Abstract
Rationale
Recent advancements have brought to light the origins, complexity, and functions of tissue-resident macrophages. However, in the context of tissue injury or disease, large numbers of monocytes infiltrate the heart and are thought to contribute to adverse remodeling and heart failure pathogenesis. Little is understood about the diversity of monocytes and monocyte-derived macrophages recruited to the heart after myocardial injury, including the mechanisms that regulate monocyte recruitment and fate specification.
Objective
We sought to test the hypothesis that distinct subsets of tissue-resident CCR2− (C-C chemokine receptor 2) and CCR2+ macrophages orchestrate monocyte recruitment and fate specification after myocardial injury.
Methods and Results
We reveal that in numerous mouse models of cardiomyocyte cell death (permanent myocardial infarction, reperfused myocardial infarction, and diphtheria toxin cardiomyocyte ablation), there is a shift in macrophage ontogeny whereby tissue-resident macrophages are predominately replaced by infiltrating monocytes and monocyte-derived macrophages. Using syngeneic cardiac transplantation to model ischemia-reperfusion injury and distinguish tissue-resident from recruited cell populations in combination with intravital 2-photon microscopy, we demonstrate that monocyte recruitment is differentially orchestrated by distinct subsets of tissue-resident cardiac macrophages. Tissue-resident CCR2+ macrophages promote monocyte recruitment through an MYD88 (myeloid differentiation primary response 88)-dependent mechanism that results in release of MCPs (monocyte chemoattractant proteins) and monocyte mobilization. In contrast, tissue-resident CCR2− macrophages inhibit monocyte recruitment. Using CD (cluster of differentiation) 169-DTR (diphtheria toxin receptor) and CCR2-DTR mice, we further show that selective depletion of either tissue-resident CCR2− or CCR2+ macrophages before myocardial infarction results in divergent effects on left ventricular function, myocardial remodeling, and monocyte recruitment. Finally, using single-cell RNA sequencing, we show that tissue-resident cardiac macrophages differentially instruct monocyte fate specification.
Conclusions
Collectively, these observations establish the mechanistic basis by which monocytes are initially recruited to the injured heart and provide new insights into the heterogeneity of monocyte-derived macrophages.
Keywords: inflammation; macrophages; monocytes; myocardial infarction; receptors, CCR2
Monocyte recruitment has been observed after various forms of tissue injury and represents a hallmark characteristic of both infectious and sterile inflammation. Within the context of cardiovascular disease, peripheral monocyte mobilization and infiltration into diseased tissues is considered predominately a maladaptive response because it is associated with adverse outcomes, including infarct expansion, left ventricular (LV) systolic dysfunction, LV dilation, and atherosclerotic plaque progression.1–6 For example, after myocardial infarction, peripheral CD (cluster of differentiation) 14+ monocyte abundance is associated with larger infarct size and deterioration in LV systolic function, despite similar extents of initial myocardial injury.7–9 Consistent with these observations, inhibition of monocyte recruitment through interruption of MCP (monocyte chemoattractant protein) 1 and CCR2 (C-C chemokine receptor 2) signaling reduces excessive inflammation and is protective in mouse models of myocardial infarction and atherosclerosis.3,10–17 As such, CCR2 inhibitors are under investigation as potential therapeutics for cardiovascular disease.18,19
However, based on studies demonstrating the remarkable plasticity of monocytes and the ability of these cells to take on reparative fates,20–22 it is unlikely that all monocyte-derived macrophages are harmful. In addition, little is understood about the initial mechanisms by which monocytes are recruited to sites of injury, the diversity of monocyte-derived macrophages, and the instructive cues that specify their fates. Thus, much remains to be learned about monocyte-derived macrophages. Understanding the identity, dynamics, and functions of these cells will likely lead to important opportunities to reduce inflammation and improve the heart’s intrinsic ability to repair after myocardial infarction.
Previously, we and others have identified functionally distinct subsets of macrophages that reside within the myocardium under steady-state conditions. Tissue-resident cardiac macrophages can be divided into CCR2− and CCR2+ subsets derived from embryonic and adult hematopoietic lineages, respectively.23–26 The existence of specialized cardiac macrophage populations is consistent with evolving literature regarding the origins of tissue-resident macrophage across diverse tissues and organs.23,24,27–37 CCR2− macrophages seed the heart during embryonic and early postnatal development, are maintained independent of peripheral monocyte input under steady-state conditions, and function to promote coronary development, cardiac regeneration, and facilitate electrical conduction within the atrioventricular node.3,4,38,39 Tissue-resident CCR2+ macrophages are derived from circulating monocytes.23,24,40 However, less is known about the functions of this subset. Functional analogous populations of CCR2− and CCR2+ macrophages have been identified in the human myocardium.41
Based on gene expression profiling revealing expression of various proinflammatory mediators and the observation that tissue-resident CCR2+ macrophages are necessary for neutrophil extravasation into the myocardium,42 we postulated that tissue-resident CCR2+ macrophages represent an integral cell type that initiates inflammation within the diseased heart. Specifically, we sought to test the hypotheses that tissue-resident CCR2+ macrophages are activated after cardiomyocyte cell death, orchestrate the recruitment of monocytes to the heart, and regulate the differentiation of monocytes into inflammatory monocyte-derived macrophage populations.
We used several complementary models of cardiomyocyte cell death, including myocardial infarction, diphtheria toxin (DT) cardiomyocyte ablation, and syngeneic heart transplantation. Using these approaches, we reveal that monocytes and monocyte-derived macrophages infiltrate the heart after cardiomyocyte injury and largely replace tissue-resident macrophage populations. We further show that recruited monocyte-derived macrophages represent an inflammatory population and are distinct from tissue-resident macrophage subsets. Finally, we demonstrate that tissue-resident CCR2− and CCR2+ macrophages differentially regulate monocyte recruitment, fate specification, and outcomes after myocardial injury.
Methods
Data on the RNA sequencing analyses will be made publicly available through ImmGen or will be provided directly from the corresponding authors on reasonable request. All other supporting data are available within the article and its Online Data Supplement.
Detailed descriptions of mouse strains, reperfused myocardial infarction, permanent myocardial infarction, DT cardiomyocyte ablation, heart transplantation, parabiosis, intravital 2-photon microscopy, echocardiography, triphenyltetrazolium chloride staining, flow cytometry, macrophage fate mapping and depletion strategies, immunostaining, ethidium homodimer staining, Picrosirius red and wheat germ agglutinin staining, RNA sequencing, RT-PCR (reverse transcription polymerase chain reaction), single-cell RNA sequencing, and statistical analysis are provided in the Online Data Supplement.
Results
Myocardial Injury Results in Shifts in Macrophage Ontogeny
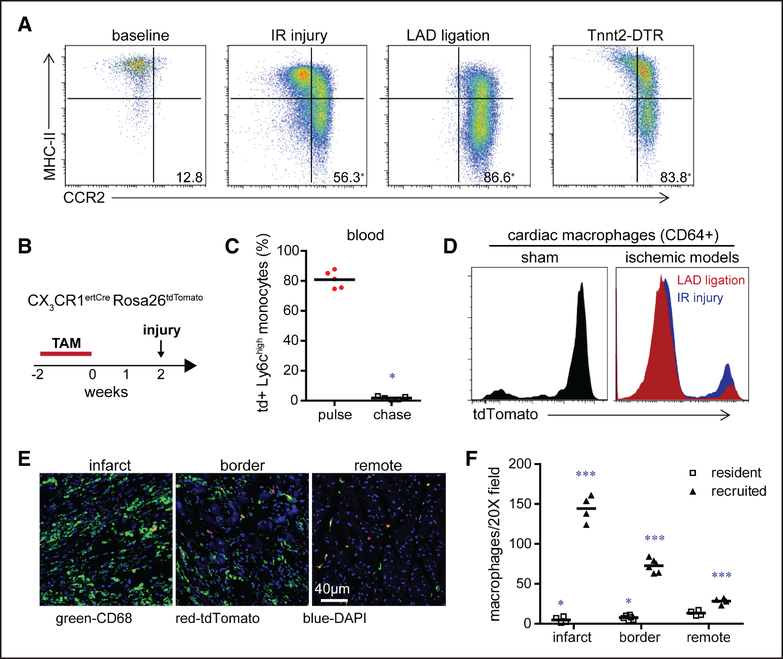
To evaluate the extent of monocyte recruitment and macrophage composition in the injured heart, we examined several mouse models of cardiomyocyte cell death, including permanent myocardial infarction (left anterior descending artery ligation), reperfused myocardial infarction (ischemia-reperfusion [IR] injury), and DT cardiomyocyte ablation. Consistent with prior studies examining cardiomyocyte ablation, myocardial infarction, and angiotensin II infusion models, we observed accumulation of Ly6ChighCCR2+MHC-II (major histocompatibility complex II)low monocytes and CCR2+MHC-IIhigh macrophages in the heart across each model indicating that CCR2+Ly6Chigh monocyte recruitment and CCR2+MHC-IIhigh macrophage accumulation represents a common and stereotyped response to cardiomyocyte cell death (Figure 1A; Online Figure I).
Figure 1. Myocardial injury triggers shifts in macrophage ontogeny.
