Abstract
Rationale and Objectives:
Multi-detector computed tomography (MDCT) is useful for measuring in the research setting single-kidney perfusion and function using iodinated contrast time-attenuation curves. Obesity promotes deposition of intrarenal fat, which might decrease tissue attenuation and thereby interfere with quantification of renal function using MDCT. The purpose of this study was to test the hypothesis that background subtraction adequately accounts for intrarenal fat deposition in mildly obese human subjects during renal contrast enhanced dynamic CT.
Materials and Methods:
We prospectively recruited seventeen human subjects stratified as lean or mildly obese based on body-mass index (BMI) below or over 30kg/m2, respectively. Renal perfusion was quantified from CT-derived indicator-dilution curves after background subtraction. DECT images were post-processed to generate iodine and virtual-non-contrast (VNC) datasets, and the ratios between kidney/aorta CT numbers and iodine values calculated as surrogates of renal function.
Results:
Subcutaneous adipose tissue was increased in obese subjects. VNC maps revealed in obese patients a decrease in basal cortical and medullary attenuation. Overall, basal attenuation inversely correlated with BMI, in line with renal fat deposition. Contrarily, the kidney/aorta CT attenuation (after background subtraction) and kidney/aorta iodine ratios were similar between lean and obese subjects and correlated directly. These observations show that following background subtraction, CT number reliably reflects basal tissue attenuation.
Conclusion:
Therefore, our findings support our hypothesis that background subtraction enables reliable assessment of kidney function in mildly obese subjects using MDCT, despite decreased basal attenuation due to renal adiposity.
Keywords: Iodine maps, Dual Energy Computed Tomography, Renal perfusion, Obesity, Intrarenal fat
Introduction
Obesity is a substantial risk factor for the progression and development of chronic kidney disease. Intrarenal fat deposition may directly impact renal function (1–3) and is an important contributor to renal disease (4, 5). Renal hemodynamic alterations like glomerular hyperfiltration frequently occur in the early stages of obesity, heralding kidney disease (5, 6). In addition, obesity may accompany other forms of kidney disease in patients requiring renal evaluation. A non-invasive and accurate method of monitoring single-kidney function could, therefore, be useful in obese patients, specifically when asymmetrical and unilateral disease is present, as traditional iothalamate and para-aminohippurate clearance only quantify glomerular filtration (GFR) and renal blood flow (RBF) in both kidneys (7).
We have previously established multi-detector computed-tomography (MDCT) as a reliable and reproducible tool to estimate single-kidney hemodynamics and function (4, 8). MDCT-derived renal function is computed from time-attenuation curves (TAC) that illustrate the transit of iodinated contrast agents through the kidney. Because the CT number of adipose tissue is highly negative (9), considerable fat deposition may confound the average CT number in a region of interest (ROI) within the kidney by underestimation of renal tissue attenuation. The solution to this problem has commonly been subtraction of the background values, which entails averaging background attenuation points prior to the arrival of contrast within the ROI. However, background subtraction might be imperfect for several reasons. These include unsatisfactory background points because of noise or other imaging artifacts, user bias or subjectivity in choosing the points to average, and bolus timing issues that result in an insufficient number of points to accurately determine the background value. For these reasons, it is not completely understood whether baseline subtraction adequately accounts for substantial fat deposition.
We have recently demonstrated in obese swine that renal adiposity does not interfere with the assessment of single-kidney function using MDCT (10). Studies in humans have shown that intrarenal fat deposition directly correlates with BMI(11) but, whether intrarenal fat deposits affect MDCT evaluation of renal function in obese human subjects remains unknown. Importantly, new advances in MDCT technology now allow addressing this question. The dual-energy capabilities of MDCT allow using two x-ray tubes with different x-ray energies for image acquisition in the helical mode, yielding two separate datasets that permit material decomposition to depict a change in iodine concentration alone (12, 13). In turn, these datasets offer a unique opportunity to isolate and quantify iodine concentration and serve as a reference for MDCT images, which are contrarily affected by attenuation coefficients of tissue constituents.
