Abstract
PURPOSE:
Breast cancer survivorship care plans (SCP) have limited content addressing women’s health issues. This trial tested if young breast cancer survivors who receive a web-based, women’s health SCP were more likely to improve on at least one of four targeted issues (hot flashes, fertility-related concerns, contraception and vaginal symptoms) compared to attention controls.
METHODS:
A randomized controlled trial recruited female survivors ages 18–45 at diagnosis, 18–50 at enrollment, completed primary cancer treatment, and had a significant women’s health issue: moderate fertility-related concerns; ≥4 hot flashes/day with ≥1 of moderate severity; ≥1 moderate vaginal atrophy symptom; or not contracepting/using less effective methods. Survivors underwent stratified, block randomization with equal allocation to intervention and control groups. The intervention group accessed the online SCP; controls accessed curated resource lists. In intention-to-treat analysis, the primary outcome of improvement in at least one issue by 24 weeks was compared by group.
RESULTS:
182 participants (86 intervention, 96 control), mean age 40.0±5.9 and 4.4±3.2 years since diagnosis, were randomized. Sixty-one intervention group participants (70.9%) improved, compared to 55 controls (57.3%) (OR 1.82, 95%CI0.99–3.4, p=0.057). The following issue-specific improvements were observed in the intervention versus control arms: fertility-related concerns (27.9% vs. 14.6%, OR 2.3, 95%CI 1.1–4.8); hot flashes (58.5% vs. 55.8%, OR 1.1, 95%CI 0.57–2.2); vaginal symptoms (42.5% vs. 40.7%, OR 1.1, 95%CI 0.6–2.0); contraception (50% vs. 42.6%, OR 1.4, 95%CI 0.74–2.5).
CONCLUSIONS:
In young breast cancer survivors, a novel, web-based SCP did not result in more change in the primary outcome of improvement in at least one of the 4 targeted women’s health issues, over the attention control condition. The intervention was associated with improved infertility concerns, supporting efficacy of disseminating accessible, evidence-based women’s health information to this population.
Keywords: breast cancer, survivorship care plan, fertility, reproductive health, randomized controlled trial
Background
Young breast cancer survivors undergo chemotherapy and/or endocrine therapy, treatments that impair ovarian function and result in significant women’s health issues in survivorship.[1] These include infertility and pregnancy concerns, limited contraception choices, hot flashes, and sexual dysfunction, which negatively impact quality of life.[2–7][8–12] Despite substantial evidence from research, practice guidelines and clinical expertise on women’s health screening and symptom management, many survivors have limited access to high quality information throughout the cancer continuum; in particular, there are substantial unmet informational needs during the prolonged time after primary cancer treatment when fertility concerns are revisited and symptoms are experienced.[13–17]
Survivorship care plans (SCPs) aim to inform cancer survivors about the effects of cancer and cancer treatment, guide follow up care and improve care coordination.[18] As general and breast cancer SCPs have limited women’s health guidance, the investigators developed an evidence-based guide for women’s health care for young breast cancer survivors who have completed primary cancer treatment. The web-based SCP intervention was developed by conducting systematic reviews of women’s health management strategies, critical appraisal of professional practice guidelines and online resources, and generation and refinement of the prototype with stakeholders, including survivors and their healthcare providers (providers).[19]
The SCP intervention was designed to be web-based and accessible to both survivors and the provider of their choice, as survivors actively seek health information from their providers and on the Internet.[20, 21] Oncology and primary care providers are the most common providers for this population, yet many do not have women’s health expertise specific to young breast cancer survivors.[22] Hence, a randomized controlled clinical trial (RCT) was conducted to test the hypothesis that young breast cancer survivors who received the SCP intervention were more likely to improve on at least one of four targeted women’s health issues, i.e. hot flashes, fertility-related concerns, contraception and vaginal symptoms, compared to attention controls who did not receive the intervention.
Methods
Study Design and Young Breast Cancer Survivor Participants
The RCT tested the efficacy of a web-based intervention in improving four women’s health issues in female young breast cancer survivors. The full protocol has been published previously.[23] Eligible survivors were diagnosed with stages 0-III breast cancer between ages 18 to 45, completed primary cancer treatment (surgery, chemotherapy and/or radiation), were ages 18 to 50 at study enrollment, and reported a clinically significant women’s health issue. Clinically significant issues were defined as: ≥4 hot flashes per day, ≥1 at least moderate in severity; moderate to high fertility-related concerns; at risk of unintended pregnancy; ≥1 vaginal symptom at least moderate in severity.[24–27] Survivors were able to enter the study any time after completion of primary cancer treatment.
The trial was conducted remotely in entirety. Survivors were recruited via emails and social media in partnership with Young Survival Coalition, the Susan G. Komen Foundation–San Diego, Army of Women, and Research Match; provider referrals; and emails to participants of the investigators’ prior observational studies.[28] Individuals were screened for eligibility, consented, completed study baseline questionnaires, and nominated a provider through the study website. Informed consent was obtained from all individual participants included in the study. Recruitment occurred between March, 2016 and June, 2017. The study was approved by the institutional review board of the University of California, San Diego ( NCT02667626).
Randomization, Follow Up, and Intervention
Survivors were randomized in a 1:1 ratio to the intervention or attention control arms and followed for 24 weeks. Computerized, block randomization was stratified by the four women’s health issues. Participants in the intervention arm received access to the full web-based SCP intervention, as frequently as they desired, and twice weekly text message women’s health action prompts. The SCP intervention encompassed four sections for each of the four issues: 1) a 2-page SCP framed in a question and answer format; 2) a detailed summary of the systematic review results, including hyperlinks to primary research articles; 3) a description of relevant clinical guidelines with hyperlinks to them; 4) curated web-based resources for survivors and providers (Supplemental Material). Text messages with reproductive health prompts encompassed tips for managing a symptom. An example is, “Anti-depressants like venlafaxine (75 mg daily) have been shown to reduce hot flash frequency and severity. Contact your healthcare provider this week to discuss venlafaxine.”
