Abstract
Aims
We sought to identify the prevalence and related outcomes of frail individuals undergoing transcatheter mitral valve repair and transcatheter aortic valve replacement (TAVR).
Methods and results
Patients aged 65 and older were included in the study if they had at least one procedural code for transcatheter mitral valve repair or TAVR between 1 January 2016 and 31 December 2016 in the Centers for Medicare and Medicaid Services Medicare Provider and Review database. The Hospital Frailty Risk Score, an International Classification of Diseases, Tenth Revision (ICD-10) claims-based score, was used to identify frailty and the primary outcome was all-cause 1-year mortality. A total of 3746 (11.6%) patients underwent transcatheter mitral valve repair and 28 531 (88.4%) underwent TAVR. In the transcatheter mitral valve repair and TAVR populations, respectively, there were 1903 (50.8%) and 14 938 (52.4%) patients defined as low risk for frailty (score <5), 1476 (39.4%) and 11 268 (39.5%) defined as intermediate risk (score 5–15), and 367 (9.8%) and 2325 (8.1%) defined as high risk (score >15). One-year mortality was 12.8% in low-risk patients, 29.7% in intermediate-risk patients, and 40.9% in high-risk patients undergoing transcatheter mitral valve repair (log rank P < 0.001). In patients undergoing TAVR, 1-year mortality rates were 7.6% in low-risk patients, 17.6% in intermediate-risk patients, and 30.1% in high-risk patients (log rank P < 0.001).
Conclusions
This study successfully identified individuals at greater risk of short- and long-term mortality after undergoing transcatheter valve therapies in an elderly population in the USA using the ICD-10 claims-based Hospital Frailty Risk Score.
Keywords: Claims data, Frailty, Transcatheter valve therapies
Introduction
Frailty is conceptually defined as a multisystem clinical syndrome that results in a decreased physiological reserve and an increased vulnerability to stressors.1 Regardless of definition, frailty is a key factor in identifying older patients’ potential for improvement after transcatheter valve therapies.2 Prior studies have shown that both short- and long-term mortality rates are significantly higher after transcatheter mitral valve repair and transcatheter aortic valve replacement (TAVR) in frail patients.3–9 National guidelines strongly recommend an objective evaluation of frailty to optimize patient selection, but the range of available measures raises issues with consistency.10,11 In clinical practice, frailty is not often measured due to the lack of consensus surrounding frailty assessment tools,12 and there are divergent prevalence estimates and effect sizes reported across different studies.4,13
Since 2012, the transcatheter valve therapy (TVT) registry has reported the outcomes of patients undergoing procedures including TAVR and transcatheter mitral valve repair in the USA.14 This robust registry allows for transparent analysis of patient outcomes after these procedures. However, the prospective collection of information on frailty is time-consuming and may not always be feasible. In addition, registries may misrepresent frailty status and prevalence by focusing on a limited definition of frailty, not including all hospitals, and defining frailty according to a single point in time. Administrative claims represent an alternative source of data by which frailty might be more easily assessed.15,16 However, prior claims-based classification systems may have been insufficiently granular to adequately characterize complex conditions such as frailty. The International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), which was introduced in many countries including the USA in 2015, greatly increased the number of codes that are available for use. Recently, an ICD-10-based frailty score has been developed and validated among the elderly population in the UK.17 No studies have yet evaluated whether the ICD-10-CM-based Hospital Frailty Risk Score can be used to evaluate frailty among patients undergoing valvular heart disease interventions. Therefore, in this study, we sought to identify the prevalence of frailty based on the recently validated ICD-10-based Hospital Frailty Risk Score, and measure the impact of frailty on outcomes of individuals undergoing TAVR or transcatheter mitral valve repair using data from the US Centers for Medicare and Medicaid Services (CMS) Medicare Provider Analysis and Review (MedPAR) data, which contains a longitudinal record of patient hospitalizations.
Materials and methods
Study population
The CMS MedPAR database utilized for this study is a 100% sample of administrative billing claims for inpatient hospitalizations, and has been used previously to study national patterns of procedure utilization in the USA.18,19 Patients aged 65 and older were included in the study if they had at least one procedural code for transcatheter mitral valve repair (ICD-10-CM code 02UG3JZ) or TAVR (ICD-10-CM codes 02RF38H or 02RF38Z), between 1 January 2016 and 31 December 2016.
Covariates
Baseline covariates were ascertained using secondary diagnosis codes that were coded as ‘present on admission’ during the index hospitalization (i.e. the hospitalization for the initial procedure), as well as from principal and secondary diagnosis codes from all hospitalizations at least 3 months prior to the date of admission for the index hospitalization in each patient (from 1 October 2015 to 1 October 2016). Elixhauser (see Supplementary material online, Table S1) and Charlson (see Supplementary material online, Table S2) comorbidity indices, both of which are validated summary comorbidity measures that have been previously shown to predict mortality in the Medicare population,20,21 were measured for each patient.
