Abstract
Duck beak atrophy and dwarfism syndrome (BADS) is a newly emerged disease in China since 2015. In October 2017, an unidentified disease occurred in Cherry Valley ducks, Chongqing municipality, the southwest of China. The affected birds showed short beak and growth retardation clinical signs. The disease caused approximately 20.00% morbidity and serious weight loss due to retarded growth. In order to identify the causative agent of BADS, liver, spleen, lung and heart samples were collected for virus isolation, hemagglutination test, PCR identification, and partial gene sequencing. The isolated virus was tentatively named SC16. Hemagglutination test indicated that the virus was negative to chicken red blood cells. Based on the PCR and sequencing results, the causative agent of BADS was a novel duck-origin goose parvovirus (DGPV) while no another co-infection pathogen was found in this case. Further analysis could provide insights into the control strategies of DGPV in ducks.
Key Words: Beak atrophy and dwarfism syndrome, China, Duck-origin goose parvovirus
Introduction
Duck beak atrophy and dwarfism syndrome (BADS), characterized by dysplasia of the beak and tongue, is a newly emerged disease in China since 2015.1
Due to the striking loss of weight and size caused by BADS, the disease causes significant economic loss to the Chinese duck industry. Based on virus isolation and experimental animal infection, a novel goose parvovirus (GPV) was identified as the causative agent of the BADS in ducklings.2 GPV is a member of the Dependovirus genus, Parvoviridae family, and is one of the causative agents of Derzsy’s disease of goslings and Muscovy ducklings.3 However, the recent occurrence of BADS in Cherry Valley ducks was proved to be caused by a new GPV-related virus, called duck-origin GPV (DGPV) or novel GPV-related virus (NGPV).2 In China, DGPV strains isolated from Cherry Valley duck and mule duck with BADS shared 90.80% to 94.60% nucleotide sequence identity with the classical GPV isolates indicated that it was a derivative strain of GPV.4
The outbreak of BADS in China was very quick and wide. In the last two years, cases of BADS were found in some mainly duck-producing provinces including Fujian, Jiangsu, Shandong, Anhui, Zhejiang, and Guangdong.3,5 Chongqing municipality, located in the southwest of China, is also a major duck-producing area where BADS has never been reported. Recently, BADS was found in a duck farm in Dazu district of Chongqing. This report describes the diagnosis and characterization of BADS identified in Chongqing, China.
Case Description
In October 2017, an outbreak of BADS in Cherry Valley ducks which characterized by the short beak and strong growth retardation (Fig. 1 A and B), occurred in a duck farm in Dazu (Chongqing municipality), southwestern China. The disease caused approximately 20.00% morbidity and serious weight loss caused by retarded growth. In order to characterize the causative agent of the disease and the evolutionary relationships between the identified agent and currently known avian parvoviruses, a phylogenetic tree was constructed. Liver, spleen, lung and heart samples were collected from diseased ducks and each tissue sample was separately homogenized in phosphate-buffered saline (PBS; Sangon Biotech, Shanghai, China) to make 10% (w/v) suspension. After freezing and thawing the samples for three times, the suspensions were clarified by centrifugation at 12,000 g for 10 min at 4 ˚C and then filtered through 0.22 μm filter (Sangon Biotech, Shanghai, China). The filtered suspensions were used for virus isolation and identification.
Fig.1.
A and B) Cherry Valley ducks with typical signs of BADS (short beak with protruding and drooping tongue, red arrows) occurred in a duck farm in Dazu (Chongqing municipality), southwestern of China
Firstly, prepared filtered suspensions were inoculated into 9-day-old SPF embryonated duck eggs via the allantoic cavity. The results showed that the duck embryos died 84 to 144 hr after inoculation through sequential passage for three times. The infected allantoic fluids were collected, concentrated and purified by polyethylene glycol -6000 (Sangon Biotech) precipitation. Then, the purified samples were examined using a hemagglutination test according to Johnston et al.6 Results indicated that the isolated pathogen could not agglutinate chicken red blood cells.