Participants in the attention control arm received access to only the curated web-based resource lists and twice weekly study adherence text messages, to standardize the number of contacts between groups. An example of an attention control text messages is, “Please take a look at (Study Web Link) to complete your study tasks.”
The two groups underwent equivalent number of contacts and study duration, but varied in the content of the web-based materials. All participants completed questionnaires at enrollment, 12 and 24 weeks and reported daily hot flashes via text messaging for 24 weeks. Daily, two text messages were pushed to all YBCS participants to ascertain hot flash frequency and severity.
Healthcare provider participants
Providers nominated by survivors as their preferred provider for managing women’s health issues were contacted by email, phone, fax and mail for study recruitment. Interested providers were directed to the study website for eligibility screening, consent, completion of the enrollment questionnaire, and access to their patient’s intervention materials, i.e. full web-based SCP intervention versus curated web-based resource lists. Providers then completed a 24-week questionnaire. Providers were also able to complete all study procedures (consent, questionnaires) and access intervention materials in paper format.
Blinding
Study staff and survivor and provider participants were blinded to treatment allocation. To preserve blinding of participants, consent forms stated that participants would receive information and tools on self-care of reproductive health symptoms but did not detail content of the SCP or state how this information differs between the intervention and control groups. Upon study completion, the website accounts of survivor and provider participants assigned to the attention control arm automatically changed to allow access to the full SCP intervention.
Assessment of Study Outcomes
Study outcomes were assessed by self-report at 12 and 24 weeks. Hot flash frequency and severity over the prior 24 hours were assessed, and the hot flash score was calculated.[25] Fertility-related concerns were measured using the fertility potential and pregnancy subscales of the Reproductive Concerns After Cancer scale (RCAC), a multidimensional scale with good internal consistency (α=0.82) and construct validity.[26] Scores >3 indicated moderate to high concern and are associated with depression.[10] Using questions and definitions derived from the National Survey for Family Growth,[27] contraception outcomes were calculated in participants at risk of unintended pregnancy, defined as having a uterus and at least one ovary, sexually active, ≤45 years old, and not attempting pregnancy. Sexual health was measured using the Vaginal Atrophy Symptoms Score, a 4-item scale on vaginal dryness, soreness, irritation and dyspareunia experienced in the prior 4 weeks.[24] The scale was summarized by averaging responses with higher scores indicating a greater level of vaginal atrophy.
For each issue, participants were also asked whether they undertook actions to improve the symptom. These actions were part of the SCP content and text message reproductive health tips for the intervention group. For example, for hot flashes, the stem of the question read, “During the past 12 weeks, have you used any of the following self-care methods or medications to help with hot flashes?”, and examples of responses included: I have not had hot flashes, dress in layers, keep a small fan with you during the day, acupuncture, and anti-depressant medications, such as venlafaxine, citalopram, or paroxetine.
Providers reported preparedness and confidence in talking to their patients about each of the four issues. Each preparedness item had a 4-point Likert scale response (very unprepared to very prepared). Each confidence item had a 7-point Likert scale response (not at all confident to extremely confident).
Statistical analysis
The primary analysis was intent-to-treat. McNemar’s test with continuity correction was used to test for differences in provider-reported preparedness and confidence. The primary composite outcome was survivor-reported improvement in at least one outcome over the 24-week study, defined as: 50% decrease in enrollment hot flash score; fertility and pregnancy concern scale scores ≤3; use of highly effective contraceptive methods (intrauterine devices, female sterilization, male partner sterilization, combined hormonal contraception, progestin implants or injections); or 50% decrease in enrollment vaginal atrophy symptom score. Linear and logistic mixed effect models were used to impute missing outcomes for the primary composite outcome for 10 participants (5.5%). Logistic regression models were used to test for differences by intervention versus control arm; odds ratios (OR) with 95% confidence intervals (CI) for intervention effect are reported.
To quantify intervention effects on each women’s health issue, secondary analyses were performed to compare changes in each issue with only participants who met the enrollment criterion for that issue were included. We compared each outcome between the intervention and control groups using linear mixed effect models for the continuous outcomes (hot flash score, vaginal atrophy symptom score, fertility-related concerns score) and logistic mixed effects models for binary outcomes (use of effective contraceptive method). Two-sided p-values <0.05 were considered statistically significant.
A priori sample-size calculations were based on detecting clinically relevant changes in hot flash score [25]. With a sample size of 50 per arm, there was 80% power to detect a 0.57 effect-size between groups on hot flash score changes from baseline to 24 weeks based on a 2-sided t-test with alpha as 0.05. Sample size was inflated for potential clustering effect from participants having the same provider via an intraclass correlation of 0.1, 20% loss during run-in, and 20% drop-out for a total enrollment of 196 participants.
