Abstract
In the present study, the antioxidant and antimicrobial effects of essential oil (EO) and extract of Pimpinella affinis on the shelf life of rainbow trout during storage at refrigerator temperature based on three indicators of microbial, chemical and sensory quality parameters were evaluated. For this purpose, fish samples were stored in zein coatings containing 1.00% EO and 2.00% extract and in combination treatment containing 1.00% of each of them in refrigerator for 12 days. All of treatments were analyzed for microbiological count (Enterobacteriaceae, psychrophilic bacteria, mesophilic bacteria and pseudomonas bacteria) and chemical including pH, thiobarbituric acid reactive substances (TBARS), total volatile base nitrogen, peroxide value (POV) and free fatty acids (FFAs) and sensory (odor, color and texture) characteristics. Results showed that the highest levels of three factors including TBARS, POV and FFAs were related to the control sample and the least amount of these parameters was observed in EO and extract combination treatments. This effect of combined treatment was also observed in the reduction of total volatile basic nitrogen and pH parameters. Total bacterial counts during storage in fish treated with the extract and EO were remained below the acceptable level and microbial spoilage was significantly decreased compared to control. According to the sensory evaluation, treatments containing EO and extract showed improvement in this index compared to control treatment during storage. Based on the results of this study, it can be concluded that the EO and extract of P. affinis as active ingredients in zein coating successfully improve the quality and shelf life of the fish in the refrigerator.
Key Words: Essential oil, Extract, Pimpinella affinis, Rainbow trout, Zein
Introduction
Fish meat is more susceptible to microbial spoilage and oxidation and deteriorates faster than other meat products due to high levels of protein, pH, free amino acids, polyunsaturated fatty acids and volatile nitrogen bases in its tissue. 1 Various factors such as endogenous enzymes activity, lipid oxidation and microorganism activity are important with regard to quality and shelf life of fish. 2 However, fish plays an important role in human diet as an important natural source of omega-3 fatty acids, hydrosoluble and liposoluble vitamins and protein. 3
Various methods have been used to maintain the quality and to increase the shelf life of fresh seafood products. In this regard, the use of edible coatings in order to protect food has increased significantly in recent years. This type of packaging has several environmental benefits compared to the synthetic materials. 4 Films and coatings originate from a variety of sources such as polysaccharides, proteins and lipids. 5 Corn proteins are unique in comparison with many of the plant proteins used for coatings and films. Zein is a prolamine protein extracted from corn gluten. This compound has a high percentage of non-polar amino acids and a small amount of acidic and basic amino acids. As a result, it is insoluble in water and is dissolving only in organic solvents. Since zein acts as a barrier against oxygen and moisture penetration, it is used as a coating for candy, dry fruits and meat.6,7
Nowadays, because of the great consumer awareness and concern regarding synthetic chemical additives, natural products of herbal origin have been welcomed.8 Addition of essential oil (EO) and extract to the coating and edible film increases the storage time of food or prevent the pathogenic foodborne bacteria growth due to their antioxidant and antimicrobial capacities.9
Pimpinella affinis is one of the most important species in Apiaceae family with proven antioxidant and antibacterial properties.10 Plant EOs and extracts have a role in spoilage and pathogenic bacteria control and food shelf life increase. Antimicrobial mechanism of the EOs is due to the effect on the lipid or phospholipid of cell membranes leading to cell membranes structure disruption and cellular components release.11,12
Vacuum packing is a method of packaging that removes air from the package resulting in product durability and quality increase.13 Therefore, the present study was conducted to investigate the antioxidant and antimicrobial effects of Pimpinella affinis EO and extract, combination of them and vacuum packaging on rainbow trout shelf life at 4.00 ± 1.00 ˚C.
Materials and Methods
Preparation of extract and EO. Pimpinella affinis was collected from Amol, Mazandaran province, Iran in spring. The aerial parts (500 g) were washed and dried at room temperature in the shade and then hydrodistillation method with Clevenger-type apparatus was used for EO extraction for 2 hr.2
For plant extract preparation, the stems and leaves of the plant were dried in shade at ambient temperature, pulverized by the mill and passed through a 60-mesh sieve. Two hundred gram of powder was dissolved in 1 L of ethanol (Merck Millipore, Burlington, USA) and water (70 to 30 V/V) and placed in a shaker (KS 4000i control; IKA Werke GmbH & Co. KG, Staufen, Germany) with a round of 150 rpm for 24 hr. Afterwards, the solution was passed through a filter paper and then placed in a rotary machine (Heidolph, Schwabach, Germany) to remove at least 90.00% of the solvent. Finally, it was placed in an oven (D-645, Heraeus, Hanau, Germany) at 45 ˚C for final condensation and stored in a refrigerator at 4 ˚C.14
Fish sample preparation. Rainbow trout with a weight of approximately 600.00 ± 50.00 g were purchased from the retail centers of Urmia, West Azerbaijan province, Iran. After discharge and evacuations, drinking water was used for washing the blood and then they were transferred to the Laboratory of the Faculty of Veterinary Medicine, Urmia University, Urmia, Iran in a cool box (4.00 ± 1.00 ˚C).