The purpose of this study was to establish the feasibility of measuring hemodynamics and function using CT in obese human subjects. We hypothesized that intrarenal fat would not impact renal perfusion quantification in obese patients. To test our hypothesis, we studied obese and lean human subjects using MDCT. We found that despite lower baseline tissue attenuation, iodinated contrast injection resulted in comparable increases above baseline values in both CT numbers (after background subtraction) and iodine concentrations compared to the aorta.
Materials and Methods
Dual-energy MDCT (DECT) data were collected from lean and obese human subjects. In each group, single-kidney function was evaluated by analysis of TACs generated during a perfusion scan, and renal size from a helical scan. In addition, DECT post-processed data were reconstructed from two MDCT detectors to obtain iodine and virtual non-contrast (VNC) (14–16) maps in the renal cortex, medulla, and aorta.
Patient studies
All studies were approved by the Internal Review Board in accordance with HIPAA, and all patients provided informed written consent. Between June 2013 and July 2018, we prospectively recruited 17 patients with unilateral renal vascular disease (RVD) enrolled in an ongoing study (17). The inclusion criteria for that study included >60% stenosis (by Doppler ultrasound velocity) in one kidney. All patients were treated with anti-hypertensive therapy consisting of blockers of the renin-angiotensin system. The exclusion criteria included diabetes mellitus, allergy to iodinated contrast, serum creatinine >2.5mg/dl, kidney transplant, pregnancy, major cardiovascular events in the past 6 months, and/or malignancy (17). The patients were then stratified based on body-mass index (BMI) into lean (<30 kg/m2) and obese (≥30 kg/m2) groups (n=10 and n=7, respectively). To examine the impact of fat accumulation on renal attenuation, the analysis focused on the non-stenotic kidney, which was unaffected by ischemia. Nevertheless, the stenotic kidney was studied as well.
All patients were admitted to Saint Marys Hospital, Clinical Research Unit, Rochester, Minnesota between March 2016 and July 2018. Using a 6F sheath via the femoral vein, a 5F pigtail Cobra catheter (Cook, Inc., Bloomington, IN) was advanced into the right atrium for contrast injections for renal hemodynamic and functional assessment. The patients were then moved to the scanning gantry of a clinical 64-slice (SOMATOM Definition, Siemens Healthcare) or 128-slice (SOMATOM Definition Flash, Siemens, Germany) MDCT. In order to determine the start time of the perfusion scan, a delay time was calculated to define the time duration from contrast injection to its arrival in the aorta. This delay was determined by a test bolus acquisition, which was comprised of 10cc of iopamidol-370 per sec, followed by 20cc of saline at 4 cc/sec breath hold. Using the DynEva application on the Siemens workstation, a TAC was generated from an ROI drawn in the aorta, and a delay then selected for subsequent scans approximately 2–3 seconds prior to contrast arrival in the aorta, allowing at least 3 baseline points for background definition. As previously described, a bolus of iopamidol-370 (0.5ml/kg at 10 mL/sec) was injected for DECT-MDCT perfusion studies (17, 18). Imaging parameters were set at 120 kV and 160 mAs, 24 X 1.2 collimation, and 0 table feed. The perfusion scan included 45 acquisitions over approximately 2.5 minutes. To limit the breath-hold and radiation dose, the initial 35 consecutive acquisitions were distributed into three ≤ 20-second breath-holds, followed by 10 additional scans at 8-second intervals. Four adjacent 7.2-mm slices were collected at each time point and reconstructed using a B35f kernel.
The flow scan was followed by a helical volume scan of the entire kidney, performed after 0.5 cc/kg of iopamidol (with a maximum dose of 40cc) at 4cc/sec, and followed by 25cc saline at the same rate. Acquisition parameters for the helical DECT scan were detector collimation 64×0.6mm, 0.6 pitch, A and B tubes at 80 kV, 80 quality reference mAs (QRM) and 140 kV, 440 QRM, respectively. Images were reconstructed using a D40f reconstruction kernel. The peak vascular phase was determined using TAC-generated from ROIs manually drawn in both the aorta and renal cortex using software built into the scanner’s workstation (DynEva, Siemens Healthcare).
