Abstract
Calpain-mediated tau cleavage into the neurotoxic tau45-230 fragment plays an important role in Alzheimer’s disease (AD). This tau fragment accumulates mainly in the cytoplasm of degenerating neurons. However, subcellular localization studies indicated that a pool of tau45-230 associates with the cytoskeleton in hippocampal neurons. In the present study, we assessed whether such localization could underlie tau45-230 neurotoxic effects. Quantitative Western blot analysis showed decreased levels of full-length tau bound to microtubules in tau45-230-expressing hippocampal neurons when compared to controls. In addition, the presence of this tau fragment induced a transient increase in tyrosinated tubulin, a marker of unstable microtubules, followed by a significant decrease in the levels of this tubulin isoform. The data obtained also showed a significant reduction in actin filaments in tau45-230-expressing neurons. These changes in microtubules and actin filaments correlated with delayed neurite elongation and axonal differentiation in the presence of this tau fragment. Together, these results suggest that tau45-230 could exert its toxic effects, at least in part, by modifying the composition of the neuronal cytoskeleton and impairing neurite elongation in neurons undergoing degeneration.
Keywords: actin, microtubule-associated protein, microtubule, neurite outgrowth, neurodegenerative diseases
INTRODUCTION
The identification of tau as the major component of neurofibrillary tangles in Alzheimer’s disease (AD) prompted numerous studies on the mechanisms by which this microtubule-associated protein (MAP) could induce neurodegeneration (Kosik et al., 1986; Wood et al., 1986; Kondo et al., 1988; Parihar and Hemnani, 2004). Initially, those studies focused on tau phosphorylation since highly phosphorylated isoforms of this MAP are present in neurofibrillary tangles (NFT) (Kosik et al., 1986; Wood et al., 1986; Grundke-Iqbal et al., 1986; Takashima et al., 1993; Ferreira et al., 1997; Alvarez et al., 1999; Ekinci et al., 1999; Parihar and Hemnani, 2004). Recently, tau proteolysis has also been implicated in AD and other tauopathies (Canu et al., 1998; Gamblin et al., 2003; Park and Ferreira, 2005; Park et al., 2007; Reinecke et al., 2011; Reifert et al., 2011; Lang et al., 2014; Zhang et al., 2014; Zhao et al., 2016; Afreen et al., 2017; Quinn et al., 2018). Multiple truncated forms of tau or small fragments of this protein generated by the activation of several proteases including caspases and asparagine endopeptidase (AEP) have been identified in AD brains (Fasulo et al., 2000; Chung et al., 2001; Gamblin et al., 2003; Rissman et al., 2004; Park and Ferreira, 2005; Park et al., 2007; Ferreira and Bigio, 2011; Reinecke et al., 2011; Reifert et al., 2011; Lang et al., 2014; Zhang et al., 2014; Zhao et al., 2016). The calcium-dependent protease calpain also cleaves tau in the context of neurodegenerative diseases. As the result of this cleavage, the tau45-230 fragment accumulates in degenerating neurons (Park and Ferreira, 2005; Reinecke et al., 2011; Lang et al., 2014; Afreen et al., 2017; Ferreira and Bigio, 2011). The mechanisms leading to this calpain-mediated tau cleavage have been extensively studied in hippocampal neurons. Thus, we showed that the abnormal activation of the of N-methyl-D-aspartate (NMD A) 2B glutamate receptors results in the enhancement of extracellular calcium influx (Kelly et al., 2005; Kelly and Ferreira, 2006). This increased Ca2+ influx is responsible, in turn, for the dysregulation of calpain in multiple neurodegenerative diseases (Kelly et al., 2005; Kelly and Ferreira, 2006).
Much is known also about the toxic effects of tau45-230. The accumulation of this fragment induced cell death and synapse loss in cultured hippocampal neurons as well as in the hippocampal region in tau45-230 transgenic mice (Park and Ferreira, 2005; Lang et al., 2014). Similar toxic effects have been described in a Drosophila model of tau-mediated degeneration (Reinecke et al., 2011). In addition to these toxic effects, behavioral deficits have been described in tau45-230 transgenic mice including anxiety-like phenotypes and memory loss (Lang et al., 2014). In contrast, the complement of mechanisms underlying the toxic effects of this fragment have not been completely elucidated. Studies on the subcellular localization of tau45-230 provided some insights into such mechanisms. Although most of this fragment accumulated in the neuronal cytosol, a pool of tau45-230 was associated with membrane-bound organelles and the cytoskeleton. The association of this tau fragment with organelles affected their anterograde and retrograde axonal transport (Afreen et al., 2017). On the other hand, no data are available on the effects of this fragment on the neuronal cytoskeleton. To get insights into such effects, we analyzed cytoskeletal changes in hippocampal neurons expressing tau45-230. Our results showed that this tau fragment reduced the levels of full-length tau bound to microtubules and induced a transient increase in tyrosinated tubulin, a marker of unstable microtubules, followed by a significant decrease in this tubulin isoform. In addition, decreased levels of F-actin were detected in tau45-230-expressing neurons. These cytoskeletal modifications were associated with impaired neurite elongation and axonal differentiation. Combined, these data suggest that tau45-230 could induce neurodegeneration, at least in part, by altering the cytoskeleton and the formation and maintenance of neuritic networks.
EXPERIMENTAL PROCEDURES
Tau45-230 transgenic mice
Transgenic mice were generated using the human cDNA coding sequence for the tau45-230 fragment cloned into the peGFP-N1 plasmid under the control of the Thy 1.2 promoter (Lang et al., 2014). The mice were generated on a C57BL/6J genetic background. The characterization of the phenotype of these mice has been previously reported (Lang et al., 2014). Briefly, our results showed a significant increase in cell death in the hippocampal pyramidal cell layer of transgenic tau45-230 mice when compared to wild type controls. In addition, significant synapse loss was detected as early as six months after birth in transgenic hippocampal neurons. These synaptic changes were accompanied by alterations in the expression of the N-methyl-D-aspartate glutamate (NMDA) receptor subunits. In addition, functional abnormalities were detected in the transgenic mice using Morris Water Maze and fear conditioning tests (Lang et al., 2014). Wild type C57BL/6J mice were used as controls. A total of 120 mice were used in this study. Mice were housed at environmentally controlled conditions (temperature: 22 ±1°C; humidity: 55±10%) and maintained under a 12-h light/dark cycle with ad libitum access to food and water. Mice were euthanized by CO2 inhalation. All animal experiments were performed according to protocols approved by the Institutional Animal Care Committee of Northwestern University.
Hippocampal culture preparation
Embryonic day E16 C57BL/6J mice wt and GFP-tau45-230-transgenic mice were used for the preparation of hippocampal cultures as described previously (Rapoport et al., 2002; Banker and Goslin, 1998). In brief, hippocampi were dissected, stripped of meninges, and trypsinized (0.25%) for 15 min at 37°C. Neurons were dissociated by pipetting gently through a fire-polished Pasteur pipette and plated (~800,000 cells/60 mm dish) in minimum essential medium (MEM) containing 10% horse serum (MEM10) on poly-L-lysine coated dishes. After 4 hr, the medium was replaced with glia-conditioned MEM containing N2 supplements, ovoalbumin 0.1%, and 0.1 mM sodium pyruvate (N2 medium, Bottenstein and Sato, 1979). For immunocytochemical analysis, neurons were plated (150,000 cells/60-mm dish) onto poly-L-lysine-coated coverslips in MEM10. After 4 hr, the coverslips were transferred to dishes containing an astroglial monolayer and maintained in N2 medium.
Preparation of astrocyte monolayer cultures.
Astrocyte cultures were prepared from the cerebral cortex of El 6 mice embryos as previously described (Ferreira and Loomis, 1998). Briefly, embryos were removed and their cerebral cortex dissected and freed of meninges. The cells were dissociated by trypsinization (0.25% for 35 min at 37°C) and then centrifuged in MEM with 10% horse serum at 500 × g for 10 min. The cells were resuspended in fresh MEM with 10% horse serum, triturated with a fire-polished pipette, and plated at high density (1,600,000 cells/60-mm dish) on non-coated culture dishes. After the cells reached ~70% confluency, the medium was replaced with N2 medium to produce N2-glial-conditioned medium or to co-culture with coverslips containing dissociated hippocampal neurons. Astrocyte cultures were prepared from the cerebral cortex of 5 E16 mice embryos.