Preparation of the solution and treatment of fish samples. Zein solution was prepared according to Kashiri et al. by dissolving 16.00 g of zein (Sigma, St. Louis, USA) powder in 80.00% (v/v) ethanol–water solution and stirring for 30 min at 80 ˚C on a magnetized hot plate until the zein was completely dissolved.7 Glycerol (Merck, Darmstadt, Germany) 15.00% (g glycerol per g dry zein powder) was added to the solution and stirred again for 8 min at 30 ˚C. Finally, the extract and EO were added to the suppository suspension and it was stirred for another 8 min.7
Randomly selected fish samples were immersed in prepared solutions for 2 min and dried. Finally, fish specimens were stored in a refrigerator after packaging in plastic bags (4.00 ± 0.50 ˚C). Fish samples were prepared in 6 treatments as follows: 1) uncoated fish without vacuum (C); 2) uncoated fish and vacuum (CV); 3) fish covered with zein and no vacuum (ZV); 4) zein coating with 2.00% extract (g extract per g zein solution; ZEX); 5) zein coating with 1.00% EO (g EO per g zein solution; ZEO) and 6) treated samples with zein coating containing 1.00% EO and 1.00% extract (ZEXO). Samples were evaluated for chemical, microbial and sensorial quality parameters after 0, 3, 6, 9 and 12 days.
Proximate composition. Proximate composition was done on fish muscle. For moisture determination, the drying method was used in an oven (D-645; Heraeus Hanau, Germany) at 103 ˚C.15 In order to determine the ash, an electric furnace (BS OV-160; Gallenkamb, London, UK) was used at a temperature of 500-550 ˚C.15 Fat measurements were performed using Soxhlet method and n-hexane (Merck Millipore) solvent.15 Total protein measurements were also performed by Kjeldahl's method.15 The nitrogen content was multiplied by 6.25 and considered as the protein content.15
pH determination. For this purpose, 10 g of sample was homogenized in 100 mL distilled water and pH of the sample was measured using a pH meter (E520, Metrohm, Herisau, Switzerland).1
Measurement of total volatile basic nitrogen (TVB-N). Total volatile basic nitrogen was determined according to a modified micro-Kjeldahl distillation technique. Ten gram of fish sample was homogeneously mixed with 300 mL distilled water and 2 g of MgO (Merck Millipore). First, the distillation was carried out in a flask containing boric acid (2.00%; Merck Millipore) and a few drops of methyl red as an indicator. Finally, the contents of the flask were titrated with a 0.01 M sulfuric acid solution (Merck Millipore). The amount of TVB-N was according to the consumed amount of sulfuric acid and expressed as mg of nitrogen per 100 g of fish.16
Determination of thiobarbituric acid reactive substances (TBARS). The TBARS test was performed according to Wrolstad et al. method.17 Briefly, 10 g of fish samples were homogenized with 1.00 mL butylated hydroxytoluene (0.10%; Merck Millipore) and 20 mL trichloroacetic acid (5.00%; Merck Millipore) at 13500 rpm for 1 min. Then, the solution was filtered with No. 42 filter (Whatman, Pleasanton, USA) and its volume was minimized with 50 mL of trichloroacetic acid (Merck Millipore). Five-mL samples were mixed with 5 mL of thiobarbituric acid (TBA) reagent (0.02M; Merck Millipore), vortexed and placed at 100 ˚C for 1 hr and the optical absorbance was measured at 532 nm with a spectrophotometer (S2100; Unico Dayton, USA,) after cooling. The TBARS values were expressed as mg malondialdehyde (MDA) per kg of fish.
Free fatty acids (FFAs) measurements. The amount of FFAs expressed as oleic acid percentages was determined by Egan et al. method.18 For this purpose, 0.50 g of fish oil was extracted and dissolved in 50 mL of solvent (a mixture of equal volume of 96% ethanol and ethyl ether; Merck Millipore). Then, the solution was titrated with 0.10 N. NaOH (Merck Millipore). The amount of FFAs was calculated according to the following equation:
FFA = (V×28.2×100)/(W×1000)
where, V is the volume of sodium hydroxide and W is sample fat content.
Determination of peroxide value (POV). This was carried out using international dairy federation (IDF) standard method (No. 74A).19 First, 0.20 g of the fat extracted from samples was mixed with 9.80 mL of chloroform-methanol (Merck Millipore; 7 to 3 ratio). Then, 0.05 mL of ammonium thiocyanate solution (Merck Millipore; 10 mM) was added and the sample was vortexed for 2-4 sec. After that, 0.05 mL of iron chloride solution II (Merck Millipore) was added to the sample. The mixture was then kept at room temperature for 5 min and absorbance was read at 500 nm using the spectro-photometer (Unico). After drawing the standard curve and using the following formula for calculation, POV was expressed as mg of peroxide per kg of fat:20
Peroxide value = (As – Ab) × m / 55.84 × m 0 × 2
where, As is absorbance of the sample, Ab is absorbance of the blank, m is the slope obtained from the calibration curve, m0 is mass of the sample (g) and 55.84 is the atomic weight of iron.
Microbiological analyses. To conduct microbial tests, 10 g of fish samples (from each treatment) were sampled with scalpel in sterile conditions, transferred to a 90-millimeter pepton water (Merck Millipore) 0.10% and homogenized in a stomacher (Seward, Sussex, UK) at 250 rpm for 2 min. 21 Then, the next dilutions were prepared in pipettes containing 0.10 percent peptone water. For microbial enumeration, different dilutions of the samples were prepared and then 100 microlitres of each dilution were spread on the desired agar medium. Counting of mesophilic bacteria and enterobacteriaceae was performed using plate count agar (Merck Millipore) and viollet red bile agar (Merck Millipore), respectively. After inoculation, the plates were incubated at 37 ˚C for 48 hr. 22 , 23 King agar and citramide agar (Merck Millipore) culture media were used for enumeration of total psychrotrophic bacteria (TPC) and Pseudomonas bacteria, respectively. Then, plates were incubated at 21 ˚C for 48 hr for psychrotrophic bacteria count and at 37 ˚C for 48 hr for pseudomonas bacteria count. 24
Sensory evaluation. In order to perform sensory evaluation, treatments in 6 groups were examined by a ten-member trained panel in terms of overall acceptability inclusive texture, odor and color. In order to score from 0 to 10, 0 was considered as the lowest and 10 as the highest score and the product with a score of less than 6 was defined as an unacceptable product. 25
Statistical analyses. Statistical analysis of the observations was performed using IBM SPSS Software (version 20.0, IBM SPSS Inc, Armonk, USA). One-way ANOVA was used to analyze the data and to determine if there was a significant difference between treatments (p ≤ 0.05). Then, Duncan test was used to group the treatments based on the statistical difference among them.