Definition of frailty
To identify frail individuals, we calculated the Hospital Frailty Risk Score for each individual. This score was recently developed and validated in a large, older (≥75 years) population in the UK, and is based on diagnoses associated with high resource use.17 It has not been used to describe frailty in the USA. For each patient, we calculated the Hospital Frailty Risk Score based on 109 ICD-10 diagnostic codes (the first three characters) from all hospitalizations occurring at least 3 months prior to the date of admission for the index hospitalization or using secondary diagnosis codes that were coded as ‘present on admission’ during the index hospitalization in each patient. The full list of diagnoses is shown in Supplementary material online, Table S3. We categorized individuals into risk categories based on their calculated Hospital Frailty Risk Score. Individuals were categorized as low (<5), intermediate (5–15), and high risk (>15) for frailty based on previously published cut-points, and patients in the intermediate-risk and high-risk categories were defined as frail.17
Outcomes
The primary outcome was all-cause 1-year mortality, determined through linkage of the MedPAR files to the Medicare Beneficiary Summary File, which includes vital status information. Time to death was calculated as the time period between the date of the index procedure and the date of death. Additionally, we identified long hospital stays (defined as >10 days in hospital), and 30-day mortality. Furthermore, we determined the number of rehospitalizations (defined as ‘0’, ‘1’, and ‘≥2’); all-cause rehospitalization rates within 1 year; and rehospitalizations due to acute myocardial infarction, acute heart failure, acute kidney failure, stroke or transient ischaemic attack, and acute post-haemorrhagic anaemia as secondary outcomes in patients discharged alive from the index procedure. Transfers to other hospitals were linked to a single index hospitalization. Time to rehospitalization was calculated as the time period between the date of discharge from the index hospitalization and the date of admission for the first subsequent hospitalization. Patients were censored if they were no longer enrolled in Medicare according to the denominator file as of 31 December 2016, which marked the end of the follow-up period for time to rehospitalization.
Statistical analysis
Continuous variables are presented as means and standard deviations or medians and inter-quartile ranges, and categorical variables are presented as counts and percentages. Restricted cubic spline curves with five knots were used to show the non-linear associations between the Hospital Frailty Risk Score and 1-year mortality. Multivariable Cox regression models were used to determine the impact of frailty (continuous and categorical) on all-cause 1-year mortality. Competing risk Cox regression analyses were used to show the performance of frailty in all-cause rehospitalization and each subgroup of rehospitalization, and to show cumulative incidence rates of rehospitalization, since mortality was a competing risk for rehospitalization. Patients who died within the study period (before 31 December 2016) represented a competing risk since they could not be rehospitalized after the date of death. Since different measures of comorbidity may be collinear with the frailty score, we used variance inflation factors (VIF) to test whether there are collinearities among the Hospital Frailty Risk Score, Elixhauser and Charlson comorbidity indices. Models were adjusted for age, sex, and Elixhauser and Charlson comorbidity indices. Multivariable Cox regression models were used to investigate the interaction between the Hospital Frailty Risk Score and transcatheter valve therapies. Harrell’s c-statistic was used to assess model discrimination, and the improvement in discrimination with the addition of the Hospital Frailty Risk Score was assessed by the change in the c-statistic and the DeLong test.22 An integrated discrimination improvement (IDI) test was also used to assess discrimination improvement.23 Unadjusted cumulative incidence curves were created to plot time to event, stratified by risk category. All statistical analyses were performed in STATA version 15.0 (Stata Corporation, College Station, TX, USA) or SAS version 9.4 (SAS Institute, Cary, NC, USA) using a two-tailed alpha <0.05 to define statistical significance.
Results
Overall results and frailty
A total of 32 986 patients treated with TAVR or transcatheter mitral valve repair were identified during the study period. After excluding 709 patients aged <65 years, a total of 32 277 patients were ultimately included in the analytic sample. Of these, 3746 underwent transcatheter mitral valve repair and 28 531 underwent TAVR. The prevalence of the 28 ICD-10 codes contributing the largest point totals (minimum two points) towards the Hospital Frailty Risk Score are presented in Table 1 (all covariates are presented in Supplementary material online, Table S3). ‘Other disorders of fluid, electrolyte and acid-base balance’ (31.4% and 26.9%), and ‘other disorders of urinary system’ including urinary tract infection and urinary incontinence (10.9% and 10.8%), were the most frequently diagnosed codes in both the transcatheter mitral valve repair and TAVR groups, respectively. Baseline characteristics of patients are presented in Table 2. The mean age of TVT recipients was 80.1 ± 8.9 years in the group undergoing transcatheter mitral valve repair, and in 81.5 ± 8.1 in the group undergoing TAVR. The majority of patients were male in both transcatheter mitral valve repair (51.8%) and TAVR (53.6%) groups.
Table 1.