Hippocampal neuron transfection.
cDNA encoding for tau45-230 was subcloned into the mammalian expression vector enhanced green fluorescent protein-N1 (p-eGFP-N1) (Invitrogen ThermoFisher Scientific, Waltham, MA) to produce a C-terminal-labeled tau45-230 (tau45-230-GFP) construct (Park and Ferreira, 2005). This construct was nucleofected into dissociated hippocampal neurons as described (Afreen et al., 2017). Briefly, dissociated neurons were resuspended in nucleofection solution containing 3 μg of the DNA transferred to an electroporation cuvette, and nucleofected using the Amaxa Nucleofection system (Lonza, Inc. Allendale, NJ) according to the manufacturer’s protocol (program O-03). Non-transfected cells as well as neurons transfected with the empty vector DNA were used as controls.
Preparation of whole cell lysates and detergent-resistant subcellular fractions.
To prepare homogenates from the hippocampi of wt and tau45-230-transgenic mice, the tissue was homogenized in chilled RIPA buffer (0.5M Tris-HCl, pH 7.4, 1.5M NaCl, 2.5% deoxycholic acid, 10mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM aminobenzamidine). The homogenate was centrifuged at 20,000 × g for 20 min at 4°C, the supernatant was collected, diluted in 2X Laemmli buffer (Laemmli, 1970), and boiled for 10 min. Cytoskeletal fractions were prepared as previously described (Lyford et al., 1995). Briefly, the tissue was homogenized in RIPA buffer containing 1% Triton X-100 (RIPA lysis buffer). The homogenate was centrifuged at 100,000 × g for 1 hr at 4°C and the pellet containing the cytoskeletal proteins was resonicated in RIPA lysis buffer, and centrifuged at 100,000 × g for 30 min at 4°C. The pellets (triton-insoluble cytoskeletal fractions) were collected in Tris-urea buffer (1 M Tris-HCL, pH 8.5, 7M urea and 2 M thiourea), diluted 1:1 in 2X Laemmli buffer and boiled for 10 min. To prepare whole cell lysates from hippocampal cultures, cells were washed once in warmed (37°C) phosphate-buffered saline (PBS), scraped in 2X Laemmli buffer, and homogenized by boiling in a water bath for 10 min. Detergent-resistant subcellular fractioning was performed to isolate cytoskeleton fractions as previously described (Paganoni et al., 2004). In brief, hippocampal neurons were washed once with warmed PBS, and stabilized by 30s incubation with warmed microtubule stabilizing buffer (MTSB: 130 mM HEPES, 4 mM MgCl2, 10 mM EGTA, pH 6.9). Cultures were extracted in MTSB containing 0.2% Triton X-100 (Sigma, St Louis, MO) for 60 s to prepare cytoskeletal fractions. Extracted cells were gently rinsed with MTSB, scraped in 2X Laemmli buffer, and homogenized in a boiling water bath for 10 min. Whole cell extracts and detergent-resistant fractions were analyzed by immunoblotting as described below. For these experiments, a total of 30 mice were used for the preparation of hippocampi homogenates. Cultures prepared from 18 pregnant mice were used for these experiments.
Determination of F/G-actin ratio.
F-actin/G-actin in vivo assay kit (BK037; Cytoskeleton, Inc., Denver, CO) was used to detect the content of F-actin in tau45-230-expressing hippocampal neurons and control ones according to the manufacturer’s instructions. Briefly, hippocampi were homogenized, and hippocampal cultures were resuspended, in lysis buffer supplemented with 1 mM ATP and protease inhibitor mixture for F-actin stabilization (Cytoskeleton, Inc.). The lysates were incubated for 10 min at 37°C and then centrifuged at 350 × g for 5 min to pellet unbroken cells and tissue debris. Supernatants were centrifuged at 100,000 × g at 37°C for 1 hr. Pellet and supernatant fractions were collected. Pellets were incubated on ice with F-actin depolymerization buffer for 1 hr to allow actin depolymerization to occur. Fractions were diluted in 5X Laemmli buffer and actin was quantified by means of quantitative Western blot analysis as described below. For these experiments, a total of 32 mice were used for the preparation of hippocampi homogenates and 28 pregnant mice were used for the preparation of hippocampal cultures.
In vitro actin co-sedimentation assay.
Full-length tau and tau45-230 binding to F-actin was assessed using the Actin Binding protein Biochem Kit (BK013, Cytoskeleton Inc.) according to the manufacture’s instructions. Briefly, F-actin was incubated in the presence of recombinant full-length tau, tau45-230, or both for 30 min at room temperature. Samples were then centrifuged at 150,000 × g for 1.5 hr at 24°C. The supernatants were carefully removed and the pellets were resuspended in Milli-Q water (30 μl) and incubated on ice for 10 min. Samples were further diluted in 2X Laemmli buffer, boiled for 10 min, and analyzed by means of Western blotting as described below.
Electrophoresis and immunoblotting.
Hippocampus homogenates, whole cell lysates, and subcellular fraction extracts prepared as described above were ran on sodium dodecyl sulfate (SDS)-polyacrylamide gels. Proteins were transferred onto Immobilon-P membranes (Millipore, ThermoFisher Scientific) and detected by immunoblotting (Towbin et al., 1979). The following primary antibodies were used for immunodetection of specific proteins: α-tubulin (1:1,000; clone DM1 A, Sigma), tau (1:1,000; clone tau5, BioSource International), anti-dephosphorylated tau (1:1,000; clone tau1, Millipore, ThermoFisher Scientific) GFP (1:500; Millipore, ThermoFisher Scientific); acetylated tubulin (1:1,000; clone 6-11-B1, Sigma), tyrosinated tubulin (1:1,000, clone TUBIA2, Sigma), and actin (1:250, Cytoskeleton Inc). Incubation with secondary antibodies conjugated with horseradish peroxidase and enhanced chemiluminescence reagent were used to detect protein expression (Yakunin and Hallenbeck, 1998). Imaging and densitometric quantification of proteins were performed using a ChemiDoc XRS system (Bio Rad Life Sciences, Hercules, CA). Lysates were loaded equally based on internal control levels of α-tubulin (for whole cell extracts) or acetylated tubulin (for cytoskeleton fractions).
Immunocytochemistry.
Hippocampal neurons cultured on coverslips were fixed in 4% paraformaldehyde in PBS containing 0.12 mM sucrose for 15 min and permeabilized in 0.3% Triton X-100 in PBS for 4 min. Coverslips were then incubated with 10% bovine serum albumin (BSA) in PBS at room temperature for 1 hr before overnight incubation with the primary antibody. The following antibodies were used: α-tubulin (clone DM1A; 1:1,000), and GFP (1:500). Anti-mouse and anti-rabbit AlexaFluor secondary antibodies (1:200; Molecular Probes, ThermoFisher Scientific) were used for protein detection. For some experiments, hippocampal neurons were stained for 1 hr at 37°C with rhodamine-phalloidin to detect actin filaments (1:1,000; Sigma). Cultures prepared from 6 E16 pregnant mice were used for these experiments.
Morphometric analysis
To determine the percentage of neurons in each developmental stage (stages 1 to 3), control and tau45-230-expressing neurons were fixed 48 hr after plating, stained with a tubulin antibody and viewed on a Nikon fluorescent microscope. The number of neurons in each developmental stage was expressed as a percentage of the total number of cells in each field. Forty fields from three independent culture preparations were analyzed. Cultures prepared from 6 E16 pregnant mice were used for these experiments.
Statistical analysis
The data presented were obtained from at least 3 independent cultures per experimental condition, as described above. Quantitative analysis and statistical comparisons were performed using Prism 5.0 (GraphPad Software, Inc. San Diego, CA). We tested for and found that all sampled distributions satisfied the normality criteria. Values of P<0.05 were considered significant. Data are presented as mean ± standard error of the mean (S.E.M.).
For quantitative Western blot analysis, the compiled data were analyzed across the experimental conditions using one-way ANOVA followed by Fisher’s LSD post hoc test. The values in the graphs represent the mean ± S.E.M. and statistical significance is indicated in the graphs. For the morphometric analysis, the data were analyzed using the Student’s T test.