Results
Participant flow
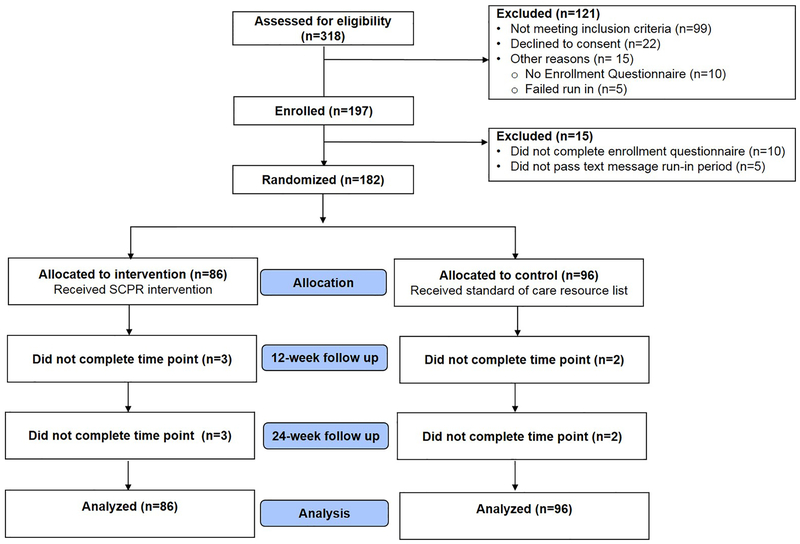
Three hundred eighteen individuals underwent eligibility screening, the majority of whom (82.7%) met criteria for at least one women’s health issue (Figure 1). One hundred eighty-two survivors underwent randomization, 86 to the intervention arm and 96 to the control arm. Participants were from 38 states in the U.S. and were recruited from cancer advocacy organizations (61.5%), previous investigator-led observational studies (13.7%), ResearchMatch (5.5%), provider referrals (3.9%), word of mouth (3.9%), and other sources (11.5%). During 6 months of follow up, one participant (0.5%) left the study due to health reasons. Two participants in each the intervention group and the control group did not complete at least one follow up (2%).
Figure 1:
Consort diagram of study flow
Baseline characteristics of survivors and providers
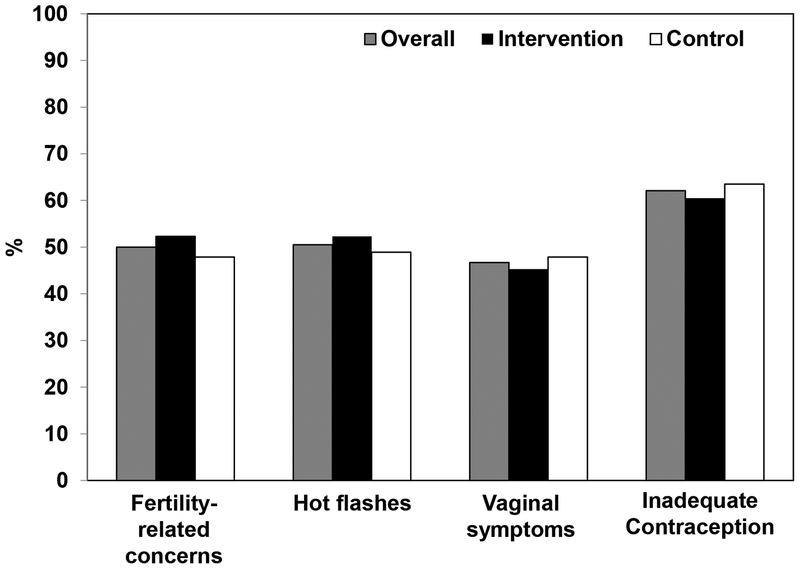
Table 1 depicts baseline characteristics of survivor participants. The mean age (standard deviation) at enrollment was 40.0 (5.9) years. The majority was white (86%), non-Hispanic (95%) and college graduates (89%). Mean age at breast cancer diagnosis was 35.6 (5.4) years, with the majority of participants undergoing surgery, chemotherapy, radiation and endocrine therapy. Significant hot flashes, fertility-related concerns, vaginal symptoms, and inadequate contraception were reported by 50 to 62.1% of participants (Figure 2), with 71% reporting more than one issue. Randomization led to similar distributions of baseline characteristics and women’s health issues between the intervention and control arms (Table 1, Figure 2).
Table 1:
Breast cancer survivor participant baseline characteristics by intervention arm
| Characteristic | Overall N (%) (N=182) |
Intervention N (%) (N=86) |
Attention Control N (%) (N=96) |
|---|---|---|---|
| Age at enrollment (y) 25–35 35–40 41–45 46–50 |
51 (28.0) 48 (26.4) 54 (29.7) 29 (15.9) |
25 (29.1) 20 (23.3) 27 (31.4) 14 (16.3) |
26 (27.1) 28 (29.2) 27 (28.1) 15 (15.6) |
| Race White Asian Black or African American American Indian or Alaskan Native Mixed and other race |
157 (86.3) 7 (3.8) 8 (4.4) 2 (1.1) 8 (4.4) |
77 (89.5) 4 (4.7) 1 (1.2) 2 (2.3) 2 (2.3) |
80 (83.3) 3 (3.1) 7 (7.3) 0 (0.0) 6 (6.3) |
| Hispanic ethnicity | 10 (5.5) | 4 (4.7) | 6 (6.3) |
| Partnered relationship status | 149 (81.9) | 74 (86.0) | 75 (78.1) |
| Education Did not complete college College graduate |
20 (11.0) 162 (89.0) |
9 (10.5) 77 (89.5) |
11 (11.5) 85 (88.5) |
| Incomea <$51,000 ≥$51,000 |
25 (14.6) 146 (85.4) |
10 (11.8) 75 (88.2) |
15 (17.4) 71 (82.6) |
| 7 (3.8) | 5 (5.8) | 2 (2.1) | |
| Co-morbidities Hypertension Diabetes Mood disorder Osteopenia or osteoporosis |
12 (6.6) 7 (3.8) 59 (32.4) 28 (15.4) |
7 (8.1) 5 (5.8) 30 (34.9) 13 (15.1) |
5 (5.2) 2 (2.1) 29 (30.2) 15 (15.6) |
| Cancer characteristics | |||
| 36–45 | 92 (50.6) | 44 (51.2) | 48 (50.0) |
| Years since diagnosis <1 1–2 3–5 >5 |
20 (11.0) 52 (28.6) 47 (25.8) 63 (34.6) |
9 (10.5) 27 (31.4) 18 (20.9) 32 (37.2) |
11 (11.5) 25 (26.0) 29 (30.2) 31 (32.3) |
| Cancer surgery | 180 (98.9) | 85 (98.8) | 95 (99.0) |
| Radiation | 124 (68.1) | 54 (62.8) | 70 (72.9) |
| Chemotherapy | 156 (85.7) | 72 (83.7) | 84 (87.5) |
| Biologic therapy | 48 (26.4) | 22 (25.6) | 26 (27.1) |
| Hormone therapy | 142 (78.0) | 67 (77.9) | 75 (78.1) |
| Cancer stagea 0 1 2 3 |
10 (5.6) 49 (27.2) 81 (45.0) 40 (22.2) |
8 (9.5) 21 (25.0) 38 (45.3) 17 (20.2) |
2 (2.1) 28 (29.1) 43 (44.8) 23 (24.0) |
| Cancer Recurrence | 7 (3.8) | 6 (7.0) | 1 (1.0) |
| Reproductive characteristics | |||
| Hysterectomy | 19 (10.4) | 9 (10.5) | 10 (10.4) |
| Bilateral oophorectomy | 32 (17.6) | 13 (15.1) | 19 (19.8) |
| Menses past year 0 1–3 4–9 10–12 |
78 (42.9) 35 (19.2) 36 (19.8) 33 (18.1) |
36 (41.9) 16 (18.6) 18 (20.9) 16 (18.6) |
42 (43.7) 19 (19.8) 18 (18.8) 17 (17.7) |
