Abstract
The association between tuberculosis (TB) and diabetes mellitus (DM) had a common place in the literature up to the first half of the 20th century, but virtually disappeared with the discovery of insulin to treat DM and antibiotics to cure TB. In the late 1990s the literature began to re-emerge with the worldwide increase in type 2 DM, particularly in TB-endemic countries. Today, type 2 DM is the most prevalent comorbidity among TB patients and the World Health Organization considers it a threat to TB control. We summarize the literature on TB and DM up to the 1960s. Then we evaluate unique aspects of this comorbidity in older times, such as the frequent diabetic comas that suggest challenges for proper DM management as insulin was being implemented, or the absence of antibiotics to cure TB. Despite the unique aspects of each study period, the literature across times is consistent in key aspects of the association. Namely, a higher TB prevalence among DM (versus non-DM patients), the importance of glucose control and chronic DM on TB susceptibility and the higher risk of death among patients with the comorbidity. From the older literature, we can infer the likely contribution of type 1 DM to TB (in addition to type 2), regardless of their differing autoimmune or metabolic pathophysiology, respectively. Furthermore, in the older literature there was a notable reporting of DM development among TB patients, even though DM usually preceded TB. This observation deserves further epidemiological and basic studies to elucidate this intriguing aspect of the relationship between TB and DM.
Keywords: tuberculosis, type 2 diabetes, type 1 diabetes, history, death, treatment outcomes
1. Introduction
The medical profession has been intrigued for centuries by the complex interactions between the alterations in the metabolism caused by diabetes mellitus (DM), its associated impairment in immune function, and its relationship to heightened susceptibility to infections, including pulmonary tuberculosis (TB). In the early and mid-years of the 20th century, hallmark events occurred in the management of patients with DM and TB: the introduction of insulin into the clinical practice in 1923 to improve glucose control, and the discovery and implementation of effective antibiotics for the treatment of TB between the 1940s and 1970s [1]. In this review, we provide a historical perspective on the epidemiology, clinical observations and management of patients with DM and TB. Then we present a summary of the literature on the association between both diseases, with emphasis on studies conducted up to the first half of the 20th century. Then we evaluate how these historical perspectives enrich our understanding of this re-emerging association.
2. Overview and brief history of DM and TB
DM is a syndrome characterized by chronic increase in blood glucose as a result of defects in insulin secretion, the action of insulin or a combination of both [2]. DM has been classically divided in two major groups: Type 1 DM is an autoimmune disease the results in the destruction of pancreatic beta cells and inability to produce insulin. Type 2 DM is characterized by insulin resistance and associated with obesity [3]. A key aspect in the history of DM was the discovery of insulin, which started in 1869 when a German medical student, Paul Langerhans , described the pancreatic islets [4]. After several failed attempts by numerous researchers to use the pancreatic islet concentrates to treat DM, Frederic Banting and John Macleod at London Western University successfully dogs after pancreatectomy [5]. They reported these findings in 1921 at the meeting of the produced pancreatic extracts that prolonged the survival of American Medical Association. There were subsequent improvements of the extract, and in 1923 the pharmaceutical company Eli Lilly began the mass production of insulin in the US, and Banting and Macleod received the Nobel price for their achievement [4].
DM is currently a global pandemic, and its worldwide prevalence has increased from 4-5% in 1980 to 8-9% in 2014 [6]. In the US, DM has also increased between 2002 and 2012 among younger populations, aged <20 years, at a relative annual rate of about 1.8% for type 1 DM and 4.8% in type 2 DM, after adjusting for confounders [7]. The diagnostic criteria for DM has evolved over time as its underlying pathophysiology is better understood. For centuries, the diagnosis was based on symptoms (e.g. thirst, polyuria, weight loss and comma) and the presence of sweet urine [8]. In the 18th century the cause of sweetness in urine was discovered to be due to excess sugar, and to be accompanied by high blood glucose [8]. In contemporary times, the biomarkers to define DM have been further evolving, with the use of fasting plasma glucose, a 2h oral glucose tolerance test, and most recently, HbA1c [2, 9]. Collectively, the introduction of new biomarkers and updated cut-off points for diabetes diagnosis is likely to increase prevalence estimates, but there is acceptance of a worldwide net increase in DM that is driven by excess population obesity [9].
Chronic hyperglycemia is characterized by glucotoxicity that affects multiple organs leading to a number of comorbidities (i.e. micro- and macrovascular diseases, gestational DM, depression). Chronic hyperglycemia also affects immune surveillance, which contributes to the higher risk of morbidity and mortality from certain types of infections. Today, type 1 DM is treated with insulin and T2D is most commonly managed with oral anti-diabetic drugs like metformin to increase peripheral glucose use and sulfonyureas to promote pancreatic insulin secretion. Insulin is ultimately added when the patient’s pancreas has lost capacity to produce insulin [2]. In this review, we focus on the association between DM (types 1 and 2) and pulmonary TB, a comorbidity reported since antiquity and a re-emerging concern in contemporary times [10].
Mycobacterium tuberculosis is the etiological agent of TB, an airborne infectious disease. In Ancient Greece, Hippocrates described TB as phthisis (“to waste away”). Avicenna (980-1037) described TB as caused by “body humors perverted on their behavior” [11]. The infectious nature of TB was demostrated by Jean-Antoine Villemin in 1865, when he observed that recruits in barracks developed TB more often, and showed that rabbits inoculated with “purulent material” from patients that died of TB had extensive disease on autopsy [12]. Robert Koch discovered the microbe behind TB, Mycobacterium tuberculosis, and presented his findings to the Society of Physiology in Berlin in 1882 [13]. TB was widespread in Europe after the decline of the Roman Empire. The industrial revolution was associated with overcrowding and poor ventilated living areas and around 1838 up to one-third of the English tradesman and employees died of TB. Today, TB is the ninth leading cause of death in the world, with about 10.4 million new TB cases and 1.67 million deaths a year [14]. TB therapy has evolved over centuries. During the 19th century, sanatoria were developed. In 1944 streptomycin and para-aminosalicilic acid were discovered. This success was followed by the discovery of isoniazid in 1951, ethambutol and rifampin in the 1960’s, and pyrazinamide in 1972 [1].