Plasma levels of total cholesterol, triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were measured at the Immunochemical Core Laboratory (Mayo Clinic, Rochester MN).
Image Processing
Low and high-energy DECT data were reconstructed using 140kV and 80kV on the 64slice scanner or 140kV (Sn) and 100kV on the 128-slice scanner, as per clinical protocol. The DECT imaging protocol was based on the differences between the two different tube potentials (i.e., energy) that are acceptable for iodine material decomposition (19). Helical studies were generated by averaging both tube potentials utilizing a 50% linear blend. Image data were reconstructed with D-kernels dedicated for quantification applications, with a slice thickness of 0.6mm, and loaded into a Siemens workstation for DECT post-processing. As previously described (10), subtraction of iodine signal to create both VNC images and iodine maps was performed using the ‘Liver-VNC’ application in Siemens Syngo.via (Siemens Healthcare). Iodine maps depicted the iodine concentration (mg/ml) within each pixel while VNC maps measure CT number (HU). VNC map measurements were utilized to compare the baseline values of both lean and obese groups (10).
CT Image Analysis
ROIs were drawn on the aorta, cortex, and medulla of helical mixed images, iodine maps, and VNC maps (10). ROIs were chosen at the same tomographic region close to the kidney hilum where perfusion slices were prescribed. This was followed by averaging the subsequent ROI value for both iodine concentration and CT number in 3 to 4 slices where cortex and medulla were readily distinguishable. This resulted in a CT number for both VNC and mixed images, as well as the pure iodine concentration of the kidney. We used the ratios of CT number after background subtraction (kidney tissue/aorta) and iodine concentration (kidney tissue/aorta) to the aortic input as a nominal index of perfusion (contrast uptake), because iodine maps were not readily generated from perfusion scans. Single-kidney perfusion measurement has been similarly quantified in previous studies where the ratio of TAC peak height of tissue and the area under the input function, i.e. aorta (20). Hence, CTRatio describes the ratio between the CT number within the kidney (medulla or cortex) and the CT number of the aorta, whereas IRatio is equivalent to the ratio of tissue iodine concentration (ml/mg) and the iodine concentration (ml/mg) of the aorta (10).
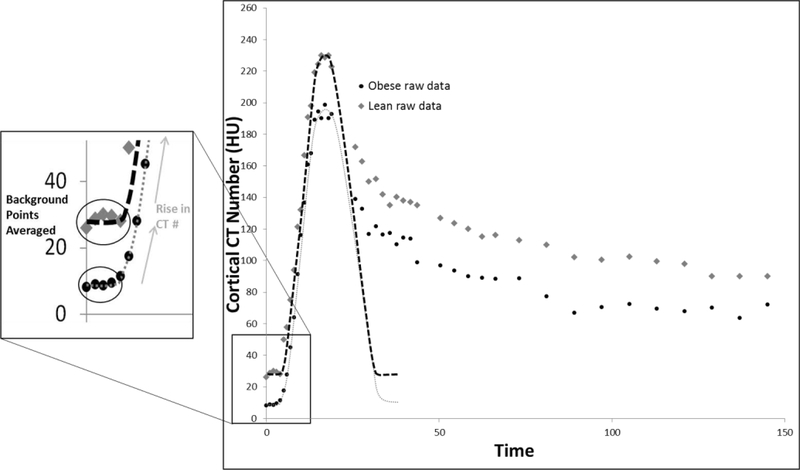
To evaluate kidney volumes, DECT images were reconstructed and then loaded into the Analyze™ software (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN). ROIs were then manually drawn in the medulla and cortex in each slice, and added together to calculate cortical and medullary volumes (7). Renal function was evaluated from TACs produced from ROIs drawn in the renal cortex, medulla, and aorta in perfusion scan images depicting the change in tissue attenuation over time. The TAC was fitted using a Γ-variate model implemented using an in-house Matlab® module (MathWorks, version R2013B) (8, 21, 22). For background subtraction, tissue attenuation was observed in at least three images immediately preceding the arrival of the contrast bolus and was averaged and set to zero (Figure 1). Then, renal perfusion and RBF were assessed from the TAC using the rise in CT numbers (HU) from baseline, as previously described (7, 18). Single-kidney MDCT-derived GFR of the non-stenotic kidney was calculated from the slope of the proximal-tubular curve (7).