A, Flow cytometry of cardiac monocyte and macrophage subsets under baseline conditions and 4 d after myocardial ischemia-reperfusion (IR) injury (90 min of ischemia), left anterior descending artery (LAD) ligation, and diphtheria toxin mediated cardiomyocyte ablation (Tnnt2 [troponin T2]-DTR [diphtheria toxin receptor]). Displayed frequencies indicate the percentage of CCR2+ (C-C chemokine receptor 2) monocytes and macrophages. n=4 per experimental group. B, Schematic describing the strategy to distinguish tissue resident from recruited macrophages. TAM: tamoxifen. C, Quantification of the percentage of tdTomato+ Ly6Chigh blood monocytes in CX3CR1ertCre (CX3C chemokine receptor 1) Rosa26tdTomato mice after 2 wk of TAM treatment (pulse) and 2 wk of TAM treatment followed by 2 wk of normal chow (chase). n=5 per experimental group. D, Flow cytometry showing the distribution of tdTomato+ cardiac macrophages (CD [cluster of differentiation] 64+) 4 d after sham surgery, IR injury, or LAD ligation in CX3CR1ertCre Rosa26tdTomato mice that underwent the TAM pulse-chase protocol. Similar results were obtained from 5 independent biological replicates. E, Immunostaining for CD68 (green), tdTomato (red), and DAPI (4’,6-diamidino-2-phenylindole; blue) showing the spatial distribution of resident and recruited macrophages 4 d after LAD ligation. ×200 magnification. F, Quantification of the absolute number of tissue-resident (tdTomato+) and recruited (tdTomato−) CD68+ macrophages in the infarct, remote, and border zones after LAD ligation. n=4 per experimental group. *P <0.05 compared with resident macrophages in the remote zone; ***P <0.05 compared with all other groups.
To verify that CCR2+Ly6Chigh monocytes and CCR2+MHC-IIhigh macrophages were derived from newly recruited cells and quantify the extent by which monocyte-derived macrophages replace resident macrophages after myocardial injury, we used a tamoxifen-inducible lineage tracing strategy (Figure 1B). Administration of tamoxifen to CX3CR1 (CX3C chemokine receptor 1)ertCre Rosa26tdTomato mice for 2 weeks results in labeling of >80% of peripheral monocytes and cardiac macrophages under steady-state conditions. After withdrawal of tamoxifen (2-week chase period), monocytes are replenished from bone marrow progenitors and are tdTomato−, whereas cardiac macrophages remain tdTomato+ (Figure 1C and 1D). We then utilized the CX3CR1ertCre Rosa26tdTomato pulse-chase model and examined tdTomato expression in monocytes and macrophages (CD45+CD64+ cells) 4 days after coronary ligation or IR injury. For these experiments, ischemia was induced immediately after the 2-week chase period. Flow cytometry revealed that the majority of CD64+ monocytes and macrophages in the heart 4 days after ischemic injury were tdTomato− indicating that they were derived from newly recruited monocytes (Figure 1D).
Immunostaining provided additional information related to where monocyte-derived macrophages accumulate in the infarcted heart. Although the overwhelming majority of CD68+ macrophages located within infarct and border zones were derived from recruited monocytes, we also observed that monocytes partially contributed to CD68+ macrophage populations located within areas remote to the site of ischemic injury (Figure 1E and 1F). Collectively, these data indicate that shortly after myocardial injury, a shift in macrophage ontogeny occurs whereby resident macrophages are largely replaced by recruited CCR2+Ly6Chigh monocytes and CCR2+ monocyte-derived macrophages.
Monocyte-Derived Macrophages Recruited to the Injured Heart Are Distinct From Tissue-Resident Macrophages
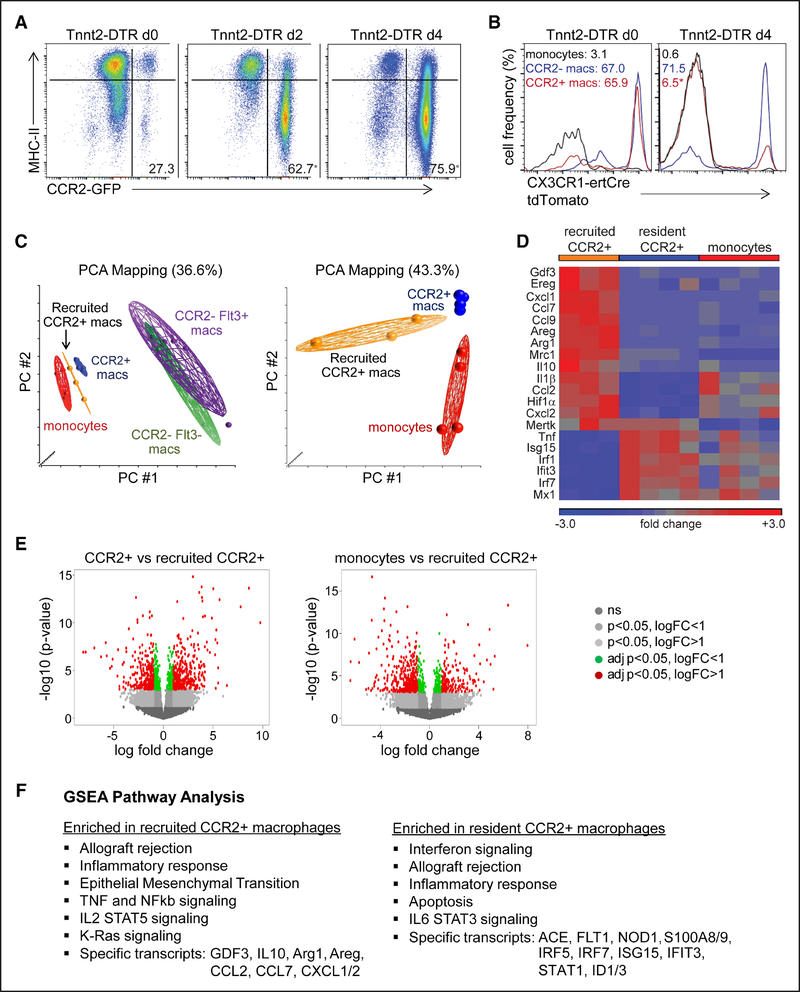
To determine whether monocyte-derived macrophages recruited to the heart after cardiomyocyte cell death differed from tissue-resident cardiac macrophages, we performed RNA sequencing on macrophage subsets isolated by flow cytometry using our DT cardiomyocyte ablation model. We chose this model because it results in robust cardiomyocyte cell death and avoids any confounding effects of inflammatory cell recruitment associated with surgical thoracotomy. To delineate the timing of monocyte recruitment and distinguish tissue resident from recruited macrophage populations in the Tnnt2 (troponin T2)-DTR (DT receptor) model, we performed flow cytometry on Tnnt2-DTR CX3CR1ertCre Rosa26tdTomato mice treated with tamoxifen using the pulse-chase strategy described above. Monocyte recruitment was first evident 2 days after DT administration and continued to increase through day 4 where the vast majority of cells were either CCR2+ monocytes or CCR2+ macrophages (Figure 2A). Examination of tdTomato expression revealed that immediately after DT administration, the majority of CCR2− and CCR2+ macrophages within the heart were tdTomato+ indicating that they are of tissue-resident origin. In contrast, 4 days after DT administration, the vast majority of CCR2+ macrophages were tdTomato− indicating they were derived from recruited monocytes. CCR2− macrophages remained tdTomato+ indicating that they were derived from tissue-resident macrophages and not recruited monocytes. Monocytes were tdTomato− at both time points (Figure 2B).
Figure 2. Recruited monocyte-derived macrophages are distinct from tissue-resident subsets.
A and B, Flow cytometry of cardiac monocyte and macrophage subsets in Tnnt2 (troponin T2)-DTR (diphtheria toxin receptor; A) and Tnnt2-DTR CX3CR1ertCre (CX3C chemokine receptor 1) Rosa26tdTomato (B) mice after diphtheria toxin administration and the TAM pulse-chase protocol. Displayed frequencies indicate the percentage of CCR2+ (C-C chemokine receptor 2) monocytes and macrophages (left) and the percentage of tdTomato+ monocytes and macrophages (right). n=4 per experimental group. C, Principal component analysis (PCA) of RNA sequencing data obtained from sorted monocyte and macrophage populations harvested from the hearts of Tnnt2-DTR Flt3-Cre Rosa26tdTomato mice after diphtheria toxin administration. Tissue-resident CCR2− and CCR2+ macrophages were isolated from hearts 36 h after diphtheria toxin (DT) treatment, recruited CCR2+ macrophages were isolated from hearts 4 d after DT treatment. Monocytes were harvested 36 h and 4 d after DT treatment. D, Heat map highlighting genes that were differentially expressed between tissue-resident CCR2+ macrophages, recruited CCR2+ macrophages, and CCR2+Ly6Chigh monocytes. E, Volcano plots showing the number of genes differentially expressed between tissue-resident CCR2+ vs recruited CCR2+ macrophages (left) and monocytes vs recruited CCR2+ macrophages (right). logFC: log based 2 fold change, adj p: adjusted P (false discovery rate analysis). F, Gene set enrichment analysis (GSEA) pathway analysis showing pathways and specific genes enriched in tissue-resident and recruited CCR2+ macrophages. *P <0.05 compared with baseline.
