Abstract
The transplanted liver can modulate the recipient immune system to induce tolerance after transplantation. This phenomenon was observed nearly five decades ago. Subsequently, the liver’s role in multivisceral transplantation was recognized, as it has a protective role in preventing rejection of simultaneously transplanted solid organs such as kidney and heart. The liver has a unique architecture and is home to many cells involved in immunity and inflammation. After transplantation, these cells migrate from the liver into the recipient. Early studies identified chimerism as an important mechanism by which the liver modulates the human immune system. Recent studies on human T-cell subtypes, cytokine expression, and gene expression in the allograft have expanded our knowledge on the potential mechanisms underlying immunomodulation. In this article, we discuss the privileged state of liver transplantation compared to other solid organ transplantation, the liver allograft’s role in multivisceral transplantation, various cells in the liver involved in immune responses, and the potential mechanisms underlying immunomodulation of host alloresponses.
Keywords: Liver transplantation, Alloimmunity, Liver-kidney transplant, Tolerance, Rejection
Core tip: The liver not only protects itself from host alloimmune responses, but also modulates alloimmune responses to other simultaneously transplanted solid organs like heart or kidney. The titer of donor specific alloantibodies decreases after liver transplantation, making transplantation of other solid organs possible even in highly sensitized high-risk patients. The immune cells from the liver allograft cross-talk with recipient immune cells and modulate the immune system towards tolerance. The cross-talk between these cells suppress the genes involved in alloimmunity and upregulate the genes involved in tissue repair and metabolism.
INTRODUCTION
The liver has baffled researchers for decades because of its complex set of functions and unique architecture. From a metabolic and anatomic standpoint, it has a dual blood supply with the portal vein carrying blood from the gastrointestinal tract and the hepatic artery carrying systemic blood. From an immunological standpoint, the liver is home to many cells of the lymphoid system. Together, the liver’s unique architecture and resident immune cells, allow it to play a key role in transplant alloimmunity. It is well recognized that the liver is an immunologically privileged organ, compared to other organs that are commonly transplanted. The liver allograft not only protects itself from the host immune system, but this protection also extends to other simultaneously transplanted solid organs from the same donor. Many researchers have investigated potential mechanisms of this donor-specific hyporesponsiveness. Recent studies on the host T-cell subtypes and gene expression in the allograft after multi-visceral transplants that include the liver, have expanded our knowledge on the liver’s role in transplant immunity[1-3]. In this article, we revisit the liver allograft’s role in modulating host alloimmunity with special emphasis on combined organ transplants.
LITERATURE SEARCH
For purpose of this review, the Embase and Ovid MEDLINE databases were searched from January 2000 through January 2019 using keywords “Liver transplant*” and “Alloimmunity”. The search included Epub ahead of print, in process, and other nonindexed citations. After removing duplicate publications, 242 studies were finally reviewed by title and abstract for selecting full text articles for current review. The studies describing liver-based modulation of cells of the immune system in solid organ solitary liver or multivisceral transplantation were selected for review.
THE LIVER IS AN IMMUNOLOGICALLY PRIVILEGED ORGAN
The liver’s immune-privileged status was first recognized in the porcine liver transplantation model[4]. As early as 1965, it was observed that pigs undergoing liver transplantation survived for prolonged periods with limited immunosuppression, whereas other organs, including skin, heart, and kidneys were quickly rejected[4]. This phenomenon has since been observed in other animal models[5].
The first reports of tolerance in human liver transplantation came from Dr. Thomas Starzl and the Pittsburgh group[6,7]. Their early experience showed that 27% of liver transplant recipients could be weaned from all immunosuppression[8]. Subsequently, many other groups tried immunosuppression weaning in patients with stable liver function[6,9-11]. In a pilot study, 60% of carefully selected pediatric liver transplant recipients could be successfully weaned off immunosuppression[10]. This approach, however, was associated with increased rejection [9-11]. Nevertheless, most liver transplant patients require less maintenance immunosuppression than recipients of other solid organs. Likewise, induction immunosuppression, other than steroids, is rarely needed in liver transplantation.
Antibody-mediated rejection
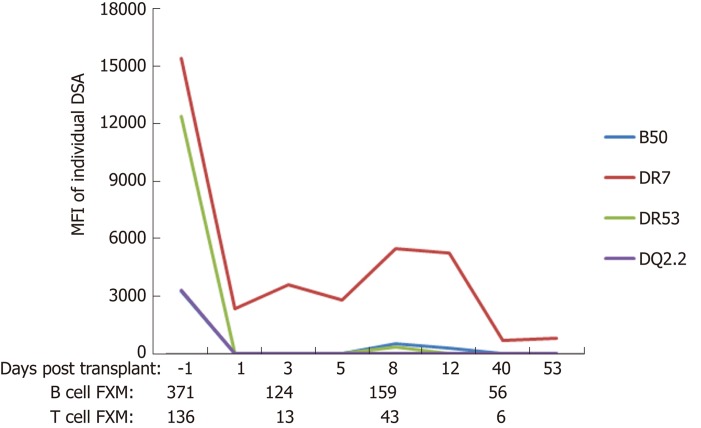
Compared to other solid organ transplants, liver transplant recipients have fewer episodes of antibody-mediated rejection (AMR). While donor specific alloantibodies can cause antibody-mediated hyperacute or acute rejection in other solid organs, their role in liver transplantation remains unclear[12-17]. Liver transplantation is often performed without a prospective cross-match and outcomes do not appear to be related to pre-transplant positive cross-matches[18,19]. The majority of recipients with preformed donor specific antibodies (DSA) show decline in their DSA levels after liver transplantation (Figure 1). InMoreover, persitent post-transplant DSA, do not appear to be negatively impact allograft survival within the first year following liver transplantation[20]. The observed decline in DSA post-transplant appears to be linked to the overall health of the liver allograft, as persistence of DSA or development of de novo DSA are observed more commonly in patients with allograft fibrosis or recurrent disease. However, this protection is not absolute and there is evidence of complement fixation in recipients with persistent DSA in protocol liver biopsies[20]. In patients with de novo DSA against class II human leukocyte antigens (HLA), overall survival is inferior when compared to those with no DSA[21]. Importantly, de novo DSA are not uncommon in patients in whom immunosuppression withdrawal is attempted, suggesting that most liver transplant patients require some, albeit minimal, immunosuppression to counter the host alloimmune responses[22].