Results
Proximate composition and chemical analyses results. The average amounts of fish composition including protein, fat, moisture and ash in raw trout were 22.08%, 2.80%, 71.80% and 1.65%, respectively.
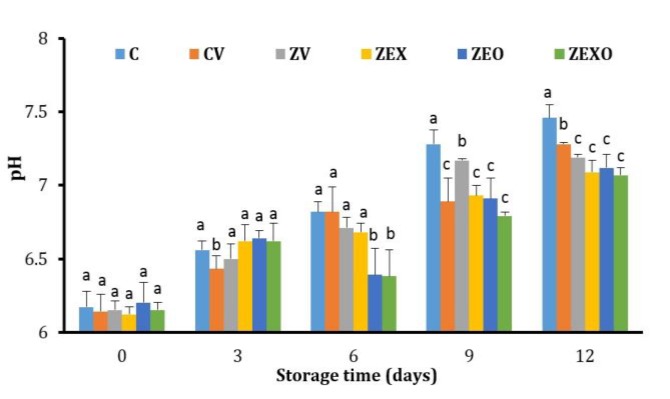
Figure 1 shows changes in rainbow trout pH during storage at 4 ˚C. An increase in the pH of all samples was observed and the highest pH increase was in the control sample. The pH in zein-coated samples containing EO or extract and the combination of them was lower than control on day 9 (7.28-6.93-6.91-6.79) and day 12 (7.46-7.09-7.12-7.07), respectively.
Fig. 1.
pH values of rainbow trout fillets during refrigerated storage C: Without coating and vacuum control; CV: Without coating and vacuumed control; ZV: Zein coating with vacuum; ZEX: Zein coating with 2.00% extract and vacuumed; ZEO: Zein coating with 1.00% essential oil (EO) and vacuumed; ZEXO: Zein coating with 1.00% EO and 1.00% extract and vacuumed.
abc Different letters indicate significant differences among treatments (p < 0.05).
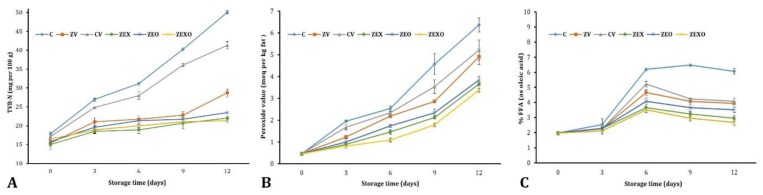
Figure 2A shows the TVB-N values for different treatments during storage. The initial values of TVB-N were from 15.05 to 17.78 mg N per100 g of muscle. The highest amount of TVB-N on the last day for the control sample and the vacuum sample was 50.05 and 41.30 mg N/100 g of muscle, respectively. The amount of TVB-N in the control and vacuum samples as well as coated samples during storage was increased significantly (p ≤ 0.05).
Fig. 2.
A) Changes in total volatile basic nitrogen (TVB-N) of rainbow trout fillets during refrigerated storage; B) Peroxide value (meq kg-1 sample) of trout fillets during refrigerated storage; C) Changes in free fatty acids (FFA) values of fish samples during refrigerated storage. C: Without coating and vacuum control; CV: Without coating and vacuumed control; ZV: Zein coating with vacuum; ZEX: Zein coating with 2.00% extract and vacuumed; ZEO: Zein coating with 1.00% essential oil (EO) and vacuumed; ZEXO: Zein coating with 1.00% EO and 1.00% extract and vacuumed
The effect of coating on fish oil POV changes is shown in Figure 2B. The initial amount of peroxide was about 0.46 milliequivalent per kg of fat.
According to the results, the amount of peroxide in different treatments was increased until the 12th day of storage. Storage life had a significant effect on POV in control and other treatments (p ≤ 0.05). At the end of storage time, there was a significant difference among control and other samples (p ≤ 0.05).
Figure 2C shows the amount of FFAs during storage. The FFAs production was increased from an initial level of 1.97 to a final value of 6.34. According to the results, FFAs in the control sample were significantly higher than other groups. The results showed that EO or extract and combination treatments had a significant difference with control group, vacuum and zein in most storage days. There was also a significant difference between the zein-containing EO and extracts.
According to the results presented in Table 1 indicating the changes in TBA, at the beginning of the experiment, the TBA of rainbow trout meat was about 0.02-0.03 in all treatments. According to the results, at the end of the storage period, the highest TBA was related to the control sample and the lowest TBA was observed in the fish containing EO and extract. Comparison between different treatments during storage showed that the increasing trend in the combined treatment was slower than others.
Table 1.
