Abstract
The significance of hepatitis E virus (HEV) as an important public health problem is rising. Until a decade ago, cases of HEV infection in Eur-ope were mainly confined to returning travelers, but nowadays, hepatitis E represents an emerging zoonotic infection in many European countries. The aim of this manuscript is to perform a systematic review of the published literature on hepatitis E distribution in humans, animals and environmental samples ("One Health" concept) in the South-Eastern European countries. Comparison of the available data showed that the anti-HEV seroprevalence in the South-Eastern Europe varies greatly, depending on the population studied, geographical area and methods used. The IgG seroprevalence rates in different population groups were found to be 1.1%-24.5% in Croatia, up to 20.9% in Bulgaria, 5.9-%17.1% in Romania, 15% in Serbia, up to 9.7% in Greece and 2%-9.7% in Albania. Among possible risk factors, older age was the most significant predictor for HEV seropositivity in most studies. Higher seroprevalence rates were found in animals. HEV IgG antibodies in domestic pigs were detected in 20%-54.5%, 29.2%-50%, 38.94%-50% and 31.1%-91.7% in Serbia, Bulgaria, Romania and Croatia, respectively. In wild boars seroprevalence rates were up to 10.3%, 30.3% and 31.1% in Romania, Slovenia and Croatia, respectively. A high HEV RNA prevalence in wild boars in some countries (Croatia and Romania) indicated that wild boars may have a key role in the HEV epidemiology. There are very few data on HEV prevalence in environmental samples. HEV RNA was detected in 3.3% and 16.7% surface waters in Slovenia and Serbia, respectively. There is no evidence of HEV RNA in sewage systems in this region. The available data on genetic characterization show that human, animal and environmental HEV strains mainly belong to the genotype 3.
Keywords: Hepatitis E virus, "One-Health", Humans, Animals, Environment, South-East Europe
Core tip: In South-East Europe, the hepatitis E virus (HEV) prevalence as in other parts of Europe varies greatly, depending on the studied population, geographical area and methods used. Seroprevalence rates were found to be 0%-36% in humans and 10.3%-54.5% in animals. Human studies showed sporadic detection of HEV RNA in patients with acute hepatitis and in transplant population. HEV RNA was detected in up to 31.6% pigs and 16.7% environmental samples. Studies on phylogenetic characterization in human, animal and environmental samples showed that HEV strains from the south-eastern European countries mainly belong to the genotype 3.
INTRODUCTION
Hepatitis E represents an important public health problem in many parts of the world. The World Health Organization estimates that 20 million hepatitis E virus (HEV) infections occur worldwide leading to an estimated 3.3 million symptomatic cases and 44000 deaths related to hepatitis E. Until a decade ago, cases of HEV infection in Europe were mainly confined to travelers returning from endemic areas, whereas nowadays, hepatitis E is endemic in many European countries. It is estimated that 5%-15% of all acute hepatitis infections of unknown origin in Europe are caused by HEV[1]. The seroprevalence ranges from 0.6% to 52.5%, depending on the population group tested and geographical region. In addition to differences in seroprevalence rates between countries, there are also differences in HEV seropositivity within the same country[2]. Changes in HEV epidemiology could be explained by the confirmation of zoonotic nature of the disease. To date, three zoonotic HEV genotypes have been confirmed to infect humans. In addition to well-known HEV-3 and HEV-4 genotypes, a new genotype HEV-7 has also been recently described to infect humans[3,4]. Domestic pigs and wild boars represent the most important animal reservoirs for HEV-3 and HEV-4 worldwide with usually high seroprevalence rates ranging between 23%-100%[5]. However, zoonotic transmission of HEV has also been reported from some other animal reservoirs such as rabbits, deer and camels[3,6-8]. In addition, HEV must be considered as a food-borne pathogen as well, since food-borne cases of hepatitis E in humans are increasingly reported. Food-borne HEV-3 and HEV-4 infections due to consumption of undercooked meat and meat products from infected animal reservoirs have been repeatedly described[4,6,9-11]. Furthermore, HEV-3 has also been found in mollusks and human consumption of contaminated shellfish has been implicated as the cause of sporadic cases of acute hepatitis E[12,13]. Other types of food such as berry fruit have rarely been suspected to act as vehicles for HEV transmission after environmental contamination with animal feces[14,15].
In this manuscript, a bibliographic review of the literature on hepatitis E was performed with the aim of gathering the latest data regarding the prevalence, risk factors and HEV genotype distribution in the South-East Europe in the multi-disciplinary "One Health" concept (Tables 1-3).
Table 1.
Prevalence of hepatitis E in different population groups
| Country | Population | Sample size | Method | anti-HEV | HEV RNA (%)/genotype (subtype) | Ref. |
| Albania | Refugees in Greece (pregnant women) | 500 | EIA | 2%1 | NT | Malamitsi-Puchner et al[16] |
| Refugees in Greece (adult population) | 350 | EIA/IB | 4.85%1 | NT | Dalekos et al[17] | |
| General population | ND | ND | IgG 9.7% | NT | Adhami et al[18] | |
| Thalassemic children | ND | ND | IgG 0% | NT | ||
| Patients with chronic liver disease | 109 | EIA | 36.6%1 | NT | Kondili et al[19] | |
| Patients with no apparent liver disease | 190 | EIA | 12.1%1 | NT | ||
| Bulgaria | Patients with symptoms of acute hepatitis | 806 | EIA | IgM/IgG 2.48% | NT | Baymakova et al[23] |
| Hospitalized patients with clinical symptoms of hepatitis and outpatients with laboratory data of liver dysfunction | 325 | EIA | IgM 13.2% | NT | Stoykova et al25] | |
| IgG 20.9% | NT | |||||
| Patients with acute hepatitis E (IgM positive) | 105 | RT-PCR | NT | G3 98% (3e 62%; 3f 24%; 3c 13%); G1 2% | Bruni et al[26] | |
| Patients conducting ambulatory examination due to various reasons | 741 | EIA | IgM/IgG 1.48% | Teoharov et al[27] | ||
| IgG 9.04% | ||||||
| Croatia | Patients with clinical symptoms of hepatitis negative for HAV/HBV/HCV | 504 | EIA/IB/RT-PCR | IgM/IgG 10.7% | IgM positive 35.7% | Đaković Rode et al[31] |
| HIV-infected patients | 88 | EIA/IB | IgG 1.1% | NT | ||
| Liver transplant recipients | 242 | EIA | IgG 24.5% | NT | Mrzljak et al[32] | |
| Alcohol abusers | 56 | EIA/IB | IgG 8.9% | NT | Vilibic-Cavlek et al[33] | |
| Patients with PTSD | 35 | EIA/IB | IgG 8.1% | NT | ||
| Injecting drug users | 49 | EIA/IB | IgG 6.1% | NT | ||
| Persons with risk sexual behaviour | 37 | EIA/IB | IgG 0% | NT | ||
| Forest workers | 37 | EIA/IB | IgG 8.1% | NT | Jeličić et al[34] | |
| Healthcare workers | 50 | EIA/IB | IgG 2.0% | NT | Jeličić et al[34] | |
| Pregnant women | 68 | EIA/IB | IgG 2.9% | NT | Jeličić et al[35] | |
| Hunters | 25 | EIA/IB | IgG 4.0% | NT | Jeličić et al[35] | |
| General population | 87 | EIA/IB | IgG 3.4% | NT | Jeličić et al[34] | |
| Blood donors | 1036 | EIA/IB EIA | IgM 1.7% IgG 20.3% | NT | Miletic Lovric et al[36] | |
| Greece | Transplant patients | 76 | RT-PCR | NT | 1 positive/1.3% G3 | Sinakos et al[45] |
| Transfusion dependent thalassaemia | 96 | EIA | IgG 0% | 0% | Klonizakis et al[44] | |
| Blood donors | 1 200 | EIA | IgG 2.9% | NT | Zervou et al[49] | |
| HIV patients | 243 | EIA | IgG 7.3% | NT | Politou et al[48] | |
| Blood donors (South Greece) | 265 | EIA | IgG 9.43 | NT | Pittaras et al[47] | |
| Patients after open-heart surgery | 204 | EIA | IgG 5.4% | NT | Zervou et al[43] | |
| Patients on haemodialysis | 351 | EIA | IgG 4.8% | NT | Stefanidis et al[46] | |
| Non-A,-B hepatitis patients | 198 | EIA/RT-PCR | IgG 7.6% IgM 1% | 1 positive | Psichogiou et al[42] | |
| Healthy controls | 316 | EIA/RT-PCR | IgM 0%/IgG 2.2% | NT | ||
| Epirus region | Healthy blood donors | 2636 | EIA/IB | IgG 0.23% | NT | Dalekos et al[17] |
| Refugees from southern Albania | 350 | EIA/IB | IgG 4.85% | NT | ||
| Children | 165 | EIA/IB | IgG 0% | NT | ||
| Injecting drug users | 65 | EIA/IB | IgG 0% | NT | ||
| Multiply transfused patients | 62 | EIA/IB | IgG 0% | NT | ||
| Patients with chronic viral hepatitis | 75 | EIA/IB | IgG 5.30% | NT | ||
| Chronic haemodialysis patients | 149 | EIA/IB | IgG 1.34% | NT | ||
| Agrinion area | Healthy blood donors | 380 | EIA/IB | IgG 0.53% | NT | |
| Chronic hemodialysis patients | 62 | EIA/IB | IgG 9.7% | NT | ||
| Kosovo | Kosovar refugees | 104 | EIA/RT-PCR | IgM 7.7% | 0% | Rey et al[56] |
| Montenegro | Patients with acute viral hepatitis | 400 | EIA | IgM 6% | NT | Terzić et al[57] |
| Romania | Patients with hepatitis B or C | 25 | EIA | IgG 12% | NT | Anita et al[59] |
| Students | 40 | EIA | IgG 12.5% | NT | Voiculescu et al[60] | |
| Doctors and nurses | 93 | EIA | IgG 13.98% | NT | Voiculescu et al[60] | |
| Persons undergoing routine hematological tests | 148 | EIA | IgG 14.86% | NT | Anita et al[61] | |
| General population | 67 | EIA | IgG 5.9% | NT | Savuta G et al[62] | |
| Serbia | Blood donors | 200 | EIA RT-PCR | IgG 15% | 0% | Petrović et al[66] |
| Slovenia | Acute/recent hepatitis E (IgM antibodies) | 10 | RT-PCR | NT | 3/10 (G3e and G1) | Steyer et al[74] |
Total anti-hepatitis E virus antibodies. HEV: Hepatitis E virus; EIA: Enzyme immunoassay; IB: Immunoblot; RT-PCR: Reverse-transcriptase polymerase chain reaction; NT: Not tested; ND: No data.
