Abstract
Piperine, the principle pungent compound in black peppers, is known to activate the capsaicin receptor TRPV1 ion channel. How piperine interacts with the channel protein, however, remains unclear. Here we show that piperine binds to the same ligand-binding pocket as capsaicin but in different poses. There was no detectable detrimental effect when T551 and E571, two major sites known to form hydrogen bond with capsaicin, were mutated to a hydrophobic amino acid. Computational structural modeling suggested that piperine makes interactions with multiple amino acids within the ligand binding pocket, including T671 on the pore-forming S6 segment. Mutations of this residue could substantially reduce or even eliminate piperine-induced activation, confirming that T671 is an important site. Our results suggest that the bound piperine may directly interact with the pore-forming S6 segment to induce channel opening. These findings help to explain why piperine is a weak agonist, and may guide future efforts to develop novel pharmaceutical reagents targeting TRPV1.
Keywords: agonist, pungency, nociception, spice, capsaicin, pepper
INTRODUCTION
Black peppercorns are one of the oldest ingredients of food spices in both eastern and western cultures; they remain a rudimental ingredient in old- and new-world cuisines[1]. Piperine (Figure 1a), as the main pungent compound of black peppercorns Piper nigrum, is an alkaloid with a stable and simple chemical structure that shows potentials for the development of new drugs. Like capsaicin in chili peppers, piperine is the principle natural compound that makes black peppers spicy. The pungent and irritating properties add attractive flavors to food. Piperine has been appreciated for medicinal usages for centuries[2]. Understanding the molecular mechanisms underlying the interactions of piperine with biological processes therefore has important implications.
Figure 1.
Piperine directly activates TRPV1 by binding to the capsaicin-binding pocket. (a) The chemical structures of piperine and capsaicin. (b) Representative live-cell calcium signal images responding to bath solution (control), piperine and ionomycin in non-transfected HEK293 cells. (c) Representative live-cell calcium signal images responding to bath solution (control) , piperine (3 μM, 10 μM) and capsaicin (10 μM). (d) Concentration-response curve of WT mTRPV1 normalized to 10 μM capsaicin response. Superimposed is a fit of Hill function; the data point at 30 μM was omitted during fitting. (e) Calcium fluorescence signal in response to 10 μM piperine with or without 10 μM capsazepine (CZP), respectively, normalized to the response of 10 μM capsaicin (n = 4). ***, Student t-test, p < 0.001.
Piperine has long been known to activate sensory neurons isolated from trigeminal ganglion of rats[3]. Subsequent studies have confirmed that piperine and capsaicin act on the same receptor[4], the capsaicin receptor TRPV1 ion channel[5]. TRPV1 is a non-selective cation channel mainly expressed in the peripheral nervous system[6]. Noxious stimuli including heat[5, 7], protons[8], animal toxins[9-11], vanilloids[5] and a variety of endogenous ligands[12] can all strongly activate TRPV1 to initiate an excitatory current in sensory neurons. TRPV1 activation is associated with various biological responses[5, 13, 14], such as: i) regulation of pain and body temperature[8]; ii) regulation of tumor formation and apoptosis[15]; iii) control of metabolism and glucose homeostasis[16]; iv) regulation of bladder function[17]; v) regulation of the respiratory system[18]. Though piperine is known to activate TRPV1, the underlying molecular mechanism remains poorly understood.
Studies in recent years have started to clarify the structural mechanism underlying capsaicin activation of TRPV1 (summarized in[19]). Capsaicin has three functional parts: the vanillyl head group and the amide neck are hydrophilic, whereas the fatty acid chain tail is hydrophobic (Figure 1a). Capsaicin binds in the TRPV1 ligand-binding pocket formed by the S3 and S4 transmembrane segments, the S5 and S6 segments from a neighboring subunit, and the S4-S5 linker. The bound capsaicin takes a “head-down tail-up” orientation stabilized by two hydrogen bonds—between the amide group in the capsaicin neck and the hydroxyl group of T551 on the S4 segment of mouse TRPV1, and between the hydroxyl group in the capsaicin head and the carboxyl group of E571 on the S4-S5 linker—as well as extensive hydrophobic interactions[20]. Although piperine can also activate TRPV1, its molecular structure is substantially different from that of capsaicin. It is challenging to predict based on chemical structure how piperine binds inside TRPV1.
Here in this study, we used computational structural modeling to explore potential piperine-TRPV1 interactions, and combined live-cell calcium imaging and patch-clamp electrophysiological recording to investigate piperine activation with key TRPV1 residues. We conclude that piperine activates TRPV1 by directly interacting with the pore-forming S6 segment, in a manner distinct from that of capsaicin.
