Abstract
This review investigates the significant role polysaccharide particles play in functional drug delivery. The importance of these systems is due to the wide variety of polysaccharides and their natural source meaning that they can provide biocompatible and biodegradable systems with a range of both biological and chemical functionality valuable for drug delivery. This functionality includes protection and presentation of working therapeutics through avoidance of the reticuloendothelial system, stabilization of biomacromolecules and increasing the bioavailability of incorporated small molecule drugs. Transport of the therapeutic is also key to the utility of polysaccharide particles, moving drugs from the site of administration through mucosal binding and transport and using chemistry, size and receptor mediated drug targeting to specific tissues. This review also scrutinizes the methods of synthesising and constructing functional polysaccharide particle drug delivery systems that maintain and extend the functionality of the natural polysaccharides.
Keywords: polysaccharide, carbohydrate, functional drug-delivery, hydrogel
1. Introduction:
Polysaccharides are a popular basis for targeted therapeutic delivery systems because as natural biomaterials they often; (i) are available at large scale and are relatively inexpensive; (ii) have high biocompatibility and biodegradability; (iii) are non-toxic and non-reactogenic. This is demonstrated in their extensive use as excipients for traditional pharmaceutical formulations and in other clinical applications (Caliceti, Salmaso & Bersani, 2009; Castelli et al., 2008; Dass & Choong, 2008; Debele, Mekuria & Tsai, 2016b; Duncan, 2003; Kang, Opatz, Landfester & Wurm, 2015; Liu et al., 2008; Wen & Oh, 2014; Yang, Du, Liu & Zhai, 2015b). They also have physicochemical properties that both provides a convenient handle for chemical modification where desired and enables easy construction of particles and hydrogels for delivery purposes (Alhaique et al., 2015; Caliceti et al., 2009; Debele et al., 2016b; Liu et al., 2008; Nicolas et al., 2013; Wen & Oh, 2014). Additionally, specific polysaccharides can provide targeting mechanisms due to receptor recognition and binding (Yadav, Mishra & Agrawal, 2008), mucosal adhesion and transport (Feng et al., 2015; Ramasamy et al., 2013), site specific enzymatic degradation (Castelli et al., 2008; Vervoort et al., 1998a; Vervoort et al., 1997) and environmental triggering (Du et al., 2005; Elzatahry, Eldin, Soliman & Hassan, 2009; Maciel et al., 2013; Xue et al., 2015).
In using polysaccharide particles for drug delivery, the drugs can be absorbed into external compartments or bound to the external surface (Liu et al., 2008). This can enhance the aqueous solubility of the drug (Kang et al., 2015; Tan et al., 2011b; Zhang et al., 2013a) and increase stability of drugs and other unstable therapeutics, such as proteins (Hasegawa et al., 2009; Kang et al., 2015; Li, Shi, Du & Tang, 2007). Nanoparticles, in particular, are often used for drug delivery. For oral or nasal administration, their small size enables them to adhere to mucosal surfaces, after which transport occurs through intracellular pathways and defects in the mucosal epithelium (Sithole et al., 2017), allowing them to penetrate cells and cross mucosal tissue gaps (Dai Hai, Jong Hoon, Yoon Ki & Ki Dong, 2011; Li et al., 2007; Liu et al., 2008; Sandri et al., 2007). The small size of nanoparticles also enables them, when parenterally administered, to traverse the smallest blood vessels and avoid the phagocyte system, thereby extending the circulation half-life of attached drugs and potentially reducing the toxic effects of burst delivery (Liu et al., 2008). Parenterally delivered nanoparticles can also be designed to be concentrated in tumors due to the enhanced permeability and retention effect in which the leaky vasculature and impaired lymphatic clearance allows the buildup of nanoparticles inside tumors compared with normal tissue (Dai Hai et al., 2011; Debele et al., 2016b; Huh et al., 2010; Kang et al., 2015; Yang et al., 2015b; Yoon et al., 2014; Zhang et al., 2013a).
2. Polysaccharides used in Drug delivery systems
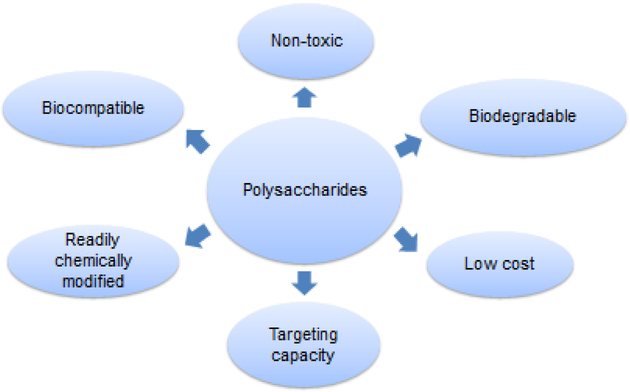
Polysaccharides are a complex collection of biopolymers isolated from plant, animal, microbial and algal sources that are built from monosaccharides linked by O-glycosidic linkages. They are often in high abundance, water soluble and easily processed and so provide relatively low cost biomaterials (Dass & Choong, 2008; Kang et al., 2015; Liu et al., 2008) that have an environmental advantage over petrochemical derived polymers and those that require non-aqueous solvents for processing (Šimkovic, 2008). Further, because polysaccharides are natural materials, cellular physiology is well adapted to them giving them generally excellent biocompatibility, biodegradability and low toxicity. This also means they have specific bioactivities and all of these factors can be exploited in the creation of drug delivery vehicles. Indeed, appropriately formulated systems are able to provide the advantages of both biodegradable (Poly[lactic-co-glycolic] acid; PLGA) and stealth (polyethylene glycol; PEG) synthetic polymers in a single component system (Liu et al., 2008; Nicolas et al., 2013). Other bioactivities of specific polysaccharides include the facility to modulate of the immune system, to stabilize labile therapeutics and their extensive and varied tissue-specific targeting capacity (Fig. 1). This varied range of functionality provides advantages for polysaccharide use in controlled release systems with regulatory issues simplified by the use of only simple polysaccharide components as a scaffold to build a sophisticated drug delivery system. An overview of polysaccharide particle use in drug delivery is summarized in Table 2 (at the end of the paper).
Figure 1:
Key benefits of polysaccharides in drug delivery systems
Table 2:
Overview of polysaccharide particle-based drug delivery systems
| Polysaccharide | Type of delivery system | Drug | Modification and benefit | Source | Reference |
|---|---|---|---|---|---|
| Chitosan | Polyelectrolyte hydrogel
|
|
Gelation with polysaccharide anions
|
N-Deacetylated chitin from shells of arthropods | (Boddohi et al., 2009; Bodnar et al., 2005; Chen et al., 2003; Cui & Mumper, 2001; Deng et al., 2014; Goycoolea et al., 2009; Huang et al., 2014; Li et al., 2007; Ramasamy et al., 2013; Sarmento et al., 2006b Feng, 2015 #143) |
Ionic crosslinking
|
|
|
(Amidi et al., 2006; Feng et al., 2015; Liu et al., 2008; Maestrelli et al., 2006; Sandri et al., 2007; Schütz et al., 2011) | ||
| Tunable size drug delivery particles | Crosslinked by peptide coupling to multi-carboxylic acid compounds | (Bodnar et al., 2005) | |||
| Hyaluronic Acid | Amphiphilic self-assembly
|
Etoposide Salinomycin Curcumin Doxorubicin Paclitaxel |
Hydrophobic modification can bind cell membrane and unpack hydrogel
|
Part of animal cell extracellular matrix | (Huang et al., 2014; Park et al., 2012; Tripodo et al., 2015; Wei et al., 2013; Yang et al., 2011) |
Polyelectrolyte hydrogel
|
Doxorubicin MicroRNA |
Gelation with chitosan
|
(Deng et al., 2014; Ramasamy et al., 2013) | ||
| Hyaluronic acid | Covalently crosslinked biodegradable hydrogel | Therapeutic peptides and proteins | Crosslinking groups
|
Part of animal cell extracellular matrix | (Hirakura et al., 2010; Tan et al., 2011a) |
| Dextran | Modified for polyelectrolyte hydrogel
|
Doxorubicin Therapeutic peptides and proteins | Sulfate group added to make anionic polysaccharide | Bacteria synthesise from sucrose | (Chen et al., 2003; Huh et al., 2017; Ramasamy et al., 2013; Sarmento et al., 2006b) |
Modified for amphiphilic self-assembly
|
Doxorubicin Bortezomib Curcumin |
Modified with drug to make amphiphile for self-assembly
|
(Liu et al., 2013; Xu et al., 2015a; Xu et al., 2015b) | ||
| Arabinogalactan | Self-assembled particles
|
Norcantharidin | Arabinogalactan, modified chitosan and drug self-assembled together into nanoparticles | Microbes and plants such as larch tress | (Caliceti et al., 2009; Dion et al., 2016; Zhang et al., 2018) |
| Starch | Hydrophobic modification binds and stabilises hydrophobic drugs | Flufenamic acid Testosterone Caffeine |
Hydrophobically modified with propyl groups for self-assembly into nanoparticles | Energy storage in many green plants | (Robyt, 2008; Santander-Ortega et al., 2010) |
| • Cyclodextrin From enzymatic degradation of starch | Hydrophobic cavity incorporated into a range of drug delivery vehicles | Co-loading of ciprofloxacin and 3-methyl benzoic acid Triclosan Furosemide Retinoic acid |
Chemically crosslinked into dextran, agar and hydroxypropyl methyl cellulose hydrogels Non-covalent association with chitosan / tripolyphosphate ionic crosslinked system. | (Blanco-Fernandez et al., 2011; Caliceti et al., 2009; Maestrelli et al., 2006; Moya-Ortega et al., 2012; Peng et al., 2010) | |
| • Cyclodextrin From enzymatic degradation of starch | |||||
| • Cycloamylose From enzymatic degradation of starch | Amphiphilic self-assembly
|
siRNA shRNA |
Cycloamylose modified with both cholesteryl and spermine groups for self-assembly into particles capable of binding RNA | (Fujii et al., 2014) | |
| Pullulan | Amphiphilic self-assembly
|
Model protein IRDye 800 | Hydrophobic modification with cholesteryl groups | Converted from starch by fungus | (Singh et al., 2017). (Akiyoshi et al., 1991; Morimoto et al., 2013; Noh et al., 2012) |
PEI modification assembles into particles
|
siRNA | Modified with PEI for DNA binding and protection | (Kang et al., 2010) | ||
| Inulin | Semi-crystalline inulin microparticles
|
Self-assembled under specific conditions and attached to drugs through bio-labile linkages | Compositae family plants | (Stevens et al., 2001; Wang et al., 2017) | |
Inulin hydrogels deliver drugs to the colon
|
Diflunisal 5-Fluorouracil |
Modified with methacryl groups - crosslinked directly or through diacrylate linkers
|
(Castelli et al., 2008; Pitarresi et al., 2012; Vervoort et al., 1998a) | ||
| Cellulose | Covalently crosslinked hydrogel with redox sensitive linkages | Hydroxylpropyl cellulose modified and crosslinked with dithiol linkages | Structural component of many plants | (Rahimian et al., 2015; Senna et al., 2014; Tan et al., 2011b) | |
Covalently crosslinked hydrogel with redox sensitive linkages
|
Doxorubicin | Carboxymethyl cellulose crosslinked with cystamine bisacrylamide to form nanohydrogels | (Qian et al., 2014) | ||
| Hemicellulose | Polyelectrolyte hydrogels | Model protein | Carboxymethyl modification followed by gelation with chitosan | In cell walls of trees and grasses | (Du et al., 2004) |
| Alginic Acid | Ionic crosslinking
|
Doxorubicin Therapeutic proteins |
Self-assembly with calcium ions
|
Brown seaweed | (Elzatahry et al., 2009; Lee et al., 2013; Pawar & Edgar, 2012; Sarmento et al., 2006a; Xue et al., 2015) |
Polyelectrolyte hydrogel
|
Isoniazid, rifampicin, pyrazinamide | Gelation with chitosan | (Li et al., 2007; Zahoor et al., 2005) | ||
| Chondroitin Sulfate | Amphiphilic self-assembly
|
Doxorubicin | Hydrophobic modification with acetate groups for self-assembly into particles | Component of animal cartilage | (Park et al., 2010; Shi et al., 2014) |
| Heparin | Polyelectrolyte hydrogel
|
Model protein Paclitaxel | Gelation with chitosan | Secreted by mast cells | (Yang et al., 2015b) (Boddohi et al., 2009; Liu et al., 2007; Yuk et al., 2012) |
Amphiphilic self-assembly
|
Dexamethasone Doxorubicin |
Modified with drug to make amphiphile for self-assembly
|
(Li et al., 2014) | ||
| Covalently crosslinked hydrogel with redox sensitive linkages | Doxorubicin | Methacrylate modified heparin crosslinked with cystamine bisacrylamide to form nanohydrogels | (Wu et al., 2015) | ||
| Gums • Pectin |
Oral delivery to colon
|
Protein and polypeptide drugs | Self-assembly with calcium ions to resist solvation | Higher plant cell walls, especially fruits and vegetables | (Sirisha & D’Souza, 2016) (Izydorczyk, Ciu & Wang, 2005) |
| • Gum Arabic | Biphasic emulsion supporting hydrophobic drugs | Metronidazole | Gelation of surfactant supported oil in water emulsion | Acacia Senegal | (Sahoo et al., 2015; Sirisha & D’Souza, 2016) |
Potential drawbacks to the use of polysaccharides in drug delivery include that they can have broad and/or mixed molecular weights, and some polysaccharide chemistry is quite variable making it difficult to precisely define the delivery vehicle. Lack of solubility of many polysaccharides in most organic solvents also limits the potential for chemical modification (Wen & Oh, 2014). Finally, polysaccharide-based drug delivery platforms often require slow enzymatic degradation of the biopolymer ideal for sustained release but not if rapid release is desired. In this case, stimuli responsive carriers are potentially valuable advancements on standard polysaccharide systems (Šimkovic, 2008).
2.1. Chitosan
Chitin is a polysaccharide contained in the cell walls of fungi and in the shells of arthropods such as crustaceans and consists of a linear chain of 2-acetaylamino-2-deoxy-β-D-glucopyranose units connected through β-1,4 linkages (Bodnar, Hartmann & Borbely, 2005). Chitin undergoes N-deacetylation to make chitosan, a non-toxic, biocompatible and enzymatically biodegradable derivative containing amino groups, making it the only essentially natural cationic polysaccharide readily isolated in high yields. The amino groups provide solubility in mildly acidic solution to the polymer and are useful for chemical modification and electrostatic interactions in drug delivery systems (Bodnar et al., 2005; Hu & Luo, 2016; Liu et al., 2017; Sarmento et al., 2006b; Valmikinathan et al., 2012; Wang, Buschle-Diller & Misra, 2015). Further, despite the amino groups and positive change, chitosan has very low immunogenicity when compared with synthetic cationic polymers and can be used as a bioadhesive, sticking to negatively charged mucosal cells and increasing the retention properties and transport into cells (Elzatahry et al., 2009; Feng et al., 2015; Goycoolea et al., 2009; Li et al., 2007; Maestrelli, Garcia-Fuentes, Mura & Alonso, 2006; Ramasamy et al., 2013; Sandri et al., 2007). Assisting this transport, chitosan’s positive charge also enables it to enhance opening of epithelial tight junctions and widen paracellular pathways, assisting delivery of drugs including hydrophilic and high molecular weight molecules (Li et al., 2007; Sandri et al., 2007) to tissues of interest (Berger et al., 2004; Goycoolea et al., 2009; Sandri et al., 2007). Finally, chitosan has bioactivity as an antioxidant, an antimicrobial and an antifungal (Bodnar et al., 2005; Divya, Smitha & Jisha, 2018). All these factors mean that chitosan has been used extensively in polysaccharide medical applications (Berger et al., 2004; Bodnar et al., 2005; Caliceti et al., 2009; Huh et al., 2017; Liu, Jiao, Liu & Zhang, 2007; Ramasamy et al., 2013; Wen & Oh, 2014).
Despite the advantages of chitosan in drug delivery, it suffers from insufficient water solubility for some applications in which case it has been further modified by degradation into shorter chains by both physical (Sieval, Thanou, Kotze & Verhoef, 1998) and chemical (Cui & Mumper, 2001; Li et al., 2007) means and/or modification with hydrophilic groups such as carboxymethyl groups (Wang et al., 2010) and quaternary ammonium groups (Liu et al., 2008). Additionally, the quaternary ammonium groups provide fixed charge independent of pH to enhance adhesion and uptake through mucosa (Li et al., 2007; Sandri et al., 2007). Also, chitosan can be modified at the C-6 hydroxyl group to form glycol chitosan, a highly water soluble, biocompatible and biodegradable polysaccharide with low toxicity (Liu & Yao, 2014).
2.2. Hyaluronic Acid/Hyaluronan
Hyaluronic acid is a linear chain of alternating D-glucuronic acid and N-acetyl-D-glucosamine units linked by β-1,4 and β-1,3 glycosidic linkages and often has very high molecular weights and predominantly is in the anionic form under physiological conditions (Luo, Kirker & Prestwich, 2000; Tripodo et al., 2015; Varghese et al., 2010). It is formed in the plasma membrane and is a key component of the extracellular matrix, contributing to cell movement and proliferation, and so is distributed widely in animal tissues (Tripodo et al., 2015). As hyaluronic acid has relatively high abundance in parts of the human body, it naturally has high biocompatibility, is degraded by native enzymes (Hachet et al., 2012) and has low immunogenicity (Luo et al., 2000; Varghese et al., 2010). Further, it’s bioactivity includes functionality that can be exploited in targeted therapeutics, most prominently its binding to CD44 receptors that are upregulated in cancer cells (Choi et al., 2014; Ramasamy et al., 2013; Tripodo et al., 2015). Its degradation products interact with receptors on macrophages and dendritic cells, resulting in innate immunity activation (Caliceti et al., 2009; Huh et al., 2017). This functionality combined with the acid group being a convenient handle for easy chemical modification using esterification and peptide coupling reactions means hyaluronic acid has been extensively used in drug delivery (Luo et al., 2000; Tripodo et al., 2015).