Table 3.
Prevalence of hepatitis E virus RNA in different environmental samples
| Country | Sample | Sample size | HEV RNA (%)/ genotype (subtype) | Ref. |
| Greece | Vegetable leafy greens | Pooled samples | 4.76%1 3.2%1 | Kokkinos et al[51] |
| Sewage | 48 | 0% | Kokkinos et al[54] | |
| Sewage | 5 | 0% | Clemente-Casares et al[53] | |
| Serbia | Vegetable leafy greens | Pooled samples | 4.76%1 3.2%1 | Kokkinos et al[51] |
| Berry fruits | Pooled samples | 2.5%1 | Maunula et al[15] | |
| Surface waters | 60 | 16.67% | Lazić et al[73] | |
| Urban sewage | 6 | 0% | ||
| Slovenia | Waste water treatment plant | 12 | 0% | Steyer et al[77] |
| Swabs from the different site on the slaughter line | 62 | 3.2% (G3) | Raspor Lainšček et al[76] | |
| Minced meat | 22 | 0% | ||
| Bratwurst | 30 | 0% | ||
| Surface water | 60 | 3.3% (G3) | Steyer et al[74] |
Pooled samples. HEV: Hepatitis E virus; EIA: Enzyme immunoassay; IB: Immunoblot; NT: Not tested.
Table 2.
Prevalence of hepatitis E in different animal species
| Country | Population | Sample size | Method | anti-HEV | HEV RNA (%)/genotype (subtype) | Ref. |
| Bulgaria | Piglets | 44 | EIA | IgG 50% | NT | Pishmisheva et al[28] |
| Fattening pigs | 41 | EIA | IgG 29.2% | NT | ||
| Croatia | Domestic pigs | 848 | RT-PCR | NT | 24.5% (G3) | Prpić et al[40] |
| Wild boars | 536 | RT-PCR | NT | 12.3% (G3) | ||
| Molluscs (mussels, oysters) | 538 | RT-PCR | NA | 0% | ||
| Cattle | 32 | RT-PCR | NT | 0% | ||
| Red fox | 50 | RT-PCR | NT | 0% | ||
| Deer | 320 | RT-PCR | NT | 0% | ||
| Muflons | 12 | RT-PCR | NT | 0% | ||
| Ferrets | 8 | RT-PCR | NT | 0% | ||
| Martens | 10 | RT-PCR | NT | 0% | ||
| Pigs (serum samples) | 60 | EIA/RT-PCR | IgG 91.7% | 13.3% 8.1% | Lipej et al [39] | |
| Pigs (bile samples) | 60 | RT-PCR | NA | 8.1% | ||
| Pigs, domestic | 1424 | EIA/RT-PCR | IgG 32.94% | 0% | Jemeršić et al[41] | |
| Wild boars | 1000 | EIA/RT-PCR | IgG 31.10% | 11.33% | ||
| Greece | Mussels | 51 | RT-PCR | NT | 0% | Diez-Valcarce et al55] |
| Black rats/Norway rats | 20 | RT-PCR | NT | 10% | Ryll et al[50] | |
| Romania | Farm pigs | 50 | EIA/IB | IgG 50% | NT | Savuta et al[62] |
| Backyard pigs | 95 | EIA/IB | IgG 38.94% | |||
| Backyard pigs | 112 | EIA | IgG 49.27% | NT | Savuta et al[58] | |
| Pigs (2-4 mo) stool samples | 19 | RT-PCR | NT | 31.58% (G3) | Anita et al[61] | |
| Wild boars | 52 | EIA | 9.61%1 | NT | Porea et al[63] | |
| Wild boars | 68 | EIA | IgG 10.29% | NT | Porea et al[63] | |
| Wild boars | 50 | RT-PCR | NT | 18% (G3) | Porea et al[65] | |
| Serbia | Farm pigs (pooled stool samples) | 30 | RT-PCR | NT | 30% | Petrovic et al[67] |
| Farm pigs (polled tissue samples) | 20 | RT-PCR | NT | 45% | ||
| Backyard pigs (pooled tissue samples) | 15 | RT-PCR | NT | 0% | ||
| Wild boars (pooled stool samples) | 10 | RT-PCR | NT | 0% | ||
| Backyard pigs | 315 | EIA | IgG 34.6% | NT | Lupulovic et al[68] | |
| Pigs (liver samples) | 50 | NT | NT | 26% | Savic et al[69] | |
| Fattening pigs | 95 | NT | NT | 7.37% | Petrović et al[71] | |
| Piglets (8 wk) | 50 | NT | NT | 64% | ||
| Pigs (blood samples) | 55 | EIA | IgG 54.54% | NT | Lupulovic et al[70] | |
| Pigs (meat juice samples) | 55 | EIA | IgG 20% | NT | ||
| Slovenia | Black rats/Norway rats | 17/1 | RT-PCR | NT | 0% | Ryll et al[50] |
| Domestic pigs | 85 | RT-PCR | NT | 20.3% | Steyer et al[74] | |
| Suckling pigs (0-3 wk) | 38 | RT-PCR | NT | 5.3% | ||
| Weanling pigs (3-10 wk) | 21 | RT-PCR | NT | 28.6% | ||
| Fattening pigs (> 10 wk) | 26 | RT-PCR | NT | 26.9% | ||
| Wild boars | 288 | EIA/RT-PCR | 30.21%1 | 0.35% | Žele et al[75] | |
| Pigs (stool samples) | 811 | RT-PCR | NT | 5.4% | Raspor Lainšček et al[76] | |
| Pigs (bile samples) | 811 | RT-PCR | NT | 4.9% | ||
| Pigs (liver samples) | 811 | RT-PCR | NT | 5.3% (G3a, 3b, 3c, 3e) |
Total anti-hepatitis E virus antibodies. HEV: Hepatitis E virus; EIA: Enzyme immunoassay; IB: Immunoblot; RT-PCR: Reverse-transcriptase polymerase chain reaction; NT: Not tested; NA: Not applicable.
Search strategy and selection criteria
A literature search was conducted in the following electronic databases: PubMed, Web of Science, Medline and Scopus along with hand-searching references of key articles and a ResearchGate and Google search with no limitations placed on year of publication and language restriction. The search included articles in peer-reviewed journals and grey literature. Books, dissertations, review articles and unpublished reports were excluded. In addition, non-relevant studies by the review of the abstracts were also excluded. Keywords searched were: Hepatitis E virus, epidemiology, human, animals, pigs, wild boars, environment, waste water, vegetables, seroprevalence, risk factors, HEV RNA, genotype. Once a comprehensive list of abstracts has been retrieved and reviewed, any studies appearing to meet inclusion criteria were reviewed in full.