MATERIALS AND METHODS
Molecular biology and Cell transfection
The mouse TRPV1 (a gift from Dr. Michael X. Zhu, University of Texas Health Science Center at Houston) was used in this study. WT cDNA as well as all the mutant cDNAs were fused with the eYFP cDNA to assist identification of expressed cells. The fluorescent tag did not affect the functional properties of the channel[21]. Mutants were constructed with overlapping PCR as previously described; all constructs were confirmed by sequencing. HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37°C with 5% CO2. Cells were plated on glass coverslips before transfection. Transient transfection was conducted with Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol. Experiments were performed 1-to-2 days after transfection.
Calcium fluorescence imaging
HEK293 cells expressing mTRPV1 channels seeded on 25 mm coverslips were washed twice with an extracellular solution (ECS) that contained 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose 1.8 mM CaCl2, and 15 mM HEPES (pH 7.4), then incubated at room temperature for 45 minutes in 2 ml of ECS supplemented with 2 μM Fluo-4/AM and 2 μM Pluronic F-127. Pluronic F-127 (2 μM) was included in all solutions to prevent Fluo-4 leakage from cells. At the end of incubation, cells were washed three times with ECS. Coverslip with dye-loaded cells in a 35-mm chamber was placed on the stage of an OLYMPUS IX73 microscope equipped with an OLYMPUS DP80 CCD camera. A blue filter (460-to-495 nm) and a long-pass filter (510 nm) were used as the excitation and emission filters, respectively, in conjunction with a dichroic mirror at 505 nm. The duration of light exposure was controlled by a computer-driven mechanical shutter. Cells were challenged sequentially with piperine of various concentrations, capsaicin (10 μM) and ionomycin (10 μM). Fluorescence video were acquired at an exposure time of 222 ms. Change in fluorescence intensity, ΔF, was calculated as the difference between the equilibrium level before and after stimulation (ΔF = F – F0), and was normalized to the response of 10 μM capsaicin, FCAP.
Electrophysiological recording
Patch-clamp recordings were performed with a HEKA EPC10 amplifier driven by PatchMaster software (HEKA). Single-channel recordings were performed under inside-out configuration. Cell membrane potential was held at 0 mV for 50 ms, from which the voltage was stepped to +80 mV for 1 s. Both the pipette solution and the bath solution contained 130 mM NaCl, 0.2 mM EDTA and 3 mM HEPES (pH 7.2). Current signals were filtered at 2.25 kHz and sampled at 10 kHz. To apply piperine or capsaicin during patch-clamp recording, we used a gravity-driven perfusion system (RSC-200, Biological Science Instruments) to achieve rapid solution switching. Each drug solution was delivered through a separate tube so that there was no cross contamination between solutions. Pipette tip was placed right in front of the perfusion tube outlet during recording to ensure that solution exchange was complete. All recordings were performed at room temperature (25°C).
Reagents
Piperine, capsaicin and capsazepine at > 98% purity were purchased from MedChem Express (China). Stock solutions of piperine, capsaicin and capsazepine were prepared in dimethylsulphoxide (DMSO) at 100 mM concentration and were diluted to working concentrations as needed. The maximum DMSO concentration used in working solutions was 0.1% which was shown in control experiments to have negligible effects (data not shown).
Molecular docking
Docking of piperine onto TRPV1 was performed using the RosettaLigand application within the Rosetta molecular modeling software suite, version 3.7[22, 23], using an XML-style script in RosettaScripts[24]. The rat TRPV1 capsaicin-bound cryo-EM structure (PDB ID: 3J5R) was first relaxed in a membrane environment using RosettaMembrane[25]. Piperine was initially placed at the center of the ligand binding pocket, and was constrained within a 10 Å diameter sphere in which it was allowed to move freely. A total of 200 conformers were generated using the Open Eye OMEGA software[26, 27]. A total of 30,000 models were generated and ranked by total energy. The top 3,000 lowest energy models were then ranked by binding energy between the ligand and the channel. To quantify the docking results for structural convergence, the binding energy value for the top 3,000 lowest energy models were plotted against its unsuperimposed ligand root-mean-squared deviation (R.M.S.D). The lowest binding energy model was used as a reference. The top 30 models were identified as candidates. All molecular graphics were rendered by UCSF Chimera software version 1.13.
To define potential atomic interactions, the hydrogen bond and van der Waals (VDW) energies (calculated by the sum of attractive and repulsive energies) for each model were determined. These energy values were mapped on a per residue basis using Rosetta’s residue_energy_breakdown function.