3. TB and DM: What we learned up to the first half of the 20th century
The earliest reports on the association between DM and TB include the descriptions by the Persian philosopher Avicenna in the 10th century, and an Indian siddhar, Yugimahamuni, who described the progression of obesity to impotence, thirst, glycosuria, and ultimately to TB [11, 15]. The association between TB and DM had a common place in the literature up the first half of the 20th century, but it diminished with the discovery and use of insulin for DM in the 1920s, and virtually disappeared with the implementation of antibiotics to treat TB in the 1960s. In the 1980s the advent of HIV/AIDS took center stage in the TB literature and remains the largest risk factor for TB at the individual level, with approximately 20-37 fold higher risk of TB [16]. But with the increasing number of DM patients worldwide, particularly in countries where TB is also endemic, there has been a re-emergence of literature on the concurrence of TB and DM since the late 1990s, with an exponential increase in the number of publications in the most recent years[19, 20, 21] (Figure 1). Details on the current state of the association between both diseases are provided elsewhere [17, 18]. In this section, we focus on the studies published in the latter part of the 19th century and first half of the 20th century, with emphasis on four studies published between 1931 and 1958 that provide the most detailed descriptions for these times. We first describe their study designs to point out differences in the methodology used during these older times (Table 1), and then compare their findings to the contemporary literature (late 1990s to today).
Figure 1. The re-emerging association between TB and DM based on indexed publications.
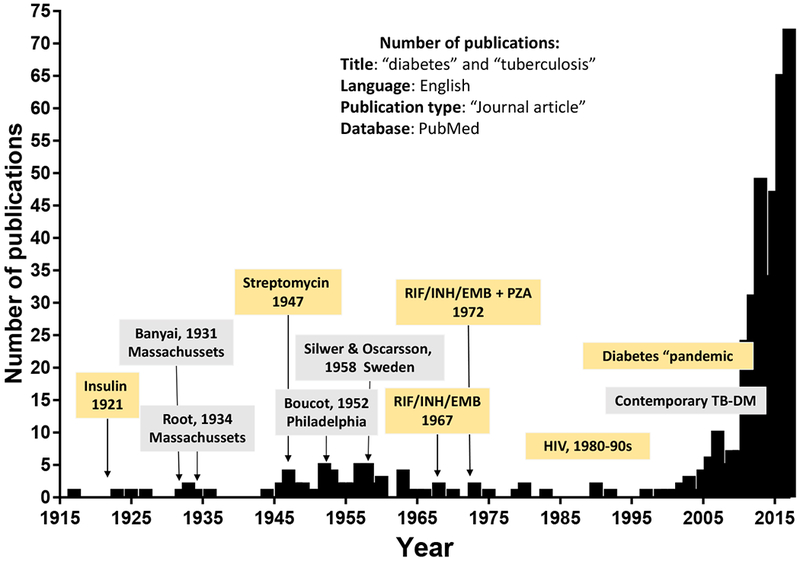
The number of “journal articles” published in English with titles containing the words “diabetes” and “tuberculosis” and indexed in PubMed between 1900 and 2017 is plotted by year. The association between TB and DM was first reported by Avicenna in the 10th century (not shown) and the comorbidity had a common place in the literature up to the first half of the 20th century. In 1921 insulin was introduced to treat DM, which appeared to reduce the prevalence of TB in these patients due improvements in glucose control. With the additional discovery of antibiotics to cure TB between the 1940s and 1970s, the literature on the comorbidity virtually disappeared. In the 1980s and 1990s the emergence of HIV took center stage in the TB literature, but in the early 2000s there was a re-emergence in publications that paralleled the contemporary DM pandemic. RIF, rifampicin; INH, isoniazid, EMB, ethambutol; PZA, pyrazinamide.
Table 1.
Study designs from four detailed studies published between 1931 and 1958
| Author (Year) | Study design | Study period | DM definition | TB definition | n | Age groups |
|---|---|---|---|---|---|---|
| A.L. Banyai (1931) | A. Prevalence of DM in TB patients admitted to the Muirdale Sanatorium, Winsconsin, USA | 1923-1929 | Not specified | Admission to sanatorium | 5,224 a | All ages, age groups not specified |
| B. Summary of previous reports on the prevalence of TB-DM | ||||||
| H. Root (1934) | A. Prevalence of TB from autopsies in DM patients at New England Deaconess Hospital | 1880-1932 | Not specified | Autopsy | 1,000 b | Not specified |
| B. Prevalence of TB by chest x-ray in DM patients at New England Deaconess Hospital | 1880-1923 | Not specified | Chest X-ray | 1,659 b | Not specified | |
| C. Summary of previous reports on the prevalence of TB in DM patients | 1859-1932 | Not specified | All based on autopsies | see Table 2 | Not specified | |
| K. Boucot et al. (1952) | Prevalence of TB in all DM patients consecutively presenting to four hospital clinics in Philadelphia. Compare to database from population survey of 71,767 Philadelphia industrial health workers 1942-9145 (not shown in table). | 1945-1947 | Screening: FBS >120mg/dl or RBS >170mg/dL with glycosuria Diagnosis:RBS > 200 mg/dL or abnormal OGTT | Roentgenography and Photofluography | 3,106 b | < 20 yrs, n=108/3106 (3.4%) 20-40yrs, n=324/3106 (10.4%) 40-60 yrs, n=1380/3106 (44.4%) 60-80 yrs, n=1245/3106 (40%) > 80 yrs, n=28/3106 (0.9%) unknown, n=23/3106 (0.07%) |
| H. Silwer & P.N. Oscarsson (1958) | A. Cross-sectional prevalence of DM in all the population of the country of Kristianstad, Sweden | 1954-1958 | Hospital records; DM referrals | not specified | 260,491 c | Unspecified |
| B. Cross sectional prevalence of TB in DM patients enrolled in the Kristianstad study | 1954-1958 | Same as study A. | Roentgenography, Fluoroscopy and Photofluorography | 1,326 b,d | < 40 yrs, n=231/1270 (18.1%) 40-60yrs, n=368/1270 (28.9%) 60-80 yrs, n=671/1270 (52.8%) |
|
TB patients only;
DM patients only;
General population;
56/1,326 not screened for TB, so total studied were 1,270; FBS, Fasting blood sugar; RBS, Random blood sugar; OGTT, oral glucose tolerance test, with cut-off for “abnormal” not specified; n= total sample size
3.1. Study designs
In 1931 Andrew L. Banyai published a literature review on the prevalence, clinical presentation and outcomes of patients with TB and DM [19]. In the same manuscript, he *described the findings from a cross-sectional study he conducted on the association between TB and DM among 5,224 patients of all ages admitted with TB to the Muirdale sanatorium in Wisconsin between 1923 and 1929 (Table 1) [19].
