Introduction
Transgender women (TW) have recently been acknowledged as a unique and important risk group in HIV research and care. Although transgender men (TM) also face specific problems related to HIV infection, less is known about risk behaviors and HIV prevalence among TM. Following the lead of public health efforts that address the high frequency of HIV infection among TW and the socio-structural factors contributing to HIV risk in this population, researchers have increasingly emphasized transgender-specific aspects of the epidemiology, prevention, treatment, and management of complications of HIV infection [1, 2]. Here, we seek to highlight key issues within these distinct areas, identify areas of overlap and importance between social scientific and biomedical disciplines, and explore future areas for HIV-related research in transgender adults.
Epidemiology of HIV in Transgender Populations
Global Epidemiology of HIV in Transgender Men and Women
Transgender women are commonly recognized as a key risk group for HIV infection, though epidemiologic patterns of HIV prevalence, incidence and associated risk factors in transgender populations remain poorly understood. Previous meta-analyses have estimated the global prevalence of HIV among TW at 19.1% (95% CI: 17.4–20.7), with the odds ratio of HIV infection among TW compared to other reproductive age adults at 48.8 (95% CI: 21.2–76.3) [3, 4]. Regional estimates of HIV infection in transgender communities vary dramatically, however, with differences attributable to individual studies’ choices of sampling methods, inclusion criteria, and techniques for determining HIV infection status [5]. In a recent systematic review, laboratory-confirmed prevalence of HIV among TW in the U.S. ranged from 2.0% in San Diego, CA to 40.1% in New York, NY with global estimates ranging from 4.0% in Spain to 34.1% in Argentina [6]. Although transmasculine individuals are estimated to represent 11% of transgender persons in HIV care, data on HIV infection among TM is scarce, with reported prevalence in the same review ranging from 0.0% to 8.0% [7, 8]. HIV and STI prevalence among sexual partners of trans persons is also poorly documented, though small samples of cisgender men with TW partners have reported both high frequencies of sexual risk behavior and a high prevalence of HIV and other sexually transmitted infections (STIs) [9, 10]. Ultimately, the lack of standardized systems for consistent monitoring, surveillance, and identification of HIV, STIs, and associated risk behaviors in representative samples of transgender populations and their partners limit current efforts to construct a comprehensive epidemiologic profile of these groups.
Sampling Methodologies
The majority of epidemiologic analyses of transgender populations have been based on convenience samples of individuals recruited from community, clinic, or commercial sex venues [11]. Although these studies provide important information on local population characteristics and identify specific areas for intervention, they may not accurately represent the epidemiologic characteristics of the larger transgender population. In an effort to improve the validity of epidemiologic data, many recent studies have used Respondent Driven Sampling (RDS) techniques [12–15]. However, while RDS may be effective in identifying members of “hidden populations” like transgender persons, relatively small cohort sizes, limited numbers of recruitment waves, and unequal distribution networks that violate the statistical assumptions underlying RDS can undermine the validity of the resulting weighted estimates [16]. Further work using rigorously applied representative sampling methods to accurately capture transgender communities in all of their diversity is critically needed for the development of culturally appropriate and epidemiologically relevant prevention interventions.
“Transgender” as an Epidemiologic Category
In order to construct epidemiologic estimates, researchers must also consider how to define the term “transgender” when designing their analysis. Using the definition of transgender as “An individual whose gender identity differs from their assigned sex at birth,” the Two-Step Method for assessment of gender identity and natal sex has been adopted as a global standard for quantifying transgender persons [17, 18]. This approach has been central to the growing acknowledgement of TW as distinct from the risk category of men who have sex with men (MSM) [19, 20]. However, the Two-Step Method does not incorporate sexual identity into the transgender experience, and frequently minimizes “sexual orientation” as a simple matter of partner gender. Particularly when defining local HIV epidemiology in diverse global contexts and developing corresponding prevention strategies, it is essential to understand and address how social constructions of gender and sexuality intersect, overlap, and diverge [21, 22]. Acknowledging and understanding the diversity of gender and sexual identities included within the term “transgender’ is essential for defining accurate epidemiologic frameworks and for developing appropriate public health responses [23].
Epidemiology of HIV/STIs in Transgender Sexual Partners and Networks
A critical component of HIV risk for transgender individuals lies in the prevalence of HIV and STIs among their sexual partners and associated dyadic- or network-level patterns of infectious disease transmission. Network mixing patterns involving transgender and cisgender individuals that structure HIV/STI risks in the population, and the pathways of disease transmission through these networks, have not yet been thoroughly defined [24, 25]. As noted above, there is scant literature documenting the HIV/STI status, sexual practices, partner concurrency patterns, and network interactions of the sexual partners of transgender individuals, and almost no data exist on how these sexual networks are integrated into epidemiologic patterns of the spread of HIV/STIs in the larger population [26, 27]. In order to accurately define HIV/STI prevalence, risk, and transmission among transgender individuals it is essential to place individual-level risk factors within the larger context of their dyadic sexual partnerships and their complex sexual networks.
Transgender-Specific HIV Prevention
Overview of HIV Prevention with Transgender People
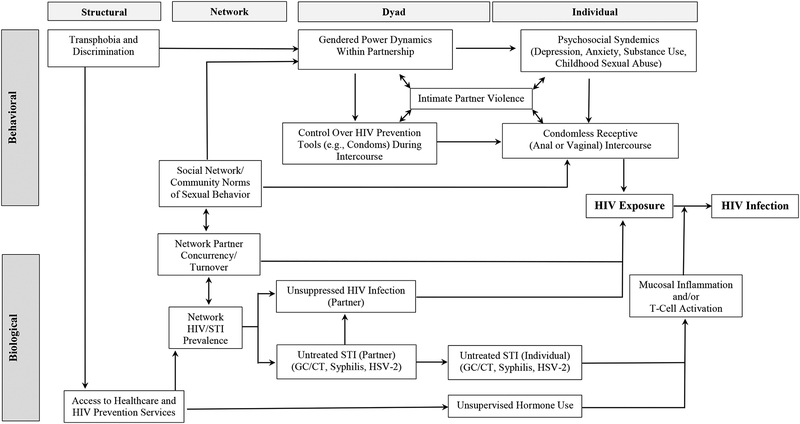
Within the context of local epidemiologic patterns, HIV prevention efforts for transgender individuals must recognize the complex interaction of biological and behavioral aspects of individual decision-making processes, dyadic partnership interactions, social and sexual network frameworks, and structural contexts that define HIV risk (Figure 1). On an individual level, syndemic patterns of depression, anxiety, substance use, childhood abuse, and trauma may negatively affect decisions regarding condom use with HIV serodiscordant or unknown status partners [28]. Adherence to gender norms (sexual roles traditionally aligned with masculinity and femininity) during partnership interactions may encourage TW to maintain a receptive role during intercourse while also limiting control, both psychologically and mechanically, over condom use [29, 30]. For TW who engage in commercial sex work, desires to maintain intimacy with romantic/non-commercial partners, to replicate traditional heterosexual norms of behavior, and to differentiate their romantic interactions from their commercial sexual contacts may lead to a higher frequency of condomless intercourse with partners who are stable and considered “safe” despite a lack of objective knowledge of HIV or STI status [31, 32]. Community and network norms of sexual behavior may influence the frequency of condomless intercourse, rates of partner change and concurrency, and adoption of and adherence to biomedical prevention interventions like pre-exposure prophylaxis (PrEP) [33, 34]. At the same time, environmental frameworks of stigma, discrimination, and harassment both define the contexts of individual and interpersonal risk behavior processes, and determine structural access to education, prevention, and treatment resources [35].
Fig. 1.
Biological and behavioral contributors to risk of HIV infection in transgender adults.