Figure 1.
A: Representative time-attenuation curve (TAC) from the cortex in lean and obese patients, demonstrating selection of background points for averaging and background subtraction. The gray diamond (lean) and circular black points (obese) are the raw data points while the black (lean) and gray (obese) dashed lines represent the fitted data after background subtraction.
Visceral and subcutaneous adipose tissue was measured by thresholding for adipose tissue (−30 to −190 CT numbers (HU)) at L3 to L4 regions of the cross-sectional MDCT helical images. The fat ratio was expressed as the ratio of adipose tissue/ total abdominal volume, as previously described (23, 24) (Supplemental Figure 1).
Statistical Analysis
Mean values and standard deviation were used to express results. Two-tailed unpaired t-tests (JMP software 10.0, SAS) were used to compare lean and obese patients. Results were considered statistically significant when p≤0.05.
Results
Obese patients had significantly higher BMI (30.5–34.0 kg/m2) than lean patients (Table 1), as well as higher systolic blood pressure. Of the lean patients, 70% were male and 30% were female. Among the obese subjects, 40% were male and 60% female, with no significance in age difference between the lean and obese groups. Lipids panels were similar among the patients, most of who were taking lipid-lowering medications. Single-kidney volume, hemodynamics, and function did not differ between the groups in either the non-stenotic or stenotic kidneys, although RBF of the stenotic kidney appeared to be higher in Lean due to one outlier kidney with high RBF. (Table 1 and Supplemental Table 1).
Table 1.
Systemic and renal characteristics of the non-stenotic kidney in lean and obese patients with renovascular disease
| Lean | Obese | |
|---|---|---|
| Number of patients/kidneys | 10 | 7 |
| Age | 70.0±10.9 | 73.1±2.3 |
| Sex (M:F) | 7:3 | 2:5 |
| BMI (kg/m2) | 25.7±2.8 | 31.9±1.4* |
| Total cholesterol (mg/dl) | 170.1±40.4 | 187.8±60.2 |
| HDL cholesterol (mg/dl) | 56.0±12.9 | 63.8±34.5 |
| LDL cholesterol (mg/dl) | 90.2±30.8 | 102.7±52.9 |
| Triglycerides (mg/dl) | 133.6±61.4 | 107.0±51.8 |
| Diastolic blood pressure (mmHg) | 68.1±15.4 | 75.6±4.4 |
| Systolic blood pressure (mmHg) | 137.5±13.0 | 151.9±11.5* |
| Mean arterial pressure (mmHg) | 91.2±12.7 | 101±4.9 |
| Lipid-lowering Drugs (% of patients) | 8 (80%) | 7 (100%) |