Figure 1.
Typical course of donor-specific antibodies and flow cytometric cross match after liver transplant in a patient with fully functional liver allograft who is maintained on triple regimen immunosuppression (tacrolimus, mycophenolate, and prednisone). DSA: Donor specific antibodies; FXM: Flow cytometric cross match.
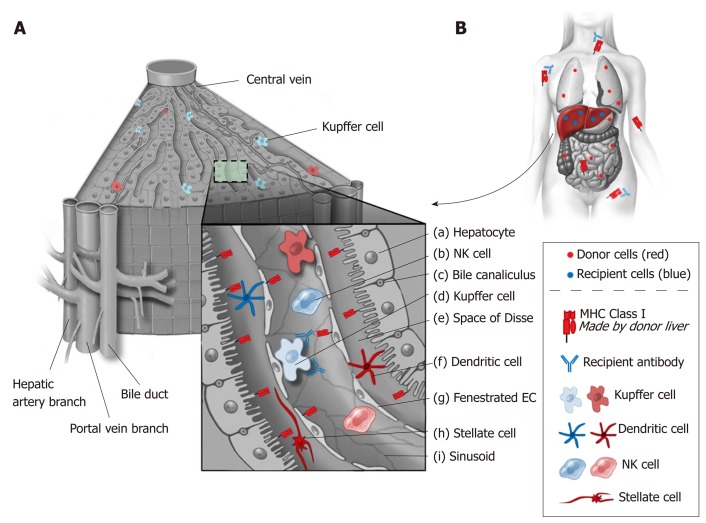
Several factors are felt to play a role in the liver’s resistance to antibody-mediated hyperacute rejection. These factors include the liver’s dual blood supply, its fenestrated sinusoidal complex, secretion of soluble major histocompatibility complex antigens, and its ability to absorb antibody (Figure 2). In contrast to other solid organs, the microvascular network of the liver is sinusoidal and lined by fenestrated endothelium with a scant underlying basement membrane (Figure 2, g)[23]. This sinusoidal network is in contrast to other organs that not only have a single afferent blood supply, but also have standard capillary microvasculature that results in ischemia when occluded by complement activated immune complexes. In the liver, only the biliary system is truly dependent on capillary microvasculature. This histological variation may result in a more limited, biliary-specific, form of injury in liver transplantation compared to other solid organs[24].
Figure 2.
Liver architecture and resident immune cells. A: The liver’s unique architecture and the large number of passenger immune cells that accompany it during transplant likely play a role in its immunologic activity. Class I major histocompatibility (MHC) antigens are strongly expressed on bile ducts (c) and to a lesser extent on sinusoidal and endothelial cells (g). By contrast, Class II MHC antigens are primarily expressed on capillary endothelium, sinusoidal cells and dendritic cells (f). It is also recognized that cell surface MHC antigens are not static and can change in response to host and allograft dynamics such as infection and rejection; B: Liver transplants secrete soluble class I MHC antigens that bind and neutralize systemically circulating antibodies. Kupffer cells (d) also are involved in neutralization of antibodies. As such, liver allografts are thought to function as sinks for circulating immune complexes. EC: Endothelial cell; NK: Natural killer; MHC: Major histocompatibility complex.
T cell-mediated rejection (TCMR)
Unlike other solid organs, cellular (T cell-mediated) rejection (TCMR) in liver transplantation follows a bimodal pattern of distribution with the majority of cellular rejections occurring very early (< 6 weeks) post-transplant[25]. When early cellular rejection episodes occur in liver transplant patients, these episodes require much less immunosuppression compared to TCMR in heart, pancreas, lungs, or kidney. Similarly, unlike other solid organs, these early episodes of TCMR do not appear to have a long-term impact of patient or allograft survival[25]. In liver transplantation, TCMR can largely be treated by increasing the dose of immunosuppression or by pulse steroids without requiring lymphocyte depleting antibody-based treatment.
THE LIVER’S ROLE IN MULTIVISCERAL TRANSPLANTATION
Liver-induced immunological tolerance to other allografts was first recognized in pigs, when liver allografts were noted to prevent rapid rejection of skin, kidney, and heart from the same donor[4]. This phenomenon was observed to be true for both orthotopic and auxiliary liver transplants[4]. Since these initial animal models, the same observation has been made in human multivisceral transplants[1,2,26-32]. Patients who undergo a combined liver-kidney transplantation (LKT) experience a lower number of kidney TCMR episodes compared to matched solitary kidney transplant recipients (4.2% vs 32.6%)[33]. The protective effect of the liver allograft on simultaneously transplanted kidneys persists long term[2,34]. In a study comparing kidney transplantation after liver or heart/lung transplantation, recipients who previously had a liver transplant had fewer episodes of TCMR in their kidneys (20% vs 36%)[34]. In addition, the observed rejection episodes were less severe (all rejection episodes were grade IA/IB), and grade II or grade III rejections were seen only after heart/lung transplantation (0% vs 16%)[34]. Similar protective effects against AMR have been reported by several groups. In Sweden, auxiliary liver transplantation was performed in a group of highly sensitized kidney patients who were otherwise deemed too risky to transplant, so as to facilitate kidney transplantation, with partial success[35]. We have also demonstrated protection of the heart allograft from AMR in highly sensitized patients by initial liver transplantation from the same donor[27]. All patients in this cohort had pre-existing DSA and positive cross-matches. In this group, there was an immediate decrease in DSA and stable cardiac and liver allograft function at mean follow up of nearly 2 years[27]. Furthermore, there was less cardiac allograft vasculopathy (assessed with 3D volumetric intravascular ultrasound), lower plaque volume, and slower plaque progression in the cardiac allografts of patients who underwent combined liver-heart transplantation[36]. In a series of 13 combined liver-lung transplants, only 3 patients experienced early rejection that was successfully treated with methylprednisolone[28]; this rate is much lower than that seen after solitary lung transplantation[28]. Similar protective effects have been observed in combined liver-intestine transplantation[26,37].