In 1932 Howard Root reported the results of two studies he conducted to evaluate TB prevalence in DM patients at the New England Deaconess Hospital in Massachusetts. One was from 1,000 autopsies and the second one was from 1,659 chest x-rays (Table 1)[20]. He studied individuals of all ages but sample sizes were not specified. The information he collected was compared to other epidemiological studies, vital statistics and TB surveillance reports in the US. He also summarized the data from previous prevalence studies of TB among DM patients or in the general population (Figure 2).
Figure 2. Meta-analysis of TB prevalence by DM status based on autopsy studies (1859-1934).
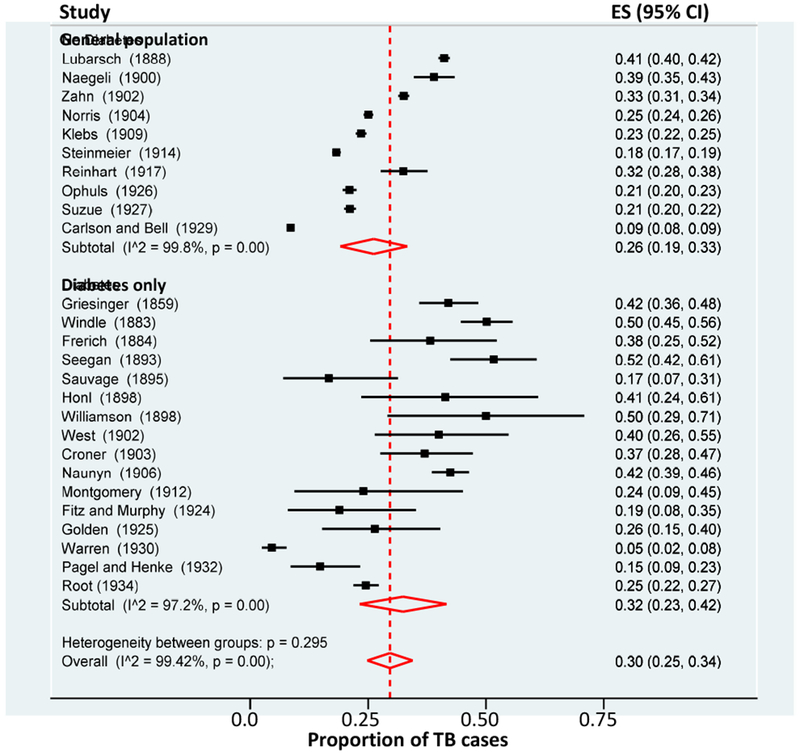
The proportion of TB in the general population or in DM patients only was evaluated for studies published between 1859 and 1934. All studies conducted autopsies to diagnose TB. Studies are listed in chronological order to show the parallel reduction in TB prevalence in the general population or in DM patients at the turn of the 20th century. TB refers to autopsy findings suggestive of active or inactive TB; ES, Estimation of proportion; Data on TB proportion and population sizes was obtained from the literature reviews from Root (1932) and the autopsy study done by Root (1932).
In 1952, Katherine Boucot and collaborators published the results of “The Philadelphia Study” conducted from December 1945 to 1957. They enrolled diabetic patients (n=3,106) of all ages that were consecutively referred by private practice providers to four hospital clinics in Philadelphia and screened them for TB. TB (active or inactive) was based on photofluorography, a photograph of x-rays under fluorescent light to show the extent of lung involvement. DM was defined as blood glucose over 200 mg/dL or an abnormal oral glucose tolerance test (Table 1). There were unique aspects of this study’s design for these times. First, they used blood glucose to diagnose DM. Second, they classified glucose control based on the last five blood sugar measurements, as follows: Poor, if at least four readings were ≥200 mg/dL; fair, if at least three measurements were ≥200 mg/dL; and good, if all are <200 mg/dL. Third, they defined DM severity based on the requirement for insulin: none, 1-40 units or more than 40 units. Fourth, the physicians reading the photofluorography were blinded regarding the DM status of the participants. Fifth, they did an age and sex-matched comparison of the prevalence of TB among DM patients against an existing database from a survey reporting the prevalence of TB in 71,767 Philadelphia industrial health workers.
In 1958, Hans Silwer and P. Oscarsson performed an epidemiological study in two phases [21]. First they evaluated the prevalence of DM in the entire county of Kristianstad in Sweden. They used several strategies to identify all the DM patients. First, they searched hospital records for patients with insulin prescription, since there was universal coverage if the patient presented a certificate of their diagnosis. Second, they sent letters to practices outside the hospitals to detect DM patients not requiring insulin. Third, they enlisted social workers and parish members to locate diabetics that would not be otherwise found. Then they used census data to calculate the prevalence of DM in the county. In the second phase, they conducted a nested study to determine the prevalence of TB among individuals with DM. From 1326 diabetics identified, 1270 underwent radiological examination to investigate evidence of TB infection.