| Total single kidney volume (cc) | 147.4±41.6 | 142.8±38.4 |
| Cortical Volume (cc) | 91.4±26.4 | 91.1±25.7 |
| Medullary Volume (cc) | 56.0±16.7 | 51.8±15.3 |
| Cortical Perfusion (ml/min/cc) | 2.7±0.9 | 2.8±0.9 |
| Medullary Perfusion (ml/min/cc) | 0.9±0.3 | 0.9±0.3 |
| RBF (ml/min) | 298.4±143.1 | 298.7±133.3 |
| MDCT-single kidney GFR (ml/min) | 55.8±19.1 | 45.4±15.2 |
p≤0.05 vs. Lean. RBF: renal blood flow, GFR: glomerular filtration rate. BMI: body mass index, LDL: low-density-lipoprotein, HDL: high-density-lipoprotein, MDCT: Multi-detector computed tomography
CT Image Analysis- Non-Stenotic Kidney
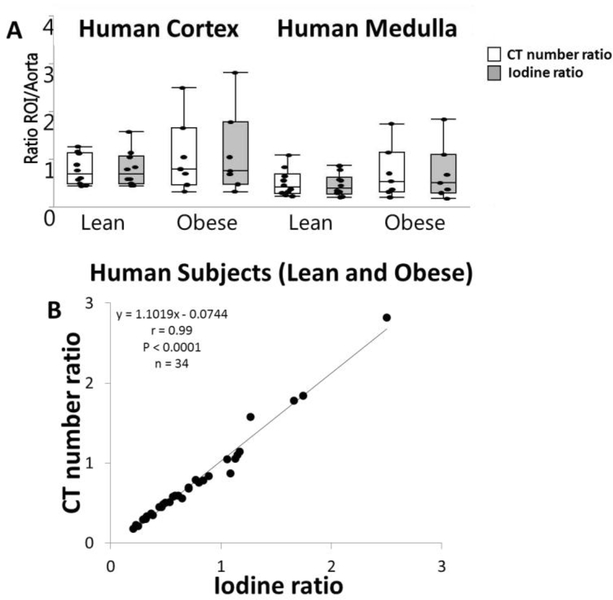
There was no significant difference in IRatio or CTRatio between lean and obese patients within either the cortex or medulla of the non-stenotic kidney (Figure 2A). CTRatio and IRatio were directly correlated (y = 1.102X-0.074,r=0.99, P<0.0001 Figure 2B).
Figure 2.
A. Standard CT number (HU, white) and iodine (mg/ml, gray) kidney/aorta ratios were unchanged in the renal cortex and medulla of obese patients. B. Direct correlations were shown between standard CT number (cortex and medulla) and iodinated kidney/aorta ratios (cortex and medulla) in both lean and obese subjects combined (y = 1.1019X-0.0744, r=0.99, p<0.0001).
Subcutaneous fat was more abundant in obese patients compared to lean, whereas visceral fat did not differ between the groups (Supplemental Figure 1).
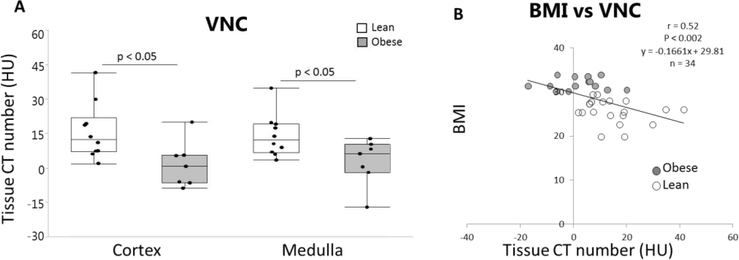
VNC images indicated lower CT numbers in obese subjects compared to lean subjects in both the cortex and medulla (P<0.05) (Figure 3A). VNC in the cortex and medulla of both obese and lean subjects inversely correlated (r=0.52, P<0.002) with all patients’ BMI (Figure 3B).
Figure 3.
A. VNC showed lower cortical and medullary CT numbers (HU) in obese compared to lean patients *p<0.05 vs. lean. B. Inverse correlation between VNC (cortex and medulla) and BMI for all patients (r=0.52, p<0.002).
CT Image Analysis - Stenotic Kidney
There was no significant difference in IRatio or CTRatio between lean and obese patients within either the stenotic kidney cortex or medulla (Supplemental Figure 2A). VNC images exhibited no difference in CT numbers in the cortex of obese compared to lean subjects in either the cortex or medulla (P<0.05) (Supplemental Figure 2B). CTRatio and IRatio were directly correlated (y = 1.038×-0.0438, r=1.0, p<0.0001, Supplemental Figure 2C).