| Prior live birth | 100 (55.0) | 52 (60.5) | 48 (50.0) |
| Desires another baby in the future | 77 (42.3) | 40 (46.5) | 37 (38.5) |
| Sexually active with male partner | 162 (89.0) | 79 (91.9) | 83 (86.5) |
| Fertility concerns score, mean (SD) | 3.2 (1.1) | 3.3 (1.1) | 3.2 (1.1) |
| Pregnancy concerns score, mean (SD) | 3.0 (1.0) | 3.0 (0.9) | 2.9 (1.1) |
| Hot flash frequency, mean (SD) | 3.4 (5.2) | 3.4 (4.7) | 3.3 (5.6) |
| Hot flash score, mean (SD) | 7.1 (13.5) | 7.1 (12.4) | 7.1 (14.6) |
| Vaginal atrophy symptom score Mean (SD) Median (range) |
3.3 (2.5) 3.0 (0–12) |
3.4 (2.6) 3.0 (0–12) |
3.3 (2.3) 3.0 (0–10) |
| Birth control Not using WHO Class I/II WHO Class III/IV |
52 (28.6) 85 (46.7) 45 (24.7) |
24 (27.9) 40 (46.5) 22 (25.6) |
28 (29.1) 45 (46.9) 23 (24.0) |
Numbers do not sum up to 182 for some variables due to missing data; WHO, World Health Organization
Figure 2:
At enrollment, proportions of breast cancer survivor participants (n=182) with hot flashes, fertility-related concerns, vaginal symptoms and inadequate contraception, by intervention arm.
Survivor participants nominated 165 unique providers, of which 54 (33%) enrolled with up to 5 recruitment attempts. Only three providers were nominated by more than one survivor participant, with each of these providers associated with survivor participants who were randomized to the same study arm. Table 2 depicts baseline characteristics of provider participants. Most providers were female (81%), white (83%) and a physician in oncology (46.3%) or gynecology (27.8%). Mean time in clinical practice was 15.4 ± 8.2 years.
Table 2:
Healthcare provider baseline characteristics assigned to their breast cancer patient, by intervention arm
| Characteristic | Overall N (%) (n=54) |
Intervention N (%) (n=31) |
Attention Control N (%) (n=23) |
|---|---|---|---|
| Age (y) 30–39 40–49 50–59 ≥60 |
12 (22.2) 20 (37.0) 17 (31.5) 5 (9.3) |
8 (25.8) 10 (32.3) 10 (32.3) 3 (9.6) |
4 (17.4) 10 (43.5) 7 (30.4) 2 (8.7) |
| Female | 44 (81.5) | 26 (83.9) | 18 (78.3) |
| Race White Asian Black or African American American Indian or Alaskan Native Mixed and other race |
45 (83.3) 5 (9.3) 2 (3.7) 0 (0.0) 2 (3.7) |
26 (83.9) 2 (6.5) 1 (3.2) 0 (0.0) 2 (6.4) |
19 (82.6) 3 (13.0) 1 (4.4) 0 (0.0) 0 (0.0) |
| Profession Physician Nurse practitioner, physician assistant or nurse |
45 (83.4) 9 (16.6) |
26 (83.9) 5 (16.1) |
19 (82.6) 4 (17.4) |
| Specialty Oncology Family or Internal medicine Obstetrics/Gynecology Psychiatry/Psychology |
25 (46.3) 12 (22.2) 15 (27.8) 2 (3.7) |
12 (38.7) 9 (29.0) 9 (29.0) 1 (3.3) |
13 (56.5) 3 (13.0) 6 (26.1) 1 (4.4) |
| Number of breast cancer patients seen per year ≤20 21–40 >40 |
23 (42.6) 10 (18.5) 21 (38.9) |
15 (48.4) 6 (19.3) 10 (32.3) |
8 (34.8) 4 (17.4) 11 (47.8) |
| Preparednessa for discussing Hot flashes Fertility-related concerns Vaginal symptoms Contraception |
49 (90.7) 37 (68.5) 47 (87.0) 47 (87.0) |
29 (93.5) 22 (71.0) 29 (93.5) 30 (96.8) |
20 (87.0) 15 (65.2) 18 (78.3) 17 (73.9) |
| Confidenceb in discussing Hot flashes Fertility-related concerns Vaginal symptoms Contraception |
38 (70.4) 27 (50.0) 37 (68.5) 40 (74.1) |
21 (67.7) 16 (51.6) 21 (67.7) 23 (74.2) |
17 (73.9) 11 (47.8) 16 (69.6) 17 (73.9) |
Preparedness indicates very prepared or prepared, versus very unprepared/unprepared
Confidence indicates extremely confident, very confident, or quite confident, versus not at all/slightly/somewhat/moderately confident
Preparedness for talking with their patient about each women’s health issue was dichotomized as prepared, versus unprepared (Table 2). The majority of providers reported that they were prepared across all 4 issues, but providers were significantly less likely to report preparedness in discussing fertility-related issues, compared to hot flashes (p=0.001), vaginal symptoms (p=0.009) and contraception (p=0.016).