List of ICD-10 codes, their prevalence in each group, and the number of points that each variable contributes to the creation of the Hospital Frailty Risk Score in patients undergoing transcatheter mitral valve repair and transcatheter aortic valve replacement
| Transcatheter mitral valve repair, n = 3746, n (%) | Transcatheter aortic valve replacement, n = 28 531, n (%) | Points | ||
|---|---|---|---|---|
| G81 | Hemiplegia | 31 (0.8) | 365 (1.3) | 4.4 |
| G30 | Alzheimer’s disease | 23 (0.6) | 312 (1.1) | 4.0 |
| I69 | Sequelae of cerebrovascular disease (secondary codes) | 96 (2.6) | 993 (3.5) | 3.7 |
| R29 | Other symptoms and signs involving the nervous and musculoskeletal systems (R29.6 Tendency to fall) | 53 (1.4) | 534 (1.9) | 3.6 |
| N39 | Other disorders of urinary system (includes urinary tract infection and urinary incontinence) | 410 (10.9) | 3080 (10.8) | 3.2 |
| F05 | Delirium, not induced by alcohol and other psychoactive substances | 49 (1.3) | 479 (1.7) | 3.2 |
| W19 | Unspecified fall | 6 (0.2) | 34 (0.1) | 3.2 |
| S00 | Superficial injury of head | 24 (0.6) | 161 (0.6) | 3.2 |
| R31 | Unspecified haematuria | 162 (4.3) | 924 (3.2) | 3.0 |
| B96 | Other bacterial agents as the cause of diseases classified to other chapters (secondary code) | 163 (4.4) | 1186 (4.2) | 2.9 |
| R41 | Other symptoms and signs involving cognitive functions and awareness | 102 (2.7) | 891 (3.1) | 2.7 |
| R26 | Abnormalities of gait and mobility | 212 (5.7) | 1559 (5.5) | 2.6 |
| I67 | Other cerebrovascular diseases | 30 (0.8) | 319 (1.1) | 2.6 |
| R56 | Convulsions, not elsewhere classified | 20 (0.5) | 141 (0.5) | 2.6 |
| R40 | Somnolence, stupor, and coma | 11 (0.3) | 124 (0.4) | 2.5 |
| T83 | Complications of genitourinary prosthetic devices, implants, and grafts | 35 (0.9) | 200 (0.7) | 2.4 |
| S06 | Intracranial injury | 7 (0.2) | 99 (0.3) | 2.4 |
| S42 | Fracture of shoulder and upper arm | 9 (0.2) | 73 (0.3) | 2.3 |
| E87 | Other disorders of fluid, electrolyte, and acid-base balance | 1176 (31.4) | 7677 (26.9) | 2.3 |
| M25 | Other joint disorders, not elsewhere classified | 78 (2.1) | 547 (1.9) | 2.3 |
| E86 | Volume depletion | 245 (6.5) | 1558 (5.5) | 2.3 |
| R54 | Senility | 149 (4.0) | 1523 (5.3) | 2.2 |
| F03 | Unspecified dementia | 162 (4.3) | 1595 (5.6) | 2.1 |
| W18 | Other fall on same level | 1 (<1) | 19 (0.1) | 2.1 |
| Z75 | Problems related to medical facilities and other health care | 4 (0.1) | 12 (<1) | 2.0 |
| F01 | Vascular dementia | 5 (0.1) | 124 (0.4) | 2.0 |
| S80 | Superficial injury of lower leg | 10 (0.3) | 84 (0.3) | 2.0 |
| L03 | Cellulitis | 114 (3.0) | 699 (2.4) | 2.0 |
The score presents the top 28 codes, each of which contributes ≥2 points. (All covariates are presented in Supplementary material online, Table S3.)
Table 2.
Characteristics of patients after transcatheter mitral valve repair and transcatheter aortic valve replacement
| Transcatheter mitral valve repair (n = 3746) | Transcatheter aortic valve replacement (n = 28 531) | |
|---|---|---|
| Age, mean (SD) | 80.1 (8.9) | 81.5 (8.1) |
| Male, no. of pts | 1941 (51.8%) | 15 304 (53.6%) |
| Charlson Index, mean (SD) | 2.9 (1.9) | 3.1 (2.0) |
| Elixhauser Comorbidity Index, mean (SD) | 5.7 (1.9) | 5.8 (1.9) |
| Hospital Frailty Index, mean (SD) | 6.6 (6.1) | 6.3 (5.7) |
| Hospital Frailty Risk Category | ||
| Low risk (<5), no. of pts | 1903 (50.8%) | 14 938 (52.4%) |
| Intermediate risk (5–15), no. of pts | 1476 (39.4%) | 11 268 (39.5%) |
| High risk (>15), no. of pts | 367 (9.8%) | 2325 (8.1%) |
| Frail (≥5 Hospital Frailty Index), no. of pts | 1843 (49.2) | 13 593 (47.6%) |
There were 1903 (50.8%) and 14 938 (52.4%) patients defined as low risk using a cut-point score of <5; while 1476 (39.4%) and 11 268 (39.5%) were defined as intermediate risk (5–15), and 367 (9.8%) and 2325 (8.1%) were defined as high risk (>15) in the transcatheter mitral valve and TAVR populations, respectively. The mean Hospital Frailty Risk Score was 6.6 ± 6.1 in the transcatheter mitral valve repair population and 6.3 ± 5.7 in the TAVR population.
