Abstract
Rationale
Exercise shows promise as a treatment option for addiction, but in order to prevent relapse, it may need to be introduced early in the course of treatment.
Objective
We propose that exercise, by upregulating dorsal medial prefrontal cortex (dmPFC)-nucleus accumbens (NAc) transmission, offsets deficits in pathways targeting glutamate, BDNF, and dopamine during early abstinence, and in doing so, normalizes neuroadaptations that underlie relapse.
Methods
We compared the effects of exercise (wheel-running, 2-hr/day) during early (days 1-7), late (days 8-14), and throughout abstinence (days 1-14) to sedentary conditions on cocaine-seeking and gene expression in the dmPFC and NAc core of male rats tested following 24-hr/day extended-access cocaine (up to 96 infusions/day) or saline self-administration and protracted abstinence (15 days). Based on these data, we then used site-specific manipulation to determine whether dmPFC metabotropic glutamate receptor-5 (mGlu5) underlies the efficacy of exercise.
Results
Exercise initiated during early, but not late abstinence, reduced cocaine-seeking; this effect was strongly associated with dmPFC Grm5 expression (gene encoding mGlu5), and modestly associated with dmPFC Grin1 and Bdnf-IV expression. Activation of mGlu5 in the dmPFC during early abstinence mimicked the efficacy of early-initiated exercise; however, inhibition of these receptors prior to the exercise sessions did not block its efficacy indicating that there may be redundancy in the mechanisms through which exercise reduces cocaine-seeking.
Conclusion
These findings indicate that addiction treatments, including exercise, should be tailored for early versus late phases of abstinence since their effectiveness will vary over abstinence due to the dynamic nature of the underlying neuroadaptations.
Keywords: Bdnf-IV, Cocaine, Exercise, Grm5, mGlu5, dmPFC, Relapse
Introduction
Exercise shows promise as a non-pharmacological intervention for addiction in both humans and animal models (Lynch et al. 2017; Wang et al. 2014a). In humans, exercise during abstinence reduces craving for cocaine and other drugs of abuse (Abrantes et al. 2011; Wang et al. 2014a), and recent findings provide preliminary support for its ability to reduce relapse (De La Garza et al. 2016; Trivedi et al. 2017). In animals, exercise, such as wheel running, during abstinence prevents the progressive increase, or incubation, of drug-seeking (Lynch et al. 2010; Zlebnik and Carroll 2015), an animal model of relapse (Grimm et al. 2003). Despite these encouraging results, evidence indicates that some exercise conditions may be ineffective or even harmful. In humans, involvement in certain team sports is associated with increased drug and alcohol use (Kwan et al 2014; Veliz et al. 2013), and while social factors likely contribute to some of these effects (Terry-McElrath et al 2011), findings in rats similarly show that certain exercise conditions (unlimited voluntary, intense forced exercise), enhance addictive behaviors, including relapse-like responding (Engelmann et al. 2016; Smith et al. 2008; Thanos et al. 2013). These findings indicate a biological basis for the effects of exercise, and underscore the need to determine exercise conditions that induce beneficial versus detrimental outcomes.
Since neuroadaptations underlying drug-seeking vary from early to later periods of abstinence (Pickens et al. 2011; Szumlinski and Shin 2018; Wolf 2016), we proposed that the efficacy of exercise as an intervention for relapse will depend on when it is initiated during abstinence (Lynch et al. 2013). With this in mind, we showed that wheel running exercise during abstinence dose-dependently suppressed subsequent cocaine-seeking when assessed following extended access self-administration and 14-days of abstinence (Peterson et al 2014), conditions known to induce high levels of drug-seeking (Grimm et al. 2003). We further showed that the timing of exercise during abstinence is critical such that exercise initiated during early abstinence decreased subsequent cocaine-seeking, whereas, exercise initiated later during abstinence was not effective and even increased certain measures of cocaine-seeking (Beiter et al. 2016). This finding is significant given that in human studies, the introduction of exercise is often delayed based on the assumption that individuals will be reluctant to exercise during early abstinence due to withdrawal symptoms (Linke and Ussher 2015).
Although the exact pathways underlying drug-seeking have yet to be fully elucidated, we do know that during early withdrawal/abstinence, when levels of drug seeking-are low, several dorsal medial prefrontal cortex (dmPFC)-nucleus accumbens (NAc) pathways, that include markers of glutamate and dopamine, are depressed, then, as drug-seeking incubates over protracted abstinence, these pathways show compensatory increases in expression/activity (Pickens et al. 2011; Szumlinski and Shin 2018; Wolf 2016). For example, glutamatergic signaling changes dramatically in this pathway from early to late abstinence (Ben-Shahar et al. 2009; 2013; Ghasemzadeh et al 2011; Hao et al. 2010; Knackstedt et al. 2010; Ploense et al. 2018; Shin et al. 2016; 2018; Szumlinski et al 2016), and results in both humans and animal models indicate an association between dmPFC hyperactivity and heightened drug-seeking/vulnerability to relapse (Goldstein and Volkow 2011; Szumlinski and Shin 2018). The expression of metabotropic glutamate receptor 5 (mGlu5) in the dmPFC appears to closely associate with cocaine-seeking with decreased expression during early abstinence (relative to short access controls; Hao et al. 2010) or when levels of cocaine-seeking are low (i.e, following short or extended access self-administration and extinction training; Ghasemzadeh et al 2011; Pomierny-Chamiolo et al. 2017), and with increased expression when levels of cocaine-seeking are high (following cocaine re-exposure or cocaine-associated cues during early or late abstinence after short or extended access self-administration; Ben-Shahar et al. 2013; Pomierny-Chamiolo et al. 2015). Markers of dopamine signaling also show time-dependent changes following extended access self-administration and abstinence (Conrad et al. 2010), and deficits in NAc dopamine during early abstinence are believed to underlie anhedonia/negative affect (Kuhar and Pilotte 1996).
It is notable, that brain-derived neurotrophic factor (BDNF), which is also strongly associated with vulnerability to relapse in humans and animal models (Grimm et al. 2003; Gueye et al. 2018; Li and Wolf 2015; McGinty et al. 2015), when infused into the dmPFC during early, but not late abstinence, prevents the development of the incubation effect and associated neuroadaptations in the mPFC and the NAc (Giannotti et al. 2018; Whitfield et al. 2011). Exercise is also known to upregulate glutamate, dopamine and BDNF signaling (Greenwood et al. 2011; Makatsori et al. 2003; Maddock et al. 2016; Meeusen et al. 1997; Neeper et al. 1996; Obici et al. 2015; Wang et al. 2014b), and our previous work shows that the incubation of cocaine-seeking and associated increases in dmPFC BDNF gene expression (Bdnf-IV) and ERK signaling, which requires coincident activation by dopamine and glutamate, can be blocked by exercise initiated during early abstinence (Lynch et al. 2010; Peterson et al. 2014). As such, we hypothesized that exercise during early, but not late abstinence, offsets deficits in dmPFC-NAc glutamate, BDNF, and/or dopamine signaling pathways during early abstinence, thus preventing compensatory increases that underlie relapse-like responding.
To address this hypothesis, we examined gene expression changes that associated with relapse-like responding, and then determined whether the efficacy of exercise corresponded to changes in gene expression. Changes were examined in the dmPFC, which includes the prelimbic region and anterior cingulate, and the NAc core, which receives glutamatergic projections from the dmPFC, since these regions are critical for the incubation of cocaine-seeking (Ishikawa et al. 2008; Kalivas and McFarland 2003; Shin et al. 2018). We focused on glutamate (AMPA type subunit 1, 2, and 3; Gria1, 2, and 3; NMDA type subunit 1; Grin1; mGlu1, 2 and 5; Grm1, 2 and 5), BDNF (Bdnf exons I, IV and IX and its receptor, Ntrk2) and dopamine-related genes (D1 and D2 receptors; Drd1 and Drd2) known to be associated with cocaine-seeking and/or exercise (Greenwood et al. 2011; Makatsori et al. 2003; Neeper et al. 1996; Obici et al. 2015; Wang et al. 2014b).
As a follow-up to these gene expression findings, which showed that dmPFC Grm5 expression corresponded to the efficacy of exercise to reduce relapse-like responding, we examined the role of dmPFC mGlu5 receptor using site-specific manipulation. Based on our prediction that exercise exerts its efficacy by upregulating deficit pathways thus preventing later compensatory changes, we predicted that stimulation of mGlu5 in the dmPFC during early abstinence would mimic the efficacy of exercise, and that blockade of mGlu5 prior to exercise would block its efficacy.
Methods
Subjects.
Adult, male Sprague-Dawley (Charles River Laboratories, Raleigh, NC) rats (N=106) weighing approximately 380 g at the start of the study were used as subjects. Upon arrival, rats were housed in individual operant conditioning chambers (Med Associates Inc., St. Albans, VT) in a temperature (20-22° C) and humidity (40-70%) controlled vivarium on a 12-hr light/dark cycle (lights on at 7-am) with ad libitum access to food and water. After a 2-day acclimation period, in order to facilitate subsequent cocaine self-administration, rats were pre-trained to lever press for sucrose pellets (45-mg, Noyes Company, Lancaster, NH) using methods previously described (fixed-ratio 1, FR1; 2 consecutive days ≥ 50 deliveries; note: no stimulus was paired with sucrose pellet delivery; Beiter et al. 2016). Each rat was then implanted with a jugular catheter using methods previously described (Beiter et al. 2016). Health was monitored daily, and body weights were recorded three times/week. All procedures were approved by the University of Virginia Animal Care and Use Committee and were conducted within guidelines set by the NIH.
