Abstract
Purpose:
To evaluate the association between pregnancy loss history and adverse pregnancy outcomes.
Methods:
Pregnancy history was captured during a computer-assisted personal interview for 21,277 women surveyed in the National Survey of Family Growth (1995–2013). History of pregnancy loss (<20 weeks) at first parity was categorized in three ways: number of losses, maximum gestational age of loss(es), and recency of last pregnancy loss. We estimated risk ratios for a composite measure of selected adverse pregnancy outcomes (preterm, stillbirth, or low birthweight) at first parity and in any future pregnancy, separately, using predicted margins from adjusted logistic regression models.
Results:
At first parity, compared with having no loss, having 3+ previous pregnancy losses (adjusted risk ratio (aRR) = 1.66 [95% CI = 1.13, 2.43]), a maximum gestational age of loss(es) at ≥10 weeks (aRR = 1.28 [1.04, 1.56]) or having experienced a loss 24+ months ago (aRR = 1.36 [1.10, 1.68]) were associated with increased risks of adverse pregnancy outcomes. For future pregnancies, only having a history of 3+ previous pregnancy losses at first parity was associated with increased risks (aRR = 1.97 [1.08, 3.60]).
Conclusion:
Number, gestational age, and recency of pregnancy loss at first parity were associated with adverse pregnancy outcomes in U.S. women.
Keywords: Pregnancy loss, Preterm birth, Low birthweight, Miscarriage
Introduction
Although pregnancy loss is a common occurrence [1,2], questions regarding its etiology and association with other reproductive outcomes remain unanswered. While associations between recurrent pregnancy loss and preterm birth in the subsequent pregnancy have been recognized for decades [3–5], the associations between nonrecurrent loss, gestational age of loss, and recency of loss and the risks of other adverse pregnancy outcomes are less clear. In part, this may be due to choice of comparison group; for example, comparing women with only pregnancy loss to women with a history of live birth may lead to inflated risk estimates associated with pregnancy loss [5–8].
Only a handful of studies have examined history of nonrecurrent pregnancy loss on risk of adverse pregnancy outcomes at subsequent pregnancy among primiparous women [7–15]. These studies have been limited by a small number of study participants [11], restriction to pregnancy losses requiring a hospital visit [8,12], or by sparse reproductive history information, including lack of data on gestational age of loss, how long ago the loss occurred, and history of induced abortions [7,9,10,13–15]. In addition, prior studies have only considered the outcome of the pregnancy subsequent to the loss and not other future pregnancies the primiparous women will experience.
The objective of our study was to estimate the risks of preterm birth, stillbirth, low birthweight, and a composite outcome of any of the above conditions by pregnancy loss history at first parity among reproductive aged women in the United States.
Material and methods
Study population
The National Survey of Family Growth (NSFG) is a cross-sectional survey conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics [16]. We included data from female respondents from four survey periods: 1995, 2002, 2006–2010, and 2011–2013. Each survey period includes a multistage, probability-based, nationally representative sample of the household population aged 15–44 years. The National Center for Health Statistics Research Ethics Review Board approved each of these NSFG data collection efforts, and no specific additional review was required for this data analysis.
Pregnancy loss history
Female respondents provided a complete pregnancy history during a computer-assisted in-person interview. Pregnancy history included, for each pregnancy, the calendar month and year at end of pregnancy, the gestational length and the pregnancy outcome (e.g., miscarriage, stillbirth, abortion, ectopic pregnancy, live birth) [17]. We defined pregnancy loss as a self-reported “miscarriage” with a gestational length <20 weeks [18].
Three aspects of pregnancy loss were assessed in separate analyses: number of losses (no loss, 1, 2, 3, or more), gestational age of pregnancy loss(es) (all <6 weeks, longest 6–9 weeks, longest ≥10 weeks), and recency of the last pregnancy loss (interpregnancy interval <6 months, 6 to 11 months, 12 to 23 months, 24 months or more [9]). In addition, we defined pregnancy loss dichotomously (no loss, at least 1). Cutpoints for gestational age of pregnancy loss(es) were based on the assumed development stage of the conceptus given the time of pregnancy loss recognition, with losses before 6 weeks representing pre-embryologic losses, 6 to 9 weeks representing embryo losses, and ≥10 weeks representing fetal losses [18,19]. Losses before 6 weeks are alternatively defined as “early pregnancy loss” and can represent pre-clinical pregnancy losses [2,20].
Adverse pregnancy outcomes
For each completed pregnancy, the following adverse pregnancy outcomes were identified: preterm birth (live birth at <37 weeks’ gestation), stillbirth (self-reported “stillbirth” or pregnancy loss at 20 weeks’ gestation or greater), and low birthweight (live birth <2500 g). In addition, a composite measure, indicating if any of the above adverse pregnancy outcome occurred, was created.
Study participant characteristics
We examined the following participant characteristics using information collected during the NSFG interview: age at conception, height, marital status at the end of each pregnancy, Hispanic origin and race, intendedness of each pregnancy at conception, number of live births from each pregnancy, any use of medical help to become pregnant ever, ever smoked, highest educational attainment and family income as percentage of poverty level at the time of the interview. We defined a yes/no variable for any history of induced abortion at the beginning of each pregnancy.
Statistical analysis
Our analysis used the first pregnancy resulting in a live birth or stillbirth, hereafter referred to as “first parity”, as the time point for defining pregnancy loss history. We focused on first parity to minimize selection bias introduced by only including women who, through choice or fecundity, achieved at least two pregnancies proceeding past 20 weeks. First parity has also been increasingly preferred as the analytical cohort to study the association between pregnancy loss and subsequent pregnancy outcomes to control for confounding by prior live birth [7–15]. We examined (1) the pregnancy outcome of the first parity and (2) the collapsed pregnancy outcomes of all pregnancies after first parity (hereafter referred to as “future pregnancies”) reported at the time of interview.