2.3. Dextran
Dextran is synthesized from sucrose by bacteria and comprises glucose units linked predominantly with α-1,6 glycosidic bonds to make a chain from which α-1,3 glycosidic linkages create branches (Caliceti et al., 2009; Huh et al., 2017). This results in a biocompatible and biodegradable biopolymer that has low cytotoxicity (Susa et al., 2010) and while low molecular weight forms are rapidly removed by the kidneys, higher molecular weight forms have prolonged residence in the circulation (Caliceti et al., 2009). Combined with these properties, the capacity of dextran to bind red blood cells and platelets, increasing their electronegativity and reducing aggregation, means it is used medically as an anticoagulant. Dextran is modified with sulfate groups to form a highly anionic polymer that retains its biocompatibility (Ramasamy et al., 2013; Sarmento et al., 2006b). The overall negative charge means dextran sulfate does not interact with cells and so assists in prolonging residence of drug delivery vehicles in the circulation (Caliceti et al., 2009).
2.4. Arabinogalactan
Arabinogalactan is a complex, branched polysaccharide of arabinose and galactose isolated from both microbes and plants. Medicinally, arabinogalactan has been used for several purposes including complexation with drug molecules to increase their bioavailability (Khvostov et al., 2017; Kong et al., 2018) and arabinogalactan from larch trees has immunomodulatory properties that enhances resistance to the common cold (Dion, Chappuis & Ripoll, 2016). The galactose termination of arabinogalactan also enables binding to asialoglycoprotein receptors and so can be used for targeting both the liver and cancer tumors (Caliceti et al., 2009; Zhang et al., 2018).
2.5. Starch
Starch is a glucose-based polysaccharide produced by many green plants to store energy. It is made up of amylose, linear chains of α-D-glucose connected through α-1,4 glycosidic bonds, and amylopectin in which branches are created in amylose chains through α-1,6 glycosidic bonds to other amylose chains (Robyt, 2008). Starch is non-toxic and non-immunogenic, biocompatible and biodegradable and is easily isolated in a highly pure form. Hence, starch is used extensively as an excipient in pharmaceutical products (Zhang et al., 2013a), either unmodified or often after partial hydrolysis (Robyt, 2008). Without hydrolysis starch is often insoluble and the semi-crystalline nanomaterials found in starch (Blazek & Gilbert, 2011) have been used to modify the properties of drug delivery systems to improve sustained release (Lin et al., 2011) and directly to enhance drug delivery (Odeniyi, Omoteso, Adepoju & Jaiyeoba, 2018). To improve the properties of starch for drug delivery, is has been modified in a range of ways (Masina et al., 2017) such as hydrophobic modification (Santander-Ortega et al., 2010) hydrophilic modification (Quadrado & Fajardo, 2018) and crosslinking (Zhang et al., 2013a).
2.5.1. Hydrolyzed Starch: Dextrin, Cyclodextrin and Cycloamylose
Compared to starch, partial hydrolysis into dextrin provides a lower molecular weight meaning it can be easier to physically and chemically manipulate and renal elimination prevents tissue accumulation (Das & Pal, 2015). Enzymatic degradation of starch forms natural cycloamyloses and smaller cyclodextrins that have 6, 7 and 8 glucopyranose linked by α-1,4 glycosidic linkages into a cyclic oligomer containing a hydrophobic cavity (Caliceti et al., 2009). The hydrophobic cavity of cyclodextrins can bind hydrophobic drugs, assisting in stabilization and solubilization of those drugs in delivery systems (Caliceti et al., 2009), enhancing their bioavailability (Maestrelli et al., 2006). Cyclodextrin has also been shown to have immunological activity and has been proposed as part of a vaccine adjuvant delivery system (Kusakabe et al., 2016; Onishi et al., 2015).
2.6. Pullulan
Pullulan is converted from starch by a fungus to make a linear polysaccharide containing maltotriose units connected by α-1,6 glycosidic linkages. Pullulan is non-toxic, has low immunogenicity and high water solubility, all valuable features for drug delivery (Kaneo, Tanaka, Nakano & Yamaguchi, 2001; Singh, Kaur, Rana & Kennedy, 2017). Drug delivery using pullulan has also been supported by derivatization of pullulans through its hydroxyl groups to provide the desired physico chemical properties (Singh et al., 2017). This includes hydrophobic modification for drug delivery particle formation using amphiphilic self-assembly (Morimoto et al., 2013) and polyethyleneimine modification for DNA binding and protection (Kang et al., 2010). It also has high affinity for asialoglycoprotein receptors overexpressed in the liver and in cancer cells and so can be used for drug targeting (Huh et al., 2017; Kaneo et al., 2001; Kang et al., 2010).
2.7. Inulin
Inulin is an energy storage polysaccharide found in plants of the Compositae family (Stevens, Meriggi & Booten, 2001) made up of linear chains of fructosyl groups linked by β-2,1 glycosidic bonds and terminated at the reducing end by an α-D-1,2 glucopyranoside ring (Barclay, Ginic-Markovic, Cooper & Petrovsky, 2010). Inulin has a GRAS regulatory classification and is used as an excipient in tablet formulations and soluble forms are used to test glomerular filtration by the kidneys (Barclay et al., 2010). Specific semicrystalline particulate forms known as delta inulin have immunomodulatory properties exploited as a vaccine adjuvant (Cooper, Barclay, Ginic-Markovic & Petrovsky, 2013; Cooper & Petrovsky, 2011). Inulin is also indigestible to humans, but bifidobacteria in the gut can metabolize inulin providing a probiotic effect as well as other benefits including colon targeted drug delivery (Castelli et al., 2008; Vervoort et al., 1998a; Vervoort et al., 1997). Modification of inulin for medical applications commonly uses periodate oxidation followed by reaction with amine chemistry (Wang et al., 2017) or functionalization through reactions with anhydrides (Afinjuomo et al., 2018).
2.8. Cellulose
Cellulose is an important structural component of many plants made up of a linear chain of D-glucopyranosyl units linked by β-1,4 glycosidic bonds and is the most abundant biopolymer on earth (Senna, Novack & Botaro, 2014). Nonetheless, the natural form readily crystallizes requiring exotic solvents for easy solubility (Kono & Fujita, 2012; Senna et al., 2014) and so has limited use in pharmaceutical products. Instead, cellulose modified to disturb its crystallinity, such as in hydroxypropyl cellulose, which is soluble in water, non-toxic and biodegradable and approved for use in medicines by the FDA (Tan et al., 2011b). Hydroxypropyl cellulose is thermally sensitive with tight aggregates prepared at high temperature forming loose aggregates as the temperature is reduced (Rahimian, Wen & Oh, 2015; Tan et al., 2011b). Also, cellulose esters such as cellulose acetate have better solubility in organic solvents than cellulose (Senna et al., 2014) and have been become common components in drug delivery systems in which some processing in organic solvents is required (Oliveira et al., 2013).
2.9. Hemicellulose
Hemicellulose is present along with cellulose in plant cell walls, in particular woods and grasses, and so is a highly abundant and renewable resource (Voepel, Edlund & Albertsson, 2009). Hemicellulose varies from cellulose in being chemically heterogeneous, and different types can be made from a range of sugar monomers and specific varieties of hemicellulose include:
Glucomannan is a random linear copolymer of glucose and mannose linked by 1,4 glycosidic bonds with a small amount of branching through 1,6 glycosidic linkages (Katsuraya et al., 2003). For drug delivery, this polysaccharide has been modified to a carboxy methyl version to provide carboxylate groups (Du et al., 2004).
Galactoglucomannan is a glucomannan chain in which the mannose residues are partially substituted with both galactose groups through 1,6 glycosidic linkages and acetyl groups at C2 and C3 hydroxyl groups of the mannose. Relatively low molecular weight makes this species soluble in water and key polar non-protic solvents.
2.10. Alginic acid
Alginic acid is part of the cell walls of brown seaweeds and comprises a linear block copolymer of β-D-mannuronic acid and α-L-guluronic acid residues all linked with 1,4 glycosidic bonds (Lee et al., 2013; Li et al., 2007; Pawar & Edgar, 2012; Zahoor, Sharma & Khuller, 2005). Alginic acid’s structure and chemistry means it readily gels aqueous solution, with gel properties determined by the arrangement of monosaccharide units (Pawar & Edgar, 2012). It is also biocompatible and biodegradable and so has been used extensively in pharmaceutical hydrogels (Goycoolea et al., 2009; Li et al., 2007; Richardson, Dettmar, Hampson & Melia, 2004). These alginate gels are bioadhesive, readily attaching to mucosal surfaces when sufficiently hydrated (Li et al., 2007; Richardson et al., 2004). Alginate can also form nanoparticles that can be stabilized by the addition of cationic polyelectrolytes that coat the surface (Rajaonarivony et al., 1993; Sarmento, Ferreira, Veiga & Ribeiro, 2006a), such as chitosan (Li et al., 2007; Zahoor et al., 2005).
2.11. Chondroitin Sulfate
Chondroitin Sulfate is a structural component of cartilage and is a linear, alternating copolymer D-glucuronic acid and N-acetyl D-galactosamine residues linked by β-1,3 glycosidic linkages and sulfated to varying extents at C-4 and C-6 of the galactosamine residue and C-2 and C-3 of the glucuronic acid residue (Shi et al., 2014). This polysaccharide is highly biocompatible and a range of bioactivities help to promote bone healing and growth and so is used in the treatment of osteoarthritis (Shi et al., 2014).
2.12. Heparin
Heparin is a negatively charged, linear, heterogeneous glycosaminoglycan of relatively low molecular weight made up of a number of different disaccharide repeating units connected through α-1,4 glycosidic bonds and containing carboxylate, acetamido, sulfamido and sulfate groups as well as occasional free amines (Liu et al., 2007; Yang et al., 2015b). It is secreted by mast cells at sites of tissue injury and may provide antibacterial defense, though its biological role is unclear. Nonetheless, as an endogenous polysaccharide it is non-toxic and has useful biological activity (Yang et al., 2015b). For example, heparin interacts with proteins associated with cancer including heparinase, an enzyme causally involved in inflammation and tumorigenesis associated with colitis (Vlodavsky et al., 2011). Hence, heparin can be used to inhibit tumor formation, growth and metastasis and to target anticancer treatments (Chung et al., 2010; Debele et al., 2016b; Wu et al., 2015; Yang et al., 2015b). Heparin is used as an anticoagulant because a unique pentasaccharide of heparin specifically binds antithrombin III (Yamazaki et al., 2000; Yang et al., 2015b). This offers an alternate route for cancer targeting as tumors tend to have networks of clotted plasma proteins that will bind heparin (Yang et al., 2015b; Yuk et al., 2012).
2.13. Natural Polysaccharide Gums
Guar gum, xanthum gum, gum Arabic, carrageenan gum and pectin are amongst polysaccharides capable of causing a large increase in the viscosity of an aqueous solution, becoming hydrocolloids (BeMiller, 2008). They often do this at very low concentration due to their ability to form junctions of intermolecular interactions between two different polymer chains, creating three dimensional networks (BeMiller, 2008). Such networks are very useful to contain and protect drugs during drug delivery (Sirisha & D’Souza, 2016). Pectin is an example that is used in oral drug delivery to the colon when bound through noncovalent interactions with calcium ions to form microspheres. This is due to the microspheres capacity to resist swelling and breakup in the stomach and small intestine while being degraded by pectinases in the colon (Sirisha & D’Souza, 2016). Also, gum Arabic has been used gelate biphasic emulsions to support hydrophobic drug transport (Sahoo et al., 2015) and carrageenan used to make polyelectrolytes with bovine serum albumin that were templated into nanotubes for drug delivery (Maldonado et al., 2019).
3. Functional Drug Delivery using Polysaccharides
A range of issues can be overcome in pharmaceutical development using functional design of drug delivery vehicles. For example, a pharmaceutical agent administered by any route will encounter a range of physical and physiological barriers to transport to sites that require treatment. It is also often important to maintain a therapeutic drug concentration at the treatment site while minimizing systemic availability and subsequent toxicity (Debele et al., 2016b). Finally, a difficult goal is to increase the bioavailability of unstable and poorly soluble therapeutics. As such, drug delivery systems that enhance the bioavailability of drugs and increase transport to target sites are highly valuable (Debele et al., 2016b). Polysaccharide-based particulate delivery systems can achieve this in a number of ways, as detailed below and summarized in Table 2 (at the end of the paper).
3.1. Preventing opsonization
Often small molecule drugs that enter the bloodstream are rapidly eliminated by the kidneys. This can be addressed by the use of particulate drug delivery systems, but these can then be opsonized and targeted by the reticuloendothelial system for destruction. As such, synthetic PEG has been used to provide a stealth coating that protects drug delivery systems from being opsonized and subsequent phagocytic destruction, thereby extending the circulatory half-life of drug delivery vehicles (Knop, Hoogenboom, Fischer & Schubert, 2010). Despite the continued extensive use of PEG in pharmaceutical products approved by regulatory authorities, PEG is known to have issues with biocompatibility and biodegradability (Kang et al., 2015; Knop et al., 2010). For example, long PEG chains can accumulate in kidney cells resulting in renal damage (Bendele & et al., 1998; Conover, Gilbert, Shurn & Shorr, 1997) and shorter chains can be enzymatically oxidized resulting in toxic byproducts (Herold, Keil & Bruns, 1989). Additionally, PEGylated drug delivery vehicles can through complement activation result in anaphylactoid reactions in up to 45% of patients (Chanan-Khan, 2003; Van Den Hoven et al., 2013).
A number of polymeric alternatives have been suggested to replace PEG in drug delivery systems, but with many synthetic alternatives having similar problems of biocompatibility and/or biodegradability to those of PEG (Knop et al., 2010). Polysaccharides offer similar abilities to PEG to reduce non-specific protein binding onto drug delivery vehicles. Further, most polysaccharides are highly biocompatible, with biocompatible products of biodegradation, and have low immunogenicity (Debele et al., 2016b; Huh et al., 2017; Kang et al., 2015) and can be used similarly to extend blood residence of drug delivery vehicles, which is essential to many treatments (Yang, Kootala, Hilborn & Ossipov, 2011).
3.2. Biomacromolecule stabilization
Therapeutic biomacromolecules such as proteins and DNA and their related species commonly suffer from poor stability in aqueous solution, limiting their shelf-life. Further, they may be expensive and difficult to produce in large scale. Hence, drug delivery vehicles that can both stabilize and target these species are very important (Amorij et al., 2008; Carpenter & Crowe, 1989; Hasegawa et al., 2009; Hinrichs, Prinsen & Frijlink, 2001; Huh et al., 2017; Huh et al., 2010; Oliver et al., 2001; Susa et al., 2010). This can be achieved with polysaccharide-based hydrogels that encapsulate unstable biomacromolecules, preventing aggregation and providing protection from degradative systems within the body (Hirakura et al., 2010; Tan et al., 2011b).
3.2.1. Proteins
One polysaccharide system extensively studied for the stabilization of proteins is nanoparticle hydrogels self-assembled from cholesterol-modified pullulan that have hydrophobic cores in which the proteins reside (Akiyoshi, Yamaguchi & Sunamoto, 1991; Hasegawa et al., 2009; Hirakura et al., 2010; Kazunari Akiyoshi & Junzo, 2001). These particles prevent aggregation of previously denatured proteins and even enabled protein refolding through a mechanism resembling molecular chaperone proteins. When released, these proteins regained almost 100% of original protein activity (Akiyoshi, Sasaki & Sunamoto, 1999). Proteins have also been protected against drying using polysaccharides that lack reducing groups and dry to glassy polymers with a low crystallization rate. These types of polysaccharides stabilize proteins and peptides as they provide multiple hydroxyl groups to interact with polar groups, helping maintain the biomacromolecules’ native conformation and preventing denaturation during the drying process (Amorij et al., 2008; Carpenter & Crowe, 1989; Hinrichs et al., 2001; Oliver et al., 2001; Slade & Levine, 1991).
3.2.2. DNA
Cationic polymers provide a method to electrostatically bind and protect negatively charged DNA and RNA strands. The resulting complex can be formed into particles or added to particles with the appropriate size for efficient uptake into the cytoplasm (Howard et al., 2006; Köping-Höggård, Tubulekas & Guan, 2001; Raemdonck et al., 2010; Susa et al., 2010). Chitosan is a suitable option for such applications as it has both lower toxicity (Howard et al., 2006; Köping-Höggård et al., 2001; Maksimenko et al., 2005) and increased stability and loading of oligonucleotides (Maksimenko et al., 2005) as compared to other positively charged polymers. Chitosan has also been modified with spermine to increase the number of cationic groups to improve transfection properties (Jiang et al., 2011). Additionally, chitosan has been subjected to N-acetylation post DNA binding to reduce the acid solubility of the chitosan to enhance transfer through the stomach when orally administered for delivery to the intestines (Kai & Ochiya, 2004). Other polysaccharides have also been modified with cationic groups to bind and protect oligonucleotides. This includes dextran modified with amino or ammonium groups (Azzam et al., 2004; Naeye et al., 2011; Raemdonck et al., 2010) and pullulan modified with polyethyleneimine. In the latter case, the toxicity of polyethyleneimine was significantly reduced by combining with pullulan without significantly effect on the efficacy of oligonucleotide delivery (Kang et al., 2010).
3.3. Recognition, binding and membrane transport
Cell communication is required for the appropriate cellular function and communication between cells requires that signals are received at the surface and then delivered internally. Carbohydrates play a key role in this signaling, with glycolipids and glycoproteins including enzymes, immunoglobulins and lectins binding carbohydrates (Kang et al., 2015). Then lectins transduce signals and in some cases promote subsequent intracellular transport (David, 2010). Consequently, glyco-targeting strategies have been developed based on lectin-mediated endocytosis of drug delivery vehicles, allowing release of the drug within the cell of interest. For example, some types of cancer cells overexpress specific receptors that can be targeted with carbohydrate-based delivery systems (David, 2010; Kang et al., 2015; Park et al., 2012; Yamazaki et al., 2000). To achieve this, direct glycosylation of drugs has been tried to enhance drug delivery, but these small molecules are generally rapidly cleared from the blood circulation by the kidneys (Davis & Robinson, 2002). Polymer conjugates can serve as better targeting vectors and small molecule carbohydrate residues bound to polymeric drug delivery vehicles have exploited lectin and related binding (Ahmed & Narain, 2015; Davis & Robinson, 2002; Sunasee, Adokoh, Darkwa & Narain, 2014). While these systems manage to avoid some of the difficulties in working with polysaccharides (Sunasee et al., 2014), they do miss out on the excellent biocompatibility and biodegradability that make polysaccharides so valuable in drug delivery (Ahmed & Narain, 2015). This again highlights the benefit of using polysaccharides for multiple purposes within one vehicle (e.g. targeting and drug encapsulation) to maximize the overall potential of the drug delivery system.
