Abstract
Rationale.
Cocaine use disorder (CUD) is associated with cognitive deficits that have been linked to poor treatment outcomes. An improved understanding of cocaine’s deleterious effects on cognition may help optimize pharmacotherapies. Emerging evidence implicates abnormalities in glutamate neurotransmission in CUD and drugs that normalize glutamatergic homeostasis (e.g., N-acetylcysteine [NAC]) may attenuate CUD-related relapse behavior.
Objectives.
The present studies examined the impact of chronic cocaine exposure on touchscreen-based models of learning (repeated acquisition) and cognitive flexibility (discrimination reversal) and, also, the ability of NAC to modulate cocaine self-administration and its capacity to reinstate drug-seeking behavior.
Methods.
First, stable repeated acquisition and discrimination reversal performance was established. Next, high levels of cocaine-taking behavior (2.13-3.03 mg/kg/session) were maintained for 150 sessions during which repeated acquisition and discrimination reversal performance was probed periodically. Finally, the effects of NAC treatment were examined on cocaine self-administration and, subsequently, extinction and reinstatement.
Results.
Cocaine self-administration significantly impaired performance under both cognitive tasks; however, discrimination reversal was disrupted considerably more than acquisition. Performance eventually approximated baseline levels during chronic exposure. NAC treatment did not perturb ongoing self-administration behavior but was associated with significantly quicker extinction of drug-lever responding. Cocaine-primed reinstatement did not significantly differ between groups.
Conclusions.
The disruptive effects of cocaine on learning and cognitive flexibility are profound but performance recovered during chronic exposure. Although the effects of NAC on models of drug-taking and drug-seeking behavior in monkeys are less robust than reported in rodents, they nevertheless suggest a role for glutamatergic modulators in CUD treatment programs.
Keywords: Self-Administration, Cocaine, N-acetylcysteine, Learning, Cognitive Flexibility, Reinstatement, Nonhuman Primates
INTRODUCTION
Cocaine use disorder (CUD) continues to be a major public health problem for which there currently is no FDA-approved pharmacotherapy (NIDA 2018). CUD is often characterized by a high probability of relapse to compulsive drug-taking behavior. Individuals with CUD also may suffer other deleterious behavioral consequences of long-term drug exposure. For example, many cocaine users exhibit increased impulsivity as well as deficits in attention, spatial processing, reversal learning, and cognitive control (reviewed extensively in Potvin et al. 2014; Spronk et al. 2013; Wood et al. 2013). Indeed, poor treatment outcomes have been linked to cognitive deficits that emerge during prolonged cocaine exposure (e.g., Aharonovich et al. 2003; 2006; Moeller et al. 2001; Turner et al. 2009; Verdejo-García et al. 2012; Winhusen et al. 2013a, b).
Studies in laboratory animals generally have supported the view that exposure to cocaine impairs cognitive function and, therefore, improving our understanding of cocaine’s deleterious effects on cognitive performance may help to optimize behavioral interventions and to develop effective medications with clinical applicability. In this regard, studies in nonhuman primates have shown that brief (2 weeks) intermittent exposure to high doses of cocaine as well as extended (up to 5 years) periods of daily intravenous (i.v.) cocaine self-administration lead to abnormalities in critical cognitive domains, including stimulus discrimination, reversal learning, and short-term memory (Gould et al. 2012; Jentsch et al. 2002; Liu et al. 2008; Porter et al. 2011). In addition, deficits in reversal learning, a widely accepted index of cognitive flexibility (Chudasama 2011; Easton 2005; Mackintosh et al. 1968), have been reported in nonhuman primates during drug-free periods following extended i.v. cocaine self-administration (Porter et al. 2013). Taken together, these findings support the idea that the cognitive deficits can be induced by chronic cocaine exposure and, as well, can persist into abstinence.
The neural mechanisms that underlie CUD have been widely studied but are not yet fully delineated. Although it has been known for some time that cocaine’s blockade of dopamine transport likely mediates its abuse-related effects (Ritz et al. 1987; Kuhar et al. 1991), a great deal of evidence indicates that abnormalities in glutamate neurotransmission and neuroplasticity also can promote CUD (Bauzo et al. 2012; Ben-Shahar et al. 2012; Kalivas and Volkow 2011; Knackstedt et al. 2014; Liu et al. 2011; Martinez et al. 2014; Yang et al. 2009). In this regard, drugs that normalize synaptic glutamate homeostasis and neurotransmission (e.g., N-acetylcysteine (NAC), ceftriaxone, clavulanic acid, topiramate) have been reported to attenuate the priming effects of a variety of self-administered drugs in reinstatement studies in rodents (e.g., Amen et al. 2011; Johnson et al. 2013; Knackstedt et al. 2010; LaRowe et al. 2013; Moussawi et al. 2011; Schmaal et al. 2010; Kim et al. 2016). For example, NAC has been shown to inhibit reinstatement of extinguished self-administration responding maintained by cocaine (Baker et al. 2003; Frankowska et al. 2014; Jastrzębska et al. 2016; Kau et al. 2008; Kupchik et al. 2012; Moran et al. 2005; Reissner et al. 2015; Reichel et al. 2011), methamphetamine (Chamtikov et al. 2018), heroin (Hodebourg et al. 2018; Zhou and Kalivas 2008), alcohol (Quintanilla et al. 2018), and nicotine (Ramirez-Nino et al. 2013). Moreover, these findings in rodents appear to be functionally consistent with two clinical reports of decreased cocaine craving in human CUD participants following NAC treatment (LaRowe et al. 2007; Mardikian et al. 2007). In addition, a recent study by Levi Bolin et al. (2017) found that attentional bias for cocaine cues was attenuated by NAC treatment in human participants. The latter study was also the first in which decreases in the reinforcing effects of cocaine self-administration were observed in NAC-treated participants. In studies with nonhuman primates, Bauzo et al. (2012) demonstrated that NAC administration was able to attenuate cocaine-induced extracellular dopamine increases in the caudate nucleus in vivo. However, NAC did not alter cocaine self-administration intake or reinstatement of previously extinguished cocaine self-administration.