Number of pregnancy losses at first parity was tabulated across participant characteristics (see supplemental tables 1 and 2 for tabulations of the two other aspects of pregnancy loss). Using χ2 tests, comparisons were made between women with no loss versus at least one loss and, for comparing aspects of pregnancy loss against each other, among only women with losses.
Risks of preterm birth, stillbirth, low birthweight, the composite measure, and mean gestational length of pregnancy were tabulated by pregnancy loss history at first parity. For the future pregnancies analysis, we restricted the data set to women who reported at least one additional pregnancy. We present the proportions of women with any future pregnancy, live birth, miscarriage, induced termination, and ectopic pregnancy in a supplemental table 3.
Risk ratios (RRs) and 95% confidence intervals (CIs) for the composite outcome measure at first parity and among any future pregnancy(ies) were estimated, separately, using predicted margins from logistic regression. Models were adjusted for factors associated with either preterm birth, stillbirth, or low birthweight [21–23] and included all the participant characteristics previously described except intendedness of pregnancy at conception and multiple live births at first parity, time-varying factors which could fall along the causal pathway from pregnancy loss to future pregnancy outcome. We assessed the significance of adding an interaction term for year of conception to evaluate if the relationship between pregnancy loss history and adverse pregnancy outcomes might be heterogeneous over time. A previous study using NSFG pregnancy data found the incidence of pregnancy loss appeared to be increasing by about 1.0% per year from 1970 to 2000 [24], which could indicate changes in the relationship between pregnancy loss and adverse pregnancy outcomes over time.
Analyses were conducted with SAS, 9.3 (SAS Institute, Cary, North Carolina), SUDAAN, 11.0 (RTI International, Research Triangle Park, North Carolina) or STATA, SE 13 (StataCorp LP, College Station, TX) and took into account the complex survey design.
Results
Study population
There were 36,370 women aged 15 to 44 years who participated in NSFG cycles 1995, 2002, 2006–2010, and 2011–2013; 23,835 (64%, standard error [SE] 0.5) women reported a total of 64,970 pregnancies, 21,277 of which were at first parity age 12 years and older. Reproductive history at the time of interview and at first parity was largely similar among survey periods (see supplemental table 4); however, the percent with at least one pregnancy loss at the time of first parity was higher in 2011–2013 compared with 1995 (13% vs. 10%, P < .01).
Pregnancy loss history
Among our study population, 88.8% (SE = 0.3) reported having no history of pregnancy loss at the time of first parity, 9.4% (SE = 0.3) reported one pregnancy loss, 1.3% (SE = 0.1) reported two pregnancy losses and 0.5% (SE = 0.1) reported three or more losses (Table 1). Women with at least one pregnancy loss were older than women with no loss (P < .001). After adjustment for maternal age at conception, having at least one loss was more common in white versus Hispanic women, married versus previously married women, intended versus mistimed and/or unwanted pregnancies, and among women who ever had any medical help to get pregnant (all P <.05). Pregnancy loss number was associated with gestational age of loss: a maximum gestational length of ≥10 weeks was more common among women with two losses and three or more losses than among those with one loss (P < .001). Number of pregnancy losses was not associated with recency of loss (P = .33).
Table 1.
Number of previous pregnancy losses among women at first parity, by participant and pregnancy loss characteristics, National Survey of Family Growth 1995 through 2011–2013
| Participant and pregnancy loss characteristics | n | No loss |
1 loss |
2 losses |
3 or more losses |
At least 1 loss |
P* | P† |
|---|---|---|---|---|---|---|---|---|
| n = 19,000 |
n = 1,917 |
n = 262 |
n = 98 |
n = 2,277 |
||||
| (88.8%) |
(9.4%) |
(1.3%) |
(0.5%) |
(11.2%) |
||||
| % (SE) | % (SE) | % (SE) | % (SE) | % (SE) | ||||
| All pregnancies at first parity | 21,277 | |||||||
| Age at first parity conception, years: mean ± SE | 21,277 | 22.9 ± 0.1 | 25.3 ± 0.2 | 25.4 ± 0.4 | 27.2 ± 0.5 | 25.4 ± 0.2 | <.001 | NA |
| Height, inches: mean ± SE | 20,585 | 64.6 ± 0.04 | 64.7 ± 0.14 | 64.4 ± 0.32 | 65.6 ± 0.51 | 64.7 ± 0.13 | .42 | .94 |
| Hispanic origin and race | <.001 | .02 | ||||||
| Hispanic or Latina | 4835 | 18.5 (0.9) | 13.4 (1.1) | 8.4 (2.2) | 10.5 (3.4) | 12.7 (0.9) | ||