Biological Risks of HIV Transmission
During condomless intercourse, biological factors contribute to increased risk for HIV exposure and transmission among TM and TW. Mucosal inflammation from undiagnosed rectal and vaginal STIs and elevated estrogen levels from exogenous hormone therapy induce T lymphocyte recruitment and chemokine activation, increasing cellular vulnerability to HIV acquisition [36, 37]. Occult STIs and untreated HIV infection in sexual partners, mirrored by high prevalences of disease in the larger sexual network, increase the likelihood of exposure to and acquisition of HIV infection during condomless intercourse [38]. Prior experiences of discrimination and mistreatment by healthcare workers, pervasive social stigma surrounding HIV and STIs, and limited access to affordable, culturally sensitive testing resources all discourage effective HIV/STI diagnosis and treatment within traditional institutional frameworks and increase the likelihood that infections in both transgender individuals and their partners will remain unrecognized and untreated [39, 40].
Pre-Exposure Prophylaxis (PrEP)
Within this intersection of behavioral risk and biological vulnerability, biomedical interventions like PrEP and topical microbicides have shown promise in addressing the prevention needs of transgender persons. By providing an effective tool for routine protection against HIV infection that neither depends on sexual contact-specific use nor is routinely controlled by one partner during a sexual interaction, PrEP offers the possibility for sustained HIV prevention across a range of different partnership contexts [41]. Although PrEP has revolutionized the field of HIV prevention, data on the effectiveness of PrEP in TW is currently limited (and no data is available on its use in TM) [42]. In the original iPrEx study, 339 TW (14% of the total sample) were enrolled, with HIV seroconversion occurring among 11 TW in the intervention arm, 10 TW in the control arm (HR 1.1, 95% CI: 0.5–2.7), and no new infections among TW with detectable serum drug levels [43]. Hypothesized reasons for the low blood levels of Truvada® among TW in iPrEx include poor adherence to study medication or potential metabolic interactions of antiretroviral medications with exogenous hormones. Subsequent acceptability studies and demonstration projects have shown high frequencies of PrEP acceptability and uptake among TW, though sustained levels of medication adherence remain low [44, 45]. Qualitative analyses have identified several reasons for the low rates of PrEP adoption and maintenance among transgender individuals, including: Prior experiences of stigma and discrimination with institutional healthcare systems that discourage routine access and follow-up; Perceptions that PrEP education and access programs are directed towards gay male populations and are not meant for TW; A lack of PrEP services offered within a comprehensive, gender-affirming healthcare context; Concerns about the possible biological interactions of PrEP medication with exogenous hormones; and Mistrust of drugs and prevention approaches that may be perceived as “experimental” or unproven [46, 47]. Building on these problems, trans-specific strategies to improve PrEP uptake and adherence could include systems to couple gender-affirming healthcare with PrEP administration and monitoring, education and advertising efforts to emphasize PrEP use in the context of TM- and TW-specific prevention needs, and community-generated strategies to promote the organic development of social norms of PrEP use and adherence among transgender individuals [48, 49].
Topical Microbicides
Similar to PrEP, microbicides offer the promise of a prevention tool that shifts control from the insertive to the receptive partner during intercourse, another option that may be useful for reducing HIV risk among some transgender individuals. Despite potential advantages, no data exist on how topical microbicides may work within the unique mucosal environment of a sigmoid neovagina, or of how exogenous feminizing hormones may influence microbicide activity in rectal mucosa. Even the risk of neovaginal HIV transmission in TW has not yet been determined, though cases of gonococcal infection in both penile inversion and sigmoid colonic neovaginal tissue have been reported [50, 51]. Formative research on acceptability and sample formulations of rectal microbicides in both cis- and transgender populations are more advanced, suggesting high levels of potential use, though likely agents remain in early stages of development [52, 53].
Partner Management and Couples-Based Prevention
On an interpersonal level, partner-based prevention strategies recognize the importance of dyadic contexts in defining sexual behavior and shaping HIV/STI risks [54, 55]. Couples-based HIV counseling and testing provides a mechanism for both HIV testing and serostatus disclosure within an established partnership framework [56]. Other strategies to promote partner notification (e.g., partner referral cards or internet-based notification systems) and treatment (e.g., Patient Delivered Partner Therapy) following HIV or STI diagnosis promote treatment of occult infections in exposed partners, limiting the index partner’s risk for (re-) exposure and reducing the prevalence of untreated disease within their sexual network [57, 58]. A central benefit of these interventions is the potential to reach sexual partners who are at high risk for HIV/STIs but do not identify as transgender or MSM, and may not consider themselves at risk for infection [59]. Potential barriers to partner notification articulated by TW in formative research studies include fears of stigma, shame, violence, and abuse in response to disclosure, though no actual events of partner violence have been reported in these trials [60]. Additional studies on whether and how the partnership contexts of transgender individuals affect the specific mechanisms, barriers and incentives to use, and outcomes of new and traditional partner management technologies are important areas for future research.
Social Networks and HIV Prevention
Parallel to the sexual network-based approach of partner management, recent and ongoing research with transgender communities has focused on the role of social networks in defining and maintaining norms of HIV risk and prevention and behavior. In one of the few examples of a successful, randomized controlled trial of a sexual risk behavior intervention for transgender people, the LifeSkills trial used small group workshops to promote collective self-esteem and empowerment among participants while improving their knowledge and behavioral skills related to HIV risk reduction [61, 62]. At the 4-month follow-up evaluation, TW in the intervention groups reported a 39.8% reduction in condomless sex acts compared to participants in the standard of care arm [63]. Other ongoing studies are assessing the role of social network support for encouraging PrEP adherence among TW in Latin America [64, 65]. These approaches seek to transform perceptions of PrEP and other prevention interventions from something imposed onto transgender participants by distant health professionals into tools that are organically generated and promoted within transgender communities and address issues important to their daily lives [66]. Problems identified during the course of this research include ambiguities in defining the social network structures and patterns of influence within transgender communities, differences between networks of social supports or influences and networks of sexual contacts or partners, and difficulty promoting collective consciousness and community formation among individuals whose lives have frequently been marked by social marginalization, rejection, and mistrust [67].
Structural Interventions
Finally, structural interventions are essential for transforming the contexts of HIV/STI risk and integrating HIV prevention into daily life. Routine transphobia manifests as stigma, discrimination, harassment, and violence in a range of different global environments and has pervasive effects on access to healthcare, gender-specific services, and HIV testing and treatment [68, 69]. TW involved in commercial sex work may also face problems of substance use, housing instability, and intimate partner violence that exacerbate risks for HIV and other health issues, and undermine the relative importance of HIV prevention in their lives [38, 70]. Partially due to the difficulty of evaluating structural interventions within the parameters of a randomized controlled trial, many of the studies addressing these issues have been project reports or program evaluations [71, 72]. Yet projects in diverse global settings have sought to address different structural aspects of health among transgender populations, including education to reduce discrimination and promote culturally sensitive healthcare environments, advocacy efforts to promote transgender rights and limit harassment and violence, and economic interventions designed to alter the contexts of daily life for transgender persons [73–75]. Through the collective integration of individual, dyadic and social contexts of behavioral, biological, and structural frameworks, transgender HIV prevention can provide a roadmap for the broader field of HIV prevention.
HIV and Gender-Affirming Treatment
Immuno-metabolic Effects of Feminizing Hormonal Therapies (FHT)
Data on the immuno-metabolic effects of FHT are mixed, and effects vary by type and route of administration. 17-α-ethinyl estradiol is associated with the greatest perturbations of inflammatory and coagulation biomarker and lipid levels [76]. 17-β estradiol has milder effects on inflammatory and atherosclerotic pathways, and is now the recommended form of estrogen for FHT. However, 17-α-ethinyl estradiol use is still observed, and TW engaging in medically unsupervised FHT use may not have access to standard of care therapies.