RESULTS
Subtle microtubule changes in hippocampal neurons obtained from tau45-230 transgenic
Tau45-230 includes the proline-rich domain of full-length tau (Park and Ferreira, 2005). This region has been implicated in the regulation of the binding of full-length tau to microtubules but does not include the microtubule-binding domains (Gustke et al., 1994). Nevertheless, we have previously shown that a pool of the neurotoxic tau45-230 fragment is associated with microtubules in hippocampal neurons (Afreen et al., 2017). The functional implications of this subcellular localization have not been completely elucidated. In the present study, we assessed whether this localization altered full-length tau binding to microtubules and/or the stability of these cytoskeletal components. For these experiments, whole hippocampal homogenates and cytoskeletal fraction extracts were prepared from transgenic tau45-230 mice at 3, 6, and 9 months after birth as described in the Experimental Procedures section. As controls, we analyzed samples obtained from wild type (wt) mice at the same developmental stages. Western blot analysis using a phosphorylation-independent antibody directed to full-length tau (clone tau5) showed strong immunoreactive bands (~50 kDa molecular weight) both in whole homogenates and in cytoskeletal fractions throughout the whole period studied. Densitometry of such bands showed similar full-length tau levels in both types of samples when transgenic tau45-230 mouse preparations were compared to the wt controls throughout the period studied (Figure 1A). Since most of tau associated with microtubules is dephosphorylated, we repeated these experiments using a specific tau antibody (clone tau1) widely used as a marker of dephosphorylated forms of this MAP. No significant differences in the levels of dephosphorylated tau were detected throughout the period analyzed when samples obtained from tau45-230 transgenic mice were compared to wt controls (Figure 1B). We next analyzed the stability of the microtubules under both experimental conditions using antibodies directed to either tyrosinated or acetylated tubulin, well-known markers of unstable and stable microtubules, respectively. Western blot analysis showed that the presence of both tyrosinated and acetylated tubulins in the hippocampus of tau45-230 transgenic and wt mice. No differences in the amount of either tubulin isoform were detected in whole homogenates or cytoskeletal fractions prepared from tau45-230 transgenic mice as compared to wt controls early in development (3-month old mice) (Figure 1C & D).
Figure 1: Tau and tubulin content in the hippocampus of tau45-230 transgenic mice.

Quantitative Western blot analysis of whole hippocampus homogenates [Triton X-100 (−)] and cytoskeletal fraction samples [Triton X-100 (+)] obtained from 3-, 6-, and 9-month postnatal wild type (WT) and tau45-230-transgenic (T) mice were reacted with tau (clone tau5) (A), dephosphorylated tau (clone tau1) (B), and tubulin antibodies (C & D). Samples were normalized to α-tubulin as protein controls. Values represent the mean ± S.E.M. from 5 independent experiments per condition. The levels of a given protein in WT controls were considered 100%.
The results described above were obtained analyzing samples prepared from whole hippocampi and, therefore, samples that contained both neurons and glial cells. Full-length tau as well as tau45-230 are expressed mostly in neurons. In contrast, α-tubulin and its post-translationally modified isoforms are highly expressed both in neurons and in glial cells. Therefore, specific neuronal differences in the parameters analyzed above might have been masked by the presence of glia in the samples studied. To rule out this possibility, neurons obtained from tau45-230 transgenic and wt mice were placed in culture for up to 14 days. Cytoskeletal fraction extracts were prepared as described in the Experimental Procedures section. As in the case of samples prepared from cells that developed in situ, total and dephosphorylated full-length tau isoforms were easily detected in cultured neurons by means of Western blot analysis using specific tau antibodies. Quantitative analysis of immunoreactive bands showed a decrease in the levels of full-length tau associated with microtubules in tau45-230 transgenic mice when compared to wt controls. These differences were statistically significant both early in development when cultured neurons were rapidly extending neurites (1 day in culture: 71 ± 9 % vs. 100%, respectively. T(4): 3.088, p< 0.05) and when neurons have already established a well-developed neuritic plexus (7 days in culture: 68 ± 10% vs. 100%. T(4): 3.321, p< 0.05. 14 days in cultures: 49 ± 15 % vs. 100%, respectively. T(4): 3.391, p< 0.05) (Figure 2A). On the other hand, no significant changes were detected when the levels of dephosphorylated tau isoforms associated with microtubules in tau45-230-expressing neurons were compared to those in wt controls (Figure 2B). We also analyzed the levels of tyrosinated and acetylated tubulin under these experimental conditions. The densitometric quantification of tubulin immunoreactive bands showed a transient increase in tyrosinated tubulin levels in 7 days in culture hippocampal neurons obtained from tau45-230 transgenic mice (150 ± 8% vs. 100%. T(4): 5.939, p< 0.01) followed by a significant decrease in 14 days in culture tau45-230-expressing neurons when compared to wt controls (59 ± 17 % vs. 100% . T(4): 2.859, p< 0.05) (Figure 2C).
Figure 2: Microtubular changes in cultured hippocampal neurons obtained from tau45-230 transgenic mice.
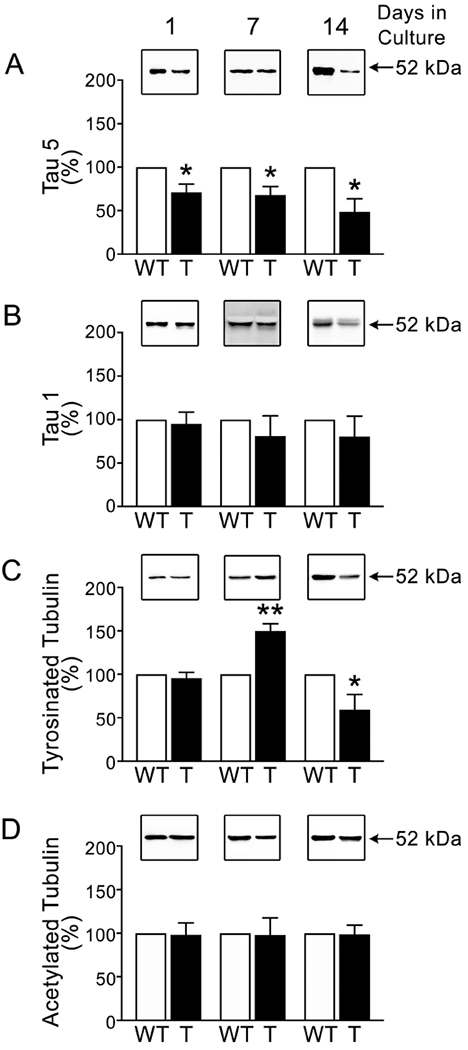
Quantitative Western blot analysis of cytoskeletal fraction samples prepared from wild type (WT) and tau45-230 transgenic (T) cultured hippocampal neurons at 1, 7, and 14 days after plating were reacted with tau (clone tau 5) (A), dephosphorylated tau (clone tau1) (B), and tubulin antibodies (C & D). Cytoskeletal fraction samples were normalized to acetylated tubulin as protein controls. Values represent the mean ± S.E.M. from 3 independent experiments per condition. The levels of a given protein in WT controls were considered 100%. *Differs from WT control values, p < 0.05.
Tau45-230 includes a region of this full-length MAP capable of binding to G-actin, promoting G-actin assembly, and bundling F-actin (He et al., 2009). To determine whether tau45-230 induces changes in actin filaments, we analyzed the ratios of F-actin/G-actin in the hippocampi of tau45-230 transgenic mice throughout development and compared them to those of wt controls. Both in wt and in tau45-230 transgenic mice, F-actin levels increased between postnatal 3 and 6 months and then remained constant up to 9 months after birth (Figure 3 A). However, the levels of F-actin were higher in tau45-230 transgenic mice (13 ± 5%, 18 ± 5 %, and 19 ± 5% vs. 35 ± 8%*, 46 ± 10%*, and 46 ± 9 %*, respectively. T(8): 2.386, 2.388, and 2.540, respectively. *Differs from wt control values, p < 0.05) (Figure 3A).
Figure 3: F-actin content in the hippocampus of tau45-230 transgenic mice.