| Non-Hispanic white | 10,474 | 60.5 (1.0) | 68.6 (1.6) | 70.2 (4.4) | 76.9 (5.0) | 69.1 (1.5) | ||
| Non-Hispanic black | 5081 | 15.7 (0.6) | 12.3 (1.1) | 14.3 (4.3) | 7.3 (2.7) | 12.4 (1.0) | ||
| Non-Hispanic other | 887 | 5.4 (0.6) | 5.7 (0.8) | 7.1 (2.6) | 5.3 (2.7) | 5.9 (0.7) | ||
| Marital status at first parity pregnancy outcome | <.001 | <.01 | ||||||
| Married | 10,875 | 56.5 (0.7) | 66.1 (1.7) | 64.9 (4.4) | 70.8 (6.1) | 66.2 (1.6) | ||
| Widowed/divorced/separated | 537 | 2.2 (0.1) | 3.7 (0.6) | 6.1 (2.0) | 11.0 (3.8) | 4.3 (0.6) | ||
| Never married | 9865 | 41.4 (0.7) | 30.2 (1.6) | 28.9 (4.5) | 18.1 (4.8) | 29.6 (1.5) | ||
| Educational attainment at time of interview | <.001 | .06 | ||||||
| No high school diploma or GED | 4374 | 17.44 (0.5) | 12.9 (1.0) | 14.2 (2.6) | 20.4 (5.1) | 13.4 (0.9) | ||
| High school diploma or GED | 7454 | 34.56 (0.6) | 32.0 (1.4) | 33.2 (3.7) | 23.7 (5.9) | 31.8 (1.3) | ||
| Some college, no bachelor’s degree | 5739 | 27.6 (0.6) | 28.7 (1.6) | 32.6 (4.6) | 29.5 (5.9) | 29.2 (1.5) | ||
| Bachelor’s degree | 2714 | 14.64 (0.5) | 18.2 (1.4) | 14.5 (3.0) | 20.0 (7.3) | 17.8 (1.2) | ||
| Master’s degree or higher | 996 | 5.77 (0.3) | 8.3 (1.1) | 5.6 (1.8) | 6.4 (2.9) | 7.9 (1.0) | ||
| Percentage of poverty level at time of interview | <.001 | .51 | ||||||
| Less than 100% | 5874 | 23.9 (0.6) | 19.2 (1.1) | 17.0 (3.0) | 25.2 (6.2) | 19.2 (1.0) | ||
| 100%–299% | 9164 | 42.5 (0.6) | 40.6 (1.7) | 46.6 (4.5) | 32.3 (5.8) | 40.9 (1.6) | ||
| 300%–399% | 2706 | 14.7 (0.4) | 16.9 (1.2) | 19.0 (3.3) | 4.3 (2.0) | 16.6 (1.1) | ||
| 400% or more | 3533 | 18.9 (0.6) | 23.3 (1.5) | 17.4 (3.1) | 38.3 (7.3) | 23.3 (1.4) | ||
| Ever smoked cigarettes‡ | .27 | .04 | ||||||
| Yes | 621 | 13.1 (0.8) | 15.0 (1.9) | 14.7 (4.8) | 21.4 (9.3) | 15.2 (1.8) | ||
| No | 3863 | 86.9 (0.8) | 85.0 (1.9) | 85.4 (4.8) | 78.6 (9.3) | 84.8 (1.8) | ||
| Intendedness of first parity pregnancy at conception | <.001 | <.001 | ||||||
| Intended | 11,867 | 56.4 (0.6) | 76.6 (1.5) | 81.9 (4.6) | 84.4 (4.2) | 77.6 (1.3) | ||
| Mistimed | 7277 | 34.5 (0.6) | 19.0 (1.3) | 9.4 (2.1) | 5.2 (2.2) | 17.3 (1.2) | ||
| Unwanted | 2125 | 9.2 (0.3) | 4.4 (0.5) | 8.7 (4.5) | 10.4 (4.4) | 5.2 (0.7) | ||
| Any medical help to become pregnant ever§ | <.001 | <.001 | ||||||
| Yes | 1477 | 7.6 (0.3) | 14.8 (1.3) | 15.0 (3.2) | 24.5 (6.1) | 15.2 (1.3) | ||
| Multiple live births at first parity‡ | .07 | .14 | ||||||
| Yes | 220 | 0.9 (0.1) | 1.9 (0.6) | 2.1 (0.8) | 0.0 (0.0) | 1.8 (0.5) | ||
| History of induced abortion‖ | <.001 | .03 | ||||||
| Yes | 2276 | 10.2 (0.4) | 14.5 (1.2) | 15.5 (2.7) | 12.7 (4.3) | 14.5 (1.1) | ||
| Gestational age of loss(es) | <.001 | NA | ||||||
| All < 6 wk | 379 | NA | 17.4 (1.2) | 5.9 (1.6) | 19.0 (6.5) | 16.1 (1.1) | ||
| Longest 6–9 wk | 896 | NA | 40.7 (1.6) | 32.1 (3.8) | 20.2 (5.6) | 38.9 (1.4) | ||
| Longest ≥ 10 wk | 1002 | NA | 41.9 (1.6) | 62.0 (3.9) | 60.8 (7.0) | 45.0 (1.6) | ||
| Recency of last loss¶ | .33 | NA | ||||||
| Within last 6 mo | 791 | NA | 35.5 (1.7) | 33.9 (3.8) | 42.0 (7.3) | 35.6 (1.5) | ||
| 6 to 11 mo ago | 390 | NA | 18.9 (1.4) | 22.9 (5.1) | 11.3 (3.5) | 19.1 (1.4) | ||
| 12 to 23 mo ago | 427 | NA | 19.2 (1.3) | 21.2 (3.8) | 12.2 (3.1) | 19.2 (1.2) | ||
| 24 mo or more ago | 585 | NA | 26.4 (1.5) | 22.0 (3.6) | 34.5 (7.7) | 26.2 (1.3) |
GED = general educational development.
Unweighted sample size with population weighted percentages and standard errors.
Missing information for height (n = 692), intendedness of first parity pregnancy at conception (n = 8), ever smoked cigarettes (n = 75), any medical help to become pregnant (n = 477) and multiple live births (n = 493).
P-value from the χ2 test for comparison of at least 1 loss versus no loss. For gestational age of loss(es) and recency of last loss categories, χ2 test compared number of pregnancy losses.
P-value from Type III test using logistic regression models adjusted for maternal age at conception.
Ever smoked at least 100 cigarettes in lifetime.
Ever received medical help to become pregnant in lifetime, not specific to first parity.
History of induced abortion only included women whose first induced abortion occurred before her first pregnancy loss.
Recency of last pregnancy loss calculated as the difference, in months, between the month of the most recent pregnancy loss and the month of conception at first parity. Recency of loss was restricted to women whose most recent pregnancy resulted in loss, which excluded 84 women whose most recent pregnancy was either an ectopic pregnancy (n = 22) or induced abortion (n = 62).