Outcomes
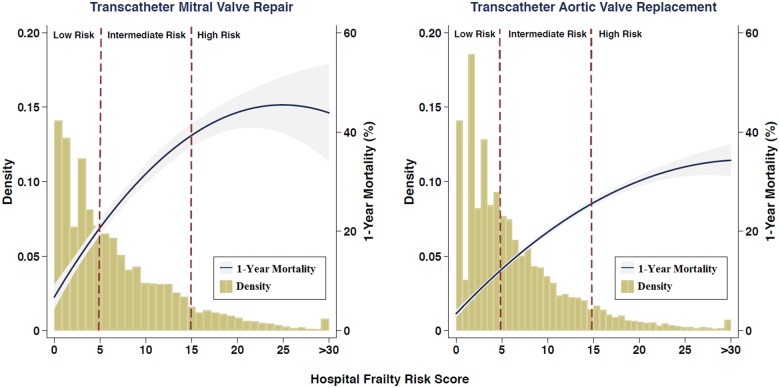
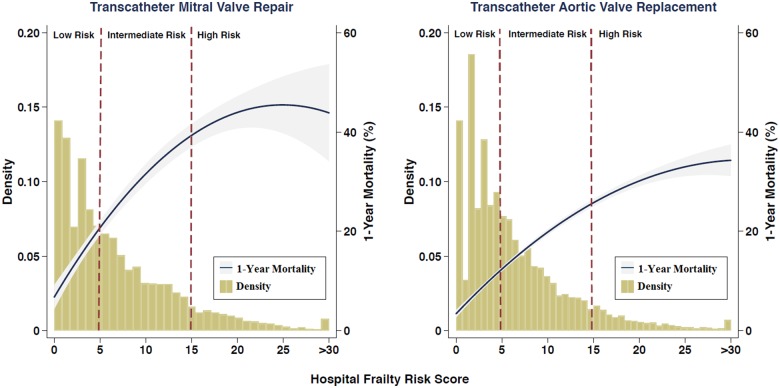
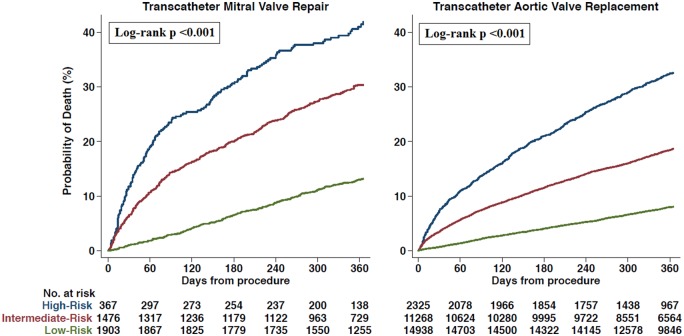
All outcomes including long length of stay, crude 30-day mortality rate, 1-year mortality rate, and rehospitalization rates for all pre-specified subgroups were consistently and significantly greater in higher Hospital Frailty Risk Score categories for patients undergoing both transcatheter mitral valve repair and TAVR (Table 3). The distribution of the Hospital Frailty Risk Score in the transcatheter mitral valve repair and TAVR populations are presented in Take home figure. The 1-year mortality rate increased with increasing values of the score in patients undergoing transcatheter mitral valve repair and TAVR (Take home figure). In Kaplan–Meier analysis (Figure 1), the 1-year mortality rate was 12.8% in the low-risk group, 29.7% in the intermediate-risk group, and 40.9% in the high-risk group for patients undergoing transcatheter mitral valve repair (log rank P < 0.001 for comparison between categories). In patients undergoing TAVR, 1-year mortality rates were 7.6% in the low-risk group, 17.6% in the intermediate-risk group, and 30.1% in the high-risk group (P-values <0.001 by log-rank test). Kaplan–Meier estimates for time to rehospitalization in transcatheter mitral valve repair and TAVR patients are also presented in Supplementary material online, Figure S1 and S2, respectively.
Table 3.
Outcomes of patients after transcatheter mitral valve repair and transcatheter aortic valve replacement
| Transcatheter mitral valve repair (n = 3746) |
Transcatheter aortic valve replacement (n = 28 531) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low risk (n = 1903) | Intermediate risk (n = 1476) | High risk (n = 367) | P-value | Low risk (n = 14 938) | Intermediate risk (n = 11 268) | High risk (n = 2325) | P-value | ||
| Long length of stay, no. of pts (%) | 40 (2.1) | 210 (14.2) | 119 (32.4) | <0.001 | 404 (2.7) | 1727 (15.3) | 629 (27.1) | <0.001 | |
| 30-day mortality, no. of pts (%) | 20 (1.1) | 99 (6.7) | 44 (12.0) | <0.001 | 143 (1.0) | 418 (3.7) | 169 (7.3) | <0.001 | |
| 1-year mortality, no. of pts (%)a | 244 (12.8) | 439 (29.7) | 150 (40.9) | <0.001 | 1142 (7.6) | 1978 (17.6) | 701 (30.1) | <0.001 | |
|
| |||||||||
|
n = 3674 (alive at discharge, index procedure) |
n = 28 111 (alive at discharge, index procedure) |
||||||||
| Low risk (n = 1899) | Intermediate risk (n = 1431) | High risk (n = 344) | Low risk (n = 14 861) | Intermediate risk (n = 10 991) | High risk (n = 2229) | ||||
|
| |||||||||
| No. of rehospitalizations, n (%) | <0.001 | <0.001 | |||||||
| 0 | 1305 (68.7) | 740 (51.7) | 133 (38.7) | 10 481 (70.5) | 5909 (53.8) | 819 (36.7) | |||
| 1 | 312 (16.4) | 338 (23.6) | 91 (26.5) | 2487 (16.7) | 2558 (23.3) | 687 (30.8) | |||
| ≥2 | 282 (14.8) | 353 (24.7) | 120 (34.9) | 1893 (12.7) | 2524 (23.0) | 723 (32.4) | |||
| All-cause rehospitalization (within 1-year), no. of pts, (%)a | 594 (47.9) | 691 (72.9) | 211 (81.3) | <0.001 | 4380 (47.7) | 5082 (68.5) | 1410 (85.3) | <0.001 | |
| Rehospitalization (within 1-year) due to | |||||||||
| Acute myocardial infarction, no. of pts, (%)a | 25 (2.4) | 35 (3.6) | 16 (8.3) | <0.001 | 218 (2.5) | 336 (3.8) | 104 (11.0) | <0.001 | |
| Acute heart failure, no. of pts, (%)a | 277 (19.4) | 350 (36.3) | 117 (54.7) | <0.001 | 1037 (10.3) | 1598 (21.6) | 486 (36.8) | <0.001 | |
| Acute renal failure, no. of pts, (%)a | 217 (16.7) | 296 (36.6) | 100 (51.3) | <0.001 | 1029 (11.0) | 1498 (22.8) | 447 (42.9) | <0.001 | |
| Transient ischaemic attack or stroke, no. of pts, (%)a | 36 (2.0) | 36 (3.5) | 21 (4.7) | <0.001 | 262 (2.6) | 338 (3.6) | 111 (12.1) | <0.001 | |
| Acute post-haemorrhagic anaemia, no. of pts, (%)a | 108 (7.7) | 117 (10.1) | 28 (12.7) | <0.001 | 735 (8.0) | 863 (10.6) | 189 (15.4) | <0.001 | |
Percentages represent Kaplan–Meier cumulative event probability (Kaplan–Meier curves for rehospitalizations are presented in Supplementary material online, Figures S1 and S2).
