Abstract
Background
A prognostic factor for patients with acute or subacute idiopathic interstitial pneumonias (IIPs) or acute exacerbation (AE) of collagen vascular diseases-related interstitial pneumonia (CVD-IP) has not been established. We aimed to determine whether the Charlson comorbidity index (CCI) could serve as a prognostic factor for patients with these patients.
Methods
We assessed baseline prognostic factors among patients with acute or subacute IIPs and AE of CVD-IP who were admitted to hospital between January 2014 and December 2017. We classified them as survivors and non-survivors at 3 months and compared their age, sex, CCI, blood parameters [lactate dehydrogenase (LDH), surfactant protein (SP)-D, Krebs von den Lungen-6, and partial pressure of oxygen in arterial blood/fraction of the inspiratory oxygen], high resolution CT (HRCT) scores and treatment.
Results
Sixty eight patients with (mean age, 75 years), were assessed. All patients received steroid pulse therapy. We found that 45 of acute or subacute IIPs and 16 of AE of CVD-IP were included. Stepwise multivariate analysis selected CCI (OR, 1.306; 95% CI, 1.090–1.573; P=0.004), serum LDH (OR, 1.003; 95% CI, 1.001–1.005; P=0.002), and sex (OR, 8.555; 95% CI, 1.729–154.978; P=0.038) as significant predictors of 3-month mortality among these patients. Three-month mortality was significantly worse among patients with high (≥4) than low (<4) CCI (mortality rates: 63.2% vs. 16.3%, P<0.001). Moreover, the composite scoring system including CCI, serum LDH, and sex was acceptable (Bootstrap AUC, 0.859; Bootstrap C-index, 0.747).
Conclusions
The composite scoring system including CCI, sex, and serum LDH could be a useful mortality prediction tool for patients with acute or subacute IIPs and AE of CVD-IP requiring steroid pulse therapy.
Keywords: Composite scoring system, sex, idiopathic pulmonary fibrosis (IPF), lactate dehydrogenase (LDH), mortality
Introduction
Acute or subacute idiopathic interstitial pneumonias (IIPs) including acute exacerbation (AE) of IIPs, acute interstitial pneumonia (AIP), and cryptogenic organizing pneumonia (COP) and AE of collagen vascular diseases-related interstitial pneumonia (CVD-IP) is a pathological condition with a poor prognosis that manifests as rapid respiratory failure (1-5).
Although the prognosis of these patients differs depending on the histological findings of the underlying type of IP, obtaining tissue samples for histological evaluation from patients with acute respiratory failure is difficult (6). Thus, non-invasive biomarkers that can accurately predict the prognosis are needed. Although serum Krebs von den Lungen-6 (KL-6), serum heat shock protein (HSP)-47 and arterial carboxyhemoglobin have been reported as biomarkers, clinical biomarkers have not been established (7-9).
The Charlson comorbidity index (CCI) is a summed score of 19 comorbidities weighted according to severity (10). The CCI was developed to assess risk of death from comorbidities and it has been widely applied as a prognostic indicator for patients with colorectal cancer, advanced non-small cell lung carcinoma and acute myocardial infarction (11-13). However, the relevance of CCI to the prediction of acute phase of IP is unknown.
The present study retrospectively investigated which of the clinical parameters of sex, age, diagnosis, blood biomarkers, high-resolution computed tomography (HRCT) scores and CCI could help predict prognosis at the time of steroid pulse therapy. In addition, we attempted to construct a scoring system that could predict 3-month mortality more accurately by combining parameters determined to be significantly prognostic in the present study.
Methods
This retrospective, observational study proceeded at Yokohama City University Hospital and Yokohama City University Medical center between November 2014 and November 2017. The medical records of 68 patients who met the following inclusion criteria were reviewed. The inclusion criteria were as follows: acute or subacute IIPs including AE of idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP), AIP, and COP, and AE of CVD-IP; treated with steroid pulse therapy. The exclusion criteria were as follows: other diffuse parenchymal lung disease including sarcoidosis; not treated steroid pulse therapy. The medical records including age, sex, CCI, diagnosis of IP, blood parameters [lactate dehydrogenase (LDH) (normal, <225 U/L), surfactant protein (SP)-D (normal, <110 ng/mL), KL-6 (normal, <500 U/mL), and partial pressure of oxygen in arterial blood (PaO2)/fraction of the inspiratory oxygen (FiO2) (PaO2/FiO2 ratio)], semi-quantitative HRCT scores which two pulmonologists and two radiologists independently assessed (14), and treatment rates. We classified them as survivors or non-survivors at three months from hospitalization and compared the collected data.