Adverse pregnancy outcomes
Number, gestational age, and recency of loss by selected birth outcomes at first parity are shown in Table 2. Approximately 11.9% (SE = 0.3) of pregnancies at first parity resulted in preterm birth, 2.1% (SE = 0.1) in stillbirth, 8.5% (SE = 0.3) in low birthweight, and 17.0% (SE = 0.4) in any of the above (Table 2).
Table 2.
Selected adverse pregnancy outcomes and gestational length by three aspects of pregnancy loss history among women at first parity, National Survey of Family Growth 1995 through 2011–2013
| Pregnancy loss history at first parity | n (%) | Preterm birth |
Stillbirth |
Low birthweight |
Any adverse outcome* |
Gestational length, weeks |
|---|---|---|---|---|---|---|
| (n = 2547) |
(n = 487) |
(n = 1952) |
(n = 3767) |
(n = 21,277) |
||
| % (SE) | % (SE) | % (SE) | % (SE) | Mean (SE) | ||
| All pregnancies at first parity | 21,277 (100.0) | 11.9 (0.3) | 2.1 (0.1) | 8.5 (0.3) | 17.0 (0.4) | 38.5 (0.03) |
| Number of pregnancy losses | ||||||
| No loss | 19,000 (88.8) | 11.7 (0.4) | 2.0 (0.2) | 8.5 (0.3) | 16.8 (0.4) | 38.5 (0.0) |
| 1 loss | 1,917 (9.4) | 12.9 (1.1) | 2.2 (0.4) | 8.3 (0.8) | 17.7 (1.2) | 38.4 (0.1) |
| 2 losses | 262 (1.3) | 17.4 (3.5) | 4.6 (1.5) | 6.2 (1.7) | 23.7 (3.8) | 38.1 (0.3) |
| 3 or more losses | 98 (0.5) | 8.2 (2.6) | 15.4 (4.6) | 10.7 (3.0) | 27.1 (5.2) | 36.6 (0.7) |
| At least 1 loss | 2,277 (11.2) | 13.2 (1.0) | 3.1 (0.5) | 8.2 (0.7) | 18.8 (1.1) | 38.3 (0.1) |
| Gestational age of loss(es) | ||||||
| All < 6 wk | 379 (1.8) | 12.1 (2.2) | 1.4 (0.8) | 8.8 (2.0) | 16.1 (2.5) | 38.4 (0.2) |
| Longest 6–9 wk | 896 (4.4) | 11.1 (1.4) | 2.5 (0.6) | 8.4 (1.3) | 16.8 (1.6) | 38.5 (0.1) |
| Longest ≥ 10 wk | 1,002 (5.1) | 15.4 (1.8) | 4.1 (0.8) | 7.8 (1.1) | 21.4 (1.9) | 38.0 (0.2) |
| Recency of last pregnancy loss† | ||||||
| Within last 6 months | 791 (3.9) | 8.9 (1.4) | 2.6 (0.7) | 7.4 (1.3) | 14.5 (1.6) | 38.8 (0.1) |
| 6 to 11 months ago | 390 (2.1) | 15.7 (3.2) | 3.4 (1.3) | 7.0 (1.6) | 20.4 (3.4) | 38.1 (0.3) |
| 12 to 23 months ago | 427 (2.1) | 14.0 (2.2) | 1.6 (0.6) | 8.6 (1.6) | 17.9 (2.3) | 38.3 (0.2) |
| 24 months or more ago | 585 (2.8) | 16.1 (2.2) | 4.6 (1.1) | 10.1 (1.7) | 23.8 (2.5) | 37.8 (0.3) |
Unweighted sample size with population weighted percentages and standard errors.
Any preterm birth, stillbirth, or low birthweight infant.
Excludes 84 pregnancies at first parity with most recent pregnancy not a pregnancy loss.
Approximately three-quarters of women reported at least one pregnancy subsequent to first parity (supplemental table 3) and were included in our analysis of adverse outcomes among future pregnancies. For future pregnancies, any preterm birth, stillbirth, low birthweight, or any of the above outcomes was reported by 14.0% (SE = 0.4), 2.6% (SE = 0.2), 9.7% (SE = 0.4) and 19.1% (SE = 0.5) of women (Table 3).
Table 3.
Selected adverse pregnancy outcomes and gestational length at any future pregnancy by three aspects of pregnancy loss history at first parity, National Survey of Family Growth 1995 through 2011–2013
| Pregnancy loss history at first parity | n (%) | Any future preterm birth |
Any future stillbirth |
Any future low birthweight |
Any future adverse outcome* |
Gestational length, weeks† | |
|---|---|---|---|---|---|---|---|
| (n = 2270) | (n = 498) | (n = 1658) | (n = 3186) | ||||
| % (SE) | % (SE) | % (SE) | % (SE) | n | Mean (SE) | ||
| All future pregnancies | 15,979 (100.0) | 14.0 (0.4) | 2.6 (0.2) | 9.7 (0.4) | 19.1 (0.5) | 14,281 | 38.6 (0.03) |
| Number of pregnancy losses | |||||||
| No loss | 14,341 (88.9) | 13.8 (0.5) | 2.6 (0.2) | 9.9 (0.4) | 19.1 (0.5) | 12,878 | 38.6 (0.0) |
| 1 loss | 1,385 (9.3) | 15.5 (1.7) | 2.4 (0.5) | 9.0 (1.3) | 19.7 (1.7) | 1194 | 38.5 (0.1) |
| 2 losses | 189 (1.3) | 12.4 (3.5) | 1.0 (0.5) | 7.0 (3.2) | 13.8 (3.6) | 159 | 38.8 (0.2) |
| 3 or more losses | 64 (0.5) | 20.2 (8.9) | 10.3 (5.4) | 7.6 (3.2) | 29.6 (9.0) | 50 | 38.0 (0.6) |
| At least 1 loss | 1,638 (11.1) | 15.3 (1.5) | 2.6 (0.5) | 8.7 (1.2) | 19.4 (1.6) | 1403 | 38.5 (0.1) |
| Gestational age of pregnancy loss(es) | |||||||
| All < 6 wk | 247 (1.6) | 13.2 (3.8) | 1.0 (0.6) | 8.4 (2.9) | 16.4 (3.8) | 221 | 38.5 (0.3) |
| Longest 6–9 wk | 669 (4.4) | 15.7 (2.1) | 1.6 (0.6) | 7.6 (1.5) | 18.3 (2.2) | 565 | 38.5 (0.1) |
| Longest ≥ 10 wk | 722 (5.1) | 15.6 (2.5) | 3.9 (0.9) | 9.8 (2.0) | 21.2 (2.7) | 617 | 38.5 (0.1) |
| Recency of last pregnancy loss‡ | |||||||
| Within last 6 mo | 601 (4.0) | 13.5 (2.4) | 2.6 (0.7) | 9.6 (2.3) | 17.7 (2.5) | 524 | 38.6 (0.1) |
| 6 to 11 mo ago | 297 (2.1) | 13.1 (2.8) | 2.3 (1.0) | 6.7 (2.3) | 17.8 (3.1) | 252 | 38.5 (0.2) |
| 12 to 23 mo ago | 303 (2.1) | 15.1 (3.0) | 3.0 (1.1) | 12.3 (2.9) | 20.4 (3.2) | 259 | 38.5 (0.3) |
| 24 mo or more ago | 377 (2.5) | 18.9 (3.0) | 2.9 (1.1) | 5.6 (1.3) | 21.8 (3.1) | 324 | 38.4 (0.2) |
Unweighted sample size with population weighted percentages and standard errors.