In general, the above findings support the view that drugs that normalize levels of synaptic glutamate, e.g., NAC, may be of clinical value in the treatment of cocaine-use disorder (reviewed in Berk et al. 2013; McClure et al. 2014). The present studies were designed to further explore 1) the effects of chronic cocaine exposure and abstinence on cognition-related behavior and 2) the role of glutamatergic mechanisms in ameliorating CUD. This was accomplished by investigating performance on touchscreen-based discrimination learning (repeated acquisition) and cognitive flexibility (discrimination reversal) tasks before, during, and after an extended period of cocaine self-administration and, additionally, the effects of NAC treatment on cocaine self-administration and reinstatement.
METHODS
Subjects.
Six adult male squirrel monkeys (Saimiri sciureus) were used in these studies. These subjects had no prior experience with drug self-administration or touchscreen procedures. Five of the subjects previously served in studies examining neural effects of passive cocaine administration but had not received cocaine or other drug treatment for approximately 2 years prior to the present studies (the 6th subject was drug and experimentally naive). Subjects were pair-housed in a temperature- and humidity-controlled vivarium with a 12-h light/dark cycle (7 am-7 pm), had unlimited access to water in the home cage, and were maintained at approximate free-feeding weights by post-session feedings of a nutritionally balanced diet of high protein biscuits (Purina Monkey Chow, St. Louis, MO). In addition, fresh fruit and environmental enrichment were provided daily. Experimental sessions were conducted 5 days a week. The protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital in a facility licensed by the US Department of Agriculture and in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, Commission on Life Sciences (National Research Council 2011).
Procedures
Figure 1 presents a procedural timeline that summarizes the present studies using the task components described in detail below. Briefly, subjects were first trained on the touchscreen-based repeated acquisition and discrimination reversal tasks. Next, cocaine dose-response functions were determined to identify the unit dose (0.32 mg/kg/inj) to use in extended self-administration conditions that also included intermittent probes of touchscreen performance. After 140 sessions of cocaine self-administration, subjects were divided into a NAC or saline treatment group and, following 10 additional self-administration sessions, reinstatement procedures began. Following reinstatement studies, a final touchscreen probe was conducted 30 days after final cocaine exposure (washout).
Figure 1.
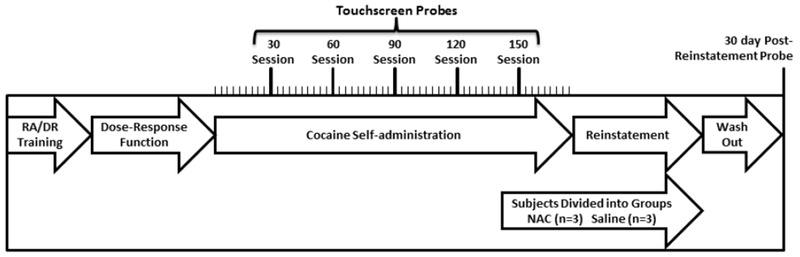
Experimental timeline. Protocols included repeated acquisition (RA) and discrimination reversal (DR) training, determinations of cocaine dose-response functions, extended cocaine self-administration conditions, intermittent touchscreen-based probes, reinstatement, and cocaine discontinuation (washout). See Procedures for additional information.
Touchscreen assays.
Repeated acquisition.
Details, schematics, and photographs of the chamber used for touchscreen studies can be found in Kangas and Bergman (2012). All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA). Previously-established methods were used to train subjects to repeatedly discriminate novel visual discriminations (Kangas and Bergman 2014, 2017). Each session began with concurrent presentation of two 7×7 cm digital photographs, each in a different randomly selected quadrant of a 17” touchscreen on the front wall of the chamber. A reservoir into which fluid could be delivered was positioned below the touchscreen and was readily accessible to the subject. A touch response on one stimulus initiated the delivery of 0.15 ml of a 30% sweetened condensed milk solution into a reservoir (S+) paired with an 880-ms yellow screen flash and followed by a 10-s intertrial interval (ITI) blackout; a touch response to the other stimulus immediately initiated the 10 s ITI (S−). The same two stimuli were presented during each of 200 trials comprising the day’s session. Photographs for each session were selected from a repository of >10,000 images. Thus, the subject was required to repeatedly learn new S+/S− discriminations based on distinguishing features of two visual stimuli that had not been viewed previously. Discrimination mastery was defined as the number of trials into a session at which the subject had performed correctly in 9 out of 10 consecutive trials (i.e., 90% correct). Subjects performed the repeated acquisition task each session until 30 unique discriminations were mastered, after which a discrimination reversal task was introduced into the daily session.
Discrimination reversal.
In the discrimination reversal task, a variant of discrimination learning for examining response inhibition (Easton 2005; Kangas and Bergman 2014; Mackintosh et al. 1968), the programmed consequences of S+ and S− were reversed after the subject learned the initial discrimination. The first 100 trials in the discrimination reversal session were conducted as described above, i.e., subjects learned a novel discrimination each session. On trial 101, the reversal occurred without signal, i.e., for the remainder of the 200-trial session the stimulus that was initially S+ was made S− and vice versa. If the subject failed to master a reversal within 100 trials, the same stimuli and contingencies were presented during a 200-trial session the following day. Subjects performed the reversal task in daily sessions until 30 reversals were mastered, after which subjects were catheterized and trained to self-administer cocaine.
Touchscreen probes.
Performance in the two touchscreen assays described above were probed following 30, 60, 90, 120, and 150 sessions of cocaine self-administration, and after a 30 day cocaine washout (forced abstinence) period. Each touchscreen probe assessed the number of trials to master 5 novel discriminations and subsequent reversals of those discriminations using the mastery criteria described above. Probe sessions occurred 22 hr following the previous self-administration session. This interval was chosen to minimize the effects of acute cocaine self-administration on performance to enable assessment of the effects of extended chronic cocaine self-administration on learning and cognitive flexibility. Thus, subjects were exposed to an approximately 1-h touchscreen session, followed by a 1-h break (spent in the subject’s home cage), and then a 1-h self-administration session. The forced abstinence probe occurred 30 days after cessation of NAC or saline treatment, at the same time of day as previous touchscreen probe sessions.
