Abstract
The optimal oxygenation target needed to prevent the extremes of hypoxia and oxygen toxicity in premature and sick newborns has been the subject of much research and debate. The advent of the pulse oximeter has allowed the continuous monitoring of oxyhemoglobin saturation and the delivery of oxygen with greater precision. Well-run, large clinical trials to determine the safest oxygen concentration have led to several revisions in guidelines for neonatal care. However, monitoring of oxyhemoglobin saturation has its limitations and does not provide a comprehensive assessment of tissue oxygenation. To identify optimal oxygen therapy, various other factors (partial pressure of arterial carbon dioxide, hemoglobin concentration, blood pH, and tissue metabolic demand) that influence perfusion and tissue oxygenation need to be considered.
Keywords: oxygen saturation, newborn, oxygen target
Résumé:
L’oxygénation cible optimale nécessaire en vue de prévenir les situations extrêmes comme l’hypoxie et la toxicité à l’oxygène chez les nouveau-nés prématurés et malades a fait l’objet de beaucoup de recherches et de débats. L’avènement de l’oxymétrie de pouls a donné le moyen de surveiller la saturation en oxyhémoglobine en continu et d’administrer de l’oxygène avec une plus grande précision. De vastes essais cliniques bien réalisés en vue d’établir la concentration d’oxygène la plus sécuritaire ont mené à plusieurs révisions des lignes directrices pour les soins en néonatalogie. Cependant, la surveillance de la saturation en oxyhémoglobine en continu ses propres limites, et ne permet pas d’obtenir une évaluation intégrale de l’oxygénation tissulaire. En vue d’établir le traitement par l’oxygène optimal, il convient de prendre en compte divers autres facteurs qui influencent la perfusion et l’oxygénation tissulaire (pression partielle du sang artériel en dioxyde de carbone, concentration d’hémoglobine, pH sanguin et demande métabolique tissulaire). [Traduit par la Rédaction]
Keywords: saturation en oxygène, nouveau-né, oxygénation cible
Introduction
Oxygen therapy is a life-sustaining intervention and remains the most commonly used drug in the care of sick newborns (Vento and Saugstad 2010). In the 1940s and 1950s, flooding the newly designed incubators with 100% oxygen contributed to the survival of some premature infants. The first clinical reports, dating back to the early 1950s, linked unrestricted use of oxygen with an increased risk of retrolental fibroplasia (now known as retinopathy of prematurity) (Campbell 1951) and prompted a change in neonatal care practice to restrict oxygen use. The resultant increase in mortality (Bolton and Cross 1974) was the first evidence of the central importance of adequate but not excessive oxygen delivery to the premature infant. New evidence from the same period suggested that oxygen can be toxic at the cellular level by generating free radicals (Gerschman et al. 1954). Over the ensuing decades, research has yielded an understanding that the immature antioxidant defense in newborns (particularly premature infants) predisposes this population to higher oxidative stress and cell damage (Vento et al. 2012; Torres-Cuevas et al. 2017). Even brief exposure to excessive oxygen used in the resuscitation of newborns in the delivery room may lead to an increased risk of childhood leukemia (Naumburg et al. 2002) as well as adverse physiological adaptation at birth (Comroe 1939; Hutchison 1987; Saugstad et al. 2008).
While the deleterious effects of administering high concentrations of oxygen to newborns in respiratory distress became apparent over half a century ago, determining the optimal fraction of inspired oxygen (FIO2) that would prevent hypoxemia was challenging in the early decades of neonatology, as no reliable method to continuously measure oxygen concentrations in the blood was available. In the 1980s, pulse oximetry measurement of oxyhemoglobin saturation provided a reliable and easier means to continuously monitor oxygenation compared with transcutaneous oxygen tension measurements (Durand and Ramanathan 1986). Soon thereafter, it became the standard of care in neonatal intensive care units (NICU) (Van Meter et al. 2017).
Recognizing that a large number of neonates admitted to the NICU receive supplemental oxygen, there has been tremendous interest in determining the safest oxyhemoglobin saturation that would ensure adequate tissue oxygenation while preventing oxygen toxicity. With growing evidence that restricted use of oxygen decreases the incidence of retinopathy of prematurity and bronchopulmonary dysplasia, the American Academy of Pediatrics stated in 2007 that an oxyhemoglobin saturation between 85% and 95% provides a pragmatic range to guide oxygen therapy (American Academy of Pediatrics and the American College of Obstetricians and Gynecologists 2007). In 2010, the International Liaison Committee on Resuscitation (ILCOR) revised their recommendations to initiate resuscitation of term newborns in room air (Perlman et al. 2010), whereas the optimal FIO2 in the resuscitation of preterm infants remains controversial (Rabi et al. 2015; Kapadia et al. 2017; Oei et al. 2017; Lui et al. 2018). The safest saturation target for this subpopulation of NICU babies continues to generate considerable debate (Sola et al. 2014; Cummings et al. 2016). Through a collaborative effort by the Neonatal Oxygenation Prospective Meta-Analysis (NeOProM) study group (Askie et al. 2011), 5 randomized trials involving a total of ~5000 premature infants examined lower (85%–89%) and higher (91%–95%) saturation targets; unfortunately, the study yielded conflicting and controversial conclusions (Carlo et al. 2010; Schmidt et al. 2013, 2017; Stenson et al. 2013; Manja et al. 2015a; Tarnow-Mordi et al. 2016; Saugstad 2018). In addition, the optimal oxygen target in term neonates with hypoxic respiratory failure or newborns undergoing hypothermia therapy remains unknown and warrants further study. Table 1 summarizes the suggested oxygen therapy in newborns (Oei et al. 2018).
Table 1.
Suggested oxygen therapy in the delivery room and during neonatal intensive care unit (NICU) stay.
| Gestational age |
Delivery room: initial FIO2 | NICU stay: SpO2 target |
|---|---|---|
| <28 weeks | 0.3* | 91%–95% |
| 28–31 weeks | 0.21–0.3* | |
| ≥32 weeks | 0.21* |
Note: FIO2, fraction of inspired oxygen; SpO2, oxyhemoglobin saturation as measured by pulse oximeter (Oei et al.2018).
Titrate FIO2 to Neonatal Resuscitation Program (NRP) target SpO2.
While the perfect oxygen saturation ranges for term and preterm infants remain uncertain, here we review data regarding the larger questions of the precision and accuracy of the current technology and the limitations of arterial oxygen saturation as a single marker of tissue oxygenation. Several common practices remain unjustified in the literature, including the tendency to more readily increase FIO2 during hypoxic events while taking a more lax approach to decreasing FIO2 when oxygen saturation is high; the continued use of the hyperoxia test in an era of readily available echocardiography; and preoxygenation (prior to intubation) in neonates to prevent oxygen debt (Sola 2008; Sola et al. 2014).