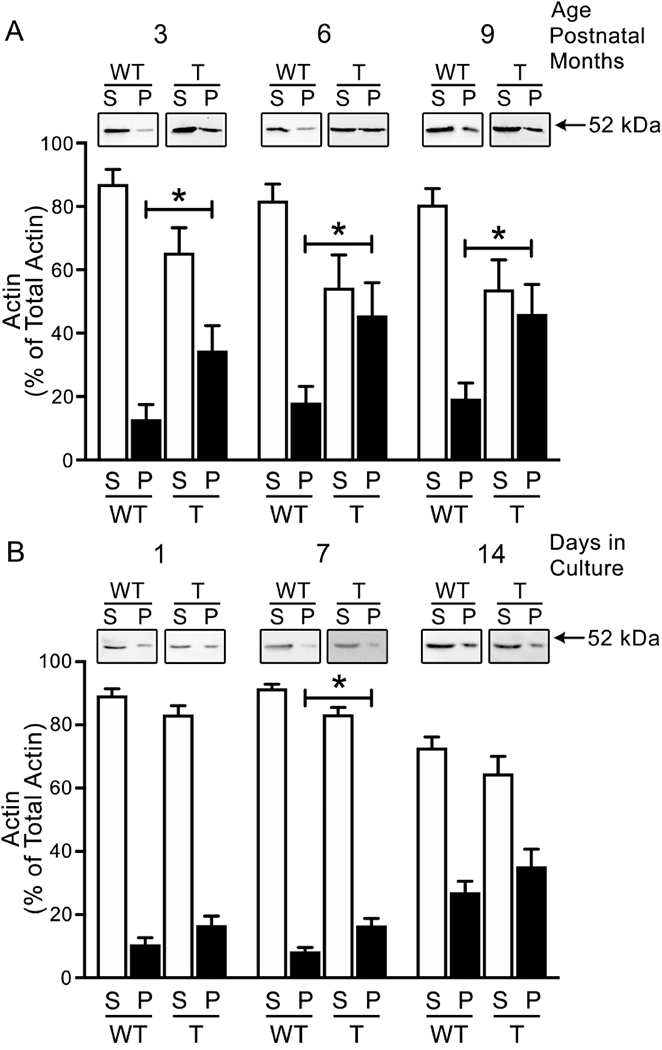
(A) Quantitative Western blot analysis of actin content in samples obtained from wild type and tau45-230 transgenic mice at 3-, 6- and 9-postnatal months. Samples were centrifuged as described in the Experimental Procedures section to separate soluble G-actin present in supernatants (S) from filamentous F-actin present in the pellets (P). (B) Quantitative Western blot analysis of actin content in supernatant and pellet samples prepared from 1, 7, and 14 days in culture hippocampal neurons obtained from wild type (WT) and tau45-230 transgenic (T) mice as described in the Experimental Procedures section. Values represent the mean ± S.E.M. from 9 independent experiments per condition. The levels of a given protein in WT controls were considered 100%. *Differs from WT control values, p < 0.05.
As in the case of the experiments described above, the homogenates analyzed contained glial cells that highly expressed actin but not tau45-230. To analyze the neuronal effects of tau45-230 on F-actin content, we cultured hippocampal neurons from these tau45-230 transgenic mice and determined the ratio of F-actin to G-actin. As previously described, a small fraction of total actin present in wt neurons corresponds to F-actin (Figure 3B). Densitometry of immunoreactive bands showed that levels of F-actin were only transiently higher in 7 days in culture tau45-230-expressing hippocampal neurons when compared to control (17 ± 2 % vs. 8 ± 1%, respectively. T(8): 3.268, p< 0.05) during the first 2 weeks in culture (Figure 3B).
Cytoskeletal changes in tau45-230-transfected hippocampal neurons.
Previous characterization of tau45-230 transgenic mice confirmed the expression of this tau fragment in hippocampal neurons by RT-PCR and immunocytochemical analysis (Lang et al., 2014). However, its expression levels were below the detection limit by Western blotting assay (Lang et al., 2014). Since tau45-230 is easily detectable by this method both in AD brains (Ferreira and Bigio, 2011) and in cultured hippocampal neurons incubated in the presence of aggregated Aβ (Park and Ferreira, 2005), we repeated the analysis described above using a model system that better reproduces the levels of expression of this tau fragment in degenerating neurons. For these experiments, cultured wt hippocampal neurons were transfected with a tau45-230-GFP construct as described in the Experimental Procedures section. Under these experimental conditions, tau45-230 was expressed in ~ 50% of the cultured neurons (Afireen et al., 2017). Furthermore, Western blot analysis showed that the levels of this fragment in transfected cultures were not significantly different when compared to Aβ-treated cultures (112 ± 7% vs. 100 %, respectively; data not shown). Non-transfected sister cultures were used as controls.
As previously described, both monomers and dimers of tau45-230 were detected by means of Western blot analysis in 2 days in culture transfected hippocampal neurons using a GFP antibody (Figure 4A; see also Afreen et al., 2017). Endogenous full-length tau was also abundant in cytoskeletal fractions obtained from control and tau45-230-transfected neurons (Figure 4 B and C). Quantitative analysis of tau immunoreactive bands showed that endogenous full-length tau associated with microtubules was significantly lower in transfected neurons as compared to untransfected controls (45 ± 7 % vs. 100%, respectively. T(10): 7.552, p< 0.01) (Figure 4B). On the other hand, no significant differences were detected in the levels of dephosphorylated tau when tau45-230-transfected neurons were compared to untransfected controls (Figure 4C). A similar analysis was conducted to quantify the levels of tyrosinated and acetylated tubulin in these cytoskeletal samples. Tyrosinated tubulin was abundant both in tau45-230-transfected neurons and in untransfected controls. However, a significant decrease in the levels of this tubulin isoform was detected in tau45-230-expressing neurons when compared to controls (62 ± 11 % vs. 100%, respectively. T(10): 3.418, p< 0.01) (Figure 4D). No changes were detected when the levels of acetylated tubulin in tau45-230-transfected neurons were compared to untransfected controls (Figure 4F).
Figure 4: Decreased levels of tyrosinated tubulin and full-length tau associated with microtubules in tau45-230-transfected hippocampal neurons.
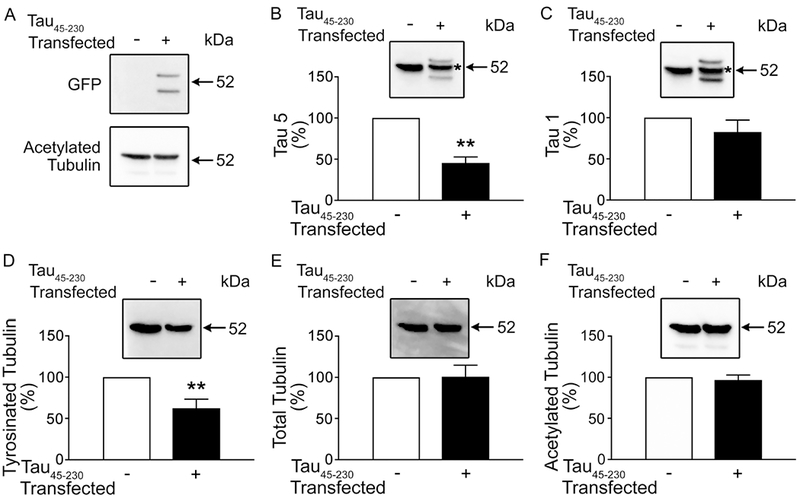
(A-F) Quantitative Western blot analysis of cytoskeletal fraction samples obtained from 2 days in culture control and tau45-230-transfected hippocampal neurons reacted with GFP (A), tau (clone tau5) (B), dephosphorylated tau (clone tau1) (C), and tubulin antibodies (D-F). Cytoskeletal fraction samples were normalized to acetylated tubulin as protein controls. *Indicates endogenous full-length tau immunoreactive band. Values represent the mean ± S.E.M. from 10 independent experiments per condition. The levels of a given protein in non-transfected controls were considered 100%. *Differs from control values p < 0.05.
We analyzed next the ratio of F-actin/G-actin in these cultures. Two days in culture control and tau45-230-transfected hippocampal neurons were collected in F-actin stabilizing buffer and centrifuged as described in the Experimental Procedures section. Western blot analysis of supernatant and pellet fractions was performed using an actin antibody. In both experimental conditions, strong immunoreactive bands corresponding to G-actin were detected in supernatant fractions. F-actin immunoreactive bands were detected in the pellet fractions (Figure 5A). Densitometry of these bands showed that the levels of F-actin were significantly lower in tau45-230-transfected hippocampal neurons when compared to controls (1 ± 0.3 % vs. 11 ± 2%, respectively. T(8): 4.540, p< 0.01) (Figure 5 B).
Figure 5: Decreased levels of F-actin in tau45-230-transfected hippocampal neurons.