Cocaine Self-administration.
During self-administration sessions, subjects sat in a Plexiglas chair (Kelleher and Morse 1968; Kangas and Bergman 2016) in a ventilated, sound-attenuating enclosure. Subjects faced a panel containing two response levers, colored stimulus lights, and a custom-designed Plexiglas receptacle (5×3.5×1.27 cm) mounted in front of the subject. Each press of the lever with a force greater than 0.25 N produced an audible click and was recorded as a response. An infusion pump (PHM-100-10, Med Associates, St. Albans, VT) outside the enclosure was used to deliver either i.v. drug infusions or, for initial training, 0.15 ml of a 30% sweetened condensed milk solution into the Plexiglas receptacle. All experimental events and data collection were controlled by Med Associates. Subjects first were trained to respond on one lever under a 10-response fixed ratio (FR10) schedule of milk delivery. Responses on the other lever had no programmed consequences. Lever assignment was counterbalanced across subjects. The completion of the FR10 on the active lever turned off the stimulus lights, delivered the reinforcer, and initiated a timeout (TO) period of 60 s during which all stimulus lights were off and responding had no scheduled consequences.
Following the establishment of robust milk-maintained responding, each subject was prepared with an intravenous catheter for drug delivery using procedures initially described by Herd et al. (1969). Under isoflurane anesthesia and in aseptic conditions, one end of a hydrophilically coated polyurethane catheter (inside diameter, 0.381 mm; outside diameter, 0.762 mm) was inserted and secured into the right femoral vein; the other end was subcutaneously threaded to exit the subject’s back. Catheterized subjects wore nylon jackets at all times to protect the catheters.
Next, i.v. infusions of cocaine (0.1 ml/infusion) gradually replaced milk deliveries as reinforcing events. Subjects self-administered cocaine with simultaneous milk delivery during 1-h sessions, beginning with 0.15 mL of milk delivery paired with 0.1 mg/kg/inj of cocaine. After 3 days of stable cocaine intake (±20 % of the 3-session mean), milk volumes were periodically decreased to 0.075 ml, 0.05 ml, 0.025 ml, and 0 ml, allowing for responding to stabilize between decreases in volume. Once cocaine intake without milk delivery was stable, a cocaine dose-response function (0.0032-0.32 mg/kg/inj) and vehicle (0.1 ml/inj saline) was determined three times in each subject. In each determination, doses were studied in a quasi-random order and each unit dose was studied for a minimum of 5 days and until session-intake across 3 consecutive sessions was within ±20 % of the 3-session mean. The unit dose that resulted in maximum average daily intake and could be studied safely (peak unit dose, 0.32 mg/kg/inj) was used during daily self-administration sessions for the remainder of the study. Throughout all studies, daily cocaine intake was limited to 3.2 mg/kg to minimize adverse effects.
NAC Treatment.
Following 140 sessions of cocaine self-administration, subjects were randomly assigned to either a NAC treatment (10 mg/kg/day, n=3) or saline vehicle treatment group (n=3). Subjects were treated with NAC or saline 7 days a week, 1 hour before daily self-administration sessions. The dose of NAC was chosen based on previous research in squirrel monkeys showing that 10 mg/kg NAC could attenuate cocaine-induced elevations in striatal dopamine levels (Bauzo et al. 2012). Following 10 cocaine self-administration sessions under NAC or saline treatment conditions, the final touchscreen probe (i.e., the 150-session probe) was conducted an hour before the daily NAC or saline treatment. Thereafter, reinstatement testing began under continued NAC or saline treatment.
Reinstatement Procedure.
Following 15 sessions of NAC or saline treatment (i.e., 10 sessions of self-administration alone followed by 5 sessions during which self-administration was preceded by the touchscreen probe), self-administration responding was extinguished by replacing cocaine infusions with saline. Sessions were otherwise identical. The criterion for extinction was defined as fewer than 10 saline infusions earned in a 1-hour session. This was based on saline infusion levels observed prior to chronic cocaine exposure. Following a minimum of 5 consecutive sessions in which extinction was observed, tests for priming-induced cocaine reinstatement began. Reinstatement tests were conducted no more than 2 times per week, with at least one session that met the extinction criterion between test days. On each test day, an i.m. priming dose of cocaine was administered 10 minutes before a saline self-administration session. Priming doses (0.032 mg/kg-1.8 mg/kg) were tested in a quasi-random order. NAC or saline treatment was stopped after the final day of reinstatement testing.
Data analysis
In i.v. cocaine self-administration studies, the primary dependent measure was total session intake of cocaine (mg/kg). Session intake was calculated by multiplying the total number of infusions in a session by the self-administered unit dose of cocaine (mg/kg/inj). Dose-response functions were constructed by averaging intake of each unit dose tested during the last 3 sessions for each subject and then presenting group averages (±SEM) for unit dose intake. The primary dependent measure used to evaluate repeated acquisition and discrimination reversal performance was trials-to-mastery. Mastery was defined as the number of trials into a session until a subject performed correctly in 9 out of 10 consecutive trials (i.e., 90% correct). Therefore, this criterion quantified the number of trials required for the subject to either learn a novel discrimination in the case of repeated acquisition or reverse response allocation in the case of discrimination reversal (Kangas and Bergman 2014). A repeated measures two-way analysis of variance (ANOVA) was conducted to evaluate group differences in the development of acquisition and reversal performance. A repeated measures one-way ANOVA followed by a Dunnett’s test was conducted to determine whether acquisition and reversal performance differed statistically in the touchscreen probe studies. The criterion for significance was set at p<.05.
Drugs
Cocaine (cocaine hydrochloride) and NAC (n-acetylcysteine) were purchased from Sigma Pharmaceuticals (St. Louis, MO), prepared for administration in 0.9% saline solution, and refrigerated and protected from light. The pH of NAC was adjusted to 7.0 using 0.1 N sodium hydroxide. Drug concentrations for self-administration experiments were prepared for each subject so as to deliver the unit dose of i.v. cocaine in a 0.1 ml infusion. For i.m. treatments, vehicle or doses of drug were administered in volumes of 0.3 ml/kg body weight or less into thigh muscle. All doses of drugs are expressed in terms of their free base.