Procedures.
Cocaine self-administration.
Rats were trained to self-administer cocaine (1.5 mg/kg/infusion) during daily sessions under an FR1 schedule with a maximum of 20 infusions available/day as previously described (Beiter et al. 2016). Briefly, sessions began with the introduction of the left lever (cocaine-associated lever) into the operant conditioning chamber, and responses on it resulted in the delivery of a cocaine infusion paired with the sound of the pump and a stimulus light located above the lever. Following acquisition (2 consecutive sessions wherein all 20 infusions were obtained), rats were given 24-hr/day access to cocaine (1.5 mg/kg/infusion) under a discrete trial procedure using methods previously described (Beiter et al. 2016). Briefly, trials initiated every 15-minutes (4-trials/hr) around the clock for a total of 10 days (up to 96 infusions/day). During trials, responding was reinforced under a FR1 schedule with infusions paired with the sound of the pump and a stimulus light. Following the last discrete trial session, two additional FR1 sessions (maximum of 20 infusions) were run to equate intake between groups prior to abstinence.
Experiment 1. Effect of exercise during abstinence on relapse-like responding and associated gene expression changes.
Wheel-running exercise during abstinence.
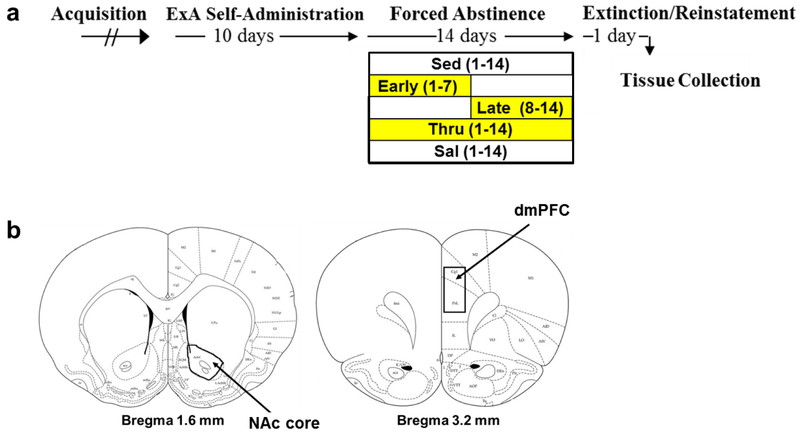
A 14-day abstinence period began following the last cocaine self-administration session wherein rats were housed in polycarbonate cages without (sedentary; n=8) or with access to a running wheel (2-hr/day) during early (days 1-7; n=14), late (days 8-14; n=12), or throughout (days 1-14; n=9) abstinence as described previously (Beiter et al. 2016; also see Fig. 1a). Behavioral data from a subset of rats within each of these groups have been previously published (6 of 14 early-initiated; 5 of 12 late-initiated; 5 of 9 throughout; 5 of 8 sedentary; Beiter et al. 2016), with tissue included here for molecular comparison. Importantly, these animals did not differ on any behavioral measure (intake, wheel running, extinction/reinstatement) as compared to the more recently run groups. All data were excluded from one rat in the throughout exercise condition due to technical issues during the final exercise session (final n=8). Additional rats were given access to saline and housed without access to a wheel during “abstinence” (n=10). These saline controls underwent the same testing and surgical procedures as those described for the sedentary cocaine group.
Fig. 1. Summary of experimental events and brain regions dissected for the gene expression analyses in Experiment 1.
(a) Effect of exercise during abstinence on relapse-like responding and associated gene expression changes: Following acquisition, rats were given 24-hr/day extended (ExA) access to cocaine under a discrete trial procedure for 10 days. After the last cocaine self-administration session, rats were housed without (sedentary, Sed, n=8) or with access to a running wheel (2-hr/day) during early (days 1-7; Early, n=14), late (days 8-14; Late, n=10), or throughout (days 1-14; Thru, n=8) a 14-day abstinence period. Additional rats were given access to saline and housed without access to a wheel during “abstinence” (saline, Sal, n=10). On day 15 of abstinence, rats underwent extinction/reinstatement testing, and on the morning following this 1-day test session, tissue was collected. (b) Schematic illustration of the brain regions dissected for the NAc core and the dmPFC.
Extinction/reinstatement testing.
Rats were returned to the same operant conditioning chambers in which they were previously housed in the afternoon on day 14 of abstinence to habituate overnight. Rats then underwent extinction/reinstatement testing on day 15 of abstinence using a within-session procedure and methods previously described (Lynch et al. 2010). Briefly, extinction responding was assessed in a minimum of 6, 1-hr sessions during which responding did not have a consequence. Sessions continued until responding extinguished (< 15 responses/session; 6-9 sessions). Cue-induced reinstatement was examined immediately after the last extinction session in a 1 -hr session wherein responses on the formerly-active lever produced a presentation of the cues associated with cocaine (stimulus light, sound of pump). All data from two rats in the late exercise condition were excluded due to technical issues during extinction/reinstatement testing (final n=10).
Gene expression.
On the morning following the extinction/reinstatement session, anesthetized rats were euthanized by rapid decapitation, and the dmPFC and NAc core were dissected from 2-mm-thick coronal brain slices based on Paxinos and Watson (2007) coordinates (3.2 and 1.6, respectively; Fig. 1b). Brain tissue was rapidly frozen and stored at −80 °C until further processing. RNA extraction, cDNA transcription, and RT-qPCR were performed as previously described (Smith et al. 2018). Oligonucleotide primers (Table S1) were chosen from prior publications and synthesized by Invitrogen (Carlsbad, CA). cDNA templates were normalized with the endogenous controls, Gapdh (Bdnf- associated genes) or B2m (glutamate and dopamine-receptor genes), which were prescreened to assure similar expression levels between groups. A small number of samples were not assayed by RT-qPCR due to contamination (dmPFC, 1 saline, 1 late; NAc, 1 saline, 1 late, 2 early); all other data were included (final groups; n=9 saline dmPFC/NAc; n=9 late dmPFC/NAc; n=14/12 early dmPFC/NAc).
Experiment 2a: Cocaine-induced changes in dmPFC Grm5 gene expression during early abstinence.
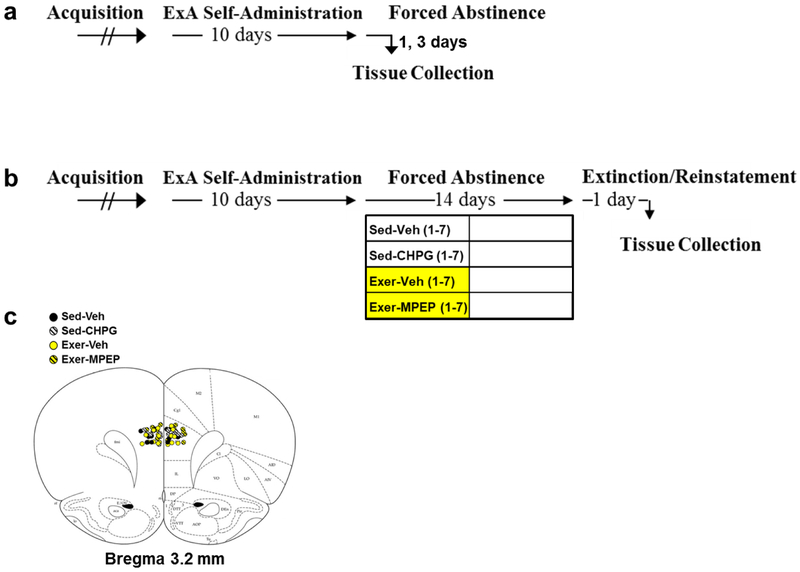
Since we found that dmPFC Grm5 expression corresponded to the efficacy of exercise to reduce relapse-like responding, we first verified that dmPFC Grm5 expression was decreased during early abstinence in additional groups of sedentary rats given extended access to cocaine (n=13) or saline (n=10). The methods used for lever pre-training, jugular catheterization, cocaine/saline self-administration, tissue collection, and Grm5 expression analysis were identical to those described above except that dmPFC tissue was obtained during early abstinence, one (n=7) to three (n=6) days following the last self-administration session, and no extinction/reinstatement testing preceded tissue collection (Fig. 2a). Because expression levels were statistically similar between abstinence days 1 and 3, data were pooled to make a single early-abstinence group.
Fig. 2. Summary of experimental events as well as cannulae track placement for Experiment 2.
(a) Cocaine-induced changes in Grm5 gene expression during early abstinence: Following acquisition, rats were given 24-hr/day extended (ExA) access to cocaine (n=13) or saline (n=10) under a discrete trial procedure for 10 days. Tissue was collected from the dmPFC on abstinence day 1 or 3. (b) Effect of dmPFC mGlu5 manipulation on relapse-like responding: Following acquisition and 24-hr/day extended (ExA) access cocaine self-administration, rats were housed without (sedentary) or with (exercise) access to a running wheel during early (days 1-7) abstinence. Rats in the sedentary condition (Sed; white bars for days 1-7) were given daily intra-dmPFC infusions of either vehicle (Sed-Veh; n=6) or the mGlu5 agonist CHPG (Sed-CHPG; n=8) during early abstinence (days 1-7), and rats in the exercise condition (Exer; yellow bars for days 1-7) were given daily intra-dmPFC infusions of either vehicle (Exer-Veh; n=8) or the mGlu5 antagonist MPEP (Exer-MPEP; n=8) during early abstinence (days 1-7). All rats were housed without access to a wheel during late abstinence (days 8-14, white bars). On day 15 of abstinence rats underwent extinction/reinstatement testing, and on the following morning, tissue was collected for cannulation placement verification. (c) Histological cannulae placements in the dmPFC for each of the rats in each of the four groups. Sed-Veh, black symbols; Sed-CHPG, black and white symbols; Exer-Veh, yellow symbols; Exer-MPEP, yellow and black symbols.