Although some data support immuno-metabolic changes with combined estrogen/anti-androgen therapy that could reduce cardiometabolic risk [77], in general estrogens modulate the body’s inflammatory and coagulation pathways and are associated with an increased risk of cardiovascular and thromboembolic events, collectively referred to here as CVD, in both cisgender women and TW [78–80]. TW experience significantly more myocardial infarctions than cisgender women, cerebrovascular disease than cisgender men, and diabetes mellitus than cisgender women or men. Of 214 TW on FHT (HIV status not reported), 10% experienced a thromboembolic event. Alarmingly, obesity, cerebrovascular disease and myocardial infarction rates dramatically increase with FHT initiation [78], although event risk persists with continued therapy. In one study, 12% of TW experienced a CVD event at a mean age of 43 years and 11 years on FHT [81]. Active FHT increases CVD death risk 3-fold [82], and TW with traditional CVD risk factors who initiate FHT are at higher risk for subsequent CVD events [78]. CVD risk in TW may be higher with combined estrogen and progesterone or anti-androgen therapy vs estrogen alone, and is higher with oral than transdermal estrogens [83].
Further supporting a relationship with metabolic disease, estrogens increase subcutaneous and visceral adipose tissue [84], reduce lean mass [85], and increase adipocyte size [86]. Enlarged adipocyte size suggests adipocyte hypertrophy rather than hyperplasia, a mechanism of fat gain associated with insulin resistance, diabetes and dysfunctional fat [87]. Of note, pre-menopausal, non-obese, biological females can modulate adipocyte size and function better than biological males, in whom detrimental responses to fat gain predominate [88]. Therefore, adipocyte hypertrophy in TW on FHT may be a maladaptive response to FHT influenced by sex. Estrogens are also generally associated with lower HDL cholesterol (particularly with oral estrogens) [89] and insulin sensitivity, [84] and higher triglycerides [90], total cholesterol,[89] cortisol, and blood pressure [84].
Oral and (to a lesser extent) transdermal estrogens have been associated with increased circulating concentrations of interleukin (IL)-1, IL-6, IL-8, and d dimer [89, 91]. Higher IL-6 and d dimer levels are associated with mortality in HIV infection [92]. Estrogens are also associated with enhanced lipopolysaccharide (LPS)-mediated liver injury. As LPS increases expression of monocyte estrogen receptor-α in biological males, [93] persistent microbial translocation in PLWH [94] could lead to enhanced effects of FHT in TW living with HIV. Women with HIV also demonstrate less reduction and systemic inflammation and immune activation than men following ART initiation [95], a finding that is not completely understood but that could have an estrogen-mediated component.
Regarding immune cells, a positive dose response between estrogen and circulating immunoglobulin levels [96] has been observed. This relationship is thought to be mediated through estrogenic stimulation of IL-10 production by monocytes and subsequent B cell stimulation [96] and differentiation [97]. Estrogens promote B cell survival and reduce B cell apoptosis [98]. At high doses, estrogens also promote T helper cell type 2 and humoral responses, decrease natural killer cell cytotoxicity and have anti-inflammatory, anti-chemotactic neutrophil effects [37]. Data also suggest important sex differences in HIV reservoir characteristics and transcriptional activity that are at least partially driven by estradiol-mediated inhibition of HIV replication through an estrogen receptor-dependent mechanism [99]. Whether FHT has effects on reservoir dynamics among TW living with HIV is unknown.
Although the above data support potential FHT-induced immuno-metabolic disturbances and negative health consequences for TW living with HIV, these data are confounded by the fact that some immuno-metabolic alterations appear transient following estrogen initiation [89], whereas clinical events may occur many years later [81]. Finally, because access to appropriate FHT is not widespread, periods of ineffective androgen suppression and/or supra-physiologic estrogen levels from intermittent or unsupervised dosing may further immuno-metabolic perturbations [100].
Immuno-metabolic Effects of HIV
This review is not meant to represent a comprehensive review of immuno-metabolic disturbances in PLWH, however, a few notable findings should be highlighted. Monocyte activation is strongly associated with non-AIDS morbidity and mortality in treated HIV infection and is linked to increased circulating LPS concentrations following gut barrier breach and gut mucosa T lymphocyte depletion during acute HIV infection [94]. LPS increases expression of monocyte estrogen receptor-α in persons assigned a male sex at birth [93], possibly enhancing FHT effects in TW living with HIV. The LPS co-receptor soluble CD14 (sCD14) is associated with all-cause mortality in PLWH [101]. Levels of both sCD14 and the monocyte activation marker soluble CD163 (sCD163) are elevated in PLWH [102, 103], higher in cisgender women than men in most studies [95, 103], and associated with CVD burden [102, 103]. Taken together, these findings suggest that effects of monocyte activation in HIV infection may be compounded by FHT in TW.
Visceral and subcutaneous fat gain are frequent with antiretroviral therapy (ART) initiation and occur with most modern regimens [104, 105]. Fat dysfunction in PLWH is well-documented and multifactorial, with HIV- and ART-specific effects and their inflammatory sequelae contributing. Loss of lean mass quantity and quality occurs with HIV infection, ART, and FHT [106, 107]. Lean mass loss has been associated with mortality in the general population [108], and lower lean mass is associated with greater subclinical obstructive coronary stenosis in men with and without HIV [109]. Thus, changes in lean and fat mass with HIV infection, ART, and FHT may converge to promote additive or synergistic immuno-metabolic and cardiometabolic disturbances in PLWH.
HIV infection increases CVD risk. Compared to persons without HIV, adult PLWH have: higher rates of acute coronary events; more subclinical atherosclerosis and endothelial dysfunction [110–112]; and predominantly non-calcified coronary plaque burden, which may be more prone to rupture than calcified plaque [113]. Chronic inflammation, including monocyte and endothelial cell activation, is believed to play an important role in CVD risk among PLWH, with degree of subclinical atherosclerosis associated with higher IL-6 [114], sCD14, sCD163 [115], and soluble vascular cell adhesion molecule-1 concentrations [114]. Interestingly, estrogen may block HIV-induced activation of the vascular endothelium [116]. However, the combination of HIV, ART, and FHT on immuno-metabolic outcomes in TW is incompletely studied, and extrapolation from pre-menopausal women and/or persons without HIV is likely inaccurate.
In total, there is a significant body of research identifying a potential modifying role of both estrogens and HIV on immunologic and metabolic pathways, both beneficial and adverse, yet a lack of research designed to prospectively assess these effects in TW living with HIV, confounding interpretation. Additionally, alterations in these pathways are multifactorial, including contributions from non-estrogen sex hormone levels, such as progesterone and testosterone, as well as other drivers of immune activation and inflammation.
Exogenous Estrogens and ART
In cisgender women of reproductive age using oral contraception, low dose ethinyl estradiol exposure is reduced approximately 30% by ritonavir- or cobicistat-containing ART [117, 118], which may reduce hormonal contraceptive (and possibly FHT) efficacy[117, 118]. Interactions with higher doses of estrogen have not been studied, nor have the effects of these interactions on the feminizing efficacy of estrogens in TW. Importantly, drug-drug interactions between ART and 17-β estradiol are often extrapolated from ethinyl estradiol data, yet metabolism differences between these two estrogens may limit generalizability. Further, exogenous and endogenous hormones may influence drug-metabolizing enzymes [119], influencing ART metabolism. In preliminary data from an ongoing study of cisgender women in Malawi on efavirenz-based ART ( NCT03153709), higher estradiol levels were associated with lower plasma and cervico-vaginal fluid efavirenz concentrations, a finding believed to be mediated by estradiol-mediated P-glycoprotein induction [120]. In a recent phase III, randomized controlled trial of HIV PrEP, lower intracellular concentrations of tenofovir diphosphate (TFVdp) were observed in dry blood spots collected from TW vs cisgender men. However, investigators could not determine whether this finding reflected a hormone-drug interaction [43] or differential ART adherence [121], and standardized FHT was not provided in the context of the study. In a small study of 20 TW receiving PrEP and initiating low-dose FHT in Thailand (estradiol 2mg daily), blood tenofovir levels were 13% lower following FHT initiation, but above the level believed to be needed to confer protection against HIV infection [122]. In a separate study of 4 TW and 4 cisgender women, rectal TFVdp levels were lower in TW and had an inverse relationship with estradiol levels [123]. This finding is critical, as many TW take 4–5x the daily dose of estradiol used in the Thai study. These preliminary findings require further exploration both in larger studies and in TW living with HIV on ART.