Several observations distinguish the study designs from the older literature. First, TB diagnosis relied largely on radiological methods, autopsy findings or referrals to sanatoriums. Thus, TB included active TB, lesions from healed TB, other forms of TB disease or latent TB infection. These methods lack specificity and the authors noted a possible over-diagnosis of TB. In current times, clinical signs and symptoms compatible with TB are complemented with the detection of acid fast bacilli by smear microscopy or pathology, and when available, by confirmation of M. tuberculosis by culture or detection of nucleic acids. Today we use the tuberculin skin test (developed in 1934) and interferon gamma release assays to help diagnose infection in non-symptomatic patients [22]. Second, a case definition for DM was rarely specified in the older literature and HbA1c was not available. In the Philadelphia study, DM severity was based on the units of insulin required, but access to insulin seemed more readily available to the higher classes. Third, studies suffered from selection bias due to the inclusion of convenience samples, with heterogeneity in host characteristics, including the inclusion of individuals dying at hospitals, a scenario more common among the lower socioeconomic classes. Today, most studies on TB and DM are done in adults, given the higher prevalence of the co-morbidity among those 35 years or older.
3.2. Results of older studies and comparison with the contemporary literature
3.2.1. Increasing DM prevalence among TB patients after the introduction of insulin
Banyai noted that around 1919 the prevalence of DM among TB patients in sanatoriums was about 0.17- 0.33% [19]. In his study in the 1930’s this proportion was about two-fold higher (0.59%) in the general population, and he attributed this change to the increased number of DM patients and the better life expectancy of TB-DM patients since the introduction of insulin. Today this proportion is significantly higher, with some communities reporting nearly 40% or 50% DM among TB patients [23–25]. This sharp surge in DM in less than one century is a reflection of the worldwide increase in obesity, particularly in lower and middle income countries where TB is endemic [17].
3.2.2. Higher TB prevalence among DM versus non-DM patients
In the 19th century, a challenge to determine if TB was more prevalent among DM versus non-DM individuals was the high background of past or present TB in the general population [20]. For example, a study examining autopsies in the general population in Zurich in the 1900s attested that 97% of those who died of any cause had evidence of active or inactive TB [20]. But the prevalence of TB in the general population was lowering at the turn of the 20th century, and a similar decrease was observed among DM patients (Figure 2). Despite these dynamics, the older studies consistently concluded a higher prevalence of TB in DM patients. For example, Root took the data from 10 studies done in general population and 15 studies done in DM patients only, and calculated the average frequency of TB in each group. He concluded for the same studies shown in Figure 2, that the prevalence of TB was higher among DM patients (35.8%) versus the general population (23.0%; Table 2). Root also concluded from his own studies that diabetic children were 13 times more likely to have evidence of pulmonary TB versus non-diabetic [20]. Banyai estimated that the prevalence of TB was about 3 times higher in patients with DM than in the general population [19]. The Philadelphia study concluded that prevalence of TB among diabetics was higher than among non-diabetics (8.4% vs 4.3%) [26], and Silwer and Oscarsson found that the prevalence of TB was 3.6% in DM and 0.9% in the non-DM patients (Table 2) [21].
Table 2.
Prevalence of TB among DM, or DM among TB patients
| Author (Year) | n All 1 | n DM | n TB | % DM in TB | % TB in DM | % TB in all 1 |
|---|---|---|---|---|---|---|
| TB diagnosis based on admission to sanatorium | ||||||
| A.L. Banyai (1931) | 31 | 5,224 | 0.59% | |||
| TB diagnosis based on autopsies | ||||||
| H. Root (1934) | 1,000 | 245 | 24.5% | |||
| Studies in Figure 2 (1888-1929) 2 | 51,705 | 11,875 | 23.0% | |||
| Studies in Figure 2 (1859-1932) 2 | 2,126 | 763 | 35.8% | |||
| TB diagnosis based on chest radiological methods3 | ||||||
| H. Root (1934) | 1,659 | 40 | 2.8% | |||
| K. Boucot et al. (1952) | 71,767 | 3,106 | 261 | 8.4% | 4.3% | |
| H Silwer & PN Oscarsson (1958) | 260,491 | 1,326 | 46 | 3.6% | 0.9% |
“All”, general population;
Same studies used for meta-analysis in Figure 2 but the TB frequencies shown in this table were done by Root (see text for details);
Chest X-rays (Root 1932), roentgenography and photofluorography (Boucot et al, 1952; Silwer & Oscarsson, 1958)
Even though these earlier studies reported that TB was more frequent among DM patients than in the general population, distinguishing a “coincidence” versus a true association between both diseases was difficult due to several limitations. First, most studies reported on the prevalence of TB among DM patients only, but did not include a non-DM group as reference [19, 20]. Therefore, comparisons were done between different studies as reported by Root, and this was further complicated by the rapid decrease of TB and parallel increase in DM in the general populations. Second, there was marked heterogeneity in study populations within and between studies with respect to age, ethnicity, socioeconomic status or diagnostic criteria for TB or DM, among others. For example, Boucot et al reported that the proportion of non-white population (surrogate for lower socio-economic strata) was about double the percentage of non-white individuals living in Philadelphia at the time, an indication that the study sample was not representative of the general population [26]. This was expected in the study population (not the general population), given that poor DM control and TB were more frequent among the lower classes. Silwer and Oscarsson reported that DM was associated with increased weight, higher standard of living and older age [21]. We noted that studies diagnosing TB based on autopsies reported higher TB prevalences when compared to those using radiological methods (Table 2). However, data was not stratified by these variable factors, and statistical tools were not available to determine if DM was an independent predictor for higher TB after controlling for possible confounders. Furthermore, the statistical analysis to determine if two proportions were significantly different was either not conducted or the method not specified. We used statistical tools available today to evaluate and interpret some of the older observations. For example, for the studies listed in Figure 2, Root estimated that the TB frequency was 23% in the general population and 35.8% in DM. He further concluded that TB was “more” prevalent in DM. We conducted a meta-analysis with random-effects on these same studies with Stata (release 15, StataCorp TX, USA). We found that the TB prevalence was 26% (95% CI: 19, 33) for general population and 32% (95% CI 23, 42) for DM, and noted that estimations had substantial overlapping on 95% confidence intervals. For these same studies, Root also noted there was a decrease in TB prevalence over time in the general population and in DM patients. To measure this change in TB prevalence in the general population or among DM patients, we performed a meta-regression of TB prevalence on year of study. We found a decrease in TB prevalence per year of 0.6% (95% CI −0.2%, −0.7%) or 0.5% (−0.2%, −0.8%) for the general population and for diabetics, respectively (Figure S1).