Experiment 2b: Effect of dmPFC mGlu5 manipulation on relapse-like responding.
In order to address the possibility that dmPFC mGlu5 mediates the efficacy of exercise, we examined the effects of site-specific receptor manipulation in additional groups of sedentary (n=15) and early-exercise rats (n=16). These rats underwent lever pre-training and jugular catheterization as described above (Fig. 2b). During the catheterization procedure, they were also implanted with a 26-gauge bilateral cannula (Plastic One, Roanoke, VA) aimed 1-mm above the prelimbic region of the dmPFC (+3.2-mm anterior-posterior, ±0.6-mm medio-lateral, −1.6-mm dorso-ventral; from the dural surface; Paxinos and Watson 2007). Guide cannulae were secured with dental cement (Stoelting Co, Wood Dale, IL) and three screws (Plastic One, Roanoke, VA). Dummy cannulae (Plastic One, Roanoke, VA) were used to prevent blockade/infection. Following a 9-day recovery period, rats were trained to self-administer cocaine and then given extended access to the drug as described above.
Following the last cocaine self-administration session, rats were habituated to the infusion procedure by inserting stylets, which extended 1-mm past the guide cannulae, into the guide cannulae. Rats were then randomly assigned to one of four abstinence conditions: 1) sedentary plus the mGlu5 agonist 2-Chloro-5-hydroxyphenylglycine (Sed-CHPG; n=9), 2) sedentary plus vehicle (Sed-Veh; n=6), 3) early-exercise plus the mGlu5 antagonist, 2-methyl-6-(phenylethynyl)pyridine hydrochloride (Exer-MPEP; n=8), or 4) early-exercise plus vehicle (Exer-Veh; n=8). The mGlu5 antagonist MPEP was selected due to its long-acting effects in the brain (~2 hours; Lindemann et al. 2011) as compared to other antagonists (such as MTEP; Anderson et al. 2003), and a moderate to high dose (3 μg/side) was selected in order to ensure blockade of exercise-induced increases in mGlu5 signaling. Specifically, considering that a 10-fold lower dose of MPEP than the mGlu5 agonist DHPG is needed to antagonize receptor activation (Renoldi et al. 2006), and considering that intra-PFC infusions of 1 to 10 μg/side of DHPG maximally elicits glutamate release (Timmer and Steketee 2013), this dose of MPEP should be maximally effective at blocking mGlu5 activation. This is also consistent with results showing that micro-infusion of MPEP at this dose, or lower, in other brain regions affects cocaine-seeking and taking without impacting other behaviors (i.e., locomotion, responding for sucrose; Bäckström and Hyytiä 2007; Kumaresan et al. 2009; Li et al. 2018a). A moderate to high dose of CHPG (3 μg/side) was similarly selected based on results showing that site-specific micro-infusion at this dose, or lower, affects cocaine-seeking (Wang et al. 2013). Daily intra-dmPFC infusions of CHPG and MPEP at this dose also did not impact body weights of the sedentary or exercise rats tested here (data not shown).
CHPG, MPEP, and vehicle were infused (0.5-μl, 2 min) between 9 and 10AM during early abstinence (days 1-7). Following delivery, infusion cannulae were left in place for an additional 2-min to prevent diffusion back up the guide cannulae tract. Rats were then returned to their polycarbonate cages for the daily exercise/sedentary session which began 15-min after infusion using the methods described above. All rats were housed in polycarbonate cages without access to a wheel during late abstinence (days 8-14). Rats were returned to their operant conditioning chambers in the afternoon on abstinence day 14, underwent extinction/reinstatement testing on abstinence day 15, and following testing were euthanized as described above. Cannula placement was determined from a coronal section of the dmPFC following methylene blue staining. One of the rats in the Sed-CHPG group had placement outside of the dmPFC; these data were excluded from all analyses (n=8). All other rats had correct cannulae placement within the dmPFC (Fig. 2c).
Drugs.
Cocaine ([−]-cocaine hydrochloride) was obtained from the National Institute on Drug Abuse (Research Triangle Park, NC), dissolved in 0.9% sterile saline (7 mg/ml), filtered (0.22 μm; Millipore, Billerica, MA), and stored in sterile bottles at 4°C. The infusion duration was adjusted three times/week to maintain a constant mg/kg dose (2 sec/100 g). CHPG and MPEP were purchased from Tocris (Ellisville, MO). CHPG was dissolved in 1M NaOH (20 μg/μl) and diluted to a final concentration of 6 μg/μl in artificial cerebrospinal fluid (ACSF). MPEP was dissolved in ACSF at a concentration of 6 μg/μl.
Data analysis.
To address behavioral differences in Experiment 1, we compared the number of infusions obtained over the 10-day extended access self-administration period between the five groups later tested under sedentary or exercise conditions using repeated measures ANOVA. Posthoc comparisons to controls (saline, cocaine sedentary) were made using Dunnett’s t-tests (Nesil et al. 2018; Ohlsson et al. 2014), and comparisons of the first and last session were made using a paired t-test. These same analyses were also used to compare daily distance run during the first seven exercise sessions, and the number of formerly-active lever responses during the first 6 extinction sessions and during the last extinction session versus the reinstatement session. Following a significant interaction of group by session, univariate ANOVAs were used to examine group differences within each session, and Holme-Bonferroni-adjusted paired t-tests were used to examine session effects within each group. Univariate ANOVAs were also used to examine group differences in average inactive lever responses during the extended access period, and total inactive and formerly-active responses during extinction. Analyses were also conducted without the saline group in order to isolate differences among the cocaine groups. These included the repeated measures ANOVA analyses, as well as a Kruskal-Wallis analysis of the number of extinction sessions run (6-9).
To address molecular differences in Experiment 1, we first determined whether variations in gene expression in the dmPFC and NAc associated with relapse-like responding by comparing the relative expression of each of the 13 targeted genes (Bdnf-I/IV/IX; Ntrk2, Drd1/2; Gria1/2/3, Grin1, and Grm1/2/5) between the saline sedentary and cocaine sedentary groups using Benjamini-Hochberg corrected t-tests (Goni et al 2009). For genes that were significantly different, we performed RT-qPCR for each of the five experimental groups as described above. Extreme group outliers (>2.5 standard deviations from the mean since 99% of a population falls within this range) were excluded from further analysis. Outliers were rare (8 of 236 data points), and no more than one outlier was ever removed from any group. Univariate ANOVAs were then used to compare expression levels between groups for data that were normally distributed (dmPFC Grm5, Grin1; NAc Ntrk2), and the non-parametric Kruskal-Wallis test was used for data that were not normally distributed (dmPFC Bdnf-IV; NAc Grm2). Post-hoc comparison to controls (saline, cocaine sedentary) were made using either a Dunnett’s two-tailed t-test (normally distributed data) or a Dunn’s (non-normally distributed data) one-tailed (for predicted differences in Bdnf-IV expression; Peterson et al. 2014) or two-tailed t-test (NAc Grm2.) Pearson correlations between relapse-like responding and gene expression were assessed by comparing gene expression normalized to saline controls (percent difference) and formerly-active lever responses during reinstatement testing. Associations were assessed within each gene across groups since there were no group differences with regard to slope of the associations.
For Experiment 2a, repeated measures ANOVA was used to compare the number of infusions obtained over the extended access period between the saline and cocaine groups; a two-tailed t-test was used for inactive lever responses, and a one-tailed t-test for predicted differences in dmPFC Grm5 expression. Experiment 2b used the same analyses as Experiment 1 to examine group differences during extended access self-administration (number of infusions), exercise (distance run), extinction (formerly-active and inactive responses, number of sessions run) and reinstatement testing (formerly-active and inactive responses), except that post-hoc Dunnett’s comparisons were made to the Sed-Veh group only. All analyses were performed using SPSS 24. Error bars on figures indicate SEM.
Results
Experiment 1. Effect of exercise during abstinence on relapse-like responding and associated gene expression changes.
Cocaine intake and exercise.