The fetus and basic concepts of oxygen physiology
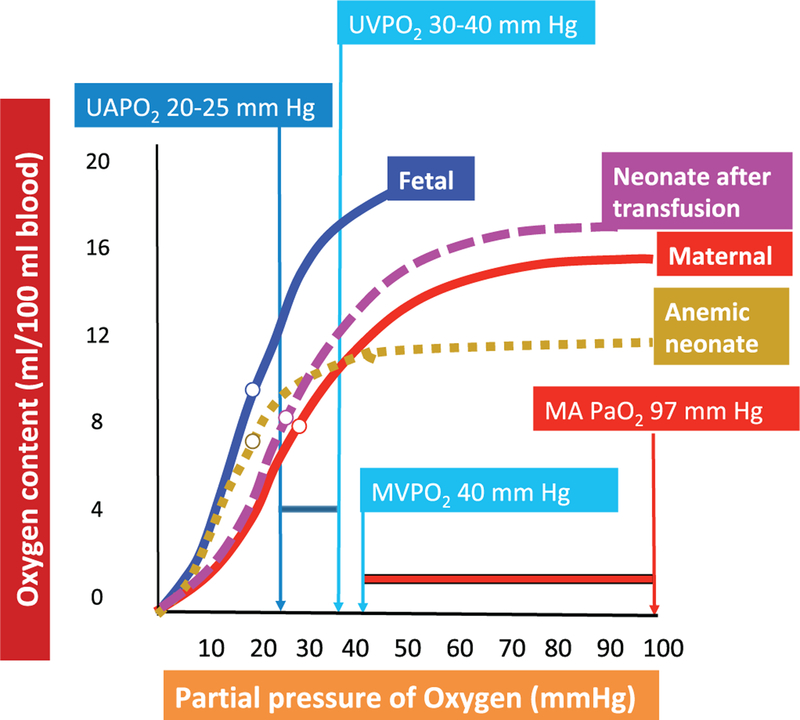
Aerobic metabolism and maintenance of satisfactory cellular homeostasis are dependent on adequate oxygen delivery to and extraction by the tissues; only when this demand cannot be met does hypoxia ensue. Oxygen delivery, in turn, depends on oxygen content in the blood — influenced primarily by the concentration and percent saturation of hemoglobin — and cardiac output. Important considerations in the fetus include the higher affinity for oxygen in fetal hemoglobin (Hgb F), high Hgb concentrations in term fetuses, and higher cardiac output, factors that provide a substantial margin of safety for oxygenation (delivery is 3 times higher than demand). In addition, properties of Hgb F facilitate unbinding of oxygen in the tissues at lower partial pressures of arterial oxygen (PaO2) (Maurer et al. 1970). Therefore, Hgb F has the ability to more efficiently bind oxygen as the fetal blood circulates through the placenta and to more readily relinquish it within the tissues (Fig. 1).
Fig. 1.
Oxyhemoglobin dissociation curve (CaO2 versus PaO2). The higher oxygen affinity of fetal hemoglobin (blue curve) shifts the oxygen dissociation curve to the left, which results in a greater release in oxygen at a lower partial pressure of oxygen (PO2) compared with adult hemoglobin (red curve). Only a small change in PO2 (≈10 mm Hg) from the higher oxygenated fetal venous blood (30–40 mm Hg) to the arterial blood (20–30 mm Hg) results in a release of ≈25% of oxygen to the tissues. In the pregnant woman, however, a similar release in oxygen from adult hemoglobin requires a drop from the arterial PO2 (97 mm Hg) to the venous PO2 (40 mm Hg) of 57 mm Hg. In a neonate, anemia (hyphenated orange curve) is associated with lower CaO2 without any change in P50 (partial pressure of oxygen resulting in 50% oxygen saturation or 50% peak CaO2). P50 is shown as an open circle along the oxygen–hemoglobin dissociation curve. Transfusion with packed red cells from an adult source (with hemoglobin (Hgb) A) results in increased CaO2 and also an increase in P50 towards adult values. MAPO2, maternal arterial PO2; MVPO2, maternal venous PO2; UAPO2, umbilical artery PO2; UVPO2, umbilical vein PO2. Data from Rudolph (2009). Note that the oxyhemoglobin dissociation curve is often presented with SaO2 on the y-axis; we opted to present these data with CaO2 on the y-axis to more clearly demonstrate the impact of blood transfusion.
Several physiological adaptations of the placenta and developing fetus ensure that the fetus is exposed to a relatively hypoxemic environment. Through an elaborate system of shunts (ductus venosus, ductus arteriosus, foramen ovale) and distribution of blood flow, the fetal circulation diverts the richest concentration of oxygenated blood to the most essential organs (the brain and heart) while minimizing blood flow to the lungs. The coronary and cerebral artery oxygen saturations in the fetus range between ≈58% and 65% (PaO2: 25–28 mm Hg) (Rudolph 1979, 2009; Prsa et al. 2014).
While PaO2 has a minimal impact on the oxygen content of blood, the fraction of oxygen dissolved in blood plays an essential role in the physiology of ventilation and cardiovascular regulation (Giussani et al. 2016). The oxygen tension in blood can exert its effect directly through a complex cascade of endogenous mediators that act directly on the vascular smooth muscle cells and by triggering chemoreflexes through the activation of chemoreceptors (Smith and Vane 1966; Ponte and Purves 1974; Giussani 2016). PaO2 has opposing effects in the systemic and pulmonary vasculature. Hypoxia results in increased cerebral blood flow (through cerebral vasodilation) but decreased pulmonary blood flow (through pulmonary vasoconstriction), while hyperoxia leads to cerebral artery vasoconstriction (Wolff 1936; Rudolph and Yuan 1966). Normoxia results in pulmonary vasodilation, but hyperoxia does not cause additional pulmonary vasodilation (Lakshminrusimha et al. 2006).
The amount of blood pumped into the pulmonary circulation is dynamic and changes during fetal development. In a clinical trial, providing 60% oxygen by face mask to pregnant women at 20–26 weeks gestation did not alter fetal pulmonary blood flow, whereas an increase in pulmonary blood flow was appreciated at 31–36 weeks gestation (Rasanen et al. 1998). Early in gestation, the cross-sectional pulmonary vasculature is low, maintaining a high pulmonary vascular resistance (PVR), and the lungs receive only ~13% of the cardiac output at 20 weeks gestation, which increases to 25%–30% at 30 weeks gestation owing to the proliferation of pulmonary vessels with a resultant drop in PVR. Cardiac output to the lungs then drops to 16%–21% near term gestation in response to active hypoxic pulmonary vasoconstriction secondary to the pulmonary vessels developing greater sensitivity to oxygen (Kinsella et al. 1994; Rudolph 2009; Rasanen et al. 1996; Prsa et al. 2014). Therefore, the effect of oxygen varies depending on the gestational age of the infant.
Oxyhemoglobin saturation, oxygen tension, and oxyhemoglobin dissociation equilibrium
Several important discoveries — including the discovery of oxygen in 1772 and hemoglobin in 1840, the demonstration in the 1850s that hemoglobin exists in 2 states (oxygenated and deoxygenated), and the development of analytical spectroscopy and the capacity to detect pulsatile (arterial) blood flow — all contributed to the development of pulse oximeters in the early 1980s (Van Meter et al. 2017). Soon thereafter, owing to its safety profile, its noninvasive nature, and the ability to continuously monitor oxygenation, the use of pulse oximeters became widespread in NICUs across the United States (Vijayakumar et al. 1997).
The differential light absorption of oxyhemoglobin (red spectrum of visible light) and deoxyhemoglobin (infrared spectrum of light) and the distinction between pulsatile and nonpulsatile flow constitute the principles of pulse oximetry. The sensor, which in newborns is applied to an extremity, contains 2 light-emitting diodes (LEDs), infrared and red light, that transmit light through the extremity and are received by a phototransistor on the opposite side of the LEDs. Pulsatile blood flow results in fluctuations in blood volume, thus changing the distance the light travels. The pulsatile component of the red to infrared light modulation ratio is calculated, and a microprocessor with built-in algorithms converts this ratio to pulse oxygen saturation (SpO2) based on a calibration curve (Chan et al. 2013; Tin and Lal 2015). Pulse oximeters are calibrated by data collected from healthy adults and correlated to arterial blood samples tested by co-oximetry (absorbance spectroscopy) that measures actual arterial oxyhemoglobin saturation (SaO2). Hgb F has an absorption comparable to that of predominant adult Hgb A, and therefore SpO2 measurement in newborns does not require a separate calibration (Harris et al. 1988; Rajadurai et al. 1992).