Take home figure.
The distribution of the Hospital Frailty Risk Score and its association with 1-year mortality in the transcatheter mitral valve repair and transcatheter aortic valve replacement populations using restricted cubic spline plots. The vertical red dashed lines show thresholds for categorizing patients as low frailty risk (score <5), intermediate frailty risk (score 5–15), or high frailty risk (score >15). Since there were very few patients with a Hospital Frailty Risk Score of >30, those patients are classified into a single group.
Figure 1.
Unadjusted Kaplan–Meier curves for all-cause mortality in the transcatheter mitral valve repair and transcatheter aortic valve replacement populations.
The c-statistic for all-cause 1-year mortality without the Hospital Frailty Risk Score was 0.65 for mitral valve repair and 0.66 for TAVR. After adding the Hospital Frailty Risk Score, the c-statistics improved to 0.70 and 0.71, respectively (DeLong P-value <0.001 for both). Furthermore, the IDI after addition of the frailty score was 0.033 (P < 0.001) for mitral valve repair and 0.024 (P < 0.001) for TAVR. The c-statistic for all-cause 1-year mortality using only the Hospital Frailty Risk Score (unadjusted) was 0.67 for mitral valve repair and 0.67 for TAVR.
There was no meaningful collinearity (mean VIF = 1.70) among the Hospital Frailty Risk Score (VIF = 1.16), Elixhauser comorbidity index (2.0) and Charlson comorbidity index (1.95). In multivariable Cox regression analyses, after adjusting for age, gender, and Elixhauser and Charlson comorbidity indices, increasing Hospital Frailty Risk Score (1 point increase) was associated with increasing all-cause mortality (HR: 1.060 for transcatheter mitral valve repair and HR: 1.062 for TAVR) and rehospitalizations (HR: 1.061 for both procedures). These associations were also present when frailty was categorized into low, intermediate, and high-risk categories for both the transcatheter mitral valve repair and TAVR groups (Table 4). There was no interaction between Hospital Frailty Risk Score and transcatheter valve therapies on all defined outcomes (Table 4).
Table 4.
Results of Cox multivariable regression models (adjusted for age, gender, Elixhauser and Charlson comorbidity indices)
| Transcatheter mitral valve repair |
Transcatheter aortic valve replacement |
P-value for interaction | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CIs) | P-value | Hazard ratio (95% CIs) | P-value | ||
| All-cause mortality | |||||
| Hospital Frailty Risk Score | 1.060 (1.050–1.070) | <0.001 | 1.062 (1.058–1.067) | <0.001 | 0.258 |
| Hospital Frailty Risk Category | <0.001 | <0.001 | |||
| Intermediate risk (5–15) | 2.257 (1.858–2.743) | <0.001 | 1.931 (1.762–2.117) | <0.001 | |
| High risk (>15) | 3.192 (2.437–4.180) | <0.001 | 3.644 (3.214–4.132) | <0.001 | |
| Rehospitalization for any cause | |||||
| Hospital Frailty Risk Score | 1.061 (1.053–1.069) | <0.001 | 1.061 (1.058–1.064) | <0.001 | 0.151 |
| Hospital Frailty Risk Category | <0.001 | <0.001 | |||
| Intermediate risk (5–15) | 1.714 (1.526–1.924) | <0.001 | 1.648 (1.579–1.719) | <0.001 | |
| High risk (>15) | 2.722 (2.306–3.214) | <0.001 | 2.920 (2.739–3.112) | <0.001 | |
| Rehospitalization due to acute myocardial infarction (I21, at subsequent index) | |||||
| Hospital Frailty Risk Score | 1.050 (1.001–1.101) | 0.047 | 1.079 (1.063–1.095) | <0.001 | 0.192 |
| Hospital Frailty Risk Category | <0.001 | <0.001 | |||
| Intermediate risk (5–15) | 2.031 (1.183–3.486) | <0.001 | 2.158 (1.800–2.587) | <0.001 | |
| High risk (>15) | 4.483 (2.257–8.904) | <0.001 | 4.250 (3.305–5.465) | <0.001 | |
| Rehospitalization due to acute renal failure (N17, at subsequent index) | |||||
| Hospital Frailty Risk Score | 1.073 (1.057–1.088) | <0.001 | 1.072 (1.064–1.079) | <0.001 | 0.355 |
| Hospital Frailty Risk Category | <0.001 | <0.001 | |||
| Intermediate risk (5–15) | 1.907 (1.585–2.295) | <0.001 | 1.813 (1.667–1.971) | <0.001 | |
| High risk (>15) | 3.391 (2.639–4.351) | <0.001 | 3.473 (3.085–3.910) | <0.001 | |
| Rehospitalization due to acute heart failure (I5021, I5031, I5041, I5023, I5033, I5043 at subsequent index) | |||||