Another difference between the older and contemporary times is the high prevalence of TB in the general population from the late 19th and early 20th centuries (26% in Figure 2). Today, active TB burden is measured by incidence (not prevalence) because it is curable. The current TB incidence is at historic low rate for the developed countries where these older studies were conducted (e.g. less than 3.0/100,000 in the US), and is highest in countries with low incomes or high HIV burdens (e.g. 781/100,000 in South Africa).[14, 27] Thus, the WHO only recommends routine screening for active TB among DM patients in high-burden countries [28, 29]. Also, today’s cohort studies aimed at evaluating the development of TB among DM versus non-DM patients require tens of thousands of participants [30].
3.2.3. Association between TB and DM by DM severity and chronicity, stratified by age
Boucot et al and Silwer and Oscarsson found that among all the DM patients, those with severe DM or more than 10 years with DM, were more likely to have active TB (Figure 3). This was observed across all age groups, but the difference was more notable in the younger DM patients, who were more likely to have severe DM. The authors noted this when measuring DM severity based on insulin requirements as shown in Figure 3B, and Boucot et al reported higher TB among diabetics younger than 40 years with a history of diabetic coma (9.6%) versus those without diabetic coma (5.3%). Similar findings were generally observed when all TB forms (active or inactive) were evaluated with respect to DM severity or duration, but results were not as consistent as for active TB forms only (data not shown). Despite the high prevalence of TB among the young TB patients with severe DM, most of the TB-DM patients were 40 years or older, for both active and inactive TB (Figure S2) [21, 26].
Figure 3. Proportion of active TB among DM patients by severity or duration of DM, stratified by age.
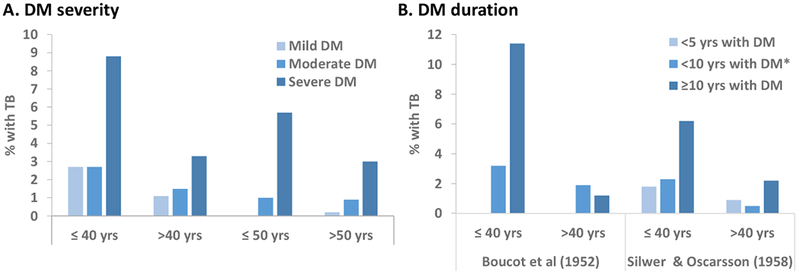
A. Severity of DM at the time of TB diagnosis, defined by Boucot et al as mild if requiring no insulin, moderate if requiring <40 U and severe if requiring ≥ 40 U. In Silwer and Oscarsson, mild DM was defined as requiring less than 20 U of insulin, severe DM if requiring ≥ 40 U, had tendency to acidosis, repeated comma attacks and was difficult to regulate, and moderate DM was the category in between mild and severe. B. History of DM prior to TB development. In Silwer and Oscarsson, < 10 yrs with DM refers to 5-9 years.
Several of these older observations are comparable with the contemporary TB-DM literature. The first is the higher prevalence of DM in TB patients who are 40 years or older, who are not as likely to have a diabetic coma or severe DM, and who have a body weight “above the normal” (51% vs 30%) [31]. Thus, most of the DM patient with TB in the older literature appeared to be type 2 DM, a finding comparable with contemporary studies. Today, type 2 DM accounts for 95% of the DM cases and most studies on TB-DM are done in adults. The second observation from the older studies is that TB was more prevalent in the DM patients with poor glucose control and/or more than 10 years of DM (Figure 3). These findings are consistent with the current epidemiological studies showing that TB is most prevalent among the DM patients with poor glucose control or a multi-year history of DM [32–35]. Basic science studies further show that the immune cells from DM patients with high HbA1c (an indication of chronic hyperglycemia when above 6.5%), versus normal HbA1c have the most notable alterations in function, and animal studies show that compromised immunity and high M. tuberculosis burden is observed only in chronic hyperglycemia [36–40]. The mechanisms by which chronic hyperglycemia leads to alterations in immune function that can compromise immune surveillance against M. tuberculosis is shown in Figure 4 [18, 41, 42].
Figure 4. From chronic glucose excess to glucotoxicity that can lead to compromised immunity.