OVERVIEW OF ALLOIMMUNITY
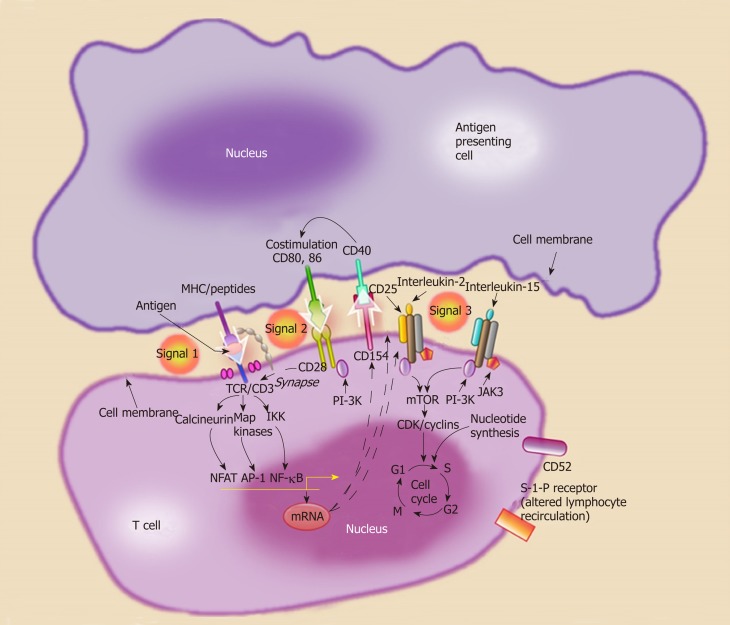
Detailed discussion of alloimmunity and downstream pathways after antigen presentation is beyond the scope of this article. Briefly, alloantigens from the transplanted organ are recognized by the host lymphocytes in the secondary lymphoid organs. Dendritic cells, macrophages, B cells, and endothelial cells can play the role of antigen-presenting cells (APC) under various circumstances. Allo-recognition occurs via three main pathways: (1) The direct pathway where T-cell receptors on host T cells directly interact with the HLA molecules on the surface of donor APC; (2) The indirect pathway where host APC process donor peptides (mostly derived from donor HLA) and present to host T cells; and (3) The semidirect pathway that involves membrane exchange between donor and host cells or extra-cellular vesicles[38,39]. T cell activation after antigen presentation (Signal 1) requires two additional signals. T-cell receptor interaction occurs through binding of costimulatory molecules on T cells (CD40, CD28) with corresponding ligands on the APCs (CD40L, CD80, CD86) (Signal 2) The presence of T cell stimulatory cytokines in the microenvironment (Signal 3) then results in T cell proliferation (Figure 3).
Figure 3.
Activation of naïve helper T cells is thought to occur through a three signal pathway. Signal 1, antigen recognition by the T cell receptor complex. Antigens are presented by major histocompatibility complex II cells [antigen presenting cells (APC) such dendritic cells]. Signal 2, co-stimulation, the interaction between the APC (CD80 and CD86) and the T cell (CD28). Signal 3, cellular proliferation and T cell differentiation into effector phenotypes (Th1, Th2), through cytokine stimulation. MHC: Major histocompatibility complex; APC: Antigen presenting cells.
THE LIVER AS A LYMPHOID AND IMMUNE-REGULATORY ORGAN
Liver architecture is uniquely adapted to provide immunomodulation after exposure to foreign antigens from the gastrointestinal tract. The liver receives a dual blood supply from the high-pressure systemic and the low-pressure portal circulation. These two circulations meet in the hepatic sinusoids resulting in low oxygen saturation, low pressure, and irregular flow facilitating interaction between antigens, T cells and other resident immune cells[40]. Although cell migration occurs in all types of solid organ transplants, the large population of migratory cells in liver allografts may explain the privileged tolerogenicity of the liver compared to other organs (Figure 2)[41]. The hepatocytes are arranged as sheets around the sinusoids. The liver is constantly exposed to microbial antigens carried through the portal circulation. In order to avoid immune activation in response to microbial antigens, liver has developed many molecular modifications. This is evident from high levels of lipopolysaccharide in the portal blood when none is detected in the systemic circulation under normal conditions[42]. Therefore, there is evolutionary advantage to the immunomodulatory role of liver parenchyma. In fact, the liver has been described as a “lymphoid”, “immunoregulatory” and “immunomodulatory” organ with various cells playing active role in supporting this function[13,42,40].
Immune cells of both lymphoid and myeloid lineage line the thin walled sinusoids, mostly in the space of Disse (Figure 2, e)[42]. These cells include Kupffer cells, dendritic cells, T cells, B cells, natural killer cells, natural killer T cells, hepatic stellate cells (HSC), and hematopoietic stem cells[42,43]. The phenotype of hepatic T cells also differs con-siderably from that observed in the periphery as reflected by a higher ratio (3.5:1 vs 1:2) of CD8+ vs CD4+ cells[44]. The unique architecture of liver sinusoids (low pressure, fenestrated system, expression of adhesion molecules) allows direct contact of circulating T cells with these cells. These alloantigen recognizing T cells are exposed to IL-10, PD-L1, and lack of co-stimulation leads to their destruction in the liver[42].
Endothelial cells
The sinusoidal endothelial cells (EC) comprise of 50% of non-parenchymal liver cells (Figure 2, g)[40,43]. The sinusoidal EC uniquely lack a basement membrane, are fenestrated, and express scavenger receptors that remove circulatory antigens[42]. ECs also express class I and II HLA and costimulatory molecules, making them potent APCs. However, their main role seems to be induction of tolerance because they respond to antigen stimulation by IL-10 secretion[42]. ECs increase their expression of FasL upon exposure to antigen and induce apoptosis of activated CD4+ T cells[45]. ECs also induce apoptosis of reactive CD8+ T cell via a pro-apoptotic Bcl-2 family member Bim[46].
Dendritic cells
The dendritic cells (DC) are professional APCs derived from bone marrow (Figure 2, f). The liver contains two types of DC: Plasmacytoid (pDC) and myeloid (mDC)[42]. The observed frequency of pDC in the liver is more than that in the lymph nodes[42]. The liver contains these cells in immature form. Under normal circumstances, these cells have low expression of the costimulatory molecule CD80[47]. Liver pDC play an important role in innate immunity, as they can produce and secrete IFN-γ[42]. On the other hand, pDC can express PD-L1 on their cell surface, and increased expression of PD-L1 on pDC in tolerant liver transplant patients has been correlated with elevated Tregs[48]. Liver mDC, unlike their counterparts isolated from other organs, appear to have a more inherent tolerant phenotype. For example, under normal circumstances, liver derived mDCs secrete IL-10 and mediate differentiation of T cells into Tregs[49]. Myeloid DC interactions with hepatic stellate cells may play a role in downregulating the immune response[50]. Hepatic stellate cells regulate mDC function by inducing signal transduction, activating transcription, and upregulating indolamine 2,3-dioxygenase (IDO)[50]. Therefore mDC, primed by hepatic stellate cells, have impaired ability to induce allogenic T cell responses[50].