Confidence in discussing each women’s health issue was dichotomized as quite confident, versus moderately confident or less. Only 50% of providers reported confidence in discussing fertility-related issues, significantly lower than proportions for hot flashes (p=0.015), vaginal symptoms (p=0.016), and contraception (p=0.002). Randomization of survivors led to similar distributions of baseline characteristics, preparedness and confidence among provider participants by intervention arm.
Outcomes
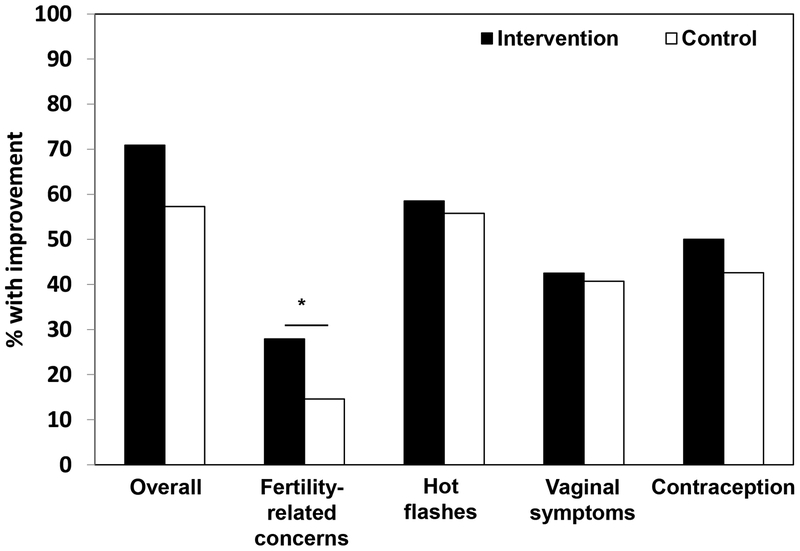
Among 182 survivor participants, 61/86 (70.9%) women in the intervention arm improved in at least one women’s health issue, compared with 55/96 (57.3%) of women in the control arm, OR 1.82, 95% CI 0.99–3.4, p=0.057 (Figure 3). For each issue, the following improvements were observed in the intervention versus control arms: 50% decrease in hot flash score (58.5% vs. 55.8%, OR 1.11, 95% CI 0.57–2.18, p=0.75); fertility-related concerns scale scores ≤3 (27.9% vs. 14.6%, OR 2.27, 95% CI 1.1–4.84, p=0.03); 50% decrease in vaginal atrophy symptom score (42.5% vs. 40.7%, OR 1.08, 95% CI 0.59–1.99, p=0.81); use of highly effective contraception (50% vs. 42.6%, OR 1.35, 95% CI 0.74–2.46, p=0.32).
Figure 3:
By 24 weeks, proportions of breast cancer survivor participants (n=182) with improvement in their women’s health issue(s) by intervention arm, * denotes p<0.05
In secondary analysis, 92 survivors reported ≥4 daily hot flashes, with ≥1 moderate in severity. Among these participants, 26/45 (57.8%) in the intervention group experienced a 50% decrease in hot flash score, compared with 24/47 (51.1%) of controls (OR 1.31, 95% CI 0.58–3.01, p=0.52), with no significant time by intervention effect. Seventy-three percent in the intervention and 42.2% in the control group reported doing a suggested tip for hot flash management (p=0.004).
Ninety-one survivors had moderate or high fertility-related concerns. In the intervention group, 24/45 (53.3%) improved with no or low fertility or pregnancy concerns, compared with 14/46 (30.4%) in the control group (OR 2.61, 95% CI 1.12–6.29, p=0.03). In the intervention group, 40/86 (46.5%) desired another baby in the future, compared to 37/96 (38.5%) in the control group (p=0.28). Thirteen percent in the intervention and 21.4% in the control group reported doing a suggested tip for fertility concerns (p=0.34).
Eighty-five survivors reported vaginal symptoms over the prior 4 weeks as at least moderate in severity. In the intervention group, 10/39 (25.6%) experienced a 50% decrease in vaginal atrophy symptom score, compared with 11/46 (23.9%) in the control group (OR 1.1, 95% CI 0.4–2.96, p=0.85). Forty-four percent in the intervention and 26.2% in the control group reported doing a suggested tip for vaginal symptom management (p=0.09).
Among participants, 113 had a uterus and at least one ovary, were sexually active and ≤45 years. In the intervention group, 23/52 (44.2%) reported using a highly effective birth control method over follow up, compared with 21/61 (34.4%) in the control group (OR 1.51, 95% CI 0.71–3.25, p=0.29). Ten percent in the intervention and 8.6% in the control group reported doing a suggested tip for birth control management (p=0.86).