Based on these findings, we performed RNA sequencing on tissue-resident CCR2− macrophages (CD64+Ly6ClowCCR2− tdTomato+), tissue-resident CCR2+ macrophages (CD64+Ly6ClowCCR2+tdTomato+), recruited CCR2+ macrophages (CD64+Ly6ClowCCR2+tdTomato−), and recruited monocytes (CD64intLy6ChighCCR2+tdTomato−) isolated by flow cytometry from the hearts of Tnnt2-DTR mice after DT administration. Tissue-resident CCR2− macrophages were further divided in Flt3 (Fms-related tyrosine kinase 3)-Cre negative and positive subsets to gain insights on whether CCR2− macrophages of primitive or definitive hematopoietic origin differentially responded to cardiac injury. Principal component analysis revealed that recruited CCR2+ macrophages were distinct from tissue-resident macrophage subsets and CCR2+Ly6Chigh monocytes (Figure 2C). When compared with tissue-resident CCR2+ macrophages, differential expression and gene set enrichment analysis and pathway analysis revealed that recruited CCR2+ macrophages expressed higher levels of inflammatory chemokines (Cxcl [chemokine (C-X-C) ligand] 1, Cxcl2, Ccl [chemokine (C-C) ligand] 2, Ccl7, and Ccl9), cytokines (Il1β and Il10), and genes implicated in adverse cardiac remodeling (Areg [amphiregulin], Ereg [epiregulin], and Gdf [growth and differentiation factor] 3). Recruited CCR2+ macrophages also expressed increased Arg1 (arginase 1), Mrc1 (mannose receptor C-type 1), and Hiflα (hypoxia-inducible factor 1α) and were enriched in pathways associated with TNF (tumor necrosis factor), NF-κB (nuclear factor κ-light chain enhancer of activated B cells), IL (interleukin)-2, and RAS signaling. These data are consistent with previous studies reporting robust monocyte infiltration and increased expression of inflammatory chemokines and cytokines in the acute phases after myocardial infarction.43 Tissue-resident CCR2+ macrophages differentially expressed type I IFN (interferon) stimulated genes and were enriched in pathways associated with IFN, IL-6, and STAT3 (signal transducer and activator of transcription 3) signaling (Figure 2D through 2F).
It is important to note that compared with tissue-resident CCR2− macrophages, tissue-resident CCR2+ macrophages also represented an inflammatory population; however, the absolute expression of many cytokines and chemokines was less than that of recruited CCR2+ macrophages (Online Figure IIA and IIB). Tissue-resident CCR2− macrophages displayed the greatest divergence from monocytes and CCR2+ macrophage subsets (Figure 2C; Online Figure IIC). Tissue-resident CCR2− macrophages differentially expressed several growth factors (Igf1 [insulin-like growth factor 1], Pdgfc [platelet-derived growth factor C], Hbegf [heparin-binding epidermal-like growth factor], and Cyr61 [cysteine-rich angiogenic inducer 61]) and genes associated with myogenesis, DNA repair, epithelial mesenchymal transitions, and RAS signaling (Online Figure IIB). Dividing CCR2− macrophages based on Flt3-Cre activity did not identify significant differences in gene expression (Online Figure IIC through IIIE). These data confirm previously established distinctions between tissue-resident CCR2− and CCR2+ macrophages and identify recruited CCR2+ macrophages as a particularly inflammatory population that is distinct from tissue-resident CCR2+ macrophages.
Tissue-Resident CCR2+ Macrophages Are Monocyte Derived and Slowly Replenished Over Time
To characterize the population dynamics of CCR2+ macrophages within the resting heart and provide evidence that these cells truly represent a tissue-resident population, we performed a series of cell dynamic and parabiosis experiments. Flow cytometry using CCR2gfp/+ reporter mice revealed that CCR2+ macrophages first entered the heart at 2 weeks of age and increased in abundance by 6 weeks of age. Previous studies have demonstrated that CCR2-deficient mice have marked reductions in blood monocyte number.44 To assess whether CCR2+ macrophages require monocyte input to maintain residence within the heart, we examined control (CCR2gfp/+) and CCR2 knockout (CCR2gfp/gfp) mice. The number of CCR2+ macrophages within the naive heart was reduced in CCR2gfp/gfp mice compared with controls, suggesting that this population arises from blood monocytes (Online Figure III).
To determine whether CCR2+ macrophages in the naive heart represent a long-lived population, we performed parabiosis experiments. Examination of cardiac macrophage populations 6 and 12 weeks after parabiosis revealed that <25% of CCR2+ macrophages were replenished by blood monocytes after 6 weeks of parabiosis and <50% of CCR2+ macrophages were replenished by blood monocytes during a 12-week period (Online Figure IV). Collectively, these data indicate that CCR2+ macrophages within the naive heart are long-lived and thus may be considered a tissue-resident population that is maintained by gradual monocyte recruitment. Consistent with previous findings, monocytes only minimally contributed to CCR2− macrophages.23,40
Tissue-Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment
Given the inflammatory potential of tissue-resident CCR2+ macrophages, we postulated that this population is activated early after cardiomyocyte injury and may orchestrate the initial recruitment of leukocytes to the injured heart through generation of chemoattractant factors. To determine whether tissue-resident CCR2+ macrophages regulate monocyte recruitment, we used a syngeneic heart transplantation model. Before transplantation, donor hearts were perfused with saline to remove circulating leukocytes and placed on ice for 1 hour to mimic ischemic injury. Donor hearts were then transplanted into a syngeneic recipient and underwent reperfusion. As a result, heart transplantation represents an IR injury. An advantage of the heart transplantation model is the ability to genetically separate donor (tissue resident) from recipient (recruited) immune subsets. As a result, this model afforded us the unique ability to investigate the requirements for tissue-resident CCR2− and CCR2+ macrophages in regulating monocyte recruitment, to dissect signaling pathways necessary for activation of tissue-resident macrophages, and to visualize monocyte recruitment in real time using 2-photon microscopy.
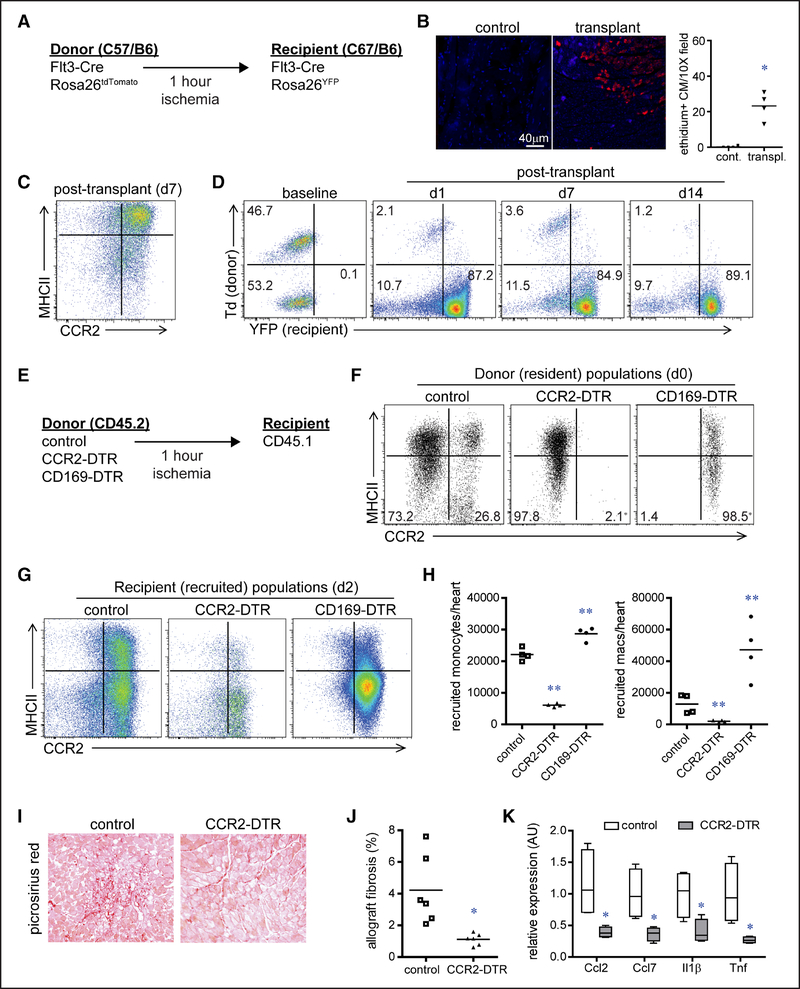
To evaluate the suitability of using our heart transplantation model to study monocyte recruitment, we transplanted Flt3-Cre Rosa26tdTomato donor hearts into syngeneic Flt3-Cre Rosa26YFP recipient mice (Figure 3A). Similar to other models of IR injury, heart transplantation resulted in cardiomyocyte cell death and accumulation of CCR2+ monocytes and CCR2+ macrophages within the donor heart (Figure 3B and 3C). Examination of tdTomato and YFP (yellow florescent protein) expression in monocytes and macrophages within the donor heart revealed that donor tissue-resident macrophages (unlabeled and tdTomato+) were replaced by recipient-derived monocytes and macrophages (YFP+; Figure 3D). Together, these data demonstrate the feasibility of using syngeneic heart transplantation model to investigate monocyte recruitment.
Figure 3. Tissue-resident CCR2− (C-C chemokine receptor 2) and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment.