Optimization of ART
It is unknown whether ART optimization recommendations for transgender persons should vary from those of the general population. Several basic considerations should be taken into account in addition to patient preference, HIV genotypic results, and ART side effect profiles. Although little-to-no prospective data exists using the FHT regimens and doses mostly likely be used by TW, unboosted integrase strand transfer inhibitors (INSTI) may be the optimal ART base for use with exogenous estrogens. This statement is based upon the fact that interactions between pharmacokinetic boosters and exogenous estrogens exist [124] such that regimens requiring ritonavir or cobicistat may be sub-optimal. In vitro evidence suggests that ritonavir may also antagonize 17-β estradiol [125], the currently preferred form of estrogen supplementation for FHT. Similarly, due to increased CVD risk in HIV and with estrogens (see above), abacavir-containing regimens may be suboptimal. While once daily dosing or single tablet regimens are preferred by most patients, they are especially important for persons with unstable food or housing and hectic daily life schedules, which are common for many PLWH, including transgender PLWH. Transgender persons also have high rates of depression, post-traumatic stress disorder, and anxiety [126, 127], suggesting that efavirenz-containing regimens may not be optimal. Given these facts, a once daily, unboosted INSTI in combination with an abacavir-sparing nucleoside reverse transcriptase inhibitor (NRTI) backbone may be the ideal ART regimen for TW living with HIV and receiving exogenous estrogens.
Alternatively, weight gain with INSTIs [128, 129], individualized resistance profiles and other factors may not permit use of unboosted regimens, and despite the potential to decrease estrogen levels [130], protease inhibitors will likely continue to play a role in ART optimization for some patients for the foreseeable future. While the effects of feminizing or masculinizing therapies on bone mineral density are likely beneficial [106, 131, 132], ART agents with improved bone health profiles, such as tenofovir alafenamide (TAF), can only be of additional benefit. In summary, an NRTI backbone with TAF and emtricitabine (FTC) or lamivudine (3TC) plus an unboosted INSTI may be the preferred choice for TW receiving FHT. Many similar factors likely apply to optimization of ART for TM, although data are even more limited, with assumptions of interactions between older protease inhibitors and testosterone extrapolated from in vitro studies [133] and/or data in cisgender men [130]. No randomized controlled trials have addressed optimization of ART for TW or TM, although at least two studies are underway ( NCT03348163, NCT03033836). Finally, whether or not actual adverse interactions between ART and hormonal therapies exist, concerns about such interactions may prevent proper medication adherence [134].
Data Specific to TM
Although the goal for testosterone therapy in TM is to achieve physiologic male dosing [135], this can require doses similar to supra-physiologic dosing in cisgender men. In men without HIV, supra-physiologic testosterone increases fat-free mass and muscle and bone strength [136] and decreases fat mass [137], but may exacerbate fatty liver disease or cause hepatotoxicity [138], cardiovascular complications, hypothyroidism, and adverse psychiatric effects [139]. In biological females, hyper-androgenic states are associated with visceral adiposity, hyperinsulinemia, insulin resistance, hyperglycemia, dyslipidemia, and increased CVD and breast cancer risk [140, 141]. However, many cisgender women living with HIV infection are hypo-androgenic [142], and whether testosterone supplementation could therefore reverse some of the negative effects associated with androgen deficiency in TM is an area that requires further study.
Interestingly, testosterone therapy in TM does not seem to be associated with the same severity of adverse events as supra-physiologic dosing in cisgender men and women [143]. Rather, limited data in persons without HIV infection suggest improved psychiatric functioning [144], minimal effect on glucose and lipid levels, potentially beneficial BMI effects [143], and no untoward aromatization of testosterone to estrogen [145]. Of note, a complicating factor for measurement of testosterone is that, while guidelines suggest targeting physiologic male levels [146, 147], these guidelines are based on total testosterone level measurement, which is less reliable in the setting of HIV infection than free testosterone measurement due to elevated sex-hormone binding globulin levels in PLWH. The optimal free testosterone level for a TM living with HIV is unknown.
Fertility Considerations
The complexities of family planning and fertility desires, including the cost and additional challenges of conception when one or more partners is living with HIV, is an important but likely under-discussed aspect of primary care for transgender persons. When feasible, initial discussion of family planning needs by the primary provider followed by referral to specialty care by a provider experienced in the care of both PLWH and transgender persons would be optimal. Briefly, cryopreservation of sperm prior to FHT is optimal for TW who desire biological children, although suppression of spermatogenesis by FHT seems to vary by dose, duration and timing of exposure [148], such that persons who do not cryopreserve sperm prior to FHT may still be able to produce functional sperm. Historical testosterone use does not seem to prevent successful pregnancy for TM who have not had hysterectomies, nor use of their own eggs for fertilization [149, 150].
Areas for Research
Huge data gaps exist to appropriately optimize care for transgender persons living with or at risk for HIV infection. The development of effective HIV prevention interventions for transgender persons depends on an accurate knowledge of epidemiologic patterns of HIV transmission. In addition to validated estimates of the size and characteristics of transgender populations worldwide, prevention research requires detailed information on how sexual risk behaviors, partnership formations, and sexual network structures affect the spread of HIV and STIs among TM, TW, and their partners. Epidemiologic data can then inform strategies to limit risk behavior within diverse partnership interactions, address the spread of HIV/STIs in larger network- and population-level contexts, and develop biomedical prevention technologies tailored to the specific biological needs of transgender persons. Social and structural frameworks for promoting the health and human rights of transgender people should address both short-term objectives of empowerment, education, and institutional reform, and long-term goals of promoting transgender representation at all levels. In clinical research, significant progress can be made by both supporting HIV-related studies specific to TW and TM and by including current gender markers (in addition to sex at birth) into data collection for new research studies, including FDA registrational trials. In this way, study-specific and pooled data can be used to inform care. Identification of appropriate control populations is another challenge: it is likely that age- and race-matched cisgender male and female controls are needed in addition to controls with and without HIV infection for both TW and TM, so that the complexities of interactions between HIV, ART, and endogenous vs exogenous hormones can be unraveled. This is no small feat, and will have to be approached incrementally, but should be a goal. Long-term cohort studies may be of great utility in addressing this need. Additional important yet unanswered questions include how aging and age-related disease onset/progression differ in transgender PLWH, and defining the long-term health trajectory of persons started on puberty blockers/gender-affirming hormonal therapies early in life. Additionally, FHT must be studied at the doses and frequencies used by TW, not extrapolated from contraception and post-menopausal hormone replacement therapy data. Finally, guidelines specific to the care of TW and TM living with or at risk for HIV infection should be repeatedly generated as data continue to evolve. Just as primary care differs somewhat for PLWH vs the general population, so may care for transgender persons by HIV serostatus.
Conclusions
Significant research is needed to optimize care for transgender persons living with or at risk for HIV infection. Epidemiologic data on the factors underlying HIV and STI prevalence among transgender persons are incomplete. Prevention research has only begun to consider the unique behavioral and biological risks for HIV infection encountered by transgender women and men, and challenges to address the social and structural contexts of HIV prevention and care remain formidable. As in vitro studies are not sufficient to recapitulate the full complexity and sequelae of HIV, ART and FHT interactions, carefully controlled in vivo trials are needed to optimally define the immunologic effects of FHT in the setting of ART-based HIV prevention and treatment. Together, these interventions will improve healthcare provision and quality of life for transgender persons.