Hgb functions to carry blood to the tissues, and its configuration changes depending on whether it is in the deoxygenated (also known as tense) or oxygenated (relaxed) state. Once oxygen binds the protein, an alteration in its configuration facilitates further oxygen binding. Conversely, as oxygen is released from Hgb, a change in configuration towards the tense state enables unloading of oxygen (Bell 1999). This ability of Hgb to alter its structure promotes the binding and unloading of oxygen. In addition, the affinity of oxygen to bind Hgb depends on the partial pressure of oxygen in the blood. At higher PO2 (as in the pulmonary circulation), oxygen freely binds Hgb until it is fully saturated, whereas at the level of the tissues, oxygen is quickly released. This relationship can be better appreciated when plotting PaO2 against SaO2/SpO2 (or CaO2: arterial oxygen content), revealing the characteristic oxyhemoglobin dissociation curve (Fig. 1).
In a study to identify a better marker of oxygenation between oxyhemoglobin saturation (SaO2) and oxygen tension (PaO2) in hypoxemic patients, Ahmed et al. made strong arguments in favor of SaO2 (Ahmed et al. 2017). Over 70 000 arterial blood gases collected from pediatric patients with cyanotic congenital heart disease were analyzed, and PaO2 was plotted against SaO2. Owing to the nonlinear relationship between PaO2 and SaO2, a narrow range of PaO2 (30–35 mm Hg) in the hypoxemic range corresponds to a wide range of SaO2 values (28%–85%). In summary, since (i) SaO2 predominantly determines oxygen content and (ii) in hypoxemia the magnitude of SaO2 changes is greater than that of PaO2 changes, SaO2 serves as the better marker of oxygenation. However, as Hgb becomes fully saturated, any further rise in PaO2 does not alter SaO2 (representing the plateau in the oxyhemoglobin dissociation curve). Therefore, patients who receive supplemental oxygen and have SpO2 values in the high 90s may reach supraphysiological PaO2 values (>100 mm Hg), putting them at risk of oxygen toxicity.
An important consideration, particularly in extremely premature infants, is the effect blood transfusions have on the PaO2–SaO2 relationship (Fig. 1). Hgb F, the predominant hemoglobin in newborns, has a high oxygen affinity and, as a result, a lower P50 (partial pressure of oxygen at which 50% of Hgb is saturated by oxygen) of ≈18–19 mm Hg, whereas adult Hgb A has a P50 of ≈26–27 mm Hg (Emond et al. 1993; Maurer et al. 1970). Following transfusion of packed red blood cells (≈27 mL/kg) in extremely premature infants, Hgb F dropped from a mean baseline of 92% to 43%, while the value of P50 increased from 18.5 to 21 mm Hg (Fig. 1) (De Halleux et al. 2002). Consequently, if SpO2 were maintained at a constant value following adult blood transfusion, the newborn’s post-transfusion PaO2 would be higher. Therefore, in premature infants, SpO2 values in the upper 90s following several adult blood transfusions pose an even greater risk for oxygen toxicity, as PaO2 may approach values >100 mm Hg (Fig. 2) (Shiao 2005).
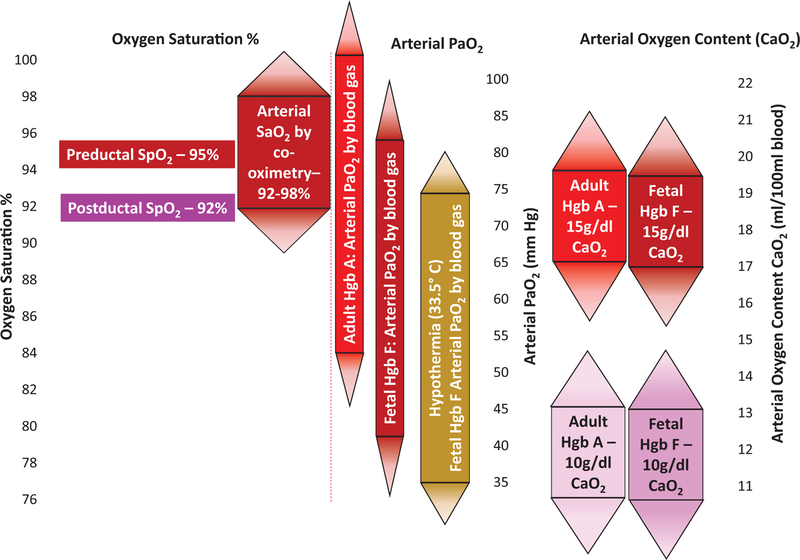
Fig. 2.
Relationship between oxyhemoglobin saturation, arterial partial pressure of oxygen (PaO2), and arterial oxygen content (CaO2). Oxyhemoglobin saturation measured by pulse oximeter (SpO2) has an accuracy of 3% when compared with the true arterial oxyhemoglobin saturation (SaO2) by co-oximetry (1 standard deviation (SD) = 3%; rectangular area represents 1 SD, and thus 68% of values; triangular areas represent 2 SD, so 95% of values). An SpO2 value of 95% can therefore represent an SaO2 between 92% and 98%. This represents a wide range in PaO2, and since adult hemoglobin (Hgb A) has a lower affinity for oxygen, for any given SaO2, the PaO2 is higher in blood containing Hgb A. Note the impact of hemoglobin on oxygen content (CaO2).
SpO2 and SaO2: pulse oximeter limitations and clinical implications
SpO2 monitoring is ubiquitous in neonatal care. All healthy term and late preterm infants are screened for critical congenital heart disease with pulse oximetry (Ewer et al. 2013; Manja et al. 2015b). Most neonatal patients admitted to the NICU have their SpO2 measured as soon as they are born. ILCOR recommends monitoring SpO2 in the delivery room for infants in need of respiratory support. While the pulse oximeter has proven to be an indispensable technology in the care of premature and sick newborns, clinicians need to be cognizant of its limitations.
Pulse oximeters do not measure oxyhemoglobin saturations directly but generate an SpO2 value using an algorithm generated from SaO2 samples. One of the most widely used pulse oximeters, Masimo Radical 7, established the accuracy of its algorithm in neonates using 79 samples collected from 16 neonates over an SpO2 range of 70%–100% (Lakshminrusimha et al. 2015a). The algorithm initially merged 2 curves (one derived from the higher saturation range and the other from the lower saturation range) that inadvertently generated higher SpO2 values by 2% in the range of 87%–90%, where the 2 curves merged (Johnston et al. 2011). The algorithm has since been revised to include a single curve. This change in algorithm had an impact on results obtained from the BOOST-II trial in the UK and Australia (Tarnow-Mordi et al. 2016).