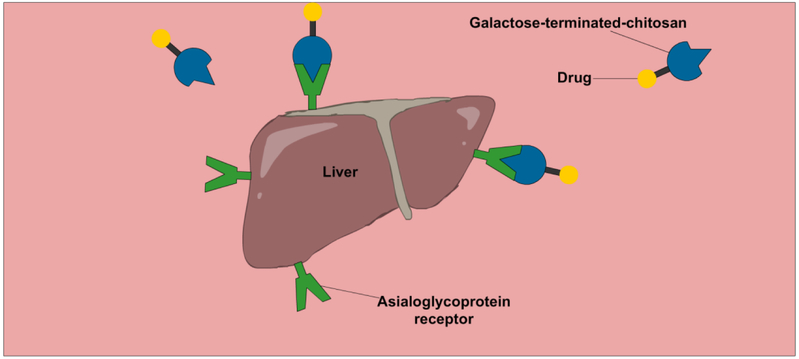
Asialoglycoprotein receptors are highly expressed in the liver. Consequently, high affinity ligands for the asialoglycoprotein receptors such as asialofetuin, N-acetyl glucosamine and galactose have been exploited for liver drug delivery (Ahmed & Narain, 2015). Chitosan has also been modified with galactose terminated disaccharides for this task (Fig.2) (Kato, Onishi & Machida, 2001; Park et al., 2000). Pullulan also binds asialoglycoproteins receptors, though more weakly, and so has similarly been used to target the liver (Ahmed & Narain, 2015; Kaneo et al., 2001; Kang et al., 2010). The asialoglycoprotein receptor, galectins, selectins, mannose receptors and hyaluronic acid receptors are all overexpressed by specific cancer cells and so associated carbohydrates can be used for targeting tumors (David, 2010; Kang et al., 2015; Park et al., 2012; Yamazaki et al., 2000). For example, hyaluronic acid has a number of binding receptors on the human cell surface (Yadav et al., 2008) of which one called CD-44 is highly overexpressed in cancer cells (Gibbs et al., 2008). As such, nanoparticles containing hyaluronic acid have been exploited for CD-44 mediated binding and endocytosis into cancer cells for targeted delivery of cancer therapeutics, providing improved anticancer efficacy (Yang et al., 2011) and delivery (Choi et al., 2014). Heparin is another polysaccharide that can target cancer cells due to its high affinity binding and transport into dividing vascular and endothelial cells that are in high abundance in tumors (Yang et al., 2015b). Also, heparin binds antithrombin III (Yamazaki et al., 2000), and so can target cancer tumors due to the associated clotting. This binding of heparin also means isolated chains have anticoagulant properties that may or may not be beneficial for drug delivery. These properties are greatly reduced in aggregated forms such as particles (Wu et al., 2015), while the anticancer activity is retained (Yang et al., 2015b).
Figure 2:
A graphic illustration of chitosan-based drug delivery system used to target asialoglycoprotein receptor
3.4. Immune system interactions
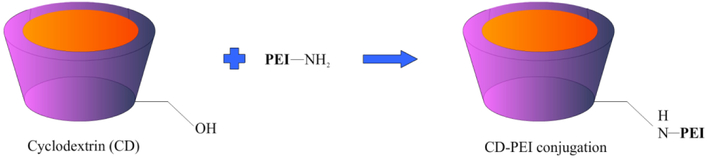
The immune system can be exploited by therapeutics to prevent and treat disease, most notably in the example of vaccination. For example, specific semi-crystalline particulate forms of inulin called delta inulin (Barclay et al., 2016; Cooper et al., 2014; Cooper et al., 2015) have immunomodulatory properties that have been extensively exploited as a vaccine adjuvant (Cooper & Petrovsky, 2011; Feinen, Petrovsky, Verma & Merkel, 2014; Gordon et al., 2014; Honda-Okubo, Kolpe, Li & Petrovsky, 2014; Saade, Honda-Okubo, Trec & Petrovsky, 2013; Silva, Cooper & Petrovsky, 2004) and could also be effective in boosting cancer treatments (Korbelik & Cooper, 2007). Their rapid uptake by monocytes and transport to lymph nodes, also suggests potential applications in drug delivery (Wang et al., 2017). Polyethyleneimine (PEI) also has adjuvant properties although its toxicity is a problem. This can be reduced by attachment to cyclodextrin that allowed the use of PEI as an adjuvant with highly efficient transport to lymph nodes (Fig. 3) (Li et al., 2017a). The toxicity of PEI has also been reduced when bound with neutral pullulan (Kang et al., 2010) and charged heparin (Liu et al., 2014; Yang et al., 2015b), exploited in these cases for therapeutic delivery. Other polysaccharides exploited in vaccines include cyclodextrin that has immunological and lymph node targeting activity (Kusakabe et al., 2016; Onishi et al., 2015). Similarly, dextran has been used for targeted delivery of CpG to lymph nodes, thereby increasing anti-tumor efficacy (Zhang, An, Xi & Liu, 2017). Notably, in all the above examples, the polysaccharide is being used for dual purposes, one being its immune modulatory effects and the other its ability to target lymphoid tissue.
Figure 3:
An example of CD-PEI complex synthesis
3.5. Mucosal adhesion and permeation
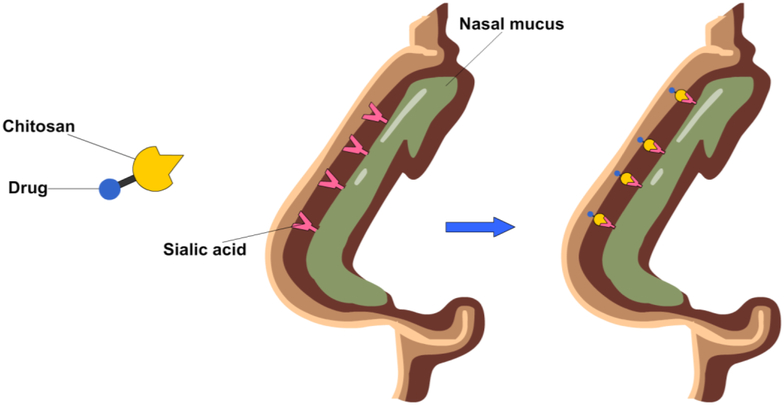
Parenteral administration of drugs has clinical advantages over other methods, such as oral and nasal administration, overcoming issues of drug stability and transport from digestive and respiratory systems into the bloodstream (Francis, Cristea & Winnik, 2004), though is not patient preferred. To improve the effectiveness of oral and nasal administration, drug delivery systems are needed that can maximize residence time and hence opportunities for the drug to cross the epithelium, increasing drug absorption into the circulatory system (Elzatahry et al., 2009; Sithole et al., 2017). Polysaccharides such as chitosan, hyaluronic acid, starch, oxidized dextran and alginate have all been exploited in this way for drug delivery (Elzatahry et al., 2009; Goycoolea et al., 2009; Huang et al., 2014; Saremi et al., 2013; Sithole et al., 2017). For example, chitosan can bind sialic acid groups (Elzatahry et al., 2009) from the mucus, enhancing mucoadhesion and mucopermeation, increasing transport through the mucus membrane (Fig. 4) (Elzatahry et al., 2009; Liang et al., 2019). The increase in transport across the intestinal barrier provided by chitosan incorporated into oral drug delivery vehicles has enabled a 10-fold increase in bioavailability and a 9-fold increase in half-life of poorly water soluble drugs in murine models (Saremi et al., 2013). Chitosan and alginate have also been used for nasal delivery, which allows these vehicles to avoid metabolism in the liver that otherwise occurs with intravenous administration (Goycoolea et al., 2009; Howard et al., 2006; Iqbal et al., 2003; Köping-Höggård et al., 2001). For such vehicles, transmucosal delivery across epithelial barriers is achieved due to chitosan’s capacity to widen cell tight junctions (Goycoolea et al., 2009; Huang et al., 2014). This enhances transport and maintains activity of bulky biomacromolecules, such as proteins (Goycoolea et al., 2009). In a contrasting example, phosphorylated polysaccharides are negatively charged and so avoid interactions with mucus when taken orally. In the gut, intestinal alkaline phosphatase cleaves the phosphate groups, changing the external charge and allowing binding to the negatively charged membrane surfaces thereby promoting drug absorption and cell uptake, improving mucus diffusion of the drug delivery vehicle by up to 3-fold (Griesser et al., 2017).
Figure 4:
An illustration of chitosan-based drug enhancing nasal delivery through increased mucosal adhesion
3.6. Swelling controlled polysaccharide hydrogels
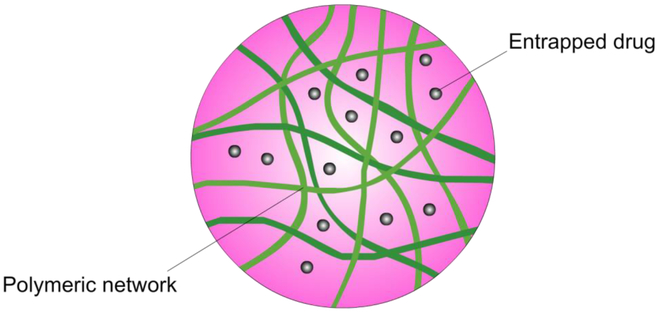
Most polysaccharides share the chemical characteristic of having multiple polar groups meaning that they tend to be hydrophilic and can form non-covalent crosslinks between chains in aqueous dispersions, creating hydrogels. Hydrogels are useful in drug delivery as they can be can loaded with drugs suspended in aqueous solutions and then dried, entrapping the drug in the network. Subsequently drugs are released as they swell on contact with aqueous bodily fluids. These systems can be tuned for desired sites and rates of release, optimizing drug delivery (Day, Barclay, Song & Garg, 2018). Many natural polysaccharides can form gels and useful gels can be formed for some polysaccharides in interaction with borax and there is synergistic gel-forming interaction between xanthan gum and locust bean gum (Copetti, Grassi, Lapasin & Pricl, 1997). However, more often the properties of the gels are not appropriate to act in drug delivery. This means that polysaccharides are often chemically modified to either improve the basic physical properties of the gel structure or tune them for optimized therapeutic uptake, storage and delivery (Liang et al., 2019; Senna et al., 2014). Such hydrogels are the overwhelmingly dominant use of polysaccharides in pharmaceutical science, including the majority of the particulate systems described in this review. Compared to the bulk swelling systems used often in oral delivery and as depots (Liang et al., 2019), polysaccharide hydrogel particulate delivery systems have benefits of using parenteral and transmucosal modes of delivery. This means the drug delivery system is mobile and can be used to target tissues of interest (Ganguly et al., 2014; Zhang, Wardwell & Bader, 2013b).
3.6.1. Specific swelling and degradation for targeting
Polysaccharide based hydrogels are useful for oral drug delivery as they have soft, porous structures with high water content and excellent biocompatibility that can mimic the microenvironment of the extracellular matrix (Fig. 5) (Lee et al., 2013; Voepel et al., 2009). The porosity, however, must be controlled for targeted delivery of encapsulated drugs and many polysaccharides and modified polysaccharides offer swelling and degradation properties that can be used for specific drug targeting. For example, most native polysaccharides are negatively charged under neutral conditions (Castelli et al., 2008; Griesser et al., 2017; Oliveira et al., 2013) and ionotropic hydrogels of positively and negatively charged polysaccharides resist swelling and dissolution under acidic conditions (Du et al., 2005; Elzatahry et al., 2009; Huang et al., 2014). This minimizes drug release from the hydrogel during the rapid transit of the stomach (Elzatahry et al., 2009). More moderate pH levels in the intestines, specifically the colon, allows negatively charged hydrogels to swell, releasing the encapsulated drugs (Castelli et al., 2008; Du et al., 2005). When chitosan was used as an outer layer coating on such ionotrophic hydrogels, transit through the intestines allowed mucoadhesion of the stable particles and increased residence time and controlled release of the drug from the delivery vehicle (Du et al., 2005; Elzatahry et al., 2009; Huang et al., 2014).
Figure 5:
Porous polysaccharide-based hydrogel
Polysaccharide-based drug delivery vehicles can also be designed with targeting provided by specific enzymatic degradation. Inulin and pectin are polysaccharides that are not degraded by endogenous human enzymes, but are digested by the enzymes of bacteria in the gut, allowing them to be exploited for colon targeted delivery (Castelli et al., 2008; Pitarresi et al., 2012; Sirisha & D’Souza, 2016; Vervoort et al., 1998a). Crosslinking inulin to form hydrogels does not prevent inulinase digestion (Vervoort et al., 1997), but varying crosslink density can be used to tune both enzyme diffusion into the hydrogel and enzyme affinity for the inulin chains, varying the degradation rate of the gel (Vervoort et al., 1998a). To prevent destructive swelling in the stomach of these inulin based hydrogels, they have included carboxylic acid groups in the crosslink chemistry, reducing premature drug release in the acidic conditions of the stomach, furthering the targeted delivery to the colon (Castelli et al., 2008). Similarly, as described in the previous section, intestinal alkaline phosphatase can be used to cleave phosphate groups from phosphorylated polysaccharides, changing the external charge and allowing enhanced mucoadhesion that promotes drug absorption and cell uptake (Griesser et al., 2017). Colon-targeted drug delivery is important as it is not just valuable for treatment of colon cancer and inflammatory bowel conditions but also is a way for less toxic delivery of non-steroidal anti-inflammatories that otherwise might irritate the gastric mucosa (Castelli et al., 2008). It can also be used for conditions that have peak symptoms in the early morning such as angina and arthritis that can benefit from delayed drug delivery (Vervoort et al., 1998a) or for drugs that are unstable in the acidic conditions of the stomach or metabolized in the small intestine (Vervoort et al., 1998a). Finally, reduced activity of brush-border peptidases and pancreatic enzymes in the colon compared to the small intestine means colon targeting is a reasonable alternative to parenteral administration for systemic release of drugs (Vervoort et al., 1998a).
In a last example, a drug delivery microcapsule has been designed sensitive to glucose concentration by covalently attaching phenylboronic acid to alginic acid (Belbekhouche, Charaabi & Carbonnier, 2019). The system is based on a porous calcium carbonate template that is loaded with drugs, such as insulin. The microcapsule is created using layer-by-layer assembly of boronic acid modified alginate and polyvinylpyrrolidone on the surface of the template, followed by dissolution of the calcium carbonate template. The boronic acid group helps to improve hydrogen bonding of the modified alginate to the polyvinylpyrrolidone, improving the stability of the capsule against changes in pH encountered during oral delivery. After administration, the boronic acid groups covalently bind glucose when present, reducing the strength of the interaction with polyvinylpyrrolidone. This releases the insulin from the drug delivery system in a glucose concentration dependent manner (Belbekhouche et al., 2019).
3.7. Particle size and its effects on targeting
The main causes of low bioavailability of orally administered drugs are poor solubility in water and low permeability into cells and through the epithelium (Maestrelli et al., 2006). Incorporation of poorly bioavailable drugs into drug delivery systems is one way to increase the bioavailability of otherwise potentially useful drugs (Maestrelli et al., 2006). Nanopolymeric drug delivery systems do this effectively with a high surface area enabling high drug encapsulation capacity (Debele et al., 2016b; Maestrelli et al., 2006). In particular, polysaccharide-based nanoparticle systems have significantly increased the bioavailability of therapeutic peptides due to their mucoadhesive properties (Maestrelli et al., 2006). Other advantages to the use of polysaccharides in therapeutic nanoparticle delivery systems include that the preparation of these nanoparticles tends to be under gentle aqueous conditions that also support protein stability and reduces the risk of toxicity (Li et al., 2007). The natural source and biodegradability means these materials can be easily cleared by the body without formation of toxic degradation byproducts (Debele et al., 2016b). Further, polysaccharide nanoparticles can be formulated and functionalized for controlled release of loaded drugs using biodegradability (Kang et al., 2015) as well as pH, redox and temperature sensitivity (Yang et al., 2015b).
For many nanoparticle therapeutic delivery systems the size and morphology of the particles is important (Barclay & Petrovsky, 2017) and the generally tunable size of polysaccharide particles enables them to be engineered to take advantage of different size effects. For example, for vaccines particles of less than 500 nm have generally better uptake (Foged, Brodin, Frokjaer & Sundblad, 2005) by dendritic cells and rod shaped particles have been shown to enhance vaccine performance (Galloway et al., 2013; Mueller, Tian & DeSimone, 2015). Also, size is important to take advantage of size dependent targeting such as the enhanced permeability and retention effect (Huh et al., 2010; Park et al., 2007; Yoon et al., 2014). Further, for oral delivery, drug permeation though the gastro-intestinal tract membrane increases with a decrease of drug delivery vehicle particle size and ideally particles from 50-300 nm in diameter are used (Desai, Labhasetwar, Amidon & Levy, 1996; Francis et al., 2004; Li et al., 2007; Sithole et al., 2017). This is because the smaller size enhances entrapment in the microvilli of the gastrointestinal tract, increasing the residence time of the drug delivery vehicle (Desai et al., 1996). Despite more limited transport, even microparticles (> 1 μm) can move through the gastro-intestinal tract membrane, mostly via immune activity through Peyer’s patches (Desai et al., 1996; Francis et al., 2004). Also, as previously mentioned, cationic surface charge is the best for mucoadhesion and mucopermeation (Sithole et al., 2017) and as uncharged polysaccharides tend to have negatively charged zeta potentials as particles (Santander-Ortega et al., 2010), this needs to be taken into account if transmucosal transport is required.
4. Polysaccharide particle construction
Polysaccharide-based particles are popular in drug delivery as they have relatively high drug loading of a range of drug types into a tunable easy to prepare biodegradable and biocompatible system that has low toxicity. There is also the potential to have drug release triggered by external stimuli (Debele, Mekuria, Lin & Tsai, 2016a). Such nanoparticles can be constructed in a number of ways as detailed below.
4.1. Polysaccharide chemistries exploited in drug delivery
Polysaccharides are chemically diverse, varying in component monosaccharides that may be neutral, negatively charged with carboxylate and/or sulfate groups and/or have positively charged amine groups. The connections between these units is variable, linear chains and branched structures are both common and molecular weight also varies significantly (Caliceti et al., 2009; Debele et al., 2016b). This enormous chemical diversity actually provides larger potential for code variation when compared to proteins and nucleic acids due to greater complexity in possible chemical linkages (Kang et al., 2015; Yamazaki et al., 2000). As a consequence, receptor mediated targeting can be highly specific (David, 2010; Kang et al., 2015; Park et al., 2012; Yamazaki et al., 2000).