Any future preterm birth, stillbirth, or low birthweight infant reported.
Among only future pregnancies that resulted in live birth.
Excludes 84 first parities with most recent pregnancy not a pregnancy loss.
Multivariable logistic regression results
Pregnancy loss history and risk of adverse pregnancy outcomes at first parity
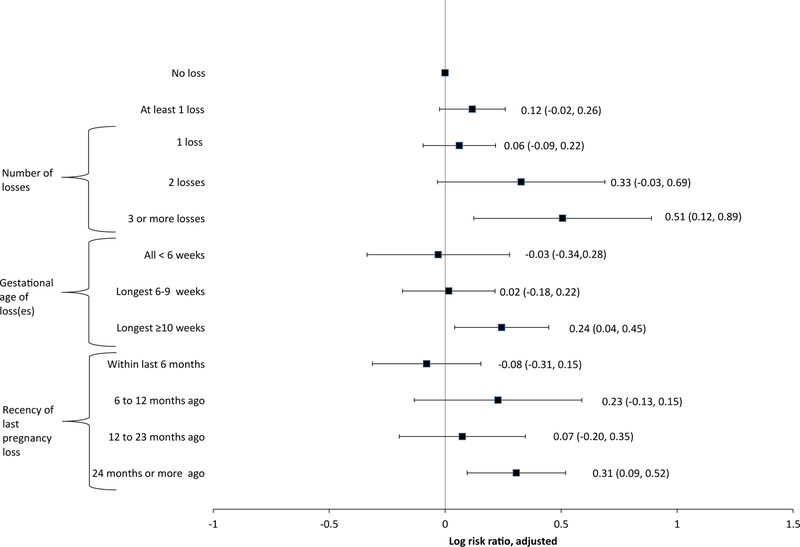
In adjusted models, having three or more losses was associated with increased risk of any selected adverse outcomes at first parity (adjusted risk ratio [aRR] = 1.66 [1.13, 2.43]; Fig. 1). Maximum gestational age of loss ≥10 weeks and having experienced a loss 24 months or more before the beginning of first parity were also associated with increased risk of adverse outcomes (aRR = 1.28 [1.04, 1.56] and 1.36 [1.10, 1.68], respectively). No other associations were statistically significant, including having at least one loss (aRR = 1.12 [95% confidence interval (CI), 0.98–1.30]) and having experienced a loss within 6 months of the beginning of first parity (aRR = 0.92 [95% CI, 0.73–1.17]). Tests for linear trend per category increase found a statistically significant increase in risk of selected adverse outcomes for number of losses (aRR = 1.13 [95% CI, 1.02–1.25]), maximum gestational age of loss(es) (aRR = 1.07 [95% CI, 1.00–1.13]), and recency of loss (aRR = 1.07 [95% CI, 1.02–1.12]).
Fig. 1.
Risk ratio for any selected adverse pregnancy outcomes at first parity by three aspects of pregnancy loss history, National Survey of Family Growth 1995–2013. Adjusted for age at conception, age at conception squared, height, race/ethnicity, marital status at pregnancy outcome, ever smoked, history of induced abortion, education, poverty level, ever received medical help getting pregnant, and year of conception. Models included the following number of observations: 20,047 (number of losses), 20,047 (gestational age of loss(es)), and 19,966 (recency of last pregnancy loss).
Pregnancy loss history and adverse pregnancy outcomes at any future pregnancy
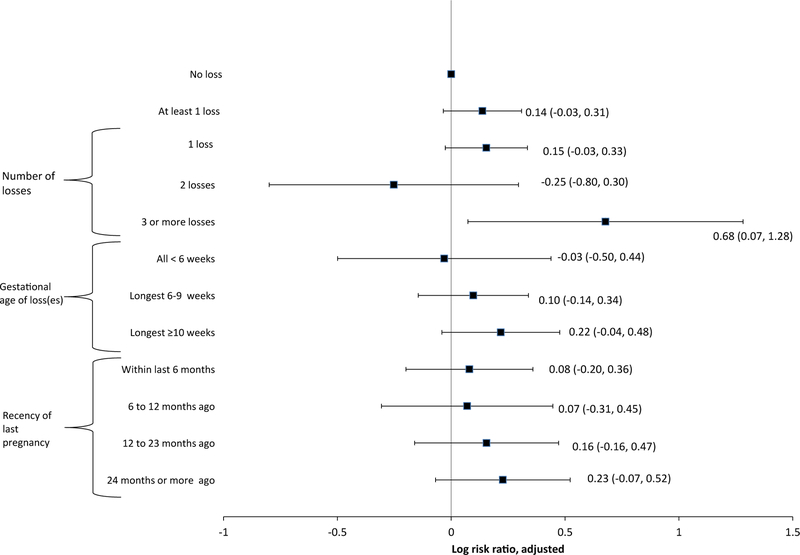
In adjusted models, having three or more losses at first parity was associated with increased risk of any selected adverse outcomes in future pregnancies (aRR = 1.97 [95% CI, 1.08–3.60]; Fig. 2).