Changes in thiobarbituric acid values of fish fillet samples during refrigerated storage
| Treatments |
Storage time (days)
|
||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | |
| C | 0.02 ± 0.01Aa* | 0.30 ± 0.11Ba | 0.77 ± 0.05Ca | 0.84 ± 0.04Ca | 1.00 ± 0.08Da |
| CV | 0.03 ± 0.02Aa | 0.20 ± 0.02Ba | 0.33 ± 0.07Cb | 0.49 ± 0.05Cb | 0.71 ± 0.00Db |
| ZV | 0.02 ± 0.00Aa | 0.28 ± 0.08Ba | 0.37 ± 0.03Cb | 0.57 ± 0.09Db | 0.81 ± 0.06Eb |
| ZEX | 0.02 ± 0.01Aa | 0.13 ± 0.02Ba | 0.28 ± 0.02Cb | 0.41 ± 0.01Db | 0.51 ± 0.07Ec |
| ZEO | 0.03 ± 0.02Aa | 0.15 ± 0.02Ba | 0.31± 0.04Cb | 0.42 ± 0.05Cb | 0.55 ± 0.07Dc |
| ZEXO | 0.02 ± 0.00Aa | 0.11 ± 0.01Ba | 0.27 ± 0.02Cb | 0.35 ± 0.03Db | 0.46 ± 0.06Ec |
C: Without coating and vacuum control; CV: Without coating and vacuumed control; ZV: Zein coating with vacuum; ZEX: Zein coating with 2.00% extract and vacuumed; ZEO: Zein coating with 1.00% essential oil (EO) and vacuumed; ZEXO: Zein coating with 1.00% EO and 1.00% extract and vacuumed.
Different lower case letters in a column indicate significant differences among treatments and different upper case letters in a row indicate significant differences during storage time (days) in each treatment (p ≤ 0.05).
Microbiological analyses results. The changes in microbial count in trout samples during storage at 4 ˚C are shown in Table 2. The control group had the highest total psychrophilic bacteria counts (TPC), so that the total viable counts in the control group were significantly higher in all days than the other groups. On the last day of the storage, the amount of pseudomonads counts from the highest to the lowest was respectively as follows: control, CV, ZV, ZEX, ZEO and ZEXO. In zein coating treatments containing EO, extract and the combination, the microbial growth process was not completely regular. Although the inhibitory effect of the combined treatment was better than the other treatments until the 9th day, on the last day of the storage, the treatment of zein coating containing EO had a greater inhibitory effect.
Table 2.
Microbial changes of fish fillet during refrigerated storage
| Bacteria | Treatments |
Storage time (days)
|
||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | ||
| Mesophilic bacteria | C | 5.11 ± 0.15a* | 6.47 ± 0.26a | 7.20 ± 0.15a | 7.82 ± 0.12a | 9.50 ± 0.40a |
| CV | 5.29 ± 0.10a | 6.69 ± 0.30a | 7.04 ± 0.16a | 7.46 ± 0.15c | 8.60 ± 0.10b | |
| ZV | 4.75 ± 0.10b | 5.30 ± 0.21b | 5.47 ± 0.22b | 5.39 ± 0.11b | 6.01 ± 0.15c | |
| ZEX | 2.04 ± 0.22d | 2.30 ± 0.15d | 2.74 ± 0.13d | 3.52 ± 0.21e | 3.67 ± 0.05e | |
| ZEO | 2.56 ± 0.09c | 3.17 ± 0.14c | 4.06 ± 0.10c | 4.92 ± 0.09d | 2.17 ± 0.16f | |
| ZEXO | 1.95 ± 0.17d | 2.39 ± 0.22d | 2.60 ± 0.21d | 3.27 ± 0.32e | 5.46 ± 0.13d | |
| Pseudomonas spp. | C | 4.01 ± 0.11a | 5.18 ± 0.14a | 5.47 ± 0.13a | 6.01 ± 0.16a | 5.91 ± 0.25a |
| CV | 3.21 ± 0.20c | 3.53 ± 0.11b | 3.98 ± 0.09c | 5.55 ± 0.05c | 5.48 ± 0.18a | |
| ZV | 2.53 ± 0.12b | 3.14 ± 0.40b | 3.56 ± 0.22b | 3.94 ± 0.13b | 3.44 ± 0.12b | |
| ZEX | 1.42 ± 0.21d | 1.59 ± 0.21c | 2.61 ± 0.24d | 2.71 ± 0.14e | 1.74 ± 0.11d | |
| ZEO | 1.01 ± 0.14d | 1.22 ± 0.14c | 2.23 ± 0.08d | 2.30 ± 0.29d | 2.32 ± 0.24c | |
| ZEXO | 1.28 ± 0.13d | 1.46 ± 0.18c | 2.32 ± 0.26d | 2.19 ± 0.17d | 1.15 ± 0.12e | |
| Enterobacteriaceae | C | 3.19 ± 0.11a | 5.30 ± 0.09a | 6.83 ± 0.02a | 7.58 ± 0.07a | 7.47 ± 0.05a |
| CV | 3.09 ± 0.21a | 4.24 ± 0.06c | 6.43 ± 0.16c | 7.11 ± 0.25c | 3.09 ± 0.12c | |
| ZV | 2.05 ± 0.25b | 2.30 ± 0.14b | 2.17 ± 0.21b | 3.57 ± 0.24b | 2.68 ± 0.13b | |
| ZEX | 1.31 ± 0.14c | 1.92 ± 0.17d | 1.40 ± 0.06d | 2.26 ± 0.13e | 1.29 ± 0.15e | |
| ZEO | 1.11 ± 0.14c | 1.40 ± 0.29e | 1.21 ± 0.07d | 1.70 ± 0.18d | 2.10 ± 0.12d | |
| ZEXO | 1.35 ± 0.15c | 1.17 ± 0.09e | 1.56 ± 0.16d | 1.78 ± 0.17d | 2.36 ± 0.14d | |
| Psychrotrophic bacteria | C | 5.17 ± 0.18a | 7.06 ± 0.06a | 8.19 ± 0.12a | 8.72 ± 0.17a | 8.65 ± 0.26a |
| CV | 3.90 ± 0.30b | 5.30 ± 0.31c | 7.38 ± 0.06d | 8.05 ± 0.15c | 8.49 ± 0.19a | |
| ZV | 2.73 ± 0.17c | 3.84 ± 0.24b | 4.66 ± 0.23c | 5.29 ± 0.23b | 8.79 ± 0.14a | |
| ZEX | 3.05 ± 0.16d | 3.82 ± 0.14b | 4.04 ± 0.18e | 3.09 ± 0.19e | 4.78 ± 0.11c | |
| ZEO | 2.51 ± 0.06c | 3.93 ± 0.11b | 4.61 ± 0.06c | 4.07 ± 0.15d | 2.30 ± 0.18b | |
| ZEXO | 3.12 ± 0.21d | 4.83 ± 0.10d | 4.38 ± 0.21e | 3.69 ± 0.31e | 5.45 ± 0.16d | |
C: Without coating and vacuum control; CV: Without coating and vacuumed control; ZV: Zein coating with vacuum; ZEX: Zein coating with 2.00% extract and vacuumed; ZEO: Zein coating with 1.00% essential oil (EO) and vacuumed; ZEXO: Zein coating with 1.00% EO and 1.00% extract and vacuumed.