Data analysis
Analysis of electrophysiological data was done with Igor Pro (WaveMatrics). The 50 Hz noise in current recordings was subtracted from the data before analyses by fitting the noise to a sine wave. We constructed all-point histograms of the single-channel recordings at each concentration of agonist. The histograms were fitted to a combination of Gaussian functions to obtain estimates of the area under the peak for the open (So) and closed state (Sc). The channel open probability was calculated as Po = So/(So + Sc). When there were two channels in the recording, the open probability was calculated as Po = 1 - SQRT(Sc/(Sc + So1 + So2)), in which SO1 and SO2 are area under the Gaussian peak for one and two channel openings, respectively. Recordings with more than two channels were discarded.
Concentration-response relationships were fitted to a Hill function to estimate the EC50 and slope factor values; all data are shown as mean ± SEM. Functional differences caused by changes in each concentrations of piperine or point mutations were tested using repeated measures two-way ANOVA followed by Tukey’s or Sidak’s multiple comparisons test. Student’s t-test was applied to examine the statistical significance of results from calcium imaging and patch clamp recordings. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Calcium imaging verified direct activation of TRPV1 by piperine
Calcium imaging was used to record fluorescence changes in HEK293 cells expressing mTRPV1 in the presence of different concentrations of piperine; 10 μM capsaicin was also administered and used for normalization (Fig. 1c). Parallel recordings were made from untransfected cells that confirmed no detectable non-specific effects of piperine (Fig. 1b). Fitting the piperine concentration-response relationship to a Hill function yielded an estimate of the EC50 value as 3.3 ± 0.7 μM (n = 5), in comparison to an EC50 value of 0.1 ± 0.002 μM for capsaicin (Fig. 1d, Table 1). The maximum activation induced by piperine was about 88% of that of capsaicin, which is known to activate mTRPV1 to a maximum open probability of above 90%[20]. We also observed that the fluorescence intensity of the cells decreased with 30 μM piperine, consistent with the occurrence of channel desensitization as previously reported[5]. Therefore, calcium imaging results indicate that piperine directly activates TRPV1, though with a lower efficacy than capsaicin.
Table 1.
Summary of calcium imaging results
| Channel type╲Drug | capsaicin | piperine | ||||
|---|---|---|---|---|---|---|
| EC50 (μM) | Hill slope factor |
n | EC50 (μM) | Hill slope factor |
n | |
| WT | 0.1 ± 0.002 | 1.9 | 6 | 3.3 ± 0.7 | 2.2 | 5 |
| T551V | 1.0 ± 0.02 | 3.3 | 6 | 5.1 ± 0.7 | 1.3 | 5 |
| E571A | 1.0 ± 0.04 | 3.0 | 6 | 3.5 ± 0.5 | 2.2 | 5 |
Does piperine activate TRPV1 by binding to the same ligand-binding pocket as capsaicin? To address this question, we used a competitive antagonist of capsaicin, capsazepine (CZP). A previous cryo-EM study has confirmed capsazepine’s overlapping binding site with capsaicin[28]. Calcium imaging results showed that the fluorescence intensity increase induced by 10 μM piperine could be almost completely inhibited in the presence of 10 μM CZP (Fig. 1e), indicating that piperine, though structurally distinct from capsaicin, binds to the same ligand-binding pocket.
Effect of TRPV1 mutations on the activation of piperine
In order to investigate the molecular mechanism underlying piperine activation of TRPV1, we individually mutated T551 and E571 from a hydrophilic residue to a hydrophobic residue, yielding T551V and E571A. These two amino acids were chosen because the specificity for capsaicin binding is bestowed by hydrogen bonds formed by its vanillyl head and amide neck with E571 and T551, respectively (Figure 1a, bottom)[20]. Functional verification by calcium imaging experiments (Fig. 2a) showed that mutating E571 had no significant effect on the EC50 value (3.5 ± 0.5 μM, n = 5, P > 0.05) or efficacy (77.1 ± 7.3%, n = 5, P > 0.05) of piperine compared to wild-type (3.3 ± 0.7 μM , 82.7 ± 5.7%, n = 5) (Fig. 2b). Similarly, changing T551 also had no obvious effect on the EC50 value (5.1 ± 0.7 μM, n = 5, P > 0.05). As was true for wild-type TRPV1, the fluorescence signal from T551V or E571A expressing cells decreased substantially when 30 μM piperine was used (Fig. 2b). In conclusion, our observations do not support the existence of a hydrogen bond between piperine and TRPV1 at these sites.
Figure 2.