Fig. 2.
Risk ratio for any selected adverse pregnancy outcomes at any future pregnancy by three aspects of pregnancy loss history at first parity, National Survey of Family Growth 1995–2013. Adjusted for age at conception, age at conception squared, height, race/ethnicity, marital status at pregnancy outcome, ever smoked, history of induced abortion, education, poverty level, ever received medical help getting pregnant, and year of conception. Models included the following number of observations: 15,008 (number of losses), 15,008 (gestational age of loss(es)), and 14,950 (recency of last pregnancy loss).
No other associations were statistically significant, including having at least one loss (aRR = 1.15 [95% CI, 0.97–1.36]), a maximum gestational age of loss ≥ 10 weeks (aRR = 1.24 [95% CI, 0.96–1.61]) and having experienced a loss within 6 months before the beginning of first parity (aRR = 1.08 [95% CI, 0.82–1.43]). Tests for linear trend did not find a statistically significant increase in risk of selected adverse outcomes for number of losses (aRR = 1.12 [95% CI, 0.98–1.28]), maximum gestational age of loss(es) (aRR = 1.04 [95% CI, 0.97–1.12]), and recency of loss (aRR = 1.04 [95% CI, 0.98–1.11]), although estimates were only slightly attenuated compared to those for outcomes at first parity.
Assessment of heterogeneous associations over time
In the adjusted model for no loss versus at least one loss on the composite outcome at first parity, an added interaction term with year of conception was not significant (P = .44), indicating that associations were not heterogeneous over the years included in our analysis.
Discussion
Among a set of nationally representative samples of reproductive aged women in the United States, certain aspects of pregnancy loss history at the time of first parity were associated with an increased risk of subsequent adverse pregnancy outcomes. Having three or more losses, a maximum gestational age of loss ≥10 weeks or having experienced a loss 24 months or more before the beginning of pregnancy were associated with an increased risk of a composite measure of preterm birth, stillbirth, and/or low birth–weight at first parity; in addition, linear trends were observed for increasing categories of pregnancy loss number, gestational age, and time since loss occurred. We also found that a history of three or more losses at first parity was associated with an increased risk of a composite measure of adverse pregnancy outcomes in pregnancies occurring after the first parity. Having at least one loss did not show an increased risk of adverse pregnancy outcomes at first parity or in future pregnancies. Our findings suggest that the number of pregnancy losses, maximum gestational age of loss(es), and recency of last pregnancy loss may be important factors to consider when assessing subsequent risk of adverse pregnancy outcomes.
Our results are generally consistent with previous studies which have found increasing risk of subsequent preterm birth, stillbirth, and other related adverse pregnancy outcomes with increasing number of pregnancy losses [3,9,10,13–15,25]; as others have observed, recurrent loss (3 or more consecutive losses), while rare, was associated with the highest risk of adverse pregnancy outcomes at first parity. The distribution of number of pregnancy losses at first parity in our population also matched those reported by at least two other retrospective cohort studies of women at first parity [10,15]. In addition, we found having at least one loss at the time of first parity was not associated with adverse outcomes, which is agreement with some, but not all, prior studies of primiparous women [7,8,11–13].
In terms of recency of loss, our finding of an increased risk of adverse pregnancy outcomes associated with a loss 24 months or more ago has not been commonly reported. Several studies have found mostly null associations between recency of loss and pregnancy outcomes [9,26], whereas others have found an interpregnancy interval <6 months associated with increased [27] or decreased risk of low birthweight and preterm birth [28,29]; one study found increasing interpregnancy interval associated with higher risks of adverse outcomes following pregnancy loss [25]. We found that an interpregnancy interval <6 months was not associated with the risk of a composite outcome of preterm birth, stillbirth, and/or low birthweight in either the index pregnancy or in any future pregnancy, however, having a pregnancy loss 24 months or more before first parity was. This finding did not change on adjustment for maternal characteristics, which included a proxy measure of underlying infertility, a potential confounder of the relationship between recency of loss and adverse pregnancy outcomes. The inconsistency of our results with previous studies may be due to different interpregnancy interval cutpoints, our choice of using first parity as the basis for defining pregnancy loss history, and differences in risk factors for adverse pregnancy outcomes across international study settings.
Our findings are in agreement with previous studies that have found higher risk of adverse pregnancy outcomes after second trimester miscarriages [30,31]. However, our gestational age categorization, which was based on assumed developmental stage at time of pregnancy loss recognition, potentially can offer more insight into the mechanisms behind pregnancy loss, for example, suggesting history of fetal deaths rather than implantation failure, aneuploidy, or abnormal placentation, may be associated with future adverse pregnancy outcomes [18]. However, we were limited to gestational age as a proxy for developmental stage, which may have presumed greater precision than was appropriate given the self-reported nature of the data.