Figure 2.
Contributions of HIV, antiretroviral therapy (ART) and feminizing hormonal therapy (FHT) to cardiometabolic disease in transgender women.
Table 1.
Selected epidemiologic studies of HIV prevalence in transgender populations.
| Methodology | Author(s) | Geographic Area | Target Population | HIV Prevalence |
|---|---|---|---|---|
|
Systematic Review/ Meta-Analysis |
||||
| Herbst et al. (2008)[1] | USA | Transgender Women (n=4; N=1,050)* |
27.7% (95% CI: 24.8–30.6%) |
|
| Operario, Soma, and Underhill (2008)[2] | USA, Europe, Asia-Pacific, Latin America (14 Countries) |
i) TW Non-Sex Workers (n=25; N=1,020) ii) TW Sex Workers (n=25; N=2,139) |
i) 14.7% (95% CI: 12.7–17.0%) ii) 27.3% (95% CI: 25.5–29.3%) |
|
| Baral et al. (2013)[3] | USA, Europe, Asia-Pacific, Latin America (15 Countries) |
Transgender Women (n=39; N=11,066) |
19.1% (95% CI: 17.4–20.7%) |
|
| Respondent-Driven Sampling | ||||
| Silva-Santisteban et al. (2012)[4] | Lima, Peru | Transgender Women (N=450) |
Crude: 29.8% RDS-Adjusted: 29.6% (95% CI: 22.6–38.7%) |
|
| Scheim, Bauer, and Travers (2017)[5] | Ontario, Canada | Trans-Masculine MSM (N=433) |
Crude: 0% RDS-Adjusted: N/A |
|
| Poteat et al. (2017)[6] | Sub-Sarahan Africa (8 Countries) |
Transgender Women (N=926) |
Crude: 25.0% RDS-Adjusted: Not Presented |
|
| Grinsztejn et al. (2017)[7] | Rio de Janeiro, Brazil | Transgender Women (N=345) |
Crude: 41.2% RDS-Adjusted: 31.2% (95% CI: 18.8–43.6%) |
|
n=Number of Studies Included in Meta-Analysis; N=Number of Subjects Included in Analysis
References
- 1.Gianella S, Sonya Haw J, Blumenthal J, Sullivan B, Smith D. The Importance of Human Immunodeficiency Virus Research for Transgender and Gender-Nonbinary Individuals. Clin Infect Dis 2018; 66(9):1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global Epidemiology of HIV Infection and Related Syndemics Affecting Transgender People. J Acquir Immune Defic Syndr 2016; 72 Suppl 3:S210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. The Lancet Infectious diseases 2013; 13(3):214–222. [DOI] [PubMed] [Google Scholar]
- 4.Poteat T, Wirtz AL, Radix A, Borquez A, Silva-Santisteban A, Deutsch MB, et al. HIV risk and preventive interventions in transgender women sex workers. Lancet 2015; 385(9964):274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav 2008; 12(1):1–17. [DOI] [PubMed] [Google Scholar]
- 6.Reisner SL, Poteat T, Keatley J, Cabral M, Mothopeng T, Dunham E, et al. Global health burden and needs of transgender populations: a review. Lancet 2016; 388(10042):412–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemons A, Beer L, Finlayson T, McCree DH, Lentine D, Shouse RL, et al. Characteristics of HIV-Positive Transgender Men Receiving Medical Care: United States, 2009–2014. Am J Public Health 2018; 108(1):128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reisner SL, Murchison GR. A global research synthesis of HIV and STI biobehavioural risks in female-to-male transgender adults. Glob Public Health 2016; 11(7–8):866–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Operario D, Burton J, Underhill K, Sevelius J. Men who have sex with transgender women: challenges to category-based HIV prevention. AIDS Behav 2008; 12(1):18–26. [DOI] [PubMed] [Google Scholar]
- 10.Bockting W, Miner M, Rosser BR. Latino men’s sexual behavior with transgender persons. Arch Sex Behav 2007; 36(6):778–786. [DOI] [PubMed] [Google Scholar]
- 11.Miller WM, Miller WC, Barrington C, Weir SS, Chen SY, Emch ME, et al. The Where and How for Reaching Transgender Women and Men Who Have Sex with Men with HIV Prevention Services in Guatemala. AIDS Behav 2017; 21(12):3279–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinsztejn B, Jalil EM, Monteiro L, Velasque L, Moreira RI, Garcia AC, et al. Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV 2017; 4(4):e169–e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Santisteban A, Raymond HF, Salazar X, Villayzan J, Leon S, McFarland W, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav 2012; 16(4):872–881. [DOI] [PubMed] [Google Scholar]
- 14.Scheim AI, Bauer GR, Travers R. HIV-Related Sexual Risk Among Transgender Men Who Are Gay, Bisexual, or Have Sex With Men. J Acquir Immune Defic Syndr 2017; 74(4):e89–e96. [DOI] [PubMed] [Google Scholar]
- 15.Poteat T, Ackerman B, Diouf D, Ceesay N, Mothopeng T, Odette KZ, et al. HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis. PLoS Med 2017; 14(11):e1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Valente TW, Shin HS, Weeks M, Zelenev A, Moothi G, et al. Overlooked Threats to Respondent Driven Sampling Estimators: Peer Recruitment Reality, Degree Measures, and Random Selection Assumption. AIDS Behav 2018; 22(7):2340–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group TG. Best Practices for Asking Questions to Identify Transgender and Other Gender Minority Resopndents on Population-Based Surveys. In. Edited by Herman JL. Los Angeles, CA: The Williams Institute; 2014. [Google Scholar]
- 18.Deutsch MB. Making It Count: Improving Estimates of the Size of Transgender and Gender Nonconforming Populations. LGBT Health 2016; 3(3):181–185. [DOI] [PubMed] [Google Scholar]
- 19.Young RM, Meyer IH. The trouble with “MSM” and “WSW”: erasure of the sexual-minority person in public health discourse. Am J Public Health 2005; 95(7):1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker R, Aggleton P, Perez-Brumer AG. The trouble with ‘Categories’: Rethinking men who have sex with men, transgender and their equivalents in HIV prevention and health promotion. Glob Public Health 2016; 11(7–8):819–823. [DOI] [PubMed] [Google Scholar]
- 21.Sandfort TG, Lane T, Dolezal C, Reddy V. Gender Expression and Risk of HIV Infection Among Black South African Men Who Have Sex with Men. AIDS Behav 2015; 19(12):2270–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stief M The Sexual Orientation and Gender Presentation of Hijra, Kothi, and Panthi in Mumbai, India. Arch Sex Behav 2017; 46(1):73–85. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Brumer AG, Oldenburg CE, Reisner SL, Clark JL, Parker RG. Towards ‘reflexive epidemiology’: Conflation of cisgender male and transgender women sex workers and implications for global understandings of HIV prevalence. Glob Public Health 2016; 11(7–8):849–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabet S, Sanchez J, Lama J, Goicochea P, Campos P, Rouillon M, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS 2002; 16(9):1271–1277. [DOI] [PubMed] [Google Scholar]
- 25.Tucker C, Arandi CG, Bolanos JH, Paz-Bailey G, Barrington C. Understanding social and sexual networks of sexual minority men and transgender women in Guatemala city to improve HIV prevention efforts. J Health Care Poor Underserved 2014; 25(4):1698–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Operario D, Nemoto T, Iwamoto M, Moore T. Risk for HIV and unprotected sexual behavior in male primary partners of transgender women. Arch Sex Behav 2011; 40(6):1255–1261. [DOI] [PubMed] [Google Scholar]
- 27.Wilson EC, Santos GM, Raymond HF. Sexual mixing and the risk environment of sexually active transgender women: data from a respondent-driven sampling study of HIV risk among transwomen in San Francisco, 2010. BMC Infect Dis 2014; 14:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisner SL, White Hughto JM, Pardee D, Sevelius J. Syndemics and gender affirmation: HIV sexual risk in female-to-male trans masculine adults reporting sexual contact with cisgender males. Int J STD AIDS 2016; 27(11):955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bockting WO, Robinson BE, Rosser BR. Transgender HIV prevention: a qualitative needs assessment. AIDS Care 1998; 10(4):505–525. [DOI] [PubMed] [Google Scholar]