There were no significant interactions between time since cancer diagnosis and intervention for both primary and secondary outcomes (data not shown). While provider visits were not required, 69% of participants reported seeing a provider to manage reproductive health issues by 24 weeks (22% in the intervention group and 13% in the control group by 12 weeks [p=0.13]; 66% in the intervention group and 71% in the control group by 24 weeks, p=0.34).
Discussion
This RCT is first to test the effects of a SCP intervention focused on fertility-related concerns, contraception, hot flash and vaginal symptom outcomes in young breast cancer survivors. Clinical improvements were observed in both the intervention and attention control arms across all four issues. The web-based SCP did not result in the hypothesized change in the primary outcome of improvement in at least one of the 4 targeted women’s health issues, over the attention control condition. For infertility concerns, the intervention group had significantly higher odds of experiencing low concerns by the end of 6 months, a clinically meaningful improvement as fertility-related distress is related to poorer quality of life and depression.[29]
The finding on fertility-related concerns was consistent with provider participants’ report of less preparedness and confidence in managing fertility; where the vast majority of providers expressed confidence in managing hot flashes, contraception and sexual health, only half felt confident in discussing fertility-related concerns. The gap in fertility care among survivors is well-described, despite longstanding oncology and reproductive medicine society guidelines and quality measures for fertility care.[30–33] Importantly, this care is needed not only at diagnosis, but also after cancer treatment because of persistent and prevalent infertility concerns and misperceptions on fertility and pregnancy risks.[9, 34] Optimal strategies to meet fertility-related concerns in survivorship have not been defined,[35] but this study supports that provision of concise, evidence-based information on fertility and pregnancy after breast cancer meets more of the informational needs than recommended resources alone. Given the observed positive effect of the fertility-related SCP, future studies can leverage the materials as one implementation strategy for fertility care.
The finding of improvement across arms may result from effective materials presented to both groups. Intervention materials were designed to improve self-efficacy by providing concise and up-to-date evidence, actionable steps for symptom management, in addition to hyperlinks to primary studies, clinical guidelines, and curated resources. However, controls had access to the curated resources, which contained hyperlinked online resources from professional organizations that investigators selected for valid and specific content on managing these issues. It is possible that these resources alone impacted participant engagement and education. Moreover, participants were engaged in daily text messaging, which may have served as a motivational prompt. However, natural remission of symptoms, with individuals entering trials when they are most symptomatic, and regression to the mean cannot be excluded.[36]
The intervention did not result in more improvements in hot flashes or vaginal symptoms than access to curated resources. However, the majority of participants who had hot flash or vaginal symptoms at enrollment had at least 50% decrease in symptom scores during follow up. This proportion for hot flash improvement is higher than the observed 1/2 to 1/3 in the placebo arms of many hot flash trials.[25] Many participants reported doing a suggested tip to improve symptoms during the study, supporting that a health behavior was undertaken on study. A limitation is that details on dose and duration of patient behaviors to improve these symptoms were not collected.
Highly effective contraceptive methods were not undertaken by most participants, even when restricted to participants at risk of unintended pregnancy. Non-contraception among cancer survivors is high compared to the general U.S. population, and may result from limited contraception choices and misperceptions of infertility risk.[17, 34] While hormone-free intrauterine devices are highly effective, reversible and minimally invasive, low uptake by the end of this study supports that delivering educational materials alone is unable to address other implementation barriers such as provider access and cost.[37]
The trial contributes new knowledge on the efficacy of cancer SCPs. A recent systematic review summarized 13 randomized studies on SCPs, observed heterogeneous interventions, timing, and outcome measures, and noted largely null effects on physical and psychological health outcomes.[38] Positive findings included increased implementation of survivorship care.[38–40] For general survivorship care, trials suggest that the addition of a SCP document does not augment the care delivered by an oncology provider.[41–43] In contrast, the current study focused on women’s and reproductive health, an area less commonly managed by oncology providers, and the data support this SCP as an effective strategy for delivering specialty expertise to young breast cancer survivors. As our intervention did not require provider counseling, it is unknown whether provider review of the intervention with their patient would result in more clinical improvement.[44] Importantly, the study supports a tailored approach to delivering information to survivors; secondary analyses showed larger effect sizes when restricted to participants who met criteria for that particular health issue.
The study’s primary strength is in intervening on women’s health, an unmet survivorship care need for young breast cancer survivors. Additional strengths include use of valid and clinically relevant outcome measures, use of a web-based format to increase reach, collaboration with breast cancer patient advocacy groups for intervention generation and study recruitment, high follow up rates, and intent-to-treat analysis to minimize bias. All aspects of the study were conducted remotely, from screening for symptoms to delivering evidence-based information, rendering future dissemination of such a tailored intervention for young survivors feasible.
A limitation of the study is that the population was primarily white and highly educated, impacting generalizability of findings to under-represented minority women and lower SES women. The attention control condition may have been active, which may result in larger than expected change in control outcomes. The enrolled provider sample was modest, limiting provider reinforcement of survivors’ actions, but similar to other survivorship care or physician-patient dyad studies.[45, 46] Even with this limitation, having a similar distribution in provider characteristics at baseline between survivors in the intervention and control arms would minimize treatment bias that could result from differences in baseline provider confidence and preparedness. We did not embed measures of the dose of use of study materials in the study platform, ask participants about how much they used these materials, or measure change in knowledge, which may be observed via usage statistics in future work. While the content did not have patient-specific treatment summaries, breast cancer treatment and management of women’s health issues are fairly homogeneous. Future studies on the women’s health SCP will need to address these limitations and how content is to be modified to retain all four issues or limit to addressing fertility alone.