It is the molecular structure that ultimately dictates the properties of the polysaccharides and modification has often been exploited in drug delivery formulations to enhance their physicochemical, biopharmaceutical and therapeutic properties (Alhaique et al., 2015; Caliceti et al., 2009; Debele et al., 2016b; Wen & Oh, 2014). In constructing particle-based drug delivery systems three main functional groups are exploited; hydroxyl, carboxylic acid and amino groups. The chemical nature of these groups makes them useful in the formation of particles and binding of drugs using both non-covalent interactions and covalent linking reactions (Alhaique et al., 2015; Caliceti et al., 2009; Wen & Oh, 2014). For covalent crosslinking and linkages to drugs the basic chemistries highlighted in Table 1.
Table 1.
Covalent linkages to polysaccharides (Debele et al., 2016b; Yang et al., 2015b)
| Functional group |
Chemical modifications used | |
|---|---|---|
| −OH | Esterification with acylating agents | |
| Etherification with alkylating agents | ||
| Oxidation of primary alcohol to COOH | Ester linkage to −OH | |
| Amide linkage to −NH2 | ||
| Oxidation of vicinal secondary −OH to aldehydes | Hydrazone linkage to −NHNH2 | |
| Schiff base linkage to −NH2 | ||
| −COOH | Ester linkage to −OH | |
| Amide linkage to −NH2 | ||
| −NH2 | Amide linkage to −COOH | |
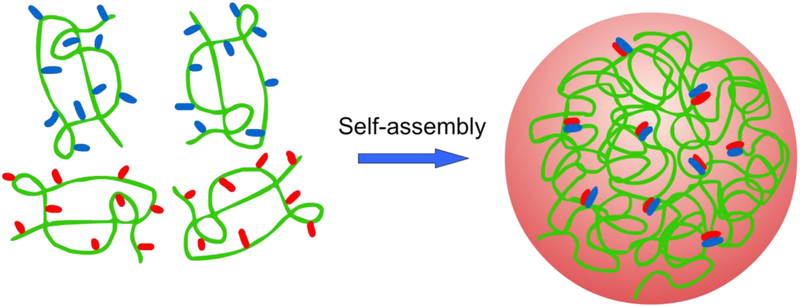
4.2. Self-assembled polysaccharide particles
For a polysaccharide to form a hydrogel that can encapsulate a therapeutic, it needs to be physically or chemically crosslinked. Ideally, a method to physically crosslink the polysaccharide is best to best maintain the biocompatibility and biodegradability of the system. Self-assembled polysaccharide drug delivery systems also benefit from mild and simple preparation conditions compared to most chemically crosslinked systems (Yang et al., 2015b). Those physically crosslinked nanohydrogels with the greatest practical application in drug delivery are often formed by ion complexation for polysaccharides containing carboxylic acid or amine groups (Fig. 6). Hydrogels, whether physically or chemically crosslinked, are how polysaccharides are overwhelmingly most often exploited in pharmaceutical science. Nonetheless, some polysaccharides, such as delta inulin, spontaneously self-assemble into biomedically useful semicrystalline microparticles that are not hydrogels.
Figure 6:
An illustration of a self-assembled polysaccharide hydrogel
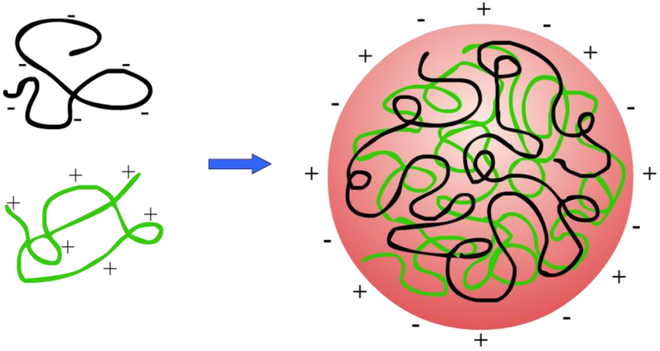
4.2.1. Polyelectrolytes
Polysaccharide polyelectrolyte systems are predominantly based on positively charged chitosan or chitosan oligomers mixed with negatively charged polyanion polysaccharides including carboxymethyl cellulose (Cui & Mumper, 2001), carboxymethyl glucomannan (Du et al., 2004), carboxymethyl chitosan, dextran sulfate (Chen, Mohanraj & Parkin, 2003; Ramasamy et al., 2013; Sarmento et al., 2006b; Tiyaboonchai & Limpeanchob, 2007), alginate (Li et al., 2007; Sarmento et al., 2006b), heparin (Boddohi, Moore, Johnson & Kipper, 2009; Liu et al., 2007; Yuk et al., 2012), octenyl succinic anhydride starch (Fang et al., 2019), carboxymethyl starch (Li et al., 2019) and hyaluronan (Fig 7) (Boddohi et al., 2009; Li et al., 2007; Ramasamy et al., 2013). Use of biocompatible chitosan and natural polysaccharide anions obviates the need for further chemical modification and the potential for toxicity of synthetic polymers (Ramasamy et al., 2013). Further, simple ionic interactions means that polyelectrolyte systems can be prepared under very gentle and biocompatible conditions (Katsuraya et al., 2003). After construction, polyelectrolyte systems generally are not neutral and retain at least sufficient charge to prevent flocculation of the particles and so can still interact with charged proteins and cells to provide the related bioactivity (Cui & Mumper, 2001; Etrych, Leclercq, Boustta & Vert, 2005). With this charge, the electrolyte systems can also be used to promote binding of charged therapeutics, such as doxorubicin (Ramasamy et al., 2013) or plasmid DNA (Cui & Mumper, 2001). In an interesting alternative delivery system, biomacromolecule polyelectrolytes have also been prepared using polysaccharides and proteins (chitosan/α-lactalbumin and carrageenan/bovine serum albumin) and templated into nanotubes for drug delivery (Maldonado et al., 2019).
Figure 7:
Formation of polysaccharide hydrogel via ionic crosslinking
The physical and pharmaceutical characteristics of polyelectrolyte based nanoparticles and their potential to encapsulate and release specific drugs can be tuned by a number of factors including the identity and molecular weight of the included polysaccharides (Debele et al., 2016b; Liu et al., 2007; Ramasamy et al., 2013), the mass ratio of the incorporated polyelectrolytes (Chen et al., 2003; Debele et al., 2016b; Du et al., 2004; Liu et al., 2007) and the pH, temperature and solvents used, including ionic strength of aqueous solutions, are all important. This is because all these aspects ultimately determine the size, charge and surface charge density and crosslinking parameters of the hydrogel, all key to the targeting, biodegradation, drug loading and release characteristics of the drug delivery vehicle (Debele et al., 2016b; Du et al., 2004; Liu et al., 2007). For example, chitosan/dextran sulfate created more stable particles than chitosan/hyaluronan, thereby increasing the residence in the circulation. However, the chitosan/hyaluronan better targeted doxorubicin for uptake by cancer cells in in vitro studies (Ramasamy et al., 2013). Additionally, insulin for oral delivery was also incorporated into pure polyelectrolyte nanohydrogels made from chitosan and alginate or dextran sulfate. Dextran sulfate proved best able to retain insulin in gastric conditions, increasing the bioavailability of the polypeptide (Sarmento et al., 2006b).
4.2.2. Ionic crosslinking
Charged polysaccharides can be crosslinked by both interactions with oppositely charged polymers, as discussed in the previous section, and interactions with simpler ions. These ionically crosslinked hydrogels are generally biocompatible and well tolerated and swelling and dissolution can be tuned for pH-controlled drug release (Berger et al., 2004; Xue et al., 2015). For example, alginate strongly chelates multivalent metal ions, often calcium ions, forming nanohydrogels that are biocompatible and nonimmunogenic and have been used for many biomedical applications including sustained drug release (Elzatahry et al., 2009; Narayanan et al., 2012; Xue et al., 2015; You & Peng, 2005). Iron (III) ions also gel alginate and were found in one study to have better cell adhesion properties than the calcium gels (Narayanan et al., 2012). Nonetheless, calcium alginate gels have been used where the charge on the alginate has been used to bind doxorubicin from solution and then calcium ions used to crosslink into nanohydrogels, with particle size tunable by varying alginate concentration (Xue et al., 2015). Calcium alginate nanohydrogel size has also been tuned by assembly of alginate inside a water-in-oil emulsion before the addition of calcium ions crosslinked the alginate into nanohydrogels (You & Peng, 2005). The use of a negatively charged alginate in this instance also provides a benefit in pH modulated protonation of the alginate and release of ionically bound drugs within the acidic conditions of endosomes (Xue et al., 2015).
Chitosan modified with carboxymethyl groups is also negatively charged and is similarly converted into nanoparticles and microparticles by precipitation with calcium ions. The size of particles are carefully controlled in narrow size distributions by controlling the addition parameters of the calcium ions and were useful for sustained drug release in in vitro investigations (Wang et al., 2010). More common is the use of ionic crosslinked nanohydrogels prepared from chitosan mixed with tripolyphosphate that has been used extensively for drug delivery (Du et al., 2019; Liu et al., 2008). These types of nanohydrogels were first prepared in 1997 (Calvo, RemunanLopez, VilaJato & Alonso, 1997), and also incorporated non-ionic Pluronic® block copolymers to improve hemocompatibility, the resultant positively charged nanoparticles able to entrap proteins and release them in vitro over time. In related experiments, model therapeutic peptides were also directly incorporated into the assembly of chitosan tripolyphosphate based on the charged peptides interactions with the system (Du et al., 2019). Chitosan/tripolyphosphate nanohydrogels have also been modifed with hydroxypropyl cyclodextrin to improve the incorporation of hydrophobic drugs (Maestrelli et al., 2006). Finally, sodium phytate with six phosphate groups has been used for ionic crosslinking of chitosan in a layer-by-layer deposition onto probiotic bacteria to support their transit through the stomach and small intestine (Wang et al., 2019).
In another application for chitosan, it has been used to form nanocomplexes through ionic crosslinking with caseinophosphopeptides (Huang et al., 2019) and polyelectrolyte formation with carboxymethyl starch (Li et al., 2019) forming nanoparticles used to stabilize Pickering high internal phase emulsions. This extended the life and increased the bioavailability of incorporated therapeutics when using an oral delivery model (Huang et al., 2019). This is one of the ways of addressing the issue of precipitation of chitosan in oral drug delivery as it deprotonates and binds together when it reaches the neutral conditions of the colon (Li et al., 2007; Maestrelli et al., 2006; Xu, Du, Huang & Gao, 2003). This situation can also be addressed, in part, by incorporation of chitosan into nanohydrogels (Maestrelli et al., 2006; Xu et al., 2003), degradation of the chitosan into shorter chains using physical (Sieval et al., 1998) and chemical means (Cui & Mumper, 2001; Li et al., 2007) or by modification of the amine to quaternary ammonium groups (Amidi et al., 2006; Li et al., 2007; Sandri et al., 2007; Sieval et al., 1998; Xu et al., 2003), providing a fixed charge (Liu et al., 2008). The use of a fixed quaternary ammonium charged group also addresses the issue that deprotonation above pH 6 that can also reduce the beneficial effect of charge on mucoadhesion (Maestrelli et al., 2006; Xu et al., 2003). As such, quaternary ammonium modified chitosan/tripolyphosphate nanoparticles were shown to enhance mucosal adhesion and uptake of nanoparticles containing high molecular weight model therapeutics through mucosa as well as penetration into epithelial intestinal cells compared with unmodified control nanoparticles (Sandri et al., 2007) and were also used to transport proteins through the nasal mucosa (Amidi et al., 2006).
4.2.3. Combining polyelectrolyte and ionic assembly
Electrostatic formation of polysaccharide based nanohydrogels has also been achieved by combining both anionic and cationic polysaccharides as well as simple ions for additional functionality and to modulate and stabilize the assembled systems (Feng et al., 2015; Goycoolea et al., 2009; Tiyaboonchai & Limpeanchob, 2007). In this case, often the particles are formed with all ingredients at once (Goycoolea et al., 2009), but can also be assembled in a layered fashion so the external surface and interior of the particles have different characteristics (Elzatahry et al., 2009; Feng et al., 2015; Schütz, Juillerat-Jeanneret, Käuper & Wandrey, 2011). For example, chitosan/tripolyphosphate systems have; (i) been targeted to cancer cells by incorporation of hyaluronic acid (Deng et al., 2014); (ii) had cancer drug uptake into cells promoted by incorporation of carboxymethyl chitosan (Feng et al., 2014); (iii) been stabilized by incorporation of alginate for transmucosal transport of encapsulated proteins in nasal delivery (Goycoolea et al., 2009); and (iv) the drug release character of quaternary ammonium modified chitosan/tripolyphosphate was altered by incorporation of sodium alginate into the drug delivery vehicle, both reducing the burst release and tuning encapsulation efficiency (Xu et al., 2003). Coatings of chitosan/tripolyphosphate nanohydrogels with carboxymethyl chitosan have also been used to stabilize the particles (Feng et al., 2015). Such coatings with anionic polysaccharides can also alleviate the issues with chitosan complexes that can be rapidly marked for degradation by phagocytes and have higher cytotoxicity than negatively charged species (Schütz et al., 2011). Consequently, alginate coated chitosan/tripolyphosphate nanogels were able to deliver protein cargoes to a range of cell types with high cytocompatibility for all cell types tested (Schütz et al., 2011).
It is not just the addition of anionic polysaccharides to chitosan/tripolyphosphate systems that are of value to improving the properties of electrostatically bound polysaccharide nanohydrogels. For example, the addition of zinc ions stabilized dextran sulfate and chitosan hydrogel nanoparticles that encapsulated amphotericin B (Tiyaboonchai & Limpeanchob, 2007). Also, calcium alginate nanohydrogels have been variously modified with cationic polymers to enhance bioavailability of encapsulated drugs (Elzatahry et al., 2009; Rajaonarivony et al., 1993; Sarmento et al., 2006a; Zahoor et al., 2005). This includes chitosan coatings on calcium alginate nanohydrogels to improve mucoadhesion for oral delivery (Elzatahry et al., 2009). Calcium alginate pre-gels have also been precipitated by the addition of chitosan solution in the presence of the therapeutic to be encapsulated (Sarmento et al., 2006a; Zahoor et al., 2005) with the nanoparticle size tuned by the ratio of polysaccharides and the pH of the particle formation (Sarmento et al., 2006a). These particles have been used to stabilize encapsulated insulin for oral delivery (Sarmento et al., 2006a) and an inhalable formulation was prepared to enhance the bioavailability and provide extended controlled release of encapsulated anti-tubercular drugs, physically targeting treatment to the lungs (Zahoor et al., 2005). Quaternary ammonium modified chitosan has also been used with these calcium alginate pre-gels (Li et al., 2007) providing pH modulated release of encapsulated proteins, that were retained in the acid conditions of the stomach and rapidly released at pH of the colon. The protein absorption and release properties were tuned by the relative concentration of components, as well as the molecular weight of and degree of substitution of the modified chitosan (Li et al., 2007).
Finally, carboxymethyl chitosan has been precipitated into nanogels using calcium chloride and then coated by ionic interactions with chitosan. These nanohydrogels were formed in the presence of doxorubicin and the positively charged gel had greater transport into colorectal cancer cells with better inhibition of cell viability than controls without chitosan coatings. The nanohydrogel with chitosan on the external surface also had better adhesion ex vivo to intestinal samples and reduced paracellular transport resulting in increased transcellular transport, beneficial to treat colon cancer (Feng et al., 2015).
4.2.4. Crystalline polysaccharide particles
Many polysaccharides lack solubility in water despite the large quantities of hydroxyl groups, usually due to a propensity for those hydroxyl groups to interact and organize the structure, leading to crystalline domains (Senna et al., 2014). This results in semi-crystalline materials such as those found in starch (Blazek & Gilbert, 2011), cellulose (Kajiwara & Miyamoto, 2004) and chitin (Lin et al., 2011). Nanocrystals of these materials have been used to alter the physical properties and drug release characteristics of alginate microspheres in composites that resulted in improved sustained release of encapsulated drugs (Lin et al., 2011). Inulin is another polysaccharide that naturally assembles into semicrystalline materials. It has relatively short chains that precipitate from aqueous solution into an antiparallel arrangement of helices orthogonal to the plane of two-dimensional nanolayers that make up a spherulite-like three-dimensional particle (Barclay et al., 2016; Cooper et al., 2014; Cooper et al., 2015). These particles have unique interactions with the immune system and have been shown to be an effective vaccine adjuvant with significantly reduced side effects compared to standard alum-based adjuvants (Cooper & Petrovsky, 2011; Feinen et al., 2014; Gordon et al., 2014; Honda-Okubo et al., 2014; Saade et al., 2013; Silva et al., 2004). Their interactions with the immune system and rapid uptake by monocytes means there has been demonstrated success in cancer treatment (Korbelik & Cooper, 2007) and proposed application in drug delivery into monocytes and using monocytes for targeting (Wang et al., 2017). In this case the nanolayered structure provides significantly more opportunity for loading than a solid particle of the same size.
4.2.5. Self-assembly of hydrophobically modified polysaccharides
For polysaccharide drug delivery vehicles, particularly those that use electrostatic interactions to promote self-assembly of drug delivery particles discussed in Sections 4.2.1-4.2.3, there is an issue that their hydrophilic nature prevents loading of hydrophobic drugs or results in significant burst release of encapsulated drugs. To address this, hydrophobically modified systems can be used. Most simply, the inclusion of cyclodextrins into polysaccharide hydrogels enables the formation of drug inclusion complexes (Blanco-Fernandez, Lopez-Viota, Concheiro & Alvarez-Lorenzo, 2011; Maestrelli et al., 2006; Moya-Ortega et al., 2012; Peng et al., 2010). In an entirely self-assembled system, hyaluronic acid was assembled with a Poly(β-amino ester) and a quenched fluorophore through electrostatic and hydrophobic interactions to form a nanohydrogel. Receptor mediated endocytosis using CD-44 enabled pH mediated breakup of the nanohydrogel and release of the unquenched fluorophore demonstrating possible use as a theranostic agent (Park et al., 2012). Chemical modification of polysaccharides with hydrophobic small molecules or polymers also creates amphiphiles that self-assemble from aqueous dispersions to form micellar nanoparticles or nanohydrogels (Alhaique et al., 2015; Peng et al., 2019), perfect for encapsulation and increasing the bioavailability of hydrophobic drugs (Alhaique et al., 2015; Debele et al., 2016b; Peng et al., 2019; Wen & Oh, 2014). The covalent linkages binding the polysaccharide and hydrophobic groups can be stable, or they can be biolabile to allow stimuli-responsive release of encapsulated drugs.