Because the BMI in our Lean group was slightly over 25kg/m2 (the upper limit of normal), we reexamined our data after excluding two patients from the lean group, generating a subgroup of 8 patients with BMI=24.9±2.58kg/m2. In this subgroup, systemic and renal characteristics remained similar to those of the entire group (shown in Table 1), with only BMI and systolic pressure being lower compared to obese patients. Similarly, imaging results in this subgroup were similar to those observed using the entire lean group, including correlation coefficients and statistical significance levels between the groups.
Discussion
In this study, we observed that intrarenal fat deposition does not impede quantification of human single-kidney perfusion using dual-energy MDCT. VNC maps in human subjects consistently revealed lower baseline cortical and medullary attenuation, and VNC from both obese and lean subjects (cortex and medulla) showed an inverse correlation with BMI, consistent with increased renal fat deposition in patients with higher BMI. Nevertheless, lower baseline kidney attenuation did not affect CTRatio or IRatio in obese subjects compared to lean subjects. Furthermore, CTRatio and IRatio correlated directly in patients. These findings extend our previous observations in swine (10) and support the adequacy of background subtraction to account for differences in baseline renal attenuation, yielding reliable assessments of single-kidney function using MDCT in obese subjects.
Obesity is a growing worldwide concern, which often accompanies coexisting disease states and is associated with a heightened risk for atherosclerosis, diabetes, hypertension (5), and chronic kidney disease. Noninvasive measurements of renal function and hemodynamics may be useful to monitor renal disease and its progression in subjects with coexisting obesity. It is especially useful to measure single-kidney perfusion in patients who have unilateral or asymmetrical disease, as traditional methods only measure RBF and GFR of total kidney mass (7).
Assessment of renal function using MDCT requires generation of cortical and medullary TAC (7, 8, 18). This method mandates adequate estimation of baseline tissue attenuation, and measurement of the rise in CT numbers secondary to increased average tissue opacity within a given ROI. We implemented background subtraction by determining the correct contrast delay time to acquire a sufficient number of background points (to subsequently average and then zero the baseline), and by coaching the patient on breath-hold to avoid motion artifacts. In addition, potential underestimation of kidney tissue attenuation due to fat deposits might hamper renal function quantification, as the negative CT numbers values of fat (25) might artificially lower the average ROI attenuation. However, whether the background subtraction method used to depict changes in attenuation above baseline is sufficient to account for renal fat deposition in obese human subjects remains unclear.
We previously utilized a reproducible animal model of early obesity, which exhibits increased deposition of fat in the kidney (26, 27) confirmed by ex-vivo Oil-Red-O staining. We found that VNC maps in obese pigs revealed decreased basal cortical attenuation, yet the CTRatio and IRatio remained similar, suggesting that background subtraction allows for adequate assessment of kidney perfusion in obese pigs using MDCT. However, whether these findings held in obese human subjects remained unknown.
Therefore, to test the relevance of our findings in human subjects, we studied a small group of patients recruited as part of an ongoing study (17) of unilateral renal artery stenosis. To study fat deposition, we focused on the non-stenotic kidney unaffected by decreased RBF. To determine the impact of intrarenal fat deposition, we compared, in similar ROIs, the iodinated contrast-induced increases in iodine concentrations (in iodine maps) vs. increase in total tissue attenuation (CT numbers in mixed images without background), both of which were normalized to concurrent iodine concentration or the CT number in the aorta. We initially examined renal VNC maps, which are post-processed datasets that reveal data on baseline (background) tissue attenuation alone, and which represent a reliable substitute for non-enhanced images (28). The lower VNC-derived CT numbers in the cortex and medulla of obese subjects are consistent with the presence of intra-renal fat, which tends to lower the average HU value in the ROI. Furthermore, within the entire cohort, VNC and BMI showed a moderate inverse correlation that was congruent with a direct correlation between triglycerides content and BMI observed in human kidneys (11). Contrarily, correlations were not observed within the individual groups, likely due to the small range of BMI values in our sample. Obesity was also evident in elevated CT-derived subcutaneous fat in obese patients. Nevertheless, despite the evident obesity and renal fat deposition, intrarenal fat did not interfere with CT measurements. We found that despite the apparent renal fat deposition suggested by lower cortical and medullary background attenuation, IRatio was similar to CTRatio in the medulla and cortex of the obese groups and exhibited a strong positive correlation in obese and lean patients.