RESULTS
Figure 2 presents dose-response functions for cocaine infusions (upper panel) and total cocaine intake (lower panel) during 1-hour i.v. self-administration sessions. The number of cocaine infusions described an inverted U-shape curve whereas total cocaine intake increased monotonically in a dose-dependent manner. The insets in Figure 2 present the same data from the larger panels divided by subjects that would, in later conditions, comprise the NAC (n=3) and saline (n=3) groups. As the insets show, there were no systematic differences in either number of injections or total intake across the two groups. Intake was maximal at the unit dose of 0.32 mg/kg/inj for all subjects (mean intake 1.75 ±0.31 SEM mg/kg). Consequently, the unit dose of 0.32 mg/kg/inj cocaine was chosen for subsequent daily self-administration sessions. Average session-wide cocaine intake increased during the first 7-10 sessions in which this unit dose was available and subsequently stabilized. Thereafter, daily intake remained highly consistent for both the group and individuals, ranging from 2.13 to 3.03 mg/kg/session across subjects.
Figure 2.
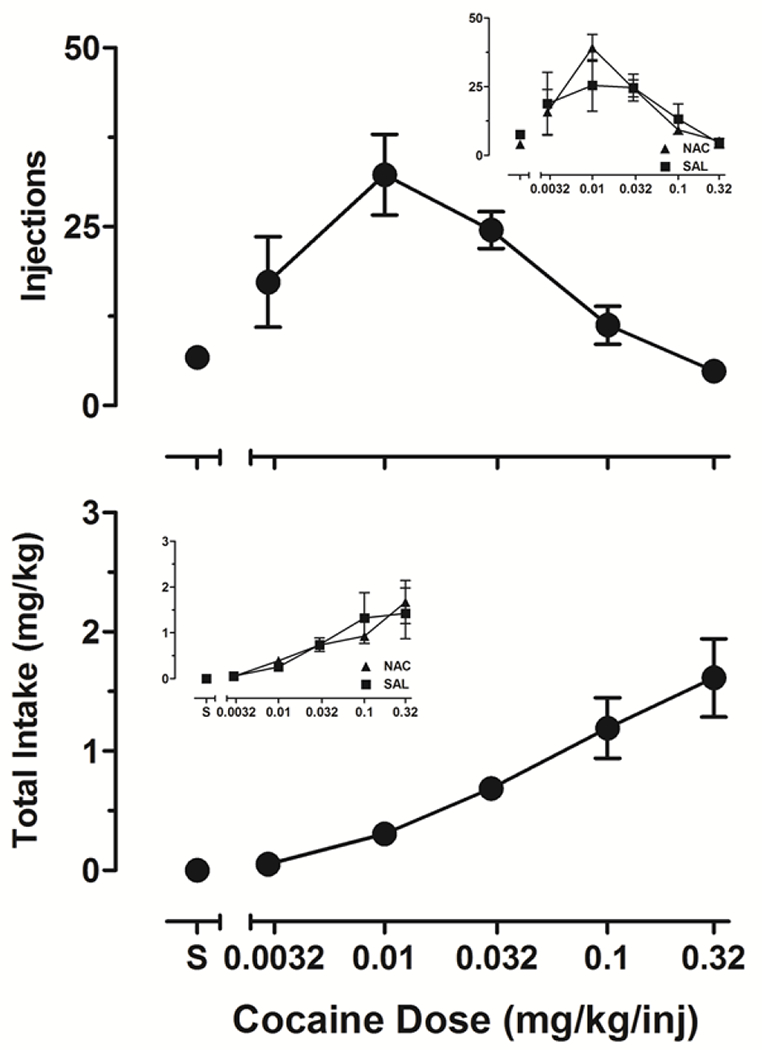
Average (±SEM) cocaine infusions (upper panel) and total cocaine intake (lower panel) during 1-h sessions in which different unit doses of cocaine (mg/kg/inj) and saline (S) were self-administered (n=6). The insets in show the same data from the larger panels divided by subjects that would, in later conditions, comprise the NAC (n=3) and saline (n=3) groups.
Figure 3 presents repeated acquisition (white bars) and discrimination reversal (gray bars) data for trials to mastery. Values are shown from sessions following training and immediately prior to the introduction of cocaine self-administration sessions (control [C]) and from the sets of touchscreen probes that occurred following 30, 60, 90, 120, and 150 sessions of cocaine self-administration and after a 30-day washout (forced abstinence [Ab]) period. During training, values for repeated acquisition plateaued at approximately 20 trials to mastery whereas values for discrimination reversal plateaued at the higher level of approximately 50 trials to mastery. These baseline learning-to-learn and learning-to-reverse data systematically replicate and closely approximate previous findings (cf. Kangas and Bergman 2014, 2016) and are represented in Figure 3 as control values. Each subsequent probe, in which 5 novel discriminations and reversals were mastered, was conducted following every 30 sessions of cocaine self-administration. After 30 sessions of cocaine self-administration, the average number of trials to master the 5 successive discriminations increased significantly from approximately 20 at baseline to 30, and the average number of trials to master each of 5 subsequent reversals increased significantly from approximately 50 at baseline to 95 (F=3.54, p<.01; F=5.37, p<.001, respectively). After 60 sessions of self-administration, the number of trials to master each initial discrimination was greater than baseline but these differences did not reach statistical significance. Reversal performance improved some but remained significantly impaired relative to baseline; subjects took an average of approximately 80 trials to master each reversal (F=3.69, p<.01). After 90 sessions of self-administration, the average number of trials to master initial discriminations again was greater than baseline but did not differ statistically from control values. Reversal performance continued to be statistically different; subjects took an average of approximately 75 trials to master each reversal (F=2.72, p<.05). Thereafter, although probe values did not differ statistically from control values, the average number of trials to master discriminations and reversals decreased steadily over the course of the remaining 60 sessions of self-administration. Probe discrimination and reversal values during the forced abstinence period (30 days following cocaine) closely approximated control values that were obtained prior to the initiation of cocaine self-administration.
Figure 3.