An early example of the use of polysaccharides in functional drug delivery vehicles is cholesterol modified pullulan that self-assembles from water into nanohydrogels with hydrophobic cores which can host hydrophobic guest molecules (Akiyoshi et al., 1991). This solubilizes, protects and transports drugs and therapeutic biomacromolecules (Alhaique et al., 2015; Hirakura et al., 2010; Kazunari Akiyoshi & Junzo, 2001), even being used to provide an environment suitable for refolding denatured proteins (Akiyoshi et al., 1999). These systems have also been further modified for additional functionality. For example, the addition of a Pluronic® polymer covalently bound to cholesterol modified pullulan provided thermosensitive properties (Deguchi & Akiyoshi, 1994). Also, the use of vinyl ether linkages between pullulan and the cholesterol groups meant the nanogel was stable at neutral pH, but swelled and degraded under mildly acidic conditions, similar to those expected in the endosome, releasing incorporated proteins (Morimoto et al., 2013). Finally, cholesterol modified pullulan was also functionalized with an Infrared dye to form 30 nm diameter nanohydrogels for rapid uptake into lymph nodes. This was used to map sentinel lymph node and to monitor lymphatic flow and could be useful in targeting cancer treatments (Noh et al., 2012).
There have been many other hydrophobically modified polysaccharide drug delivery systems that follow this basic model. This includes hydrophobically modified starch (Santander-Ortega et al., 2010) and dextran (Daoud-Mahammed et al., 2009) but more often charged systems such as chitosan (Chung et al., 2010; Huh et al., 2010; Liu & Yao, 2014; Park et al., 2007; Yoon et al., 2014), quaternary ammonium modified chitin (Peng et al., 2019), heparin (Chung et al., 2010; Liu et al., 2014; Yang et al., 2015b), hyaluronic acid (Huang et al., 2014; Park et al., 2012), chondroitin sulfate (Park, Park & Na, 2010), rhamnogalacturonoglycan (Sombra et al., 2019) and spermine modified cycloamylose (Fujii et al., 2014). The charged systems provided additional binding for charged anticancer therapeutics (Fujii et al., 2014) including amphiphiles such as doxorubicin (Park et al., 2010; Peng et al., 2019) as well as increasing the circulation half-life of the system (Huh et al., 2010; Liu et al., 2014; Park et al., 2007; Yang et al., 2015b; Yoon et al., 2014). These systems were also used for targeting delivery of therapeutics and theranostic agents to cancer cells by taking advantage of size-dependent targeting, such as the enhanced permeability and retention effect (Huh et al., 2010; Park et al., 2007; Yoon et al., 2014) as well as heparin and hyaluronic acid receptor binding (Chung et al., 2010; Huang et al., 2014; Liu et al., 2014; Park et al., 2012; Yang et al., 2015b). Interesting examples of targeting and release mechanisms of these systems includes a hydrophobically modified hyaluronic acid system was subsequently electrostatically bound to chitosan, the chitosan layer used to act as a mucoadhesive to prolong the residence of the vehicle in the small intestine and mediate opening of tight junctions in intestinal epithelium. As the pH increased to pH 7.4, the chitosan detaches from the surface of the drug delivery vehicle exposing the hyaluronic acid that transports the vehicle into cancer cells through the CD-44 receptor. The hyaluronic acid particle is then degraded by hyaluronidase-1 triggering the release of the drug within cancer cells (Huang et al., 2014). Hyaluronic acid has also been covalently modified with cholesteryl groups and with hydrophobic drug molecules using pH sensitive ester bonds and then self-assembled into nanohydrogels suitable for drug delivery using CD44 receptor mediated endocytosis (Wei, Senanayake, Warren & Vinogradov, 2013). In this case the cholesteryl units were proposed to be anchored into the membrane of the endosome, unpacking the hydrogel and allowing more efficient drug delivery to cancer cells and higher cancer cell toxicity than related systems (Wei et al., 2013).
Polysaccharides have also been modified with therapeutics containing hydrophobic moieties for direct self-assembly into nanoparticles. This includes acid labile linkages between dextran and both doxorubicin (Xu et al., 2015b) and bortezomib (Xu et al., 2015a) that self-assemble into nanoparticles that release the drug under the acidic conditions found inside tumors and in endosomes. Similarly, an adipic acid dihydrazide link between heparin and dexamethasone provided a hydrophobic core to accommodate loading of doxorubicin. This vehicle is targeted to tumors using heparin after which there was acid-mediated cleavage of the linkages and release of the drugs (Li, Lin, Di & Zhang, 2014). Dextran has also been modified with both polyethyleneimine and disulfide linkages to doxorubicin. The polyethyleneimine promoted endosomal release of endocytosed assembled nanohydrogels and the disulfide bridge released the drug under the reducing conditions of the cytoplasm (Liu et al., 2013). The endosomal escape also helps to prevent drug resistance through P-glycoprotein drug efflux transporters (Liu et al., 2013). Finally, curcumin was covalently bound to dextran and subsequently self-assembled into nanoparticles from aqueous solution. The nanoparticles were successfully delivered into HeLa cells in preference to normal cells. The fluorescence of the curcumin suggests potential use as a cancer theranostic agent (Nagahama, Sano & Kumano, 2015).
4.3. Chemical crosslinking
Covalent crosslinks used to stabilize polysaccharide nanohydrogels avoid the issue of dilution upon administration in which dynamically stable self-assembled structures can fall below the critical aggregation concentration and come apart (Bae & Yin, 2008; Li et al., 2015; Qian et al., 2014; Rahimian et al., 2015; Yang et al., 2011). This enables more predictable drug release that can be diffusion controlled (Bae & Yin, 2008; Berger et al., 2004; Qian et al., 2014). It is also considered that chemically crosslinked particles have better tunability of the mechanical and physical properties of the resultant hydrogels by varying crosslink length, chemistry and density of connections (Khanlari, Detamore & Gehrke, 2013; Sithole et al., 2017; Wen & Oh, 2014). Despite the stabilizing benefits of covalent crosslinks, some particle instability may be required for the desired release characteristics. As such, biodegradable crosslinks have been used to provide stimuli triggered drug release (Wen & Oh, 2014). For example disulfide linkages are stable in the oxidizing extracellular space, but cleaved under the reducing conditions in the cytoplasm and tumors and by enzymes in lysosomes, releasing the drug payload within the cell (Debele et al., 2016a; Liu et al., 2013; Qian et al., 2014; Tan et al., 2011b; Wu et al., 2015; Zhang et al., 2013a). Several linkage chemistries are also acid labile such as esters, hydrazones and Schiff base and so can be cleaved under the acidic conditions found in tumors and inside lysosomes.
Crosslinking reactions without prior organization can lead to broad size distribution of particles. Pre-organizing the components before crosslinking in self-assembled systems can be a way of narrowing this distribution (Bodnar et al., 2005; Wen & Oh, 2014). For example, polysaccharides can be assembled in emulsions prior to crosslinking (Li et al., 2015; Maciel et al., 2013; Manchun, Dass, Cheewatanakornkool & Sriamornsak, 2015; Moya-Ortega et al., 2012; Xi, Zhou & Dai, 2012) and the temperature sensitive aggregation of hydroxypropyl cellulose (Rahimian et al., 2015; Tan et al., 2011b) and carboxymethyl cellulose (Wen & Oh, 2015) was exploited to create tight nanogels that were subsequently stabilized by chemical crosslinking. Perhaps more common is the modification of polysaccharides such as pullulan (Hasegawa et al., 2009), hyaluronic acid (Yang et al., 2011) and starch (Zhang et al., 2013a) with both reactive groups for covalent crosslinking and hydrophobic groups. Subsequently, the system can be preassembled into nanoparticles based on the amphiphilic properties before chemical crosslinking occurs (Hasegawa et al., 2009; Yang et al., 2011; Zhang et al., 2013a). As discussed in Section 4.2.5, the resultant hydrophobic cores have additional use in the loading of hydrophobic therapeutics as well as stabilizing therapeutic proteins to degradation during the delivery process (Hasegawa et al., 2009).
As the polysaccharide modification and crosslinking strategies become more complex and introduce a range of new chemistries into the drug delivery vehicle (Östmark, Nyström & Malmström, 2008), care must be taken not to induce toxicity or detrimentally affect biodegradability and biocompatibility (Ahmed & Narain, 2015; Kang et al., 2015). Early work on chitosan nanoparticles used glutaraldehyde to crosslink the amino groups (Ohya, Shiratani, Kobayashi & Ouchi, 1994) but the toxicity immunogenicity of glutaraldehyde means it is now avoided (Calvo et al., 1997; Liu et al., 2008). More promising is to use naturally occurring modifying chemistry and linkages that result in biocompatible and biodegradable byproducts so this key advantage of using polysaccharides is not lost (Bodnar et al., 2005).
4.3.1. Alkenyl modification for crosslinking
Due to the large amounts of the various functional groups, polysaccharides are readily chemically crosslinked by a number of reagents. This includes modification of polysaccharides with alkenyl chemistry that can subsequently be crosslinked, such as vinyl, maleoyl, acrylate and methacrylate groups. This has occurred using 2-amino ethyl methacrylate to bind carboxylic acid groups (Valmikinathan et al., 2012; Wu et al., 2015) and methacrylic acid to bind amines (Tan, Luan, Hu & Hu, 2012) as well as anhydride- (Castelli et al., 2008; Hachet et al., 2012; Qian et al., 2014; Xi et al., 2012), epoxide- (Khanlari et al., 2013; Li et al., 2015; Vervoort et al., 1998a; Vervoort, Van den Mooter, Augustijns & Kinget, 1998b; Yang et al., 2015a) and imidazoyl-activated (Pescosolido et al., 2011; Van Thienen, Raemdonck, Demeester & De Smedt, 2007; Voepel et al., 2009) compounds and the ring opening polymerization of dl-lactide (Das et al., 2016) that can readily modify polysaccharide hydroxyl groups under relatively gentle conditions (Khanlari et al., 2013). The pendant alkenyl groups can then be crosslinked directly or through multifunctional functional linkers (Das et al., 2016; Qian et al., 2014; Wen & Oh, 2015; Wu et al., 2015; Yang et al., 2015a) using free radical crosslinking chemistry through redox- (Das et al., 2016; Vervoort et al., 1998a; Vervoort et al., 1997; Vervoort et al., 1998b; Voepel et al., 2009; Xi et al., 2012; Yang et al., 2015a) or photo-initiation (Castelli et al., 2008; Hachet et al., 2012; Khanlari et al., 2013; Li et al., 2015; Tan et al., 2012; Voepel et al., 2009) strategies to create hydrogels. The linkage chemistry of the attached alkenyl groups (Voepel et al., 2009) and the use of various multi-alkenyl crosslinkers (Khanlari et al., 2013; Pitarresi et al., 2012; Qian et al., 2014) along with crosslink density (Hachet et al., 2012; Valmikinathan et al., 2012; Vervoort et al., 1997; Vervoort et al., 1998b; Xi et al., 2012) have all been used to tune the properties of the resultant gel.
4.3.2. Multifunctional reagents for crosslinking
Multi-functional reagents are also available to crosslink polysaccharides directly in a one step process. Carboxylic acid containing polysaccharides, such as heparin, have been often crosslinked through multi-amine containing linkers such as ethylenediamine, dihydrazides (Luo et al., 2000) and polyethyleneimine to form nanohydrogels using peptide coupling techniques (Yang et al., 2015b). Amine containing polysaccharides such as chitosan can also be crosslinked with peptide coupling strategies using multi-carboxylic acid compounds like succinic acid and citric acid (Bodnar et al., 2005). Hydroxyl group reactive multifunctional reagents such as dianhydrides (Kono & Fujita, 2012; Oliveira et al., 2013; Senna et al., 2014) and diepoxides (Blanco-Fernandez et al., 2011; Moya-Ortega et al., 2012; Simkovic, 2004; Tomihata & Ikada, 1997) are also popular. The use of dianhydrides means that the formation of each ester linkage with the polysaccharide also creates a carboxylic acid group that can be beneficial in increasing the water capacity of the hydrogel, particularly for poorly soluble polysaccharides and those modified with hydrophobic groups (Kono & Fujita, 2012; Oliveira et al., 2013; Senna et al., 2014). Also, the introduction of acid groups reduces swelling of the hydrogel under the acidic conditions of the stomach, allowing targeted oral delivery to the colon (Castelli et al., 2008). If these linkages are unsuitable, polysaccharides activated by oxidation of vicinal hydroxyl groups to aldehydes using sodium periodate can be crosslinked by reaction to multi-amine compounds (Sarker et al., 2014) or dihydrazides (Bouhadir, Alsberg & Mooney, 2001; Luo et al., 2000). This results in relatively unstable Schiff base and hydrazone linkages respectively that can be exploited for drug release (Sarker et al., 2014) or can be stabilized by being reduced to amines. Schiff base linkages can also be stabilized by surrounding chemistry such as with carbodihydrazides (Oommen et al., 2012).
4.3.3. Disulfide bridges
Disulfide bridges are a popular crosslinking strategy for polysaccharide nanohydrogels as the linkages are biologically stable until exposed to the reducing conditions of the cytosol, inside tumors or by reducing enzymes in lysosomes (Dai Hai et al., 2011; Debele et al., 2016a; Maciel et al., 2013; Qian et al., 2014; Rahimian et al., 2015; Wu et al., 2015). For example, carboxylic acid containing polysaccharides have been modified with cysteamine and cysteine using peptide coupling strategies followed by crosslinking of the thiols to disulfide bridges under oxidizing conditions to make cytocompatible nanohydrogels (Dai Hai et al., 2011; Debele et al., 2016a; Khatun et al., 2015; Maciel et al., 2013; Tan et al., 2011b). Methacrylate modified heparin and carboxymethyl cellulose have also been crosslinked with cysteamine bisacrylamide to form nanohydrogels resulting in redox sensitive nanohydrogel degradation and resultant drug release (Qian et al., 2014; Wu et al., 2015). Finally, in a complex system involving grafting from hydroxypropyl cellulose of polymethylmethacrylates containing both oligoethyleneoxide and disulfide units, the disulfide units were catalyzed to cleave and reform as crosslinking bridges by the addition of dithiothreitol (Rahimian et al., 2015).
4.3.4. Click chemistry crosslinking
Click chemistry has been used to crosslink polysaccharides into hydrogels because of the high specificity of the reactions under gentle conditions (Dong et al., 2011; Li et al., 2017b; Takahashi et al., 2013). This includes nanohydrogel formation using the thiol-ene click reaction between maleimido modified dextran and PEG dithiol (Fig. 8)(Peng et al., 2010) and methacrylate modified hyaluronic acid crosslinked with dithiothreitol (Hirakura et al., 2010) as well as Diels-Alder cycloaddition linking of hyaluronic acid chains separately modified with maleimide and furan groups (Tan, Rubin & Marra, 2011a).
Figure 8:
Nanohydrogel formation using the thiol-ene click reaction
5. Conclusions and future perspectives
Polysaccharides represent an extraordinarily versatile platform for therapeutic delivery applications (Alhaique et al., 2015). Firstly, polysaccharides are renewable and tend to have high biocompatibility and biodegradability and low toxicity, immunogenicity and cost compared to synthetic polymers with otherwise similar functionality. Secondly, as described in Section 3, the functionality of polysaccharides has a large range that benefits drug delivery applications. This includes; (i) a range of targeting strategies suitable to specifically deliver treatment to different organs and for different conditions; (ii) the ability to protect and increase the bioavailability of incorporated therapeutics; (iii) capacity to avoid the reticuloendothelial system and extend therapeutic circulation half-life of incorporated therapeutics; (iv) flexible chemistry that allows ready chemical modification and a range of construction methods resulting in tunable platforms that can be made suitable for a range of therapeutic delivery requirements; and (v) a history of pharmaceutical and food use demonstrating the safety of the systems.
The weaknesses of polysaccharides in drug delivery are that the natural source means that their structure is difficult to define with molecular weight and chemistry often variable with the seasons and the environment. Also, the lack of solubility in most solvents for many polysaccharides restricts the chemical modifications and drug loading conditions to which polysaccharides can be applied. Finally, when modifying polysaccharides to improve their properties and increase their functionality for drug delivery, care must be taken not to significantly change the properties that make polysaccharides so valuable, such as high biocompatibility and low toxicity. Indeed, while many of these systems have been evaluated for these properties and their pharmaceutical benefit using in vitro and relatively simple in vivo tests, there needs to be deeper investigation of how these systems interact with the whole human body as a drug delivery vehicle in the future (Sirisha & D’Souza, 2016). This is particularly the case as many of these systems are nanoparticles that bring additional size-based functionality that has not needed to be considered for traditional pharmaceuticals (Barclay & Petrovsky, 2017).
The highlighted faults of polysaccharides in drug delivery mean that they are not a universal solution for all therapeutic delivery applications. Despite this, their positive points more often outweigh the negatives and polysaccharides are already used extensively in pharmaceutical science as excipients and in other clinical applications, using simple physical and biological properties. This exploitation of these natural and renewable resources is only likely to increase in the future. However, as modern medicine becomes more sophisticated, new drugs often require the support of a delivery system to enhance multiple factors. This includes increasing the stability and bioavailability of the therapeutic and providing targeted delivery. These factors can increase the efficacy of the overall system and reduce cost and toxicity of the drugs. In the case of polysaccharides, the fact that multiple functionalities can be exploited in a single simple system means they can provide highly elegant solutions to the complex issue of therapeutic and drug delivery as exploited in a number of applications presented in this review (Ahmed & Narain, 2015; Alhaique et al., 2015; Huang et al., 2014; Zhang et al., 2017). It is to these more advanced purposes that polysaccharides are likely to be increasingly applied in the future.
Highlights.
Polysaccharides have physical and chemical properties suitable for drug delivery systems
Polysaccharides provide a range of drug stabilization, delivery and release mechanisms
Polysaccharide drug delivery systems are biocompatible, biodegradable and non-toxic
Particle-based drug delivery systems are easily constructed from polysaccharides
Polysaccharide particles can deliver protein, oligonucleotide and small molecule drug forms
Acknowledgements
This work was supported by the Australian Research Council’s Linkage Projects funding scheme (Project number LP140100142). Development of delta inulin was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, under Contracts No. HHSN272200800039C and U01 AI061142 and NP is supported by Contract HHSN272201400053C.