Pulse oximeters have been shown to have an accuracy of ~3%, which represents 1 standard deviation (SD) (Johnston et al. 2011; Milner and Mathews 2012). Therefore, a displayed SpO2 of 90% may represent a true SaO2 anywhere from 87% to 93% in 68% of cases, but may fall outside the range of 84%–96% in 5% of patients. Similarly, an SpO2 of 95% may represent a true SaO2 between 92% and 98% in 68% of cases, and results in hyperoxia (with SaO2 at 99% and 100%) in 16% of cases (Fig. 2). Furthermore, in a study of preterm infants comparing postductal SpO2 to umbilical artery SaO2, SpO2 between 85% and 89% was associated with an SaO2 <85% in 39% of samples (Rosychuk et al. 2012). Also, in critically ill adults, changes in SpO2 have been shown to overestimate changes in SaO2, and this discrepancy worsened at lower Hgb concentrations (Perkins et al. 2003). As noted previously, SaO2 is associated with a wide range of PaO2 values depending on the oxyhemoglobin dissociation curve (Figs. 1 and 2). A shift to the left (as seen with Hgb F, hypothermia, and alkalosis) will result in lower PaO2 for a given SaO2 and can potentially influence vascular regulation (such as PVR). However, as dissolved oxygen contributes to a very small component of the oxygen content in arterial blood (CaO2), this value is influenced mainly by Hgb content and SaO2 (Fig. 2). Finally, to avoid erratic responses to aberrant signals, pulse oximeters do not display instantaneous readings, but rather use time-averaging to smooth out the signal. The default manufacturer time-averaging is set at 8 s, which may be adjusted to between 2 and 32 s. Longer averaging times reduce the detection of brief or severe desaturations (Ahmed et al. 2010; Vagedes et al. 2013), while short averaging times run the risk of worsening alarm fatigue (Johnson et al. 2017). However, for the purpose of SpO2 monitoring in the delivery room, shorter averaging times may lead to quicker adjustments in FIO2 in an attempt to prevent hyperoxemia.
While oxyhemoglobin saturation is currently the best marker to assess oxygenation, various other factors, including the partial pressure of arterial carbon dioxide (PaCO2), Hgb concentration, blood pH, and tissue metabolic demand, play a crucial role in perfusion and tissue oxygenation (Fig. 3). Attempting to identify the optimal SpO2 while not concurrently evaluating PaCO2 and pH values, which are known to significantly alter cerebral and pulmonary blood flow (Rudolph and Yuan 1966; Yoon et al. 2012), may paint only part of the bigger picture. Continuous CO2 monitoring by means of capnography and (or) transcutaneous monitors is a helpful surrogate for this information with less frequent blood gas monitoring. Near-infrared spectroscopy (NIRS) is a newer technology that differs from pulse oximetry in that near-infrared light is reflected from Hgb in tissues and capillaries and returns to the sensor to provide a continuous noninvasive estimate of tissue oxygenation, thus serving as a surrogate marker for tissue oxygen consumption. The distance between the light source and the sensor determines the depth of the tissue interrogated. With careful selection of an appropriate probe, regional tissue saturation can be measured in organs such as the brain, kidney, liver, intestine, and skeletal muscle, while automated oxygen-controlling systems may help to maintain SpO2 in a target range and thus assist in achieving an optimal FIO2 (Claure and Bancalari 2013; Sood et al. 2015; Waitz et al. 2015); this advance is not designed to incorporate the additional factors noted previously.
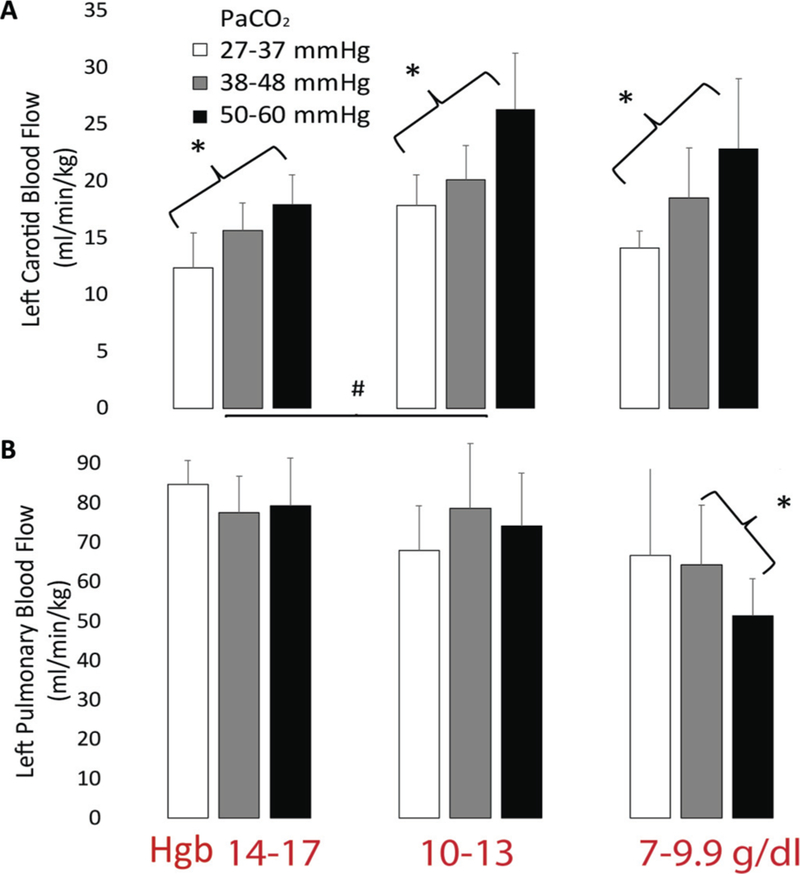
Fig. 3.
Effect of hemoglobin (Hgb) and arterial partial pressure of carbon dioxide (PaCO2) on pulmonary and left carotid blood flow. Pooled data from 15 lambs sorted by Hgb and PaCO2 showing changes in carotid (A) and pulmonary (B) blood flow. Carotid blood flow is more sensitive than pulmonary blood flow to changes in PaCO2. *, P < 0.05 by analysis of variance (ANOVA) post hoc test with change in PaCO2; #, P < 0.05 between Hgb groups.
In experimental studies in our laboratory, 15 term lambs with meconium aspiration syndrome Lakshminrusimha et al. 2015b; Rawat et al. 2016) were ventilated with different Hgb concentrations, and ventilator parameters were altered to adjust PaCO2. A drop in Hgb concentration from 14–17 g/dL to 10–13 g/dL resulted in an increase in carotid blood flow. Further reduction in Hgb to 7–9.9 g/dL did not alter carotid flow. A rise in PaCO2 significantly increased carotid blood flow at all levels of Hgb. Interestingly, Hgb did not alter pulmonary flow, albeit an increase in PaCO2 from 38–48 mm Hg to 50–60 mm Hg decreased pulmonary flow only in the severely anemic lambs (Hgb of 7–9.9 g/dL). In the Hgb range of 10–17 g/dL, cerebral blood flow appears to be more sensitive to PaCO2 than pulmonary blood flow (Fig. 3). Hence, mild hypercapnia may promote oxygen delivery to the brain without markedly increasing PVR and may be an effective strategy in the management of conditions such as congenital diaphragmatic hernia and persistent pulmonary hypertension of the newborn (in the absence of anemia) (Gupta et al. 2002; Puligandla et al. 2015).