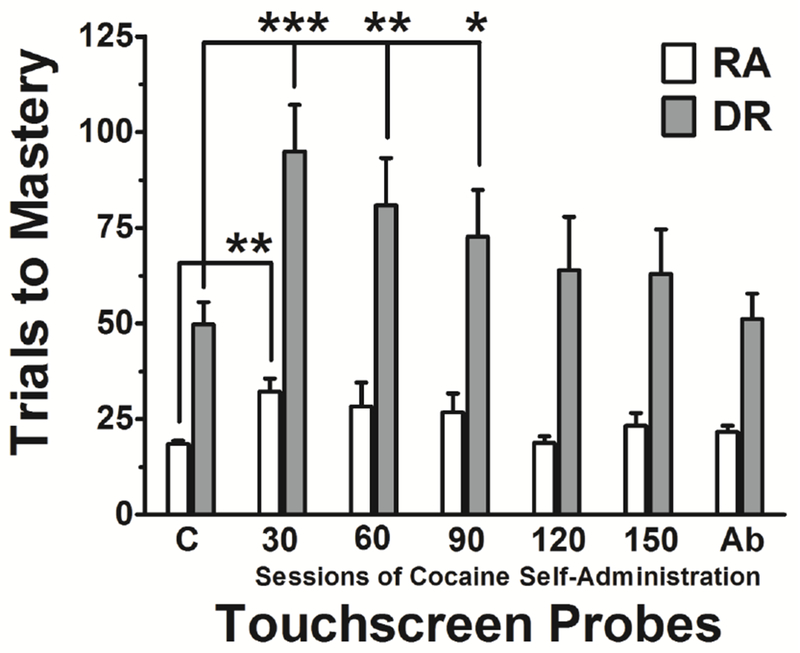
Average (±SEM) number of trials to master five discriminations (Repeated Acquisition [RA], open bars) and subsequent reversals (Discrimination Reversal [DR], filled bars) during probe sessions conducted before cocaine exposure (C), following each 30th session of cocaine self-administration, and after a 30-d washout (abstinence) period (Ab) (n=6). (*p<.05; **p<.01; ***p<.001)
Although cocaine intake was highly consistent within-subject across the extended self-administration condition, there were individual differences in levels of cocaine intake across subjects. The possible relationship between daily intake and trials to master discriminations and reversals was examined using data from the 30-session touchscreen probe when the effects on touchscreen performance were most apparent. The left panel of Figure 4 presents data from individual subjects and shows the percent change from baseline in the average number of trials to master 5 discriminations as a function of average daily cocaine intake during the sessions in which those discriminations were mastered. Figure 4 indicates a high correlation between cocaine intake and percent change in trials to master initial discriminations (R2=.75). In contrast, however, there was no correlation between cocaine intake and percent change in trials to master reversals, shown in the right panel of Figure 4 (R2=.02).
Figure 4.
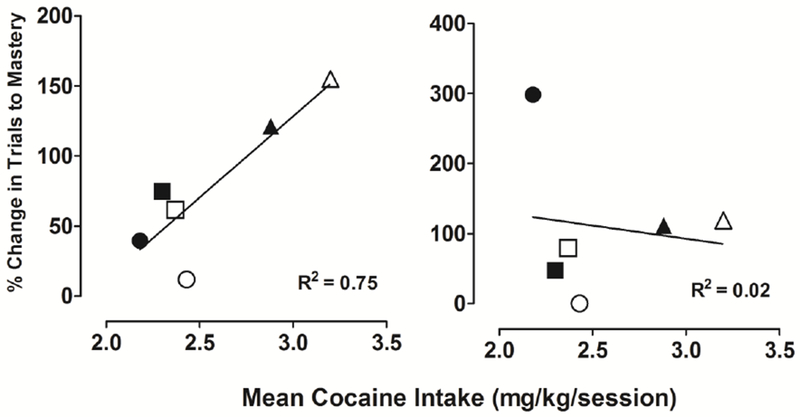
Percent change from baseline in average trials to master 5 discriminations (left panel) and 5 reversals (right panel) following 30 days of cocaine self-administration, plotted as a function of average daily cocaine intake during the sessions in which those 5 discriminations were mastered. Each data point represents that relationship for an individual subject. The solid line represents a standard linear regression fit to the data.
After 140 sessions of cocaine self-administration, subjects were treated with NAC or saline daily. The left panel of Figure 5 shows group average session-wide cocaine intake (mg/kg) during the last 10 sessions of cocaine self-administration for all subjects before saline or NAC treatment (gray bar, n=6), and during the 10 sessions of saline (white bar, n=3) or NAC pretreatment (black bar, n=3). A two-way repeated measures ANOVA did not reveal a significant effect of NAC treatment on cocaine intake during the final ten sessions of cocaine self-administration (t=0.37, p=.78). However, when cocaine was replaced with saline, subjects treated with NAC required significantly fewer sessions to meet extinction criteria than did saline-treated subjects (Figure 5, right panel; t=3.43, p=.03).
Figure 5.