Kupffer cells
The Kupffer cells (KC) are macrophages present in the intrasinusoidal space and comprise of 15% of all liver cells and 20% of non-parenchymal liver cells (Figure 2, d)[40,43]. Their main role is phagocytosis and cytokine secretion[40]. They also express HLA and costimulatory molecules, therefore they can present antigens to T cells[42]. However, compared to DC, their expression of HLA and costimulatory molecules is low[40]. KC secrete IL-10 and downregulate secretion of proinflammatory cytokines IL-6 and TNF-γ after exposure to lipopolysaccharide[51]. KC have also been found to secrete prostaglandin E2 (PGE2) and 15-deoxy-delta 12,14-PGJ2 (15d-PGJ2)[52]. PGE2 and 15d-PGJ2 inhibit activation of CD4+ T cells[52]. KC can also stimulate Tregs to secrete IL-10[53].
Natural killer cells
The liver contains a high percentage of natural killer (NK) cells (50% of liver lymphocytes) compared to peripheral blood (Figure 2, b)[40,42]. Two type of NK cells exist in the liver: CD3 - CD56dimCD16+CD27- (cytotoxic phenotype) and CD3-CD56brightCD16- CD27+ (cytokine secreting phenotype)[40]. Their role in alloimmunity and rejection appears to be influenced by their origin, such that the NK cells derived from the recipient are involved in rejection while donor-derived NK cells induce tolerance[54]. NK cells have been found to overexpress certain genes in tolerant liver transplant recipients signifying their important role in tolerance induction[55]. This is consistent with the upregulation of NK cell transcripts in tolerant liver transplant patients[56]. In addition, natural killer T cells (NKT), which express markers of NK cells along with the T-cell receptor Vα chain, appear to have a role in liver-induced tolerance, as tolerance is reversed in mice deficient in Vα14 NKT cells [57].
Hepatic stellate cells
Hepatic stellate cells (HSC) (Figure 2, h) are located in the subendothelial space and comprise 10% of the liver cells[43]. Known also as Ito cells, HSC store vitamin A and are involved in various fibrotic processes[43,58]. They express HLA class I, HLA class II and can activate T cells[58]. However, they also express PD-L1 that can lead to tolerance by inactivating activated T cells[42,59,60]. There is evidence that both parenchymal and nonparenchymal cells in the liver cause activation followed by apoptosis of the T cells in the liver allograft as well as in vitro[42,61]. Allogenic HSC can migrate to lymph nodes and induce expression of Tregs[62].
Mesenchymal stromal cells
The mesenchymal stromal cells (MSC) have also been localized in the liver[63]. MSC were first described by Friedenstein et al[64] as fibroblast like colonies in the bone marrow cultures[64]. Subsequently, MSC have been identified in various organs such as adipose tissue and the liver. These cells are characterized by their ability of trilineage differentiation, plastic adherence, and expression of certain markers on their surface[65]. Though liver derived MSC have not been well characterized yet, extensive research on the bone marrow and adipose tissue-derived MSC shows that MSC have the ability to modulate every cell of the immune system including macrophages, DC, NK cells, B cells, and T cells[66]. Interaction of APC with MSC program the former towards a tolerant phenotype as evident from increased IL-10 secretion[66]. MSC may modulate these responses by IDO[67]. Liver MSC appear to be more potent than the bone marrow- and adipose-derived MSC in their capacity to modulate alloimmune T-cell responses, at least in vitro[68].
THE LIVER’S ROLE IN TOLERANCE DEVELOPMENT AND THE UNDERLYING MECHANISMS
“True tolerance” is long-term acceptance of the allograft in the absence of any immunosuppression and without evidence of any DSA or signs of lymphocyte activation on biopsy[38]. True tolerance in human beings is a rare phenomenon. A more common scenario in clinical transplantation is stable graft function for at least 1 year in the absence of immunosuppression (“operational tolerance”) or with minimal immunosuppression (“prope tolerance”)[38,69-71]. Nearly 25% of adult and 60% of pedi-atric liver transplantation recipients can achieve operational tolerance[55,72]. While different tolerance mechanisms have been demonstrated in animal models and limited clinical studies, how exactly the liver allograft dampens the host alloimmune responses remains unknown.
Chimerism
The liver contains a population of hematopoietic stem cells[73]. In fact, liver transplants can behave like bone marrow transplants and rare cases of graft versus host disease have been described after liver transplantation[74-76]. In the earliest era of clinical liver transplantation, presence of donor cells in the recipient circulation was observed (chimerism), and chimerism was thought to lead to tolerance. Chimerism was first demonstrated in 1968, with karyotyping studies of male donor livers that had been transplanted into female recipients[77]. It was observed that the majority of the allograft retained its donor specificity, but the bone marrow derived passenger leukocytes, including KCs, were largely replaced with recipient female cells within 100 days. There was also evidence of adoptive immunity, with demonstration of newly acquired immunoglobulin types of donor specificity and donor derived anti-erythrocyte isoagglutinin-associated hemolysis. Despite these subtle clues, it would not be until almost two decades later, that the conviction that donor cells were wholly eliminated by the immune system would be challenged[7]. In 1992, decisive steps were taken to search for donor leukocytes in the blood and tissue of thirty human recipients of successful liver transplants performed up to 29 years prior. Female recipients from male donor were found to have microchimerism in their allografts and extrahepatic tissues 10 to 19 years post-transplant[7].