Goals of oxygen therapy
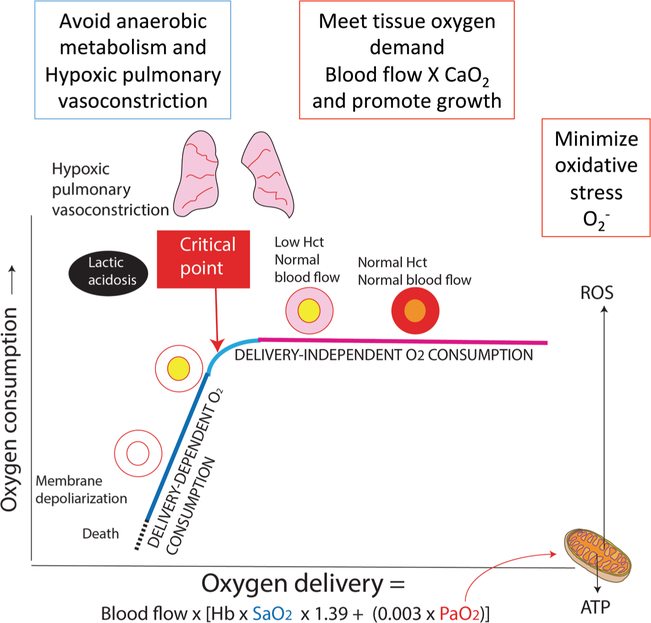
The role of oxygen therapy in hypoxemia is to (i) provide sufficient oxygen to the tissues, thus avoiding anaerobic metabolism and lactic acidosis; (ii) prevent hypoxic pulmonary vasoconstriction, which would further exacerbate hypoxemia and lead to hypoxia; and (iii) promote brain and somatic growth. The ideal oxygen therapy would meet these goals while minimizing formation of oxygen free radicals (Fig. 4).
Fig. 4.
Relationship between oxygen delivery (DO2) and oxygen consumption (VO2). DO2 is a product of blood flow and arterial oxygen content (CaO2). The driving force for oxygen from alveoli to mitochondria is the partial pressure of oxygen (PO2). Increased mitochondrial PO2 can lead to formation of reactive oxygen species (ROS). When DO2 decreases below a critical point, VO2 becomes dependent on delivery. Hypoxemia leads to hypoxic pulmonary vasoconstriction, anaerobic metabolism, and lactic acidosis. Persistent hypoxemia can result in cell death.
In determining the lower oxygenation limit, 2 main factors need to be considered: (i) the oxygen level when hypoxic PVR becomes significant and (ii) the critical point when oxygen delivery (DO2) cannot sustain oxygen consumption (VO2). The alveolar partial pressure of oxygen (PAO2) has the greatest impact on pulmonary vascular reactivity (Moudgil et al. 2005). However, as PAO2 is not directly measured in clinical settings, PaO2 and SpO2 cutoffs have been evaluated in newborn animal models, which have shown a significant rise in PVR below a PaO2 of ≈45–50 mm Hg (Rudolph and Yuan 1966; Lakshminrusimha et al. 2009); in a lamb model of pulmonary hypertension induced by fetal ductus arteriosus ligation, maintaining SpO2 between 90% and 97% resulted in low PVR (Lakshminrusimha et al. 2009). The oxygenation target to identify the critical point below which VO2 decreases as DO2 decreases is more difficult to ascertain given that Hgb concentration has a greater impact on arterial oxygen content (CaO2) than oxyhemoglobin saturation, and because there are currently no reliable means to measure cardiac output continuously. Unpublished pooled data from newborn lamb experiments identified the CaO2 at ≈12 mL O2/mL when there is a notable reduction in the arteriovenous difference in oxygen. Once DO2 falls below the critical point, anaerobic metabolism and lactic acidosis ensue, consistent with tissue hypoxia. For example, a patient with a Hgb concentration of 10 g/dL who has an SpO2 of 85% (CaO2 ≈ 11.5 mL O2/mL) may fall below the critical point if cardiac output is low. An SpO2 of 93% under the same conditions would yield a CaO2 of ≈12.5 mL O2/mL, whereas an increase in Hgb to 13 g/dL (following a blood transfusion) would increase the CaO2 to ≈16 mL O2/mL. One needs to be mindful, however, that as Hgb drops, a compensatory increase in cardiac output can maintain oxygen delivery and thus prevent reaching of the critical point. An ongoing study funded by the NICHD (through the neonatal research network) and the NHLBI, the Transfusion of Prematures (TOP) trial (https://clinicaltrials.gov NCT01702805), aims to evaluate whether a liberal Hgb threshold (13 g/dL during the first week of postnatal life on respiratory support) or a restrictive strategy (Hgb of 11 g/dL) for blood transfusion improves neurological outcomes in preterm infants.
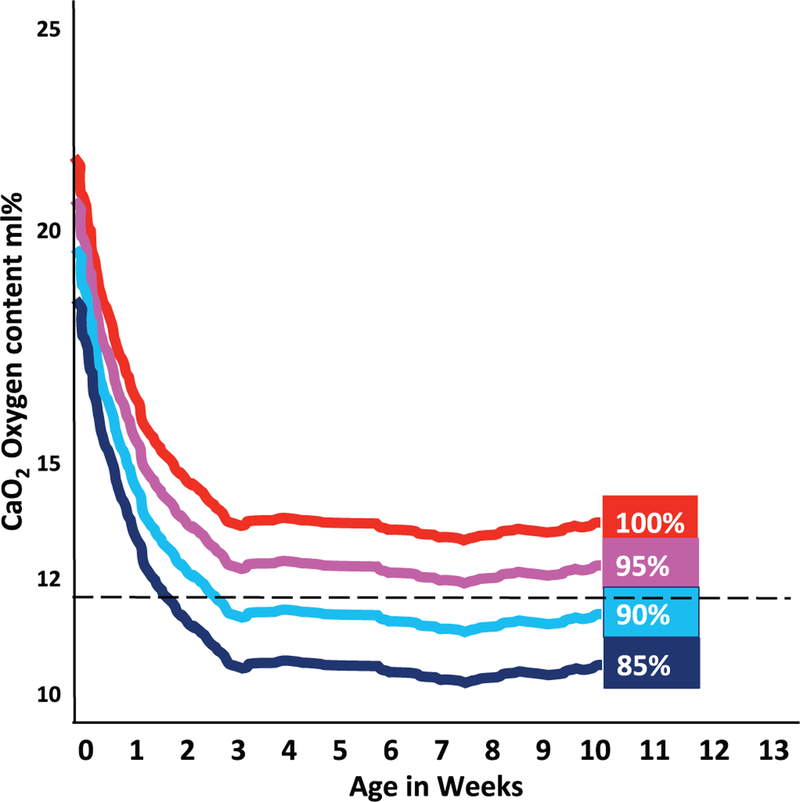
An interesting observation in the SUPPORT trial was that the increased mortality among infants randomized to the lower target SpO2 did not occur until after the first 2 postnatal weeks (Carlo et al. 2010). Vento et al. have speculated that the postnatal decrease in Hgb (especially in NICUs where there is low Hgb transfusion threshold) may result in low CaO2 values in preterm infants managed with low target SpO2 values (Fig. 5) (Vento 2014). Promoting placental transfusion at birth, minimizing iatrogenic blood loss, and using strategies to maintain higher Hgb levels should increase CaO2.
Fig. 5.
Plausible explanation for late mortality in oxygen saturation trials in preterm infants. The increased mortality observed in extremely preterm infants randomized to the 85%–89% SpO2 arm of the Neonatal Oxygenation Prospective Meta-Analysis (NeOProM) trials occurs after the first 2 weeks of postnatal life. Vento et al. (2012) have speculated that a gradual postnatal decrease in hemoglobin (especially if threshold for transfusion is low in the neonatal intensive care unit and hemoglobin gradually decreases with time) results in decreased CaO2, which may lead to an oxygen delivery below the critical point (dashed line), as shown in Fig. 4. Suboptimal oxygen delivery can potentially result in necrotizing enterocolitis and mortality, as has been observed in the low saturation target group of the NeOProM trials.