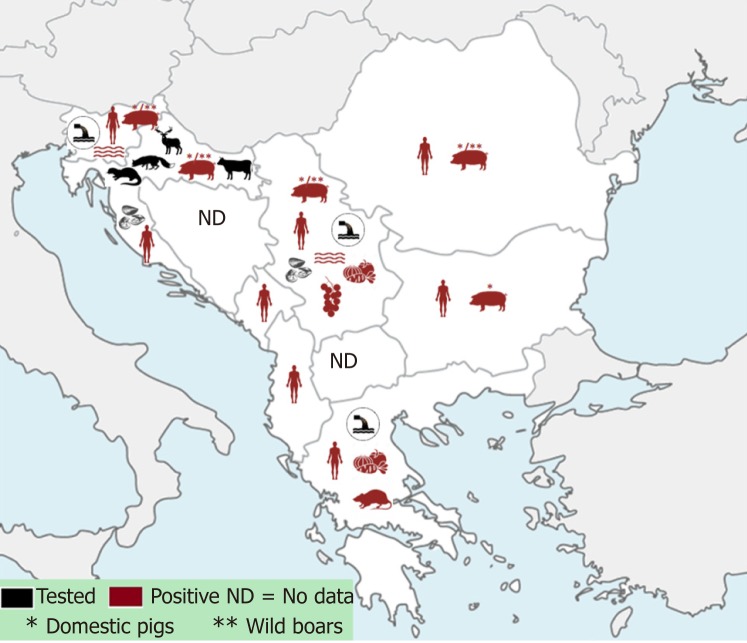
Out of 11 south-eastern European countries, data for Albania, Bulgaria, Croatia, Greece, Kosovo, Montenegro, Romania, Serbia and Slovenia are available and presented in this review (Figure 1). So far, there have been no data published on HEV for Bosnia and Herzegovina and the Republic of North Macedonia.
Figure 1.
Data on hepatitis E prevalence in South-East Europe in humans, animals and environmental samples ("One health" concept).
ALBANIA
There are few studies on hepatitis E in Albania published in 1990s and 2000s. In the studies conducted among Albanian refugees in Greece, the anti-HEV prevalence was 2% among pregnant women[16] and 4.85% in the adult population from southern Albania[17]. In 2000s, acute HEV in Albania made up 2.4% of all acute viral hepatitis cases. HEV infections occurred more frequently in women (female to male ratio 2:1), as well as in adults over 35 years (73.3%). In the general population, the prevalence of anti-HEV antibodies was 9.7% and increased progressively with age, from 1.2% in children less than 9 years to 17.7% in persons older than 60 years. There was no difference in the prevalence between the general population and patients with chronic liver disease (10.5%). The prevalence of anti-HEV antibodies in pregnant women was lower than in the general population (1.1%). No positive cases were found among children with thalassemia receiving multiple transfusions[18]. In contrast, in the other Albanian case-control study, HEV antibodies were found in 36.6% patients with chronic liver disease compared to 12.1% in patients with no apparent liver disease. In the univariate analysis, anti-HEV prevalence was found to be associated with the age (> 50 years), lower education status (≤ 8 years of formal education) and positivity for hepatitis B surface antigen (HBsAg), while no association was observed in people who lived in villages and/or those who were occupationally involved with handling animals. In the multivariate analysis, significant associations remained for age and HBsAg positivity[19].
Since there are no recent data on HEV infection in Albania, the burden of diseases caused by HEV is not known. Moreover, no data on the role of animals in the epidemiology of HEV infections in Albania is currently available. Therefore, more research should be done to evaluate a local HEV epidemiology in Albania.
BULGARIA
In Bulgaria, the first human cases of hepatitis E were reported in 1995[20]. Several studies reported cases and hospitalized patients with acute hepatitis E[21,22]. A retrospective study of acute viral hepatitis performed in Sofia (2004-2012) found anti-HEV IgM/IgG antibodies in 2.48% patients[23]. HEV infection more frequently affected males (61%-69%), with the highest incidence in persons over 50 years of age[23,24]. Another study in the North-Eastern Bulgaria (2012-2016) analyzed the prevalence of HEV infection in hospitalized patients with clinical symptoms of acute hepatitis and outpatients with laboratory data of liver dysfunction. Acute infection was documented in 13.2% patients, while 20.9% patients showed only IgG antibodies indicating past HEV infection[25]. A study conducted from 2013 to 2015 analyzed HEV genotypes in patients with acute hepatitis E. Phylogenetic analysis showed HEV-3 in 98% and HEV-1 in 2% of cases. Subtyping of HEV-3 sequences showed 3e (62%), 3f (24%) and 3c (13%) subtypes. There were differences in the geographical distribution of genotypes. Subtypes 3f and 3c were scattered throughout the country, while 3e subtype was restricted to the South-West area[26]. A seroepidemiological study conducted in the general Bulgarian population from Plovdiv region during 2012-2013 found anti-HEV IgG seroprevalence of 9.04%, while 1.48% participants showed both IgM and IgG antibodies. Seropositivity did not differ significantly between males (9.87%) and females (8.47%), but increased with the age from 3.53% in the age group 1-9 years to 19.23% in the group over 60 years[27].
In 2016, serum samples from clinically healthy pigs from five industrial farms in two districts in South Bulgaria were collected and tested for the presence of anti-HEV antibodies. The overall HEV seroprevalence was 40.0% with 50.0% seropositive piglets and 29.2% fattening pigs. Seropositivity differed significantly among regions from 0% (Peshtera) to 100% (Nova Zagora)[28]. In a recently published study, genetic diversity and the phylogenetic relationships among different strains of human HEV genotype 3 were analyzed to estimate the date of origin and the demographic history of HEV epidemic in Bulgaria. The root of the Bayesian tree showed at least two different epidemic entrances for HEV genotype 3e strains[29].
Seroprevalence data and the high prevalence of clinical cases registered indicate that HEV infection in Bulgaria is endemic. Phylogenetic analysis showed that human HEV isolates in 98% of cases belonged to HEV 3 genotype.
CROATIA
In Croatia, the first autochthonous human case of hepatitis E was reported in 2012[30]. A study conducted from 2011 to 2013 in patients with clinical symptoms of hepatitis and elevated liver enzymes who tested negative for hepatitis A-C, detected HEV IgM/IgG antibodies in 10.7% patients. Among IgM positive patients, 35.7% were positive for HEV RNA[31].
Several studies analyzed the seroprevalence of HEV infection in different population groups in Croatia. HEV IgG antibodies were detected in 24.5% liver transplant recipients[32], 8.9% alcohol abusers[33], 8.6% patients with war-related posttraumatic stress disorder[33], 8.1% forest workers[34], 6.1% injecting drug users[33], 4.0% hunters[35], 3.4% general population[34], 2.9% pregnant women[35], 2.0% healthcare workers[34] and 1.1% HIV-infected patients[31]. No person with high-risk sexual behavior was HEV seropositive[33]. One study conducted among blood donors in 2016 showed a high IgG seroprevalence rate of 20.3% with 1.7% IgM positive. None of IgM positive samples was positive for HEV RNA[36]. HEV IgG positivity increased significantly with the age[33,37]. In addition, seroprevalence rates were higher in residents of suburban and rural areas compared with residents of urban areas, sub-jects living in families with more household members, persons who use wells as a source of drinking water[33] and those who use a sewage system connected to a septic tank[37]. Gender, marital status, educational level, history of blood transfusions, surgical procedures, tattooing and traveling were not associated with HEV seropositivity[33]. In 2018, the first documented case of HEV induced acute-on-chronic liver failure was reported with severe clinical course considered for liver tran-splantation. The patient was treated with ribavirin and subsequently recovered[38].
Several studies addressed the HEV distribution in domestic animals and wildlife in Croatia[39,40]. During 2009-2010, a comprehensive survey was carried out based on HEV RNA detection in blood, spleen and liver samples originating from different domestic and wild animals from all Croatian counties. Furthermore, digestive gland samples from molluscs were also analyzed. A high HEV RNA prevalence was found in domestic pigs (24.5%) and wild boars (12.3%), whereas cattle, molluscs, ruminant and carnivore wildlife samples tested negative. Molecular characterization confirmed the phylogenetic clustering of the obtained sequences into HEV genotype 3[40]. In 2012, the epidemiology of naturally occurring hepatitis E was investigated in swine herds from three large pig farms in continental Croatia. Nearly all animals (91.7%) tested seropositive for HEV. In addition, active infection was detected in all age groups by detection of HEV RNA in 13.3% serum samples and 8.1% bile samples[39]. In 2016, the overall HEV seroprevalence was shown to be 32.94% (range 8.33%-60.00%) in domestic pigs from 11 counties and 31.10% (range 7.70%-50.60%) in wild boars from six Croatian counties. While no positive HEV RNA samples were detected in domestic pigs, 11.33% seropositive wild boars were found to be HEV RNA positive indicating that wild boars may have a key role in HEV epidemiology[41].
Data on genetic characterization of HEV are available only for animal species, confirming genotype 3 in pigs and wild boars. It is important to note a relatively small sample size of some tested population groups in humans. Therefore, studies on larger samples as well as species comparison studies on HEV genotypes are needed to obtain better insight into HEV epidemiology/molecular epidemiology in Croatia.
GREECE
In 1995, autochthonous hepatitis E virus was documented in Greece. Several studies investigated HEV infection in various population groups in different regions in Greece. A study conducted among patients with clinical signs of hepatitis showed a significantly higher anti-HEV IgG seroprevalence among acute non-A, non-B hepatitis patients (7.6%) compared to healthy controls (2.2%). Acute HEV infection (IgM antibodies) was confirmed in 1.0% of acute non-A, non-B hepatitis patients, while one patient was HEV RNA positive. None of anti-HEV IgM positive patients reported any possible risk factors[42].