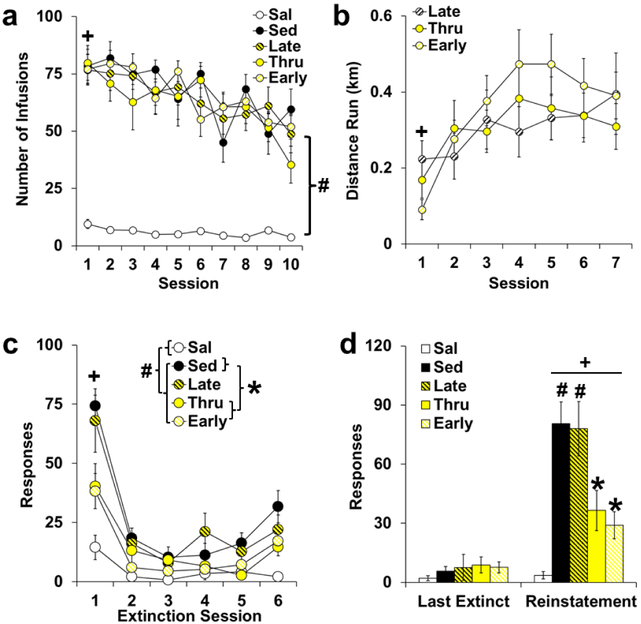
Rats self-administered high levels of cocaine during the 10-day extended access period, and each of the cocaine groups obtained significantly more infusions as compared to the saline group (Fig. 3a, group: F(4,45) = 112.00, P < 0.001; versus saline: P’s < 0.001). Notably, however, no differences were observed for posthoc comparisons to sedentary controls (P’s > 0.05). Patterns of intake were also similar between the groups (group by session: P > 0.05), with the highest intake on the first versus last session (session: F(9,405) = 8.20, P < 0.001; session 1 versus 10: t(49) = 5.18, P < 0.001). Analysis within the cocaine groups also revealed non-significant overall and interactive effects of group (P’s > 0.05) indicating that levels and patterns of cocaine intake were similar between the cocaine groups prior to abstinence. Inactive lever responding during the extended access period was highly variable (mean ± SEM: 8.5 ± 2.2, saline; 26.1 ± 10.9, sedentary; 26.8 ± 8.4, late; 29.9 ± 15.1, throughout; 26.3 ±7.3, early), and did not differ significantly between groups (P > 0.05). Levels of running were also similar between the early, late, and throughout exercise groups (Fig. 3b; group: P > 0.05), and each group showed a similar increase in running over time (session: F(6,174) = 9.55, P < 0.001; session by group: P > 0.05; session 1 versus 7: t(31) = 5.46, P < 0.001). Thus, prior to extinction/reinstatement testing, levels of cocaine intake and exercise were similar between groups.
Fig. 3. Exercise initiated during early, but not late abstinence, reduces relapse-like responding.
(a) No differences were observed between the cocaine groups that were later tested under sedentary (Sed) versus exercise during early (Early), late (Late), or throughout (Thru) abstinence. Significant difference from the last session (+) and from saline (bracket and #). (b) Levels of running (km) during the first seven exercise sessions were similar between the groups given access to exercise during early, late, and throughout abstinence. Significant difference from the last session (+). (c) Exercise during early and throughout, but not during late abstinence, decreased cocaine-seeking during extinction. Data are plotted as formerly-active lever responses for the first six extinction sessions. Significant difference from the last session (+), from saline (bracket and #), and from sedentary controls (bracket and *). (d) Each of the cocaine groups showed an increase in responding from the last extinction (Extinct) session to the reinstatement session, and this increase was markedly reduced by exercise during early and throughout, but not late abstinence. Data are plotted as formerly-active lever responses for the last extinction session versus the reinstatement session. Significant difference from the last extinction session (bar and +), from saline (#), and from sedentary controls (*). Sal, open symbols, n=10; Sed, filled symbols, n=8; Late, yellow and black symbols, n=10; Thru, yellow symbols, n=8; Early, yellow and white symbols, n=14.
Timing of exercise availability during abstinence on relapse-like responding.
As predicted, cocaine-seeking during extinction was significantly affected by the timing of wheel-running availability during abstinence (Fig. 3c, group: F(4,45) = 11.47, P < 0.001) with the highest levels of responding (formerly-active lever) observed in the sedentary and late groups (versus saline, P’s < 0.01). Responding was also increased from saline control levels in the early and throughout exercise groups (P < 0.05), but were markedly lower than sedentary control levels (P’s < 0.01), and were highest for all groups during the first session (session: F(52,225) = 47.07, P < 0.001; session 1 versus 6: t(4) = 6.55, P < 0.001). We also observed a significant interaction of group by session (F(20,225) = 2.73, P < 0.001), but this effect appears to be attributable to differences from saline as analysis within the cocaine groups revealed a significant effect of group (F(3,36) = 9.09, P < 0.001) and session (F(5,180) = 43.61, P < 0.001), but a non-significant interaction of group by session (P > 0.05). The number of extinction sessions run were also similar between the sedentary (7.63 ± 0.42), late (7.40 ± 0.43), throughout (7.25 ± 0.45), and early (6.79 ± 0.28) groups (group: P > 0.05) indicating that while levels of extinction responding differed, rates of extinction were similar between the groups. Total extinction responses (formerly-active lever) over all extinction sessions run were also significantly higher than saline control levels (27.3 ± 6.3) for the sedentary (206.6 ± 12.2; group: F(4,45) = 11.47, P 0.001; P < 0.001) and late groups (199.9 ± 35.5; P < 0.001), and were reduced from sedentary control levels for the throughout (114.9 ± 19.4, P < 0.05) and early exercise groups (94.43 ± 19.3, P <0.01). Levels of responding were also higher than saline control levels for the throughout group (P < 0.05), but the difference did not reach statistical significance for the early group (P < 0.10). Inactive responses during extinction were low for each of the groups (mean ± SEM: 4.40 ± 2.09, saline; 3.25 ± 1.33, sedentary; 8.20 ± 5.32, late; 3.38 ± 1.33, throughout; 3.14 ± 1.24, early), and did not differ significantly between groups (P > 0.05).
Cue-induced cocaine-seeking was similarly affected by the timing of exercise availability during abstinence (Fig. 3d). Although responding (formerly-active lever) was similar between groups during the last extinction session (P > 0.05), the sedentary and late groups showed a marked increase in responding during the cue-induced reinstatement session, and this increase was greatly attenuated by exercise during early and throughout abstinence (group by session: F(4,45) = 14.10, P < 0.001; within reinstatement, group: F(4,45) = 12.17, P < 0.001). Analysis within each of the groups showed that responding was significantly higher during the reinstatement session versus the last extinction session for each of the groups (P’s < 0.05), except saline (P > 0.05). Analysis within the reinstatement session revealed a significant difference from saline for the sedentary and late groups (P’s < 0.001), and a significant difference from sedentary controls for the throughout and early groups (P < 0.05 and 0.001, respectively). While levels of reinstatement responding tended to be higher than saline control levels in the throughout group (P = 0.075), the difference was not significant for the early group indicating that early-initiated (P > 0.1) exercise blocked the development of relapse-like responding to saline control levels. Analysis within the cocaine groups also revealed a significant interaction of session by group (F(3,36) = 9.21, P < 0.001) indicating that differences are attributable to effects of exercise within the cocaine groups. Inactive lever responses during reinstatement were low for each of the groups (mean ± SEM: 1.30 ± 0.65, saline; 3.88 ± 1.38, sedentary; 2.70 ± 2.16, late; 1.75 ± 1.35, throughout; 1.79 ± 0.74, early), and did not differ significantly between groups (P > 0.05). These results confirm our earlier findings (Beiter et al. 2016), and show in a larger cohort that exercise initiated during early abstinence is critical for reducing relapse-like responding.
Gene expression changes associated with relapse-like responding.
As summarized in Table 1, relapse-like responding was associated with changes in glutamate- and BDNF-, but not dopamine-related, gene expression in the dmPFC and NAc. In the dmPFC, effects on glutamate gene expression were evident for both Grm5 (t(15) = 3.29, P < 0.01) and Grin1 (t(15) = 2.58, P < 0.05), which were significantly upregulated in the cocaine versus saline group. As predicted, dmPFC Bdnf-IV expression was upregulated in the cocaine versus saline group (t(14)) = 2.95, P < 0.01), whereas no differences were observed for other Bdnf isoforms or its receptor, Ntrk2. These findings contrast to those observed in the NAc where both Grm2 (t(13) = 3.32, P < 0.01) and Ntrk2 (t(15) = 2.72, P < 0.05) mRNA were significantly downregulated in the cocaine versus saline groups. No differences were found for either region for genes encoding AMPA (Grial, Gria2 and Gria3) or dopamine receptors (Drd1 and Drd2). Each of the genes that showed differentially expression in the dmPFC or NAc in the cocaine versus saline groups (i.e., Grm5, Grm2, Grin1, Bdnf-IV, and Ntrk2), were further examined to determine how exercise during abstinence influences their expression as discussed below.
Table 1:
Cocaine-induced changes in glutamate-, BDNF-, and dopamine-related gene expression changes in the dmPFC and NAc.*
| Gene Family | Gene | dmPFC | NAc |
|---|---|---|---|
| Glutamate | Grm1 | NS | NS |
| Grm2 | NS | ↓ Sed vs Sal; P < 0.01 | |
| Grm5 | ↑ Sed vs Sal; P < 0.01 | NS | |
| Grin1 | ↑ Sed vs Sal; P < 0.05 | NS | |
| Gria1 | NS | NS | |
| Gria2 | NS | NS | |
| Gria3 | NS | NS | |
| BDNF | Bdnf-I | NS | NS |
| Bdnf-IV | ↑ Sed vs Sal; P < 0.01 | NS | |
| Bdnf-IX | NS | NS | |
| Ntrk2 | NS | ↓ Sed vs Sal; P < 0.05 | |
| Dopamine | Drd1 | NS | NS |
| Drd2 | NS | NS |
Genes showing a probability value (P) were significantly impacted by cocaine versus not affected (NS, non-significant) as determined using an FDR-adjusted t-test comparison of the sedentary cocaine (Sed) versus saline (Sal) groups.
Gene expression changes associated with the efficacy of exercise.