The early alloresponse after liver transplantation is characterized by recruitment of CD4+ T cells to the allograft and by their proliferation and IFN-γ production[78]. However, later there is selective reduction of T cells in the recipient[78]. At the same time the donor hematopoietic and T cells migrate from the allograft into the recipient. These donor-derived cells may survive in the recipient for a prolonged period of time and lead to chimerism observed after liver transplantation[79]. If donor hematopoietic cells constitute more than 1% of the recipient tissue, this is termed macrochimerism, and if they are < 1%, microchimerism[79]. In one study, all patients showed chimerism initially after liver transplantation, however chimerism decreased to variable degrees in the first year[79]. The rejection episodes in this study correlated with the lower degree of chimerism[79]. The patients with high degrees of chimerism had measurable in vitro alloreactive response after one year suggesting that chimerism did not lead to complete depletion of cytotoxic T cells[79]. Passenger cells in the liver may play a role in tolerance induction[6,7,79,80], as strategies to reduce the number of these passenger cells before transplantation prevents tolerance induction in experimental models[81].
T cell deletion
The unique architecture of the liver and the cross-talk between alloreactive T cells and liver inhabitant cells may play a significant role in tolerance induction by destroying host T cells[42,45,61]. The fenestrated endothelium of hepatic sinusoids facilitates direct contact between T cells and parenchymal cells leading to T cell deletion[74]. There is distinct expression of genes for T cell recruiting cytokines after LKT in tolerant patients[82]. This study found large number of CD3+ T cells and macrophages in the liver allograft but only a few in the simultaneously implanted kidney allograft[82]. It seems that increased expression of chemokines in the liver attracts alloreactive T cells that are subsequently destroyed by coming in contact with various liver cells inherently programmed towards tolerance induction. Another study found donor specific hypo-responsiveness, down regulation of T helper type I cytokine (IFN-γ) and no change in T helper type 2 cytokine (IL10) in the in vitro mixed lymphocyte reaction in recipients who achieved operational tolerance[83]. A similar cytokine pattern was found in the allograft on real time reverse transcriptase polymerase reaction (RT-PCR)[83]. Animal experiments have shown that T cells activated in the lymph nodes are capable of mediating immune response but T cells activated in the liver are short lived, defective, and are not able to mount immune responses[84].
Peripheral Tregs
An alternative model is the development of regulatory T cells (Treg) that actively regulate alloreactive T cells[62]. The liver cells secrete cytokines after antigen presentation that differentiates host T cells into a regulatory phenotype[48,49,53,62].
DSA neutralization
Under normal circumstances, liver has strong expression of class I HLA, secretes class I HLA antigens, and has weak class II expression[13,85]. Soluble class I HLA may absorb anti-HLA type I DSA leading to lower risk of antibody mediated rejection. We studied DSA levels in the serum of liver transplant recipients who did not receive any antibody-targeting induction[20]. Nearly 20% of recipients had preformed DSA that markedly decreased in all but three recipients 7 days after transplantation[20]. In rare instances, when DSA persisted, there was compliment activation and C4d deposition in the liver[20]. One year follow-up showed stable function despite antibody-mediated complement activation in patients with persistent DSA[20]. The unique architecture of hepatic sinusoids (fenestrated endothelium, lack of basement membrane, wider lumen) may confer resistance to complement activity. When endothelial injury does occur, it is seen in the microvasculature but not in the sinusoids[86]. This may be the reason for the increased susceptibility of peribiliary plexus to immunological or ischemic damage as its blood supply is derived from the hepatic artery[43,86]. DSA levels in the recipient seem to be the net result of two opposing factors: Host memory cells mounting immune attack and liver mediated neutralization of alloantibodies. Though protection against de novo class II DSA is less, incidence of de novo class II DSA is lower in liver transplantation compared to kidney transplantation[13].
At Mayo Clinic, we perform nearly 400 solid organ transplants in a year and many are combined liver-kidney transplants. Our group has investigated the liver’s role in modulating host alloimmune responses in these combined transplant recipients. Our program also employs protocol kidney biopsies to investigate the extent of subclinical and chronic alloreactivity. In our work, we have found that liver allografts from LKT protect the kidney from hyperacute/acute antibody mediated rejection [odds ratio 0.11, 95% confidence interval (CI) 0.03-0.32] and acute cellular rejection (odds ratio 0.13; 95%CI 0.06-0.27). Moreover, in assessing variables, the presence of a functioning liver allograft was the most predictive factor for protecting kidney allografts from the chronic injury (odds ratio 0.22, 95%CI 0.06-0.59)[1]. Solitary kidney transplant patients with positive DSA had 44% decline in GFR by 5 years while LKT patients with positive DSA had stable GFR[1]. The LKT recipients had a lower frequency of circulating CD8+, activated CD4+, and effector memory T cells, compared to kidney transplant alone (KTA) recipients[2]. Moreover, surviving T cells in LKT patients had a lower proliferative response to the donor cells (11.9% vs 42.9) [2,87]. This donor-specific hypo-responsiveness persisted after the first year of transplant[2]. We further compared molecular changes in the kidney allograft after LKT and KTA by doing RT-PCR on the protocol kidney biopsies[3]. We found that mechanisms underlying the liver’s protective role do not only operate inside the liver but extend to the kidney as there were distinct gene expressions seen on the RT-PCR[3]. The kidneys in LKT showed markedly increased expression of genes associated with tissue integrity/metabolism, even in cross-match positive transplants[3]. We hypothesize that liver inhabitant cells migrate into the circulation after liver transplant transplantation to home at the site of inflammation in the second co-transplanted solid organ and modulate host immune cells. While the key cell type is not yet known, this hypothesis is supported by our work and previously published studies[2,3,7,80,88].
Tolerance can be conceptualized as a state of fine balance between two opposing forces: Host immune system and liver mediated immune-regulation. This balance can be tilted towards rejection by stimulation of the immune system by tissue damage[89]. After exposure to endotoxins from infectious agents or Toll Like Receptors resulting from ischemia-reperfusion injury, there is upregulation of class II HLA on hepatic EC[13]. While operational tolerance has been demonstrated in a small number of patients, the majority of liver transplant patients require small amounts of immuno-suppression to counter the effect of host alloimmune response.
CONCLUSION
The liver microenvironment is inherently programmed towards induction of tolerance as a result of evolution to avoid immune activation on exposure to the gut delivered antigens. This has important implications for alloimmunity in the context of liver transplantation alone or in multivisceral transplants. Liver-induced protection against the host immune system is likely the result of a multitude of effects including microchimerism, deficient antigen presentation due to lack of costimulation, expression of inhibitory molecules, deletion of activated recipient T cells in the liver, a large antigen load in liver, active secretion of HLA molecules neutralizing alloantibody, and generation of Tregs in peripheral lymph nodes. Liver parenchymal as well as non-parenchymal cells, including MSC, may play a crucial role in some or all of the effects of the liver on the host immune system.