In the north-western Greece (Epirus and Agrinion region), a very low anti-HEV IgG prevalence was reported in various populations. In Epirus region, none of different population groups such as children, injecting drug users or multiply transfused patients tested positive for HEV antibodies. Seroprevalence in healthy blood donors was only 0.23%. In contrast, higher anti-HEV prevalence was found among patients with chronic viral hepatitis (5.3%) and patients on hemodialysis (HD) (1.34%). Even higher anti-HEV prevalence was found among HD patients in the Agrinion region (9.7%). No association between the seroprevalence and duration of hemodialysis, seropositivity for hepatitis B or C, history of hepatitis, increased alanine amino-transferase, renal transplantation, history of transfusion or number of units transfused was detected[17]. Another study from the Epirius region (Ioannina) analyzed the risk of blood borne HEV transmission among patients after open-heart surgery who had received more than three blood units perioperatively. Past HEV infections were documented in 5.2% of surgical patients and 0% of healthy controls[43].
Another study from northern Greece analyzed the presence of antibodies and HEV RNA in transfusion dependent thalassaemia patients. The study confirmed no past or acute HEV infection in this multi-transfused population[44].
The most recent data from northern Greece (Thessaloniki) found that 1.3% of liver transplant patients were positive for HEV RNA. Phylogenetic analysis showed that the sequences clustered into the HEV genotype 3 clade. The only patient that tested RNA positive experienced acute hepatitis flare without progression into chronic form. The HEV RNA prevalence in the Greek transplant population (1.3%) corresponds with previously reported data in immunocompromised patients from other European countries[45].
Study from the semi-rural region of central Greece (Thessalia region) on HD patients in three different HD units found a total seroprevalence rate of 4.8% (varying from 1.8%-9.8% according to the unit). The highest anti-HEV prevalence was found in Karditsa unit (9.8%), which finding the authors assume to have been a result of a local infection in the past. Risk factors for HEV seropositivity (age, sex, duration of HD, hepatitis B or C virus infection markers, previously elevated aminotransferase levels or history of transfusion) were not identified[46].
Data from southern Greece showed even higher seroprevalence rates. In 2014, a study among blood donors from urban population of Athens demonstrated a seroprevalence of 9.43%[47]. Furthermore, a study on HIV patients in Athens indicated 7.3% anti-HEV IgG seroprevalence[48].
A recent study from 11 Blood Services throughout Greece revealed 2.9% and 3.6% of anti-HEV IgG seroprevalence among blood donors and multi-transfused patients, respectively. The highest seroprevalence rate (13.3%) had male blood donors from Heraklion, Crete. The seroprevalence was higher in persons older than 50 years (5.9%) compared to younger group (1.8%)[49].
There is only one study on HEV infection among animals in Greece. The animals tested included Norway rats (Rattus norvegicus) and Black rats (R. rattus) from northern Greece, with the total prevalence of 10% in both species[50].
HEV was reported in a leafy green vegetable supply chain in Greece, showing that HEV RNA was found in 4.76% and 3.2% samples from the primary production and point-of-sale phases, respectively[51]. Later, the same authors investigated the presence of HEV in irrigation water samples used for the leafy green vegetables production and showed that one out of 20 samples tested positive[52]. The sample which was found HEV positive was a groundwater sample collected from the depth of 100 m, indicating well contamination unlikely[51].
Further two studies tested sewage samples from Patras (western Greece) for the presence of HEV. HEV RNA was not found in any of the samples[53,54]. Likewise, commercial mussels tested at the retail in Greece showed no samples positive for HEV RNA[55].
In conclusion, higher seroprevalence rates were detected in southern parts of Greece, the highest so far being in Crete, in contrary to the seroprevalence rates in the northern regions. Moreover, the prevalence of anti-HEV in risk populations (transplant, HIV, HD patients) was higher than in healthy blood donors. Phylogenetic analysis of human HEV isolates in Greece showed that the sequences clustered into the HEV genotype 3 clade. Further researches are needed, especially in animals and environmental samples in individual regions in Greece to gain a better insight in local HEV epidemiology.
KOSOVO
An outbreak of acute hepatitis E occurred among the Kosovar refugees in July 1999, after the return to their country. Several field surveys were undertaken as well as a serological study in patients with symptoms of hepatitis to assess the possible risk factors for HEV infection. A higher incidence of HEV infection was found among well-water consumers compared to those drinking network waters. An acute HEV infection (anti-HEV IgM positive) was found in 7.7% of refugees that had been tested. Only four persons had clinical symptoms, while the others were asymptomatic. There are no other published data on HEV prevalence/seroprevalence in Kosovo[56].
MONTENEGRO
The first available investigation about HEV infection in Montenegro was published in 2009. Data included hospitalized and out-patients with acute viral hepatitis collected from 2000 to 2007, showing that 6% of patients had acute hepatitis E. Although a higher seropositivity was found in males (62.5%) than in females (37.5%), the difference was not statistically significant. Epidemiological data showed that the majority of patients had never travelled out of the country, indicating an autochthonous HEV infection in Montenegro. An asymptomatic form of the disease was serologically confirmed in 7/24 patients, whereas the mild or short course of subclinical disease in 5/7 patients. No one patient had severe, fulminant or chronic course of disease[57].
ROMANIA
There are several studies on HEV infection in humans in Romania. The earliest human studies analyzed anti-HEV IgG prevalence in eastern Romania. The seroprevalence rate of 5.9% was found in the general population[58] and of 12% among patients with hepatitis B or C[59].
A study from 2010 analyzed the prevalence rates in low risk population groups in South and South-Eastern Romania. The seroprevalence of anti-HEV IgG among students (mean age: 22.91 ± 0.56 years) was 12.5%, whereas among doctors and nurses (mean age: 36.71 ± 8.88 years) was 13.98%[60].
A following study from the North-Eastern Romania among persons undergoing routine haematological tests with no signs of hepatitis showed anti-HEV IgG seroprevalence of 17.1% and 12.82% in 2011 and 2012, respectively. In 2011, the youngest age group (9-20 years) tested negative, whereas as older age groups (> 20 and > 40 years) had seroprevalence of 28.6% and 12.9%, respectively. In 2012, the youngest age group (18-45 years) had seroprevalence of 6.25%, whereas the older groups had the seroprevalence up to 28%. These results indicate that HEV infections in this region mainly affect middle-aged adults. Moreover, anti-HEV IgG was more prevalent in women (15.78%) than in men (10%)[61].
One of the earliest animal studies investigated swine population for the presence of anti-HEV IgG in farm and backyard pigs. The immunoblot results for the backyard pigs showed seroprevalence rates of 50%[58,62] and for the farm pigs of 38.94%[62].
A more recent study tested fecal samples from five swine farms (pigs aged between 2 and 4 mo) for HEV RNA, and 6 out of 19 samples tested positive. Phylogenetic analysis showed that Romanian swine isolates grouped into genotype 3 and were closely related to the swine and human HEV isolates identified in other European countries[61]. In 2015 and 2016, serological studies investigated HEV prevalence in wild boars from eastern Romania. The seroprevalence rates were 9.61% (5/52)[63] and 10.29% (7/68), respectively[64]. A recent study on molecular detection of HEV in liver and spleen samples from wild boars aged > 1 year from eastern Romania showed an overall prevalence of 18%. All isolates belonged to the genotype 3 subtypes 3a and 3h[65].
Studies in humans are scarce and new data are needed to define the seroprevalence of HEV infection. Studies conducted in animal species show high seroprevalence rates. Phylogenetic analyses show that Romanian swine and wild boar isolates grouped into genotype 3.
SERBIA
There is only one published study on the HEV seroprevalence in humans in Serbia. The study was conducted among volunteer blood donors aged 19 to 65 years (average age 39.3 years), of whom 15% tested positive for anti-HEV IgG. No significant difference in anti-HEV IgG seropositivity was found between men and women (14.6% and 16.7%, respectively). HEV seroprevalence increased with age, as higher rates were recorded in subjects older than 51 years (21.5%) when compared with younger age groups (< 50 years or < 30 years)[66].
Several studies on HEV prevalence were conducted in Serbia in different animal species. The first study on HEV in swine population in 2007, tested pooled swine stool and tissue samples (spleen, mesenteric lymph node and liver). Thirty percent of stool and 45% of tissue samples tested HEV positive. HEV RNA was detected in four out of five pig farms examined. Simultaneously, all samples from backyard pigs and wild boars were negative[67]. Further studies also confirmed the high prevalence of HEV among pigs in Serbia. The first HEV serology testing in pigs was done on 315 serum samples collected from 3-4 mo old backyard pigs in 63 herds from 28 towns and villages of four different districts in northern Serbia (Vojvodina province), demonstrating seroprevalence rate of 34.6%. The prevalence of anti-HEV antibodies varied widely between municipalities (range 16.7%-75.0%) and herds (range (0-100%)[68].