Furthermore, we also examined at CTRatio and IRatio in the stenotic kidney of both the obese and lean patients. Our findings in the stenotic kidneys closely replicated those in the non-stenotic kidney, as CTRatio and IRatio were similar, with no difference between obese and lean groups in either the cortex or the medulla, and with a direct correlation between CTRatio and IRatio. However, unlike non-stenotic kidneys, we observed no difference in VNC background values. This is likely due to lower perfusion in the stenotic kidney, which comparably to fat deposits, decreases CT numbers. Collectively, this suggest that the background subtraction method is adequate even in varying degrees of renovascular disease, although it should be validated in further studies with larger patient cohorts.
Limitations
Our study is limited by the small number of patients enrolled in the study. Systemic lipid levels were not elevated, likely because the majority of patients were treated with lipid-lowering medications. However, given that obese patients exhibited higher BMI and adiposity, a decrease in circulating cholesterol levels clearly does not rule out the deposition of tissue fat. The BMI in our lean group was marginally higher than normal limits (18.5–24.958kg/m2), yet subgroup analysis including lean subjects with a mean BMI<25kg/m2 showed similar statistical significance levels compared to the obese group, underscoring the validity of our comparisons. The obesity in our cohort was significant but mild, and we cannot rule out the possibility that far greater renal fat retention would ultimately impact CTRatio. While unlikely, the use of a non-stenotic kidney or the slightly higher blood pressure in obese subjects might have affected our assessments. In addition, current studies are attempting to develop methods to derive iodine maps directly from perfusion scans to generate iodine (rather than attenuation)-based indicatordilution curves for a more direct comparison with CT-number TAC. Moreover, unavailability of human tissue samples precluded direct demonstration of kidney fat deposits, but this has been previously validated in animals (10). Lastly, it is important to know that estimation of renal function with MDCT is currently confined to the research arena, given the use of iodinated contrast agents and high radiation exposure (total effective dose of 26 mSv in men and 27 mSv in women, including localization and volume scans). Nevertheless, recent developments allow substantial decreases in radiation dose (29).
Conclusion
In summary, we found that CTRatio and IRatio were comparable in mildly obese human subjects, suggesting that intrarenal adiposity does not impact quantification of single-kidney function using MDCT-derived TAC in these patients. Therefore, the method of background subtraction used for CT renal function assessment is sufficient for eliminating the potentially confounding attenuation of intrarenal fat. Taken together, these observations support the use of background subtraction assessment of dynamic changes in iodinated contrast concentration in kidneys of obese human subjects.
Supplementary Material
Acknowledgments
Funding:
This study was partly supported by National Institutes of Health Grants Numbers DK104273, DK100081, HL123160, DK102325, and C06 RR018898.