- 30.Melendez RM, Pinto R. ‘It’s really a hard life’: love, gender and HIV risk among male-to-female transgender persons. Cult Health Sex 2007; 9(3):233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson EC, Garofalo R, Harris RD, Herrick A, Martinez M, Martinez J, et al. Transgender female youth and sex work: HIV risk and a comparison of life factors related to engagement in sex work. AIDS Behav 2009; 13(5):902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satcher MF, Segura ER, Silva-Santisteban A, Sanchez J, Lama JR, Clark JL. Partner-Level Factors Associated with Insertive and Receptive Condomless Anal Intercourse Among Transgender Women in Lima, Peru. AIDS Behav 2017; 21(8):2439–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reisner SL, Mimiaga MJ, Bland S, Mayer KH, Perkovich B, Safren SA. HIV risk and social networks among male-to-female transgender sex workers in Boston, Massachusetts. J Assoc Nurses AIDS Care 2009; 20(5):373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitt HM, Horne SG, Freeman-Coppadge D, Roberts T. HIV Prevention in Gay Family and House Networks: Fostering Self-Determination and Sexual Safety. AIDS Behav 2017; 21(10):2973–2986. [DOI] [PubMed] [Google Scholar]
- 35.Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med 2014; 47(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schust DJ, Quayle AJ, Amedee AM. Mucosal co-infections and HIV-1 transmission and pathogenesis. Current HIV research 2012; 10(3):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8(9):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Operario D, Nemoto T. HIV in transgender communities: syndemic dynamics and a need for multicomponent interventions. J Acquir Immune Defic Syndr 2010; 55 Suppl 2:S91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheim AI, Travers R. Barriers and facilitators to HIV and sexually transmitted infections testing for gay, bisexual, and other transgender men who have sex with men. AIDS Care 2017; 29(8):990–995. [DOI] [PubMed] [Google Scholar]
- 40.Socias ME, Marshall BD, Aristegui I, Romero M, Cahn P, Kerr T, et al. Factors associated with healthcare avoidance among transgender women in Argentina. Int J Equity Health 2014; 13(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant RM, Sevelius JM, Guanira JV, Aguilar JV, Chariyalertsak S, Deutsch MB. Transgender Women in Clinical Trials of Pre-Exposure Prophylaxis. J Acquir Immune Defic Syndr 2016; 72 Suppl 3:S226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2(12):e512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinsztejn B, Hoagland B, Moreira RI, Kallas EG, Madruga JV, Goulart S, et al. Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV 2018; 5(3):e136–e145. [DOI] [PubMed] [Google Scholar]
- 45.Rowniak S, Ong-Flaherty C, Selix N, Kowell N. Attitudes, Beliefs, and Barriers to PrEP Among Trans Men. AIDS Educ Prev 2017; 29(4):302–314. [DOI] [PubMed] [Google Scholar]
- 46.Sevelius JM, Keatley J, Calma N, Arnold E. ‘I am not a man’: Trans-specific barriers and facilitators to PrEP acceptability among transgender women. Glob Public Health 2016; 11(7–8):1060–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood SM, Lee S, Barg FK, Castillo M, Dowshen N. Young Transgender Women’s Attitudes Toward HIV Pre-exposure Prophylaxis. J Adolesc Health 2017; 60(5):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sevelius JM, Deutsch MB, Grant R. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc 2016; 19(7(Suppl 6)):21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reisner SL, Perez-Brumer AG, McLean SA, Lama JR, Silva-Santisteban A, Huerta L, et al. Perceived Barriers and Facilitators to Integrating HIV Prevention and Treatment with Cross-Sex Hormone Therapy for Transgender Women in Lima, Peru. AIDS Behav 2017; 21(12):3299–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bodsworth NJ, Price R, Davies SC. Gonococcal infection of the neovagina in a male-to-female transsexual. Sex Transm Dis 1994; 21(4):211–212. [DOI] [PubMed] [Google Scholar]
- 51.van der Sluis WB, Bouman MB, Gijs L, van Bodegraven AA. Gonorrhoea of the sigmoid neovagina in a male-to-female transgender. Int J STD AIDS 2015; 26(8):595–598. [DOI] [PubMed] [Google Scholar]
- 52.McGowan I, Cranston RD, Mayer KH, Febo I, Duffill K, Siegel A, et al. Project Gel a Randomized Rectal Microbicide Safety and Acceptability Study in Young Men and Transgender Women. PLoS One 2016; 11(6):e0158310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman PA, Roungprakhon S, Tepjan S. A social ecology of rectal microbicide acceptability among young men who have sex with men and transgender women in Thailand. J Int AIDS Soc 2013; 16:18476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gamarel KE, Reisner SL, Laurenceau JP, Nemoto T, Operario D. Gender minority stress, mental health, and relationship quality: a dyadic investigation of transgender women and their cisgender male partners. J Fam Psychol 2014; 28(4):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gamarel KE, Reisner SL, Darbes LA, Hoff CC, Chakravarty D, Nemoto T, et al. Dyadic dynamics of HIV risk among transgender women and their primary male sexual partners: the role of sexual agreement types and motivations. AIDS Care 2016; 28(1):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Operario D, Gamarel KE, Iwamoto M, Suzuki S, Suico S, Darbes L, et al. Couples-Focused Prevention Program to Reduce HIV Risk Among Transgender Women and Their Primary Male Partners: Feasibility and Promise of the Couples HIV Intervention Program. AIDS Behav 2017; 21(8):2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark JL, Segura ER, Oldenburg CE, Rios J, Montano SM, Perez-Brumer A, et al. Expedited Partner Therapy (EPT) increases the frequency of partner notification among MSM in Lima, Peru: a pilot randomized controlled trial. BMC Med 2017; 15(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark JL, Segura ER, Oldenburg CE, Salvatierra HJ, Rios J, Perez-Brumer AG, et al. Traditional and Web-Based Technologies to Improve Partner Notification Following Syphilis Diagnosis Among Men Who Have Sex With Men in Lima, Peru: Pilot Randomized Controlled Trial. J Med Internet Res 2018; 20(7):e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munoz-Laboy M, Severson N, Levine E, Martinez O. Latino men who have sex with transgender women: the influence of heteronormativity, homonegativity and transphobia on gender and sexual scripts. Cult Health Sex 2017; 19(9):964–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark JL, Perez-Brumer AG, Segura ER, Salvatierra HJ, Sanchez J, Lama JR. Anticipated Notification of Sexual Partners following STD Diagnosis among Men Who Have Sex with Men and Transgender Women in Lima, Peru: A Mixed Methods Analysis. PLoS One 2016; 11(9):e0163905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garofalo R, Johnson AK, Kuhns LM, Cotten C, Joseph H, Margolis A. Life skills: evaluation of a theory-driven behavioral HIV prevention intervention for young transgender women. J Urban Health 2012; 89(3):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reisner SL, Hughto JM, Pardee DJ, Kuhns L, Garofalo R, Mimiaga MJ. LifeSkills for Men (LS4M): Pilot Evaluation of a Gender-Affirmative HIV and STI Prevention Intervention for Young Adult Transgender Men Who Have Sex with Men. J Urban Health 2016; 93(1):189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garofalo R, Kuhns LM, Reisner SL, Biello K, Mimiaga MJ. Efficacy of an Empowerment-Based, Group-Delivered HIV Prevention Intervention for Young Transgender Women: The Project LifeSkills Randomized Clinical Trial. JAMA Pediatr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark JL, Salazar X, Perez-Brumer AG, Silva-Santisteban A, McLean S, Weintraub B, et al. Leveraging existing social networks to introduce and disseminate PrEP among transgender women in LIma, Peru: a qualitative inquiry In: International AIDS Society Durban, South Africa; 2016. [Google Scholar]