Furthermore, a study in 2010 analyzed liver tissue samples from 50 dead farm pigs aged 7 to 15 wk which died on pig farms from different regions in Serbia, and detected HEV RNA in 13 of samples (26%)[69]. In 2013, a study on 55 serum samples and meat juice samples collected from three slaughterhouses were analyzed for the presence of anti-HEV IgG antibodies. The mean seroprevalence in the pig serum and meat juice samples was 54.54% and 20%, respectively[70]. In addition, stool, liver, bile and meat samples from 145 animals (95 fatteners and 50 eight weeks piglets) collected on during slaughter were tested for HEV RNA. Among fatteners, HEV has been detected in 7.37% of the stool samples. In piglets, HEV RNA prevalence was high and detected in 54%, 26%, 16% and 10% samples of stool, bile, liver and meat, respectively[71]. The presence of HEV IgG and HEV RNA was examined in 201 blood and 298 liver samples from wild boars culled during hunting season from January 2010 until February 2011. The samples were collected from 27 hunting grounds located on the territory of 7 counties of the country. The overall seroprevalence rate was 34.33%. HEV RNA prevalence was 9.40% with marked regional differences. A high proportion of adult wild sow and wild boars were found positive for HEV RNA[72].
In addition to animal studies, there are few data regarding the environment re-porting the contamination of vegetable supply chain in Serbia. Namely, HEV RNA was found in 4.76 % and 3.2% of samples of leafy green vegetable from the primary production and point-of-sale phases, respectively[51]. Another report also documented the presence of HEV RNA in frozen raspberries (2.6%)[15]. Lately, HEV RNA has also been detected in surface waters in northern Serbia (Vojvodina Province) where 16.67% of samples tested positive for HEV RNA, during summer sampling occasion, whereas none of the tested urban sewage systems tested positive[73].
In Serbia, different population groups should be studied in future in order to reflect local epidemiology, modes of transmission and risk factors for HEV infection. Animal and environmental studies on larger samples as well as studies on HEV genotyping are needed to obtain better insight into HEV epidemiology.
SLOVENIA
Human studies on HEV infection in Slovenia are scarce. Steyer et al[74] tested 10 serum samples of patients with the diagnosis of acute/recent hepatitis E based on detection of HEV IgM antibodies, and three samples tested HEV RNA positive. One sample was typed as genotype 3, clustered in the lineage "e" and the second was genotype 1 strain. Slovenian human HEV strains were not related to porcine HEV strains identified in this study[74].
There are several studies in different animal species in Slovenia. In 2011[74], 20.3% stool samples of domestic pigs were positive for HEV RNA, of which 5.3%, 28.6% and 26.9% were from suckling, weanling and fattening pigs, respectively. All HEV strains were analyzed at 5’ ORF1 and 5’ ORF2 regions and both genome regions confirmed that Slovenian HEV strains represent a distinct genotype 3 lineage. All but one HEV strains detected in pigs in Slovenia represent a monophyletic branch in phylogenetic trees, with a high degree of sequence identity. One human HEV strain belonged to genotype 1 and two to genotype 3 but did not match the new genotype 3 lineage detected in Slovenian pig herds.
HEV RNA was tested in Norway rat and Black rats from one site close to Ljubljana and none of the rats tested HEV-RNA positive[50]. The HEV presence in the wild boar population in Slovenia was first documented in 2016, showing HEV antibodies in 30.2% of animals tested, whereas HEV RNA was detected in only one sample (1/ 288)[75].
Recently, the possibility of HEV entering into the food supply chain was investigated in a large study analyzing pigs entering a slaughterhouse. The study covered three different age groups and three different samples (feces, bile and liver), showing the overall HEV RNA (sample) prevalence of 5.4%, 4.9% and 5.3%, respectively[76]. In the group of three months old pigs, 13.7% of feces, 13.0% of bile and 2.1% of liver samples were HEV RNA positive. The youngest group originating from Slovenia (none imported), was proven to be a group for highest risk for HEV infection. In the group of six months old pigs (imported from Austria), only one liver and one bile sample out of 400 tested positive. In the category of sows, no positive samples were found. The same study analyzed swabs collected at three different sites on the slaughter line (the hooks that were used to hang the liver and the lung, the containers in which livers were stored and the hooks for hanging carcasses in the cooling room). Of 62 swab samples, two were positive (a swab from the hook that carried liver and one from the liver container). However, all minced meat and bratwurst samples from these studies tested HEV RNA negative[76]. The phylogenetic analysis revealed that detected strains were clustered into subtypes 3a, 3b, 3c and 3e.
Further environmental studies investigated surface water and waste water treatment plant. Out of 60 surface water samples tested throughout the country, only two (3.3%) were HEV RNA positive, one of them in the near vicinity of a pig farm. HEV sequences detected in the surface water belonged to genotype 3[74]. In another study, the waste water treatment plant samples were collected from the effluent on a monthly basis from January to December 2012 and tested for HEV RNA. No HEV RNA was detected during the whole length of the study, neither before nor after the concentration step[77].
There are several studies in different animal species showing high prevalence of HEV RNA in Slovenia, with the genotype 3 being the most common. On the other hand, studies in humans are lacking.
CONCLUSION
In the South-East Europe, anti-HEV seroprevalence as in other parts of Europe varies greatly, depending on the population studied, geographical area and assays used for the detection. Studies on HEV RNA detection and phylogenetic characterization in human samples showed that human HEV strains from the South-East European countries mainly belong to the genotype 3. Detection of the same genotype in animals as well as in the environment emphasizes the need of the multidisciplinary collaboration ("One Health" approach) in the surveillance and control of this emerging infection.
Although significant progress in HEV epidemiology has been made in the past decade, many important questions remain[9]. The origin of HEV is still largely unknown. Swine and wild boars were proven to be the primary natural HEV reservoir, but recent studies indicate that rabbits may serve as an additional reservoir of HEV[78]. Some hepatitis E cases observed in immunocompromised patients clustered with rabbit HEV strains confirming their contribution to zoonotic HEV transmission[79,80]. It is still unclear whether HEV strains present in other animals can cross the species barrier and infect humans. However, it is likely that the known host range of HEV has increased and novel strains continue to be identified. Transmission of HEV-3 from deer to humans has been described, although deer most probably undergoes spillover infections from wild boars, rather than being a natural HEV reservoir[7]. It seems that recently described new HEV-7 has been widely distributed in dromedary camels from the Middle East[3,8], but was also detected in an immunocompromised transplant patient who regularly consumed camel milk and meat[4]. While HEV transmission by breast milk was confirmed in humans[81], a recent study from China indicates that viral RNA of HEV-4 can be excreted by cow milk[82]. Although cattle have been rarely described to be infected with HEV, these findings implicate possible HEV transmission through milk or milk products. Human contacts with other animals, especially pet animals should be investigated in future for better understanding of HEV epidemiology. Rabbits are commonly farmed in many countries for meat consumption and fur production but also as pet and possible transmission from pet house rabbits to humans should be considered as another possible transmission route[83]. Infection of dogs and cats with HEV is confirmed serologically[84], but their importance in HEV epidemiology need to be further investigated.
Although HEV infection is not an economically important pig disease, development of a vaccine against the zoonotic genotypes 3 and 4 and vaccination of swine should be considered as a possible public health measure. Such vaccine should also be useful for high-risk populations such as organ transplant recipients since the majority of the chronic hepatitis E are caused by the zoonotic HEV-3[9].
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Peer-review started: March 11, 2019
First decision: May 9, 2019
Article in press: June 1, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Komatsu H, Sergi C S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
Contributor Information
Anna Mrzljak, Department of Medicine, Merkur University Hospital; School of Medicine, University of Zagreb, Zagreb 10000, Grad Zagreb, Croatia. anna.mrzljak@mef.hr.
Petra Dinjar-Kujundzic, Department of Medicine, Merkur University Hospital, Zagreb 10000, Grad Zagreb, Croatia.
Lorena Jemersic, Croatian Veterinary Institute, Zagreb 10000, Grad Zagreb, Croatia.
Jelena Prpic, Croatian Veterinary Institute, Zagreb 10000, Grad Zagreb, Croatia.
Ljubo Barbic, Faculty of Veterinary Medicine, University of Zagreb, Zagreb 10000, Grad Zagreb, Croatia.
Vladimir Savic, Croatian Veterinary Institute, Zagreb 10000, Grad Zagreb, Croatia.
Vladimir Stevanovic, Faculty of Veterinary Medicine, University of Zagreb, Zagreb 10000, Grad Zagreb, Croatia.
Tatjana Vilibic-Cavlek, Department of Virology, Croatian Institute of Public Health; School of Medicine, University of Zagreb, Zagreb 10000, Grad Zagreb, Croatia.