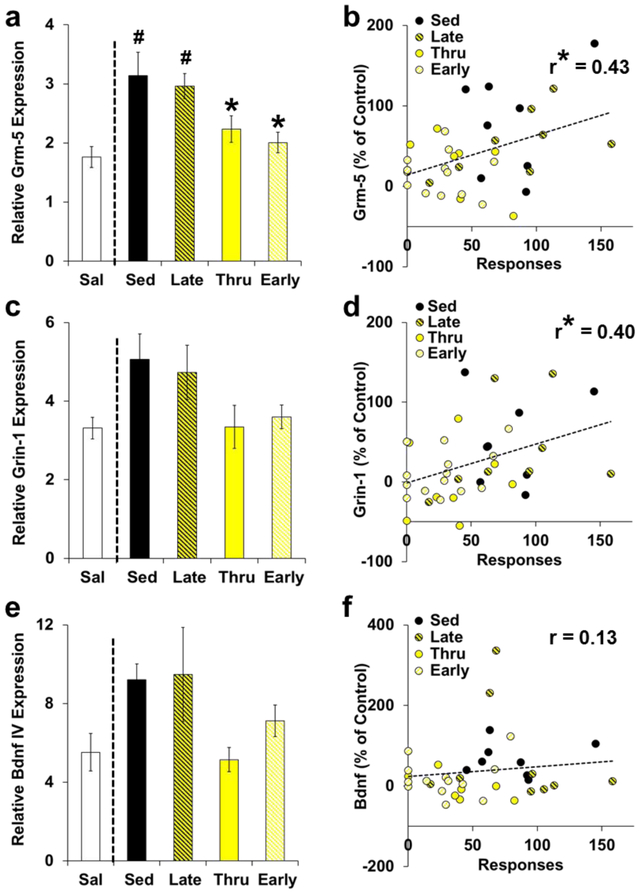
Grm5 expression in the dmPFC robustly mirrored the efficacy of exercise to reduce cocaine-seeking (Fig. 4a). Specifically, exercise beginning during early, but not late abstinence, normalized cocaine-induced elevations in dmPFC Grm5 expression (group: F(4,41) = 6.09, P < 0.001). Posthoc comparisons to saline revealed significant differences for the sedentary and late groups (P’s < 0.01), but not for the throughout and early groups (P’s > 0.05), and posthoc comparisons to sedentary revealed significant differences for the throughout (P < 0.05) and early groups (P < 0.01), but not for the late group (P > 0.05). Notably, dmPFC Grm5 expression significantly correlated with reinstatement responding (Fig. 4b; r(35) = 0.43; P < 0.01). A similar, but more variable, pattern of change was observed in the dmPFC for Grin1, which encodes glutamate ionotropic receptor NMDA Type Subunit 1, and while the overall effect of group (Fig. 4c, F(4,42) = 2.70, P < 0.05) and the correlation between cocaine-seeking and expression levels were significant (Fig. 4d, r(36) = 0.40; P < 0.05), posthoc comparisons to saline and sedentary controls were not (P’s > 0.05). These data suggest that exercise may exert its efficacy in the dmPFC via interactions with glutamate via Grm5, and to a lesser extent Grin1, during early abstinence.
Fig. 4. The efficacy of exercise at reducing relapse-like responding is associated with Grm5 expression in the dmPFC.
(a) Exercise during early (Early; n=13) and throughout (Thru; n=8), but not during late abstinence (Late; n=8), normalized cocaine-induced increases in dmPFC Grm5 expression (as observed in sedentary, Sed, controls; n=8) to saline (Sal) control levels (n=9). Significant difference from saline (#) and sedentary controls (*). (b) dmPFC Grm5 expression was associated with cocaine-seeking as indicated by a significant correlation (*) between expression levels and reinstatement responding (plotted as percent difference from control for responses on the formerly-active lever) within the cocaine groups (n=37). (c) Relative expression levels of Grin1 in the dmPFC modestly, though non-significantly, corresponded to the efficacy of exercise with exercise during early (n=14) and throughout (n=8), but not late abstinence (n=8), offsetting cocaine-induced increases in expression (as observed in sedentary controls; n=8) to saline control levels (n=9). (d) Grin1 expression was positively associated with relapse-like responding as indicated by a significant correlation (*) between Grin1 expression levels and reinstatement responding (plotted as percent difference from control for responses on the formerly-active lever) within the cocaine groups (n=38). (e) Relative expression levels of dmPFC Bdnf-IV modestly, though non-significantly, corresponded to the efficacy of exercise with exercise during early (n=13) and throughout (n=7), but not late abstinence (n=9), offsetting cocaine-induced increases in expression (as observed in sedentary controls; n=8) to saline control levels (n=8). (f) dmPFC Bdnf-IV expression was not associated with cocaine-seeking (plotted as percent difference from control on the formerly-active lever) within the cocaine groups (n=37). Sal, open bars; Sed, filled symbols; Late, yellow and black symbols; Thru, yellow symbols; Early, yellow and white symbols.
Levels of Bdnf-IV gene expression also corresponded to the efficacy of exercise, but expression levels were variable, particularly within the early and late exercise groups, and the overall group effect did not reach significance (Fig. 4e, Kruskal-Wallis: P = 0.07). Levels of Bdnf-IV expression in the dmPFC also were not significantly associated with levels of cocaine-seeking (Fig. 4f). It is notable, however, that comparisons of the throughout, saline, and sedentary groups replicate our previous findings (Peterson et al. 2014) for both the overall effect of exercise (Kruskal-Wallis, group: H(2) = 9.259, P = 0.01) and pairwise differences (e.g., throughout exercise normalized cocaine-induced increases in expression; throughout versus sedentary, P < 0.05; throughout versus saline, P > 0.05), suggesting that Bdnf-IV expression in the dmPFC is associated with the efficacy of exercise, but only modestly.
Our findings in the NAc show that while Grm2, which encodes mGlu2, was differentially expressed between groups (Fig. 5a, group: H(4) = 10.57, P < 0.05), exercise-induced changes in expression did not correspond to its behavioral effects as exercise during early, but not late or throughout abstinence, increased Grm2 expression as compared to sedentary cocaine (P < 0.05). Cocaine-induced changes in NAc Grm2 expression were also modest, since despite a significant difference for the initial comparison to saline, the post-hoc comparison in the full five group model did not reach significance (versus saline: P = 0.076). The relationship between Grm2 gene expression and cocaine-seeking was also not significant (Fig. 5b; P > 0.05). We also observed changes in NAc expression of Ntrk2 (Fig. 5c; group: F(4,41) = 2.90, P < 0.05), but like NAc Grm2 expression, effects were variable since despite a significant difference from saline during the initial screening, the post-hoc comparison in the full five group model was not significant (versus saline: P > 0.05). Changes in Ntrk2 expression also did not correspond to behavioral effects as both early- and late-initiated exercise similarly increased expression (versus sedentary: P’s < 0.05). Ntrk2 expression also was not associated with cocaine-seeking (Fig. 5d). These findings indicate that NAc Grm2 and Ntrk2 expression do not predict the efficacy of exercise to reduce relapse-like responding.
Fig. 5. The efficacy of exercise to prevent relapse-like responding is not associated with gene expression in the NAc.
(a) Exercise-induced changes in NAc Grm2 expression did not correspond to its behavioral effects since exercise during early (Early; n=12), but not late (n=9) or throughout (n=7) abstinence, increased Grm2 expression as compared to sedentary controls (n=7). Significant difference from sedentary controls (*). (b) Grm2 expression was not associated with cocaine-seeking (plotted as percent difference from control for responses on the formerly-active lever) and Grm2 expression levels within the cocaine groups (n=35). (c) Exercise-induced changes in NAc Ntrk2 expression did not correspond to its behavioral effects since exercise during both early (Early; n=12) and late (n=9), but not throughout (n=7) abstinence, increased Ntrk2 expression as compared to sedentary controls (n=7). Significant difference from sedentary controls (*). (d) NAc Ntrk2 expression was also not associated with cocaine-seeking (plotted as percent difference from control for responses on the formerly-active lever) within the cocaine groups (n=36). Sal, open bars; Sed, filled symbols; Late, yellow and black symbols; Thru, yellow symbols; Early, yellow and white symbols.
Experiment 2a: Cocaine-induced changes in dmPFC Grm5 gene expression.
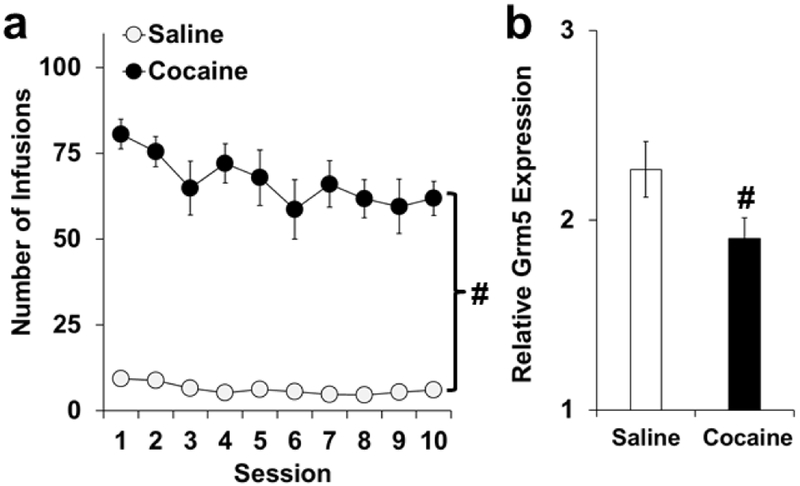
As expected, rats in the cocaine group self-administered more infusions over the 10-day extended period as compared to rats in the saline group (Fig. 6a; F(1,21) = 335.7, P < 0.001). Responding on the inactive lever was highly variable for both the cocaine and saline groups (average ± SEM responses: 45.0 ± 48.4 and 10.6 ± 9.3, respectively), but was significantly higher in the cocaine group (t(21) = 4.4, P < 0.05). As predicted, rats in the cocaine group had lower levels of dmPFC Grm5 expression as compared to saline controls (Fig. 6b; t(21) = 3.8, P < 0.05). Thus, dmPFC Grm5 expression was decreased during early abstinence following extended access cocaine self-administration.