Our sample of women is the largest population-based study in the United States to look at the association between pregnancy loss and subsequent pregnancy outcomes. In addition, because many miscarriages do not require medical intervention, self-reported information collected in NSFG may provide a more comprehensive history of pregnancy loss compared with medical records. The NSFG also collects information on induced abortions, allowing for adjustment of this potentially important confounder, which has been either unavailable in other studies [15] or indistinguishable from spontaneous abortion [27,32]. However, underreporting of induced abortions in NSFG has been documented [33], which could potentially have led to residual confounding. Finally, we chose to examine pregnancy loss history at first parity; comparisons with no pregnancy loss history in parous women, of proven fertility, may artificially inflate risks associated with pregnancy loss [6–8].
We were unable to differentiate between spontaneous and medically indicated preterm birth or ascertain other pregnancy outcomes, such as preeclampsia, placental abruption, or intrauterine growth restriction because of limited clinical information collected. Similarly, pregnancy loss <20 weeks comprised a heterogenous group with varied pregnancy loss etiologies; more detailed clinical information regarding the losses would have enhanced the interpretation of our findings. Furthermore, self-reported information on pregnancy loss number and timing is subject to exposure misclassification (particularly since self-reported pregnancy loss comprises only approximately half of all human chorionic gonadotropin–detected pregnancy loss [2]) and coupled with the self-reported information on pregnancy outcomes could lead to dependent misclassification [34]. Although internal validation studies were not performed for either our exposure or outcomes, maternal self-report of gestational age and birthweight are considered fairly accurate [35–40], particularly for firstborn children [35], even when these data are collected many years after the index pregnancy [35,36,39–41]. In addition, we pooled data across four NSFG surveys, which might have obscured differences over time in the relationship between pregnancy loss and subsequent adverse pregnancy outcomes. Yet, we found no significant multiplicative interaction with year of conception (P = .44). While we chose to examine pregnancy loss history at first parity rather than all pregnancy loss patterns, further research could evaluate the complex relationship between pregnancy loss and adverse pregnancy outcomes over time among all pregnancies experienced by reproductive aged women. Finally, findings for pregnancy loss history at first parity and adverse future pregnancy outcomes may not be generalizable to other pregnancy loss history patterns (e.g., pregnancy loss after a live birth).
In conclusion, our findings from a set of large, nationally representative samples of reproductive aged women in the United States found that specific aspects of pregnancy loss history, including the number, gestational age, and recency of losses, may be associated with adverse pregnancy outcomes in subsequent pregnancies. Findings may help to inform research related to increased antenatal surveillance and targeted treatment for women with history of pregnancy loss at first continuing pregnancy.
Supplementary Material
Acknowledgments
We thank Alan Simon, MD, and Anjani Chandra, PhD, for their input and advice on this manuscript. Dr. Simon and Dr. Chandra are employees of the National Center for Health Statistics in Hyattsville, MD, and their work was performed under employment of the U.S. federal government; they did not receive any other compensatory funding.
Financial support: This work was performed under employment by the U.S. federal government; the authors did not receive any outside funding.
Footnotes
The authors report no conflict of interest.
Paper presentation: A preliminary version of this analysis was presented at the 27th annual meeting of the Society of Pediatric and Perinatal Epidemiologic Research (SPER) in Denver, Colorado, on June 15–17, 2015.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, Centers for Disease Control and Prevention.
References
- [1].Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14(5):839–54. [DOI] [PubMed] [Google Scholar]
- [2].Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med 1988;319(4):189–94. [DOI] [PubMed] [Google Scholar]
- [3].Swingle HM, Colaizy TT, Zimmerman MB, Morriss FH Jr. Abortion and the risk of subsequent preterm birth: a systematic review with meta-analyses. J Reprod Med 2009;54(2):95–108. [PubMed] [Google Scholar]
- [4].Speert H Pregnancy prognosis following repeated abortion. Am J Obstet Gynecol 1954;68(2):665–73. [DOI] [PubMed] [Google Scholar]
- [5].Bhattacharya S, Bhattacharya S. Effect of miscarriage on future pregnancies. Women’s Health (Lond Engl) 2009;5(1):5–8. [DOI] [PubMed] [Google Scholar]
- [6].Basso O, Baird DD. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Hum Reprod 2003;18(11):2478–84. [DOI] [PubMed] [Google Scholar]
- [7].Hiersch L, Ashwal E, Aviram A, Rayman S, Wiznitzer A, Yogev Y. The association between previous single first trimester abortion and pregnancy outcome in nulliparous women. J Matern Fetal Neonatal Med 2016;29:1457–61. [DOI] [PubMed] [Google Scholar]
- [8].Bhattacharya S, Townend J, Shetty A, Campbell D, Bhattacharya S. Does miscarriage in an initial pregnancy lead to adverse obstetric and perinatal outcomes in the next continuing pregnancy? BJOG 2008;115(13):1623–9. [DOI] [PubMed] [Google Scholar]
- [9].Makhlouf MA, Clifton RG, Roberts JM, Myatt L, Hauth JC, Leveno KJ, et al. Adverse pregnancy outcomes among women with prior spontaneous or induced abortions. Am J Perinatol 2014;31(9):765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hammoud AO, Merhi ZO, Diamond M, Baumann P. Recurrent pregnancy loss and obstetric outcome. Int J Gynaecol Obstet 2007;96(1):28–9. [DOI] [PubMed] [Google Scholar]
- [11].Kashanian M, Akbarian AR, Baradaran H, Shabandoust SH. Pregnancy outcome following a previous spontaneous abortion (miscarriage). Gynecol Obstet Invest 2006;61(3):167–70. [DOI] [PubMed] [Google Scholar]
- [12].Bhattacharya S, Townend J, Bhattacharya S. Recurrent miscarriage: Are three miscarriages one too many? Analysis of a Scottish population-based database of 151,021 pregnancies. Eur J Obstet Gynecol Reprod Biol 2010;150(1):24–7. [DOI] [PubMed] [Google Scholar]
- [13].Weintraub AY, Sergienko R, Harlev A, Holcberg G, Mazor M, Wiznitzer A, et al. An initial miscarriage is associated with adverse pregnancy outcomes in the following pregnancy. Am J Obstet Gynecol 2011;205(3):286.e1–5. [DOI] [PubMed] [Google Scholar]
- [14].Oliver-Williams C, Fleming M, Wood A, Smith G. Previous miscarriage and the subsequent risk of preterm birth in Scotland, 1980–2008: a historical cohort study. BJOG 2015;122:1525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gunnarsdottir J, Stephansson O, Cnattingius S, Akerud H, Wikstrom AK. Risk of placental dysfunction disorders after prior miscarriages: a population-based study. Am J Obstet Gynecol 2014;211(1):34.e1–8. [DOI] [PubMed] [Google Scholar]
- [16].National Survey of Family Growth. Questionnaires, Datasets and Related Documentation 2015. [cited 2015 September]; Available at: http://www.cdc.gov/nchs/nsfg.htm.