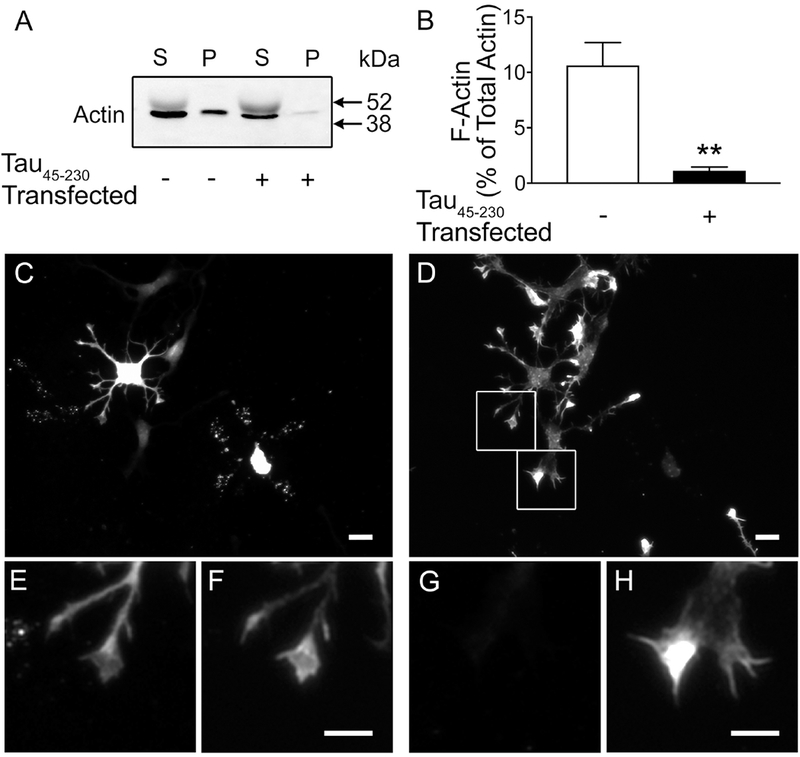
(A) Immunoblots of supernatant (S) and pellet (P) fraction samples obtained from 2 days in culture control and tau45-230-transfected hippocampal neurons reacted with an actin antibody. (B) Quantitative analysis of the levels of F-actin recovered in the pellets. Values represent the mean ± S.E.M. from 8 independent experiments per condition. The levels of a given protein in non-transfected controls were considered 100%. *Differs from control values, p < 0.05. (C & D) Tau45-230-transfected cultures were fixed and stained using a GFP antibody (C, E, G) and counterstained using rhodamine-phalloidin to detect F-actin (D, F, H). GFP (+) neurons showed significant decrease in F-actin when compared to GFP (−) ones. (E-H) High power magnification of growth cones in boxed areas in D. Note the fainter phalloidin staining (F) in growth cones of tau45-230 (+) neurons (E) as compared to growth cones of tau45-230 (−) ones (G) Scale bar: C & D = 20 μm; E-H = 10 μm
To further assess changes in F-actin in the presence of this tau fragment, dissociated tau45-230-transfected hippocampal neurons cultured for 2 days were fixed, and immunostained using a GFP antibody and counterstained using rhodamine-phalloidin, a marker of F-actin. Filamentous actin was highly concentrated in the peripheral area of growth cones of untransfected hippocampal neurons (Figure 5 D & H). On the other hand, faint phalloidin staining was detected in growth cones of tau45-230 (+) neurons. (Figure 5 D, F).
The binding of tau45-230 to F-actin was independent of the presence of full-length tau.
We next determined whether tau45-230 could bind directly to F-actin and/or interfere with the binding of full-length tau to F-actin by means of co-sedimentation assays. For these experiments, we incubated F-actin with increasing concentration (4, 8 and 16 μg) of recombinant tau45-230 and separated the pellet from the supernatant fractions. As expected, almost all of the F-actin was recovered in the pellet fraction in the presence or in the absence of tau45-230. (Figure 6A). A pool of tau45-230 (~20%) was recovered in the pellet fraction when incubated separately with F-actin at all concentration studied. The percentage of this tau fragment recovered in the pellet fraction was significantly lower than the one of full-length tau (~43%) present in the pellet fractions under these experimental conditions. On the other hand, the incubation of F-actin with both tau forms together resulted in a significant increase (~30%) in tau45-230 (19 ± 3 vs. 29 ± 3%, respectively. T(6): 2.401 p< 0.05) and a significant decrease (~25%) in full-length tau (43 ± 4 vs. 25 ± 7 % T(6): 2.417, p<0.05) associated with F-actin when 8 and 16 μg of tau45-230 were used for these experiments (Figure 6). No significant changes on in the full-length tau bound to F-actin were detected when the lowest dose of tau45-230 was used (data not shown).
Figure 6: Tau45-230 reduced full-length tau binding to F-actin.
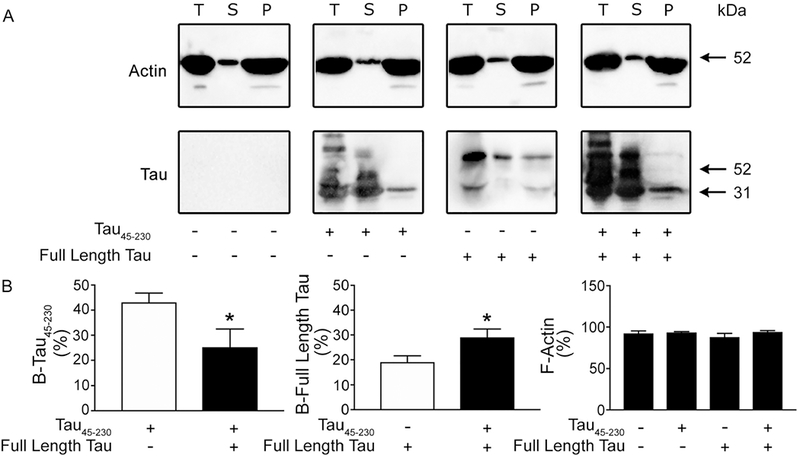
(A) Western blot analysis of total (T) F-actin content in the reaction mixture as well as in the supernatant (S) and pellet (P) fractions after F-actin was incubated in the presence of tau45-230 (16 μg), full-length tau, or both for an hour and centrifuged as described in the Experimental Procedures section. Immobilon membranes were reacted using actin and tau antibodies. (B) Quantitative analysis of immunoreactive bands. Values represent the mean ± S.E.M. from 4 independent experiments per condition. *Differs from control values, p < 0.05. B-tau45-230: tau45-230 bound to F-actin. B-Full-length tau: Full-length tau bound to F-actin.
Delayed neurite elongation in tau45-230-expressing hippocampal neurons.
The results described above suggested that tau45-230 affected both microtubules and actin filaments in hippocampal neurons. Since the interaction of these elements of the cytoskeleton are essential for neuritogenesis, we investigated next whether tau45-230 could alter the formation and/or elongation of processes in cultured hippocampal neurons. Under control conditions, cultured hippocampal neurons extend neurites following a sequence of well characterized morphological changes (Dotti et al., 1988). Upon plating, hippocampal neurons are surrounded by a lamellipodial veil (stage 1). Within the first 12 hours, the majority of the cells extend several undifferentiated neurites or minor processes (stage 2) and by 1 day in culture they become polarized, i.e. they extend one axon and several short processes (stage 3) (Dotti et al., 1988). To evaluate the effects of tau45-230 on the establishment of polarity, dissociated hippocampal neurons were nucleofected with the tau45-230-GFP construct, fixed 48 hr after plating, and double-stained using GFP and tubulin antibodies. As previously described, approximately half of the hippocampal neurons analyzed displayed intense GFP immunoreactivity (Figure 7A, see also Afreen et al., 2017). Quantitative analysis of the number of tau45-230-transfected and untransfected control neurons in stage 1-3 was performed using 3 independent culture preparations. As previously described, the majority of non-transfected hippocampal neurons had extended neurites during the first day in culture. By contrast, a significantly higher number of tau45-230 (+) cells had failed to elongate processes and remained in stage 1 when compared to controls (stage 1: 10 ± 6 % vs. 31 ± 1 % in untransfected controls and tau45-230 (+) neurons, respectively. T(4): 3,4, p< 0.01) (Figure 7). In addition, fewer tau45-230 (+) neurons extended axons (stage 3) when compared to untransfected controls (stage 3: 10 ± 5 % vs. 47 ± 6 %*. T(4): 5.9, p< 0.01) (Figure 7). To rule out the possibility that the inhibitory effects on neurite elongation and axonal differentiation observed in GFP-tau45-230 expressing neurons were due to the expression of GFP and not of the expression of this tau fragment, we repeated the experiments described above using cultures nucleofected with the GFP empty vector as an additional control. Quantitative analysis indicated that the number of tau45-230 (+) neurons in stage 1 was also significantly higher than in GFP (+) cells (stage 1:31 α 1% vs. 20 ± 3 % in GFP-tau45-230 (+), and GFP (+) neurons, respectively. T(4): 3.05, p< 0.01). On the other hand, the number of tau45-230 (+) neurons extending axons was significantly lower than the GFP (+) ones (stage 3: 10 ± 5 %* vs. 49 ± 10 %. T(4): 3.39, p< 0.01). These results indicated that the morphological changes observed in transfected neurons were due to the expression of tau45-230 and not due to the presence of GFP.