Footnotes
Conflict-of-interest statement: No conflicts of interest. No financial support.
Peer-review started: March 15, 2019
First decision: April 11, 2019
Article in press: May 18, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Guo JS, Ramos E S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
Contributor Information
Nitin Abrol, William J. von Liebig Center for Transplantation and Clinical Regeneration, Massyo Clinic, Rochester, MN 55905, United States.
Caroline C Jadlowiec, Transplant Center, Mayo Clinic, Phoenix, AZ 85259, United States.
Timucin Taner, William J. von Liebig Center for Transplantation and Clinical Regeneration, Massyo Clinic, Rochester, MN 55905, United States. taner.timucin@mayo.edu.
References
- 1.Taner T, Heimbach JK, Rosen CB, Nyberg SL, Park WD, Stegall MD. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int. 2016;89:909–917. doi: 10.1016/j.kint.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Taner T, Gustafson MP, Hansen MJ, Park WD, Bornschlegl S, Dietz AB, Stegall MD. Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year. Kidney Int. 2018;93:1465–1474. doi: 10.1016/j.kint.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Taner T, Park WD, Stegall MD. Unique molecular changes in kidney allografts after simultaneous liver-kidney compared with solitary kidney transplantation. Kidney Int. 2017;91:1193–1202. doi: 10.1016/j.kint.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 5.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes J, Zeevi A, Ramos H, Tzakis A, Todo S, Demetris AJ, Nour B, Nalesnik M, Trucco M, Abu-Elmagd K. Frequent achievement of a drug-free state after orthotopic liver transplantation. Transplant Proc. 1993;25:3315–3319. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, Kocova M, Ricordi C, Ildstad S, Murase N. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos HC, Reyes J, Abu-Elmagd K, Zeevi A, Reinsmoen N, Tzakis A, Demetris AJ, Fung JJ, Flynn B, McMichael J. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: Is it worth the risk? Transplantation. 2005;79:1157–1159. doi: 10.1097/01.tp.0000162084.46555.10. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM, Bridges ND, Turka LA, Tchao NK. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 11.Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, Hayashi M, Kanematsu T, Tanaka K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]
- 12.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: Implications for treatment. Am J Transplant. 2008;8:1367–1373. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng EY. The Role of Humoral Alloreactivity in Liver Transplantation: Lessons Learned and New Perspectives. J Immunol Res. 2017;2017:3234906. doi: 10.1155/2017/3234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 15.Jakab SS, Navarro VJ, Colombe BW, Daskalakis C, Herrine SK, Rossi S. Human leukocyte antigen and adult living-donor liver transplantation outcomes: An analysis of the organ procurement and transplantation network database. Liver Transpl. 2007;13:1405–1413. doi: 10.1002/lt.21264. [DOI] [PubMed] [Google Scholar]
- 16.Opelz G, Döhler B. Effect of human leukocyte antigen compatibility on kidney graft survival: Comparative analysis of two decades. Transplantation. 2007;84:137–143. doi: 10.1097/01.tp.0000269725.74189.b9. [DOI] [PubMed] [Google Scholar]
- 17.Watson R, Kozlowski T, Nickeleit V, Woosley JT, Schmitz JL, Zacks SL, Fair JH, Gerber DA, Andreoni KA. Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant. 2006;6:3022–3029. doi: 10.1111/j.1600-6143.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 18.Doyle HR, Marino IR, Morelli F, Doria C, Aldrighetti L, McMichael J, Martell J, Gayowski T, Starzl TE. Assessing risk in liver transplantation. Special reference to the significance of a positive cytotoxic crossmatch. Ann Surg. 1996;224:168–177. doi: 10.1097/00000658-199608000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon RD, Fung JJ, Markus B, Fox I, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. The antibody crossmatch in liver transplantation. Surgery. 1986;100:705–715. [PMC free article] [PubMed] [Google Scholar]
- 20.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, Heimbach JK. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Leary JG, Demetris AJ, Friedman LS, Gebel HM, Halloran PF, Kirk AD, Knechtle SJ, McDiarmid SV, Shaked A, Terasaki PI, Tinckam KJ, Tomlanovich SJ, Wood KJ, Woodle ES, Zachary AA, Klintmalm GB. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779–787. doi: 10.1111/ajt.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng S, Demetris AJ, Spain KM, Kanaparthi S, Burrell BE, Ekong UD, Alonso EM, Rosenthal P, Turka LA, Ikle D, Tchao NK. Five-year histological and serological follow-up of operationally tolerant pediatric liver transplant recipients enrolled in WISP-R. Hepatology. 2017;65:647–660. doi: 10.1002/hep.28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanless IR. Physioanatomical Considerations. In: Schiff ER, Meddrey WC, Reddy KR, editors. Schiff’s Diseases of the Liver. 12th ed. Chichester (West Sussex): Wiley-Blackwell; 2018. p. 86. [Google Scholar]
- 24.Batts KP, Moore SB, Perkins JD, Wiesner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988;45:376–379. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Jadlowiec CC, Morgan PE, Nehra AK, Hathcock MA, Kremers WK, Heimbach JK, Wiesner RH, Taner T. Not All Cellular Rejections Are the Same: Differences in Early and Late Hepatic Allograft Rejection. Liver Transpl. 2019;25:425–435. doi: 10.1002/lt.25411. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Elmagd K, Reyes J, Todo S, Rao A, Lee R, Irish W, Furukawa H, Bueno J, McMichael J, Fawzy AT, Murase N, Demetris J, Rakela J, Fung JJ, Starzl TE. Clinical intestinal transplantation: New perspectives and immunologic considerations. J Am Coll Surg. 1998;186:512–25; discussion 525-7. doi: 10.1016/s1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daly RC, Pereira NL, Taner T, Gandhi MJ, Heimbach JK, Dearani JA, Edwards BS, Kushwaha SS. Combined Heart and Liver Transplantation in Highly Sensitized Patients: Protection of the Cardiac Allograft from Antibody Mediated Rejection by Initial Liver Implantation. J Heart Lung Transplant. 2017;36:S200. [Google Scholar]