Calcium imaging indicates that T551 and E571 do not interact with piperine. (a) Representative calcium imaging data of mTRPV1 WT and mutant channels responding to bath solution, piperine and capsacin. (b) Concentration-dependent activation of WT and mutant channels fitted to a Hill function; data points at 30 μM were omitted during fitting.
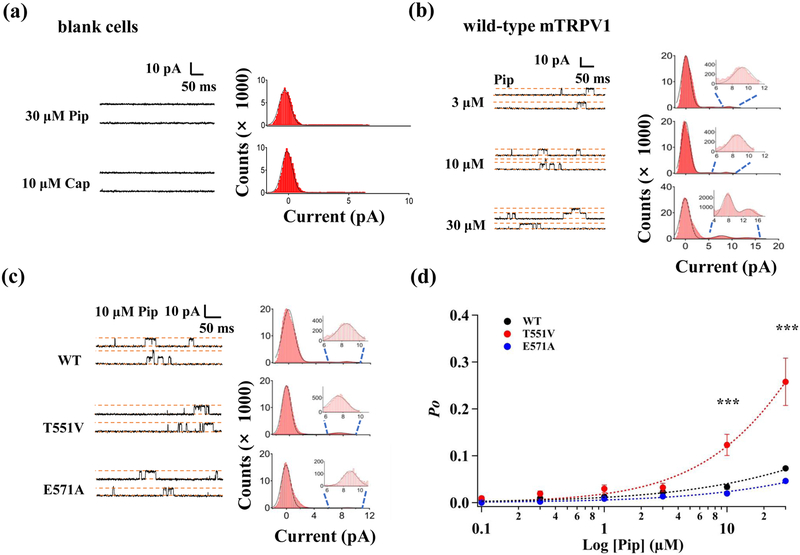
Patch-clamp recording showed that piperine weakly activates TRPV1
Calcium imaging results described above showed that piperine had a lower activation capacity for TRPV1 than capsaicin. Single-channel recording results confirmed this observation, demonstrating that the channel open probability arose with increasing concentrations of piperine but reached quite low levels (Fig. 3b&d). Even in the presence of 30 μM piperine, the Po was only 10% (Fig. 3b&d), with the maximal Po being about 36% predicted by extrapolation. In contrast to the calcium imaging results, the open probability at 30 μM piperine was not lower than that at 10 μM piperine. Note that for patch-clamp experiments, Ca2+ was removed from the recording solutions; Ca2+ is known to mediate rapid channel desensitization[29, 30]. The estimated EC50 value, at 252.3 ± 38.1 μM (n = 4), was much higher than the estimated EC50 value from calcium imaging. Similar observations were previously reported and attributed to differences in the two detection methods[31].
Figure 3.
Preventing hydrogen bond formation at T551 and E571 does not reduce piperine’s potency and efficacy. Representative single-channel traces recorded from (a) non-transfected HEK293, (b) WT TRPV1, and (c) T551V and E571A mutant TRPV1 expressing cells in the presence of piperine or capsaicin (left panel) with the corresponding all-point histograms (right panel). Superimposed is a fit of a double- or triple-Gaussian function. Blue dash lines correspond to the inset of the open state peaks. Upward deflection indicates channel opening. (d) Concentration-dependent activation of WT and mutant channels fitted to a Hill function. Repeated measures two-way ANOVA followed by Tukey's multiple comparison test was used, where asterisks indicate statistical differences between the WT and T551V mutant channels. n = 4, ***, p < 0.001.
Effect of TRPV1 mutations on the channel open probability induced by piperine
We recorded piperine responses of the T551V and E571A mutant channels by single-channel recordings, from which channel open probabilities at various concentrations were determined (Fig. 3c&d). It can be seen that the E571A mutation had no detectable effect on the EC50 value (EC50 = 276.8 ± 36.5 μM, n = 4, P > 0.05). The maximum activation open probability was estimated to be only 22%. Interestingly, the T551V mutant has an estimated EC50 value of 93.5 ± 15.1 μM (n = 4), which is significantly smaller than that of the wild-type (P < 0.01). The open probabilities at both 10 μM (12.3 ± 2.2%, n = 4) and 30 μM (25.8 ± 5.1%, n = 4) were significantly higher than that of the wild-type (3.3 ± 0.5%, n = 4) (concentration, F(5, 15) = 141.5, P < 0.001; mutation, F(1, 3) = 35.7, P < 0.001; concentration × mutation interaction, F(5, 15) = 24.9, P < 0.01; repeated measures two-way ANOVA followed by Tukey’s multiple comparisons test; Figure 3d). These observations suggest that piperine does not interact directly with E571 but it does interact with T551, though likely not via a hydrogen bond.