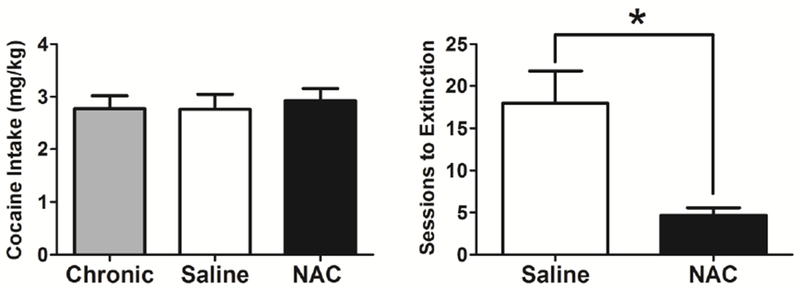
Left panel: Mean cocaine intake (mg/kg) during the last 10 sessions of self-administration for all subjects prior to saline or NAC treatment (gray bar, n=6), and during the 10 sessions of saline (white bar, n=3) or NAC pretreatment (black bar, n=3). Right panel: Mean sessions required to meet extinction criteria. Extinction criteria were defined as a 1-hour session with fewer than 10 saline infusions earned. (p<.05)
Finally, after extinction criteria were met, reinstatement procedures began. As shown in Figure 6, pre-session priming with cocaine produced a dose-dependent reinstatement of previously extinguished cocaine-lever responding in both the saline (open symbols) and NAC group (filled symbols). A one-way ANOVA revealed a significant main effect of the priming dose of cocaine on the number of saline infusions that were earned (F=7.43, p<.0001). The average number of saline infusions earned after priming doses of 0.1 mg/kg and 0.32 mg/kg cocaine was significantly greater than after priming infusions of saline (p<.01, p<.001, respectively). The average number of saline infusions earned after the priming dose of 0.32 mg/kg cocaine was also significantly greater than after priming doses of 0.032 mg/kg and 1.8 mg/kg (p<.01, p<.05, respectively). Thus, the number of saline infusions earned in a session was an inverted-U shape function of the priming dose of cocaine. Although the dose-response function for the NAC-treated group was lower than that of the saline-treated group at the group-average level, a two-way repeated measures ANOVA revealed no significant main effect or interaction of NAC treatment on saline infusions earned (F=1.21, p=.33; F= 1.45, p=.24, respectively). The modest suppression of the NAC dose-response function primarily reflected a flattened dose-response function for one of the NAC-treated subjects.
Figure 6.
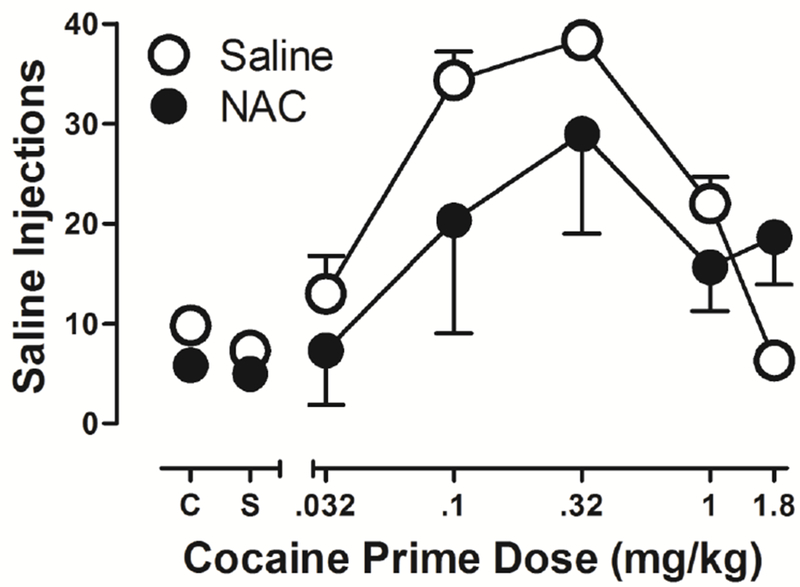
Effect of NAC treatment (filled circles, n=3) and saline treatment (open circles, n=3) on reinstatement of previously extinguished self-administration behavior induced by pre-session cocaine primes. Data are expressed as average number of saline infusions (±SEM) earned during a 1-h session after an i.m. cocaine prime (mg/kg), saline (S), or no injection (C).
DISCUSSION
In the present studies, learning and cognitive flexibility, assayed in nonhuman primates using touchscreen-based repeated acquisition and discrimination reversal tasks, were adversely affected by daily self-administration of high dosages of cocaine. These findings are consistent with the results of previous research (e.g., Gould et al. 2012; Liu et al. 2008; Porter et al. 2011), strengthening the view that the deleterious effects of cocaine abuse include degraded cognitive function. Additionally, the effects of cocaine exposure on discrimination reversal in the present work were more pronounced than its effects on repeated acquisition—a relative sensitivity that also has been reported in previous studies with methamphetamine (Groman et al. 2012, 2013; Kangas and Bergman 2016). Interestingly, the sensitivity of this measure of cognitive flexibility to the effects of psychoactive drugs is not observed only with psychomotor stimulants; similar findings have been observed following the administration of cannabinoids (Kangas et al. 2016). Therefore, the present and previous data suggest that the loss of inhibitory control that characterizes discrimination reversal performance may be a deficit resulting from the use of abused drugs more generally (reviewed in Izquierdo and Jentsch 2012). However, it is also important to note that, even under control conditions, discrimination reversal is more difficult than discrimination learning (see pre-cocaine control values in Fig. 3), and the relative magnitude of cocaine-induced deficits may be dependent on baseline levels of task performance. That is, persistently difficult cognitive tasks may be relatively more vulnerable to the adverse effects of abused drugs, regardless of drug class.
Of note, performance on both cognitive tasks gradually recovered over the course of the extended regimen of daily cocaine self-administration sessions and data from probe assessments eventually approximated pre-chronic values. These findings are not consistent with much of the clinical literature on cocaine’s adverse effects on cognition (reviewed in Potvin et al. 2014; Spronk et al. 2013; Wood et al. 2013). The mechanisms responsible for the development of tolerance to cocaine’s adverse effects on performance are currently unclear. One possibility is that chronic cocaine exposure alone lessened its impact on behavior through neuroadaptation, reflected in surmounted deficits in task performance. However, it is also possible that probe assessments were essential for subjects to reacquire learning and reversal performance under chronic cocaine conditions, and that the rate of performance recovery reflects such remedial state-dependent training or behavioral tolerance across tasks (see Woolverton and Schuster 1978). The relative contribution of cellular or neurochemical adaptations or state-dependent training to the recovery of cognitive performance during chronic cocaine exposure as well as the range of cognitive tasks to which such tolerance occurs are questions that remain to be determined. Two additional caveats warrant discussion. First, because these studies did not include a non-cocaine control group, e.g., a group of subjects in which task performance was intermittently probed during extended saline self-administration conditions, the role of, simply, the passage of time on touchscreen performance is undetermined. Second, it is important to make the distinction between recovery of repeated acquisition and discrimination reversal task performance and the more general recovery of learning and cognitive flexibility during extended cocaine self-administration. Previous studies highlighted above have reported persistent deficits in cognitive function and it is certainly possible that the recovery of cognitive performance in the present studies was limited to specific tasks rather than reflecting a recovery of general cognitive function. Regardless, the present findings suggest that cocaine-induced impairments of cognition-related behavior are not necessarily a permanent deficit and, indeed, may be overcome during extended chronic exposure.