References
- 1.World Health Organization. Hepatitis E. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
- 2.Hartl J, Otto B, Madden RG, Webb G, Woolson KL, Kriston L, Vettorazzi E, Lohse AW, Dalton HR, Pischke S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses. 2016:8. doi: 10.3390/v8080211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R, Wernery R, Wernery U, Yuen KY. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis. 2014;20:1044–1048. doi: 10.3201/eid2006.140140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK, Purdy MA, Teo CG. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355–7.e3. doi: 10.1053/j.gastro.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2010;140:256–265. doi: 10.1016/j.vetmic.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abravanel F, Lhomme S, El Costa H, Schvartz B, Peron JM, Kamar N, Izopet J. Rabbit Hepatitis E Virus Infections in Humans, France. Emerg Infect Dis. 2017;23:1191–1193. doi: 10.3201/eid2307.170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anheyer-Behmenburg HE, Szabo K, Schotte U, Binder A, Klein G, Johne R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013-2015. Emerg Infect Dis. 2017;23:130–133. doi: 10.3201/eid2301.161169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasche A, Saqib M, Liljander AM, Bornstein S, Zohaib A, Renneker S, Steinhagen K, Wernery R, Younan M, Gluecks I, Hilali M, Musa BE, Jores J, Wernery U, Drexler JF, Drosten C, Corman VM. Hepatitis E Virus Infection in Dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983-2015. Emerg Infect Dis. 2016;22:1249–1252. doi: 10.3201/eid2207.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yugo DM, Meng XJ. Hepatitis E virus: foodborne, waterborne and zoonotic transmission. Int J Environ Res Public Health. 2013;10:4507–4533. doi: 10.3390/ijerph10104507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, Shinohara Y, Kurata Y, Ishida M, Sakamoto S, Takeda N, Miyamura T. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis. 2005;11:1958–1960. doi: 10.3201/eid1112.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JY, Lee JM, Jo YW, Min HJ, Kim HJ, Jung WT, Lee OJ, Yun H, Yoon YS. Genotype-4 hepatitis E in a human after ingesting roe deer meat in South Korea. Clin Mol Hepatol. 2013;19:309–314. doi: 10.3350/cmh.2013.19.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao S, Li D, Zha E, Zhou T, Wang S, Yue X. Surveillance of hepatitis E virus contamination in shellfish in China. Int J Environ Res Public Health. 2015;12:2026–2036. doi: 10.3390/ijerph120202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crossan C, Baker PJ, Craft J, Takeuchi Y, Dalton HR, Scobie L. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg Infect Dis. 2012;18:2085–2087. doi: 10.3201/eid1812.120924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brassard J, Gagné MJ, Généreux M, Côté C. Detection of human food-borne and zoonotic viruses on irrigated, field-grown strawberries. Appl Environ Microbiol. 2012;78:3763–3766. doi: 10.1128/AEM.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maunula L, Kaupke A, Vasickova P, Söderberg K, Kozyra I, Lazic S, van der Poel WH, Bouwknegt M, Rutjes S, Willems KA, Moloney R, D'Agostino M, de Roda Husman AM, von Bonsdorff CH, Rzeżutka A, Pavlik I, Petrovic T, Cook N. Tracing enteric viruses in the European berry fruit supply chain. Int J Food Microbiol. 2013;167:177–185. doi: 10.1016/j.ijfoodmicro.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Malamitsi-Puchner A, Papacharitonos S, Sotos D, Tzala L, Psichogiou M, Hatzakis A, Evangelopoulou A, Michalas S. Prevalence study of different hepatitis markers among pregnant Albanian refugees in Greece. Eur J Epidemiol. 1996;12:297–301. doi: 10.1007/BF00145420. [DOI] [PubMed] [Google Scholar]
- 17.Dalekos GN, Zervou E, Elisaf M, Germanos N, Galanakis E, Bourantas K, Siamopoulos KC, Tsianos EV. Antibodies to hepatitis E virus among several populations in Greece: increased prevalence in an hemodialysis unit. Transfusion. 1998;38:589–595. doi: 10.1046/j.1537-2995.1998.38698326339.x. [DOI] [PubMed] [Google Scholar]
- 18.Adhami JE, Angoni R. [Hepatitis E virus infection in Albania] Sante. 2001;11:13–15. [PubMed] [Google Scholar]
- 19.Kondili LA, Chionne P, Porcaro A, Madonna E, Taffon S, Resuli B, Taliani G, Rapicetta M. Seroprevalence of hepatitis E virus (HEV) antibody and the possible association with chronic liver disease: a case-control study in Albania. Epidemiol Infect. 2006;134:95–101. doi: 10.1017/S095026880500470X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teoharov P, Tiholova M, Draganov P. First cases of hepatitis E virus infection in Bulgaria. Infectol (Sofia) 1995;32:17–18. [Google Scholar]
- 21.Teoharov P, Pishmisheva M, Kovaleva V. Hepatitis E virus infection: a case report. Infectol (Sofia) 2008;45:43–44. [Google Scholar]
- 22.Rouseva A, Nikolovska D. Two cases of hepatitis E in Bulgaria. Pediatr Infect Dis (Sofia) 2010;2:26–28. [Google Scholar]
- 23.Baymakova M, Sakem B, Plochev K, Popov GT, Mihaylova-Garnizova R, Kovaleva V, Kundurdjiev T. Epidemiological characteristics and clinical manifestations of hepatitis E virus infection in Bulgaria: A report on 20 patients. Srp Arh Celok Lek. 2016;144:63–68. doi: 10.2298/sarh1602063b. [DOI] [PubMed] [Google Scholar]
- 24.Baymakova M, Popov GT, Pepovich R, Tsachev I. Hepatitis E virus infection in Bulgaria: A brief analysis of the situation in the country. Open Access Maced J Med Sci. 2019;7:458–460. doi: 10.3889/oamjms.2019.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoykova Z, Ivanova L, Tsaneva-Damyanova D, Kostadinova T. Hepatitis E virus infection in Northeastern Bulgaria. Med Review. 2017;53:30–34. [Google Scholar]
- 26.Bruni R, Villano U, Equestre M, Chionne P, Madonna E, Trandeva-Bankova D, Peleva-Pishmisheva M, Tenev T, Cella E, Ciccozzi M, Pisani G, Golkocheva-Markova E, Ciccaglione AR. Hepatitis E virus genotypes and subgenotypes causing acute hepatitis, Bulgaria, 2013-2015. PLoS One. 2018;13:e0198045. doi: 10.1371/journal.pone.0198045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teoharov P, Kevorkyan A, Raycheva R, Golkocheva-Markova E, Trandeva-Bankova D, Andonov A. Data on the prevalence of hepatitis E in Bulgaria. C R Acad Bulg Sci. 2014;67:1429–1432. [Google Scholar]
- 28.Pismisheva M, Baymakova M, Golkocheva-Markova E, Kundurzhiev T, Pepovich R, Popov GT, Tsachev I. First serological study of hepatitis E virus infection in pigs in Bulgaria. C R Acad Bulg Sci. 2018;71:1001–1008. [Google Scholar]
- 29.Cella E, Golkocheva-Markova E, Sagnelli C, Scolamacchia V, Bruni R, Villano U, Ciccaglione AR, Equestre M, Sagnelli E, Angeletti S, Ciccozzi M. Human hepatitis E virus circulation in Bulgaria: Deep Bayesian phylogenetic analysis for viral spread control in the country. J Med Virol. 2019;91:132–138. doi: 10.1002/jmv.25296. [DOI] [PubMed] [Google Scholar]
- 30.Čivljak R, Đaković-Rode O, Jemeršić L, Topić A, Turalija M, Čačić M, Kuzman I. Autochthonous hepatitis E in a patient from Zagreb: a case report. Croatian J Infect. 2013;33:35–39. [Google Scholar]
- 31.Ðaković Rode O, Jemeršić L, Brnić D, Pandak N, Mikulić R, Begovac J, Vince A. Hepatitis E in patients with hepatic disorders and HIV-infected patients in Croatia: is one diagnostic method enough for hepatitis E diagnosis? Eur J Clin Microbiol Infect Dis. 2014;33:2231–2236. doi: 10.1007/s10096-014-2187-7. [DOI] [PubMed] [Google Scholar]
- 32.Mrzljak A, Djakovic Rode O, Dinjar Kujundžić P, Perkov S, Vince A. Seroprevalence of hepatitis E virus among liver transplant patients in Croatia: preliminary pilot study data. 27th ECCMID, Vienna, 22-25 April, 2017, EV0274 [Google Scholar]