Figure 7: Tau45-230 delayed neurite elongation in cultured hippocampal neurons.

(A & B) Tau45-230-transfected cultures were fixed and double-stained using GFP (A) and tubulin (B) antibodies. Most of GFP-tau45-230 (+) neurons (arrows) remained in stage 1 and 2, failing to elongate their axons after 2 days in culture. Scale bar: 20 μm. (C) Quantification of tau45-230 (−) and tau45-230 (+) neurons in stage 1, 2, and 3. Neurons in 30 fields from 3 independent culture preparations per experimental condition were counted. **Differs from untransfected controls, p < 0.01. ax: axon
DISCUSSION
The data described above showed that tau45-230 induced changes in the microtubular and actin cytoskeletons affecting neurite elongation and axonal differentiation in hippocampal neurons. These results provide further insights into the molecular mechanisms underlying the toxic effects of this tau fragment in the context of neurodegenerative diseases.
The association of tau45-230 with degenerating neurons prompted several studies to assess its potential toxic effects (Park and Ferreira, 2005; Park et al., 2007; Reinecke et al., 2011; Ferreira and Bigio, 2011). The data obtained indicated that the presence of tau45-230 in hippocampal neurons induces progressive axonal and dendritic degeneration, synapse loss, decreased neuronal survival, and behavioral defects (Park and Ferreira, 2005; Park et al., 2007; Lang et al., 2014; Nicholson and Ferreira, 2009). However, the mechanisms underlying the toxicity of this tau fragment remain an open question. Recently, subcellular localization studies have shown that a pool of tau45-230 was recovered in detergent-resistant cytoskeletal fractions (Afreen et al., 2017). In the present study, we addressed to what extent such association with the cytoskeleton could contribute to the toxic effects of this tau fragment. Our results identified several cytoskeletal changes induced by tau45-230 in hippocampal neurons. Quantitative Western blot analysis showed that the levels of full-length tau bound to microtubules were lower in the presence of tau45-230 than in control neurons. None of the four microtubule binding domains of this MAP are present in tau45-230 (Kanai et al., 1992; Park and Ferreira, 2005). Therefore, it is unlikely that this fragment could compete with full-length tau for similar binding sites along microtubules thereby decreasing the levels of this MAP recovered in cytoskeletal fractions. In contrast, tau45-230 includes the sequence involved in the dimerization of full-length tau (Rosenberg et al., 2008; Feinstein et al., 2016). In vitro studies have shown that the N-terminal and central tau regions form dimers in an antiparallel configuration by an electrostatic zipper of complementary salt bridges (Kanai et al., 1992). It has been proposed that full-length tau homodimers could bind, stabilize, and bundle tubulin subunits promoting microtubule growth essential for the formation and maintenance of neuritic processes (Feinstein et al., 2016). We speculate that the formation of tau45-230/full-length tau heterodimers could reduce the interaction of these dimers with tubulin subunits since microtubule-binding domains are present in only one of the components of these heterodimers. In addition, the formation of this type of dimers could reduce the pool of monomeric full-length tau in the cytosol readily available for its binding to microtubules. Through these mechanisms, tau45-230 not only reduces the binding of this MAP to microtubules but also affects their dynamic instability. The neuronal cytoskeleton is composed of subpopulations of microtubules that differ in their content of α-tubulin isoforms, turnover rates, and their resistance to depolymerization (Gundersen et al., 1984; Wehland and Weber, 1987; Piperno et al., 1987; Robson et al., 1989; Kresi, 1987; Schulze at al., 1987; Webster et al., 1987; Cambray-Deakin and Burgoyne, 1987a & b; Cambray-Deakin et al., 1988). Unstable microtubules are enriched in tyrosinated tubulin. On the other hand, microtubules containing detyrosinated and/or acetylated tubulin isoforms are considered stable ones (Gundersen et al., 1984; Wehland and Weber, 1987; Piperno et al., 1987; Robson et al., 1989). Using specific antibodies to these post-translationally-modified tubulin isoforms as markers of microtubule stability, we showed a significant increase in the levels of tyrosinated tubulin when tau45-230-transgenic hippocampal neurons were cultured for up to 7 days. In contrast, tau45-230 transgenic hippocampal neurons kept in culture for 14 days and transiently tau45-230-transfected neurons showed a significant decrease in tyrosinated tubulin. These results suggested that low levels of tau45-230 during periods of active neurite outgrowth are associated with an increase in unstable microtubules. On the other hand, the accumulation of this tau fragment for longer periods of time and/or at higher levels are associated with a significant decrease in this microtubule subtype.
This bi-modal effect of tau45-230 on tyrosinated microtubules could underlie defects on the establishment and maintenance of neuritic networks. A transient increase in unstable microtubules during periods of de novo neurite elongation during early stages of development as well as during regeneration could favor the elongation of primary neurites and their branches. However, the concomitant decrease in detyrosinated tubulin could prevent the stabilization of microtubules needed to support this rapid net neurite elongation and its maintenance (Ferreira and Caceres, 1989). On the contrary, a significant decrease in tyrosinated tubulin once neuronal processes have been extended could result in a less dynamic composition of the microtubular system in mature neurons. Such composition could have deleterious effects under conditions of degeneration. Thus, we have previously shown that an increase in tyrosinated tubulin in mature neurons is responsible, at least in part, for the resistance of mature hippocampal neurons obtained from tau knockout mice to Aβ toxicity (Rapoport et al., 2002). Conversely, taxol-induced stabilization of their microtubules increased their degeneration in the presence of this peptide (Rapoport et al., 2002). Therefore, a tau45-230-induced decrease in dynamic microtubules as neurons age could result in their increased susceptibility to Aβ-induced degeneration. In addition to the structural deleterious effects of a more stable microtubular system, a decrease in dynamic microtubules could underlie behavioral defects since it has been shown that increased stabilization of microtubules prevented memory formation in mice (Fanara et al., 2010; Atarod et al., 2015). Finally, a conserved balance between unstable and stable microtubules is also required for normal axonal transport in central neurons (Fanara et al., 2007; Dubey et al., 2015). Thus, it has been shown that reduction in microtubule dynamics using a Parkinsonism-inducing neurotoxin could be responsible, at least in part, for abnormal axonal transport (Cartelli et al., 2010). Recently, we have shown that a pool of tau45-230 was associated with membrane-bound organelles altering their anterograde and retrograde transport along axons (Afreen et al., 2017). Collectively, the data discussed above suggest that tau45-230-induced changes in the microtubular system could also contribute to the impairment of organelle transport observed in hippocampal neurons expressing this tau fragment. It is worth noting that tau45-230 only affected the levels of the subset of stable microtubules that contained detyrosinated tubulin. On the other hand, stable microtubules containing acetylated microtubules were not affected by the presence of this tau fragment. These results are consistent with previous findings showing that detyrosinated and acetylated tubulin isoforms define two different subsets of stable microtubules in central neurons (Robson et al., 1989). Moreover, our data agree with studies on the effects of full-length tau depletion on the stability of microtubules showing that only the levels of tyrosinated/detyrosinated microtubules, but not acetylated ones, were affected in hippocampal neurons from tau knockout mice (Rapoport et al., 2002). Regardless of the mechanism, our results suggest that by altering the balance between unstable and stable microtubules, tau45-230 could compromise neuronal functions essential for the normal function of the central nervous system.
In addition to the changes in the microtubular system discussed above, tau45-230 modified the actin cytoskeleton. Two actin binding domains have been identified in full-length tau (He et al., 2009). The main actin binding domain is localized at the C-terminal half of the tau molecule. The second one is located in the proline-rich portion of this MAP (He et al., 2008). Both domains are involved in the regulation of actin filament formation and bundling (Yu and Rasenick, 2006; Farias et al., 2002). Since tau45-230 includes the second actin binding domain, it was not surprising to detect changes in actin filaments in hippocampal neurons expressing this tau fragment. The low levels of tau45-230 present in hippocampal neurons from transgenic mice induced a slight increase in F-actin content both in neurons that develop in situ and in culture. On the other hand, the accumulation of higher amounts of this neurotoxic fragment as detected in nucleofected neurons resulted in a significant decrease in F-actin content. Cell-free experiments provided insight into the potential mechanism(s) underlying this effect of tau45-230 on actin filaments. These experiments showed both a significant decrease in the binding of full-length tau to F-actin and a significant increase in tau45-230 bound to actin filaments when F-actin was incubated with both forms of tau together. These results suggest that tau45-230 could bind directly to actin filaments as a monomer through the second binding domain. This tau fragment could also bind to actin indirectly forming dimers with full-length tau. As in the case of the microtubules, the formation of these heterodimers could reduce the ability of full-length tau to induce the formation and bundling of actin filaments.