| Hospital Frailty Risk Score | 1.058 (1.044–1.072) | <0.001 | 1.068 (1.062–1.075) | <0.001 | 0.160 |
| Hospital Frailty Risk Category | <0.001 | <0.001 | |||
| Intermediate risk (5–15) | 1.738 (1.471–2.054) | <0.001 | 1.892 (1.742–2.054) | <0.001 | |
| High risk (>15) | 2.869 (2.279–3.613) | <0.001 | 3.509 (3.128–3.936) | <0.001 | |
| Rehospitalization due to stroke or TIA (I63 and G45, at subsequent index) | |||||
| Hospital Frailty Risk Score | 1.065 (1.021–1.110) | 0.003 | 1.074 (1.059–1.089) | <0.001 | 0.821 |
| Hospital Frailty Risk Category | 0.035 | <0.001 | |||
| Intermediate risk (5–15) | 1.511 (0.927–2.462) | 0.097 | 1.918 (1.619–2.274) | <0.001 | |
| High risk (>15) | 4.546 (2.534–8.161) | <0.001 | 4.061 (3.204–5.146) | <0.001 | |
| Rehospitalization due to acute post-haemorrhagic anaemia (D62, at subsequent index) | |||||
| Hospital Frailty Risk Score | 1.043 (1.015–1.071) | 0.002 | 1.043 (1.032–1.058) | <0.001 | 0.806 |
| Hospital Frailty Risk Category | 0.001 | <0.001 | |||
| Intermediate risk (5–15) | 1.620 (1.228–2.137) | 0.001 | 1.636 (1.474–1.816) | <0.001 | |
| High risk (>15) | 2.049 (1.325–3.168) | 0.001 | 2.266 (1.914–2.683) | <0.001 | |
For each outcome, two separate (continuous and categorical scale) models were built (low-risk group handled as a reference for category groups; hazard ratio = 1).
Discussion
This nationwide cohort study demonstrates that almost half of patients undergoing transcatheter valve therapies in the USA have intermediate or high frailty levels, according to a frailty scoring system recently derived and validated in administrative claims data. The addition of the ICD-10-based Hospital Frailty Risk Score effectively stratified patients undergoing valvular interventions based on their risk of multiple patient-oriented endpoints, including short- and long-term mortality, long length of stay, and all-cause rehospitalization, and provided predictive information significantly above commonly used claims-based comorbidity assessments. Our study adds to the existing literature suggesting that frailty is an important predictor of outcomes for patients with valvular heart disease who are scheduled to undergo catheter-based interventions. It also demonstrates that a novel ICD-10-based frailty score developed in a patient population over the age of 75 years in the UK can provide accurate prognostic information on patient frailty in a younger (≥65 years) population undergoing transcatheter valve therapies in the USA.
Our study expands on similar findings demonstrating that adding frailty to summary measures of comorbidities can improve the discrimination of 1-year mortality in the TAVR population.24 However, the assessment of frailty and its exact prevalence remains unclear due to substantial disagreement among 35 existing frailty scales, including commonly used scales such those by Fried and Rockwood.25 For example, the rate of disagreement among seven of these scales ranged between 35% and 74% in the largest prospective study to date specifically designed to investigate frailty in older patients undergoing TAVR.4 This range of estimates illustrates the challenge of using any one frailty scale to diagnose an individual as frail. Furthermore, prior studies suggest that in order to be used as a measure that captures the dynamic nature of frailty, a continuous or ordinal scoring system might be better than a dichotomous one (frail vs. non-frail).26 This idea is supported by our study findings, which demonstrate a graded increase in the risk of adverse events across most of the frailty-score spectrum. In addition to the association between frailty and long-term mortality, the current study demonstrates a strong association between frailty and long-term rehospitalizations. Thus, identifying frail patients and stratifying risk categories using the Hospital Frailty Risk Score may also help physicians inform patients and families about the incidence of potential outcomes during the follow-up period. In addition, patients identified as having higher frailty risk could be targeted for strategies such as more intensive follow-up in an effort to prevent costly readmissions.