- 33.Vilibic-Cavlek T, Vilibic M, Kolaric B, Jemersic L, Kucinar J, Barbic L, Bagaric A, Stevanovic V, Tabain I, Sviben M, Jukic V, Mlinaric-Galinovic G. Seroepidemiology of Hepatitis E in Selected Population Groups in Croatia: A Prospective Pilot Study. Zoonoses Public Health. 2016;63:494–502. doi: 10.1111/zph.12254. [DOI] [PubMed] [Google Scholar]
- 34.Jeličić P, Jemeršić L, Brumen V, Janev-Holcer N, Prohić A, Barbić Lj, Tabain I, Stevanović V, Vilibić-Čavlek V. Seroprevalence of hepatitis E in professionally exposed groups in Croatia: preliminary results. 7th International Congress "Veterinary Science and Profession", Zagreb, 5-7 October, 2017, p.55 [Google Scholar]
- 35.Jeličić P, Vilibić-Čavlek T, Vilibić M, Jemeršić L, Kolarić B, Jemeršić L, Kučinar J, Barbić Lj, Stevanović V, Janev-Holcer N, Tabain I, Brumen V, Djaković I, Prohić A, Košec V, Kaić B. Seroprevalence of hepatitis E in different population groups in Croatia. 7th Congress of Slovenian Microbiological Society, Bled, Slovenia, 20-22 September, 2017, p.155 [Google Scholar]
- 36.Miletic Lovric M, Stojic Vidovic M, Hecimovic A, Mihaljevic I, Jemersic L, Strauss-Patko M, Jukic I. Seroprevalence of hepatitis E among Croatian blood donors. Vox Sang. 2016;111:309. [Google Scholar]
- 37.Mrzljak A, Dinjar Kujundžić P, Djakovic Rode O, Kolarić B, Vince A. Socio-demografic risk factors for high seroprevalence among liver transplant recipients in Croatia. 4th Central and Eastern European Meeting on Hepatitis and HIV, Prague, 10-12. October; Rev Antivir Ther Infect Dis. 2018;12:6. [Google Scholar]
- 38.Dinjar Kujundžić P, PavičićŠarić J, Betica Radić Lj, Mrzljak A. Fulminant hepatitis E on a liver transplantation list. Proceeding from the “Emerging and neglected zoonoses in the One Health context”, Zagreb, 18-19 October; 2018. p. 83. [Google Scholar]
- 39.Lipej Z, Novosel D, Vojta L, Roić B, Simpraga M, Vojta A. Detection and characterisation of hepatitis E virus in naturally infected swine in Croatia. Acta Vet Hung. 2013;61:517–528. doi: 10.1556/AVet.2013.031. [DOI] [PubMed] [Google Scholar]
- 40.Prpić J. Černi S, Škorić D, Keros T, Brnić D, Cvetnić Ž, Jemeršić L. Distribution and Molecular Characterization of Hepatitis E virus in Domestic Animals and Wildlife in Croatia. Food Environ Virol. 2015;7:195–205. doi: 10.1007/s12560-015-9193-5. [DOI] [PubMed] [Google Scholar]
- 41.Jemeršić L. Keros T, Maltar Lj, Barbić Lj, Vilibić-Čavlek T, Jeličić P, Rode Đaković O, Prpić J. Differences in hepatitis E virus (HEV) presence in naturally infected seropositive domestic pigs and wild boars - confirmation of wild boars having a key role in HEV epidemiology. Vet Arh. 2017;87:651–663. [Google Scholar]
- 42.Psichogiou MA, Tassopoulos NC, Papatheodoridis GV, Tzala E, Klarmann R, Witteler H, Schlauder GG, Troonen H, Hatzakis A. Hepatitis E virus infection in a cohort of patients with acute non-A, non-B hepatitis. J Hepatol. 1995;23:668–673. doi: 10.1016/0168-8278(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 43.Zervou EK, Georgiadou SP, Liapi GK, Karabini F, Giogiakas V, Zisiadis K, Gatselis NK, Goudevenos I, Dalekos GN. Markers of hepatitis viruses and human T-lymphotropic virus types I/II in patients who have undergone open-heart surgery: evidence of increased risk for exposure to HBV and HEV. Eur J Intern Med. 2005;16:424–428. doi: 10.1016/j.ejim.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Klonizakis P, Gioula G, Exindari M, Apostolou C, Kotsiafti A, Vlachaki E. Hepatitis E in transfusion-dependent thalassaemia patients, in Greece: a single centre experience. Vox Sang. 2017;112:678–679. doi: 10.1111/vox.12572. [DOI] [PubMed] [Google Scholar]
- 45.Sinakos Ε. Gioula G, Liava C, Papa A, Papadopoulou E, Tsakni E, Fouzas I, Akriviadis E. Prevalence of hepatitis E in liver transplant recipients in Greece. Epidemiol Infect. 2018;146:1619–1621. doi: 10.1017/S0950268818001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanidis I, Zervou EK, Rizos C, Syrganis C, Patsidis E, Kyriakopoulos G, Sdrakas L, Tsianas N, Rigopoulou EI, Liakopoulos V, Dalekos GN. Hepatitis E virus antibodies in hemodialysis patients: an epidemiological survey in central Greece. Int J Artif Organs. 2004;27:842–847. doi: 10.1177/039139880402701005. [DOI] [PubMed] [Google Scholar]
- 47.Pittaras T, Valsami S, Mavrouli M, Kapsimali V, Tsakris A, Politou M. Seroprevalence of hepatitis E virus in blood donors in Greece. Vox Sang. 2014;106:387. doi: 10.1111/vox.12122. [DOI] [PubMed] [Google Scholar]
- 48.Politou M, Boti S, Androutsakos T, Valsami S, Pittaras T, Kapsimali V. Seroprevalence of hepatitis E in HIV infected patients in Greece. J Med Virol. 2015;87:1517–1520. doi: 10.1002/jmv.24214. [DOI] [PubMed] [Google Scholar]
- 49.Zervou EZ, Politis CP, Hassapopoulou EH, Vini MV, Parara MP, Kavallierou LK, Fountouli KF, Zaxarioudaki AZ, Hatzitaki MH, Martinis GM, Katopi DK, Megalou AM, Avrami DA, Halkia PH, Aggelou EA, Tsironi ET, Lafiatis IL, Richardson CR. Prevalence of hepatitis E virus (HEV) infection in blood donors and multi-transfused patients in Greece. Vox Sang. 2015;109:242–243. [Google Scholar]
- 50.Ryll R. Bernstein S, Heuser E, Schlegel M, Dremsek P, Zumpe M, Wolf S, Pépin M, Bajomi D, Müller G, Heiberg AC, Spahr C, Lang J, Groschup MH, Ansorge H, Freise J, Guenther S, Baert K, Ruiz-Fons F, Pikula J, Knap N, Tsakmakidis Ι, Dovas C, Zanet S, Imholt C, Heckel G, Johne R, Ulrich RG. Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet Microbiol. 2017;208:58–68. doi: 10.1016/j.vetmic.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Kokkinos P, Kozyra I, Lazic S, Bouwknegt M, Rutjes S, Willems K, Moloney R, de Roda Husman AM, Kaupke A, Legaki E, D'Agostino M, Cook N, Rzeżutka A, Petrovic T, Vantarakis A. Harmonised investigation of the occurrence of human enteric viruses in the leafy green vegetable supply chain in three European countries. Food Environ Virol. 2012;4:179–191. doi: 10.1007/s12560-012-9087-8. [DOI] [PubMed] [Google Scholar]
- 52.Kokkinos P, Kozyra I, Lazic S, Söderberg K, Vasickova P, Bouwknegt M, Rutjes S, Willems K, Moloney R, de Roda Husman AM, Kaupke A, Legaki E, D'Agostino M, Cook N, von Bonsdorff CH, Rzeżutka A, Petrovic T, Maunula L, Pavlik I, Vantarakis A. Virological Quality of Irrigation Water in Leafy Green Vegetables and Berry Fruits Production Chains. Food Environ Virol. 2017;9:72–78. doi: 10.1007/s12560-016-9264-2. [DOI] [PubMed] [Google Scholar]
- 53.Clemente-Casares P, Pina S, Buti M, Jardi R, MartIn M, Bofill-Mas S, Girones R. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis. 2003;9:448–454. doi: 10.3201/eid0904.020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokkinos P, Ziros P, Meri D, Filippidou S, Kolla S, Galanis A, Vantarakis A. Environmental surveillance. An additional/alternative approach for virological surveillance in Greece? Int J Environ Res Public Health. 2011;8:1914–1922. doi: 10.3390/ijerph8061914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diez-Valcarce M, Kokkinos P, Söderberg K, Bouwknegt M, Willems K, de Roda-Husman AM, von Bonsdorff CH, Bellou M, Hernández M, Maunula L, Vantarakis A, Rodríguez-Lázaro D. Occurrence of human enteric viruses in commercial mussels at retail level in three European countries. Food Environ Virol. 2012;4:73–80. doi: 10.1007/s12560-012-9078-9. [DOI] [PubMed] [Google Scholar]
- 56.Rey JL, Ramadani Q, Soarès JL, Nicand E, Ibrahime D, Preteni E, Buisson Y, Teyssou R. [Sero-epidemiological study of the hepatitis epidemic in Mitrovica in the aftermath of the war in Kosovo (1999)] Bull Soc Pathol Exot. 2002;95:3–7. [PubMed] [Google Scholar]