A, Schematic describing the strategy to distinguish tissue-resident from recruited macrophages in our model of syngeneic heart transplantation. B, Ethidium homodimer-1 (red) staining showing cardiomyocyte cell death 2 h after heart transplantation. C, Flow cytometry showing the cell surface phenotype of CD (cluster of differentiation) 45+CD64+ monocytes and macrophages 7 d after heart transplantation. D, Flow cytometry characterizing the lineage of cardiac CD45+CD64+ monocytes and macrophages after heart transplantation: no reporter (donor, Flt3-Cre negative), dTomato+ (donor, Flt3-Cre positive), and YFP+ (yellow florescent protein; recipient Flt3-Cre positive). Displayed frequencies indicate the percentage of each population. n=4 per experimental group. E, Schematic describing the strategy to investigate whether tissue-resident (donor) macrophages influence recipient monocyte recruitment after syngeneic heart transplantation. F, Flow cytometry of CD45+CD64+ cardiac macrophages in diphtheria toxin-treated control, CCR2-DTR (diphtheria toxin receptor), and CD169-DTR donor hearts before transplantation. Displayed frequencies indicate the percentage of tissue-resident CCR2− and CCR2+ macrophages. *P <0.05 compared with baseline. n=4 per experimental group. G, Flow cytometry of recipient CD45.1+CD64+ monocytes and macrophages 2 d after heart transplantation. H, Quantification of the number of recipient monocytes (CD45+CD64+Ly6C+CCR2+MHC-II [major histocompatibility complex II]low) and macrophages (CD45+CD64+Ly6Clow) recruited to the heart 2 d after transplantation. I, Picrosirius red staining of cardiac allografts 28 d after transplantation (×100 magnification). J, Quantification of Picrosirius red staining in control and CCR2-DTR allografts. K, Quantitative RT-PCR (reverse transcription polymerase chain reaction) measuring chemokine and cytokine mRNA expression in control and CCR2-DTR allografts 4 d after transplantation. Data are displayed as a box and whiskers plot. Line indicates the mean value. n=4 per experimental group. *P <0.05 compared with control; **P <0.05 compared with all other groups.
To delineate whether cardiac tissue-resident macrophages influence monocyte recruitment in our heart transplantation model, we treated CCR2-DTR and CD169-DTR donor mice with DT for 4 days before transplantation to specifically deplete tissue-resident CCR2+ and CCR2− macrophages within the donor heart, respectively (Figure 3E). CCR2-DTR mice have previously been described to effectively deplete monocytes, macrophages, and dendritic cells without significant effects on neutrophil or lymphocyte populations.45 Examination of immune cell subsets in the naive heart demonstrated that macrophages represent the predominant myeloid cell type with few monocytes and rare dendritic or T cells present (Online Figure I). Importantly, neutrophils, monocytes, and lymphocytes are contained within the vasculature compartment and are removed after saline perfusion.23 After DT treatment, CD169-DTR mice displayed no reductions in monocyte, lymphocyte, or neutrophil abundance in the heart compared with controls, consistent with prior studies showing that this mouse strain does not deplete lymphocytes, monocytes, neutrophils, or dendritic cells (Online Figure VA).46
Compared with controls, donor hearts lacking tissue-resident CCR2+ macrophages displayed substantially reduced recruitment of recipient monocytes and macrophages 2 days after transplantation. In contrast, donor hearts lacking tissue-resident CCR2− macrophages displayed marked increase in monocyte and macrophage recruitment (Figure 3G and 3H). Depletion of donor-resident CCR2+ macrophages also resulted in reductions in interstitial fibrosis 28 days after transplantation, as well as decreased neutrophil influx and inflammatory chemokine and cytokine expression 2 days after transplantation (Figure 3I through 3K; Online Figure VB). Collectively, these findings indicate that tissue-resident CCR2− and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and that depletion of tissue-resident CCR2+ cardiac macrophages is sufficient to reduce inflammation and interstitial graft fibrosis after transplantation.
MYD88 Signaling Is Required to Activate Tissue-Resident CCR2+ Macrophages
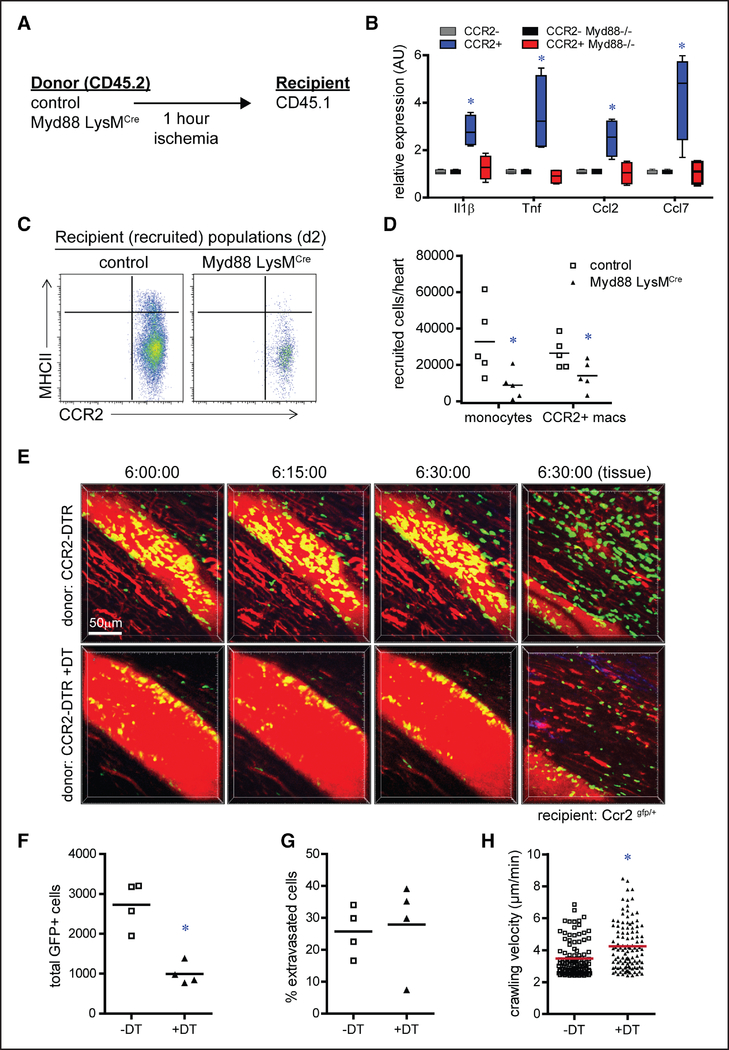
To test the hypothesis that tissue-resident CCR2+ macrophages promote monocyte recruitment through MYD88 (myeloid differentiation primary response 88)-dependent chemokine expression, we transplanted either CD45.2 control or Myd88flox/flox LysM (lysozyme M)-Cre donor hearts into recipient CD45.1 mice (Figure 4A). Within this model, Myd88 expression was eliminated from cardiac macrophages (Online Figure VC), and previous studies have shown that Myd88flox/ flox LysM-Cre mice display normal LV systolic function and LV chamber dimensions under baseline conditions.47 RT-PCR analysis of CCR2− and CCR2+ donor macrophages sorted from control and Myd88flox/flox LysM-Cre donor hearts 2 hours after transplantation revealed that donor CCR2+ macrophages expressed Il1β, Tnf, Ccl2, and Ccl7 in an MYD88-dependent manner (Figure 4B). Flow cytometry performed 2 days after transplantation demonstrated diminished recruitment of recipient monocytes and CCR2+ macrophages into Myd88flox/flox LysM-Cre donor hearts compared with control donor hearts (Figure 4C and 4D). These results indicate that tissue-resident CCR2+ macrophages promote monocyte recruitment through an MYD88-dependent mechanism presumably via the production of monocyte chemokines (CCL2/MCP1 and CCL7/MCP3).
Figure 4. Tissue-resident CCR2+ (C-C chemokine receptor 2) cardiac macrophages promote monocyte recruitment through an MYD88 (myeloid differentiation primary response 88)-dependent pathway regulating monocyte mobilization.
A, Schematic describing the strategy to investigate whether MYD88 is required for activation of tissue-resident (donor) cardiac macrophages after syngeneic heart transplantation. B, Quantitative RT-PCR (reverse transcription polymerase chain reaction) measuring chemokine and cytokine mRNA abundance in tissue-resident (CD [cluster of differentiation] 45.2+) CCR2− and CCR2+ macrophages isolated from control and Myd88 LysMCre (lysozyme M) donor hearts by FACS (fluorescence-activated cell sorting) 2 h after transplantation. Data are displayed as a box and whiskers plot. Line indicates the mean value. n=4 per experimental group. C, Flow cytometry of recipient CD45.1+CD64+ monocytes and macrophages isolated from control and CCR2-DTR (diphtheria toxin receptor) donor hearts day 2 after transplantation. D, Quantification of recipient monocytes (CD45+CD64+Ly6C+CCR2+MHC-II [major histocompatibility complex II]low) and macrophages (CD45+CD64+Ly6Clow) recruited to control and Myd88 LysMCre donor hearts 2 d after transplantation. E, Intravital 2-photon microscopy images obtained 6 h after transplantation of CCR2-DTR donor hearts into CCR2-GFP recipients. Control group: CCR2-DTR donors; experimental group: CCR2-DTR donors treated with diphtheria toxin (DT) before transplantation. Images are focused on coronary veins or adjacent myocardial tissue (far right). red: intravascular Qdot; green: recipient monocytes. F, Quantification of total number of CCR2+ cells. G, Quantification of the percent of CCR2+ cells that extravasated into the myocardium. H, Quantification of crawling velocity. *P <0.05 compared with control.