Previous studies have demonstrated the value of using administrative data in the assessment of frailty based on ICD-9 codes.9,15,16,27 Now, the use of ICD-10 codes routinely used in current administrative databases provides hospitals with a systematic method to prospectively screen for frailty risk. The Hospital Frailty Risk Score developed and validated in the UK performs at least as well as or better than existing frailty measures or risk stratification tools.17 Recently, this score was also externally validated to predict outcomes including long length of stay, 30-day rehospitalization and 1-year mortality in elderly patients from Canada.28 In addition, administrative data include a longitudinal record and demonstrate how the frailty phenotype influences risk indirectly, as evidenced by multiple hospitalizations for conditions related to elevated medical risk rather than geriatric-specific risk (e.g. falls, failure to thrive, etc.). Claims data represent an inexpensive alternative source of data in the absence of prospectively collected information on frailty, which is often not routinely collected in the course of care. The ubiquity of claims data may make it a useful alternative to clinical risk scoring systems in determining procedural outcomes.
There are several potential benefits to routinely identifying older people at risk of adverse clinical outcomes after transcatheter valve therapies. First, we believe that by merging claims-based frailty data with ongoing registries of transcatheter valve therapies, we might enhance the ability to define patient risk and understand long-term outcomes, improve hospital and physician benchmarking, and increase the completeness of data collection by incorporating variables that were not previously being collected. In addition, the score could be applied to hospital information systems prospectively, potentially removing the inter-operator variability and implementation burden associated with manual scoring systems. A uniform and easily implemented method of identifying frail patients can help highlight the magnitude of the challenge associated with their care, enable services to evolve and provide frailty-attuned care, and improve patient- and facility-level outcomes. Identifying frailty in patients with valvular heart disease may also assist physicians in determining appropriateness of treatment and in discussing a patient’s prognosis with the patient and family members.
There are notable limitations to the current study. Physiological measures of frailty are not captured in administrative claims data, and thus the claims-based definition of frailty may not comprehensively quantify frailty for all patients. It is also unclear whether the frailty risk score is a truly a measurement of frailty or simply another comorbidity index. The indications and criteria for patient selection are not available, and administrative coding may misclassify some comorbidities and complications compared with prospective collection using standard clinical trial definitions. Future studies could validate the variables used in the frailty score in a small sample of patients undergoing valve therapies. Claims codes also do not always capture the severity of a given condition or its change post-procedure, and we are not able to determine the cause of death. Additionally, because the study population was limited to Medicare beneficiaries, we did not have information on all patients who might have undergone transcatheter valve therapies in the USA. Since the model’s discrimination is still modest, the true clinical utility of this score is still unclear, and should be assessed prospectively. Because ICD-10 codes have only been used in the USA since October 2015, we selected a relatively short 3 month lookback period for frailty assessment. As more data become available, longer historical periods for frailty assessment may be useful. Such data would allow for more rigorous assessments regarding changing frailty scores over time—either increases due to accumulation of deficits, or decreases to successful treatment of reversible conditions. Future analyses examining longitudinal changes in frailty and its impact of outcomes are warranted. Also, due to the level of granularity in the claims data, we did not have the variables necessary to calculate traditional risk scores such as the EuroSCORE29 or STS-PROM30 score.
Conclusions
The Hospital Frailty Risk Score, which is readily available and relatively inexpensive to implement, provides hospitals and health systems with a systematic way to identify frailty in patients undergoing transcatheter valve therapies. The score effectively classifies patients at high risk for adverse events including mortality, rehospitalizations and long length of stay. Use of a claims-based frailty score may facilitate the prospective assessment of frailty among patients undergoing transcatheter valve therapies.
Funding
Members of the study team are supported by funding from the National Heart, Lung, and Blood Institute [1F32HL1407-11 (J.B.S.) and R01HS024520-01 (C.S.) and 1R01HL136708-01 (R.W.Y.)].
Conflict of interest: The following authors have no conflicts of interest to declare: H.K., J.J.P., M.R.R., J.B.S., D.S.P., L.R.V., C.S., and E.C. J.J.P. reports grants from Medtronic, Abbott Vascular, and Direct Flow Medical and personal fees from Boston Scientific, Cordis, and Direct Flow Medical, outside the submitted work. R.W.Y. reports investigator initiated grant funding from Abiomed, grant support from Boston Scientific, and consulting from Abbott, Medtronic, and Teleflex, outside the submitted work.