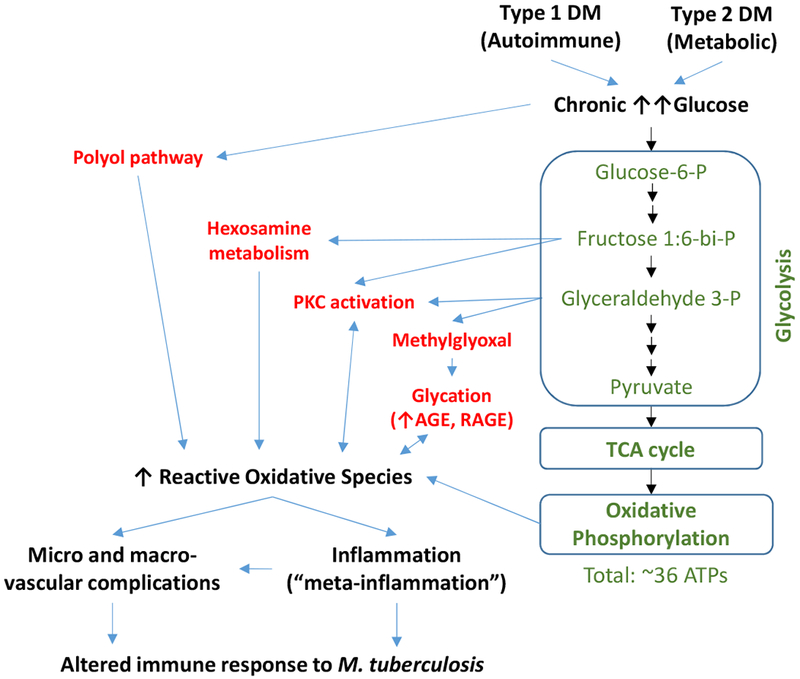
During normoglycemia, glucose is oxidized through the glycolysis, tricarboxylic acid cycle and oxidative phosphorylation for efficient generation of ATP and reducing power (green text). During the chronic hyperglycemia resulting from type 1 or type 2 DM, excess glucose swamps the glycolytic pathway and inhibits the catabolism of glyceraldehydes, leading to the abnormal and excess shunting of the up-stream metabolic intermediates to other pathways (red font). These shunting pathways enhance the accumulation of reactive oxygen species that cause chronic oxidative stress. The reactive oxidative species cause micro- and macrovascular damage and induce chronic inflammation (referred to meta-inflammation in the case of metabolic alterations associated with type 2 DM). We posit that these alterations jointly contribute to compromised immune responses, contributing to the higher risk of DM patients to TB, particularly those with poor glucose control. P, phosphate group; PKC, protein kinase C; AGE, Advanced glycation end product; RAGE, receptor for AGE.; TCA, tricarboxylic acid cycle; Only selected intermediates of the glycolysis pathway are shown.
The older literature also provides additional knowledge to our contemporary understanding of the TB-DM association given the higher prevalence of TB among young patients with a history of diabetic coma or requiring high doses of insulin. This clinical presentation is characteristic of type 1 DM and its frequent reporting in the older literature provides further support for the contribution of type 1 DM to TB. This is a poorly studied aspect of the TB-DM association today due to the low frequency of type 1 DM (~5%) [43]. The underlying pathology in type 1 DM is autoimmune in nature, while in type 2 DM it is metabolic and associated with obesity. Given the apparent contribution of both types of DM to TB, then one can speculate that the higher risk of TB in DM patients is attributed to the negative downstream consequences of a chronic and poorly controlled hyperglycemia (Figure 4). These findings are in line with the immune response alterations noted in humans and in animal models of TB-DM [18, 39, 40, 44, 45]. However, the additional contribution to TB risk conferred by autoimmune or metabolic abnormalities in type 1 or type 2 DM, respectively, cannot be ruled out [43, 46].
3.2.4. Is the association between TB and DM bi-directional?
Banyai reported that although TB was more common among DM patients, the relationship between DM and TB may be bidirectional with carbohydrate intolerance developing during the later stages of TB disease [19]. He reported on previous experimental studies in rabbits, where “toxemia” caused carbohydrate intolerance, for example, when rabbits were exposed to diphtheria toxin. He also reported cases of experimental models on rabbits and guinea pigs that became hypoglycemic when inoculated with TB, and later some became hyperglycemic due to atrophy and necrosis of the pancreas [19]. Root examined the coinfection of TB and DM, and proposed that TB developed more frequently among DM patients, but he did report that 1.5% of the TB patients developed DM as a complication of TB [20]. Silwer reported that the relationship between TB and DM seemed to be bidirectional, with DM patients showing a higher prevalence of TB, and vice versa [21].
Together, most evidence supported a progression from DM to TB, which is consistent with the current literature [30]. However, there are also frequent reports of TB exacerbating glucose control among patients who already had DM, or predisposing to the development of DM. These observations have two probable explanations. The first is that “DM” in these cases is in fact a transient hyperglycemia as a consequence of the febrile presentation of TB [42, 47]. The second possibility is that there is indeed a higher risk for DM development among individuals with TB. In this regard, Banyai summarized published observations on experimental studies with rabbits suggesting that an infection with M. tuberculosis can promote necrosis and atrophy of the pancreas resulting in a permanent reduction of functional capacity[19]. In contemporary times, the higher risk of DM among individuals with a history of TB has also been suggested but evidence is scanty [17]. A plausible mechanism is that the febrile process associated with TB induces insulin resistance, with overstimulation of the pancreatic β-cells to produce insulin. If this process is severe and chronic, the prolonged stress induces pancreatic β-cell apoptosis that results in permanent damage [48]. Given the lack of antibiotics during the early part of the 20th century, we predict that patients who survived chronic episodes of TB would indeed be more vulnerable to this pathology when compared to the TB patients from today who have access to anti-mycobacterial treatment with shorter episodes of TB and a permanent cure in most cases. However, in contemporary times this can also happen, particularly in individuals in the lowest socio-economic strata who do not have ready access to healthcare, and may have delayed TB diagnosis and treatment.