Dysregulation of glutamatergic mechanisms has been proposed to play a role in a variety of neuropsychiatric conditions (reviewed in Skvarc et al. 2017) including drug addiction (Kalivas and Volkow 2011; McClure et al. 2014). However, this view must be considered cautiously. The failure of NAC treatment to perturb ongoing cocaine self-administration behavior in nonhuman primates in the present and previous studies (Bauzo et al. 2012) is not consistent with a glutamatergic role in the reinforcing effects of self-administered drugs. On the other hand, as reviewed above, NAC has displayed efficacy in reinstatement models across drug classes in rodents, presumably through normalizing glutamatergic function and, since it received FDA approval in 1963 for the treatment of pulmonary disorders, has a long-established safety record in patient populations (e.g., Grandjean et al. 2000; Repine et al. 1997). Future studies should examine whether this is purely a species difference or whether different conditions of NAC administration (e.g., dose/duration) are able to reduce cocaine self-administration behavior.
Although NAC treatment did not significantly alter the direct reinforcing effects of cocaine, its effects on extinction behavior indicate that it was behaviorally active. The quicker extinction of drug-lever responding in NAC-treated subjects compared to vehicle-treated subjects is consistent with the results of previous cocaine self-administration studies in rodents (e.g., Jastrzębska et al. 2016; Reichel et al. 2011) and may be predictive of a NAC-induced reduction in overall drug-seeking behavior. In agreement with this idea, extinguished drug-lever responding in the present reinstatement experiments was not as fully restored in the NAC-treated group as in the saline-treated group, despite pre-session cocaine injections as the priming stimulus. However, it is important to note that this latter effect did not reach statistical significance and was largely driven by data in one of the three NAC-treated subjects. Moreover, it remains to be determined whether such NAC effects on extinction rate would be observed during extinction of behavior maintained by other reinforcers such as food. In the absence of additional data, the idea that NAC treatment can reduce drug-seeking behavior must remain speculative.
In summary, the present studies indicate that the disruptive effects of cocaine on measures of learning and cognitive flexibility, while acutely pronounced, were not sustained over the course of chronic exposure. The present studies also reveal effects of NAC treatment that, although not as robust as previous findings in rodents, may offer some promise for the development of glutamatergic modulators for relapse prevention. NAC is relatively lipophilic and does not easily cross the blood brain barrier (Samuni et al. 2013), and once in the brain, is subject to extrusion by organic anion transporters (Hagos et al. 2016). Accordingly, its limited brain bioavailability also may limit its behavioral efficacy—a view that is supported by findings with an NAC analogue with higher bioavailability than NAC (e.g., N-acetylcysteine amide; NACA) that also extinguishes cocaine seeking with greater potency (Jastrzębska et al. 2016). Additional studies employing regulators of glutamate transport with better brain bioavailability, e.g., NACA or clavulanic acid (Kim et al. 2016; Hakami and Sari 2017; Hakami et al. 2017) will be useful for further determining how normalizing glutamatergic activity affects ongoing self-administration of cocaine, extinction of cocaine self-administration, and/or attenuation of the reinstatement of drug-seeking behavior following extinction.
Acknowledgements
This research was supported by grants K01-DA035974 (BDK) and R21-DA039301 (MJK) from the National Institute on Drug Abuse. The authors thank Roger Spealman for comments on a previous version of this manuscript.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
REFERENCES
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV (2006) Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend 81:313–322. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D (2003) Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend 71:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA (2011) Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 36:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW (2003) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749. [DOI] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL (2012) The cystine-glutamate transporter enhancer N-acetyl-L-cysteine attenuates cocaine-induced changes in striatal dopamine but not self-administration in squirrel monkeys. Pharmacol Biochem Behav 101:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, DeMartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N (2012) Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol 17:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM (2013) The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 34:167–177. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Pudiak CM, Bevins RA (2018) The effect of N-acetylcysteine or bupropion on methamphetamine self-administration and methamphetamine-triggered reinstatement of female rats. Neuropharmacology 135:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y (2011) Animal models of prefrontal-executive function. Behav Neurosci 125:327–343. [DOI] [PubMed] [Google Scholar]
- Easton A (2005) Behavioural flexibility, social learning, and the frontal cortex In: Easton A, Emery NJ (eds) The cognitive neuroscience of social behavior. New York: Psychology Press, pp 59–80. [Google Scholar]
- Frankowska M, Jastrzębska J, Nowak E, Białko M, Przcgaliński E, Filip M (2014) The effects of N-acetylcysteine on cocaine reward and seeking behaviors in a rat model of depression. Behav Brain Res 266:108–118. [DOI] [PubMed] [Google Scholar]
- Gould RW, Gage HD, Nader MA (2012) Effects of chronic cocaine self-administration on cognition and cerebral glucose utilization in Rhesus monkeys. Biol Psychiatry 72:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean EM, Berthet P, Ruffmann R, Leuenberger P (2000) Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebocontrolled clinical trials. Clin Ther 22:209–221. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkem MA, London ED, Jentsch JD (2012) Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. JNeurosci 32:5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD (2013) Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology (Berl) 229:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagos FT, Daood MJ, Ocque JA, Nolin TD, Bayir H, Poloyac SM, Kochanek PM, Clark RS, Empey PE (2017) Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica 47:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Alshehri FS, Sari Y (2018) β-lactams modulate astroglial glutamate transporters and attenuate dependence to CP 55,940, a CB1 receptor agonist, in rat model. Behav Brain Res S0166-4328(18)30743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Sari Y (2017) β-Lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neurosci Lett 657:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd JA, Morse WH, Kelleher RT, Jones LG (1969) Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol 217:24–29. [DOI] [PubMed] [Google Scholar]