The low-cost, web-based intervention did not result in a statistically significant increase in the composite outcome across four targeted women’s health issues, compared to the attention control condition. The intervention was associated with greater improvement in infertility concerns. Follow up work is needed on how the intervention can help to disseminate evidence-based women’s health information to more survivors with the goal of meeting the reproductive health and quality of life needs of this population.
Supplementary Material
Acknowledgments
This study was conducted in collaboration with Young Survival Coalition, Susan B. Komen Foundation – San Diego, Army of Women.
Funding
This work was supported by the California Breast Cancer Research Program 20OB-0144; National Cancer Institute P30-CA008748.
Footnotes
Supplemental materials
Sample of SCP content (SCP and Resources) on fertility-related concerns for participants randomized to the Intervention arm. Similar materials are provided for hot flashes, contraception, and sexual health. Participants randomized to the attention control arm were only able to access the list of curated web-based resources.
Ethical Standards
The clinical trial complies with the current laws of the United States. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors declare that they have not conflicts of interest.
References
- 1.Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, Freeman EW, Gracia CR, DeMichele A: Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer 2010, 116(3):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold EB, Flatt SW, Pierce JP, Bardwell WA, Hajek RA, Newman VA, Rock CL, Stefanick ML: Dietary factors and vasomotor symptoms in breast cancer survivors: the WHEL Study. Menopause 2006, 13(3):423–433. [DOI] [PubMed] [Google Scholar]
- 3.Caan BJ, Emond JA, Su HI, Patterson RE, Flatt SW, Gold EB, Newman VA, Rock CL, Thomson CA, Pierce JP: Effect of postdiagnosis weight change on hot flash status among early-stage breast cancer survivors. J Clin Oncol 2012, 30(13):1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cvancarova M, Samuelsen SO, Magelssen H, Fossa SD: Reproduction rates after cancer treatment: experience from the Norwegian radium hospital. J Clin Oncol 2009, 27(3):334–343. [DOI] [PubMed] [Google Scholar]
- 5.Safarinejad MR, Shafiei N, Safarinejad S: Quality of life and sexual functioning in young women with early-stage breast cancer 1 year after lumpectomy. Psychooncology 2013, 22(6):1242–1248. [DOI] [PubMed] [Google Scholar]
- 6.Cook-Andersen H, Flatt SW, Komrokian S, DeMichele A, Su HI: Breast cancer patients have lower rates of contraception use: a prospective cohort study. In: American Society of Reproductive Medicine Annual Meeting: 2011; 2011. [Google Scholar]
- 7.Patel A, Schwarz EB, Society of Family P: Cancer and contraception. Release date May 2012. SFP Guideline #20121. Contraception 2012, 86(3):191–198. [DOI] [PubMed] [Google Scholar]
- 8.Yoo C, Yun MR, Ahn JH, Jung KH, Kim HJ, Kim JE, Park JY, Park KO, Yoon DH, Kim SB: Chemotherapy-induced amenorrhea, menopause-specific quality of life, and endocrine profiles in premenopausal women with breast cancer who received adjuvant anthracycline-based chemotherapy: a prospective cohort study. Cancer chemotherapy and pharmacology 2013, 72(3):565–575. [DOI] [PubMed] [Google Scholar]
- 9.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP: Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 2004, 22(20):4174–4183. [DOI] [PubMed] [Google Scholar]
- 10.Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP: Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat 2010, 123(2):477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP: Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012, 118(6):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL: Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 2012, 104(5):386–405. [DOI] [PubMed] [Google Scholar]
- 13.Johns C, Seav SM, Dominick SA, Gorman JR, Li H, Natarajan L, Mao JJ, Irene Su H: Informing hot flash treatment decisions for breast cancer survivors: a systematic review of randomized trials comparing active interventions. Breast Cancer Res Treat 2016, 156(3):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seav S, Dominick S, Stepanyuk B, Gorman J, Chingos D, Ehren J, Krychman M, Su HI: Management of sexual dysfunction in breast cancer survivors: a systematic review. Women’s Midlife Health 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorman JR, Bailey S, Pierce JP, Su HI: How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv 2012, 6(2):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartula I, Sherman KA: Screening for sexual dysfunction in women diagnosed with breast cancer: systematic review and recommendations. Breast Cancer Res Treat 2013, 141(2):173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominick SA, McLean MR, Whitcomb BW, Gorman JR, Mersereau JE, Bouknight JM, Su HI: Contraceptive Practices Among Female Cancer Survivors of Reproductive Age. Obstet Gynecol 2015, 126(3):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medicine Io: Implementing Cancer Survivorship Care Planning: Workshop Summary. Washington, D.C.: National Academy of Sciences; 2006. [Google Scholar]
- 19.Gorman JR, Julian AK, Roberts SA, Romero SAD, Ehren JL, Krychman ML, Boles SG, Mao J, Irene Su H: Developing a post-treatment survivorship care plan to help breast cancer survivors understand their fertility. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan AS, Moldovan-Johnson M, Gray SW, Hornik RC, Armstrong K: An analysis of the association between cancer-related information seeking and adherence to breast cancer surveillance procedures. Cancer Epidemiol Biomarkers Prev 2013, 22(1):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen SK, Ingledew PA: Tangled in the breast cancer web: an evaluation of the usage of web-based information resources by breast cancer patients. Journal of cancer education : the official journal of the American Association for Cancer Education 2013, 28(4):662–668. [DOI] [PubMed] [Google Scholar]