The upper limit of oxygenation should represent the level of oxygenation that results in toxicity, which in return leads to adverse effects (retinopathy of prematurity, bronchopulmonary dysplasia, and poor neurodevelopment). Experimental studies have shown that targeting PaO2 >80 mm Hg does not result in additional pulmonary vasodilation (Rudolph 1979; Lakshminrusimha et al. 2006, 2009). Furthermore, PaO2 >100 mm Hg following excessive use of oxygen in the resuscitation of babies with hypoxic–ischemic encephalopathy has been shown to be associated with poor neurodevelopmental outcomes (Kapadia et al. 2013).
SpO2 target limits
The final results from the NeOProM collaboration were recently reported (Askie et al. 2018). In this analysis, there was no significant difference between the lower SpO2 (85%–89%) and higher SpO2 (91%–95%) target range on the primary composite outcome of death or major disability at a corrected age of 18–24 months. However, patients who were randomized to the lower SpO2 target range were shown to have a higher risk of death and necrotizing enterocolitis, but a lower risk of retinopathy of prematurity (Askie et al. 2018). The results from this study suggest that an SpO2 target range of 91%–95% may be safer than a range of 85%–89% in extremely preterm infants (<28 weeks gestation) based primarily on an observed increase in the risk of death associated with the lower range (Bizzarro 2018).
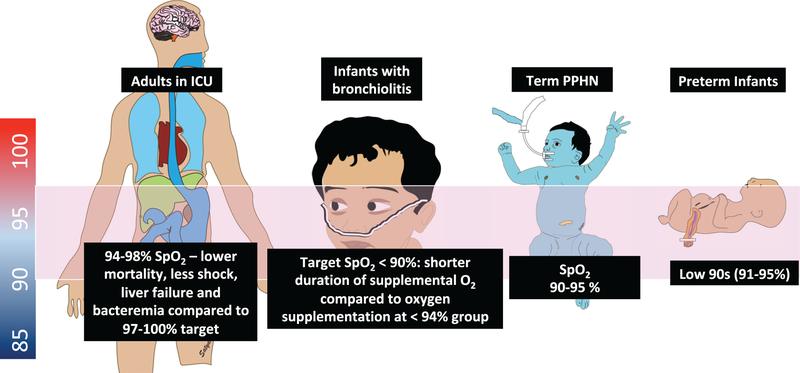
Current evidence suggests that the safest SpO2 range in term infants with hypoxic respiratory failure and persistent hypertension of the newborn is 90%–95% (Vali and Lakshminrusimha 2017). A multi-center randomized study comparing SpO2 ≥94% to ≥90% in infants suffering from bronchiolitis has demonstrated that infants who were maintained at an SpO2 closer to 90% had less need for oxygen supplementation (56% versus 73%), a lower duration of oxygen use (5.7 h versus 27.6 h), and were discharged from the hospital sooner (40.9 h vs. 50.9 h) (Cunningham et al. 2015). Finally, in a large adult trial, patients who were expected to be admitted for >72 h in the intensive care unit were randomized to receive oxygen therapy to maintain PaO2 between 70 and 100 mm Hg (SpO2 between 94% and 98%; conservative group) or to allow PaO2 values up to 150 mm Hg (Spo2 values between 97% and 100%; conventional control group) (Girardis et al. 2016). Patients who were maintained at the lower oxygen range had lower risk of mortality, shock, liver failure, and bacteremia. Although children are not small adults and neonates (especially preterm) are not small children, from a physiological standpoint there may be some similarities in oxygen saturation targets across age groups while individuals receive supplemental oxygen (Fig. 6).
Fig. 6.
Optimal oxyhemoglobin saturation across age groups. Current evidence suggests that maintaining an SpO2 target range ~90%–95% has the most favorable outcomes in extremely premature infants, term newborns suffering from pulmonary hypertension, older infants admitted with bronchiolitis, as well as critically ill adults (94%–98%) admitted to the intensive care unit (ICU). PPHN, pulmonary hypertension of the newborn.
Conclusion
The human body has an extraordinary ability to maintain homeostasis. When this equilibrium is disturbed, restoring balance by means of medical therapies may lead to unexpected adverse effects. In premature and sick newborns, many of whom suffer from respiratory distress, one of the biggest challenges is balancing the optimal oxygen supplementation to ensure adequate tissue metabolism while avoiding hypoxia and oxygen toxicity. The advent of the pulse oximeter to provide continuous, reliable, noninvasive, and safe measurements of SpO2 has radically influenced oxygen therapy in neonates. While the optimal oxyhemoglobin saturation target to preserve the well-being of these patients needs further elucidation, future studies should include other variables (pH, PaCO2, Hgb concentration) that influence perfusion and tissue oxygenation.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest associated with this work.
This Review is part of a Special Issue entitled “Connecting Maternal, Fetal, and Newborn Physiology”.
References
- Ahmed H, Tang X, Polizzotti B, Gauvreau K, Kellogg M, DiNardo J, and Kheir J 2017. Use of oxyhemoglobin saturation, rather than oxygen tension, as a marker of oxygenation in cyanotic patients. JAMA Pediatr. 171(10): 1012–1014. doi: 10.1001/jamapediatrics.2017.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SJ, Rich W, and Finer NN 2010. The effect of averaging time on oximetry values in the premature infant. Pediatrics, 125(1): e115–e121. doi: 10.1542/peds.2008-1749. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. 2007. Guidelines for perinatal care. 6th ed. American Academy of Pediatrics, Elk Grove Village, Ill. [Google Scholar]
- Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W, and the NeOProM Collaborative Group. 2011. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr. 11: 6. doi: 10.1186/1471-2431-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. 2018. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA, 319(21): 2190–2201. doi: 10.1001/jama.2018.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SG 1999. An introduction to hemoglobin physiology. Neonatal Netw. 18(2):9–15. doi: 10.1891/0730-0832.18.2.9. [DOI] [PubMed] [Google Scholar]
- Bizzarro MJ 2018. Optimizing oxygen saturation targets in extremely preterm infants. JAMA, 319(21): 2173–2174. doi: 10.1001/jama.2018.5724. [DOI] [PubMed] [Google Scholar]
- Bolton DP, and Cross KW 1974. Further observations on cost of preventing retrolental fibroplasia. Lancet, 1(7855): 445–448. [DOI] [PubMed] [Google Scholar]
- Campbell K 1951. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Med. J. Aust 2(2): 48–50. [PubMed] [Google Scholar]
- Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. 2010. Target ranges of oxygen saturation in extremely preterm infants. N. Engl. J. Med 362(21): 1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Chan MM, and Chan MM 2013. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir. Med 107(6): 789–799. doi: 10.1016/j.rmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Claure N, and Bancalari E 2013. Automated closed loop control of inspired oxygen concentration. Respir. Care, 58(1): 151–161. doi: 10.4187/respcare.01955. [DOI] [PubMed] [Google Scholar]
- Comroe JH Jr. 1939. The location and function of the chemoreceptors of the aorta. Am. J. Physiol 127(1): 176–191. doi: 10.1152/ajplegacy.1939.127.1.176. [DOI] [Google Scholar]
- Cummings JJ, Polin RA, and Committee on Fetus and Newborn. 2016. Oxygen targeting in extremely low birth weight infants. Pediatrics, 138(2): e20161576. doi: 10.1542/peds.2016-1576. [DOI] [PubMed] [Google Scholar]
- Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, and BIDS Collaborators Group. 2015. Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol. Assess 19(71): i–xxiii, 1–172. doi: 10.3310/hta19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Halleux V, Truttmann A, Gagnon C, and Bard H 2002. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Semin. Perinatol 26(6): 411–415. doi: 10.1053/sper.2002.37313. [DOI] [PubMed] [Google Scholar]