3.2.5. Are TB outcomes worse in patients with TB and DM?
Banyai attributed the poor outcomes among patients with DM who developed TB to a number of factors: i) the late detection of TB among DM cases, ii) the late diagnosis and difficult treatment of DM, iii) the delay in admission to a sanatorium to treat TB, iv) the lack of proper TB therapy (including surgical care), v) the complications of advanced TB such as dissemination to extra-pulmonary sites and pneumothorax, vi) the poor socioeconomic status of the patient, and vii) the non-TB and non-DM complications associated with older age such as coronary artery disease [19]. The calculated life expectancy of TB-DM patients at the time of TB diagnosis was 63% at one year, 23% at 3 years, 13% more than 3 years, and only 1% completely resolved. From his own series (n=31), Banyai reported that 32% of the patients died, 45% did not improve, 16% improved and 6% became quiescent. TB treatment consisted of admission to a sanatorium and increase protein, fat and carbohydrate intake, and DM was managed with insulin [19]. Root reported that the overall TB death rate among diabetics increased over time from 4.7% before June 1919 to 6.7% between 1922 and 1931, despite decrease in mortality in the community. He observed that most of the young patients with TB would die at an early age. Root raised the possibility that inadequate reporting and bias in the literature could contribute to inaccurate measurement of TB mortality. For example, he reported that individuals from poor socioeconomic strata tended to die more often in hospitals, and these were usually the reported cases. He also indicated that before the availability of therapy for DM, most of the diabetics who survived the complications of DM were likely to die from TB [20].
The Philadelphia survey’s authors noted that TB tended to present a more acute course among patients with DM [26]. Silwer and Oscarsson reported that death due to TB at one year was 21% and this progressed to 65% at 10 years, while death due to other causes among TB-no DM patients was only 9% at 10 years [21].
In summary, the cross-sectional design of these four studies are not ideal to determine whether outcomes were more severe in the TB-DM patients versus TB without DM. However, based on the reported comparisons and prevalence of death among the co-infected patients, in the 19th century up to the latter part of the 20th century, TB was a regular accompaniment of DM and among those patients who did not die in coma, TB was a common cause of death. The coincidence of TB and DM was particularly common in the poorer classes. Today, most studies suggest that DM patients are also more likely to die during the course of TB treatment.
However, as indicated in this older literature, the cause of death may not necessarily be TB, but rather, DM complications such as cardiovascular diseases [17, 46, 49]. Today there is also evidence for other adverse outcomes in TB-DM patients (versus TB-no DM), such as delayed clearance of M. tuberculosis during the course of treatment based on AFB smears or cultures, but these protocols were not established in the early 20th century [50]. Today we also know that TB-DM patients are more likely to fail treatment or relapse [17, 51].
4. Concluding remarks
Even though the older literature suffered from a number of limitations in their study designs, conclusions can be draw given the overall consistency in the observations between studies, and the high number of individuals studied. A unique aspect of this literature when compared to contemporary times was the absence of antibiotics to treat TB, and the frequent reporting of diabetic comas despite the implementation of insulin to manage DM. This literature reiterates the higher frequency of the co-morbidity, the higher risk among those DM patients with poor glucose control and multi-year history of DM, regardless of their age, and the higher risk of complications in these patients, notably death. This literature also provides support for the higher risk of TB among patients with type 1 DM, which is difficult to study today. Furthermore, it also provides support for the higher risk of DM development among individuals with a chronic history of TB. This is an intriguing observation that warrants further epidemiological and basic science studies in contemporary times [17].
Supplementary Material
Each article needs to have a financial disclosure line, usually within the author bio section. Publication of this supplement was supported by The University of Texas Health Science Center at Houston.
Acknowledgments
This work was presented in part at the Texas Tuberculosis Research Symposium (TTRS) 2018, El Paso, Texas, USA, sponsored by the Texas Tech University Health Sciences Center El Paso.
Funding
This work was did not receive specific funding. BIR is supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID) [grant number R01AI116039] and National Institutes of Aging [grant number P01 AG044298].
Role of funding source. Not applicable.
List of abbreviations
- DM
Diabetes mellitus
- TB
Tuberculosis
- HIV
Human immunodeficiency virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Competing interests. The authors declare that they have no conflicts of interest. The views expressed on this document are those of the authors and do not necessarily represent the views of their employees or the US government.
References
- [1].Riva MA. From milk to rifampicin and back again: history of failures and successes in the treatment for tuberculosis. J Antibiot (Tokyo). 2014;67(9):661–5. [DOI] [PubMed] [Google Scholar]
- [2].American-Diabetes-Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–S80. [DOI] [PubMed] [Google Scholar]
- [3].Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–9. [DOI] [PubMed] [Google Scholar]
- [4].Karamitsos DT. The story of insulin discovery. Diabetes Res Clin Pract. 2011;93 Suppl 1:S2–8. [DOI] [PubMed] [Google Scholar]
- [5].Rosenfeld L Insulin: discovery and controversy. Clin Chem. 2002;48(12):2270–88. [PubMed] [Google Scholar]
- [6].International-Diabetes-Federation. IDF Diabetes Atlas- 8th Edition. http://wwwdiabetesatlasorg/resources/2017-atlashtml. [Internet] 2017 05/25/2018. Available from: http://www.diabetesatlas.org/resources/2017-atlas.html. [PubMed]
- [7].Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gutteridge IF. Diabetes mellitus: a brief history, epidemiology, definition and classification. Clin Exp Optom. 1999;82(2-3):102–6. [DOI] [PubMed] [Google Scholar]
- [9].Collaboration NCDRF. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331,288 participants. Lancet Diabetes Endocrinol. 2015;3(8):624–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS One. 2018;13(3):e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sharma OA. Avicenna’s description of tuberculosis. Bull Indian Inst Hist Med Hyderabad. 1981;1-4:83–6. [PubMed] [Google Scholar]
- [12].Daniel TM. The history of tuberculosis. Respir Med. 2006;100(11):1862–70. [DOI] [PubMed] [Google Scholar]
- [13].Barberis I, Bragazzi NL, Galluzzo L, Martini M. The history of tuberculosis: from the first historical records to the isolation of Koch’s bacillus. J Prev Med Hyg. 2017;58(1):E9–E12. [PMC free article] [PubMed] [Google Scholar]
- [14].WHO. Global tuberculosis report 2017. http://wwwwhoint/tb/publications/global_report/en/ [Internet]. 2017.