Different lower case letters in a column indicate significant differences among treatments (p ≤ 0.05).
In relation to the entero-bacteriaceae count results, similar to other microbial tests, the control treatment had significantly more bacteria than other treatments. There was a significant difference among treated treatments in the last day and ZEX, ZEO and ZEXO had significantly lower total viable counts than controls, vacuum and zein coating treatment. According to the psychrotrophic bacteria results, the control samples were reached to maximum limit before the day 6, while the amounts of these bacteria in the treated samples were below the proposed limit at the end of the storage period being significantly lower than the control sample. Comparison of psychrotrophic bacteria levels among control and other treatments showed that the control sample had more bacteria in all studied periods.
Sensory evaluation results. The results are presented in Table 3. There was no significant difference among the treatments in the first 3 days of storage. However, a significant difference was observed after 6 days of storage; the control sample had lower score than the other treatments at this time. The average score of the three parameters examined in the sensory evaluation showed fish general acceptance reduction during the storage time. Score 6 was considered as the lowest score regarding consumption and few treatments were considered beyond the scope of admission.
Table 3.
Changes in sensory attributes (average of texture, color and odor) of fish fillet during refrigerated storage
| Treatments |
Storage time (days)
|
||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | |
| C | 9.14 ± 0.32a | 8.21 ± 0.45a | 4.32 ± 0.40a | 2.78 ± 0.62a | 1.60 ± 0.86a |
| CV | 9.17 ± 0.24a | 8.53 ± 0.32a | 6.14 ± 0.20b | 3.67 ± 0.93a | 2.17 ± 0.88a |
| ZV | 9.42 ± 0.32a | 8.57 ± 0.37a | 7.10 ± 0.65c | 4.56 ± 0.51a | 3.12 ± 0.50a |
| ZEX | 8.78 ± 0.87a | 8.67 ± 0.90a | 7.71 ± 0.80c | 7.03 ± 0.83b | 5.25 ± 0.84b |
| ZEO | 9.46 ± 0.63a | 9.10 ± 0.83a | 7.92 ± 0.89c | 6.75 ± 0.87b | 5.78 ± 0.91b |
| ZEXO | 9.28 ± 0.65a | 8.85 ± 0.89a | 8.10 ± 0.78c | 7.57 ± 0.92b | 5.60 ± 0.95b |
C: Without coating and vacuum control; CV: Without coating and vacuumed control; ZV: Zein coating with vacuum; ZEX: Zein coating with 2.00% extract and vacuumed; ZEO: Zein coating with 1.00% essential oil (EO) and vacuumed; ZEXO: Zein coating with 1.00% EO and 1.00% extract and vacuumed.
Different lower case letters in a column indicate significant differences among treatments (p ≤ 0.05).
Discussion
Differences in the approximate amount of rainbow trout compounds, especially fat, have been reported in several studies.2,26 Several factors such as nutrition, fishing season, gender differences, fish size and area as well as other environmental conditions are factors influencing the differences in the chemical composition of fish. 1 Following the effects of the above-mentioned factors on fish compounds, changes may be created in sensory properties such as taste, smell, color, texture and fish appearance, which may ultimately affect the microbial growth and final fish acceptance.1,27
One of the most varied factors during the fish storage period is pH, which can be considered as an indicator of the fish freshness being low in its early storage, but it has been shown to increase in fish muscle during storage due to the development of nitrogen compounds such as ammonia, trimethylamine or other nitrogen compounds through the act of internal or microbial enzymes. 28 After death, the pH changes in the range of 5.40 to 7.20 depending on the fish species. 26 The difference among the control sample and other samples on day 9 and day 12 was significant. On the other hand, the difference among these treatments was not significant throughout the storage period. In relation with pH, it has been reported that the increasing trend of pH is inhibited in trout coated with chitosan-containing thyme EO. 28 Also, the use of clove EO has been able to keep the cod fish fillet pH below 7.00. 8
The TVB-N in fish consists mainly of trimethylamine, dimethyl, ammonia and other volatile nitrogen compounds. 1 It is a parameter measuring the amount of compounds composed of ammonia and primary, secondary and tertiary amines widely used as indicators of muscle tissue damage. 29 This parameter indicates the spoilage level in seafood. The fish has a large number of gastrointestinal bacteria and strong digestive enzymes produced during the feeding period resulting in rapid post-mortem autolysis during the storage; this may lead to a strong flavor, which is often related to the protein breakup and nitrogen-containing volatiles production.30
The amount of TVB-N is generally between 5 and 20 mg N per 100 g of muscle in fresh fish. 3 The initial values of TVB-N were consistent with the results of previous studies.31,32 An increase in this index during storage in the refrigerator temperature may also be due to the deamylation of amino acids. 33 At the end of the storage time, the zein coating containing EO and extract and combination of them was significantly different from other treatments, but this difference was not seen between treatments containing EO or extract. In a previous study on the effect of grape seed enriched with oregano and thyme EO on the survival time of salmon at refrigerator temperatures, the treatments significantly reduced the volatile nitrogen bases production at the time of storage. 34 The amount of 35 mg N per 100 g is considered as the highest acceptable level for the fresh fish by the European Commission. It has been suggested that the amount of 25 mg nitrogen per 100 g would be the beginning of corruption for the rainbow trout fillet. 35 In the present study, the level did not exceed in the treatments containing EO, extract and the combination of them. In our study, the TVB-N for coated treatments was less than acceptable level (35 mg N per 100 g), while it was unacceptable for control and vacuum treatment from day 6.