Fig. 6. Cocaine-induced decreases in Grm5 expression during early abstinence.
(a) The cocaine group self-administered significantly more infusions over the 10 extended access sessions as compared to the saline group (as indicated by a bracket and #). (b) dmPFC Grm5 expression was significantly decreased in the cocaine versus saline group (as indicated by #). Saline, open bars; Cocaine, black symbols.
Experiment 2b: Effect of dmPFC mGlu5 manipulation on relapse-like responding.
Cocaine intake and exercise.
Prior to abstinence, cocaine intake over the 10-day extended access period was similar between groups later tested under exercise and sedentary conditions (Fig. 7a; P > 0.05). Patterns of intake were also similar between groups (group by session: P > 0.05), with the greatest intake observed during the first session (session: F(9,223) = 2.8, P < 0.01; session 1 versus 10: t(29) = 2.72, P < 0.05). Inactive lever responding was highly variable over the 10-day extended access period (mean ± SEM: 1.30 ± 0.28, Sed-Veh; 20.70 ± 10.64, Sed-CHPG; 15.8 ± 7.58, Exer-Veh; 38.71 ± 39.15, Exer-MPEP), and did not differ significantly between groups (group: P > 0.05). Levels of running were also similar between the Exer-Veh and Exer-MPEP groups (Fig. 7b; group: P > 0.05), and both groups showed a similar increase in running over time (session: F(6,84) = 11.49, P < 0.001; session by group: P > 0.05; session 1 versus 7: t(15) = 3.7, P < 0.01), indicating that MPEP did not impact levels or patterns of running. Thus, prior to extinction/reinstatement testing, levels of cocaine intake were similar between the four groups and levels of running were similar between the two exercise groups.
Fig. 7. Stimulation of mGlu5 in the dmPFC during early abstinence mimics the efficacy of exercise.
(a)No differences were observed for the number of infusions obtained over the 10 extended access sessions between the groups that were later tested under sedentary (Sed-Veh and Sed-CHPG) versus exercise (Exer-Veh and Exer-MPEP) conditions. Significant difference from the last session (+) (b) Levels of running (km) during the seven exercise sessions were similar between groups given daily intra-dmPFC infusions of vehicle (Exer-Veh) or MPEP (Exer-Veh). Significant difference from the last session (+) (c) No differences were observed for cocaine-seeking during extinction. Data are plotted as formerly-active lever responses for the first six extinction sessions. Significant difference from the last session (+). (d) Exercise during early abstinence (Exer-Veh) blocked the reinstatement of cocaine-seeking (as observed in the Sed-Veh group); this effect of exercise was mimicked in sedentary rats given intra-dmPFC infusions during early abstinence (Sed-CHPG), but not affected by intra-dmPFC infusions of MPEP prior to the exercise sessions (Exer-MPEP). Data are plotted as formerly-active lever responses for the last extinction (Extinct) session versus the reinstatement session. Significant difference from the last extinction session (+) and from sedentary controls (*). (e) Representative micrographs from rats that received daily bilateral dmPFC infusions within each of the groups. Sed-Veh, filled symbols, n=6; Sed-CHPG, black and white symbols, n=8; Exer-Veh, yellow symbols, n=8; Exer-MPEP, yellow and white symbols, n=8.
dmPFC mGlu5 manipulation on relapse-like responding.
In contrast to Experiment 1, responding (formerly active) during extinction were similar between the exercise and sedentary groups for both the first six sessions (Fig. 7c; group; P > 0.05), and for all sessions run (mean ± SEM; 130.33 ± 13.98, Sed-Veh; 92.75 ± 12.84, Sed-CHPG; 117.13 ± 22.16, Exer-Veh; 109.38 ± 15.46, Exer-MPEP; P > 0.05). Each group also showed a similar pattern of responding over the first six extinction sessions (session by group, P > 0.05), with the greatest number of responses observed during the first session (session: F(5,130) = 73.76, P < 0.001; session 1 versus 6, P < 0.001). Inactive lever responses were low during extinction for each group (mean ± SEM: 1.67 ± 0.98, Sed-Veh; 1.5 ± 0.68, Sed-CHPG; 1.00 ± 0.33, Exer-Veh; 1.5 ± 0.85, Exer-MPEP), and did not differ significantly between groups (group: P > 0.05). The number of extinction sessions run were also similar between the Sed- Veh (6.67 ± 0.42), Sed-CHPG (6.00 ± 0.00), Exer-Veh (6.25 ± 0.16), and Exer-MPEP (6.38 ± 0.26) groups (P > 0.05) indicating that levels and rates of extinction were similar between groups.
As with Experiment 1, however, exercise during early abstinence blocked cue-induced reinstatement of cocaine-seeking (Fig. 7d). Notably, this effect of exercise was mimicked in sedentary rats given infusions of CHPG during early abstinence, but was not blocked by infusions of MPEP prior to exercise (session: F(1,26) = 45.09, P < 0.001; group: F(3,26) = 4.71, P < 0.01; session by group: F(3,26 = 5.83, P < 0.01). Prior to the reinstatement test, responding was low for each groups indicating that a similar level of non-responsiveness had been achieved (within last extinction session, group: P > 0.05). Robust differences, however, were observed within the reinstatement session (group: F(3,26) = 5.45, P < 0.01), with the Sed-CHPG, Exer-Veh, and Exer-MPEP groups showing a marked reduction in responding in comparison to the Sed-Veh group (P’s <0.01). Analysis within each of these groups showed that while responding was reinstated by the cues in the Sed-Veh group (last extinction versus reinstatement: t(5) = 5.38, P < 0.05), this effect did not reach significance for the Sed-CHPG group (P = 0.08), and was not significant for the Exer-Veh or Exer-MPEP groups (P’s > 0.10). Daily intra- PFC infusions of MPEP, CHPG, and Veh did not appear to induce significant tissue damage (e.g., Fig. 7e). Inactive lever responding was low during reinstatement testing for each group (mean ± SEM: 0.5 ± 0.34, 0.5 ± 0.33, 0.38 ± 0.38, and 0.38 ± 0.38 for the Sed-Veh, Sed-CHPG, Exer-Veh, and Exer-MPEP groups, respectively), and was not significantly different between groups (group: P > 0.05). These findings indicate that the efficacy of exercise to decrease relapse-like responding can be mimicked by dmPFC mGlu5 stimulation during early abstinence, but not blocked by antagonizing these receptors.
Discussion
Exercise initiated during early, but not late abstinence, reduced cocaine-seeking following protracted abstinence, and this effect was strongly associated with dmPFC Grm5 expression, and modestly associated with dmPFC Grin1 and Bdnf-IV expression. Activation of mGlu5 in the dmPFC of sedentary rats during early abstinence mimicked the efficacy of exercise; however, inhibition of these receptors prior to the exercise sessions did not block its efficacy indicating that stimulation of mGlu5 during early abstinence is sufficient for reducing relapse-like responding, but not necessary for exercise-induced decreases. These findings support our neurobiological framework for the efficacy of exercise to reduce relapse vulnerability (Lynch et al. 2013). Specifically, we predicted that during early abstinence, when signaling in some dmPFC-NAc pathways are depressed, the upregulation in signaling induced by exercise normalizes expression thereby preventing the subsequent compensatory changes that incubate over abstinence and trigger relapse. Our findings suggest that such an effect may occur through interactions with dmPFC glutamate via Grm5, and possibly Grin1, and to a lesser extent through interactions with BDNF via Bdnf-IV. These findings also indicate that addiction treatments, including exercise, should be tailored for early versus late phases of abstinence since their therapeutic effectiveness will vary over abstinence due to the dynamic nature of the underlying neuroadaptations.
Gene expression changes associated with relapse-like responding.
As expected, following extended access cocaine self-administration and protracted abstinence, levels of cocaine-seeking were elevated (in the cocaine sedentary group) and were associated with changes in glutamate and BDNF-related gene expression in the dmPFC (Grm5, Grin1, and Bdnf-IV) and the NAc (Grm2 and Ntrk2). Our findings with Grm5 are consistent with changes in protein expression in this region which indicate that increased expression of mGlu5 corresponds to enhanced relapse-like responding (Pomiemy-Chamiolo et al. 2015). They are also consistent with numerous reports indicating that BDNF protein and gene expression are increased following protracted abstinence and correspond to heightened cocaine-seeking (Hearing et al. 2008; Li et al. 2013). Recent findings with Grin1 show that it is upregulated during early abstinence (24-hr following extended access self-administration; Ploense et al. 2018), and given that it was also upregulated here following protracted abstinence, it is possible that the increase persists throughout abstinence and represents a long-term adaptation.
Our findings in the NAc with Grm2, which encodes mGlu2, are also consistent with results from a series of studies demonstrating that enhanced cocaine-seeking following protracted abstinence corresponds to decreased mGlu2/3 receptor functioning in the NAc core (for review see Moussawi and Kalivas 2010). Our finding showing downregulation of Ntrk2, the gene encoding the Trkb receptor, in the NAc core is also consistent with findings showing that blocking receptor activity enhances cocaine-seeking (Li et al. 2013), and our result showing no effect of cocaine on Bdnf expression is consistent with findings showing that while protein levels of BNDF incubate in the NAc core over abstinence (Gueye et al. 2018), gene expression does not change (Li et al. 2018b). Although we did not observe changes in dmPFC or NAc dopamine receptor gene expression, we cannot rule out the possibility that protein, rather than gene expression changes occurred, and would be predictive of enhanced relapse-like responding. Future studies are needed to examine this possibility.