Intravital Imaging of Monocyte Recruitment
To gain further insights into how tissue-resident CCR2+ macrophages orchestrate monocyte recruitment, we performed intravital 2-photon imaging of CCR2-DTR donor hearts transplanted into CCR2gfp/+ recipients. Six hours after transplantation, recipient CCR2+ monocytes were observed migrating from the coronary venous vasculature into the myocardium of vehicle-treated CCR2-DTR donor hearts. In contrast, significantly fewer CCR2+ monocytes were visualized within both the vasculature and myocardium of DT-treated CCR2-DTR donor hearts (Figure 4E and 4F; Online Movies I through IV). Interestingly, transendothelial migration of monocytes was not adversely affected in DT-treated CCR2-DTR donor hearts. Quantification of the percentage of CCR2+ monocytes that extravasated into the myocardium revealed no significant differences between vehicle and DT-treated groups (Figure 4G). Furthermore, crawling velocity was not reduced in DT-treated CCR2-DTR donor hearts, indicating that the efficiency of transendothelial migration was not impaired (Figure 4H). Collectively, these data suggest that the mechanism by which tissue-resident CCR2+ macrophages govern monocyte recruitment differs substantially from how they regulate neutrophil recruitment.42 Specifically, tissue-resident CCR2+ macrophages promote monocyte infiltration into the myocardium through peripheral monocyte mobilization or recruitment to the heart rather than enhancing endothelial cell adhesion or transendothelial migration, consistent with established functions of CCL2 and CCL7.44,48
Tissue-Resident Cardiac Macrophages Govern Outcomes After Myocardial Infarction
To decipher whether manipulation of tissue-resident cardiac macrophage populations might represent a clinically relevant approach to improve outcomes after myocardial infarction, we investigated the effects of depleting tissue-resident CCR2− and CCR2+ macrophages in a reperfused myocardial infarction model. We chose to use a closed-chest IR (90 minutes of ischemia) model to minimize confounding effects of inflammation associated with surgical thoracotomy.49,50 Tissue-resident CCR2− macrophages were depleted by treating CD169-DTR mice with DT for 1 week before IR injury (Figure 3E). CD169-DTR mice did not display significant reductions in monocyte, neutrophil, or lymphocyte numbers within the blood, spleen, or heart after DT treatment (Online Figure VA). Tissue-resident CCR2+ macrophages were depleted by treating CCR2-DTR mice with a single injection of DT 4 days before IR injury. Immediately after DT treatment, CCR2+ monocytes (peripheral and cardiac), tissue-resident CCR2+ macrophages, and rare cardiac dendritic cells were readily depleted. However, 4 days after DT treatment, CCR2+ monocytes and cardiac dendritic cells were repopulated, whereas cardiac tissue-resident CCR2+ macrophages remained absent (Online Figure VIA through VIC). These data are consistent with prior studies showing that both monocytes and dendritic cells are repopulated 4 days after DT administration into CCR2-DTR mice.45 Echocardiography further demonstrated that DT-treated CD169-DTR and CCR2-DTR mice displayed no impairments in LV systolic function or altered chamber dimensions compared with controls (Online Figure VID).
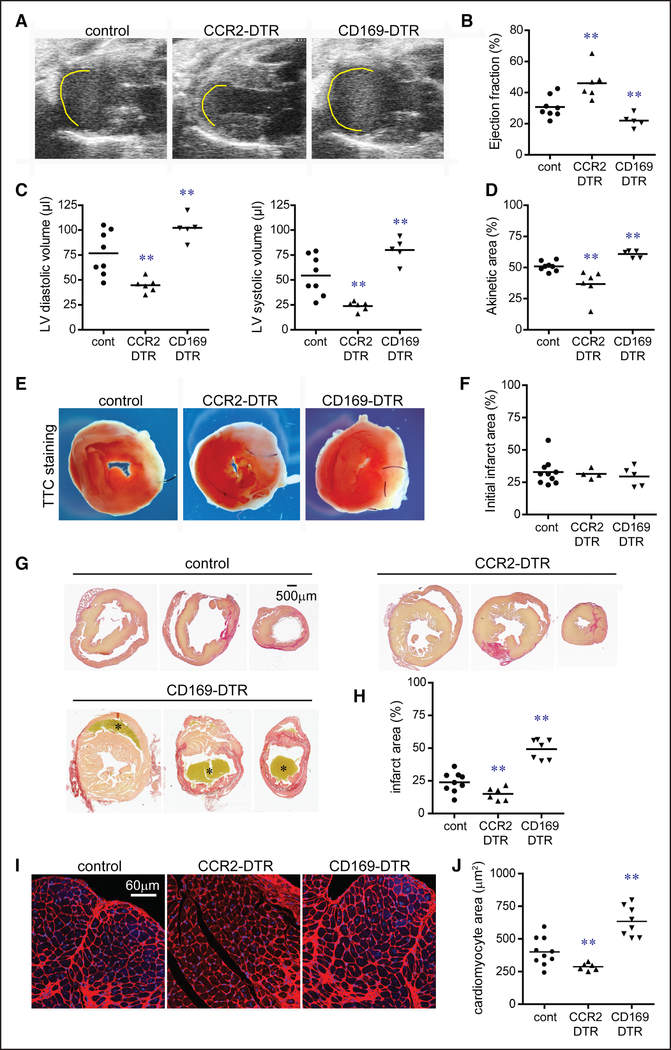
Echocardiography performed 28 days after IR injury revealed that depletion of tissue-resident CCR2+ macrophages before IR injury resulted in improved LV systolic function, smaller LV chamber dimensions, and reduced akinetic myocardium compared with controls. In contrast, depletion of tissue-resident CCR2− macrophages resulted in diminished LV systolic function, larger LV chamber dimensions, and increased akinetic myocardium compared with controls and mice lacking tissue-resident CCR2+ macrophages (Figure 5A through 5D). Pathological measurement of infarct size 2 and 28 days after IR injury showed that although initial infarct area did not differ between experimental groups, depletion of tissue-resident CCR2+ macrophages resulted in smaller sized infarcts at later time points compared with controls. Depletion of tissue-resident CCR2− macrophages led to larger sized infarcts compared with all other groups (Figure 5E through 5H). These findings indicate that tissue-resident macrophages do not affect initial infarct size but rather influence postinfarction LV remodeling.
Figure 5. Tissue-resident cardiac macrophages govern outcomes after myocardial infarction.
A, Echocardiographic images of control, CCR2 (C-C chemokine receptor 2)-DTR (diphtheria toxin receptor), and CD (cluster of differentiation) 169-DTR hearts 28 d after closed-chest ischemia-reperfusion injury. Diphtheria toxin (DT) was administered before ischemia-reperfusion injury. Yellow line denotes akinetic myocardial segments. B–D, Quantification of ejection fraction (B), left ventricular (LV) diastolic and systolic volumes (C), and akinetic area (D) 28 d after ischemia-reperfusion injury. E, Triphenyltetrazolium chloride (TTC) staining of control, CCR2-DTR, and CD169-DTR hearts 48 h after ischemia-reperfusion injury. DT was administered before ischemia-reperfusion injury. White area denotes the infarcted region, and red area indicates viable myocardial tissue. F, Quantification of infarct area at 48 h based on TTC staining. G, Picrosirius red staining of control, CCR2-DTR, and CD169-DTR hearts 28 d after ischemia-reperfusion injury. DT was administered before ischemia-reperfusion injury. Red staining highlights the infarcted region, and yellow staining indicates viable myocardial tissue. Asterisks denote a thrombus. H, Quantification of infarct area 28 d after ischemia-reperfusion injury based on Picrosirius red staining. I, Wheat germ agglutinin (WGA; red) staining demonstrating differential effects on cardiomyocyte hypertrophy within the borderzone of control, CCR2-DTR, and CD169-DTR hearts 28 d after ischemia-reperfusion injury. DT was administered before ischemia-reperfusion injury. Blue: DAPI (4’,6-diamidino-2-phenylindole). ×200 magnification. J, Quantification of cardiomyocyte cross-sectional area 28 d after ischemia-reperfusion injury based on WGA staining. **P <0.05 compared with all other groups.
Quantification of cardiomyocyte cell size 28 days after myocardial infarction revealed that hearts depleted of tissue-resident CCR2+ macrophages before IR injury had reduced cardiomyocyte hypertrophy compared with controls. Hearts depleted of tissue-resident CCR2− macrophages before IR injury displayed increased cardiomyocyte hypertrophy compared with all other groups (Figure 5I and 5J; Online Figure VII). Consistent with our heart transplant model, monocyte and neutrophil accumulation within the infarct 2 days after IR injury was reduced in hearts lacking tissue-resident CCR2+ macrophages (Online Figure VIII).
RNA Sequencing Reveals the Diversity of Macrophages in the Infarcted Heart
Provocative studies have suggested that monocytes display tremendous plasticity and can adopt a broad spectrum of cell fates in response to different environmental stimuli.20–22 To determine whether tissue-resident cardiac macrophages influence monocyte cell fate decisions, we performed single-cell RNA sequencing of CD45+Ly6G-CD11b+CD64+ monocytes and macrophages isolated by flow cytometry from control, CCR2-DTR, and CD169-DTR hearts 4 days after IR injury (Online Figure IXA). At this time point, the majority of macrophage populations are derived from recruited monocytes (Figure 1D). DT was administered to CCR2-DTR and CD169-DTR mice as outlined above to deplete tissue-resident CCR2+ and CCR2− macrophages, respectively.