- 28.Grannas G, Neipp M, Hoeper MM, Gottlieb J, Lück R, Becker T, Simon A, Strassburg CP, Manns MP, Welte T, Haverich A, Klempnauer J, Nashan B, Strueber M. Indications for and outcomes after combined lung and liver transplantation: A single-center experience on 13 consecutive cases. Transplantation. 2008;85:524–531. doi: 10.1097/TP.0b013e3181636f3f. [DOI] [PubMed] [Google Scholar]
- 29.Gonwa TA, Nery JR, Husberg BS, Klintmalm GB. Simultaneous liver and renal transplantation in man. Transplantation. 1988;46:690–693. doi: 10.1097/00007890-198811000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Olausson M, Mjörnstedt L, Nordén G, Rydberg L, Mölne J, Bäckman L, Friman S. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7:130–136. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 31.Fung J, Makowka L, Tzakis A, Klintmalm G, Duquesnoy R, Gordon R, Todo S, Griffin M, Starzl T. Combined liver-kidney transplantation: Analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20:88–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Fong TL, Bunnapradist S, Jordan SC, Selby RR, Cho YW. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76:348–353. doi: 10.1097/01.TP.0000071204.03720.BB. [DOI] [PubMed] [Google Scholar]
- 33.Creput C, Durrbach A, Samuel D, Eschwege P, Amor M, Kriaa F, Kreis H, Benoit G, Bismuth H, Charpentier B. Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation. Am J Transplant. 2003;3:348–356. doi: 10.1034/j.1600-6143.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 34.Schachtner T, Stein M, Reinke P. Kidney transplant recipients after nonrenal solid organ transplantation show low alloreactivity but an increased risk of infection. Transpl Int. 2016;29:1296–1306. doi: 10.1111/tri.12856. [DOI] [PubMed] [Google Scholar]
- 35.Fung J, Griffin M, Duquesnoy R, Shaw B, Starzl T. Successful sequential liver-kidney transplantation in a patient with performed lymphocytotoxic antibodies. Transplant Proc. 1987;19:767–768. [PMC free article] [PubMed] [Google Scholar]
- 36.Topilsky Y, Raichlin E, Hasin T, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP, Gandhi MJ, Maltais S, Park SJ, Daly RC, Lerman A, Kushwaha SS. Combined heart and liver transplant attenuates cardiac allograft vasculopathy compared with isolated heart transplantation. Transplantation. 2013;95:859–865. doi: 10.1097/TP.0b013e31827eef7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T Intestinal Transplant Association. Intestinal transplant registry report: Global activity and trends. Am J Transplant. 2015;15:210–219. doi: 10.1111/ajt.12979. [DOI] [PubMed] [Google Scholar]
- 38.Beaudreuil S, Samuel D, Rouas-Freiss N, Durrbach A. New aspect of immunosuppressive treatment in liver transplantation. How could you induce tolerance in liver transplantation? Transpl Immunol. 2007;17:98–107. doi: 10.1016/j.trim.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 40.Huang H, Lu Y, Zhou T, Gu G, Xia Q. Innate Immune Cells in Immune Tolerance After Liver Transplantation. Front Immunol. 2018;9:2401. doi: 10.3389/fimmu.2018.02401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 43.Demetris AJ, Bellamy CO, Gandhi CR, Prost S, Nakanuma Y, Stolz DB. Functional Immune Anatomy of the Liver-As an Allograft. Am J Transplant. 2016;16:1653–1680. doi: 10.1111/ajt.13749. [DOI] [PubMed] [Google Scholar]
- 44.Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: The enemy within. Am J Transplant. 2004;4:466–474. doi: 10.1111/j.1600-6143.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 45.Tokita D, Shishida M, Ohdan H, Onoe T, Hara H, Tanaka Y, Ishiyama K, Mitsuta H, Ide K, Arihiro K, Asahara T. Liver sinusoidal endothelial cells that endocytose allogeneic cells suppress T cells with indirect allospecificity. J Immunol. 2006;177:3615–3624. doi: 10.4049/jimmunol.177.6.3615. [DOI] [PubMed] [Google Scholar]
- 46.Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, Carbone FR, Heath WR. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosma BM, Metselaar HJ, Mancham S, Boor PP, Kusters JG, Kazemier G, Tilanus HW, Kuipers EJ, Kwekkeboom J. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl. 2006;12:384–393. doi: 10.1002/lt.20659. [DOI] [PubMed] [Google Scholar]
- 48.Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, Thomson AW. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–377. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 49.Kushwah R, Wu J, Oliver JR, Jiang G, Zhang J, Siminovitch KA, Hu J. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur J Immunol. 2010;40:1022–1035. doi: 10.1002/eji.200939782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, Thomson AW, Gandhi CR. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189:3848–3858. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Büschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 52.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmon C, Sanchez-Fueyo A, O'Farrelly C, Houlihan DD. Natural Killer Cells and Liver Transplantation: Orchestrators of Rejection or Tolerance? Am J Transplant. 2016;16:751–757. doi: 10.1111/ajt.13565. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Wozniak LJ, Rodder S, Heish S, Talisetti A, Wang Q, Esquivel C, Cox K, Chen R, McDiarmid SV, Sarwal MM. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. Am J Transplant. 2012;12:1218–1228. doi: 10.1111/j.1600-6143.2011.03928.x. [DOI] [PubMed] [Google Scholar]
- 56.Sarwal MM. Fingerprints of transplant tolerance suggest opportunities for immunosuppression minimization. Clin Biochem. 2016;49:404–410. doi: 10.1016/j.clinbiochem.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, Taniguchi M, Ikeda S. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761–1767. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 60.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 61.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, Fung JJ, Thomson AW. Apoptosis within spontaneously accepted mouse liver allografts: Evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 62.Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, Vodovotz Y, Huang C, Thomson AW, Gandhi CR. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: Role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007;16:717–728. doi: 10.3727/000000007783465154. [DOI] [PubMed] [Google Scholar]