Gene expression changes associated with the efficacy of exercise.
Of the genes regulated by cocaine in the dmPFC and NAc, only one, Grm5, was found to robustly correspond to the efficacy of exercise, with two additional genes, Grin1 and Bdnf-IV, showing modest correspondence. The pattern of change was similar for each of these genes -- exercise during early, but not late abstinence, normalized (or tended to normalize) cocaine-induced increases in dmPFC expression. Grm5 and Grin1 expression in the dmPFC were also positively associated with relapse-like responding. These data, combined with our findings showing that Grm5 expression is decreased during early abstinence, provide strong support for the idea that exercise upregulates Grm5 expression in the dmPFC, and in doing so, prevents later compensatory changes that trigger relapse-like responding. While it’s possible that exercise was still able to activate dmPFC mGlu5 following infusion with MPEP, this seems unlikely since the MPEP dose tested was selected to maximally block mGlu5 (Renoldi et al. 2006; Timmer and Steketee 2013). It is also possible that the effects of exercise are mediated independent of Grm5/mGlu5. However, considering the strong correspondence between Grm5 gene expression and the efficacy of exercise, a more likely possibility is that multiple, possibly overlapping mechanisms mediate the efficacy of exercise. In support, we previously showed that exercise activates genes associated with multiple mechanisms including synaptic plasticity, cell signaling, and epigenetic regulation (Abel and Rissman 2013). Such mechanisms may in fact underlie our current findings showing that exercise initiated during early abstinence, presumably associated with gene activation, increases the expression of genes that are normally depressed at this time (i.e., Grm5, Bdnf). However, since we did not include a saline exercise group in this study, we can only speculate that exercise was responsible for this change.
Given that the pattern of Grm5 change in dmPFC was also similar for Grin1 and Bdnf-IV, it is possible that these genes play a similar or related role in relapse and the efficacy of exercise to reduce relapse vulnerability. For Grin1, however, this possibility seems less likely considering recent findings showing that expression is increased, rather than decreased, in the dmPFC following extended access self-administration during early abstinence (Ploense et al. 2018). In contrast, for Bdnf-IV, there is considerable support for the idea that it mediates the efficacy of exercise to reduce cocaine-seeking: expression is decreased in the dmPFC during early abstinence (McGinty et al. 2010), and exercise normally increases both BDNF protein and mRNA expression (Berchtold et al 2002, Abel and Rissman 2013). In fact, we were surprised to observe only modest correspondence between dmPFC Bdnf-IV expression and the efficacy of exercise to reduce relapse vulnerability considering our previous findings showing that when exercise is available throughout abstinence, it dose-dependently attenuates cocaine-seeking and dmPFC Bdnf-IV expression (Peterson et al. 2014). It is notable, that in our previous study that while 6 and 24-hr/day exercise access robustly impacted Bdnf-IV expression in the dmPFC, the effect of 2-hr/day access, the condition used in the present study, was modest. In the NAc, Ntrk2 and Grm2 expression significantly changed in response to both cocaine and exercise; however, effects did not correspond with behavior indicating that these genes did not influence relapse-like responding in our exercise model. These findings also indicate that gene changes in the dmPFC, rather than the NAc, underlie the efficacy of exercise to reduce relapse-like responding.
Role of mGlu5 in the dmPFC in relapse-like responding.
Our finding showing that stimulation of mGlu5 in the dmPFC during early abstinence mimics the efficacy of exercise to reduce relapse-like responding adds to a growing body of work implicating mGlu5 as a target for treating addiction (Bames et al. 2018). Specifically, numerous previous studies have shown that systemic administration of mGlu5 antagonists, as well as negative allosteric modulators, decrease cocaine-seeking when administered during protracted abstinence (Keck et al. 2013; Keck et al. 2014; Knackstedt et al. 2016). While these previous studies point to the potential therapeutic effects of mGlu5 antagonism/negative allosteric modulation, our findings indicate that an agonist, rather than an antagonist, would be effective for treatment initiated during early abstinence. This idea is also consistent with previous findings showing that the effects of mGlu5 manipulations during abstinence are time-dependent (Caprioli et al. 2017). For example, daily antagonist administration during early abstinence (days 1-9) blocks the protective effects of extinction training on subsequent relapse-like responding; whereas, treatment with the agonist during this time period, decreases subsequent cocaine-seeking (Kim et al. 2015; Perry et al. 2016). However, it’s important to point out that mGlu5 agonist treatment in the vmPFC region of rats during protracted abstinence (30 days), facilitated extinction learning leading to a reduction in cocaine-seeking (Ben-Shahar et al 2013). Therefore, effects of mGlu5 manipulation seem to depend not only on timing during abstinence, but also brain region with even dorsal versus ventral parts of the PFC inducing different effects.
While these findings support the idea that exercise exerts its efficacy via Grm5 expression in the dmPFC, one major caveat to this interpretation is that we were not able to block the efficacy of exercise by inhibiting dmPFC mGlu5 prior to exercise sessions. As discussed above, one possible explanation is that there are multiple, possibly redundant, mechanisms underlying the efficacy of exercise to reduce relapse-like responding. For example, intra-dmPFC BDNF infusion during early abstinence prevents the incubation of cocaine-seeking and this mechanism appears to be mediated by TrkB activation of Src family kinases (SFKs) and the subsequent phosphorylation of GluN2 subunits of NMDA receptors (Barry and McGinty 2017). SFKs act as a convergence point for many signaling pathways to regulate NMDA receptor activity, and one of the most well characterized pathways upstream of SFKs are G-protein coupled receptors including mGlu5 (Salter and Kalia 2004). Thus, one possibility is that BDNF and mGlu5 signaling pathways converge on SFK to regulate NMDA receptor function such that if mGlu5 is blocked, BDNF is still available to activate SFK, thereby stimulating NMDA receptors and suppressing cocaine-seeking. Future studies are needed to investigate this possibility.
In conclusion, our results show that moderate exercise, timed properly during abstinence, normalizes neuroadaptations in the dmPFC associated with enhanced relapse-like responding. While many studies have focused on BDNF, we show for the first time that the efficacy of exercise to reduce relapse-like responding corresponds to Grm5 expression in the dmPFC, and that stimulation of its receptor product, mGlu5, during early abstinence mimics this protective effect of exercise. However, given that the mGlu5 antagonist did not block the efficacy of exercise, it is likely that other pathways, converge on the same downstream target to suppress cocaine-seeking. Our results also have implications for the development of exercise- and medication-based treatments for cocaine addiction. For medications development, these findings suggest that outcome will depend on the timing of treatment during abstinence such that while an agonist is effective during early abstinence, an antagonist is necessary during late abstinence (or vice versa). For exercise-based interventions, our findings indicate a persistent beneficial effect of exercise initiated during early abstinence and suggest that the timing of its availability during abstinence is even more critical than length of exposure. Since a majority of drug abusers are willing to exercise during early abstinence (Abrantes et al. 2017), we believe that moderate exercise initiated during this period has significant therapeutic potential to prevent cocaine relapse, without side effects. It may also be possible to increase the efficacy of exercise by combining it with medications that target mGlu5 signaling, but at low doses, with presumably few side effects.
Supplementary Material
Acknowledgments
This research was supported by NIDA R01 grants DA039093 and DA024716 (WJL).
Footnotes
The authors declare no competing interests.