Supplementary Material
See page 2240 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz382)
References
- 1. Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E.. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013;68:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Talbot‐Hamon C, Afilalo J.. Transcatheter aortic valve replacement in the care of older persons with aortic stenosis. J Am Geriatr Soc 2017;65:693–698. [DOI] [PubMed] [Google Scholar]
- 3. Metze C, Matzik A-S, Scherner M, Körber MI, Michels G, Baldus S, Rudolph V, Pfister R.. Impact of frailty on outcomes in patients undergoing percutaneous mitral valve repair. JACC: Cardiovasc Interv 2017;10:1920–1929. [DOI] [PubMed] [Google Scholar]
- 4. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin J-F, Popma JJ, Webb JG, Perrault LP.. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 5. Arnold SV, Afilalo J, Spertus JA, Tang Y, Baron SJ, Jones PG, Reardon MJ, Yakubov SJ, Adams DH, Cohen DJ.. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol 2016;68:1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, Tsuchikane E, Suzuki T, Otsuka T, Kohsaka S, Tada N, Yamanaka F, Naganuma T, Araki M, Shirai S, Watanabe Y, Hayashida K, Yashima F, Inohara T, Kakefuda Y, Arai T, Yanagisawa R, Tanaka M, Kawakami T, Maekawa Y, Takashi K, Yoshitake A, Iida Y, Yamazaki M, Shimizu H, Yamada Y, Jinzaki M, Tsuruta H, Itabashi Y, Murata M, Kawakami M, Fukui S, Sano M, Fukuda K, Hosoba S, Sato H, Teramoto T, Kimura M, Sago M, Tsunaki T, Watarai S, Tsuzuki M, Irokawa K, Shimizu K, Kobayashi T, Okawa Y, Miyasaka M, Enta Y, Shishido K, Ochiai T, Yamabe T, Noguchi K, Saito S, Kawamoto H, Onishi H, Yabushita H, Mitomo S, Nakamura S, Yamawaki M, Akatsu Y, Honda Y, Takama T, Isotani A, Hayashi M, Kamioka N, Miura M, Morinaga T, Kawaguchi T, Yano M, Hanyu M, Arai Y, Tsubota H, Kudo M, Kuroda Y, Kataoka A, Hioki H, Nara Y, Kawashima H, Nagura F, Nakashima M, Sasaki K, Nishikawa J, Shimokawa T, Harada T, Kozuma K.. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation 2017;135:2013–2024. [DOI] [PubMed] [Google Scholar]
- 7. Martin GP, Sperrin M, Ludman PF, deBelder MA, Gunning M, Townend J, Redwood SR, Kadam UT, Buchan I, Mamas MA.. Do frailty measures improve prediction of mortality and morbidity following transcatheter aortic valve implantation? An analysis of the UK TAVI registry. BMJ Open 2018;8:e022543.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers T, Alraies MC, Moussa Pacha H, Bond E, Buchanan KD, Steinvil A, Gai J, Torguson R, Ben-Dor I, Satler LF, Waksman R.. Clinical frailty as an outcome predictor after transcatheter aortic valve implantation. Am J Cardiol 2018;121:850–855. [DOI] [PubMed] [Google Scholar]
- 9. Kundi H, Valsdottir LR, Popma JJ, Cohen DJ, Strom JB, Pinto DS, Shen C, Yeh RW.. Impact of a claims-based frailty indicator on the prediction of long-term mortality after transcatheter aortic valve replacement in medicare beneficiaries. Circ Cardiovasc Qual Outcomes 2018;11:e005048.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM, Thompson A.. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–289. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791.28886619 [Google Scholar]
- 12. Van Mieghem NM, Dumonteil N, Chieffo A, Roux Y, van der Boon RMA, Giustino G, Hartman E, Aga Y, de Jong L, Abi Ghanem M, Marcheix B, Cavazza C, Carrié D, Colombo A, Kappetein A-P, de Jaegere PPT, Tchetche D.. Current decision making and short-term outcome in patients with degenerative aortic stenosis: the Pooled-RotterdAm-Milano-Toulouse In Collaboration Aortic Stenosis survey. EuroIntervention 2016;11:e1305–e1313. [DOI] [PubMed] [Google Scholar]
- 13. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE.. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, Hanzel G, Bavaria JE, Tuzcu EM, Peterson ED, Fitzgerald S, Kourtis M, Michaels J, Christensen B, Seward WF, Hewitt K, Holmes DR.. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Ann Thorac Surg 2017;103:1021–1035. [DOI] [PubMed] [Google Scholar]
- 15. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J.. Measuring frailty in medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci 2018;73:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segal JB, Chang H-Y, Du Y, Walston JD, Carlson MC, Varadhan R.. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care 2017;55:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, Bardsley M, Conroy S.. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krumholz HM, Nuti SV, Downing NS, Normand S-L, Wang Y.. Mortality, hospitalizations, and expenditures for the Medicare population aged 65 years or older, 1999-2013. JAMA 2015;314:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schermerhorn j, Buck DB, O’malley AJ, Curran T, McCallum JC, Darling J, Landon BE.. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med 2015;373:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore BJ, White S, Washington R, Coenen N, Elixhauser A.. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data. Med Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 21. Zhang JX, Iwashyna TJ, Christakis NA.. The performance of different lookback periods and sources of information for Charlson comorbidity adjustment in Medicare claims. Med Care 1999;37:1128–1139. [DOI] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 23. Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS.. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 24. Schoenenberger AW, Moser A, Bertschi D, Wenaweser P, Windecker S, Carrel T, Stuck AE, Stortecky S.. Improvement of risk prediction after transcatheter aortic valve replacement by combining frailty with conventional risk scores. JACC: Cardiovasc Interv 2018;11:395–403. [DOI] [PubMed] [Google Scholar]
- 25. Aguayo GA, Donneau A-F, Vaillant MT, Schritz A, Franco OH, Stranges S, Malisoux L, Guillaume M, Witte DR.. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017;186:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitnitski AB, Mogilner AJ, Rockwood K.. Accumulation of deficits as a proxy measure of aging. Sci World J 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Stürmer T.. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 2015;24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McAlister F, van Walraven C.. External validation of the Hospital Frailty Risk Score and comparison with the Hospital-patient One-year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf 2019;28:284–288. [DOI] [PubMed] [Google Scholar]
- 29. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R.. European system for cardiac operative risk evaluation (Euro SCORE). Eur J Cardiothorac Surg 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 30. Sm, Shahian Dm O, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg 2009;88:23–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.