- 57.Terzic D, Dupanovic B, Mugosa B, Draskovic N, Svirtlih N. Acute hepatitis E in Montenegro: epidemiology, clinical and laboratory features. Ann Hepatol. 2009;8:203–206. [PubMed] [Google Scholar]
- 58.Savuţa G, Aniţă A, Aniţă D, Ludu L, Duca E, Pavio N. Seroepidemiological researches regarding swine and human hepatits E in Romania. Lucr St Med Vet USAMVB Timisoara. 2008;XLI:309–313. [Google Scholar]
- 59.Anita A, Anita D, Ludu L, Savuta G. Seroepidemiological investigation of human and swine hepatitis in Botosani County. Bull UASVM Vet Med. 2010;67:19–22. [Google Scholar]
- 60.Voiculescu M, Iliescu L, Ionescu C, Micu L, Ismail G, Zilisteanu D, Radasan A, Micu G, Pertache I. A cross-sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South-Eastern regions of Romania. J Gastrointestin Liver Dis. 2010;19:43–48. doi: 10.1007/s11749-009-0177-3. [DOI] [PubMed] [Google Scholar]
- 61.Aniţă A, Gorgan L, Aniţă D, Oşlobanu L, Pavio N, Savuţa G. Evidence of hepatitis E infection in swine and humans in the East Region of Romania. Int J Infect Dis. 2014;29:232–237. doi: 10.1016/j.ijid.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Savuţa G, Aniţă A, Aniţă D, Ludu L, Pavio N. Preliminary epidemiological investigations regarding hepatitis E virus infection in swine form the North-east of Romania. Bull USAMV-CN. 2007;64:356–358. [Google Scholar]
- 63.Porea D, Anita A, Paslaru A, Savuta G. Serological evidence of wild boar hepatitis E infection in three counties from eastern Romania. Lucr St Med Vet USAMVB Timisoara. 2015;48:174–178. [Google Scholar]
- 64.Porea D, Anita A, Paslaru A, Savuta G. Wild boar hepatitis E seroprevalence in hunting funds from Buzau and Galati counties. Bull UASVM Vet Med. 2016;73:44–48. [Google Scholar]
- 65.Porea D, Anita A, Demange A, Raileanu C, Oslobanu Ludu L, Anita D, Savuta G, Pavio N. Molecular detection of hepatitis E virus in wild boar population in eastern Romania. Transbound Emerg Dis. 2018;65:527–533. doi: 10.1111/tbed.12736. [DOI] [PubMed] [Google Scholar]
- 66.Petrović T, Lupulović D, Jiménez de Oya N, Vojvodić S, Blázquez AB, Escribano-Romero E, Martín-Acebes MA, Potkonjak A, Milošević V, Lazić S, Saiz JC. Prevalence of hepatitis E virus (HEV) antibodies in Serbian blood donors. J Infect Dev Ctries. 2014;8:1322–1327. doi: 10.3855/jidc.4369. [DOI] [PubMed] [Google Scholar]
- 67.Petrovic T, Prodanov J, Lazic S. First preliminary results on the presence of Hepatitis E virus in swine population in Serbia. Symposium on Current Developments in Food and Environmental Virology, Pisa, Italy 2008 October 9-11, pp. 52-53 [Google Scholar]
- 68.Lupulovic D, Lazic S, Prodanova Radulovic J, Jimenez de Oya N, Escribano-Romero E, Saiz Juan-Carslos, Petrovic R. First Serological Study of Hepatitis E Virus Infection in Backyard Prigs from Serbia. Food Environ Virol. 2010;2:110–113. [Google Scholar]
- 69.Savic B, Milicevic V, Bojkovski J, Kureljusic B, Ivetic V, Pavlovic I. Detection rates of the swine torque teno viruses (TTVs), porcine circovirus type 2 (PCV2) and hepatitis E virus (HEV) in the livers of pigs with hepatitis. Vet Res Commun. 2010;34:641–648. doi: 10.1007/s11259-010-9432-z. [DOI] [PubMed] [Google Scholar]
- 70.Lupulovic D, Grgic Z, Lazic G, Prodanov-Radulovic J, Potkonjak A, Lazic S, Petrovic T. 2013. Detection of hepatitis E virus antibodies in blood and meat juice samples in slaughtered pigs in Serbia. Proceedings of the 16th International Symposiom on the World Association of Veterinary Laboratory Diagnosticians (WAVLD), 2013, June 5-8; Berlin, Germany; p. 372. [Google Scholar]
- 71.Petrovic T, Lupulovic D, Zivoslav G, Lazic G, Vidanovic D, Dosen R, Savic S. 2013. The preliminary survey of HEV presence in samples of pigs – pork production in slaughthouses in Serbia. Proceedings of the 16th International Symposiom on the World Association of Veterinary Laboratory Diagnosticians (WAVLD), 2013, June 5-8; Berlin, Germany; p. 310. [Google Scholar]
- 72.Petrović T, Lupulović D, Lazić S, Lazić G, Blázquez AB, Escribano-Romero E, Saiz JC. 2012. Presence of hepatitis E virus infection in wild board population in Serbia. Proceedings of the IX International Congress of Veternary Virology; pp. September 4–7, Madrid, Spain; p241. [Google Scholar]
- 73.Lazić G, Grubač S, Lupulović D, Bugarski D, Lazić S, Knežević P, Petrović T. Presence of Human and Animal Viruses in Surface Waters in Vojvodina Province of Serbia. Food Environ Virol. 2015 doi: 10.1007/s12560-015-9187-3. [DOI] [PubMed] [Google Scholar]
- 74.Steyer A, Naglič T, Močilnik T, Poljšak-Prijatelj M, Poljak M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: prevalence and molecular characterization of a novel genotype 3 lineage. Infect Genet Evol. 2011;11:1732–1737. doi: 10.1016/j.meegid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Žele D, Barry AF, Hakze-van der Honing RW, Vengušt G, van der Poel WH. Prevalence of Anti-Hepatitis E Virus Antibodies and First Detection of Hepatitis E Virus in Wild Boar in Slovenia. Vector Borne Zoonotic Dis. 2016;16:71–74. doi: 10.1089/vbz.2015.1819. [DOI] [PubMed] [Google Scholar]
- 76.Raspor Lainšček P, Toplak I, Kirbiš A. A comprehensive study of hepatitis E virus infection in pigs entering a slaughterhouse in Slovenia. Vet Microbiol. 2017;212:52–58. doi: 10.1016/j.vetmic.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Steyer A, Gutiérrez-Aguirre I, Rački N, Beigot Glaser S, Brajer Humar B, Stražar M, Škrjanc I, Poljšak-Prijatelj M, Ravnikar M, Rupnik M. The Detection Rate of Enteric Viruses and Clostridium difficile in a Waste Water Treatment Plant Effluent. Food Environ Virol. 2015 doi: 10.1007/s12560-015-9183-7. [DOI] [PubMed] [Google Scholar]
- 78.Lhomme S, Dubois M, Abravanel F, Top S, Bertagnoli S, Guerin JL, Izopet J. Risk of zoonotic transmission of HEV from rabbits. J Clin Virol. 2013;58:357–362. doi: 10.1016/j.jcv.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Izopet J, Dubois M, Bertagnoli S, Lhomme S, Marchandeau S, Boucher S, Kamar N, Abravanel F, Guérin JL. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg Infect Dis. 2012;18:1274–1281. doi: 10.3201/eid1808.120057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaiser M, Delaune D, Chazouillères O, Blümel J, Roque-Afonso AM, Baylis SA. A World Health Organization Human Hepatitis E Virus Reference Strain Related to Similar Strains Isolated from Rabbits. Genome Announc. 2018;6 doi: 10.1128/genomeA.00292-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivero-Juarez A, Frias M, Rodriguez-Cano D, Cuenca-López F, Rivero A. Isolation of Hepatitis E Virus From Breast Milk During Acute Infection. Clin Infect Dis. 2016;62:1464. doi: 10.1093/cid/ciw186. [DOI] [PubMed] [Google Scholar]
- 82.Huang F, Li Y, Yu W, Jing S, Wang J, Long F, He Z, Yang C, Bi Y, Cao W, Liu C, Hua X, Pan Q. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology. 2016;64:350–359. doi: 10.1002/hep.28668. [DOI] [PubMed] [Google Scholar]
- 83.Caruso C, Modesto P, Prato R, Scaglione FE, De Marco L, Bollo E, Acutis PL, Masoero L, Peletto S. Hepatitis E Virus: First Description in a Pet House Rabbit. A New Transmission Route for Human? Transbound Emerg Dis. 2015;62:229–232. doi: 10.1111/tbed.12348. [DOI] [PubMed] [Google Scholar]
- 84.Liang H, Chen J, Xie J, Sun L, Ji F, He S, Zheng Y, Liang C, Zhang G, Su S, Li S. Hepatitis E virus serosurvey among pet dogs and cats in several developed cities in China. PLoS One. 2014;9:e98068. doi: 10.1371/journal.pone.0098068. [DOI] [PMC free article] [PubMed] [Google Scholar]