Fatty fish are more susceptible to lipid oxidation due to unsaturated fatty acids (USFAs). Lipid oxidation is a major cause of fish rancidity and flavor deterioration. In this regard, the POV is used to determine hydro peroxides as the primary products of fat oxidation in fish.1,25 The acceptable proposed level is 10-20 mg peroxide per kg body fat. 35 The POV was increased during storage for all treatments. This increase was more intense in the control treatment, which is consistent with other researchers’ findings in this regard.1,36,37 The EO and extract coatings have the ability to reduce the amount of fish oxidation, which could be due to the antioxidant properties of the extract and EO and their antimicrobial effects on bacterial enzymatic reactions related to fish fat oxidation. In addition, the amount of peroxide in combination treatment was significantly less than EO and extracts treatment. It has been reported that the amount of peroxide in the fish covered with nisin and rosemary extract is significantly lower than the control treatment. These findings are in agreement with the current research results. 37
Free fatty acids are produced during storage due to the enzymatic hydrolysis of lipid esters. After further oxidative changes, FFAs turn into compounds with a low molecular weight having an unpleasant smell and taste in fish. In addition, FFAs are smaller than triglyceride and phospholipid and their oxidation rate is higher. Additionally, FFAs have a great influence on protein denaturation and tissue degradation through the interaction with proteins.38,39 Figure 2C shows the amount of FFAs during storage with a gradual increase in the FFAs formation in all samples due to the phospholipids and triglycerides hydrolysis by lipases and phospholipases. 40 In other studies including the study of Özogul et al., the amount of FFAs increased during storage. 3 Rezaei et al. have reported an increase in FFAs in rainbow trout during the 20 days of storage. 23 Although the FFAs formation alone does not completely reduce nutritional quality, their evaluation is important to investigate fish corruption due to the effect of FFAs lipid peroxidation.
Another indicator widely used to examine the degree of lipids oxidation is TBARS using to measure MDA. The MDA is formed by hydroperoxides, which are the primary product of the reaction of USFAs with oxygen. 41 The TBA content of the samples gradually increased during storage. The increase in this index during storage may be due to a partial dehydration of the fish and an increase in the USFAs oxidation. 42 There was no significant difference among groups until the third day and significant changes in control treatment and other treatments started from day 6. It has been shown that the antioxidant activity of EO and extracts is related to the antioxidant activity of phenolic compounds through mechanisms such as radical chains formation prevention, attaching to the intermediate metal ion catalysts, peroxides decomposition and reacting with free radicals. 43 The antioxidant activity of Pimpinella affinis also relates to the high levels of phenolic compounds. The range of 1 to 2 mg MDA per kg of fat is considered as acceptable levels of TBA in fish.27 In this study, except for the control, other treatments did not reach this range during the period. It has been shown that nisin and rosemary extract treatments significantly lead to lower TBA level compared to control treatment. 38
Microorganisms are the main reasons of food spoilage. As regards, it is accepted that the initial microbial load of freshwater fish depends on the water and temperature conditions. Several reports have indicated the initial count of total viable counts for different species of freshwater fish (including rainbow trout and silver perch) within the range of 102 to 106 log CFU g-1. 45 There are different reports about the time to reach the acceptable level of fish microbial load. It has been reported that the total bacterial load of salmon fillet reaches 7 log CFU g-1 during the four days of storage in the refrigerator. 30 It has also been reported that the total microbial count in the salmon fillet reaches more than 7 logs after 12 days. 2 In the former report, the TPC for trout fillets were more than 7 logs on the 7th day.33 In the present study, the total viable counts in the control sample were 7 logs after 6 days of storage, which is in agreement with previous findings.33,38
The most important fish spoilage microorganisms are Gram-negative bacteria, which are initially pseudomonas species that anaerobic condition inhibits their growth. However, Gram-negative bacteria such as Photobacteriumn phosphoreum are known to be responsible for fish spoilage in anaerobic conditions. 45 Pseudomonads count was higher in the control group than other groups. As is known, the levels of Pseudomonas bacteria in the samples treated with EO or extract were significantly lower than other treatments and the inhibitory effects of EO and extracts on bacteria were visible. This was probably due to the combined effects of EO and extract. The results of this study are similar to the results of researches investigating the effect of EO and plant extracts on fish meat spoilage.32,37,46 Difference was not significant among ZEX, ZEO and ZEXO treatments until day 9, but in the final days of the storage, combined treatment had greater inhibitory effect.