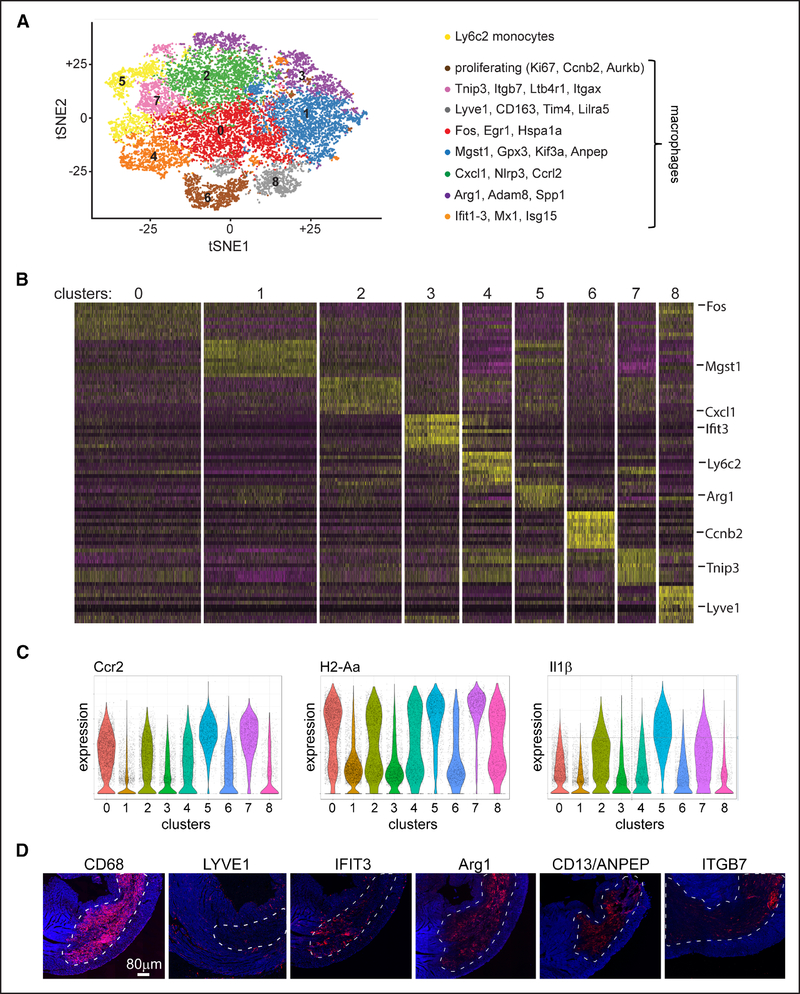
Combined analysis of 17 931 cells (100 000 reads per cell) revealed the presence of 1 monocyte cluster and 7 distinct macrophage clusters (Figure 6A and 6B; Online Figure X). In addition, we found 1 macrophage cluster that was comprised of proliferating cells. Our previous gating scheme focused on CCR2 and MHC-II expression was not sufficient to fully resolve each of these putative cell types, and the expression of important inflammatory cytokines, such as Il1β, differed between clusters (Figure 6C). Monocytes were readily distinguished from macrophages based on the expression of Ly6C2 (lymphocyte antigen 6C2), Hp (haptoglobin), Plac8 (placenta-specific 8), and Thbs (thrombospondin; Online Figure IXB and IXC). Macrophage markers were robustly expressed in all but one of the remaining clusters (Online Figure IXD). This cluster was demarcated by the expression of Tnip3 (TNFAIP3-interacting protein 3) and Itgb7 (integrin subunit β7) and lacked classic monocyte markers. Because dendritic cells were largely excluded from our gating scheme, we did not observe dendritic cell exclusive clusters (Online Figure IXE). However, dendritic cell-specific transcripts were expressed in a subset of cells within the Tnip3/Itgb7 cluster (Online Figure XI). Immunostaining of myocardial tissue 4 days after IR injury confirmed the presence of LYVE1+ (lymphatic vessel endothelial hyaluronan receptor 1), IFIT3+ (IFN-induced protein with tetratricopeptide repeats 3), ARG1+, ITGB7+, ANPEP+ (alanyl aminopeptidase), and CCRL2+ (C-C chemokine receptor-like 2) macrophages each with distinct cell morphologies and locations within the infarcted heart (Figure 6D; Online Figure XII).
Figure 6. Single-cell RNA sequencing of monocytes and macrophages after myocardial infarction.
A, Unsupervised clustering of CD (cluster of differentiation) 45+Ly6G-CD11b+CD64+ cells isolated by flow cytometry from control, CCR2 (C-C chemokine receptor 2)-DTR (diphtheria toxin receptor), and CD169-DTR hearts 4 d after ischemia-reperfusion injury. Diphtheria toxin (DT) was administered before ischemia-reperfusion injury. Each experimental group consists of a pool of 4 biologically independent samples. Data are displayed as a tSNE (t-distributed stochastic neighbor embedding) plot. B, Seurat-generated heat map showing the top 10 genes by P expressed in each cluster. Selected genes are noted in the right column. C, Violin plots demonstrating that CCR2 and H2-Aa (MHC-II [major histocompatibility complex II]) expression does not resolve clusters identified by unsupervised clustering. Il1β expression is also displayed. D, Immunostaining of myocardial tissue 4 d after ischemia-reperfusion injury. 100× tile scans showing the relationship of macrophage subsets to the infarct area (dashed white line). Blue: DAPI (4’,6-diamidino-2-phenylindole).
Tissue-Resident Cardiac Macrophages Differentially Influence Monocyte Fate Specification
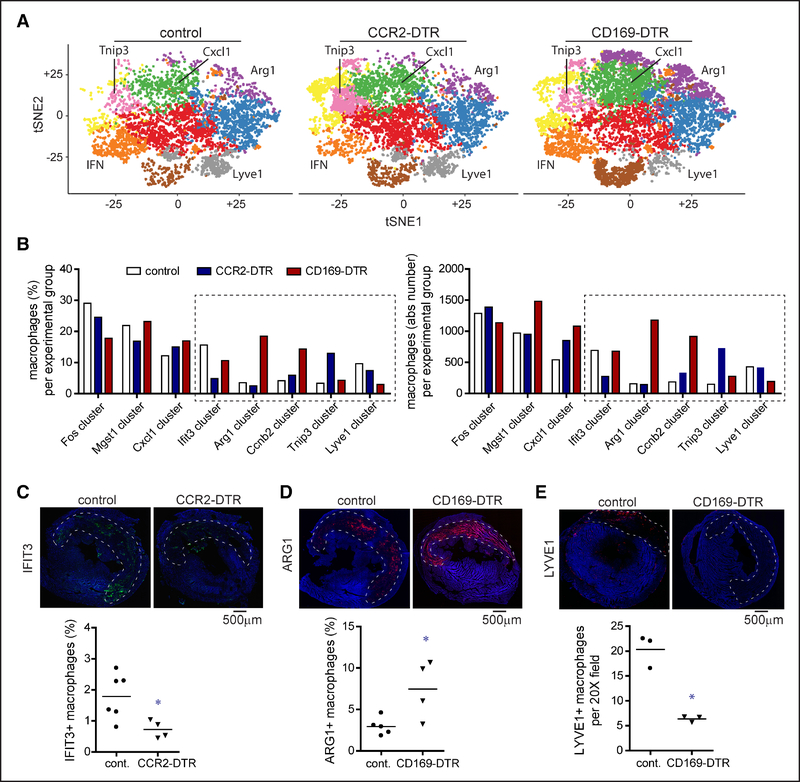
Comparative analysis between genotypes revealed significant alterations in macrophage subsets after reperfused myocardial infarction. Depletion of tissue-resident CCR2+ macrophages before IR injury resulted in reduced numbers of Ifit3+ macrophages and increased numbers of Tnip3/Itgb7+ macrophages. In contrast, depletion of tissue-resident CCR2− macrophages before IR injury led to increased numbers of Arg1+, Cxcl1/Ccrl2+, and proliferating macrophages and reduced numbers of Lyve1+ macrophages (Figure 7A and 7B). Of note, Lyve1 is expressed on resident CCR2− cardiac macrophages and may reflect a compilation of resident and recruited macrophage populations.26,51 Immunostaining analysis confirmed alterations in the abundance and distribution of macrophage subsets between genotypes (Figure 7C through 7E).
Figure 7. Tissue-resident cardiac macrophages influence monocyte fate specification after myocardial infarction.
A, tSNE plots separated by experimental group highlighting differences in cell abundance within each cluster. Clusters showing the greatest differences between experimental groups are labeled. B, Quantification of the percentage and absolute number of macrophages assigned to each macrophage cluster in control, CCR2 (C-C chemokine receptor 2)-DTR (diphtheria toxin receptor), and CD (cluster of differentiation) 169-DTR hearts on day 4 after ischemia-reperfusion injury. Diphtheria toxin (DT) was administered before ischemia-reperfusion injury. Dotted box denotes clusters that showed differences in both the percentage and absolute number of macrophages between experimental groups. C–E, Immunostaining of control, CCR2-DTR, and CD169-DTR hearts 4 d after ischemia-reperfusion injury for IFIT3 (IFN-induced protein with tetratricopeptide repeats 3; C), ARG1 (arginase 1; D), and LYVE1 (E). DT was administered before ischemia-reperfusion injury. Dashed lines indicated the infarct area. Tile scan of ×100 magnification images. Blue: DAPI (4’,6-diamidino-2-phenylindole). Quantification of the number of IFIT3+, ARG1+, and LYVE1+ macrophages between experimental groups is shown below the immunostaining images. **P <0.05 compared with all other groups.