It is worth noting that the changes in microtubules and actin filaments discussed above were subtler in tau45-230 expressing neurons that developed in situ than in hippocampal neurons obtained from tau45-230 transgenic mice and placed in culture and/or when tau45-230-transfected hippocampal neurons were compared to untransfected controls. Several factors could account for these differences. First, the expression level of tau45-230 in early stages of the development in situ of hippocampal neurons in these transgenic mice is significantly lower than in degenerating neurons and/or in tau45-230 nucleofected ones (Lang et al., 2014). Second, extracts prepared from the hippocampi of these mice also contained glial cells that do not express this tau fragment since its expression is controlled by a neuron-specific promoter (Lang et al., 2014). This mixed population of cell types could mask specific effects of this fragment on the neuronal microtubules. In contrast, ~96% of the cells present in hippocampal cultures are pyramidal neurons (Banker and Cowal, 1977). Finally, the chronic expression of tau45-230 in transgenic mice could trigger compensatory mechanisms to correct transient abnormalities in the cytoskeleton. Such mechanisms might not be present in the culture system.
The changes in the cytoskeleton induced by tau45-230 could have some bearing on the phenotypes of neurons in which this fragment accumulates. The role of full-length tau as a crosslinker of microtubules and actin filaments is essential for neurite elongation (Sharma et al., 1995; Henriquez et al., 1995; Elie, 2015). Thus, live cell imaging showed that full-length tau colocalized with both microtubules and actin filaments in growth cones of central neurons and it is located in the interface of actin filament bundles and dynamic microtubules in filopodia (Biswas and Kalil, 2018). Reduction in the expression of tau results in disruption of microtubule bundling and prevents the dynamic microtubule extension into filipodia along actin filaments (Biswas and Kalil, 2018). By impairing the binding of full-length tau to both microtubules and actin filaments, tau45-230 could mimic a full-length tau loss of function phenotype. This seems to be the case in tau45-230-transfected neurons. Thus, we showed that the expression of this fragment results in a delayed neurite elongation and axonal differentiation in hippocampal neurons. It is worth mentioning that a similar phenotype has been described in experimental conditions that block the expression of full-length tau by means of antisense oligonucleotides or homologous recombinant techniques (Caceres and Kosik, 1990; Dawson et al., 2001).
Taken together, our results suggest that the presence of tau45-230 interferes with the normal functions of full-length tau in both microtubular stabilization and actin filament formation. Tau45-230-altered cytoskeleton leads to delays in neurite elongation and axonal differentiation. This partial loss of full-length tau functions could underlie, at least in part, the toxic effects of this tau fragment in the context of neurodegenerative diseases.
HIGHLIGHTS.
Tau45-230 decreases full-length tau bound to neuronal microtubules
Decreased levels of F-actin were detected in tau45-230 expressing hippocampal neurons
Tau45-230 alters the establishment of neuronal polarity
ACKNOWLEDGEMENTS
The authors are grateful to Ashlee Rubino for her participation in the initial stages of this study. This work was supported by the National Institutes of Health grant # RO1NS090993 to AF. Sana Afreen performed the experiments, analyzed the data, and participated in the preparation of the manuscript. Adriana Ferreira designed the experiments, performed some of the experiments, analyzed the data, and wrote the manuscript.
ABBREVIATIONS
- MAP
Microtubule Associated Protein
- Aβ
Beta amyloid
- AD
Alzheimer’s Disease
- NFT
Neurofibrillary Tangles
- AEP
Asparagine Endopeptidase
- NMDA
N-methyl-D-aspartate
- MEM
Minimum Essential Medium
- GFP
Green Fluorescent Protein
- PBS
Phosphate Buffered Saline
- MTSB
Microtubule Stabilizing Buffer
- SDS
Sodium Dodecyl Sulfate
- BSA
Bovine Serum Albumin
- kDa
kilo Dalton
- E16
Embryonic Day 16
- wt
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
REFERENCES
- -Afreen S, Riherd Methner DN and Ferreira A (2017) Tau45-230 association with the cytoskeleton and membrane-bound organelles: Functional implications in neurodegeneration. Neurosci. 362: 104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Alvarez A, Toro R, Caceres A and Maccioni RB (1999) Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett. 459: 421–426. [DOI] [PubMed] [Google Scholar]
- -Atarod D, Eskandari-Sedighi G, Pazhoohi F, Karimian SM, Khajeloo M and Riazi GH (2015) Microtubule dynamicity is more important than stability in memory formation: an in vivo study. J. Mol. Neurosci 56: 313–319. [DOI] [PubMed] [Google Scholar]
- -Banker GA and Cowal M (1977) Rat hippocampal neurons in dispersed cell culture. Brain Res. 126: 397–425. [DOI] [PubMed] [Google Scholar]
- -Banker G and Goslin K (1998) Rat hippocampal neurons in low-density culture In: Culturing nerve cells (Banker G, Goslin K, eds), pp339–370. Cambridge: MIT PS. [Google Scholar]
- -Biswas S and Kalil K (2018) The microtubule-associated protein tau mediates the organization of microtubules and their dynamic exploration of actin-rich lamellipodia and filopodia of cortical growth cones. J. Neurosci 38: 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Bottenstein JE and Sato GH (1979) Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA 76: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Caceres A and Kosik KS (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343: 461–463. [DOI] [PubMed] [Google Scholar]
- -Cambray-Deakin MA and Burgoyne RD (1987a): Post-translational modifications of cx-tubulin: Acetylated and detyrosinated forms in axons of rat cerebellum. J. Cell Biol 104: 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Cambray-Deakin MA and Burgoyne RD (1987b) Acetylated and detyrosinated α-tubulins are co-localized in stable micro-tubules in rat meningeal fibroblasts. Cell Motil. 8: 284–291. [DOI] [PubMed] [Google Scholar]
- -Cambray-Deakin MA, Robson SJ and Burgoyne RD (1988) Colocalisation of acetylated microtubules, glial filaments, and mitochondria in astrocytes in vitro. Cell Motil. 10: 439–449. [DOI] [PubMed] [Google Scholar]
- -Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A and Calissano P (1998) Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J. Neurosci 18: 7061–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Cartelli D, Ronchi MG, Maggioni MG, Rodighiero S, Giavini E and Cappelletti G (2010) Microtubule dynamics precedes transport impairment and mitochondria damage in MPP (−) induced neurodegeneration. J. Neurochem 115: 247–258. [DOI] [PubMed] [Google Scholar]
- -Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo ΗN, Kwon YK, Kim ΗH, Gwag BJ, Mook-Jung IH and Jung YK (2001) Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol. Dis 8: 162–172. [DOI] [PubMed] [Google Scholar]
- -Dawson ΗN, Ferreira A, Eyster MV, Binder LI and Vitek MP (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci 114: 1179–1187. [DOI] [PubMed] [Google Scholar]
- -Doth CG, Sullivan CA and Banker GA (1988) The establishment of neuronal polarity by hippocampal neurons in culture. J. Neurosci 8: 1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Dubey J, Ratnakaran N and Koushika S (2015) Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front. Cell. Neurosci 9: 343 doi: 10.3389/fncel.2015.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Ekinci FJ, Malik KU and Shea TB (1999) Activation of the L voltage-sensitive calcium channel by mitogen-activated protein (MAP) kinase following exposure of neuronal cells to beta-amyloid. MAP kinase mediates beta-amyloid-induced neurodegeneration. J. Biol. Chem 274: 30322–30327. [DOI] [PubMed] [Google Scholar]
- -Elie A, Prezel E, Guerin C, Denarier E, Ramirez-Rios S, Serre L, Andriex A, Fourest-Lieuvin A, Blanchioin L and Arnal F (2015) Tau co-organizes dynamic microtubule and actin networks. Sci. Rep 5: 09964; doi: 10.1038/srep09964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Fanara P, Banerjee J, Hueck RV, Harper MR, Awada M, Turner H, Husted KH, Brandt R and Hellerstein MK (2007). Stabilization of hyperdynamic microtubules is neuroprotective in amyotrophic lateral sclerosis. J. Biol. Chem 282: 23465–23472. [DOI] [PubMed] [Google Scholar]
- -Fanara P, Husted KH, Selle K, Wong P-YA, Baneijee J, Brandt R and Hellerstein MK (2010). Changes in microtubule turnover accompany synaptic plasticity and memory formation in response to contextual fear conditioning in mice. Neurosci. 168: 167–178. [DOI] [PubMed] [Google Scholar]
