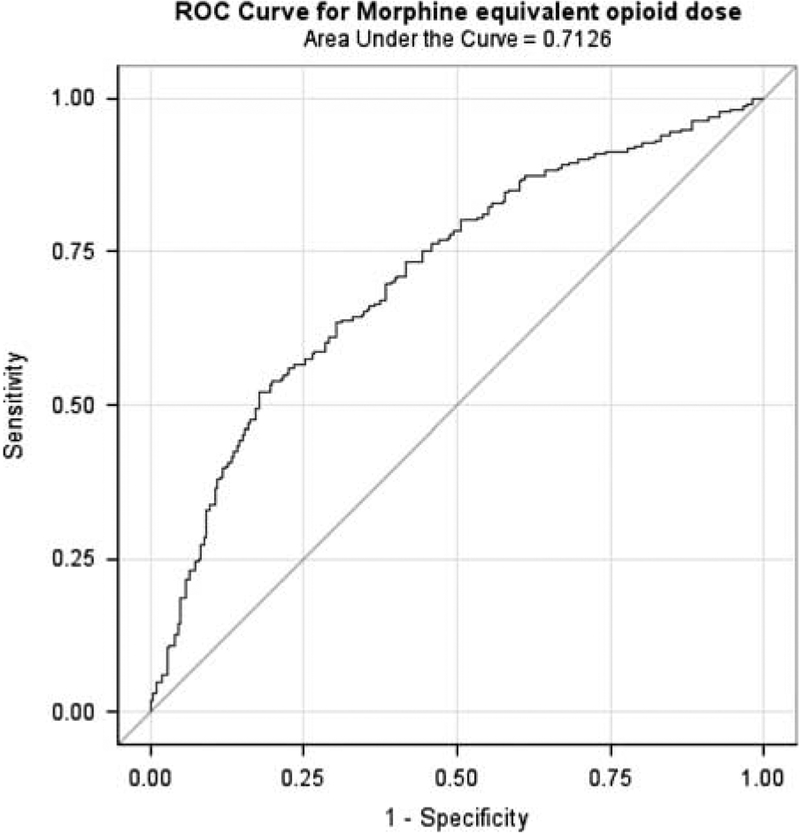
ROC curve analysis was based on a logistic regression model predicting case status (overdose death vs. control) with the independent variables of prescribed opioid dosage and all matching variables.36 We found that prescribed opioid dosage was a moderately good “predictor” of overdose death in this sample; with an Area Under the Curve of 0.71 (95% CI, 0.66–0.76), which was significantly better than expected by chance (P < 0.001). This indicates that, on average, overdose cases had a prescribed opioid dosage higher than 71% of controls (Fig. 3). A Hosmer-Lemeshow goodness-of-fit test indicated an adequate model fit (χ2 = 13.37, P = < 0.01), suggesting that differences between cases and controls were reasonably well explained by prescribed opioid dosage and the factors used in matching.
FIGURE 3.
Receiver operating characteristic curve for opioid dosage in relation to opioid overdose death (case status).
DISCUSSION
This study examined the association of prescribed opioid dosage and opioid overdose death and represented a significant innovation over prior research by treating dosage as a continuous variable rather than categorical. This allowed for an examination of the relationship of interest without imposing artificial cutoffs between opioid dosage levels. We found that cases had significantly higher prescribed opioid dosages than controls, on average. However, the analyses did not suggest that there was a clear, specific cutpoint in dosage above which there was a preponderance of opioid overdose cases and few controls.
Despite not finding a clear cutpoint, we did find that almost half of overdose cases were prescribed >60 MEM/day and nearly 60% of cases were prescribed >50 MEM/day. Seeking to lower dosages to 50 MEM/day or less would affect <25% of control patients in this sample, who were demographically and clinically similar to the cases. It is notable that some cases had dosages at the lower end of the range at the time of their death. Thus, lower prescribed opioid dosages are associated with reduced risk of overdose, but risk is not completely absent at low dosages. More specifically, there is not a threshold below which risk is eliminated, and clinicians should be aware that there are risks of opioid overdose even at lower dosages. These data suggest instead that a gradual increasing degree of caution should be considered as dosage increases. Future research could explore the circumstances of overdoses that occur when patients are prescribed relatively low dosages of opioids to better elucidate this finding.