Discussion
Collectively, our findings indicate that recruitment of monocytes and monocyte-derived macrophages represents a hallmark response to cardiomyocyte cell death and establishes tissue-resident cardiac CCR2+ macrophages as essential upstream mediators of the inflammatory response to myocardial injury. MYD88-dependent activation of tissue-resident CCR2+ macrophages results in the elaboration of inflammatory chemokines and cytokines that orchestrate monocyte and neutrophil recruitment and influence monocyte fate decisions. Furthermore, we conceptually demonstrate that therapeutic strategies that target tissue-resident CCR2+ macrophages have the potential to improve outcomes after myocardial infarction. By using intravital 2-photon imaging, we additionally show that tissue-resident CCR2+ macrophages regulate myocardial infiltration of neutrophils and monocytes through distinct mechanisms. Tissue-resident CCR2+ macrophages govern neutrophil infiltration at the level of transendothelial migration42 and control monocyte infiltration by regulating peripheral monocyte mobilization and recruitment to the heart.
In addition to providing further evidence that cardiac monocytes and macrophages are critical determinants of LV remodeling in the context of reperfused myocardial infarction, our results exemplify the importance of carefully dissecting macrophage populations to identify specific subsets that might serve as therapeutic targets to limit inflammation and postinfarct LV remodeling. Based on our findings, it is possible that agents that inhibit activation of tissue-resident CCR2+ macrophages or neutralize effector cytokines elaborated by tissue-resident CCR2+ macrophages would provide benefit after myocardial infarction. Intriguingly, aging is associated with replacement of embryonic-derived tissue-resident macrophages with monocyte-derived subsets.52 As such, it is likely that the relative abundance of tissue-resident CCR2+ macrophages increases with age. Consequently, the aged heart may be primed to generate exaggerated inflammatory responses after myocardial injury. This may represent one potential explanation for why aging is associated with excessive inflammation and worse outcomes after myocardial infarction53 and highlight the possibility that therapeutics that target tissue-resident CCR2+ macrophage may have preferential efficacy in older populations.
Although this study clarifies key elements involved in the initiation of myocardial inflammation and highlights previously unrecognized requirements for distinct tissue-resident cardiac macrophage populations, several questions remain to be answered. MYD88 signaling is universally recognized as a key pathway mediating the effects of danger-associated molecular patterns released from dying cells.54 However, because numerous danger-associated molecular pattern signaling pathways, including TLRs (Toll-like receptors; TLR2, TLR4, and TLR9) and the IL-1 receptor, may signal through MYD88,55 it is not immediately clear which receptor(s) regulate the activation of tissue-resident CCR2+ macrophages in the context of myocardial IR injury. In fact, it is likely that a combination of danger-associated molecular patterns and danger-associated molecular pattern receptors are physiologically relevant, and future studies will be required to resolve these complexities.
Depletion of tissue-resident CCR2− macrophages resulted in increased recruitment of monocytes in our heart transplant model. Interestingly, a similar result was not observed in our myocardial infarction model. Instead, we found that depletion of tissue-resident CCR2− macrophages before myocardial infarction resulted in significant shifts in monocyte fate specification, augmentation of macrophage proliferation, increased infarct area, reduced LV systolic function, and exaggerated LV remodeling. The mechanistic basis by which tissue-resident CCR2− macrophages differentially influence monocyte recruitment and fate decisions in these models is unclear. Possibilities include modulation of resident CCR2+ macrophage activation, release of anti-inflammatory mediators, and direct signaling to recruited monocytes. These findings do suggest that modulation of monocyte fate decisions represents an important mechanism that likely impacts outcomes after ischemic cardiac injury. However, the exact mechanistic basis by which manipulation of resident cardiac macrophages influences LV remodeling after myocardial infarction is likely multifactorial with potentially important contributions from neutrophil and monocyte recruitment, monocyte fate specification, cytokine signaling, and possibly other mechanisms yet to be identified.
Single RNA sequencing uncovered at least 7 distinct macrophage subsets within the infarcted heart. Each of these subsets displayed differing spatial-temporal dynamics and cell morphologies consistent with concept that they represent unique cell types. Intriguingly, tissue-resident macrophages had profound effects on monocyte fate decisions suggesting that resident macrophages have the potential to not only influence outcomes after myocardial infarction through controlling monocyte recruitment but also by regulating monocyte differentiation. For example, depletion of tissue-resident CCR2+ macrophages resulted in both reduced monocyte recruitment and decreased accumulation of type I IFN-biased macrophages—a population previously implicated in adverse LV remodeling.56 The exact mechanisms by which tissue-resident macrophages orchestrate monocyte fate decisions and the relevant functions of each macrophage subset are yet to be fully defined. Future studies will undoubtedly tackle these important questions and provide critical insights into new potential therapies for patients with ischemic heart disease.
This study is not without limitations. The strategies used to deplete tissue-resident CCR2− and CCR2+ macrophages from the heart are imprecise. Although CD169-DTR specifically depletes tissue-resident CCR2− macrophages from the heart, macrophage subsets present within other tissues are also targeted, including intestinal, alveolar, and splenic subsets.31,46,57 Therefore, it is possible that macrophage populations outside of the heart may be responsible for some of the observed phenotypes. However, by using our syngeneic heart transplantation model, we were able to overcome this obstacle and show that tissue-resident CCR2− macrophages in the heart inhibit monocyte recruitment. CCR2-DTR depletes tissue-resident CCR2+ macrophages, monocytes, and dendritic cells.45 To selectively deplete tissue-resident CCR2+ macrophages, we took advantage of the rapid repopulation kinetics of circulating monocytes and dendritic cells after DT administration. Four days after DT treatment, only tissue-resident CCR2+ macrophages were depleted from the heart. However, it is possible that CCR2+ cells located within non-cardiac tissues contribute to some of the phenotypes observed after myocardial infarction. Again, use of the syngeneic heart transplantation model allowed us to circumvent this issue and demonstrate a specific requirement for cardiac tissue-resident CCR2+ macrophages in orchestrating monocyte and neutrophil recruitment.
In conclusion, we demonstrate that cardiac tissue-resident macrophages differentially control monocyte recruitment and fate specification after cardiomyocyte cell death. We identify tissue-resident CCR2+ macrophages as critical drivers of monocyte recruitment, fate specification, inflammation, and adverse LV remodeling. Through single-cell RNA sequencing of monocytes and monocyte-derived macrophages that infiltrate the heart after myocardial infarction, we reveal unprecedented cell heterogeneity and uncover new avenues to modulate inflammation in the injured heart. Our findings establish the mechanistic basis by which monocytes are initially recruited to the injured heart and provide novel insights into the functional diversity of monocyte-derived macrophages.
Supplementary Material
Novelty and Significance.
What Is Known?
After myocardial tissue injury, monocytes are recruited to the heart in large numbers.
In general, monocytes are considered an inflammatory population of cells that contribute to collateral cardiomyocyte cell death, maladaptive tissue remodeling, and heart failure pathogenesis.
Little is understood about the mechanistic basis by which monocytes are recruited to the injured heart and how they adopt inflammatory phenotypes.
What New Information Does This Article Contribute?
As a stereotyped response to cardiomyocyte cell death, monocytes are recruited to the heart and largely replace tissue-resident macrophages.
Tissue-resident CCR2+ (C-C chemokine receptor 2) macrophages are responsible for the initial recruitment of monocytes to the injured through an MYD88 (myeloid differentiation primary response 88)-dependent pathway that regulates the expression of chemoattractant chemokines; tissue-resident CCR2− macrophages inhibit monocyte recruitment.
Selective depletion of tissue-resident CCR2+ macrophages before myocardial infarction results in divergent effects on left ventricular function, myocardial remodeling, and monocyte recruitment.
Single-cell RNA sequencing reveals that monocytes recruited to the injured heart differentiate into heterogeneous populations of macrophages and that tissue-resident macrophages are important determinants of monocyte fate specification.
Recruitment of monocytes to the injured heart is thought to be an important source of inflammation contributing to the development of heart failure. However, little is understood on how monocyte recruitment is regulated and why these cells adopt inflammatory behaviors. Using numerous models of cardiomyocyte injury, we show that CCR2+ macrophages resident within the heart orchestrate monocyte recruitment, bias monocytes toward inflammatory phenotypes, and contribute to heart failure pathogenesis. Our findings implicate tissue-resident CCR2+ as a target for therapy after myocardial infarction and provide new insights into the heterogeneity of monocyte-derived macrophages.