- [17].National Survey of Family Growth, WebDoc. Recode for PRGLNGTH [cited 2015 April]; Available at: http://www.icpsr.umich.edu/webdocs/jsp/documents/recode_specs/preg/PRGLNGTH.pdf.
- [18].Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA. Nomenclature for pregnancy outcomes: time for a change. Obstet Gynecol 2011;118(6):1402–8. [DOI] [PubMed] [Google Scholar]
- [19].Zarek SM, Mitchell EM, Sjaarda LA, Mumford SL, Silver RM, Stanford JB, et al. Antimullerian hormone and pregnancy loss from the Effects of Aspirin in Gestation and Reproduction trial. Fertil Steril 2016;105:946–952.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, et al. Urinary Concentrations of Phthalate Metabolites and Bisphenol A and Associations with Follicular-Phase Length, Luteal-Phase Length, Fecundability, and Early Pregnancy Loss. Environ Health Perspect 2016;124:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goldenberg R, Culhane J, Iams J, Romero R. Epidemiology and causes of pre-term birth. Lancet 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hinkle SN, Albert PS, Mendola P, Sjaarda LA, Boghossian NS, Yeung E, et al. Differences in risk factors for incident and recurrent small-for-gestational-age birthweight: a hospital-based cohort study. BJOG 2014;121(9):1080–8. discussion 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306(22):2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lang K, Nuevo-Chiquero A. Trends in self-reported spontaneous abortions: 1970–2000. Demography 2012;49(3):989–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Basso O, Olsen J, Christensen K. Risk of preterm delivery, low birthweight and growth retardation following spontaneous abortion: a registry-based study in Denmark. Int J Epidemiol 1998;27(4):642–6. [DOI] [PubMed] [Google Scholar]
- [26].Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med 1994;22(3):235–41. [DOI] [PubMed] [Google Scholar]
- [27].Conde-Agudelo A, Belizán JM, Breman R, Brockman SC, Rosas-Bermudez A. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet 2005;89(Suppl 1(0)):S34–40. [DOI] [PubMed] [Google Scholar]
- [28].Love ER, Bhattacharya S, Smith NC, Bhattacharya S. Effect of interpregnancy interval on outcomes of pregnancy after miscarriage: retrospective analysis of hospital episode statistics in Scotland. BMJ 2010;341:c3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goldstein RR, Croughan MS, Robertson PA. Neonatal outcomes in immediate versus delayed conceptions after spontaneous abortion: a retrospective case series. Am J Obstet Gynecol 2002;186(6):1230–4. discussion 1234–6. [DOI] [PubMed] [Google Scholar]
- [30].Goldenberg RL, Mayberry SK, Copper RL, Dubard MB, Hauth JC. Pregnancy outcome following a second-trimester loss. Obstet Gynecol 1993;81(3):444–6. [PubMed] [Google Scholar]
- [31].Edlow AG, Srinivas SK, Elovitz MA. Second-trimester loss and subsequent pregnancy outcomes: What is the real risk? Am J Obstet Gynecol 2007;197(6):581.e1–6. [DOI] [PubMed] [Google Scholar]
- [32].Brown JS Jr, Adera T, Masho SW. Previous abortion and the risk of low birth weight and preterm births. J Epidemiol Community Health 2008;62(1):16–22. [DOI] [PubMed] [Google Scholar]
- [33].Jones RK, Kost K. Underreporting of induced and spontaneous abortion in the United States: an analysis of the 2002 National Survey of Family Growth. Stud Fam Plann 2007;38(3):187–97. [DOI] [PubMed] [Google Scholar]
- [34].Kristensen P Bias from nondifferential but dependent misclassification of exposure and outcome. Epidemiology 1992;3(3):210–5. [DOI] [PubMed] [Google Scholar]
- [35].Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers’ recall of birthweight and gestational age. Br J Obstet Gynaecol 1987;94(8):731–5. [DOI] [PubMed] [Google Scholar]
- [36].Jensen CB, Gamborg M, Heitmann B, Sorensen TI, Baker JL. Comparison of birth weight between school health records and medical birth records in Denmark: determinants of discrepancies. BMJ Open 2015;5(11):e008628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children’s Cancer Group. Am J Epidemiol 1997;145(1):58–67. [DOI] [PubMed] [Google Scholar]
- [38].Elliott JP, Desch C, Istwan NB, Rhea D, Collins AM, Stanziano GJ. The reliability of patient-reported pregnancy outcome data. Popul Health Manag 2010;13(1):27–32. [DOI] [PubMed] [Google Scholar]
- [39].O’Sullivan JJ, Pearce MS, Parker L. Parental recall of birth weight: how accurate is it? Arch Dis Child 2000;82(3):202–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu J, Tuvblad C, Li L, Raine A, Baker LA. Medical record validation of maternal recall of pregnancy and birth events from a twin cohort. Twin Res Hum Genet 2013;16(4):845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaufmann RB, Clouse TL, Olson DR, Matte TD. Elevated blood lead levels and blood lead screening among US children aged one to five years: 1988–1994. Pediatrics 2000;106(6):E79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.