- 22.Mayer EL, Gropper AB, Neville BA, Partridge AH, Cameron DB, Winer EP, Earle CC: Breast cancer survivors’ perceptions of survivorship care options. J Clin Oncol 2012, 30(2):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark SS, Natarajan L, Chingos D, Ehren J, Gorman JR, Krychman M, Kwan B, Mao JJ, Myers E, Walpole T et al. : Design of a randomized controlled trial on the efficacy of a reproductive health survivorship care plan in young breast cancer survivors. Contemporary clinical trials 2018, 77:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davila GW, Singh A, Karapanagiotou I, Woodhouse S, Huber K, Zimberg S, Seiler J, Kopka SL: Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol 2003, 188(2):382–388. [DOI] [PubMed] [Google Scholar]
- 25.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H: Methodologic lessons learned from hot flash studies. J Clin Oncol 2001, 19(23):4280–4290. [DOI] [PubMed] [Google Scholar]
- 26.Gorman JR, Malcarne V, Pierce JP, Su HI: A multidimensional scale to measure the reproductive concerns of young adult cancer survivors. Journal of Cancer Survivorship 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Survey of Family Growth [http://www.cdc.gov/nchs/nsfg/nsfg_2006_2010_puf.htm]
- 28.Gorman JR, Roberts SC, Dominick SA, Malcarne VL, Dietz AC, Su HI: A Diversified Recruitment Approach Incorporating Social Media Leads to Research Participation Among Young Adult-Aged Female Cancer Survivors. Journal of adolescent and young adult oncology 2014, 3(2):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan S, Perz J, Ussher JM, Peate M, Anazodo A: Systematic review of fertility-related psychological distress in cancer patients: Informing on an improved model of care. Psychooncology 2018. [DOI] [PubMed] [Google Scholar]
- 30.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW: Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018, 36(19):1994–2001. [DOI] [PubMed] [Google Scholar]
- 31.Fallat ME, Hutter J: Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics 2008, 121(5):e1461–1469. [DOI] [PubMed] [Google Scholar]
- 32.NCCN Clinical Practice Guidelines in Oncology: Adolescent and Young Adult (AYA) Oncology, Version 1. 2019-October 3, 2018
- 33.Ethics Committee of the American Society for Reproductive Medicine. Electronic address Aao: Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril 2018, 110(3):380–386. [DOI] [PubMed] [Google Scholar]
- 34.Hadnott TN, Stark SS, Medica ACO, Dietz AC, Martinez ME, Whitcomb BW, Su HI: Perceived Infertility and Contraceptive Use in the Female, Reproductive Age Cancer Survivor. Fertility Sterility, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anazodo A, Sullivan E, Travaglia J, Saunders C, Bradford N, Barr R, Birdsall M, McDowell S, Woodruff T, Sender L et al. : How Can We Improve Oncofertility Care For Patients? A Systematic Scoping Review of Current International Practice and Models. Human Reproduction Update In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnett AG, van der Pols JC, Dobson AJ: Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005, 34(1):215–220. [DOI] [PubMed] [Google Scholar]
- 37.Secura GM, Madden T, McNicholas C, Mullersman J, Buckel CM, Zhao Q, Peipert JF: Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014, 371(14):1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen PB, DeRosa AP, Henderson TO, Mayer DK, Moskowitz CS, Paskett ED, Rowland JH: Systematic Review of the Impact of Cancer Survivorship Care Plans on Health Outcomes and Health Care Delivery. J Clin Oncol 2018, 36(20):2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolaije KA, Ezendam NP, Vos MC, Pijnenborg JM, Boll D, Boss EA, Hermans RH, Engelhart KC, Haartsen JE, Pijlman BM et al. : Impact of an Automatically Generated Cancer Survivorship Care Plan on Patient-Reported Outcomes in Routine Clinical Practice: Longitudinal Outcomes of a Pragmatic, Cluster Randomized Trial. J Clin Oncol 2015, 33(31):3550–3559. [DOI] [PubMed] [Google Scholar]
- 40.Maly RC, Liang LJ, Liu Y, Griggs JJ, Ganz PA: Randomized Controlled Trial of Survivorship Care Plans Among Low-Income, Predominantly Latina Breast Cancer Survivors. J Clin Oncol 2017, 35(16):1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emery JD, Jefford M, King M, Hayne D, Martin A, Doorey J, Hyatt A, Habgood E, Lim T, Hawks C et al. : ProCare Trial: a phase II randomized controlled trial of shared care for follow-up of men with prostate cancer. BJU Int 2017, 119(3):381–389. [DOI] [PubMed] [Google Scholar]
- 42.Boekhout AH, Vincent AD, Dalesio OB, van den Bosch J, Foekema-Tons JH, Adriaansz S, Sprangers S, Nuijen B, Beijnen JH, Schellens JH: Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011, 29(29):3862–3868. [DOI] [PubMed] [Google Scholar]
- 43.Brothers BM, Easley A, Salani R, Andersen BL: Do survivorship care plans impact patients’ evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecologic oncology 2013, 129(3):554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hudson MM, Leisenring W, Stratton KK, Tinner N, Steen BD, Ogg S, Barnes L, Oeffinger KC, Robison LL, Cox CL: Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: a randomized controlled trial. J Clin Oncol 2014, 32(35):3974–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KC, Tolbert E, Hannum SM, Radhakrishnan A, Zorn K, Blackford A, Greco S, Smith K, Snyder CF: Comparing Web-Based Provider-Initiated and Patient-Initiated Survivorship Care Planning for Cancer Patients: A Randomized Controlled Trial. JMIR Cancer 2016, 2(2):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulda KG, Hahn KA, Young RA, Marshall JD, Moore BJ, Espinoza AM, Beltran NM, McFadden P, Crim AD, Cardarelli R: Recruiting Practice-based Research Network (PBRN) physicians to be research participants: lessons learned from the North Texas (NorTex) needs assessment study. J Am Board Fam Med 2011, 24(5):610–615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.