Molecular docking indicated multiple binding poses of piperine
In order to explore potential piperine-TRPV1 interactions, we conducted computational structural modeling. Using an updated version of the RosettaLigand application[22, 23, 32, 33], we generated 30,000 binding models, from which the top 3,000 models were put forward for the determination of the binding energy between the ligand and the channel. The lowest binding energy models were identified and compared. It was noticed that among these lowest binding energy models piperine took quite different poses (Fig. 4a, left panel). Many residues within the ligand-binding pocket were indicated to make contact with piperine (Fig. 4a, right panel); yet, most of the piperine poses do not resemble a bound capsaicin molecule as previously reported, having specific interactions with T551 and E571[20]. This can be seen in Figure 4a, where there is little indication that stable hydrogen bonds could be formed with either T551 or E571. These observations are in close agreement with our experimental data.
Figure 4.
Molecular modeling reveals T671 as a potential residue for mediating piperine-induced TRPV1 activation. (a) Representative binding pose observed within the top 0.1% lowest energy models (left panel, top), clusters of top binding poses (left panel, middle), and the capsaicin binding pose (left panel, bottom); hydrogen bond and VDW interaction energies mapped on a per residue basis (top 0.1% lowest energy models) between the channel and piperine (right panel). Unit of energy is Rosetta Energy Unit (R.E.U.). (b) Binding energy plotted versus root-mean-squared deviation over the top 10% lowest energy models. The lowest energy model was used as the reference. Red dots represent the top 0.1% lowest energy models. (c) Representative single-channel traces recorded from T671S in the presence of 10 μM and 30 μM piperine (top left panel) and the corresponding all-point histograms (top right panel). Representative single-channel traces recorded from T671V in the presence of 30 μM piperine and 10 μM capsaicin (bottom left panel) and the corresponding all-point histograms (bottom right panel). (d) Concentration-dependent activation of WT and mutant channels fitted to a Hill function. Repeated measures two-way ANOVA followed by Tukey's multiple comparison test was used; asterisks indicate statistical differences between the WT and mutant channels. n = 4, *, p < 0.05, **, p < 0.01, ***, p< 0.001.
Among those residues that were indicated to interact with the bound piperine, one particular residue stood out from the structural analysis. This is T671, residing in the middle of the S6 segment: the residue was seen to have the highest average hydrogen bond energy and also contribute substantially the VDW energy (Fig. 4a, right panel). If the bound piperine indeed interacts with T671, this interaction would allow piperine to directly affect the S6 activation gate. This prediction was tested by functional analysis.
T671S mutation strongly disrupts piperine activation of TRPV1
To test whether T671 has direct interaction with piperine, we conducted functional recordings of mutant channels at this position. We found that piperine was able to induce single-channel activities from T671S channels in a concentration-dependent manner (Fig. 4c). However, whereas we have previously shown that channels with the conserved T671S mutation exhibited wildtype-like activities when stimulated with capsaicin[20], the level of activity induced by piperine was much reduced from these channels compared to the wildtype channels (Fig. 4d). The open probability of T671S was significantly lower than that of the wildtype channels at most of piperine concentrations we have tested (concentration, F(5, 15) = 141.5, P < 0.001; mutation, F(1, 3) = 35.7, P < 0.001; concentration × mutation interaction, F(5, 15) = 24.9, P < 0.01; repeated measures two-way ANOVA followed by Sidak’s multiple comparisons test; Figure 4d). To further confirm these observations, we tested a more dramatic mutation at this position, T671V. We observed no channel activity even when 30 μM of piperine was used, though capsaicin induced robust currents from the same patches (n = 4). These results support the observations from structural modeling, suggesting that piperine may directly interact with T671 to induce channel activation.
DISCUSSION
The molecular structure of piperine is distinct from that of capsaicin in several aspects (Fig. 1a). Although piperine has an aromatic heterocyclic ring and a fatty chain that mirror capsaicin’s structural components, there are a number of structural deviations that would be detrimental for activity according to the knowledge of capsaicin-TRPV1 interactions. The hydroxyl group in capsaicin known to participate in hydrogen bond formation with TRPV1 is missing, and the equivalent dioxol group is further away from the neck carbonyl which in capsaicin participates in another hydrogen bond. In addition, the long aliphatic tail of capsaicin known to have a substantial contribution to hydrophobic interactions is replaced by a piperidine ring. Furthermore, two C=C double bonds constitute a strong conjugate system linking the head and tail, limiting the structural flexibility of piperine. These molecular characteristics directly determine the structural stability of piperine. Interactions between TRPV1 and piperine have been studied by modifications of the piperine molecule before the TRPV1 structures were known[34]. Our present study provides the missing information on how different channel residues may interact with piperine, allowing interpretation of the molecular mechanism underlying piperine-TRPV1 interactions.