- [15].Rajalakshmi S, Veluchamy G. Yugi’s pramegam and diebetes mellitus: an analogue. Bull Indian Inst Hist Med Hyderabad. 1999;29(1):83–7. [PubMed] [Google Scholar]
- [16].Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50 Suppl 3:S201–S7. [DOI] [PubMed] [Google Scholar]
- [17].Critchley JA, Restrepo BI, Ronacher K, Kapur A, Bremer AA, Schlesinger LS, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 1: Epidemiology and Clinical Management. Chest. 2017;152(1):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ronacher K, van Crevel R, Critchley JA, Bremer AA, Schlesinger LS, Kapur A, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 2: Underlying Biologic Mechanisms. Chest. 2017;152(1):174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Banyai A Diabetes and pulmonary tuberculosis. The American Review of Tuberculosis. 1931;24:650–67. [Google Scholar]
- [20].Root H The Association of Diabetes and Tuberculosis. New Engl J Med. 1934;210(1):1–13. [Google Scholar]
- [21].Silwer H, Oscarsson PN. Incidence and coincidence of diabetes mellitus and pulmonary tuberculosis in a Swedish county. Acta Med Scand Suppl. 1958;335:1–48. [PubMed] [Google Scholar]
- [22].Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. 2017;64(2):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Restrepo BI, Camerlin AJ, Rahbar MH, Wang W, Restrepo MA, Zarate I, et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull WHO. 2011;89(5):352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Viney K, Brostrom R, Nasa J, Defang R, Kienene T. Diabetes and tuberculosis in the Pacific Islands region. Lancet Diabetes Endocrinol. 2014;2(12):932. [DOI] [PubMed] [Google Scholar]
- [25].Kornfeld H, West K, Kane K, Kumpatla S, Zacharias RR, Martinez-Balzano C, et al. High Prevalence and Heterogeneity of Diabetes in Patients With TB in South India: A Report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study. Chest. 2016;149(6):1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R. Tuberculosis among diabetics: The Philadelphia survey. Am Rev Tuberc. 1952;65(1:2):1–50. [PubMed] [Google Scholar]
- [27].Harries AD, Kumar AM, Satyanarayana S, Lin Y, Zachariah R, Lonnroth K, et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg. 2016;110(3):173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harries AD, Murray MB, Jeon CY, Ottmani SE, Lonnroth K, Barreto ML, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health. 2010;15(6):659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].WHO, Disease IUATL. Collaborative framework for care and control of tuberculosis and diabetes. 2011. [updated 2011]; Available from: http://www.who.int/diabetes/publications/tb_diabetes2011/en/. [PubMed]
- [30].Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS One. 2017;12(11):e0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Czerniawski AM. From average to ideal - The evolution of the height and weight table in the United States, 1836-1943. Soc Sci Hist. 2007;31(2):273–96. [Google Scholar]
- [32].Baker MA, Lin HH, Chang HY, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis. 2012;54(6):818–25. [DOI] [PubMed] [Google Scholar]
- [33].Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167(12):1486–94. [DOI] [PubMed] [Google Scholar]
- [34].Kuo MC, Lin SH, Lin CH, Mao IC, Chang SJ, Hsieh MC. Type 2 diabetes: an independent risk factor for tuberculosis: a nationwide population-based study. PLoS One. 2013;8(11):e78924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135(3):483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis. 2013;93(2):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Restrepo BI, Fisher-Hoch S, Pino P, Salinas A, Rahbar MH, Mora F, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cheekatla SS, Tripathi D, Venkatasubramanian S, Nathella PK, Paidipally P, Ishibashi M, et al. NK-CD11c+ Cell Crosstalk in Diabetes Enhances IL-6-Mediated Inflammation during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12(10):e1005972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Podell BK, Ackart DF, Obregon-Henao A, Eck SP, Henao-Tamayo M, Richardson M, et al. Increased severity of tuberculosis in Guinea pigs with type 2 diabetes: a model of diabetes-tuberculosis comorbidity. Am J Pathol. 2014;184(4):1104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb ). 2013;93(S1):S10–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kumpatla S, Aravindalochanan V, Rajan R, Viswanathan V, Kapur A. Evaluation of performance of A1c and FPG tests for screening newly diagnosed diabetes defined by an OGTT among tuberculosis patients-a study from India. Diabetes Res Clin Pract. 2013;102(1):60–4. [DOI] [PubMed] [Google Scholar]
- [43].Webb EA, Hesseling AC, Schaaf HS, Gie RP, Lombard CJ, Spitaels A, et al. High prevalence of Mycobacterium tuberculosis infection and disease in children and adolescents with type 1 diabetes mellitus. Int J Tuberc Lung Dis. 2009;13(7):868–74. [PubMed] [Google Scholar]
- [44].Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184(11):6275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Martinez N, Ketheesan N, West K, Vallerskog T, Kornfeld H. Impaired Recognition of Mycobacterium tuberculosis by Alveolar Macrophages From Diabetic Mice. J Infect Dis. 2016;214(11):1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Restrepo BI, Kleynhans L, Salinas AB, Abdelbary BE, Tshivhula H, Aguillon G, et al. Diabetes screen during tuberculosis contact investigations highlights opportunity for diabetes diagnosis and reveals metabolic differences between ethnic groups. Tuberculosis (Edinb). 2018;113:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Boillat-Blanco N, Ramaiya KL, Mganga M, Minja LT, Bovet P, Schindler C, et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Implications for Diabetes Screening Algorithms. J Infect Dis. 2016;213(7):1163–72. [DOI] [PubMed] [Google Scholar]
- [48].Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. [DOI] [PubMed] [Google Scholar]
- [49].Prada-Medina CA, Fukutani KF, Pavan Kumar N, Gil-Santana L, Babu S, Lichtenstein F, et al. Systems Immunology of Diabetes-Tuberculosis Comorbidity Reveals Signatures of Disease Complications. Sci Rep. 2017;7(1):1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Restrepo BI, Fisher-Hoch S, Smith B, Jeon S, Rahbar MH, McCormick J. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79(4):541–4. [PMC free article] [PubMed] [Google Scholar]
- [51].Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Medicine. 2011;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