- 65.Mehrotra ML, Rivet Amico K, McMahan V, Glidden DV, Defechereux P, Guanira JV, et al. The Role of Social Relationships in PrEP Uptake and Use Among Transgender Women and Men Who Have Sex with Men. AIDS Behav 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwahng SJ, Allen B, Zadoretzky C, Barber H, McKnight C, Des Jarlais D. Alternative kinship structures, resilience and social support among immigrant trans Latinas in the USA. Cult Health Sex 2018:1–15. [DOI] [PubMed] [Google Scholar]
- 67.Perez-Brumer A, Silva-Santisteban A, McLean S, Salazar X, Prenner J, Lama JR, et al. “Como conejillo de indias”: critical role of medical and research mistrust in acceptability of PrEP among transgender women in lima, Peru In: International AIDS Society. Durban, South Africa; 2016. [Google Scholar]
- 68.White Hughto JM, Reisner SL, Pachankis JE. Transgender stigma and health: A critical review of stigma determinants, mechanisms, and interventions. Soc Sci Med 2015; 147:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa AB, Fontanari AMV, Catelan RF, Schwarz K, Stucky JL, da Rosa Filho HT, et al. HIV-Related Healthcare Needs and Access Barriers for Brazilian Transgender and Gender Diverse People. AIDS Behav 2018; 22(8):2534–2542. [DOI] [PubMed] [Google Scholar]
- 70.Reback CJ, Clark K, Holloway IW, Fletcher JB. Health Disparities, Risk Behaviors and Healthcare Utilization Among Transgender Women in Los Angeles County: A Comparison from 1998–1999 to 2015–2016. AIDS Behav 2018; 22(8):2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemoto T, Operario D, Keatley J, Nguyen H, Sugano E. Promoting health for transgender women: Transgender Resources and Neighborhood Space (TRANS) program in San Francisco. Am J Public Health 2005; 95(3):382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schulden JD, Song B, Barros A, Mares-DelGrasso A, Martin CW, Ramirez R, et al. Rapid HIV testing in transgender communities by community-based organizations in three cities. Public Health Rep 2008; 123 Suppl 3:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrington C, Acevedo R, Donastorg Y, Perez M, Kerrigan D. ‘HIV and work don’t go together’: Employment as a social determinant of HIV outcomes among men who have sex with men and transgender women in the Dominican Republic. Glob Public Health 2017; 12(12):1506–1521. [DOI] [PubMed] [Google Scholar]
- 74.Hill BJ, Crosby R, Bouris A, Brown R, Bak T, Rosentel K, et al. Exploring transgender legal name change as a potential structural intervention for mitigating social determinants of health among transgender women of color. Sex Res Social Policy 2018; 15(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Logie CH, Wang Y, Lacombe-Duncan A, Jones N, Ahmed U, Levermore K, et al. Factors associated with sex work involvement among transgender women in Jamaica: a cross-sectional study. J Int AIDS Soc 2017; 20(1):21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol 2014; 170(6):809–819. [DOI] [PubMed] [Google Scholar]
- 77.Wilson R, Jenkins C, Miller H, Carr S. The effect of oestrogen on cytokine and antioxidant levels in male to female transsexual patients. Maturitas 2006; 55(1):14–18. [DOI] [PubMed] [Google Scholar]
- 78.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol 2013; 169(4):471–478. [DOI] [PubMed] [Google Scholar]
- 79.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. European heart journal 2008; 29(16):2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA 2000; 284(1):72–78. [DOI] [PubMed] [Google Scholar]
- 81.Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012; 9(10):2641–2651. [DOI] [PubMed] [Google Scholar]
- 82.Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 2011; 164(4):635–642. [DOI] [PubMed] [Google Scholar]
- 83.Gooren L, Lips P. Conjectures concerning cross-sex hormone treatment of aging transsexual persons. J Sex Med 2014; 11(8):2012–2019. [DOI] [PubMed] [Google Scholar]
- 84.Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003; 58(5):562–571. [DOI] [PubMed] [Google Scholar]
- 85.Klaver M, Dekker M, de Mutsert R, Twisk JWR, den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia 2017; 49(5). [DOI] [PubMed] [Google Scholar]
- 86.Elbers JM, de Jong S, Teerlink T, Asscheman H, Seidell JC, Gooren LJ. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism 1999; 48(11):1371–1377. [DOI] [PubMed] [Google Scholar]
- 87.Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 2014; 15(4):277–287. [DOI] [PubMed] [Google Scholar]
- 88.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015; 23(7):1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson R, Spiers A, Ewan J, Johnson P, Jenkins C, Carr S. Effects of high dose oestrogen therapy on circulating inflammatory markers. Maturitas 2009; 62(3):281–286. [DOI] [PubMed] [Google Scholar]
- 90.Elamin MB, Garcia MZ, Murad MH, Erwin PJ, Montori VM. Effect of sex steroid use on cardiovascular risk in transsexual individuals: a systematic review and meta-analyses. Clin Endocrinol (Oxf) 2010; 72(1):1–10. [DOI] [PubMed] [Google Scholar]
- 91.Kluft C, Meijer P, LaGuardia KD, Fisher AC. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception 2008; 77(2):77–83. [DOI] [PubMed] [Google Scholar]
- 92.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gibert CL, et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? J Acquir Immune Defic Syndr 2016; 72(2):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campesi I, Marino M, Montella A, Pais S, Franconi F. Sex Differences in Estrogen Receptor alpha and beta Levels and Activation Status in LPS-Stimulated Human Macrophages. J Cell Physiol 2017; 232(2):340–345. [DOI] [PubMed] [Google Scholar]
- 94.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 2013; 26(1):2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, et al. Sex-Related Differences in Inflammatory and Immune Activation Markers Before and After Combined Antiretroviral Therapy Initiation. J Acquir Immune Defic Syndr 2016; 73(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. The Journal of allergy and clinical immunology 1999; 103(2 Pt 1):282–288. [DOI] [PubMed] [Google Scholar]
- 97.Straub RH. The Complex Role of Estrogens in Inflammation. Endocrine Reviews 2007; 28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 98.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007; 28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 99.The United States President’s Emergency Plan for AIDS Relief, Key Populations: Ensuring Human Rights & Leaving No One Behind. In; 2013.