The diagnosis of IIPs was confirmed by physical findings, serological testing, HRCT finding, and lung biopsy specimens, based on the official statement for IIPs including IPF (1,15). However, patients whose lung biopsy could not be performed due to acute respiratory failure were diagnosed based on the radiological classification (1,15). The diagnosis of CVD-IP was confirmed by physical findings, serological testing, and HRCT findings that were consistent with IP. Histological evaluation of lung biopsy specimens was undertaken for the exclusion of other specific diseases. The diagnosis of drug-induced lung injury and acute hypersensitivity pneumonitis (AHP) were based on the previous reported criteria (16,17). AE of IIPs including IPF and NSIP and AE of CVD-IP was defined as unexplained worsening of dyspnea; hypoxemia or worsening or severely impaired gas exchange; new alveolar infiltrates on radiograph; and absence of an alternative explanation such as infection, pulmonary embolism, pneumothorax, or heart failure (1-5). The cause of AE was divided into idiopathic, infection, and aspiration (18).
Statistical analysis
Data were statistically analyzed using JMP12 (SAS Institute Inc., Cary, NC, USA) and are expressed as means ± SD. Groups were compared using Wilcoxon rank-sum tests. Optimal parameter cut-off values were determined from receiver operator characteristics (ROC) curves. Survival curves were generated using the Kaplan-Meier method and compared using log rank tests. Predictors of the 3-month mortality were determined using multiple stepwise regression analysis. The predictive performance of the scoring systems was investigated using ROC and concordance index (C-index). The reported area under the ROC curve (AUC) and C-index is the mean values calculated from 10,000 bootstrap samples. Values with P<0.05 were considered significant.
Ethics approval
The Institutional Review Board at Yokohama City University Hospital approved this study (approval number B171100003). In all patients, consent for participation of this retrospective study was obtained by disclosing a clinical study including the description of opt-out (https://www.yokohama-cu.ac.jp/amedrc/ethics/ethical/fuzoku_optout.html).
Results
Patients’ characteristics
Among 68 patients, 45 were diagnosed with acute or subacute IIPs including 17 of AE of IPF and 28 of other IIPs (AE of idiopathic NSIP, n=18; AIP, n=6; COP, n=4). The remaining 23 patients had 16 of AE of CVD-IP and 7 of other IPs (drug-induced, n=6; AHP, n=1). CVD-IP included 6 of rheumatoid arthritis, 5 of anti-neutrophil cytoplasmic antibodies associated vasculitis, 4 of polymyositis/dermatomyositis, and 1 of Sjögren syndrome. The causes of AE were 44 of idiopathic, 5 of infection, and 2 of aspiration. All patients were treated with steroid pulse therapy. Table 1 shows the clinical characteristics of the patients. Table 2 showed clinical difference between patients with IPF, other IIPs, and CVD-IP groups. There were no significant differences in clinical parameters other than honeycomb score.
Table 1. Patients’ characteristics.
| Characteristics | Died within 3 months (n=20) | Survived 3 months (n=48) | Total patients (n=68) | P |
|---|---|---|---|---|
| Age, y | 76.5 [72.3–81.5] | 75.0 [71.5–80.8] | 76.0 [72.3–80.6] | 0.513 |
| Male sex | 19 (95%) | 30 (63%) | 49 (72%) | 0.007 |
| CCI | 4 [2–7] | 2 [1–3] | 2 [1–4] | 0.004 |
| Diagnosis of IP | 0.630 | |||
| Acute/subacute IIPs | ||||
| AE of IPF | 6 (30%) | 11 (23%) | 17 (25%) | |
| Other IIPs | 10 (50%) | 18 (38%) | 28 (41%) | |
| AE of CVD-IP | 3 (15%) | 13 (27%) | 16 (24%) | |
| Other IPs | 1 (5%) | 6 (13%) | 7 (10%) | |
| Blood biomarkers | ||||
| PaO2/FiO2 ratio | 240 [129–290] | 283 [205–312] | 266 [187–308] | 0.184 |
| Serum LDH, U/L | 379 [259–504] | 277 [231–373] | 281 [240–423] | 0.016 |
| Serum SP-D, ng/mL | 335 [137–663] | 262 [166–408] | 276 [155–429] | 0.481 |
| Serum KL-6, U/mL | 897 [573–1,718] | 955 [574–1,835] | 936 [573–1,718] | 0.972 |
| HRCT scores | ||||
| Ground glass opacity | 12 [8–16] | 9 [7–12] | 10 [7–13] | 0.073 |
| Honeycomb | 4 [0–9] | 2 [0–7] | 2.5 [0–7] | 0.418 |
| Duration from IP diagnosis to steroid pulse, days | 78 [2.5–708] | 201 [10.8–1,046.3] | 177.5 [7.5–867.5] | 0.447 |
| Treatment | ||||
| Steroid use before hospitalization | 7 (35%) | 6 (13%) | 13 (19%) | 0.053 |
| Steroid pulse | 20 (100%) | 48 (100%) | 68 (100%) | 1.000 |
| Anti-coagulant | 9 (45%) | 5 (10%) | 14 (21%) | 0.001 |
| Neutrophil elastase inhibitor | 4 (20%) | 4 (8%) | 8 (12%) | 0.174 |
Results are shown as medians with 25th–75th percentiles or n (%). Serum SP-D could be measured in 64 patients. Serum KL-6 could be measured in 67 patients. Biopsy proven cases are 27 including 7 of IPF, 12 of non-IPF IIPs, 7 of CVD-IP, and 1 of other IP. AE, acute exacerbation; CCI, Charlson comorbidity index; CVD-IP, collagen vascular diseases-related interstitial pneumonia; HRCT, high-resolution computed tomography; IIPs, idiopathic interstitial pneumonias; IP, interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; KL-6, Krebs von den Lungen; LDH, lactate dehydrogenase; PaO2/FiO2 ratio; partial pressure of oxygen in arterial blood/fraction of the inspiratory oxygen; SP-D, surfactant protein-D.