References
- Abel JL, Rissman EF (2013) Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci 31:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes AM, Battle CL, Strong DR, Ing E, Dubreuil ME, Gordon A, Brown RA (2011) Exercise preferences of patients in substance abuse treatment. Ment Health Phys Act 4:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. (2003) In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur J Pharmacol 18:35–40. [DOI] [PubMed] [Google Scholar]
- Bäckström P and Hyytiä P (2007) Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 192:571–580. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Sheffler DJ, Semenova S, Cosford NDP, Bespalov A. (2018) Metabotropic glutamate receptor 5 as a target for the treatment of depression and smoking: Robust preclinical data but inconclusive clinical efficacy. Biol Psychiatry 83:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SM, McGinty JF (2017) Role of src family kinases in BDNF-mediated suppression of cocaine-seeking and prevention of cocaine-induced ERK, GluN2A, and GluN2B dephosphorylation in the prelimbic cortex. Neuropsychopharmacology 42:1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiter RM, Peterson AB, Abel J, Lynch WJ (2016) Exercise during early, but not late abstinence, attenuates subsequent relapse vulnerability in a rat model. Transl Psychiatry 6:e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK, (2009) Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 63:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, and Szumlinski KK (2013) Deficits in ventromedial prefrontal cortex Group1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci 33:495–506a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Cotman CW (2002) Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res 68(5):511–21. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Justinova Z, Venniro M, Shaham Y (2017) Effect of novel allosteric modulators of metabotropic glutamate receptors on drug self-administration and relapse: A review of preclinical studies and their clinical implications. Biol Psychiatry 84:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Ford K, Marinelli M, Wolf ME (2010) Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience 169:182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R 2nd, Yoon JH, Thompson-Lake DG, Haile CN, Eisenhofer JD, Newton TF, Mahoney JJ 3rd (2016) Treadmill exercise improves fitness and reduces craving and use of cocaine in individuals with concurrent cocaine and tobacco-use disorder. Psychiatry Res 245: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD (2014) Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct 219(2):657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR (2011) Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res 1413:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti G, Barry SM, Siemsen BM, Peters J, McGinty JF (2018) Divergent Prelimbic Cortical Pathways Interact with BDNF to Regulate Cocaine-seeking. J Neurosci 38:8956–8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni R, Garcia P, Foissac S (2009) The qPCR data statistical analysis. Integromics White Paper 1–9. [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M (2011) Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res 217:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y (2003) Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci 23:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueye AB, Allain F, Samaha AN (2018) Intermittent intake of rapid cocaine injections promotes the risk of relapse and increases mesocorticolimbic BDNF levels during abstinence. Neuropsychopharmacology. October 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F (2010) Behavioral and functional evidence of mGlu2/3 and mGlu5 metabotropic glutamate receptor dysregulation in cocaine escalated rats: Factor in the transition to dependence. Biol Psychiatry 68:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF (2008) Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology 198:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008) Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci 28:5088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168:44–56. [DOI] [PubMed] [Google Scholar]
- Keck TM, Yang HJ, Bi GH, Huang Y, Zhang HY, Srivastava R, Gardner EL, Newman AH, Xi ZX (2013) Fenobam sulfate inhibits cocaine-taking and cocaine-seeking behavior in rats: implications for addiction treatment in humans. Psychopharmacology 229:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck TM, Zou MF, Bi GH, Zhang HY, Wang XF, Yang HJ, Srivastava R, Gardner EL, Xi ZX, Newman AH (2014) A novel mGlu5 antagonist, MFZ 10-7, inhibits cocaine-taking and cocaine-seeking behavior in rats. Addict Biol 19:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Perry C, Luikinga S, Zbukvic I, Brown RM, Lawrence AJ (2015) Extinction of a cocaine-taking context that protects against drug-primed reinstatement is dependent on the metabotropic glutamate 5 receptor. Addict Biol 20:482–489. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Schwendt M (2016) mGlu5 receptors and relapse to cocaine-seeking: The role of receptor trafficking in postrelapse extinction learning deficits. Neural Plast 2016:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW (2010) Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking J Neurosci 30:7984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Pilotte NS (1996) Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci 17:260–264. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. (2009) Metabotropic glutamate receptor 5 (mGlu5) antagonists attenuate cocaine priming- and cue- induced reinstatement of cocaine seeking. Behav Brain Res 202:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan M, Bobko S, Faulkner G, Donnelly P, Cairney J (2014) Sport participation and alcohol and illicit drug use in adolescents and young adults: a systematic review of longitudinal studies. Addict Behav 39:497–506 [DOI] [PubMed] [Google Scholar]
- Li X, Peng XQ, Jordan CJ, Li J, Bi GH, He Y, et al. (2018a). mGlu5 antagonism inhibits cocaine reinforcement and relapse by elevation of extracellular glutamate in the nucleus accumbens via a CB1 receptor mechanism. Sci Rep 8:3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, White AC, Schochet T, McGinty JF, Frantz KJ (2018b) ARC and BDNF expression after cocaine self-administration or cue-induced reinstatement of cocaine seeking in adolescent and adult male rats. Addict Biol 23:1233–1241. [DOI] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME (2013) Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci 33:1130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME (2015) Multiple faces of BDNF in cocaine addiction. Behav Brain Res 279:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Jaeschke G, Michalon A, Vieira E, Honer M, Spooren W, Porter R, Hartung T, Kolczewski S, Büttelmann B, Flament C, Diener C, Fischer C, Gatti S, Prinssen EP, Parrott N, Hoffmann G, Wettstein JG (2011) CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther 339:474–86. [DOI] [PubMed] [Google Scholar]
- Linke SE, Ussher M (2015) Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am J Drug Alcohol Abuse 41:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Abel J, Robinson AM, Smith MA (2017) Exercise as a sex-specific treatment for substance use disorder. Curr Addict Rep 4:467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (2013) Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev 37:1622–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE (2010) Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry 68:774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Casazza GA, Fernandez DH, Maddock MI (2016) Acute Modulation of Cortical Glutamate and GABA Content by Physical Activity. J. Neurosci 36:2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, Jezova D (2003) Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology 28:702–714. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW Jr, Berglind WJ (2010) Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Zelek-Molik A, Sun WL (2015) Cocaine self-administration causes signaling deficits in corticostriatal circuitry that are reversed by BDNF in early withdrawal. Brain Res 1628(Pt A):82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, Ebinger G, Michotte Y (1997) Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol Scand 159(4) :335–41. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW (2010) Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol 639:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW (1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726:49–56. [PubMed] [Google Scholar]
- Nesil T, Narmeen S, Bakhti-Suroosh A, Lynch WJ (2018) Effect of menthol on nicotine intake and relapse vulnerability in a rat model of concurrent intravenous menthol/nicotine self-administration. Psychopharmacology [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Magrisso IJ, Ghazarian AS, Shirazian A, Miller JR, Loyd CM, Begg DP, Krawczewski Carhuatanta KA, Haas MK, Davis JF, Woods SC, Sandoval DA, Seeley RJ, Goodyear LJ, Pothos EN, Mul JD (2015) Moderate voluntary exercise attenuates the metabolic syndrome in melanocortin-4 receptor-deficient rats showing central dopaminergic dysregulation. Mol Metab 4:692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Moverare-Skrtic S, Islander U, Sjogren K (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9:e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier: Amsterdam; Boston. [Google Scholar]
- Perry CJ, Reed F, Zbukvic IC, Kim JH, Lawrence AJ (2016) The metabotropic glutamate 5 receptor is necessary for extinction of cocaine-associated cues. Br J Pharmacol 173:1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ (2014) Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology 231:1305–1314. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploense KL, Vieira P, Bubalo L, Olivarria G, Carr AE, Szumlinski KK, Kippin TE (2018) Contributions of prolonged contingent and non-contingent cocaine exposure to escalation of cocaine intake and glutamatergic gene expression. Psychopharmacology 235:1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Miszkiel J, Frankowska M, Pomierny B, Niedzielska E, Smaga I, Fumagalli F, Filip M (2015) Withdrawal from cocaine self-administration and yoked cocaine delivery dysregulates glutamatergic mGlu5 and NMDA receptors in the rat brain. Neurotox Res 27:246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Miszkiel J, Frankowska M, Bystrowska B, Filip M (2017) Cocaine self-administration, extinction training and drug-induced relapse change metabotropic glutamate mGlu5 receptors expression: Evidence from radioligand binding and immunohistochemistry assays. Brain Res 1655:66–76. [DOI] [PubMed] [Google Scholar]
- Renoldi G, Calcagno E, Borsini F, Invernizzi RW (2006) Stimulation of group I mGlu receptors in the ventrotegmental area enhances extracellular dopamine in the rat medial prefrontal cortex. J Neurochem 100:1658–66. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5:317–328. [DOI] [PubMed] [Google Scholar]
- Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK (2016) Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology 102:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CB, Templeton TJ, Chiu AS, Kim J, Gable ES, Vieira PA, Kippin TE, Szumlinski KK (2018) Endogenous glutamate within the prelimbic and infralimbic cortices regulates the incubation of cocaine-seeking in rats. Neuropharmacology 128:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Gergans SR, Iordanou JC, Lyle MA (2008) Chronic exercise increases sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Rep 60(4):561–5. [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Fronk GE, Abel JM, Lacy RT, Bills SE, Lynch WJ (2018) Resistance exercise decreases heroin self-administration and alters gene expression in the nucleus accumbens of heroin-exposed rats. Psychopharmacology 235:1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Wroten MG, Miller BW, Sacramento AD, Cohen M, Ben-Shahar O, Kippin TE (2016) Cocaine self-administration elevates GluN2B within dmPFC mediating heightened cue- elicited operant responding. J Drug Abuse 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Shin CB (2018) Kinase interest you in treating incubated cocaine-craving? A hypothetical model for treatment intervention during protracted withdrawal from cocaine. Genes Brain Behav 17:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM, Johnston LD (2011) Exercise and substance use among American youth, 1991-2009. Am J Prev Med 40:530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Stamos J, Robison LS, Heyman G, Tucci A, Wang GJ, Robinson JK, Anderson BJ, Volkow ND (2013) Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behav Brain Res 239:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer KM, Steketee JD (2013) Group I metabotropic glutamate receptors in the medial prefrontal cortex: role in mesocorticolimbic glutamate release in cocaine sensitization. Synapse 67:887–896. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Rethorst CD, Carmody T, Grannemann BD, Walker R, Warden D, Shores-Wilson K, Stoutenberg M, Oden N, Silverstein M, Hodgkins C, Love L, Seamans C, Stotts A, Causey, Szucs-Reed RP, Rinaldi P, Myrick H, Straus M, Liu D, Lindblad R, Church T, Blair SN, Nunes EV. (2017) Randomized controlled trial comparing exercise to health education for stimulant use disorder: Results from STimulant Reduction Intervention using dosed exercise (CTN-0037; STRIDE). J Clin Psychiatry 78:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliz PT, Boyd C, McCabe SE (2013) Playing through pain: sports participation and nonmedical use of opioid medications among adolescents. Am J Public Health 103: e28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW (2013) Role of mGlu5 neurotransmission in reinstated cocaine-seeking. Addict Biol 18:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang Y, Wang Y, Li R, Zhou C (2014a) Impact of physical exercise on substance use disorders: A meta-analysis. PLoS One 9:e110728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang M, Yang SD, Li WB, Ren SQ, Zhang J, Zhang F (2014b) Pre-ischemic treadmill training alleviates brain damage via GLT-1-mediated signal pathway after ischemic stroke in rats. Neuroscience 274:393–402. [DOI] [PubMed] [Google Scholar]
- Whitfield TW Jr, Shi X, Sun WL, McGinty JF (2011) The suppressive effect of an intra- prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal- regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci 31:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 6:351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME (2015) Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology 232:3507–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.