Acknowledgments
This work benefitted from data assembled by the ImmGen consortium. We acknowledge the McDonnell Genome Institute for their assistance in designing and performing the single-cell RNA sequencing analysis. G. Bajpai performed the flow cytometry and RNA sequencing experiments. A. Bredemeyer performed immunostaining and single-cell RNA sequencing experiments. W. Li performed the heart transplantation and intravital 2-photon imaging studies. K. Zaitsev, A. Koenig, and M. Artyomov processed and analyzed the single-cell RNA sequencing data. I. Lokshina, J. Mohan, and B. Ivey performed the pathological analysis of myocardial specimens. H.-M. Hsiao performed RT-PCR (reverse transcription polymerase chain reaction) assays. C. Weinheimer provided surgical services, including coronary ligation, ischemia-reperfusion injury, and transverse aortic constriction. A. Kovacs performed echocardiography. S. Epelman, M. Artyomov, and D. Kreisel assisted with experimental design and critical review of the manuscript. K.J. Lavine is responsible for all aspects of this manuscript, including experimental design, data analysis, and manuscript production.
Sources of Funding
This project was made possible by funding provided from the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CH-II-2015-462 and CH-II-2017-628), Foundation of Barnes-Jewish Hospital (8038-88, 4695), Mid-America Transplant Services, and the National Heart, Lung, and Blood Institute (R01 HL138466 and R01 HL139714). K.J. Lavine is supported by NIH K08 HL123519 and Burroughs Welcome Fund (1014782). Histology was performed in the Digestive Diseases Research Core Centers advanced imaging and tissue analysis core supported by grant No. P30 DK52574. D. Kreisel is supported by NiH P01AI116501 and R01 HL094601, Veterans Administration Merit Review grant 1I01BX002730, and The Foundation for Barnes-Jewish Hospital.
Nonstandard Abbreviations and Acronyms
- Areg
amphiregulin
- Anpep
alanyl aminopeptidase
- Arg1
arginase 1
- CCR2
C-C chemokine receptor 2
- Ccrl2
C-C chemokine receptor-like 2
- CD
cluster of differentiation
- CX3CR1
CX3C chemokine receptor 1
- CCL
chemokine (C-C) ligand
- CXCL
chemokine (C-X-C) ligand
- Cyr61
cysteine-rich angiogenic inducer 61
- DTR
diphtheria toxin receptor
- DT
diphtheria toxin
- Ereg
epiregulin
- FLT3
Fms-related tyrosine kinase 3
- GDF
growth and differentiation factor
- Hif1α
hypoxia-inducible factor 1α
- Hp
haptoglobin
- Hbegf
heparin-binding epidermal-like growth factor
- IL
interleukin
- IFN
interferon
- IR
ischemia-reperfusion
- Igf1
insulin-like growth factor 1
- Itgb7
integrin subunit β7
- Ifit3
IFN-induced protein with tetratricopeptide repeats 3
- LV
left ventricle
- LysM
lysozyme M
- Lyve1
lymphatic vessel endothelial hyaluronan receptor 1
- MYD88
myeloid differentiation primary response 88
- MHC-II
major histocompatibility complex II
- MCP
monocyte chemoattractant protein
- Mrc1
mannose receptor C-type 1
- NF-κB
nuclear factor κ-light chain enhancer of activated B cells
- Pdgfc
platelet-derived growth factor C
- Plac8
placenta-specific 8
- STAT
signal transducer and activator of transcription 3
- Tnnt2
troponin T2
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- Thbs
thrombospondin
- Tnip3
TNFAIP3-interacting protein 3
- YFP
yellow florescent protein
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.118.314028.
Contributor Information
Geetika Bajpai, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
Andrea Bredemeyer, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
Wenjun Li, Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Konstantin Zaitsev, Department of Immunology and Pathology, Washington University School of Medicine, St. Louis, MO.
Andrew L. Koenig, Department of Medicine, Washington University School of Medicine, St. Louis, MO
Inessa Lokshina, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
Jayaram Mohan, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
Brooke Ivey, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
His-Min Hsiao, Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Carla Weinheimer, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
Attila Kovacs, Department of Medicine, Washington University School of Medicine, St. Louis, MO.
Slava Epelman, Toronto General Hospital Research Institute, Division of Cardiology, University Health Network, ON, Canada.
Maxim Artyomov, Department of Immunology and Pathology, Washington University School of Medicine, St. Louis, MO.
Daniel Kreisel, Department of Immunology and Pathology, Washington University School of Medicine, St. Louis, MO; Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Kory J. Lavine, Department of Medicine, Washington University School of Medicine, St. Louis, MO Department of Surgery, Washington University School of Medicine, St. Louis, MO; Department of Immunology and Pathology, Washington University School of Medicine, St. Louis, MO; Department of Developmental Biology, Washington University School of Medicine, St. Louis, MO.
References
- 1.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta P, Sager HB, Stengel KR, et al. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;16:477–487. doi: 10.1016/j.stem.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102:240–248. doi: 10.1093/cvr/cvu025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki S, Nakagomi A, Asai K, Takano H, Yasutake M, Seino Y, Mizuno K. Elevated peripheral blood mononuclear cell count is an independent predictor of left ventricular remodeling in patients with acute myocardial infarction. J Cardiol. 2011;57:202–207. doi: 10.1016/j.jjcc.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 8.Mariani M, Fetiveau R, Rossetti E, Poli A, Poletti F, Vandoni P, D’Urbano M, Cafiero F, Mariani G, Klersy C, De Servi S. Significance of total and differential leucocyte count in patients with acute myocardial infarction treated with primary coronary angioplasty. Eur Heart J. 2006;27:2511–2515. doi: 10.1093/eurheartj/ehl191 [DOI] [PubMed] [Google Scholar]
- 9.van der Laan AM, Hirsch A, Robbers LF, Nijveldt R, Lommerse I, Delewi R, van der Vleuten PA, Biemond BJ, Zwaginga JJ, van der Giessen WJ, Zijlstra F, van Rossum AC, Voermans C, van der Schoot CE, Piek JJ. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J. 2012;163:57–65.e2. doi: 10.1016/j.ahj.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 10.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a [DOI] [PubMed] [Google Scholar]
- 12.Goser S, Ottl R, Brodner A, Dengler TJ, Torzewski J, Egashira K, Rose NR, Katus HA, Kaya Z. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112:3400–3407. doi: 10.1161/CIRCULATIONAHA.105.572396 [DOI] [PubMed] [Google Scholar]
- 13.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liehn EA, Piccinini AM, Koenen RR, Soehnlein O, Adage T, Fatu R, Curaj A, Popescu A, Zernecke A, Kungl AJ, Weber C. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol. 2010;56:1847–1857. doi: 10.1016/j.jacc.2010.04.066 [DOI] [PubMed] [Google Scholar]
- 16.Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayasaki T, Kaikita K, Okuma T, Yamamoto E, Kuziel WA, Ogawa H, Takeya M. CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myocardial ischemia-reperfusion in mice. Circ J. 2006;70:342–351. [DOI] [PubMed] [Google Scholar]
- 18.Struthers M, Pasternak A. CCR2 antagonists. Curr Top Med Chem. 2010;10:1278–1298. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Qin L, Zacarías NV, et al. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature. 2016;540:458–461. doi: 10.1038/nature20605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119:414–417. doi: 10.1161/CIRCRESAHA.116.309194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27:237–248. doi: 10.1016/j.smim.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 23.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res. 2016;118:1498–1511. doi: 10.1161/CIRCRESAHA.115.308270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautier EL, Shay T, Miller J, et al. ; Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012;13:1118–1128. doi: 10.1038/ni.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoeffel G, Chen J, Lavin Y, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725 [DOI] [PubMed] [Google Scholar]
- 35.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- 36.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017; 169:510–522.e20. doi: 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, van der Lahn AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24:1234–1245. doi: 10.1038/s41591-018-0059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Hsiao HM, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, Krupnick AS, Gelman AE, Lavine KJ, Kreisel D. Heart-resident CCR2(+) macrophages promote neutrophil extravasation through TLR9/MyD88/ CXCL5 signaling. JCI Insight. 2016;1:e87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol. 2018;113:26. doi: 10.1007/s00395-018-0686-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309 [DOI] [PubMed] [Google Scholar]
- 45.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Y, Zou L, Chen C, Li D, Chao W. Role of cardiac- and myeloid-MyD88 signaling in endotoxin shock: a study with tissue-specific deletion models. Anesthesiology. 2014;121:1258–1269. doi: 10.1097/ALN.0000000000000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, Entman ML. Brief murine myocardial I/R induces chemokines in a TNF-alpha-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol. 2001;281:H2549–H2558. doi: 10.1152/ajpheart.2001.281.6.H2549 [DOI] [PubMed] [Google Scholar]
- 51.Stevens SM, von Gise A, VanDusen N, Zhou B, Pu WT. Epicardium is required for cardiac seeding by yolk sac macrophages, precursors of resident macrophages of the adult heart. Dev Biol. 2016;413:153–159. doi: 10.1016/j.ydbio.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28. doi: 10.1016/j.trsl.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164 [DOI] [PubMed] [Google Scholar]
- 55.Warner N, Nunez G. MyD88: a critical adaptor protein in innate immunity signal transduction. J Immunol. 2013;190:3–4. doi: 10.4049/jimmunol.1203103 [DOI] [PubMed] [Google Scholar]
- 56.King KR, Aguirre AD, Ye YX, et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med. 2017;23:1481–1487. doi: 10.1038/nm.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asano K, Takahashi N, Ushiki M, Monya M, Aihara F, Kuboki E, Moriyama S, Iida M, Kitamura H, Qiu CH, Watanabe T, Tanaka M. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun. 2015;6:7802. doi: 10.1038/ncomms8802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.