Enterobacteriaceae were also another group of bacteria involved in rainbow trout spoilage during refrigerated storage. The results showed that storage time has been effective in the bacterial population, therefore, the amount of bacteria in the treatments increased compared to the day 0, but this increase was irregular. In a previous study on the combined effect of salting, oregano EO and vacuum packaging on the storage time of salmon stored in the refrigerator, bacteria were reduced in salt-based treatments and vacuum-packaging containing oregano EO. 32
Psychrotrophic bacteria as Gram-negative bacteria are the most important group of aerobic microorganisms responsible for spoilage of fresh fish stored in cold temperatures.1 Psychrotrophic bacteria are so important because they are the major factors causing changes in odor, texture and flavor as a result of various metabolic compounds such as ketones, aldehydes and amines biogenic volatile sulfides. 25 The maximum limit proposed for psychrotrophic bacteria is seven logs in rainbow trout. Also, by comparing the results, it can be concluded that in treatment with zein coating with EO, the psychrotrophic bacteria had a slower growth than other treatments. At the end of the storage period, the zein coating containing EO had the least amount of bacteria. This is due to the antimicrobial effects of the EO. The major antimicrobial compounds of plants are phenolic compounds having phenolic groups.
These compounds apply their antimicrobial activity as follows: first, they disrupt cell membrane phospholipid bilayer leading to cells permeability increase and some cellular components loss. Second, they cause cells enzymatic system destruction, where enzymes play roles in energy production and cells structural compounds synthesis. Third, these antimicrobial compounds cause cells genetic material destruction. 47
Sensory evaluation is a simple method that can provide information about the fish freshness and quality in a short term. The sensory properties of the fish are clearly visible to the customer and play an important role in obtaining customer consent. 1 The results of this study are consistent with the results of Mexis et al., and Ojagh et al.2, 48 In general, during this period, the control group received less privilege than other treatments. Meanwhile, treatments covered with zein containing EO and extracts of P. affinis were accepted by panelists until the 12th day and this confirms the results of microbial and chemical factors, because in all chemical and microbiological experiments, the EO and extract treatments quality was significantly less decreased compared to the control group. Coating of fish fillets using biopolymer and incorporation active plant intercepts quality loss signs including off-flavor and odor developments and sensory defects.
This study showed that the use of biodegradable zein coatings containing EO and extracts of P. affinis had a significant inhibitory effect on microbial growth and decreased undesirable changes of sensory and chemical qualities. It maintained the sensory properties of fish and extended the shelf life of fish in at least 6 days compared to control group. Further studies are necessary and useful to evaluate the antioxidant and antimicrobial activity of EO and plant extracts in fish and marine products. These compounds can be a good alternative to synthetic materials to preserve fish meat.
Acknowledgements
This study was funded by the Faculty of Veterinary Medicine, University of Urmia, Urmia, Iran.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Sallam KI. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food control. 2007;18(5):566–575. doi: 10.1016/j.foodcont.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojagh SM, Rezaei M, Razavi SH, et al. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120(1):193–198. [Google Scholar]
- 3.Özogul Y, Özogul F, Kuley E, et al. Biochemical, sensory and microbiological attributes of wild turbot (Scophthalmus maximus) from the Black Sea, during chilled storage. Food Chem. 2006;99(4):752–758. [Google Scholar]
- 4.Quintavalla S, Vicini L. Antimicrobial food packaging in meat industry. Meat Sci. 2002;62(3):373–380. doi: 10.1016/s0309-1740(02)00121-3. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Estaca J, De Lacey AL, Lopez-Caballero M, et al. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27(7):889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Shukla R, Cheryan M. Zein: The industrial protein from corn. Ind Crops Prod. 2001;13(3):171–192. [Google Scholar]
- 7.Kashiri M, Cerisuelo JP, Dominguez I, et al. Zein films and coatings as carriers and release systems of Zataria multiflora Boiss essential oil for antimicrobial food packaging. Food Hydrocoll. 2017;70:260–268. [Google Scholar]
- 8.Gomez-Estaca J, Montero P, Gimenez B, et al. Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus) Food Chem. 2007;105(2):511–520. [Google Scholar]
- 9.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Gulcın İ, Oktay M, Kıreccı E, et al. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L) seed extracts. Food Chem. 2003;83(3):371–382. [Google Scholar]
- 11.Singh N, Singh R, Bhunia A, et al. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157: H7 on lettuce and baby carrots. LWT-Food Sci Technol. 2002;35(8):720–729. [Google Scholar]
- 12.Pol IE, Krommer J, Smid EJ. Bioenergetic consequences of nisin combined with carvacrol towards Bacillus cereus. Innov Food Sci Emerg Technol. 2002;3(1):55–61. [Google Scholar]
- 13.Sawant S, Sawant D, Shrangdher S, et al. Effect of vacuum packaging on shelf life of frozen shrimp. CIBTech J Biotechnol. 2012;1(1):27–35. [Google Scholar]
- 14.Barros L, Heleno SA, Carvalho AM, et al. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill from Portugal. Food Chem Toxicol. 2009;47(10):2458–2464. doi: 10.1016/j.fct.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.AOAC International. Official Methods of Analysis of AOAC International. 16th ed. Virginia, USA: Association of official analytical chemists; 1995. [Google Scholar]