Footnotes
Conflict of interest: Dr. McCollough: Grant recipient, Siemens Healthcare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang T, Wang Z, Proctor G, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005; 280(37):32317–25. [DOI] [PubMed] [Google Scholar]
- 2.Kume S, Uzu T, Araki S, et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol. 2007; 18(10):2715–23. [DOI] [PubMed] [Google Scholar]
- 3.Keane WF, Kasiske BL, O’Donnell MP, Kim Y. The role of altered lipid metabolism in the progression of renal disease: experimental evidence. Am J Kidney Dis. 1991; 17(5 Suppl 1):38–42. [PubMed] [Google Scholar]
- 4.Romero JC, Lerman LO. Novel noninvasive techniques for studying renal function in man. Semin Nephrol. 2000; 20(5):456–62. [PubMed] [Google Scholar]
- 5.Li Z, Woollard JR, Wang S, et al. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol. 2011; 301(5):F1078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagby SP. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol. 2004; 15(11):2775–91. [DOI] [PubMed] [Google Scholar]
- 7.Kwon SH, Saad A, Herrmann SM, Textor SC, Lerman LO. Determination of Single-Kidney Glomerular Filtration Rate in Human Subjects by Using CT. Radiology. 2015; 276(2):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007; 243(2):405–12. [DOI] [PubMed] [Google Scholar]
- 9.Kim WH, Kim CG, Kim DW. Optimal CT Number Range for Adipose Tissue When Determining Lean Body Mass in Whole-Body F-18 FDG PET/CT Studies. Nucl Med Mol Imaging. 2012; 46(4):294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson CM, Eirin A, Michalak GJ, et al. Intrarenal fat deposition does not interfere with the measurement of single-kidney perfusion in obese swine using multi-detector computed tomography. J Cardiovasc Comput Tomogr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobulescu IA, Lotan Y, Zhang J, et al. Triglycerides in the human kidney cortex: relationship with body size. PLoS One. 2014; 9(8):e101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patino M, Prochowski A, Agrawal MD, et al. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics. 2016; 36(4):1087–105. [DOI] [PubMed] [Google Scholar]
- 13.Marin D, Boll DT, Mileto A, Nelson RC. State of the art: dual-energy CT of the abdomen. Radiology. 2014; 271(2):327–42. [DOI] [PubMed] [Google Scholar]
- 14.McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology. 2015; 276(3):637–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson TR, Krauss B, Sedlmair M, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007; 17(6):1510–7. [DOI] [PubMed] [Google Scholar]
- 16.Toepker M, Moritz T, Krauss B, et al. Virtual non-contrast in second-generation, dual-energy computed tomography: reliability of attenuation values. Eur J Radiol. 2012; 81(3):e398–405. [DOI] [PubMed] [Google Scholar]
- 17.Saad A, Dietz AB, Herrmann SMS, et al. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J Am Soc Nephrol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daghini E, Juillard L, Haas JA, Krier JD, Romero JC, Lerman LO. Comparison of mathematic models for assessment of glomerular filtration rate with electron-beam CT in pigs. Radiology. 2007; 242(2):417–24. [DOI] [PubMed] [Google Scholar]
- 19.Primak AN, Ramirez Giraldo JC, Liu X, Yu L, McCollough CH. Improved dual-energy material discrimination for dual-source CT by means of additional spectral filtration. Med Phys. 2009; 36(4):135969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerman LO, Bell MR, Lahera V, et al. Quantification of global and regional renal blood flow with electron beam computed tomography. Am J Hypertens. 1994; 7(9 Pt 1):829–37. [DOI] [PubMed] [Google Scholar]
- 21.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002; 106(9):1165–71. [DOI] [PubMed] [Google Scholar]
- 22.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001; 281(4):F630–8. [DOI] [PubMed] [Google Scholar]
- 23.Kim SR, Lerman LO. Diagnostic imaging in the management of patients with metabolic syndrome. Transl Res. 2018; 194:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onuma T, Kamishima T, Sasaki T, Sakata M. Absolute reliability of adipose tissue volume measurement by computed tomography: application of low-dose scan and minimal detectable change--a phantom study. Radiol Phys Technol. 2015; 8(2):312–9. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez RE, Macovski A. Energy-selective reconstructions in X-ray computerized tomography. Phys Med Biol. 1976; 21(5):733–44. [DOI] [PubMed] [Google Scholar]
- 26.Pawar AS, Zhu XY, Eirin A, et al. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring). 2015; 23(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eirin A, Woollard JR, Ferguson CM, et al. The metabolic syndrome induces early changes in the swine renal medullary mitochondria. Transl Res. 2017; 184:45–56 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau D, Yang H, Kei PL. Dual-energy 4-phase CT scan in primary hyperparathyroidism. AJNR Am J Neuroradiol. 2013; 34(8):E91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Primak AN, Krier JD, Yu L, Lerman LO, McCollough CH. Renal perfusion and hemodynamics: accurate in vivo determination at CT with a 10-fold decrease in radiation dose and HYPR noise reduction. Radiology. 2009; 253(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.