Psychophysical tests show that piperine has a perceived pungency level 10-to-100 times lower than capsaicin (its relative pungency-taste threshold being 10.5 μM vis-a-vis 0.6 μM for capsaicin)[35]. Our results from calcium imaging and patch-clamp recording consistently showed that the EC50 value for piperine activation of TRPV1 was significantly higher than that of capsaicin. Extrapolation from the experimentally accessible concentration range indicated that the maximal level of channel activation induced by piperine is substantially lower than the level reached by saturating concentrations of capsaicin. As piperine binds to the same ligand-binding pocket, its low potency and distinct chemical structure imply that piperine may interact with TRPV1 in a way substantially different from capsaicin.
Capsaicin is a potent agonist of TRPV1 partially because of its specific binding to TRPV1 via two hydrogen bonds and substantial van der Waals interactions[19]. In piperine, both types of atomic interactions are altered. No stable hydrogen bond could be detected at T551 or E571. The carbonyl in piperine is seen to interact with T551 but the nature of this interaction appears different from that of the carbonyl in capsaicin. Piperine also lacks an extended hydrophobic structure like the capsaicin tail to exploit the heavily hydrophobic environment of the upper ligand-binding pocket. Compared with capsaicin, piperine has a conjugated system throughout the molecule. This rigid structure greatly reduces the mutually induced rearrangement between piperine and the channel; however, the structural rigidity may allow piperine to perturb local channel structures in unique ways. Indeed, our combined computational and functional results suggest that piperine can directly interact with the pore-forming S6 segment via T671. T671 and its adjacent residue Y672 are rare polar residues in the S6 segment that have been previously suggested to contribute to activation gating by capsaicin[36, 37]. In our previous studies these two residues were not found to directly interact with capsaicin[20]. Instead, the region participates in activation conformational changes that follow capsaicin-induced S4-S5 linker movement[38]. Data from the present study revealed that piperine does not interact with E571 on the S4-S5 linker, but may directly interact with T671. In this way, piperine skips the S4-S5 linker and uses a “shortcut pathway” to induce TRPV1 activation. All factors discussed above may contribute to the less stable binding of piperine in the ligand-binding pocket of TRPV1, resulting in its low potency. Indeed, it has been proposed that some of piperine’s biological effects may be due to its interaction with the TRPA1 channel[39] and the GABA-A receptor[40, 41].
The unstable binding and the associated weaker activation of TRPV1 by piperine likely contribute to the unique perception from eating black pepper-flavored food. As a weak agonist of TRPV1, piperine may offer certain advantages as a starting compound for developing novel drugs. Indeed, capsaicin has been explored for its anti-obesity property[42], for which its strong pungency is an unfavorable trait. Capsiate, a much less potent capsaicin analog produced by the sweet peppers, is a popular choice that has been intensively studied in recent years[43, 44]. It can be envisioned that piperine might be a potential alternative for anti-obesity and other purposes.
In summary, multiple lines of evidence support the view that piperine binds weakly to TRPV1 in orientations distinct from that of capsaicin, yielding a new way of activating the channel but a much reduced level of channel activity. These findings should be useful to better understand black peppers as food spices and offer a starting point for developing new pharmaceutical applications.
Table 2.
Summary of electrophysiological measurements
| Channel type╲Drug | capsaicin | piperine | ||||
|---|---|---|---|---|---|---|
| EC50 (μM) | Hill slope factor |
n | EC50 (μM) | Hill slope factor |
n | |
| WT | 0.1 ± 0.003 | 1.9 | 6 | 252.3 ± 38.1 | 2.2 | 4 |
| T551V | 1.4 ± 0.03 | 3.3 | 6 | 93.5 ± 15.1 | 0.9 | 4 |
| E571A | 1.3 ± 0.03 | 3.0 | 6 | 276.8 ±36.5 | 0.7 | 4 |
Highlights.
Piperine does not form hydrogen bonds with T551 on S4 and E571 on the S4-S5 linker like capsaicin
Piperine interacts with T671 on the pore-forming S6
Piperine may activate TRPV1 by directly interacting with the pore
Acknowledgements
We thank our current and formal lab members for their generous help and insightful discussion. This work was supported by the Qingdao Postdoctoral Research Project to YHT and the National Institutes of Health (R01NS103954) to JZ and VYY.