Table 2. Clinical difference between patients with IPF, other IIPs, and CVD-IP groups.
| Characteristics | IPF group (n=17) | Other IIPs group (n=28) | CVD-IP group (n=16) | P |
|---|---|---|---|---|
| Age, y | 75 [72–79.5] | 77.5 [73–82] | 76 [71.5–79.8] | 0.719 |
| Male sex | 14 (82%) | 23 (82%) | 8 (50%) | 0.042 |
| CCI | 2 [1–3] | 2 [1–4] | 2 [1.3–3] | 0.944 |
| Blood biomarkers | ||||
| PaO2/FiO2 ratio | 266 [204–290] | 259 [154–313] | 296 [148–343] | 0.744 |
| Serum LDH, U/L | 294 [259–425] | 302 [219–488] | 254 [231–337] | 0.322 |
| Serum SP-D, ng/mL | 266 [166–495] | 328 [146–430] | 220 [140–456] | 0.649 |
| Serum KL-6, U/mL | 995 [690–1,846] | 936 [572–2,480] | 884 [642–1,240] | 0.814 |
| HRCT scores | ||||
| Ground glass opacity | 10 [6.5–12] | 9 [6.3–12.8] | 10.5 [8–13.8] | 0.625 |
| Honeycomb | 7 [4–9] | 0 [0–3.8] | 3 [1.3–8.5] | <0.001 |
| 3-month morality rates, % | 35.3 | 35.7 | 18.8 | 0.460 |
Results are shown as medians with 25th–75th percentiles or n (%). CCI, Charlson comorbidity index; CVD-IP, collagen vascular diseases-related interstitial pneumonia; HRCT, high-resolution computed tomography; IIPs, idiopathic interstitial pneumonias; IPF, idiopathic pulmonary fibrosis; KL-6, Krebs von den Lungen; LDH, lactate dehydrogenase; PaO2/FiO2 ratio; partial pressure of oxygen in arterial blood/fraction of the inspiratory oxygen; SP-D, surfactant protein-D.
Stepwise multivariate analysis
The variables of age, sex, IPF (vs. non-IPF), serum LDH, serum KL-6, serum SP-D, PaO2/FiO2 ratio, CCI, honeycomb and ground glass opacity (GGO) scores were assessed using stepwise multiple logistic regression. CCI (OR, 1.306; 95% CI, 1.090–1.573; P=0.004), serum LDH (OR, 1.003; 95% CI, 1.001–1.005; P=0.002) and sex (OR, 8.555; 95% CI, 1.729–154.978; P=0.038) were significant predictors of 3-month mortality (Table 3).
Table 3. Multiple stepwise regression analysis of primary predictor of 3-month mortality.
| Variable | Odds ratio | 95% confidence interval | P |
|---|---|---|---|
| CCI | 1.306 | 1.090–1.573 | 0.004 |
| Sex, male vs. female | 8.555 | 1.729–154.978 | 0.038 |
| Serum LDH | 1.003 | 1.001–1.005 | 0.002 |
| Honeycomb score | 1.063 | 0.937–1.120 | 0.334 |
CCI, Charlson comorbidity index; LDH, lactate dehydrogenase.