Abbreviations
- FDA
Food and Drug Administration
- PEG
Polyethylene glycol
- PEI
Polyethyleneimine
- PLGA
Poly(lactic-co-glycolic) acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afinjuomo F, Barclay TG, Song Y, Parikh A, Petrovsky N, & Garg S (2018). Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. Reactive and Functional Polymers, 134, 104–111. [Google Scholar]
- Ahmed M, & Narain R (2015). Carbohydrate-based materials for targeted delivery of drugs and genes to the liver. Nanomedicine, 10(14), 2263–2288. [DOI] [PubMed] [Google Scholar]
- Akiyoshi K, Sasaki Y, & Sunamoto J (1999). Molecular chaperone-like activity of hydrogel nanoparticles of hydrophobized pullulan: Thermal stabilization with refolding of carbonic anhydrase b. Bioconjugate Chemistry, 10(3), 321–324. [DOI] [PubMed] [Google Scholar]
- Akiyoshi K, Yamaguchi S, & Sunamoto J (1991). Self-aggregates of hydrophobic polysaccharide derivatives. Chemistry Letters, 1263–1266 [Google Scholar]
- Alhaique F, Pietro M, Di Meo C, Coviello T, & Montanari E (2015). Polysaccharide-based self-assembling nanohydrogels: An overview on 25-years research on pullulan. Journal of Drug Delivery Science and Technology, 30(Part B), 300–309. [Google Scholar]
- Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, & Jiskoot W (2006). Preparation and characterization of protein-loaded n-trimethyl chitosan nanoparticles as nasal delivery system. Journal of Controlled Release, 111(1–2), 107–116. [DOI] [PubMed] [Google Scholar]
- Amorij JP, Huckriede A, Wilschut J, Frijlink H, & Hinrichs W (2008). Development of stable influenza vaccine powder formulations: Challenges and possibilities. Pharmaceutical Research, 25(6), 1256–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam T, Eliyahu H, Makovitzki A, Linial M, & Domb AJ (2004). Hydrophobized dextran-spermine conjugate as potential vector for in vitro gene transfection. Journal of Controlled Release, 96(2), 309–323. [DOI] [PubMed] [Google Scholar]
- Bae YH, & Yin H (2008). Stability issues of polymeric micelles. Journal of Controlled Release, 131(1), 2–4. [DOI] [PubMed] [Google Scholar]
- Barclay T, Ginic-Markovic M, Cooper P, & Petrovsky N (2010). Inulin-a versatile polysaccharide with multiple pharmaceutical and food chemical uses. Journal of Excipients and Food Chemicals, 1(3), 27–50. [Google Scholar]
- Barclay TG, & Petrovsky N (2017). Vaccine adjuvant nanotechnologies In Skwarczynski M & Toth I (Eds.). Micro- and Nanotechnology in Vaccine Development (pp. 127–148): William Andrew. [Google Scholar]
- Barclay TG, Rajapaksha H, Thilagam A, Qian G, Ginic-Markovic M, Cooper PD, Gerson A, & Petrovsky N (2016). Physical characterization and in silico modeling of inulin polymer conformation during vaccine adjuvant particle formation. Carbohydrate Polymers, 143, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belbekhouche S, Charaabi S, & Carbonnier B (2019). Glucose-sensitive capsules based on hydrogen-bonded (polyvinylpyrrolidone / phenylboronic –modified alginate) system. Colloids and Surfaces B-Biointerfaces, 177, 416–424. [DOI] [PubMed] [Google Scholar]
- BeMiller JN (2008). Gums and related polysaccharides In Fraser-Reid BO, Tatsuta K & Thiem J (Eds.). Glycoscience: Chemistry and chemical biology (pp. 1513–1533). Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Bendele A, Seely J, Richey C, Sennello G, & Shopp G (1998). Short communication: Renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicolological Sciences, 42, 152–157. [DOI] [PubMed] [Google Scholar]
- Berger J, Reist M, Mayer JM, Felt O, Peppas NA, & Gurny R (2004). Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 57(1), 19–34. [DOI] [PubMed] [Google Scholar]
- Blanco-Fernandez B, Lopez-Viota M, Concheiro A, & Alvarez-Lorenzo C (2011). Synergistic performance of cyclodextrin-agar hydrogels for ciprofloxacin delivery and antimicrobial effect. Carbohydrate Polymers, 85(4), 765–774. [Google Scholar]
- Blazek J, & Gilbert EP (2011). Application of small-angle x-ray and neutron scattering techniques to the characterisation of starch structure: A review. Carbohydrate Polymers, 85, 281–293. [Google Scholar]
- Boddohi S, Moore N, Johnson PA, & Kipper MJ (2009). Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules, 10(6), 1402–1409. [DOI] [PubMed] [Google Scholar]
- Bodnar M, Hartmann JF, & Borbely J (2005). Preparation and characterization of chitosan-based nanoparticles. Biomacromolecules, 6(5), 2521–2527. [DOI] [PubMed] [Google Scholar]
- Bouhadir KH, Alsberg E, & Mooney DJ (2001). Hydrogels for combination delivery of antineoplastic agents. Biomaterials, 22(19), 2625–2633. [DOI] [PubMed] [Google Scholar]
- Caliceti P, Salmaso S, & Bersani S (2009). Polysaccharide-based anticancer prodrugs In Reddy LH & Couvreur P (Eds.). Macromolecular anticancer therapies: Cancer drug discovery and development (Vol. 2, pp. 163–219). New York, NY: Springer New York. [Google Scholar]
- Calvo P, RemunanLopez C, VilaJato JL, & Alonso MJ (1997). Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. Journal of Applied Polymer Science, 63(1), 125–132. [Google Scholar]
- Carpenter JF, & Crowe JH (1989). An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry, 28(9), 3916–3922. [DOI] [PubMed] [Google Scholar]
- Castelli F, Sarpietro MG, Micieli D, Ottimo S, Pitarresi G, Tripodo G, Carlisi B, & Giammona G (2008). Differential scanning calorimetry study on drug release from an inulin-based hydrogel and its interaction with a biomembrane model: pH and loading effect. European Journal of Pharmaceutical Sciences, 35(1–2), 76–85. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A (2003). Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil®): Possible role in hypersensitivity reactions. Annals of Oncology, 14(9), 1430–1437. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mohanraj VJ, & Parkin JE (2003). Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Letters in Peptide Science, 10(5–6), 621–629. [Google Scholar]
- Choi KY, Silvestre OF, Huang X, Min KH, Howard GP, Hida N, Jin AJ, Carvajal N, Lee SW, Hong J-I, & Chen X (2014). Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano, 8(5), 4559–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y-I, Kim JC, Kim YH, Tae G, Lee SY, Kim K, & Kwon IC (2010). The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated pluronic on tumor targeting. Journal of Controlled Release, 143(3), 374–382. [DOI] [PubMed] [Google Scholar]
- Conover CD, Gilbert CW, Shurn KL, & Shorr RGL (1997). The impact of polyethylene glycol conjugation on bovine hemoglobin’s circulatory half-life and renal effects in a rabbit top-loaded transfusion model. Artificial Organs, 21(8), 907–915. [DOI] [PubMed] [Google Scholar]
- Cooper PD, Barclay TG, Ginic-Markovic M, Gerson AR, & Petrovsky N (2014). Inulin isoforms differ by repeated additions of one crystal unit cell. Carbohydrate Polymers, 103, 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PD, Barclay TG, Ginic-Markovic M, & Petrovsky N (2013). The polysaccharide inulin is characterised by an extensive series of periodic isoforms with varying biological actions. Glycobiology, 23(10), 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PD, & Petrovsky N (2011). Delta inulin: A novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology, 21(5), 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PD, Rajapaksha KH, Barclay TG, Ginic-Markovic M, Gerson AR, & Petrovsky N (2015). Inulin crystal initiation via a glucose-fructose cross-link of adjacent polymer chains: Atomic force microscopy and static molecular modelling. Carbohydrate Polymers, 117, 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copetti G, Grassi M, Lapasin R, & Pricl S (1997). Synergistic gelation of xanthan gum with locust bean gum: A rheological investigation. Glycoconjugate Journal, 14(8), 951–961. [DOI] [PubMed] [Google Scholar]
- Cui ZR, & Mumper RJ (2001). Chitosan-based nanoparticles for topical genetic immunization. Journal of Controlled Release, 75(3), 409–419. [DOI] [PubMed] [Google Scholar]
- Dai Hai N, Jong Hoon C, Yoon Ki J, & Ki Dong P (2011). Disulfide-crosslinked heparin-pluronic nanogels as a redox-sensitive nanocarrier for intracellular protein delivery. Journal of Bioactive and Compatible Polymers, 26(3), 287–300. [Google Scholar]
- Daoud-Mahammed S, Couvreur P, Bouchemal K, Chéron M, Lebas G. v., Amiel C, & Gref R (2009). Cyclodextrin and polysaccharide-based nanogels: Entrapment of two hydrophobic molecules, benzophenone and tamoxifen. Biomacromolecules, 10(3), 547–554. [DOI] [PubMed] [Google Scholar]
- Das D, & Pal S (2015). Modified biopolymer-dextrin based crosslinked hydrogels: Application in controlled drug delivery. RSC Advances, 5(32), 25014–25050. [Google Scholar]
- Das D, Patra P, Ghosh P, Rameshbabu AP, Dhara S, & Pal S (2016). Dextrin and poly(lactide)-based biocompatible and biodegradable nanogel for cancer targeted delivery of doxorubicin hydrochloride. Polymer Chemistry, 7(17), 2965–2975. [DOI] [PubMed] [Google Scholar]
- Dass CR, & Choong PFM (2008). The use of chitosan formulations in cancer therapy. Journal of Microencapsulation, 25(4), 275–279. [DOI] [PubMed] [Google Scholar]
- David A (2010). Carbohydrate-based biomedical copolymers for targeted delivery of anticancer drugs. Israel Journal of Chemistry, 50(2), 204–219. [Google Scholar]
- Davis BG, & Robinson MA (2002). Drug delivery systems based on sugar-macromolecule conjugates. Current Opinion in Drug Discovery & Development, 5(2), 279–288. [PubMed] [Google Scholar]
- Day CM, Barclay TG, Song Y, & Garg S (2018). Swelling-controlled drug delivery systems In Singh A, and Amiji MM (Eds.). Stimuli-responsive drug delivery systems, (pp. 232–264). Cambridge: Royal Society of Chemistry. [Google Scholar]
- Debele TA, Mekuria SL, Lin S-Y, & Tsai H-C (2016a). Synthesis and characterization of bioreducible heparin-polyethyleneimine nanogels: Application as imaging-guided photosensitizer delivery vehicle in photodynamic therapy. RSC Advances, 6(18), 14692–14704. [Google Scholar]
- Debele TA, Mekuria SL, & Tsai H-C (2016b). Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Materials Science and Engineering: C, 68(C), 964–981. [DOI] [PubMed] [Google Scholar]
- Deguchi S, & Akiyoshi K (1994). Solution property of hydrophobized pullulan conjugated with poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymer. Formation of nanoparticles and their thermosensitivity. Macromolecular Rapid Communications, 15, 705–711 [Google Scholar]
- Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, Xiao X, Yang Y, Sheng W, Wu Y, & Zeng Y (2014). Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials, 35(14), 4333–4344. [DOI] [PubMed] [Google Scholar]
- Desai MP, Labhasetwar V, Amidon GL, & Levy RJ (1996). Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharmaceutical Research, 13(12), 1838–1845. [DOI] [PubMed] [Google Scholar]
- Dion C, Chappuis E, & Ripoll C (2016). Does larch arabinogalactan enhance immune function? A review of mechanistic and clinical trials. Nutrition & Metabolism, 13(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya K, Smitha V, & Jisha MS (2018). Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. International Journal of Biological Macromolecules, 114, 572–577. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saeed AO, Hassan W, Keigher C, Zheng Y, Tai H, Pandit A, & Wang W (2011). “One-step” preparation of thiol-ene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromolecular Rapid Communications, 33(2), 120–126. [DOI] [PubMed] [Google Scholar]
- Du J, Sun R, Zhang S, Govender T, Zhang L-F, Xiong C-D, & Peng Y-X (2004). Novel polyelectrolyte carboxymethyl konjac glucomannan–chitosan nanoparticles for drug delivery. Macromolecular Rapid Communications, 25(9), 954–958. [Google Scholar]
- Du J, Zhang S, Sun R, Zhang L-F, Xiong C-D, & Peng Y-X (2005). Novel polyelectrolyte carboxymethyl konjac glucomannan-chitosan nanoparticles for drug delivery. II. Release of albuminin vitro. Journal of Biomedical Materials Research, 72B(2), 299–304. [DOI] [PubMed] [Google Scholar]
- Du Z, Liu J, Zhang T, Yu Y, Zhang Y, Zhai J, Huang H, Wei S, Ding L, & Liu B (2019). A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chemistry, 286, 530–536. [DOI] [PubMed] [Google Scholar]
- Duncan R (2003). The dawning era of polymer therapeutics. Nature Reviews Drug Discovery, 2(5), 347–360. [DOI] [PubMed] [Google Scholar]
- Elzatahry AA, Eldin MSM, Soliman EA, & Hassan EA (2009). Evaluation of alginate-chitosan bioadhesive beads as a drug delivery system for the controlled release of theophylline. Journal of Applied Polymer Science, 111(5), 2452–2459. [Google Scholar]
- Etrych T, Leclercq L, Boustta M, & Vert M (2005). Polyelectrolyte complex formation and stability when mixing polyanions and polycations in salted media: A model study related to the case of body fluids. European Journal of Pharmaceutical Sciences, 25(2–3), 281–288. [DOI] [PubMed] [Google Scholar]
- Fang S, Zhao X, Liu Y, Liang X, & Yang Y (2019). Fabricating multilayer emulsions by using OSA starch and chitosan suitable for spray drying: Application in the encapsulation of β-carotene. Food Hydrocolloids, 93, 102–110. [Google Scholar]
- Feinen B, Petrovsky N, Verma A, & Merkel TJ (2014). Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clinical and Vaccine Immunology, 21(4), 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Li J, Kong M, Liu Y, Cheng XJ, Li Y, Park HJ, & Chen XG (2015). Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids And Surfaces B-Biointerfaces, 128, 439–447. [DOI] [PubMed] [Google Scholar]
- Feng C, Sun G, Wang Z, Cheng X, Park H, Cha D, Kong M, & Chen X (2014). Transport mechanism of doxorubicin loaded chitosan based nanogels across intestinal epithelium. European Journal of Pharmaceutics and Biopharmaceutics, 87(1), 197–207. [DOI] [PubMed] [Google Scholar]
- Foged C, Brodin B, Frokjaer S, & Sundblad A (2005). Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International Journal of Pharmaceutics, 298(2), 315–322. [DOI] [PubMed] [Google Scholar]
- Francis MF, Cristea M, & Winnik FM (2004). Polymeric micelles for oral drug delivery: Why and how. Pure and Applied Chemistry, 76(7–8), 1321–1335. [Google Scholar]
- Fujii H, Shin-Ya M, Takeda S, Hashimoto Y, Mukai S-A, Sawada S-I, Adachi T, Akiyoshi K, Miki T, & Mazda O (2014). Cycloamylose-nanogel drug delivery system-mediated intratumor silencing of the vascular endothelial growth factor regulates neovascularization in tumor microenvironment. Cancer Science, 105(12), 1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway AL, Murphy A, DeSimone JM, Di J, Herrmann JP, Hunter ME, Kindig JP, Malinoski FJ, Rumley MA, Stoltz D, Templeman T, & Hubby B (2013). Development of a nanoparticle-based influenza vaccine using the print® technology. Nanomedicine: Nanotechnology, Biology and Medicine, 9(A), 523–531. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Chaturvedi K, More UA, Nadagouda MN, & Aminabhavi TM (2014). Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. Journal of Controlled Release, 193, 162–173. [DOI] [PubMed] [Google Scholar]
- Gibbs P, Brown TJ, Ng R, Jennens R, Cinc E, Pho M, Michael M, & Fox RM (2008). A pilot human evaluation of a formulation of irinotecan and hyaluronic acid in 5-fluorouracil-refractory metastatic colorectal cancer patients. Chemotherapy, 55(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Gordon D, Kelley P, Heinzel S, Cooper P, & Petrovsky N (2014). Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled phase 1 study. Vaccine, 32(48), 6469–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goycoolea FM, Lollo G, Remuñán-López C, Quaglia F, & Alonso M. a. J. (2009). Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules, 10(7), 1736–1743. [DOI] [PubMed] [Google Scholar]
- Griesser J, Burtscher S, Köllner S, Nardin I, Prüfert F, & Bernkop-Schnürch A (2017). Zeta potential changing self-emulsifying drug delivery systems containing phosphorylated polysaccharides. European Journal of Pharmaceutics and Biopharmaceutics, 119, 264–270. [DOI] [PubMed] [Google Scholar]
- Hachet E, Van Den Berghe H, Bayma E, Block MR, & Auzély-Velty R (2012). Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules, 13(6), 1818–1827. [DOI] [PubMed] [Google Scholar]
- Hasegawa U, Sawada S.-i., Shimizu T, Kishida T, Otsuji E, Mazda O, & Akiyoshi K (2009). Raspberry-like assembly of cross-linked nanogels for protein delivery. Journal of Controlled Release, 140(3), 312–317. [DOI] [PubMed] [Google Scholar]
- Herold DA, Keil K, & Bruns DE (1989). Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochemical Pharmacology, 38(1), 73–76. [DOI] [PubMed] [Google Scholar]