- 16.Jeon YJ, Kamil JY, Shahidi F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J Agric Food Chem. 2002;50(18):5167–5178. doi: 10.1021/jf011693l. [DOI] [PubMed] [Google Scholar]
- 17.Wrolstad RE, Acree TE, Decker EA, et al. Handbook of food analytical chemistry, vol 1 Water proteins enzymes lipids and carbohydrates. 1st ed. Indianapolis, USA: John Wiley & Sons; 2005. pp. 525–527. [Google Scholar]
- 18.Egan H, Kirk R, Sawyer R. Pearson's chemical analysis of food. 9th ed. Edinburgh, UK: Churchill Livingstone; 1997. pp. 609–634. [Google Scholar]
- 19.International IDF standards. International dairy federation, IDF-Square, Vergote 41. Brussels, Belgium: IDF; 1991. section 74A. [Google Scholar]
- 20.Shantha NC, Decker EA. Rapid, sensitive, iron-based spectrophotometric methods for determination of perorlride values of food lipids. J AOAC Int. 1994;77(2):421–424. [PubMed] [Google Scholar]
- 21.Erkan N. The effect of thyme and garlic oil on the preservation of vacuum-packaged hot smoked rainbow trout (Oncorhynchus mykiss) Food Bioprocess Tech. 2012;5(4):1246–1254. [Google Scholar]
- 22.Can OP. Combine effect of salting and thyme (Thymus vulgaris) essential oil on shelf life of rainbow trout (Oncorhynchus mykiss) stored at 4 ˚C. Bull Vet Inst Pulawy. 2011;55:435–441. [Google Scholar]
- 23.Rezaei M, Jafari H, Sahari MA, et al. Relation of biogenic amines and bacterial changes in ice‐stored southern Caspian kutum (Rutilus frisii kutum) J Food Biochem. 2007;31(4):541–550. [Google Scholar]
- 24.Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005;93(3):511–520. [Google Scholar]
- 25.Özogul F, Kus B, Kuley E. The impact of strawflower and mistletoe extract on quality properties of rainbow trout fillets. Int J Food Sci Technol. 2013;48(11):2228–2238. [Google Scholar]
- 26.Gonzalez-Fandos E, Villarino-Rodrıguez A, García-Linares M, et al. Microbiological safety and sensory characteristics of salmon slices processed by the sous vide method. Food Control. 2005;16(1):77–85. [Google Scholar]
- 27.Lu F, Ding Y, Ye X, et al. Cinnamon and nisin in alginate–calcium coating maintain quality of fresh northern snakehead fish fillets. LWT-Food Sci Technol. 2010;43(9):1331–1335. [Google Scholar]
- 28.Chamanara V, Shabanpour B, Gorgin S, et al. An investigation on characteristics of rainbow trout coated using chitosan assisted with thyme essential oil. Int J Biol Macromol. 2012;50(3):540–544. doi: 10.1016/j.ijbiomac.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Fan W, Sun J, Chen Y, et al. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009;115(1):66–70. [Google Scholar]
- 30.Souza BW, Cerqueira MA, Ruiz HA, et al. Effect of chitosan-based coatings on the shelf life of salmon (Salmo salar) J Agric Food Chem. 2010;58(21):11456–11462. doi: 10.1021/jf102366k. [DOI] [PubMed] [Google Scholar]
- 31.Nowzari F, Shabanpour B, Ojagh SM. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013;141(3):1667–1672. doi: 10.1016/j.foodchem.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Frangos L, Pyrgotou N, Giatrakou V, et al. Combined effects of salting, oregano oil and vacuum-packaging on the shelf-life of refrigerated trout fillets. Food Microbiol. 2010;27(1):115–121. doi: 10.1016/j.fm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Fan W, Chi Y, Zhang S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypo-phthalmicthys molitrix) during storage in ice. Food Chem. 2008;108(1):148–153. [Google Scholar]
- 34.Jouki M, Yazdi FT, Mortazavi SA, et al. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int J Food Microbiol. 2014;174:88–97. doi: 10.1016/j.ijfoodmicro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Gimenez B, Roncales P, Beltran JA. Modified atmosphere packaging of filleted rainbow trout. J Sci Food Agric. 2002;82(10):1154–1159. [Google Scholar]
- 36.FAO. Training for fish quality improvement: training needs analysis. [Accessed May 22, 2019]. Available at: http://www.fao.org/3/a-az380e.pdf.
- 37.Pezeshk S, Rezaei M, Hosseini H. Effects of turmeric, shallot extracts, and their combination on quality characteristics of vacuum‐packaged rainbow trout stored at 4 ± 1 ˚C. J Food Sci. 2011;76(6):M387–389. doi: 10.1111/j.1750-3841.2011.02242.x. [DOI] [PubMed] [Google Scholar]
- 38.Gao M, Feng L, Jiang T, et al. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control. 2014;37:1–8. [Google Scholar]
- 39.Özyurt G, Kuley E, Özkütük S, et al. Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food Chem. 2009;114(2):505–510. [Google Scholar]
- 40.Refsgaard HH, Brockhoff PM, Jensen B. Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. J Agric Food Chem. 2000;48(8):3280–3285. doi: 10.1021/jf000021c. [DOI] [PubMed] [Google Scholar]
- 41.Rostamzad H, Shabanpour B, Kashaninejad M, et al. Inhibitory impacts of natural antioxidants (ascorbic and citric acid) and vacuum packaging on lipid oxidation in frozen Persian sturgeon fillets. Iran J Fish Sci. 2010;9(2):279–292. [Google Scholar]
- 42.Goulas AE, Kontominas MG. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chem. 2007;100(1):287–296. [Google Scholar]
- 43.Kilincceker O, Dogan IS, Kucukoner E. Effect of edible coatings on the quality of frozen fish fillets. LWT-Food Sci Technol. 2009;42(4):868–873. [Google Scholar]
- 44.Perumalla A, Hettiarachchy NS. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res Int. 2011;44(4):827–839. [Google Scholar]
- 45.Gram L, Huss HH. Microbiological spoilage of fish and fish products. Int J Food Microbiol, 1996;33(1):121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- 46.Gelman A, Glatman L, Drabkin V, et al. Effects of storage temperature and preservative treatment on shelf life of the pond-raised freshwater fish, silver perch (Bidyanus bidyanus) J Food Prot. 2001;64(10):1584–1591. doi: 10.4315/0362-028x-64.10.1584. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Marshall MR, Wei CI. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 1995;43(11):2839–2845. [Google Scholar]
- 48.Mexis S, Chouliara E, Kontominas M. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 ˚C. Food Microbiol. 2009;26(6):598–605. doi: 10.1016/j.fm.2009.04.002. [DOI] [PubMed] [Google Scholar]