- -Farias GA, Munoz JP, Garrido J and Maccioni RB (2002) Tubulin, actin, and tau protein interactions and the study of their assemblies. J. Cell. Biochem 85: 315–324. [DOI] [PubMed] [Google Scholar]
- -Fasulo L, Cigolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V, Novak M and Cattaneo A (2000) The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J. Neurochem 75: 624–633. [DOI] [PubMed] [Google Scholar]
- -Feinstein ΗE, Benbow SJ, LaPointe NE, Patel N, Ramachandran S, Do TD, Gaylord MR, Huskey NE, Dressier N, Korff M, Quon B, Cantrell KL, Bowers MT, LaL R and Feinstein SC (2016) Oligomerization of the microtubule-associated protein tau is mediated by its N-terminal sequences: implications for normal and pathological tau action. J. Neurochem 137: 939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Ferreira A and Loomis PA (1998) Isolation and culture of primary neural cells In: Cells: A laboratory manual (Spector D, Goldman R, Leinwand L, eds), pp9.1–9. Woodbury, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- -Ferreira A and Caceres A (1989) The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Dev. Brain. Res 49: 205–213. [DOI] [PubMed] [Google Scholar]
- -Ferreira A and Bigio EH (2011) Calpain-mediated tau cleavage: a mechanism leading to neurodegeneration shared by multiple tauopathies. Mol. Med 17: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Ferreira A, Lu Q, Orecchio L and Kosik KS (1997) Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A beta. Mol. Cell. Neurosci 9: 220–234. [DOI] [PubMed] [Google Scholar]
- -Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI and Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 100: 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Gundersen GG, Kalnoski MH and Bulinski JC (1984) Distinct populations of microtubules: Tyrosinated and non-tyrosinated alpha tubulin are distributed differently in vivo. Cell 38: 779–789. [DOI] [PubMed] [Google Scholar]
- -Gustke N, Trinczek B, Biemat J, Mandelkow E-M and Mandelkow E (1994) Domains of tau protein and interactions with microtubules. Biochem. 39: 9511–9522. [DOI] [PubMed] [Google Scholar]
- -Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski ΗM and Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 83: 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -He HJ, Wang XS, Pan R, Wang DL, Liu MN and He RQ (2009) The proline-rich domain of tau plays a role in interactions with actin. BioMedic. Central Cell Biology 10: 81. doi: 10.1186/471-2121-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Henriquez JP, Cross D, Vial C and Maccioni RB (1995) Subpopulations of tau interact with microtubules and actin filaments in various cell types. Cell. Biochem. Funct 13: 239–250. [DOI] [PubMed] [Google Scholar]
- -Kanai Y, Chen J and Hirokawa N (1992) Microtubule bundling by tau proteins in vivo: analysis of functional domains. EMBO J. 11: 3953–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Kelly BL, Vassar R and Ferreira A (2005) Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J. Biol. Chem 280: 31746–31753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Kelly B and Ferreira A (2006) Beta-amyloid-induced dynamin 1 degradation is mediated by NMDA receptors in hippocampal neurons. J. Biol. Chem 281: 28079–28089. [DOI] [PubMed] [Google Scholar]
- -Kondo J, Honda T, Mori H, Hamada Y, Miura R, Ogawara M and Ihara Y (1988) The carboxyl third of tau is tightly bound to paired helical filaments. Neuron 1: 827–834. [DOI] [PubMed] [Google Scholar]
- -Kosik KS, Joachim CL and Selkoe DJ (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 83: 4044–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Kreis TE (1987): Microtubules containing detyrosinated tubulin are less dynamic. EMBO J 6: 2597–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- -Lang AE, Riherd-Methner DN and Ferreira A (2014) Neuronal degeneration, synaptic defects and behavioral deficits in tau45-230 transgenic mice. Neurosci. 275: 322–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Lyford GL, Yamagata K, Kaufman WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA and Worley PF (1995) Arc a growth factor and activity regulated gene encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14: 433–445. [DOI] [PubMed] [Google Scholar]
- -Nicholson AM and Ferreira A (2009) Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J. Neurosci 29: 4640–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Paganoni S, Anderson KL and Ferreira A (2004) Differential subcellular localization of Ror tyrosine kinase receptors in cultured astrocytes. Glia 46: 456–466. [DOI] [PubMed] [Google Scholar]
- -Parihar MS and Hemnani T (2004) Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci 11: 456–467. [DOI] [PubMed] [Google Scholar]
- -Park SY and Ferreira A (2005) The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J. Neurosci 25: 5365–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Park SY, Tournell C, Sinjoanu RC and Ferreira A (2007) Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neurosci. 144: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Piperno G, Le Dizet M and Chang X (1987) Microtubules containing acetylated cx-tubulin in mammalian cells in culture. J. Cell Biol 104: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Quinn JP, Corbett NJ, Kellett KAB and Hooper NM (2018) Tau proteolysis in the pathogenesis of tauopathies: Neurotoxic fragments and novel biomarkers. J. Alzh. Dis 63: 13–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Rapoport M, Dawson ΗN, Binder LI, Vitek MP and Ferreira A (2002) Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 99: 6364–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Reinecke JB, DeVos SL, McGrath JP, Shepard AM, Goncharoff DK, Tait D,N, Fleming SR, Vincent MP and Steinhilb ML (2011) Implicating calpain in tau-mediated toxicity in vivo. PLoS One 6:e23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Reifert J, Hartung-Cranston D and Feinstein SC (2011) Amyloid β-mediated cell death of cultured hippocampal neurons reveals extensive tau fragmentation without increased full-length tau phosphorylation. J. Biol. Chem 286: 207907–20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT and Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest 114: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Robson SJ and Burgoyne RD (1989) Differential localisation of tyrosinated, detyrosinated, and acetylated α-tubulins in neurites and growth cones of dorsal root ganglion neurons. Cell Motil. Cytosk 12: 273–282. [DOI] [PubMed] [Google Scholar]
- -Rosenberg KJ, Ross JL, Feinstein E, Feinstein SC and Israelachvili J (2008) Complementary dimerization of microtubule-associated tau protein: implications for microtubule bundling and tau-mediated pathogenesis. Proc. Natl. Acad. Sci. USA 105: 7445–7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Schulze E, Asai DJ, Bulinski JC and Kirschner M (1987): Post-translational modification and microtubule stability. J. Cell Biol 105: 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Sharma VM, Litersky JM, Bhaskar K and Lee G (2007) Tau impacts on growth factor-stimulated actin modeling. J. Cell Sci 120: 748–757. [DOI] [PubMed] [Google Scholar]
- -Takashima A, Noguchi K, Sato K, Hoshino T and Imahori K (1993), Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 90: 7789–7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Towbin H, Staehelin T and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Webster DR, Gundersen GG, Bulinski JC andBorisy GG (1987): Differential turnover of tyrosinated and detyrosinated microtubules. Proc. Natl. Acad. Sci. USA 84: 9040–9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Wehland J and Weber K (1987): Turnover of the carboxy-terminal tyrosine of α-tubulin and means of reaching elevated levels of detyrosination in living cells. J. Cell Sci 88: 185–203. [DOI] [PubMed] [Google Scholar]
- -Wood JG, Mirra SS, Pollock NJ and Binder LI (1986), Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc. Natl. Acad. Sci. USA 83: 4040–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Yakunin AF and Hallenbeck PC (1998) A luminol/iodophenol chemiluminescent detection system for Western immunoblots. Anal. Biochem 258: 146–149. [DOI] [PubMed] [Google Scholar]
- -Yu JZ and Rasenick MM (2006) Tau associates with actin in differentiating PC12 cells. FASEB J. 20: 1452–1461. [DOI] [PubMed] [Google Scholar]
- -Zhang Z, Song M, Liu X, Kang SS, Kwon IS, Duong DM, Seyfried NT, Hu WT, Liu Z, Wang JZ, Cheng L, Sun YE, Yu SP, Levey AT and Ye K (2014) Cleavage of tan by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat. Med 20: 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -Zhao X, Kotilinek LA, Smith B, Hlynialuk C, Zahs K, Ramsden M, Clearly J and Ashe KH (2016) Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med 22: 1268–1276. [DOI] [PubMed] [Google Scholar]