The abbreviations used are:
- CPZ
capsazepine
- ECS
extracellular solution
- mTRPV1
mouse transient receptor potential cation channel, subfamily V, member 1
- TRPA1
transient receptor potential cation channel, subfamily A, member 1
- VDW
van der Waals
Footnotes
Authors have no competing interests to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Parry JW, Spices, Chemical Publishing Co Inc., New York, 1969. [Google Scholar]
- [2].Srinivasan K, Black pepper and its pungent principle-piperine: a review of diverse physiological effects, Critical reviews in food science and nutrition, 47 (2007) 735–748. [DOI] [PubMed] [Google Scholar]
- [3].Liu L, Simon SA, Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells, Journal of neurophysiology, 76 (1996) 1858–1869. [DOI] [PubMed] [Google Scholar]
- [4].McNamara FN, Randall A, Gunthorpe MJ, Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1), British journal of pharmacology, 144 (2005) 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D, The capsaicin receptor: a heat-activated ion channel in the pain pathway, Nature, 389 (1997) 816–824. [DOI] [PubMed] [Google Scholar]
- [6].Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJ, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB, Cloning and functional expression of a human orthologue of rat vanilloid receptor-1, Pain, 88 (2000) 205–215. [DOI] [PubMed] [Google Scholar]
- [7].Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D, TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids, Neuron, 77 (2013) 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D, The cloned capsaicin receptor integrates multiple pain-producing stimuli, Neuron, 21 (1998) 531–543. [DOI] [PubMed] [Google Scholar]
- [9].Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D, A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain, Cell, 141 (2010) 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D, Spider toxins activate the capsaicin receptor to produce inflammatory pain, Nature, 444 (2006) 208–212. [DOI] [PubMed] [Google Scholar]
- [11].Yang S, Yang F, Wei N, Hong J, Li B, Luo L, Rong M, Yarov-Yarovoy V, Zheng J, Wang K, Lai R, A pain-inducing centipede toxin targets the heat activation machinery of nociceptor TRPV1, Nature communications, 6 (2015) 8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakagawa H, Hiura A, Capsaicin, transient receptor potential (TRP) protein subfamilies and the particular relationship between capsaicin receptors and small primary sensory neurons, Anatomical science international, 81 (2006) 135–155. [DOI] [PubMed] [Google Scholar]
- [13].Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB, Ca2+ signaling, TRP channels, and endothelial permeability, Microcirculation (New York, N.Y. : 1994), 13 (2006) 693–708. [DOI] [PubMed] [Google Scholar]
- [14].Gunthorpe MJ, Szallasi A, Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms, Current pharmaceutical design, 14 (2008) 32–41. [DOI] [PubMed] [Google Scholar]
- [15].Omari SA, Adams MJ, Geraghty DP, TRPV1 Channels in Immune Cells and Hematological Malignancies, Advances in pharmacology (San Diego, Calif.), 79 (2017) 173–198. [DOI] [PubMed] [Google Scholar]
- [16].Suri A, Szallasi A, The emerging role of TRPV1 in diabetes and obesity, Trends in pharmacological sciences, 29 (2008) 29–36. [DOI] [PubMed] [Google Scholar]
- [17].Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ, Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells, Proc Natl Acad Sci U S A, 98 (2001) 13396–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Logu F, Patacchini R, Fontana G, Geppetti P, TRP functions in the broncho-pulmonary system, Seminars in immunopathology, 38 (2016) 321–329. [DOI] [PubMed] [Google Scholar]
- [19].Yang F, Zheng J, Understand spiciness: mechanism of TRPV1 channel activation by capsaicin, Protein & cell, 8 (2017) 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang F, Xiao X, Cheng W, Yang W, Yu P, Song Z, Yarov-Yarovoy Y, Zheng J, Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel, Nature chemical biology, 11 (2015) 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng W, Yang F, Takanishi CL, Zheng J, Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties, J Gen Physiol, 129 (2007) 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davis IW, Baker D, RosettaLigand docking with full ligand and receptor flexibility, Journal of molecular biology, 385 (2009) 381–392. [DOI] [PubMed] [Google Scholar]
- [23].Meiler J, Baker D, ROSETTALIGAND: protein-small molecule docking with full side-chain flexibility, Proteins, 65 (2006) 538–548. [DOI] [PubMed] [Google Scholar]