- Hinrichs WLJ, Prinsen MG, & Frijlink HW (2001). Inulin glasses for the stabilization of therapeutic proteins. International Journal of Pharmaceutics, 215(1–2), 163–174. [DOI] [PubMed] [Google Scholar]
- Hirakura T, Yasugi K, Nemoto T, Sato M, Shimoboji T, Aso Y, Morimoto N, & Akiyoshi K (2010). Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. Journal of Controlled Release, 142(3), 483–489. [DOI] [PubMed] [Google Scholar]
- Honda-Okubo Y, Kolpe A, Li L, & Petrovsky N (2014). A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax™) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine, 32, 4651–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen M, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, & Kjems J (2006). RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Molecular Therapy, 14(4), 476–484. [DOI] [PubMed] [Google Scholar]
- Hu Q, & Luo Y (2016). Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydrate Polymers, 151, 624–639. [DOI] [PubMed] [Google Scholar]
- Huang P, Yang C, Liu J, Wang W, Guo S, Li J, Sun Y, Dong H, Deng L, Zhang J, Liu J, & Dong A (2014). Improving the oral delivery efficiency of anticancer drugs by chitosan coated polycaprolactone-grafted hyaluronic acid nanoparticles. Journal of Materials Chemistry B, 2(25), 4021–4033. [DOI] [PubMed] [Google Scholar]
- Huang X-N, Zhou F-Z, Yang T, Yin S-W, Tang C-H, & Yang X-Q (2019). Fabrication and characterization of pickering high internal phase emulsions (HIPES) stabilized by chitosan-caseinophosphopeptides nanocomplexes as oral delivery vehicles. Food Hydrocolloids, 93, 34–45. [Google Scholar]
- Huh MS, Lee EJ, Koo H, Yhee JY, Oh KS, Son S, Lee S, Kim SH, Kwon IC, & Kim K (2017). Polysaccharide-based nanoparticles for gene delivery. Topics in Current Chemistry, 375(2), 1–19. [DOI] [PubMed] [Google Scholar]
- Huh MS, Lee SY, Park S, Lee S, Chung H, Lee S, Choi Y, Oh Y-K, Park JH, Jeong SY, Choi K, Kim K, & Kwon IC (2010). Tumor-homing glycol chitosan/polyethylenimine nanoparticles for the systemic delivery of sirna in tumor-bearing mice. Journal of Controlled Release, 144(2), 134–143. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Lin W, Jabbal-Gill I, Davis SS, Steward MW, & Illum L (2003). Nasal delivery of chitosan-DNA plasmid expressing epitopes of respiratory syncytial virus (RSV) induces protective CTL responses in BALB/c mice. Vaccine, 21(13–14), 1478–1485. [DOI] [PubMed] [Google Scholar]
- Izydorczyk M, Ciu SW, & Wang Q (2005). Polysaccharide gums: Structures, functional properties, and applications In Cui SW (Ed.). Food carbohydrates: Chemistry, Physical Properties, and Applications (pp. 269–313): CRC Press. [Google Scholar]
- Jiang HL, Lim HT, Kim YK, Arote R, Shin JY, Kwon JT, Kim JE, Kim JH, Kim D, Chae C, Nah JW, Choi YJ, Cho CS, & Cho MH (2011). Chitosan-graft-spermine as a gene carrier in vitro and in vivo. European Journal of Pharmaceutics and Biopharmaceutics, 77(1), 36–42. [DOI] [PubMed] [Google Scholar]
- Kai E, & Ochiya T (2004). A method for oral DNA delivery with n-acetylated chitosan. Pharmaceutical Research, 21(5), 838–843. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, & Miyamoto T (2004). Progress in structural characterization of functional polysaccharides In Dumitriu S (Ed.). Polysaccharides: Structural Diversity and Functional Versatility: Marcel Dekker. [Google Scholar]
- Kaneo Y, Tanaka T, Nakano T, & Yamaguchi Y (2001). Evidence for receptor-mediated hepatic uptake of pullulan in rats. Journal of Controlled Release, 70(3), 365–373. [DOI] [PubMed] [Google Scholar]
- Kang B, Opatz T, Landfester K, & Wurm FR (2015). Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chemical Society Reviews, 44(22), 8301–8325. [DOI] [PubMed] [Google Scholar]
- Kang JH, Tachibana Y, Kamata W, Mahara A, Harada-Shiba M, & Yamaoka T (2010). Liver-targeted siRNA delivery by polyethylenimine (PEI)-pullulan carrier. Bioorganic & Medicinal Chemistry, 18(11), 3946–3950. [DOI] [PubMed] [Google Scholar]
- Kato Y, Onishi H, & Machida Y (2001). Biological characteristics of lactosaminated n-succinyl-chitosan as a liver-specific drug carrier in mice. Journal of Controlled Release, 70(3), 295–307. [DOI] [PubMed] [Google Scholar]
- Katsuraya K, Okuyama K, Hatanaka K, Oshima R, Sato T, & Matsuzaki K (2003). Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydrate Polymers, 53(2), 183–189. [Google Scholar]
- Kazunari Akiyoshi SDNMSY, & Junzo S (2001). Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules, 26(12), 1–8. [Google Scholar]
- Khanlari A, Detamore MS, & Gehrke SH (2013). Increasing cross-linking efficiency of methacrylated chondroitin sulfate hydrogels by copolymerization with oligo(ethylene glycol) diacrylates. Macromolecules, 46(24), 9609–9617. [Google Scholar]
- Khatun Z, Nurunnabi M, Nafiujjaman M, Reeck GR, Khan HA, Cho KJ, & Lee Y.-k. (2015). A hyaluronic acid nanogel for photo–chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale, 7(24), 10680–10689. [DOI] [PubMed] [Google Scholar]
- Khvostov MV, Borisov SA, Tolstikova TG, Dushkin AV, Tsyrenova BD, Chistyachenko YS, Polyakov NE, Dultseva GG, Onischuk AA, & An’kov SV (2017). Supramolecular complex of ibuprofen with larch polysaccharide arabinogalactan: Studies on bioavailability and pharmacokinetics. European Journal of Drug Metabolism and Pharmacokinetics, 42(3), 431–440. [DOI] [PubMed] [Google Scholar]
- Knop K, Hoogenboom R, Fischer D, & Schubert US (2010). Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angewandte Chemie International Edition, 49(36), 6288–6308. [DOI] [PubMed] [Google Scholar]
- Kong R, Zhu X, Meteleva ES, Polyakov NE, Khvostov MV, Baev DS, Tolstikova TG, Dushkin AV, & Su W (2018). Atorvastatin calcium inclusion complexation with polysaccharide arabinogalactan and saponin disodium glycyrrhizate for increasing of solubility and bioavailability. Drug Delivery and Translational Research, 8(5), 1200–1213. [DOI] [PubMed] [Google Scholar]
- Kono H, & Fujita S (2012). Biodegradable superabsorbent hydrogels derived from cellulose by esterification crosslinking with 1,2,3,4-butanetetracarboxylic dianhydride. Carbohydrate Polymers, 87(4), 2582–2588. [Google Scholar]
- Köping-Höggård M, Tubulekas I, & Guan H (2001). Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Therapy, 8(14), 1108–1121 [DOI] [PubMed] [Google Scholar]
- Korbelik M, & Cooper PD (2007). Potentiation of photodynamic therapy of cancer by complement: The effect of gamma-inulin. British Journal of Cancer, 96(1), 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T, Ozasa K, Kobari S, Momota M, Kishishita N, Kobiyama K, Kuroda E, & Ishii KJ (2016). Intranasal hydroxypropyl-β-cyclodextrin-adjuvanted influenza vaccine protects against sub-heterologous virus infection. Vaccine, 34(27), 3191–3198. [DOI] [PubMed] [Google Scholar]
- Lee C, Shin J, Lee JS, Byun E, Ryu JH, Um SH, Kim D-I, Lee H, & Cho S-W (2013). Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules, 14(6), 2004–2013. [DOI] [PubMed] [Google Scholar]
- Li D, Kordalivand N, Fransen MF, Ossendorp F, Raemdonck K, Vermonden T, Hennink WE, & van Nostrum CF (2015). Reduction-sensitive dextran nanogels aimed for intracellular delivery of antigens. Advanced Functional Materials, 25(20), 2993–3003. [Google Scholar]
- Li M, Li Y, Peng K, Wang Y, Gong T, Zhang Z, He Q, & Sun X (2017a). Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomaterialia, 1–12. [DOI] [PubMed] [Google Scholar]
- Li N-N, Lin J, Di G, & Zhang L-M (2014). A macromolecular prodrug strategy for combinatorial drug delivery. Journal of Colloid and Interface Science, 417(C), 301–309. [DOI] [PubMed] [Google Scholar]
- Li R, Cai Z, Li Z, Zhang Q, Zhang S, Deng L, Lu L, Li L, & Zhou C (2017b). Synthesis of in-situ formable hydrogels with collagen and hyaluronan through facile Michael addition. Materials Science and Engineering: C, 77(C), 1035–1043. [DOI] [PubMed] [Google Scholar]
- Li T, Shi X-W, Du Y-M, & Tang Y-F (2007). Quaternized chitosan/alginate nanoparticles for protein delivery. Journal of Biomedical Materials Research, 83A(2), 383–390. [DOI] [PubMed] [Google Scholar]
- Li X-M, Xie Q-T, Zhu J, Pan Y, Meng R, Zhang B, Chen H-Q, & Jin Z-Y (2019). Chitosan hydrochloride/carboxymethyl starch complex nanogels as novel pickering stabilizers: Physical stability and rheological properties. Food Hydrocolloids, 93, 215–225. [Google Scholar]
- Liang Y, Zhao X, Ma PX, Guo B, Du Y, & Han X (2019). pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. Journal of Colloid and Interface Science, 536, 224–234. [DOI] [PubMed] [Google Scholar]
- Lin N, Huang J, Chang PR, Feng L, & Yu J (2011). Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids and Surfaces B-Biointerfaces, 85(2), 270–279. [DOI] [PubMed] [Google Scholar]
- Liu J, Pu H, Liu S, Kan J, & Jin C (2017). Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydrate Polymers, 174, 999–1017. [DOI] [PubMed] [Google Scholar]
- Liu L, Gou M, Yi TAO, Bai YU, Wei Y, & Zhao XIA (2014). Antitumor effects of heparin-polyethyleneimine nanogels delivering claudin-3-targeted short hairpin RNA combined with low-dose cisplatin on ovarian cancer. Oncology Reports, 31(4), 1623–1628. [DOI] [PubMed] [Google Scholar]
- Liu P, Shi B, Yue C, Gao G, Li P, Yi H, Li M, Wang B, Ma Y, & Cai L (2013). Dextran-based redox-responsive doxorubicin prodrug micelles for overcoming multidrug resistance. Polymer Chemistry, 4(24), 5793–5797. [Google Scholar]
- Liu Z, Jiao Y, Liu F, & Zhang Z (2007). Heparin/chitosan nanoparticle carriers prepared by polyelectrolyte complexation. Journal of Biomedical Materials Research, 83A(3), 806–812. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jiao Y, Wang Y, Zhou C, & Zhang Z (2008). Polysaccharides-based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews, 60(15), 1650–1662. [DOI] [PubMed] [Google Scholar]
- Liu Z, & Yao P (2014). Versatile injectable supramolecular hydrogels containing drug loaded micelles for delivery of various drugs. Polymer Chemistry, 5(3), 1072–1081. [Google Scholar]
- Luo Y, Kirker KR, & Prestwich GD (2000). Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. Journal of Controlled Release, 69(1), 169–184. [DOI] [PubMed] [Google Scholar]
- Maciel D, Figueira P, Xiao S, Hu D, Shi X, Rodrigues J, Tomás H, & Li Y (2013). Redox-responsive alginate nanogels with enhanced anticancer cytotoxicity. Biomacromolecules, 14(9), 3140–3146. [DOI] [PubMed] [Google Scholar]
- Maestrelli F, Garcia-Fuentes M, Mura P, & Alonso MJ (2006). A new drug nanocarrier consisting of chitosan and hydoxypropylcyclodextrin. European Journal of Pharmaceutics and Biopharmaceutics, 63(2), 79–86. [DOI] [PubMed] [Google Scholar]
- Maksimenko A, Polard V, Villemeur M, Elhamess H, Couvreur P, Bertrand JR, Aboubakar M, Gottikh M, & Malvy C (2005). In vivo potentialities of EWS-Fli-1 targeted antisense oligonucleotides-nanospheres complexes. Annals of the New York Academy of Sciences, 1058(1), 52–61. [DOI] [PubMed] [Google Scholar]
- Maldonado L, Chough S, Bonilla J, Kim KH, & Kokini J (2019). Mechanism of fabrication and nano-mechanical properties of α-lactalbumin/chitosan and BSA/κ-carrageenan nanotubes through layer-by-layer assembly for curcumin encapsulation and determination of in vitro cytotoxicity. Food Hydrocolloids, 93, 293–307. [Google Scholar]
- Manchun S, Dass CR, Cheewatanakornkool K, & Sriamornsak P (2015). Enhanced anti-tumor effect of pH-responsive dextrin nanogels delivering doxorubicin on colorectal cancer. Carbohydrate Polymers, 126, 222–230. [DOI] [PubMed] [Google Scholar]
- Masina N, Choonara YE, Kumar P, du Toit LC, Govender M, Indermun S, & Pillay V (2017). A review of the chemical modification techniques of starch. Carbohydrate Polymers, 157, 1226–1236. [DOI] [PubMed] [Google Scholar]
- Morimoto N, Hirano S, Takahashi H, Loethen S, Thompson DH, & Akiyoshi K (2013). Self-assembled ph-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules, 14(1), 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Ortega MD, Alvarez-Lorenzo C, Sigurdsson HH, Concheiro A, & Loftsson T (2012). Cross-linked hydroxypropyl-β-cyclodextrin and γ-cyclodextrin nanogels for drug delivery: Physicochemical and loading/release properties. Carbohydrate Polymers, 87(3), 2344–2351. [Google Scholar]
- Mueller SN, Tian S, & DeSimone JM (2015). Rapid and persistent delivery of antigen by lymph node targeting print nanoparticle vaccine carrier to promote humoral immunity. Molecular Pharmaceutics, 12(5), 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeye B, Deschout H, Röding M, Rudemo M, Delanghe J, Devreese K, Demeester J, Braeckmans K, De Smedt SC, & Raemdonck K (2011). Hemocompatibility of siRNA loaded dextran nanogels. Biomaterials, 32(34), 9120–9127. [DOI] [PubMed] [Google Scholar]
- Nagahama K, Sano Y, & Kumano T (2015). Anticancer drug-based multifunctional nanogels through self-assembly of dextran-curcumin conjugates toward cancer theranostics. Bioorganic & Medicinal Chemistry Letters, 25(12), 2519–2522. [DOI] [PubMed] [Google Scholar]
- Narayanan RP, Melman G, Letourneau NJ, Mendelson NL, & Melman A (2012). Photodegradable iron(III) cross-linked alginate gels. Biomacromolecules, 13(8), 2465–2471. [DOI] [PubMed] [Google Scholar]
- Nicolas J, Mura S, Brambilla D, Mackiewicz N, & Couvreur P (2013). Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chemical Society Reviews, 42(3), 1147. [DOI] [PubMed] [Google Scholar]
- Noh Y-W, Kong S-H, Choi D-Y, Park HS, Yang H-K, Lee H-J, Kim HC, Kang KW, Sung M-H, & Lim YT (2012). Near-infrared emitting polymer nanogels for efficient sentinel lymph node mapping. ACS Nano, 6(9), 7820–7831. [DOI] [PubMed] [Google Scholar]
- Odeniyi M, Omoteso O, Adepoju A, & Jaiyeoba K (2018). Starch nanoparticles in drug delivery: A review. Polymers in Medicine, 48(1), 41–45. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Shiratani M, Kobayashi H, & Ouchi T (1994). Release behavior of 5-fluorouracil from chitosan-gel nanospheres immobilizing 5-fluorouracil coated with polysaccharides and their cell specific cytotoxicity. Journal of Macromolecular Science, Part A, 31(5), 629–642. [Google Scholar]
- Oliveira VA, Veloso TC, Leao VA, dos Santos CG, & Botaro VR (2013). Hydrogels of cellulose acetate crosslinked with pyromellitic dianhydride - part I: Synthesis and swelling kinetics. Quimica Nova, 36(1), 102–106. [Google Scholar]
- Oliver AE, Leprince O, Wolkers WF, Hincha DK, Heyer AG, & Crowe JH (2001). Non-disaccharide-based mechanisms of protection during drying. Cryobiology, 43(2), 151–167. [DOI] [PubMed] [Google Scholar]
- Onishi M, Ozasa K, Kobiyama K, Ohata K, Kitano M, Taniguchi K, Homma T, Kobayashi M, Sato A, Katakai Y, Yasutomi Y, Wijaya E, Igarashi Y, Nakatsu N, Ise W, Inoue T, Yamada H, Vandenbon A, Standley DM, Kurosaki T, Coban C, Aoshi T, Kuroda E, & Ishii KJ (2015). Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. The Journal of Immunology, 194(6), 2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oommen OP, Wang S, Kisiel M, Sloff M, Hilborn J, & Varghese OP (2012). Smart design of stable extracellular matrix mimetic hydrogel: Synthesis, characterization, and in vitro and in vivo evaluation for tissue engineering. Advanced Functional Materials, 23(10), 1273–1280. [Google Scholar]
- Östmark E, Nyström D, & Malmström E (2008). Unimolecular nanocontainers prepared by rop and subsequent atrp from hydroxypropylcellulose. Macromolecules, 41(12), 4405–4415. [Google Scholar]
- Park HS, Lee JE, Cho MY, Hong JH, Cho SH, & Lim YT (2012). Hyaluronic acid/poly(β-amino ester) polymer nanogels for cancer-cell-specific NIR fluorescence switch. Macromolecular Rapid Communications, 33(18), 1549–1555. [DOI] [PubMed] [Google Scholar]
- Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim IS, Choi K, Kim SY, & Kwon IC (2007). Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. Journal of Controlled Release, 122(3), 305–314. [DOI] [PubMed] [Google Scholar]
- Park W, Park S-J, & Na K (2010). Potential of self-organizing nanogel with acetylated chondroitin sulfate as an anti-cancer drug carrier. Colloids And Surfaces B-Biointerfaces, 79(2), 501–508. [DOI] [PubMed] [Google Scholar]
- Park YK, Park YH, Shin BA, Choi ES, Park YR, Akaike T, & Cho CS (2000). Galactosylated chitosan-graft-dextran as hepatocyte-targeting DNA carrier. Journal of Controlled Release, 69(1), 97–108. [DOI] [PubMed] [Google Scholar]
- Pawar SN, & Edgar KJ (2012). Alginate derivatization: A review of chemistry, properties and applications. Biomaterials, 33(11), 3279–3305. [DOI] [PubMed] [Google Scholar]
- Peng K, Cui C, Tomatsu I, Porta F, Meijer AH, Spaink HP, & Kros A (2010). Cyclodextrin/dextran based drug carriers for a controlled release of hydrophobic drugs in zebrafish embryos. Soft Matter, 6(16), 3778–3776. [Google Scholar]