Some preliminary evidence suggests that reducing high dosage opioid prescribing as part of a comprehensive strategy to reduce risky prescribing and patient behavior may result in lowered rates of opioid overdose. An evaluation of a Washington State Interagency Guideline on Opioid Dosing, which recommended that opioid dosages >120 MEM only be prescribed with consultation from a pain medicine expert, found decreases in opioid-related deaths starting 3 years after implementation.38 In addition, a recent evaluation of efforts in Staten Island to reduce high dosage (> 100 MEM daily) prescribing and educate both providers and the community indicated that implementation was followed by a decrease in opioid overdose deaths, which was not seen in adjacent communities during the same time.39 However, the design and analyses of these evaluations are relatively preliminary; for example, it is unknown whether changes were due to reductions in high dosage prescribing, other concurrent initiatives, or both. More rigorous studies are needed to understand the degree to which lowering prescribed opioid dosages among patients already on chronic opioid therapy and/or reducing the number of patients initiating opioid therapy who escalate to high dosages results in fewer adverse outcomes.
An additional innovation of this study was the nested case control design with matching. This sampling strategy resulted in a set of cases and comparative controls who were similar in terms of clinical and demographic characteristics. Those cases who were unlike any available controls were excluded from analyses, reducing the degree to which the finding may be due to the influence of cases who are unusual in their clinical presentation. Although adequately adjusting analyses when only medical records data are available is challenging,40 model diagnostics indicated reasonably good fit, suggesting that there were not substantial unmeasured factors that explained differences between cases and controls. Nonetheless, caution should be taken in drawing causal inferences from this observational study. In addition, the decision to exclude cases who did not match a control on a relatively large set of characteristics reduced the generalizability to all overdose deaths.
There are several other limitations. Overdose death was defined based on cause of death and drug involvement from death certificates. The relatively precise definition of opioid overdose death for this study resulted in the exclusion of deaths that were undetected unintentional opioid overdoses (eg, misclassified as suicide, or opioids involved but not detected or recorded by medical examiner/coroner). The undetermined overdose cases are more similar to unintentional overdose than suicide,41 but likely include some suicides. There are also several limitations related to measurement of opioid exposure. This study focused on prescribed opioid dosage on a key date of interest. The benefit of this approach is that prescribed morphine equivalent dosages are relatively easy to calculate as part of routine clinical care, but other research has indicated that other measures of opioid use, such as cumulative opioid exposure over a specified period of time provide additional value in identifying overdose risk in some cases.42 In addition, available information on opioid treatment could not be used to determine how patients actually took their medication. Thus inferences are most relevant to prescribing decisions rather than patient education.
This study is the first to provide a detailed examination of prescription opioid overdose and prescribed opioid dosage treated as a continuous variable rather than with artificially imposed dosage categories. A clear cutpoint to distinguish between overdose cases and controls was not found. However, a 100 MEM dosage threshold has been used in many recent opioid prescribing guidelines based on earlier studies that used this level to define the highest dosage category,9 and these data suggest that even lower recommended dosage thresholds would affect proportionately few patients not at risk for overdose while potentially benefitting many patients who are at risk. In the absence of evidence of benefit for high prescribed opioid dosages, the present findings provide further support for caution in increasing dosages during opioid therapy.
Supplementary Material
Acknowledgments
Support through an Intergovernmental Personnel Act assignment agreement from the CDC to A.S.B.B., VA grant CDA 09–204, and VA grant RRP 13–251.
Footnotes
The study received internal review from the CDC before submission. The study also received review from the VHA Office of Mental Health Operations to ensure compliance with data use agreements.
The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the CDC or VHA.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.lww-medicalcare.com.
The authors declare no conflict of interest.
REFERENCES
- 1.Paulozzi LJ, Weisler RH, Patkar AA. A national epidemic of unintentional prescription opioid overdose deaths: how physicians can help control it. J Clin Psychiatry. 2011;72:589–592. [DOI] [PubMed] [Google Scholar]
- 2.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300: 2613–2620. [DOI] [PubMed] [Google Scholar]
- 3.Okie S A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. [DOI] [PubMed] [Google Scholar]
- 4.Nelson LS, Juurlink DN, Perrone J. Addressing the opioid epidemic. JAMA. 2015;314:1453–1454. [DOI] [PubMed] [Google Scholar]
- 5.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. [DOI] [PubMed] [Google Scholar]
- 6.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. [DOI] [PubMed] [Google Scholar]
- 9.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohio Governors’ Cabinet Opiate Action Team. Guidelines for prescribing opioids for the treatment of chronic, non-terminal pain. 2013. Available at: http://www.opioidprescribing.ohio.gov/OOAT_RX_Guidelines.html. Accessed January 12, 2016.