- [24].Lemmon G, Meiler J, Rosetta Ligand docking with flexible XML protocols, Methods in molecular biology (Clifton, N.J.), 819 (2012) 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yarov-Yarovoy V, Schonbrun J, Baker D, Multipass membrane protein structure prediction using Rosetta, Proteins, 62 (2006) 1010–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hawkins PC, Nicholls A, Conformer generation with OMEGA: learning from the data set and the analysis of failures, Journal of chemical information and modeling, 52 (2012) 2919–2936. [DOI] [PubMed] [Google Scholar]
- [27].Hawkins PC, Skillman AG, Warren GL, Ellingson BA, Stahl MT, Conformer generation with OMEGA: algorithm and validation using high quality structures from the Protein Databank and Cambridge Structural Database, Journal of chemical information and modeling, 50 (2010) 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao Y, Cao E, Julius D, Cheng Y, TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action, Nature, 534 (2016) 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R, The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity, Neuron, 54 (2007) 905–918. [DOI] [PubMed] [Google Scholar]
- [30].Ma L, Yang F, Vu S, Zheng J, Exploring functional roles of TRPV1 intracellular domains with unstructured peptide-insertion screening, Scientific reports, 6 (2016) 33827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Geron M, Kumar R, Zhou W, Faraldo-Gomez JD, Vasquez V, Priel A, TRPV1 pore turret dictates distinct DkTx and capsaicin gating, Proc Natl Acad Sci U S A, 115 (2018) E11837–e11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bender BJ, Cisneros A 3rd, Duran AM, Finn JA, Fu D, Lokits AD, Mueller BK, Sangha AK, Sauer MF, Sevy AM, Sliwoski G, Sheehan JH, DiMaio F, Meiler J, Moretti R, Protocols for Molecular Modeling with Rosetta3 and RosettaScripts, Biochemistry, 55 (2016) 4748–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davis IW, Raha K, Head MS, Baker D, Blind docking of pharmaceutically relevant compounds using RosettaLigand, Protein science : a publication of the Protein Society, 18 (2009) 1998–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Correa EA, Hogestatt ED, Sterner O, Echeverri F, Zygmunt PM, In vitro TRPV1 activity of piperine derived amides, Bioorganic & medicinal chemistry, 18 (2010) 3299–3306. [DOI] [PubMed] [Google Scholar]
- [35].Szolcsányi J, Jancso-Gabor A, Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency of pungent agents, Arzneimitteiforschung 25(12) (1975) 1877–1881. [PubMed] [Google Scholar]
- [36].Steinberg X, Kasimova MA, Cabezas-Bratesco D, Galpin JD, Ladron-de-Guevara E, Villa F, Carnevale V, Islas L, Ahem CA, Brauchi SE, Conformational dynamics in TRPV1 channels reported by an encoded coumarin amino acid, eLife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kasimova MA, Yazici AT, Yudin Y, Granata D, Klein ML, Rohacs T, Carnevale V, A hypothetical molecular mechanism for TRPV1 activation that invokes rotation of an S6 asparagine, The Journal of general physiology, 150 (2018) 1554–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang F, Xiao X, Lee BH, Vu S, Yang W, Yarov-Yarovoy V, Zheng J, The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel, Nature communications, 9 (2018) 2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Okumura Y, Narukawa M, Iwasaki Y, Ishikawa A, Matsuda H, Yoshikawa M, Watanabe T, Activation of TRPV1 and TRPA1 by black pepper components, Bioscience, biotechnology, and biochemistry, 74 (2010) 1068–1072. [DOI] [PubMed] [Google Scholar]
- [40].Khom S, Strommer B, Schoffmann A, Hintersteiner J, Baburin I, Erker T, Schwarz T, Schwarzer C, Zaugg J, Hamburger M, Hering S, GABAA receptor modulation by piperine and a non-TRPV1 activating derivative, Biochemical pharmacology, 85 (2013) 1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schoffmann A, Wimmer L, Goldmann D, Khom S, Hintersteiner J, Baburin I, Schwarz T, Hintersteininger M, Pakfeifer P, Oufir M, Hamburger M, Erker T, Ecker GF, Mihovilovic MD, Hering S, Efficient modulation of gamma-aminobutyric acid type A receptors by piperine derivatives, Journal of medicinal chemistry, 57 (2014) 5602–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M, Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity, Circulation research, 100 (2007) 1063–1070. [DOI] [PubMed] [Google Scholar]
- [43].Haramizu S, Kawabata F, Masuda Y, Ohnuki K, Watanabe T, Yazawa S, Fushiki T, Capsinoids, non-pungent capsaicin analogs, reduce body fat accumulation without weight rebound unlike dietary restriction in mice, Bioscience, biotechnology, and biochemistry, 75 (2011)95–99. [DOI] [PubMed] [Google Scholar]
- [44].Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T, Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice, Bioscience, biotechnology, and biochemistry, 65 (2001) 2735–2740. [DOI] [PubMed] [Google Scholar]