- 64.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 65.Dominici M. Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 66.Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy. 2016;18:160–171. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 68.Taner T, Gustafson M, Hansen M, Dietz A, Stegall M. Liver Mesenchymal Stem Cells Inhibit T Cell Alloresponses. Am J Transplant. 2017:17. [Google Scholar]
- 69.Calne RY. Prope tolerance: The future of organ transplantation--from the laboratory to the clinic. Transplantation. 2004;77:930–932. doi: 10.1097/01.tp.0000117776.14277.03. [DOI] [PubMed] [Google Scholar]
- 70.Cortesini R, Renna-Molajoni E, Cinti P, Pretagostini R, Ho E, Rossi P, Suciu-Foca Cortesini N. Tailoring of immunosuppression in renal and liver allograft recipients displaying donor specific T-suppressor cells. Hum Immunol. 2002;63:1010–1018. doi: 10.1016/s0198-8859(02)00442-1. [DOI] [PubMed] [Google Scholar]
- 71.Cortesini R, Suciu-Foca N. The concept of "partial" clinical tolerance. Transpl Immunol. 2004;13:101–104. doi: 10.1016/j.trim.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Martínez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, Lerut J, Latinne D, Margarit C, Bilbao I, Brouard S, Hernández-Fuentes M, Soulillou JP, Sánchez-Fueyo A. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–319. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 73.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 74.Bishop GA, Ierino FL, Sharland AF, Hall BM, Alexander SI, Sandrin MS, Coates PT, McCaughan GW. Approaching the promise of operational tolerance in clinical transplantation. Transplantation. 2011;91:1065–1074. doi: 10.1097/TP.0b013e318215e742. [DOI] [PubMed] [Google Scholar]
- 75.Chaib E, Silva FD, Figueira ER, Lima FR, Andraus W, D'Albuquerque LA. Graft-versus-host disease after liver transplantation. Clinics (Sao Paulo) 2011;66:1115–1118. doi: 10.1590/S1807-59322011000600035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanaan Z, Tse W. A novel strategy in managing post-liver transplant acute graft-versus-host-disease: The new era (T)rojan horse. Biol Blood Marrow Transplant. 2016;22:S401–S2. [Google Scholar]
- 77.Kashiwagi N, Porter KA, Penn I, Brettschneider L, Starzl TE. Studies of homograft sex and of gamma globulin phenotypes after orthotopic homotransplantation of the human liver. Surg Forum. 1969;20:374–376. [PMC free article] [PubMed] [Google Scholar]
- 78.Ahearn AJ, Klein I, Hayden T, Lui F, Lee K, Tang Q, Kang S. Liver transplant tolerance: Differences in the persistence of various alloreactive t cell subsets: 2486. Transplantation. 2010:90. [Google Scholar]
- 79.Bettens F, Tiercy JM, Campanile N, Giostra E, Majno P, Rubbia L, Roosnek E, Mentha G, Villard J. Microchimerism after liver transplantation: Absence of rejection without abrogation of anti-donor cytotoxic T-lymphocyte-mediated alloreactivity. Liver Transpl. 2005;11:290–297. doi: 10.1002/lt.20360. [DOI] [PubMed] [Google Scholar]
- 80.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 81.Sun J, McCaughan GW, Gallagher ND, Sheil AG, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60:233–236. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Ingelsten M, Karlsson-Parra A, Granqvist AB, Mölne J, Olausson M, Haraldsson B, Nyström J. Postischemic inflammatory response in an auxiliary liver graft predicts renal graft outcome in sensitized patients. Transplantation. 2011;91:888–894. doi: 10.1097/TP.0b013e3182100f19. [DOI] [PubMed] [Google Scholar]
- 83.Takatsuki M, Uemoto S, Inomata Y, Sakamoto S, Hayashi M, Ueda M, Kanematsu T, Tanaka K. Analysis of alloreactivity and intragraft cytokine profiles in living donor liver transplant recipients with graft acceptance. Transpl Immunol. 2001;8:279–286. doi: 10.1016/s0966-3274(01)00027-2. [DOI] [PubMed] [Google Scholar]
- 84.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 86.Demetris AJ. Bellamy C, Hübscher SG, O'Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ, Del Bello A, Reinholt FP, Haga H, Adeyi O, Czaja AJ, Schiano T, Fiel MI, Smith ML, Sebagh M, Tanigawa RY, Yilmaz F, Alexander G, Baiocchi L, Balasubramanian M, Batal I, Bhan AK, Bucuvalas J, Cerski CTS, Charlotte F, de Vera ME, ElMonayeri M, Fontes P, Furth EE, Gouw ASH, Hafezi-Bakhtiari S, Hart J, Honsova E, Ismail W, Itoh T, Jhala NC, Khettry U, Klintmalm GB, Knechtle S, Koshiba T, Kozlowski T, Lassman CR, Lerut J, Levitsky J, Licini L, Liotta R, Mazariegos G, Minervini MI, Misdraji J, Mohanakumar T, Mölne J, Nasser I, Neuberger J, O'Neil M, Pappo O, Petrovic L, Ruiz P, Sağol Ö, Sanchez Fueyo A, Sasatomi E, Shaked A, Shiller M, Shimizu T, Sis B, Sonzogni A, Stevenson HL, Thung SN, Tisone G, Tsamandas AC, Wernerson A, Wu T, Zeevi A, Zen Y. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816–2835. doi: 10.1111/ajt.13909. [DOI] [PubMed] [Google Scholar]
- 87.Taner T, Hansen M, Park W, Stegall M. Phenotypic and functional assessment of t cell alloimmunity after liver transplantation. Am J Transplant. 2017:17. [Google Scholar]
- 88.Pan Q, Fouraschen SM, Kaya FS, Verstegen MM, Pescatori M, Stubbs AP, van Ijcken W, van der Sloot A, Smits R, Kwekkeboom J, Metselaar HJ, Kazemier G, de Jonge J, Tilanus HW, Wagemaker G, Janssen HL, van der Laan LJ. Mobilization of hepatic mesenchymal stem cells from human liver grafts. Liver Transpl. 2011;17:596–609. doi: 10.1002/lt.22260. [DOI] [PubMed] [Google Scholar]
- 89.Testro AG, Visvanathan K, Skinner N, Markovska V, Crowley P, Angus PW, Gow PJ. Acute allograft rejection in human liver transplant recipients is associated with signaling through toll-like receptor 4. J Gastroenterol Hepatol. 2011;26:155–163. doi: 10.1111/j.1440-1746.2010.06324.x. [DOI] [PubMed] [Google Scholar]