- 11.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2—guidance. Pain Physician. 2012;15:S67–S116. [PubMed] [Google Scholar]
- 12.Paone D, Dowell D, Heller D. Preventing misuse of prescription opioid drugs. City Health Inf. 2011;30:23–30. [Google Scholar]
- 13.The Management of Opioid Therapy for Chronic Pain Working Group. VA/DoD Clinical Practice Guideline: Management of Opioid Therapy for Chronic Pain. Washington, DC: Department of Veterans Affairs & Department of Defense; 2010. [Google Scholar]
- 14.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10: 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlan AD, Reardon R, Weppler C, et al. Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ. 2010;182:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arizona Department of Health Services. Arizona opioid prescribing guidelines. 2014. Available at: http://azdhs.gov/audiences/clinicians/index.php#guidelines-recommendations-home.
- 18.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 19.World Health Organization. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Geneva: World Health Organization; 2008. [Google Scholar]
- 20.Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30:605–612. [DOI] [PubMed] [Google Scholar]
- 21.Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among veterans health administration patients. Pain Med. 2014;15:1911–1129. [DOI] [PubMed] [Google Scholar]
- 22.Nelson LS, Juurlink DN. Tramadol and hypoglycemia: one more thing to worry about. JAMA Intern Med. 2015;175:194–195. [DOI] [PubMed] [Google Scholar]
- 23.Goodman F, Gordon A, Kivlahan D, et al. Critieria for use of Buprenorphine/Naloxone and Buprenorphine Sublingual Tablets. In: VHA Pharmacy Benefits Management; 2007. Available at: http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Buprenorphine_for_Opioid_Dependence_or_Opioid_Use_Disorder_Criteria_for_Use.pdf. [Google Scholar]
- 24.Centers for Disease Control and Prevention. National Death Index. Atlanta: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 25.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy JF, Valenstein M, Kim HM, et al. Suicide mortality among patients receiving care in the veterans health administration health system. Am J Epidemiol. 2009;169:1033–1038. [DOI] [PubMed] [Google Scholar]
- 27.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darke S Opioid overdose and the power of old myths: what we thought we knew, what we do know and why it matters. Drug Alcohol Rev. 2014;33:109–114. [DOI] [PubMed] [Google Scholar]
- 29.Darke S, Zador D. Fatal heroin ‘overdose’: a review. Addiction. 1996;91:1765–1772. [DOI] [PubMed] [Google Scholar]
- 30.National Center for Injury Prevention and Control. CDC Compilation of Opioid Analgesic Formulations With Morphine Milligram Equivalent Conversion Factors, 2014 Version. Atlanta: Center for Disease Control and Prevention; 2014. Available at: http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. [Google Scholar]
- 31.Valenstein M, Taylor KK, Austin K, et al. Benzodiazepine use among depressed patients treated in mental health settings. Am J Psychiatry. 2004;161:654–661. [DOI] [PubMed] [Google Scholar]
- 32.Bohnert AS, Ilgen MA, Ignacio RV, et al. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169:64–70. [DOI] [PubMed] [Google Scholar]
- 33.Park TW, Saitz R, Ganoczy D, et al. Benzodiazepine prescribing patterns and drug overdose deaths among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698 DOI: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 35.Edelman EJ, Gordon K, Becker WC, et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med. 2013;28:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pepe MS, Fan J, Seymour CW. Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol. 2013;20:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh R, Parikh S, Arun E, et al. Likelihood ratios: clinical application in day-to-day practice. Indian J Ophthalmol. 2009;57:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franklin GM, Mai J, Turner J, et al. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55:325–331. [DOI] [PubMed] [Google Scholar]
- 39.Paone D, Tuazon E, Kattan J, et al. Decrease in rate of opioid analgesic overdose deaths—Staten Island, New York City, 2011–2013. Morb Mortal Wkly Rep. 2015;64:491–494. [PMC free article] [PubMed] [Google Scholar]
- 40.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohnert AS, McCarthy JF, Ignacio RV, et al. Misclassification of suicide deaths: examining the psychiatric history of overdose decedents. Inj Prev. 2013;19:326–330. [DOI] [PubMed] [Google Scholar]
- 42.Liang Y, Turner BJ. Assessing risk for drug overdose in a national cohort: role for both daily and total opioid dose? J Pain. 2015;16: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.