- 100.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ 3rd, Spack NP, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94(9):3132–3154. [DOI] [PubMed] [Google Scholar]
- 101.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211(8):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208(11):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dube MP, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis 2016; 62(7):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis 2011; 53(2):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fighera TM, da Silva E, Lindenau JD, Spritzer PM. Impact of Cross-Sex Hormone Therapy on Bone Mineral Density and Body Composition in Transwomen. Clin Endocrinol (Oxf) 2018. [DOI] [PubMed] [Google Scholar]
- 107.Erlandson KM, Fiorillo S, Masawi F, Scherzinger A, McComsey GA, Lake JE, et al. Antiretroviral initiation is associated with increased skeletal muscle area and fat content. Aids 2017; 31(13):1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F, Bartels SJ. Low Lean Mass With and Without Obesity, and Mortality: Results From the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci 2017; 72(10):1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tibuakuu M, Zhao D, Saxena A, Brown TT, Jacobson LP, Palella FJ Jr., et al. Low thigh muscle mass is associated with coronary artery stenosis among HIV-infected and HIV-uninfected men: The Multicenter AIDS Cohort Study (MACS). Journal of cardiovascular computed tomography 2018; 12(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. Aids 2010; 24(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13(8):453–468. [DOI] [PubMed] [Google Scholar]
- 112.Mazzuca P, Caruso A, Caccuri F. HIV-1 infection, microenvironment and endothelial cell dysfunction. The new microbiologica 2016; 39(3):163–173. [PubMed] [Google Scholar]
- 113.Pundziute G, Schuijf JD, Jukema JW, Decramer I, Sarno G, Vanhoenacker PK, et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. European heart journal 2008; 29(19):2373–2381. [DOI] [PubMed] [Google Scholar]
- 114.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009; 49(7):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204(8):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee YW, Eum SY, Nath A, Toborek M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc Res 2004; 63(1):139–148. [DOI] [PubMed] [Google Scholar]
- 117.Scarsi KK, Darin KM, Chappell CA, Nitz SM, Lamorde M. Drug-Drug Interactions, Effectiveness, and Safety of Hormonal Contraceptives in Women Living with HIV. Drug Saf 2016; 39(11):1053–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nanda K, Stuart GS, Robinson J, Gray AL, Tepper NK, Gaffield ME. Drug interactions between hormonal contraceptives and antiretrovirals. Aids 2017; 31(7):917–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams ET, Leyk M, Wrighton SA, Davies PJ, Loose DS, Shipley GL, et al. Estrogen regulation of the cytochrome P450 3A subfamily in humans. The Journal of pharmacology and experimental therapeutics 2004; 311(2):728–735. [DOI] [PubMed] [Google Scholar]
- 120.Cottrell ML CA, Chinula L, Msika A, Tegha G, Stanczyk F, Kourtis AP, Tang JH. Female genital tract efavirenz exposure negatively correlates with serum estradiol levels in Malawian women. Abstracts of AIDS 2018 2018. [Google Scholar]
- 121.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet infectious diseases 2014; 14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hiransuthikul AHK, Kerr S, Thammajaruk N, Pankam T, Janamnuaysook R, Mills S, Vannakit S, Phanuphak P, Phanuphak N, iFACT Study Team. Drug-drug interactions between the use of feminizing hormone therapy and pre-exposure prophylaxis among transgender women: The iFACT study. Abstracts of AIDS 2018 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cottrell MLPH, Maffuid K, Poliseno A, White N, Sykes C, Nelson JA, Peery A, Dellon E, Hightow-Weidman L, Adams JL, Gay C, Kashuba AD. Altered TDF/FTC pharmacology in a transgender female cohort: Implications for PrEP. Abstracts of AIDS 2018 2018. [Google Scholar]
- 124.Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. British journal of clinical pharmacology 1998; 46(2):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiang J, Wang Y, Su K, Liu M, Hu PC, Ma T, et al. Ritonavir binds to and downregulates estrogen receptors: molecular mechanism of promoting early atherosclerosis. Exp Cell Res 2014; 327(2):318–330. [DOI] [PubMed] [Google Scholar]
- 126.Brown GR, Jones KT. Mental Health and Medical Health Disparities in 5135 Transgender Veterans Receiving Healthcare in the Veterans Health Administration: A Case-Control Study. LGBT Health 2016; 3(2):122–131. [DOI] [PubMed] [Google Scholar]
- 127.Budge SL, Adelson JL, Howard KA. Anxiety and depression in transgender individuals: the roles of transition status, loss, social support, and coping. J Consult Clin Psychol 2013; 81(3):545–557. [DOI] [PubMed] [Google Scholar]
- 128.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr 2017; 76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bakal DCL, Luz PM, Clark JL, De Boni RB, Cardoso SW, Veloso VG, Lake JE, Grinsztejn B. HIV Disease Severity and Integrase Inhibitor Use are Drivers of Obesity Following Antiretroviral Therapy Initiation. Journal of Antimicrobial Chemotherapy 2018. [Google Scholar]
- 130.Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2017.
- 131.Wiepjes CM, Vlot MC, Klaver M, Nota NM, de Blok CJ, de Jongh RT, et al. Bone Mineral Density Increases in Trans Persons After 1 Year of Hormonal Treatment: A Multicenter Prospective Observational Study. J Bone Miner Res 2017; 32(6):1252–1260. [DOI] [PubMed] [Google Scholar]
- 132.Dolan Looby SE, Collins M, Lee H, Grinspoon S. Effects of long-term testosterone administration in HIV-infected women: a randomized, placebo-controlled trial. Aids 2009; 23(8):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Inaba T, Fischer NE, Riddick DS, Stewart DJ, Hidaka T. HIV protease inhibitors, saquinavir, indinavir and ritonavir: inhibition of CYP3A4-mediated metabolism of testosterone and benzoxazinorifamycin, KRM-1648, in human liver microsomes. Toxicol Lett 1997; 93(2–3):215–219. [DOI] [PubMed] [Google Scholar]
- 134.Braun HM, Candelario J, Hanlon CL, Segura ER, Clark JL, Currier JS, et al. Transgender Women Living with HIV Frequently Take Antiretroviral Therapy and/or Feminizing Hormone Therapy Differently Than Prescribed Due to Drug-Drug Interaction Concerns. LGBT Health 2017; 4(5):371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Irving A, Lehault WB. Clinical pearls of gender-affirming hormone therapy in transgender patients. Ment Health Clin 2017; 7(4):164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 1996; 335(1):1–7. [DOI] [PubMed] [Google Scholar]
- 137.Klaver M, de Blok CJM, Wiepjes CM, Nota NM, Dekker M, de Mutsert R, et al. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol 2018; 178(2):165–173. [DOI] [PubMed] [Google Scholar]
- 138.Niedfeldt MW. Anabolic Steroid Effect on the Liver. Curr Sports Med Rep 2018; 17(3):97–102. [DOI] [PubMed] [Google Scholar]
- 139.Hall RC, Hall RC. Abuse of supraphysiologic doses of anabolic steroids. Southern medical journal 2005; 98(5):550–555. [DOI] [PubMed] [Google Scholar]
- 140.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2005; 97(10):755–765. [DOI] [PubMed] [Google Scholar]
- 141.Tripathy P, Sahu A, Sahu M, Nagy A. Ultrasonographic evaluation of intra-abdominal fat distribution and study of its influence on subclinical atherosclerosis in women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol 2017; 217:18–22. [DOI] [PubMed] [Google Scholar]
- 142.Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, et al. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 1998; 83(4):1312–1318. [DOI] [PubMed] [Google Scholar]
- 143.Chan KJ, Liang JJ, Jolly D, Weinand JD, Safer JD. Exogenous Testosterone Does Not Induce or Exacerbate the Metabolic Features Associated with Pcos among Transgender Men. Endocr Pract 2018; 24(6):565–572. [DOI] [PubMed] [Google Scholar]
- 144.Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol 2015; 83(1):143–156. [DOI] [PubMed] [Google Scholar]
- 145.Chan KJ, Jolly D, Liang JJ, Weinand JD, Safer JD. Estrogen Levels Do Not Rise with Testosterone Treatment for Transgender Men. Endocr Pract 2018; 24(4):329–333. [DOI] [PubMed] [Google Scholar]
- 146.Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016.
- 147.Hembree WCC-KP, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. JCEM 2017; 102(11):3869–3903. [DOI] [PubMed] [Google Scholar]
- 148.Schneider F, Kliesch S, Schlatt S, Neuhaus N. Andrology of male-to-female transsexuals: influence of cross-sex hormone therapy on testicular function. Andrology 2017; 5(5):873–880. [DOI] [PubMed] [Google Scholar]
- 149.Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol 2014; 124(6):1120–1127. [DOI] [PubMed] [Google Scholar]
- 150.Light A, Wang LF, Zeymo A, Gomez-Lobo V. Family planning and contraception use in transgender men. Contraception 2018. [DOI] [PubMed] [Google Scholar]