- Peng N, Yang M, Tang Y, Zou T, Guo F, Wu K, Wang X, Li X, & Liu Y (2019). Amphiphilic hexadecyl-quaternized chitin micelles for doxorubicin delivery. International Journal of Biological Macromolecules, 130, 1–7. [DOI] [PubMed] [Google Scholar]
- Pescosolido L, Vermonden T, Malda J, Censi R, Dhert WJA, Alhaique F, Hennink WE, & Matricardi P (2011). In situ forming ipn hydrogels of calcium alginate and dextran-hema for biomedical applications. Acta Biomaterialia, 7(4), 1627–1633. [DOI] [PubMed] [Google Scholar]
- Pitarresi G, Giacomazza D, Triolo D, Giammona G, & Biagio PLS (2012). Rheological characterization and release properties of inulin-based hydrogels. Carbohydrate Polymers, 88(3), 1033–1040. [Google Scholar]
- Qian H, Wang X, Yuan K, Xie C, Wu W, Jiang X, & Hu L (2014). Delivery of doxorubicin in vitro and in vivo using bio-reductive cellulose nanogels. Biomaterials Science, 2(2), 220–232. [DOI] [PubMed] [Google Scholar]
- Quadrado RFN, & Fajardo AR (2018). Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arabian Journal of Chemistry. [Google Scholar]
- Raemdonck K, Naeye B, Høgset A, Demeester J, & De Smedt SC (2010). Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. Journal of Controlled Release, 145(3), 281–288. [DOI] [PubMed] [Google Scholar]
- Rahimian K, Wen Y, & Oh JK (2015). Redox-responsive cellulose-based thermoresponsive grafted copolymers and in-situ disulfide crosslinked nanogels. Polymer, 72(C), 387–394. [Google Scholar]
- Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, & Couvreur P (1993). Development of a new drug carrier made from alginate. Journal of Pharmaceutical Sciences, 82(9), 912–917. [DOI] [PubMed] [Google Scholar]
- Ramasamy T, Tran TH, Cho HJ, Kim JH, Kim YI, Jeon JY, Choi H-G, Yong CS, & Kim JO (2013). Chitosan-based polyelectrolyte complexes as potential nanoparticulate carriers: Physicochemical and biological characterization. Pharmaceutical Research, 31(5), 1302–1314. [DOI] [PubMed] [Google Scholar]
- Richardson JC, Dettmar PW, Hampson FC, & Melia CD (2004). The bioadhesive and swelling characteristics of sodium alginate suspensions Gums and Stabilisers for the Food Industry 12 (pp. 75–83). Cambridge: Royal Society of Chemistry. [Google Scholar]
- Robyt JF (2008). Starch: Structure, properties, chemistry, and enzymology In Fraser-Reid B, Tatsuta K & Thiem J (Eds.). Glycoscience (pp. 1437–1472). Berlin: Springer-Verlag. [Google Scholar]
- Saade F, Honda-Okubo Y, Trec S, & Petrovsky N (2013). A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine, 31(5), 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S, Singh VK, Uvanesh K, Biswal D, Anis A, Rana UA, Al-Zahrani SM, & Pal K (2015). Development of ionic and non-ionic natural gum-based bigels: Prospects for drug delivery application. Journal of Applied Polymer Science, 132(38). [Google Scholar]
- Sandri G, Bonferoni MC, Rossi S, Ferrari F, Gibin S, Zambito Y, Di Colo G, & Caramella C (2007). Nanoparticles based on n-trimethylchitosan: Evaluation of absorption properties using in vitro (caco-2 cells) and ex vivo (excised rat jejunum) models. European Journal of Pharmaceutics and Biopharmaceutics, 65(1), 68–77. [DOI] [PubMed] [Google Scholar]
- Santander-Ortega MJ, Stauner T, Loretz B, Ortega-Vinuesa JL, Bastos-González D, Wenz G, Schaefer UF, & Lehr CM (2010). Nanoparticles made from novel starch derivatives for transdermal drug delivery. Journal of Controlled Release, 141(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Saremi S, Dinarvand R, Kebriaeezadeh A, Ostad SN, & Atyabi F (2013). Enhanced oral delivery of docetaxel using thiolated chitosan nanoparticles: Preparation, in vitro and in vivo studies. BioMed Research International, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker B, Papageorgiou DG, Silva R, Zehnder T, Gul-E-Noor F, Bertmer M, Kaschta J, Chrissafis K, Detsch R, & Boccaccini AR (2014). Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. Journal of Materials Chemistry B, 2(11), 1470–1413. [DOI] [PubMed] [Google Scholar]
- Sarmento B, Ferreira D, Veiga F, & Ribeiro A (2006a). Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through dsc and ftir studies. Carbohydrate Polymers, 66(1), 1–7. [Google Scholar]
- Sarmento B, Martins S, Ribeiro A, Veiga F, Neufeld R, & Ferreira D (2006b). Development and comparison of different nanoparticulate polyelectrolyte complexes as insulin carriers. International Journal of Peptide Research and Therapeutics, 12(2), 131–138. [Google Scholar]
- Schütz CA, Juillerat-Jeanneret L, Käuper P, & Wandrey C (2011). Cell response to the exposure to chitosan-TPP//alginate nanogels. Biomacromolecules, 12(11), 4153–4161. [DOI] [PubMed] [Google Scholar]
- Senna AM, Novack KM, & Botaro VR (2014). Synthesis and characterization of hydrogels from cellulose acetate by esterification crosslinking with EDTA dianhydride. Carbohydrate Polymers, 114, 260–268. [DOI] [PubMed] [Google Scholar]
- Shi Y-G, Meng Y-C, Li J-R, Chen J, Liu Y-H, & Bai X (2014). Chondroitin sulfate: Extraction, purification, microbial and chemical synthesis. Journal of Chemical Technology & Biotechnology, 89(10), 1445–1465. [Google Scholar]
- Sieval AB, Thanou M, Kotze AF, & Verhoef JC (1998). Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydrate Polymers, 36(2–3), 157–165. [Google Scholar]
- Silva DG, Cooper PD, & Petrovsky N (2004). Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunology and Cell Biology, 82(6), 611–616. [DOI] [PubMed] [Google Scholar]
- Šimkovic I (2004). Cross-linking of starch with 1, 2, 3, 4-diepoxybutane or 1, 2, 7, 8-diepoxyoctane. Carbohydrate Polymers, 55(3), 299–305. [Google Scholar]
- Simkovic I (2008). What could be greener than composites made from polysaccharides? Carbohydrate Polymers, 74(4), 759–762. [Google Scholar]
- Singh RS, Kaur N, Rana V, & Kennedy JF (2017). Pullulan: A novel molecule for biomedical applications. Carbohydrate Polymers, 171, 102–121. [DOI] [PubMed] [Google Scholar]
- Sirisha VL, & D’Souza JS (2016). Polysaccharide-based nanoparticles as drug delivery systems In Kim SK (Ed.). Marine Omics: Principles and Applications (pp. 663–702): CRC Press. [Google Scholar]
- Sithole MN, Choonara YE, du Toit LC, Kumar P, & Pillay V (2017). A review of semi-synthetic biopolymer complexes: Modified polysaccharide nano-carriers for enhancement of oral drug bioavailability. Pharmaceutical Development and Technology, 22(2), 283–295. [DOI] [PubMed] [Google Scholar]
- Slade L, & Levine H (1991). Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Critical Reviews in Food Science & Nutrition, 30(2–3), 115–360. [DOI] [PubMed] [Google Scholar]
- Sombra FM, Richter AR, de Araújo AR, de Oliveira Silva Ribeiro F, de Fátima Souza Mendes J, dos Santos Fontenelle RO, da Silva DA, de Paula HCB, de Andrade Feitosa JP, Goycoolea FM, & de Paula RCM (2019). Nanocapsules of sterculia striata acetylated polysaccharide as a potential monomeric amphotericin B delivery matrix. International Journal of Biological Macromolecules, 130, 1–9. [DOI] [PubMed] [Google Scholar]
- Stevens CV, Meriggi A, & Booten K (2001). Chemical modification of inulin, a valuable renewable resource, and its industrial applications. Biomacromolecules, 2(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Sunasee R, Adokoh CK, Darkwa J, & Narain R (2014). Therapeutic potential of carbohydrate-based polymeric and nanoparticle systems. Expert Opinion on Drug Delivery, 11(6), 867–884. [DOI] [PubMed] [Google Scholar]
- Susa M, Iyer AK, Ryu K, Choy E, Hornicek FJ, Mankin H, Milane L, Amiji MM, & Duan Z (2010). Inhibition of ABCD1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS ONE, 5(5), e10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Suzuki Y, Suhara T, Omichi K, Shimizu A, Hasegawa K, Kokudo N, Ohta S, & Ito T (2013). In situ cross-linkable hydrogel of hyaluronan produced via copper-free click chemistry. Biomacromolecules, 14(10), 3581–3588. [DOI] [PubMed] [Google Scholar]
- Tan H, Luan H, Hu Y, & Hu X (2012). Covalently crosslinked chitosan-poly(ethylene glycol) hybrid hydrogels to deliver insulin for adipose-derived stem cells encapsulation. Macromolecular Research, 21(4), 392–399. [Google Scholar]
- Tan H, Rubin JP, & Marra KG (2011a). Direct synthesis of biodegradable polysaccharide derivative hydrogels through aqueous diels-alder chemistry. Macromolecular Rapid Communications, 32(12), 905–911. [DOI] [PubMed] [Google Scholar]
- Tan J, Kang H, Liu R, Wang D, Jin X, Li Q, & Huang Y (2011b). Dual-stimuli sensitive nanogels fabricated by self-association of thiolated hydroxypropyl cellulose. Polymer Chemistry, 2(3), 672–678. [Google Scholar]
- Tiyaboonchai W, & Limpeanchob N (2007). Formulation and characterization of amphotericin b–chitosan–dextran sulfate nanoparticles. International Journal of Pharmaceutics, 329(1–2), 142–149. [DOI] [PubMed] [Google Scholar]
- Tomihata K, & Ikada Y (1997). Preparation of cross-linked hyaluronic acid films of low water content. Biomaterials, 18(3), 189–195. [DOI] [PubMed] [Google Scholar]
- Tripodo G, Trapani A, Torre ML, Giammona G, Trapani G, & Mandracchia D (2015). Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. European Journal of Pharmaceutics and Biopharmaceutics, 97, 1–17. [DOI] [PubMed] [Google Scholar]
- Valmikinathan CM, Mukhatyar VJ, Jain A, Karumbaiah L, Dasari M, & Bellamkonda RV (2012). Photocrosslinkable chitosan based hydrogels for neural tissue engineering. Soft Matter, 8(6), 1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hoven JM, Nemes R, Metselaar JM, Nuijen B, Beijnen JH, Storm G, & Szebeni J (2013). Complement activation by pegylated liposomes containing prednisolone. European Journal of Pharmaceutical Sciences, 49(2), 265–271. [DOI] [PubMed] [Google Scholar]
- Van Thienen TG, Raemdonck K, Demeester J, & De Smedt SC (2007). Protein release from biodegradable dextran nanogels. Langmuir, 23(19), 9794–9801. [DOI] [PubMed] [Google Scholar]
- Varghese OP, Kisiel M, Martínez-Sanz E, Ossipov DA, & Hilborn J (2010). Synthesis of guanidinium-modified hyaluronic acid hydrogel. Macromolecular Rapid Communications, 31(13), 1175–1180. [DOI] [PubMed] [Google Scholar]
- Vervoort L, Rombaut P, Van den Mooter G, Augustijns P, & Kinget R (1998a). Inulin hydrogels. II. In vitro degradation study. International Journal of Pharmaceutics, 172(1–2), 137–145. [Google Scholar]
- Vervoort L, Van den Mooter G, Augustijns P, Bousson R, Toppet S, & Kinget R (1997). Inulin hydrogels as carriers for colonic drug targeting: I. Synthesis and characterization of methacrylated inulin and hydrogen formation. Pharmaceutical Research, 14(12), 1730–1737. [DOI] [PubMed] [Google Scholar]
- Vervoort L, Van den Mooter G, Augustijns P, & Kinget R (1998b). Inulin hydrogels. I. Dynamic and equilibrium swelling properties. International Journal Of Pharmaceutics, 172(1– 2), 127–135. [Google Scholar]
- Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, & Elkin M (2011). Significance of heparanase in cancer and inflammation. Cancer Microenvironment, 5(2), 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voepel J, Edlund U, & Albertsson A-C (2009). Alkenyl-functionalized precursors for renewable hydrogels design. Journal Of Polymer Science Part A-Polymer Chemistry, 47(14), 3595–3606. [Google Scholar]
- Wang J, Chen J-S, Zong J-Y, Zhao D, Li F, Zhuo R-X, & Cheng S-X (2010). Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. The Journal of Physical Chemistry C, 114(44), 18940–18945. [Google Scholar]
- Wang K, Buschle-Diller G, & Misra RDK (2015). Chitosan-based injectable hydrogels for biomedical applications. Materials Technology, 1–9. [Google Scholar]
- Wang L, Barclay T, Song Y, Joyce P, Sakala IG, Petrovsky N, & Garg S (2017). Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine, 35(34), 4382–4388. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang J, Li M, Wang Y, Wu H, Xiong L, & Sun Q (2019). Enhanced viability of layer-by-layer encapsulated lactobacillus pentosus using chitosan and sodium phytate. Food Chemistry, 285, 260–265. [DOI] [PubMed] [Google Scholar]
- Wei X, Senanayake TH, Warren G, & Vinogradov SV (2013). Hyaluronic acid-based nanogel–drug conjugates with enhanced anticancer activity designed for the targeting of CD544-positive and drug-resistant tumors. Bioconjugate chemistry, 24(4), 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, & Oh JK (2014). Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromolecular Rapid Communications, 3, 1819–1832. [DOI] [PubMed] [Google Scholar]
- Wen Y, & Oh JK (2015). Intracellular delivery cellulose-based bionanogels with dual temperature/pH-response for cancer therapy. Colloids and Surfaces B-Biointerfaces, 133, 246–253. [DOI] [PubMed] [Google Scholar]
- Wu W, Yao W, Wang X, Xie C, Zhang J, & Jiang X (2015). Bioreducible heparin-based nanogel drug delivery system. Biomaterials, 39(C), 260–268. [DOI] [PubMed] [Google Scholar]
- Xi J, Zhou L, & Dai H (2012). Drug-loaded chondroitin sulfate-based nanogels: Preparation and characterization. Colloids And Surfaces B-Biointerfaces, 100, 107–115. [DOI] [PubMed] [Google Scholar]
- Xu W, Ding J, Li L, Xiao C, Zhuang X, & Chen X (2015a). Acid-labile boronate-bridged dextran-bortezomib conjugate with up-regulated hypoxic tumor suppression. Chemical Communications, 51(31), 6812–6815. [DOI] [PubMed] [Google Scholar]
- Xu W, Ding J, Xiao C, Li L, Zhuang X, & Chen X (2015b). Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials, 54(C), 72–86. [DOI] [PubMed] [Google Scholar]
- Xu Y, Du Y, Huang R, & Gao L (2003). Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials, 24(27), 5015–5022. [DOI] [PubMed] [Google Scholar]
- Xue Y, Xia X, Yu B, Luo X, Cai N, Long S, & Yu F (2015). A green and facile method for the preparation of a pH-responsive alginate nanogel for subcellular delivery of doxorubicin. RSC Advances, 5, 73416–73423. [Google Scholar]
- Yadav AK, Mishra P, & Agrawal GP (2008). An insight on hyaluronic acid in drug targeting and drug delivery. Journal of Drug Targeting, 16(2), 91–107. [DOI] [PubMed] [Google Scholar]
- Yamazaki N, Kojima S, Bovin NV, Andre S, Gabius S, & Gabius HJ (2000). Endogenous lectins as targets for drug delivery. Advanced Drug Delivery Reviews, 43(2–3), 225–244. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang X, Yao X, Zhang Y, Wu W, & Jiang X (2015a). Hyaluronic acid nanogels with enzyme-sensitive cross-linking group for drug delivery. Journal of Controlled Release, 205(C), 206–217. [DOI] [PubMed] [Google Scholar]
- Yang X, Du H, Liu J, & Zhai G (2015b). Advanced nanocarriers based on heparin and its derivatives for cancer management. Biomacromolecules, 16(2), 423–436. [DOI] [PubMed] [Google Scholar]
- Yang X, Kootala S, Hilborn J, & Ossipov DA (2011). Preparation of hyaluronic acid nanoparticles via hydrophobic association assisted chemical cross-linking - an orthogonal modular approach. Soft Matter, 7(16), 7517–7519. [Google Scholar]
- Yoon HY, Son S, Lee SJ, You DG, Yhee JY, Park JH, Swierczewska M, Lee S, Kwon IC, Kim SH, Kim K, & Pomper MG (2014). Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Scientific Reports, 4, 6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J-O, & Peng C-A (2005). Calcium-alginate nanoparticles formed by reverse microemulsion as gene carriers. Macromolecular Symposia, 219(1), 147–153. [Google Scholar]
- Yuk SH, Oh KS, Cho SH, Kim SY, Oh S, Lee JH, Kim K, & Kwon IC (2012). Enhancement of the targeting capabilities of the paclitaxel-loaded pluronic nanoparticles with a glycol chitosan/heparin composite. Molecular Pharmaceutics, 9(2), 230–236. [DOI] [PubMed] [Google Scholar]
- Zahoor A, Sharma S, & Khuller GK (2005). Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. International Journal of Antimicrobial Agents, 26(4), 298–303. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zhang Z, Shi F, Ding J, Xiao C, Zhuang X, He C, Chen L, & Chen X (2013a). Disulfide crosslinked pegylated starch micelles as efficient intracellular drug delivery platforms. Soft Matter, 9(7), 2224–2210. [Google Scholar]
- Zhang N, Wardwell P, & Bader R (2013b). Polysaccharide-based micelles for drug delivery. Pharmaceutics, 5(2), 329–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, An M, Xi J, & Liu H (2017). Targeting cpg adjuvant to lymph node via dextran conjugate enhances antitumor immunotherapy. Bioconjugate Chemistry, 28(7), 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang L, Hou J, Xia X, Wang J, Ning Q, & Jiang S (2018). Promising positive liver targeting delivery system based on arabinogalactan-anchored polymeric micelles of norcantharidin. Artificial Cells, Nanomedicine, and Biotechnology, 46(S3), S630–S640. [DOI] [PubMed] [Google Scholar]