To further identify the pathogen, total nucleic acid was isolated from the filtered suspensions using All Prep DNA/RNA mini kit (Qiagen, Shanghai, China) according to the manufacturers’ instructions. A pair of primers (F: 5’-ATGGCAGAGGGAGGAGGCGG-3’; R : 5’-TGTGCATTGTGCA ATACCCG-3’) based on the VP3 gene of GPV was designed and synthesized by Sangon Biotech Co., Ltd. The PCR reaction was carried out in 25.00 µL volume containing 2 × Premix Taq™ (Takara, Dalian, China), 0.15 mM each of forward and reverse primers and 1.50 µL of DNA extract. The reaction mixture was treated at 95 ˚C for 5 min, followed by 30 cycles of 94 ˚C for 1 min, 55 ˚C for 1min, 72 ˚C for 1min, followed by a final extension at 72 ˚C for 5 min. Simultaneously, PCR and RT-PCR-based methods were employed to exclude the presence of viral contaminant of the samples which including avian influenza virus (AIV), Newcastle disease virus (NDV), duck reovirus (DRV), duck hepatitis A virus (DHAV), duck tembusu virus (DTMUV), duck circovirus (DuCV), Muscovy duck parvovirus (MDRV), chicken embryo lethal orphan virus (CELOV) and reticuloendotheliosis virus (REV). The RT-PCR results indicated that only a GPV-specific band was detected. The target fragments were cloned into pMD-19T vector and GPV was confirmed by sequencing (Invitrogen, Shanghai, China). The results revealed that the causative agent of the disease was a DGPV-related virus and named SC16 isolate.
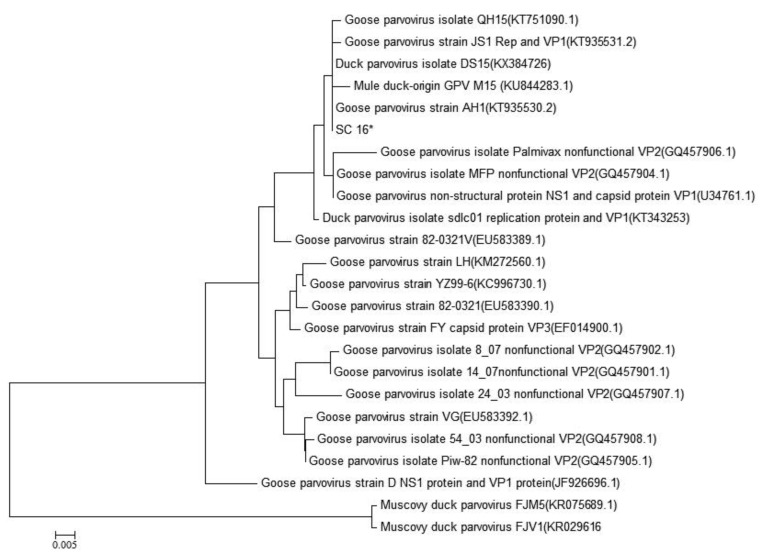
To investigate the genetic relationship between SC16 isolate and the known waterfowl parvoviruses, the VP3 protein sequences-based phylogenetic tree was generated using the neighbor-joining method based on a Tamura 3-parameter model and bootstrap analysis in MEGA 5.0 software (Biodesign Institute, Tempe, USA).7
The results clearly showed that SC16 isolate closely clustered with DGPVs (Fig. 2), confirming that it belonged to the genus parvovirus and was related to DGPV strains.
Fig. 2.
The generated phylogenetic tree of the GPV, DPV, MDPV and SC16 isolate based on partial sequences of the VP3 gene. Phylogenetic relationship was assessed using the neighbor-joining method based on a Tamura 3-parameter model and bootstrap analysis (1000 replicates) as implemented using MEGA5 software. The scale bar represents 0.005% nucleotide divergences. GenBank accession numbers of the sequences were indicated in brackets. The SC16 virus isolated from Cherry Valley ducks in this study (*) was closely related to Duck parvovirus (Cherry Valley duck, GenBank Accession No. KX384726), mull duck-origin GPV (GenBank Accession No.KU844283.1) and Peking duck-origin GPV (GenBank Accession No. KT751090.1, KT935530.2 and KT935531.2)
To examine the pathogenicity of the SC16 isolate, 105 ELD50 per 0.20 mL was inoculated into 9-day-old SPF embryonated duck eggs via the allantoic cavity. Subsequently, twenty 2-day-old ducks were selected and purchased from the Chongqing Medical University, Chongqing, and experimentally infected with the SC16 isolate by intramuscular inoculation with a dose of 5 × 105 ELD50 in 1.00 mL per bird. The main clinical signs in the infected ducks were observed on the tenth day post-infection (PI) and continue to exist during the whole experiment period (30 days). The clinical signs, including short beak with protruding and drooping tongue and strong growth retardation, were consistent with those displayed in naturally infected ducks and in line with reported findings by Xiao et al.5
Discussion
The BADS, also named duck short beak and dwarfism syndrome (SBDS) was first reported in the mule duck flocks in the early 1970s in south-western France.3 Similar case was found in Taiwan with higher morbidity and mortality than that reported in France during a co-infection of ducks with duck parvovirus and duck hepatitis virus in 1989.8 Since 2015, BADS was widely emerged in the eastern of China, especially in the Shandong, Anhui and Jiangsu provinces.3,9,10 Although Chongqing was one of the main breeding areas of ducks in the southwest of China, but there was not any report of BADS in this area. The reasons for such a low infection rate in this area is still unknown.