- Hodebourg R, Murray JE, Fouyssac M, Puaud M, Everitt BJ, Belin D (2018) Heroin seeking becomes dependent on dorsal striatal dopaminergic mechanisms and can be decreased by N-acetylcysteine. Eur J Neurosci doi: 10.1111/ejn.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD (2012) Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 219:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzębska J, Frankowska M, Filip M, Atlas D (2016) N-acetylcysteine amide (AD4) reduces cocaine-induced reinstatement. Psychopharmacology (Berl) 233:3437–3448. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R 2nd, Taylor JR (2002) Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 26:183–190. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Wang XQ, Penberthy JK, Javors MA, Seneviratne C, Liu L (2013) Topiramate for the Treatment of Cocaine Addiction: A Randomized Clinical Trial. JAMA Psychiatry 70:1338–1346. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2012) A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys J Neurosci Meth 209:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2014) Repeated acquisition and discrimination reversal in the squirrel monkey (Saimiri sciureus). Anim Cogn 17:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2016) Effects of self-administered methamphetamine on discrimination learning and reversal in nonhuman primates. Psychopharmacology (Berl) 233:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2017) Touchscreen technology in the study of cognition-related behavior. Behav Pharmacol 28:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J (2016) Comparisons of Δ9-Tetrahydrocannabinol and Anandamide on a Battery of Cognition-Related Behavior in Nonhuman Primates. J Pharmacol Exp Ther 357:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA (2008) Blunted cysteine-glutamate antiporter function in the nucleus accumbens promotes cocaine induced drug seeking. Neuroscience 155:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH (1968) Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol Biol Chem Exp Pharmakol 60:1–56. [DOI] [PubMed] [Google Scholar]
- Kim J, John J, Langford D, Walker E, Ward S, Rawls SM (2016) Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino Acids 48:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW (2010). Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M (2014) The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol 19:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14:299–302. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW (2012) The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry 71:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ (2013) A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict 22:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R (2007) Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry 164:1115–1117. [DOI] [PubMed] [Google Scholar]
- Levi Bolin B, Alcorn JL 3rd, Lile JA, Rush CR, Rayapati AO, Hays LR, Stoops WW (2017) N-Acetylcysteine reduces cocaine-cue attentional bias and differentially alters cocaine self-administration based on dosing order. Drug Alcohol Depend 178:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Sampson AR, Zhang W, Bradberry CW (2008) Evidence of temporal cortical dysfunction in rhesus monkeys following chronic cocaine self-administration. Cereb Cortex 18:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jensen JE, Gillis TE, Zuo CS, Prescot AP, Brimson M, Cayetano K, Renshaw PF, Kaufman MJ (2011) Chronic cocaine exposure induces putamen glutamate and glutamine metabolite abnormalities in squirrel monkeys. Psychopharmacology (Berl) 217:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ, McGonigle B, Holgate V, Vanderver V (1968) Factors underlying improvement in serial reversal learning. Can J Psychol 22:85–95. [DOI] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ (2007) An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31:389–394. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NB, Perez A, Liu F, Lin SF, Ropchan J, Mao X, Kegeles LS, Shungu DC, Carson RE, Huang Y (2014) Imaging Glutamate Homeostasis in Cocaine Addiction with the Metabotropic Glutamate Receptor 5 Positron Emission Tomography Radiotracer [11C]ABP688 and Magnetic Resonance Spectroscopy. Biol Psychiatry 75:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM (2014) Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs 28:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J (2001) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK (2005) Cystine/glutamate exchange regulatesmetabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25:6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW (2011). Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A 108:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA); National Institutes of Health; U.S. Department of Health and Human Services. Cocaine: Drug Facts, July 2018. www.drugabuse.gov/publications/drugfacts/cocaine; accessed 18Nov2018.
- National Research Council (2011) Guide for the care and use of laboratory animals: eighth edition. National Academy Press, Washington DC. [Google Scholar]
- Porter JN, Gurnsey K, Jedema HP, Bradberry CW (2013) Latent vulnerability in cognitive performance following chronic cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 226:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW (2011) Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci 31:4926–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stavro K, Rizkallah E, Pelletier J (2014) Cocaine and cognition: a systematic quantitative review. J Addict Med 8:368–376. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Morales P, Ezquer F, Ezquer M, Herrera-Marschitz M, Israel Y (2018) Commonality of Ethanol and Nicotine Reinforcement and Relapse in Wistar-Derived UChB Rats: Inhibition by N-Acetylcysteine. Alcohol Clin Exp Res 42:1988–1999. [DOI] [PubMed] [Google Scholar]
- Ramirez-Niño AM, D’Souza MS, Markou A (2013) N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 225:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE (2011) Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther 337:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol 20:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine JE, Bast A, Lankhorst I (1997) Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Resp Crit Care Med 156:341–357. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Goldstein S, Dean OM, Berk M (2013) The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta 1830:4117–4129. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE (2012) N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology 37:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvarc DR, Dean OM, Byrne LK, Gray L, Lane S, Lewis M, Fernandes BS, Berk M, Marriott A (2017) The effect of N-acetylcysteine (NAC) on human cognition - A systematic review. Neurosci Biobehav Rev 78:44–56. [DOI] [PubMed] [Google Scholar]
- Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ (2013). Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev 37:1838–59. [DOI] [PubMed] [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R (2009) Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J Subst Abuse Treat 37:328–334. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, González-Saiz F, Fernández-Calderón F, Bilbao-Acedos I, Pérez-García M (2012) Self-regulation and treatment retention in cocaine dependent individuals: a longitudinal study. Drug Alcohol Depend 122:142–148. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, Seamans CL, Hodgkins CC, Dicenzo JC, Botero CL, Jones DR, Somoza E (2013a) Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. J Subst Abuse Treat 44:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Lewis DF, Kropp FB, Horigian VE, Adinoff B (2013b) Frontal systems deficits in stimulant-dependent patients: evidence of pre-illness dysfunction and relationship to treatment response. Drug Alcohol Depend 127:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Sage JR, Shuman T, Anagnostaras SG (2013) Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev 66:193–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Schuster CR (1978) Behavioral tolerance to cocaine. NIDA Res Monogr. 18:127–141. [DOI] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y (2009) Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res 174:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]