- Durand M, and Ramanathan R 1986. Pulse oximetry for continuous oxygen monitoring in sick newborn infants. J. Pediatr 109(6): 1052–1056. doi: 10.1016/S0022-3476(86)80298-0. [DOI] [PubMed] [Google Scholar]
- Emond D, Lachance C, Gagnon J, and Bard H 1993. Arterial partial pressure of oxygen required to achieve 90% saturation of hemoglobin in very low birth weight newborns. Pediatrics, 91(3): 602–604. [PubMed] [Google Scholar]
- Ewer AK, Granelli AD, Manzoni P, Sánchez Luna M, and Martin GR 2013. Pulse oximetry screening for congenital heart defects. Lancet, 382(9895): 856–857. doi: 10.1016/S0140-6736(13)61859-0. [DOI] [PubMed] [Google Scholar]
- Gerschman R, Gilbert DL, Nye SW, Dwyer P, and Fenn WO 1954. Oxygen poisoning and x-irradiation: a mechanism in common. Science, 119(3097): 623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. 2016. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU randomized clinical trial. JAMA, 316(15): 1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- Giussani DA 2016. The fetal brain sparing response to hypoxia: physiological mechanisms. J. Physiol 594(5): 1215–1230. doi: 10.1113/JP271099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Bennet L, Sferruzzi-Perri AN, Vaughan OR, and Fowden AL 2016. Hypoxia, fetal and neonatal physiology: 100 years on from Sir Joseph Barcroft. J. Physiol 594(5): 1105–1111. doi: 10.1113/JP272000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Rastogi S, Sahni R, Bhutada A, Bateman D, Rastogi D, et al. 2002. Inhaled nitric oxide and gentle ventilation in the treatment of pulmonary hypertension of the newborn – a single-center, 5-year experience. J. Perinatol 22(6): 435–441. doi: 10.1038/sj.jp.7210761. [DOI] [PubMed] [Google Scholar]
- Harris AP, Sendak MJ, Donham RT, Thomas M, and Duncan D 1988. Absorption characteristics of human fetal hemoglobin at wavelengths used in pulse oximetry. J. Clin. Monit 4(3): 175–177. doi: 10.1007/BF01621812. [DOI] [PubMed] [Google Scholar]
- Hutchison AA 1987. Recovery from hypopnea in preterm lambs: effects of breathing air or oxygen. Pediatr. Pulmonol 3(5): 317–323. doi: 10.1002/ppul.1950030507. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Hagadorn JI, and Sink DW 2017. Alarm safety and alarm fatigue. Clin. Perinatol 44(3): 713–728. doi: 10.1016/j.clp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Johnston ED, Boyle B, Juszczak E, King A, Brocklehurst P, and Stenson BJ 2011. Oxygen targeting in preterm infants using the Masimo SET Radical pulse oximeter. Arch. Dis. Child. Fetal Neonatal Ed. 96(6): F429–F433. doi: 10.1136/adc.2010.206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia VS, Chalak LF, DuPont TL, Rollins NK, Brion LP, and Wyckoff MH 2013. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J. Pediatr 163(4): 949–954. doi: 10.1016/j.jpeds.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Kapadia VS, Lal CV, Kakkilaya V, Heyne R, Savani RC, and Wyckoff MH 2017. Impact of the Neonatal Resuscitation Program-recommended low oxygen strategy on outcomes of infants born preterm. J. Pediatr 191: 35–41. doi: 10.1016/j.jpeds.2017.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella JP, Ivy DD, and Abman SH 1994. Ontogeny of NO activity and response to inhaled NO in the developing ovine pulmonary circulation. Am. J. Physiol 267(5 Pt 2): H1955–H1961. [DOI] [PubMed] [Google Scholar]
- Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, et al. 2006. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr. Res 59(1): 137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, et al. 2009. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr. Res 66(5): 539–544. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Manja V, Mathew B, and Suresh GK 2015a. Oxygen targeting in preterm infants: a physiological interpretation. J. Perinatol 35(1): 8–15. doi: 10.1038/jp.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Mathew B, Nair J, Gugino SF, Koenigsknecht C, Rawat M, et al. 2015b. Tracheal suctioning improves gas exchange but not hemodynamics in asphyxiated lambs with meconium aspiration. Pediatr. Res 77(2): 347–355. doi: 10.1038/pr.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui K, Jones LJ, Foster JP, Davis PG, Ching SK, Oei JL, and Osborn DA 2018. Lower versus higher oxygen concentrations titrated to target oxygen saturations during resuscitation of preterm infants at birth. Cochrane Database Syst. Rev 5: CD010239. doi: 10.1002/14651858.CD010239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manja V, Lakshminrusimha S, and Cook DJ 2015a. Oxygen saturation target range for extremely preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 169(4): 332–340. doi: 10.1001/jamapediatrics.2014.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manja V, Mathew B, Carrion V, and Lakshminrusimha S 2015b. Critical congenital heart disease screening by pulse oximetry in a neonatal intensive care unit. J. Perinatol 35(1): 67–71. doi: 10.1038/jp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer HS, Behrman RE, and Honig GR 1970. Dependence of the oxygen affinity of blood on the presence of foetal or adult haemoglobin. Nature, 227(5256): 388–390. doi: 10.1038/227388a0. [DOI] [PubMed] [Google Scholar]
- Milner QJ, and Mathews GR 2012. An assessment of the accuracy of pulse oximeters. Anaesthesia, 67(4): 396–401. doi: 10.1111/j.1365-2044.2011.07021.x. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, and Archer SL 2005. Hypoxic pulmonary vasoconstriction. J. Appl. Physiol 98(1): 390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- Naumburg E, Bellocco R, Cnattingius S, Jonzon A, and Ekbom A 2002Supplementary oxygen and risk of childhood lymphatic leukaemia. Acta Paediatr. 91(12): 1328–1333. doi: 10.1111/j.1651-2227.2002.tb02829.x. [DOI] [PubMed] [Google Scholar]
- Oei JL, Vento M, Rabi Y, Wright I, Finer N, Rich W, et al. 2017. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 102(1): F24–F30. doi: 10.1136/archdischild-2016-310435. [DOI] [PubMed] [Google Scholar]
- Oei JL, Saugstad OD, and Vento M 2018. Oxygen and preterm infant resuscitation: what else do we need to know? Curr. Opin. Pediatr 30(2): 192–198. doi: 10.1097/MOP.0000000000000610. [DOI] [PubMed] [Google Scholar]
- Perkins GD, McAuley DF, Giles S, Routledge H, and Gao F 2003. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit. Care, 7(4): R67. doi: 10.1186/cc2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. 2010. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation, 122(16 Suppl. 2): S516–S538. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- Ponte J, and Purves MJ 1974. The role of the carotid body chemoreceptors and carotid sinus baroreceptors in the control of cerebral blood vessels. J. Physiol 237(2): 315–340. doi: 10.1113/jphysiol.1974.sp010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prsa M, Sun L, van Amerom J, Yoo SJ, Grosse-Wortmann L, Jaeggi E, et al. 2014. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ. Cardiovasc. Imaging, 7(4): 663–670. doi: 10.1161/CIRCIMAGING.113. [DOI] [PubMed] [Google Scholar]