In a previous study, DuCV was considered as a co-infection pathogen of BADS disease.11 In another study it was found that 60.00% of the samples (102/170) were positive for DuCV (type 1 and 2) in the NGPV-infected ducks indicated that DuCV might play a great role in the BADS disease.9 However, in the present study, DuCV was not detected. Meanwhile, the examined ducks were also negative for the other eight pathogens including AIV, NDV, DRV, DHAV, DTMUV, MDRV, CELOV and REV. Therefore, only DGPV was found in the current BADS outbreak, and the possibility of the co-infection with the other unknown pathogens requires further investigation.
As reported before, the capsid proteins VP1 and VP3 were adequate for molecular analysis to determine the genetic relatedness of different strains of GPV and MDPV.4,12 Therefore, the genetic relationship between SC16 isolate and the other known waterfowl parvoviruses was assessed based on the VP3 protein sequence in the present study. Although these findings could reveal that the causative agent is a novel DGPV, the whole genome sequence data are still needed to further characterize the newly identified virus.
Acknowledgments
This work was supported by Local Science and Technology Development Fund of Chongqing (2018), Basic Research and Frontier Exploration Project of Chongqing (cstc2018jcyjAX0264) and the Key Research Projects of Technology Innovation and Application Demonstration of Chongqing (cstc2018jszx-cyzd0026).
Conflict of interest
The authors declare that there was not any conflict of interest.
References
- 1.Chen H, Dou Y, Tang Y, et al. Isolation and genomic characterization of a duck-origin GPV-related parvovirus from Cherry Valley ducklings in China. PLoS One. 2015;10(10):272–284. doi: 10.1371/journal.pone.0140284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Tang Y, Dou Y, et al. Evidence for vertical transmission of novel duck-origin goose parvovirus-related parvovirus. Transbound Emerg Dis. 2016;63(3):243–247. doi: 10.1111/tbed.12487. [DOI] [PubMed] [Google Scholar]
- 3.Li PF, Zhang RH, Chen JH, et al. Development of a duplex semi-nested PCR assay for detection of classical goose parvovirus and novel goose parvovirus-related virus in sick or dead ducks with short beak and dwarfism syndrome. J Virol Methods. 2017;249:165–169. doi: 10.1016/j.jviromet.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Cheng XX, Chen SL, et al. Identification of a novel goose parvovirus (GPV) recombinant associated with short beak and warfism syndrome in Mainland China, 2015. Infect Genet Evol. 2016;41:289–291. doi: 10.1016/j.meegid.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Xiao S, Chen S, Cheng X, et al. The newly emerging duck-origin goose parvovirus in China exhibits a wide range of pathogenicity to main domesticated waterfowl. Vet Microbiol. 2017;203:252–256. doi: 10.1016/j.vetmic.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SL, Wellens K, Siegel C. Comparison of hemagglutination and hemadsorption tests for influenza detection. Diagn Microbiol Infect Dis. 1992;15(4):363–365. doi: 10.1016/0732-8893(92)90025-o. [DOI] [PubMed] [Google Scholar]
- 7.Tamura K, Peterson D, Peterson N, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu YS, Lin DF, Lee YL et al. Infectious bill atrophy syndrome caused by parvovirus in a co-outbreak with duck viral hepatitis in ducks in Taiwan. Avian Dis. 1993;37(2):591–596. [PubMed] [Google Scholar]
- 9.Yu K, Ma X, Sheng Z, et al. Identification of goose-origin parvovirus as a cause of newly emerging beak atrophy and dwarfism syndrome in ducklings. J Clin Microbiol. 2016;54(8):1999–2007. doi: 10.1128/JCM.03244-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Li Q, Chen Z, et al. Novel duck parvovirus identified in Cherry Valley ducks (Anas platyrhynchos domesticus), China. Infect Genet Evol. 2016;44:278–280. doi: 10.1016/j.meegid.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Li J, Zhang R et al. Duck “beak atrophy and dwarfism syndrome” disease complex: Interplay of novel goose parvovirus-related virus and duck circovirus? Transbound Emerg Dis. 2018;65(2):345–351. doi: 10.1111/tbed.12812. [DOI] [PubMed] [Google Scholar]
- 12.Ning K, Liang T, Wang MH, et al. Pathogenicity of a variant goose parvovirus, from short beak and dwarfism syndrome of Pekin ducks, in goose embryos and goslings. Avian Pathol. 2018;47(4):391–399. doi: 10.1080/03079457.2018.1459040. [DOI] [PubMed] [Google Scholar]