- Puligandla PS, Grabowski J, Austin M, Hedrick H, Renaud E, Arnold M, et al. 2015. Management of congenital diaphragmatic hernia: a systematic review from the APSA outcomes and evidence based practice committee.J. Pediatr. Surg 50(11): 1958–1970. doi: 10.1016/j.jpedsurg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Rabi Y, Lodha A, Soraisham A, Singhal N, Barrington K, and Shah PS 2015. Outcomes of preterm infants following the introduction of room air resuscitation. Resuscitation, 96: 252–259. doi: 10.1016/j.resuscitation.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Rajadurai VS, Walker AM, Yu VY, and Oates A 1992. Effect of fetal haemoglobin on the accuracy of pulse oximetry in preterm infants. J. Paediatr. Child Health, 28(1): 43–46. doi: 10.1111/j.1440-1754.1992.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Rasanen J, Wood DC, Weiner S, Ludomirski A, and Huhta JC 1996. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation, 94(5): 1068–1073. doi: 10.1161/01.CIR.94.5.1068. [DOI] [PubMed] [Google Scholar]
- Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, and Huhta JC 1998. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation, 97(3): 257–262. doi: 10.1161/01.CIR.97.3.257. [DOI] [PubMed] [Google Scholar]
- Rawat M, Chandrasekharan PK, Swartz DD, Mathew B, Nair J, Gugino SF, et al. 2016. Neonatal resuscitation adhering to oxygen saturation guidelines in asphyxiated lambs with meconium aspiration. Pediatr. Res 79: 583–588. doi: 10.1038/pr.2015.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosychuk RJ, Hudson-Mason A, Eklund D, and Lacaze-Masmonteil T 2012. Discrepancies between arterial oxygen saturation and functional oxygen saturation measured with pulse oximetry in very preterm infants. Neonatology, 101(1): 14–19. doi: 10.1159/000326797. [DOI] [PubMed] [Google Scholar]
- Rudolph AM 1979. Fetal and neonatal pulmonary circulation. Annu. Rev. Physiol 41: 383–395. doi: 10.1146/annurev.ph.41.030179.002123. [DOI] [PubMed] [Google Scholar]
- Rudolph AM 2009. The fetal circulation. In Congenital diseases of the heart: clinical-physiological considerations. 3rd ed. Wiley-Blackwell, Hoboken, N.J: pp. 1–24. doi: 10.1002/9781444311822.ch1. [DOI] [Google Scholar]
- Rudolph AM, and Yuan S 1966. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J. Clin. Invest 45(3): 399–411. doi: 10.1172/JCI105355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD 2018. Oxygenation of the immature infant: a commentary and recommendations for oxygen saturation targets and alarm limits. Neonatology, 114(1): 69–75. doi: 10.1159/000486751. [DOI] [PubMed] [Google Scholar]
- Saugstad OD, Ramji S, Soll RF, and Vento M 2008. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology, 94(3): 176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. 2013. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA, 309(20): 2111–2120. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Whyte RK, and Roberts RS 2017. Oxygen targeting in infants born extremely preterm who are small for gestational age: a need for heightened vigilance. J. Pediatr 186: 9–10. doi: 10.1016/j.jpeds.2017.02.071. [DOI] [PubMed] [Google Scholar]
- Shiao SY 2005. Effects of fetal hemoglobin on accurate measurements of oxygen saturation in neonates. J. Perinat. Neonatal Nurs. 19(4): 348–361. doi: 10.1097/00005237-200510000-00010. [DOI] [PubMed] [Google Scholar]
- Smith DJ, and Vane JR 1966. Effects of oxygen tension on vascular and other smooth muscle. J. Physiol 186(2): 284–294. doi: 10.1113/jphysiol.1966.sp008034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola A 2008. Oxygen in neonatal anesthesia: friend or foe? Curr. Opin. Anaesthesiol 21(3): 332–339. doi: 10.1097/ACO.0b013e3282f8ad8d. [DOI] [PubMed] [Google Scholar]
- Sola A, Golombek SG, Montes Bueno MT, Lemus-Varela L, Zuluaga C, Domínguez F, et al. 2014. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 103(10): 1009–1018. doi: 10.1111/apa.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood BG, McLaughlin K, and Cortez J 2015. Near-infrared spectroscopy: applications in neonates. Semin. Fetal Neonatal Med. 20(3): 164–172. doi: 10.1016/j.siny.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. 2013. Oxygen saturation and outcomes in preterm infants. N. Engl. J. Med 368(22): 2094–2104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- Tarnow-Mordi W, Stenson B, Kirby A, Juszczak E, Donoghoe M, Deshpande S, et al. 2016. Outcomes of two trials of oxygen-saturation targets in preterm infants. N. Engl. J. Med 374(8): 749–760. doi: 10.1056/NEJMoa1514212. [DOI] [PubMed] [Google Scholar]
- Tin W, and Lal M 2015. Principles of pulse oximetry and its clinical application in neonatal medicine. Semin. Fetal Neonatal Med. 20(3): 192–197. doi: 10.1016/j.siny.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Torres-Cuevas I, Parra-Llorca A, Sánchez-Illana A, Nuñez-Ramiro A, Kuligowski J, Cháfer-Pericás C, et al. 2017. Oxygen and oxidative stress in the perinatal period. Redox Biol. 12: 674–681. doi: 10.1016/j.redox.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagedes J, Poets CF, and Dietz K 2013. Averaging time, desaturation level, duration and extent. Arch. Dis. Child. Fetal Neonatal Ed. 98(3): F265–F266. doi: 10.1136/archdischild-2012-302543. [DOI] [PubMed] [Google Scholar]
- Vali P, and Lakshminrusimha S 2017. The fetus can teach us: oxygen and the pulmonary vasculature. Children, 4(8): 67. doi: 10.3390/children4080067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter A, Williams U, Zavala A, Kee J, Rebello E, Tsai J, et al. 2017. Beat to beat: a measured look at the history of pulse oximetry. J. Anesth. Hist 3(1): 24–26. doi: 10.1016/j.janh.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Vento M 2014. Oxygen supplementation in the neonatal period: changing the paradigm. Neonatology, 105(4): 323–331. doi: 10.1159/000360646. [DOI] [PubMed] [Google Scholar]
- Vento M, and Saugstad OD 2010. Oxygen as a therapeutic agent in neonatology: a comprehensive approach. Semin. Fetal Neonatal Med. 15(4): 185. doi: 10.1016/j.siny.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Vento M, Escobar J, Cernada M, Escrig R, and Aguar M 2012. The use and misuse of oxygen during the neonatal period. Clin. Perinatol 39(1): 165–176. doi: 10.1016/j.clp.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Vijayakumar E, Ward GJ, Bullock CE, and Patterson ML 1997. Pulse oximetry in infants of <1500 gm birth weight on supplemental oxygen: a national survey. J. Perinatol 17(5): 341–345. [PubMed] [Google Scholar]
- Waitz M, Schmid MB, Fuchs H, Mendler MR, Dreyhaupt J, and Hummler HD 2015. Effects of automated adjustment of the inspired oxygen on fluctuations of arterial and regional cerebral tissue oxygenation in preterm infants with frequent desaturations. J. Pediatr 166(2): 240–244.e1. doi: 10.1016/j.jpeds.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Wolff HG 1936. The cerebral circulation. Physiol. Rev 16: 545–596. doi: 10.1152/physrev.1936.16.4.545. [DOI] [Google Scholar]
- Yoon S, Zuccarello M, and Rapoport RM 2012. pCO(2) and pH regulation of cerebral blood flow. Front. Physiol 3: 365. doi: 10.3389/fphys.2012.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]