Survival curves for each clinical parameter including CCI, serum LDH, and sex
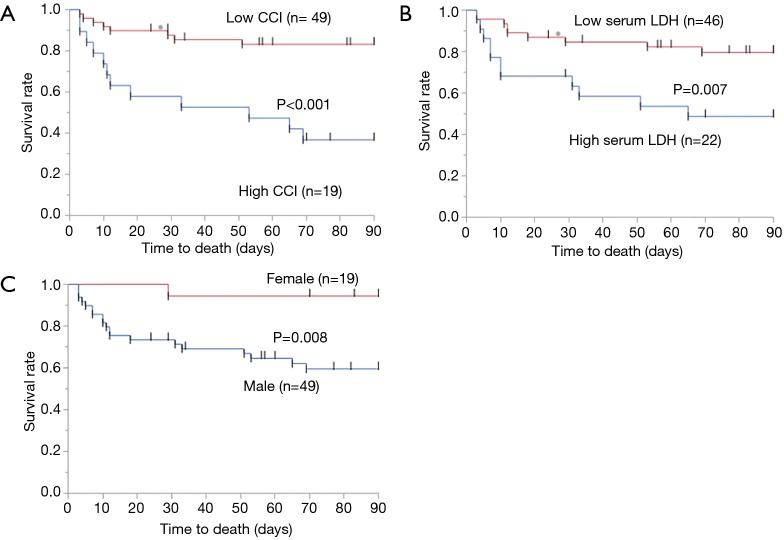
The AUC value was 0.722 in the evaluation of CCI as a predictor of 3-month mortality (Table 4). The optimal cut-off CCI for estimating 3-month mortality was 4, with 60% sensitivity and 85% specificity. We assigned the 68 patients to groups with either low CCI (n=49) or high group (n=19) CCI based on this cut-off. Log-rank tests showed that Kaplan–Meier survival curves of these groups significantly differed (P<0.001; Figure 1A). The 3-month mortality rates were 16.3% and 63.2% for the groups with low and high CCI, respectively.
Table 4. Analysis of ROC curves to predict 3-month mortality.
| Variable | AUC | Best cut-off | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|
| CCI | 0.722 | 4 | 60 | 85 | 0.001 |
| PaO2/FiO2 ratio | 0.606 | 290 | 84 | 44 | 0.223 |
| Serum LDH (ng/mL) | 0.688 | 377 | 55 | 77 | <0.001 |
| Serum SP-D (ng/mL) | 0.557 | 290 | 63 | 60 | 0.061 |
| Serum KL-6 (U/mL) | 0.497 | 766 | 68 | 42 | 0.702 |
| Ground glass opacity score | 0.639 | 11 | 70 | 56 | 0.074 |
| Honeycomb score | 0.562 | 4 | 55 | 65 | 0.325 |
CCI, Charlson comorbidity index; KL-6, Krebs von den Lungen; LDH, lactate dehydrogenase; PaO2/FiO2 ratio; partial pressure of oxygen in arterial blood/fraction of the inspiratory oxygen; SP-D, surfactant protein-D.
Figure 1.
Comparison of patients of 3-month mortality according to Charlson comorbidity index, serum LDH, and sex. Three-month mortality rates are significantly better in group with low (n=49) than high (n=19) CCI (P<0.001) (A). Also, 3-month mortality rates are significantly better in group with serum LDH, female than high serum LDH, male (B, serum LDH; C, sex). CCI, Charlson comorbidity index; LDH, lactate dehydrogenase.
Similar to CCI, the optimal cut-off was calculated for serum LDH using ROC curve analysis (Table 4). The cut-off value of serum LDH for estimating 3-month mortality was 377 ng/mL, with 55% sensitivity and 77% specificity. Log-rank tests showed that Kaplan-Meier survival curves of these groups significantly differed (P=0.007; Figure 1B). Also, Kaplan-Meier survival curves of male or female groups significantly differed (P=0.008; Figure 1C).
The clinical relevance of CCI according to types of IP
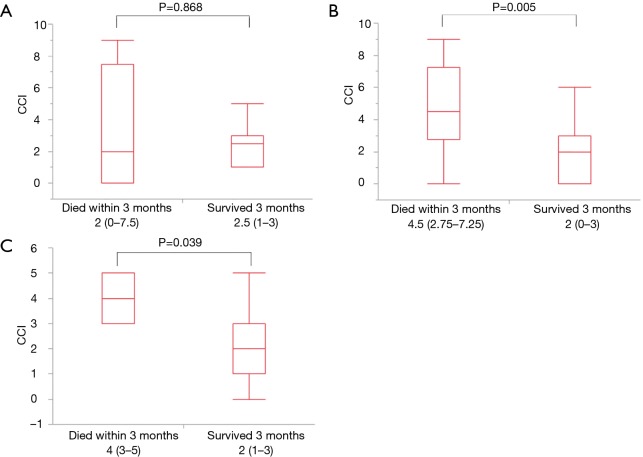
In patients with AE of IPF, CCI was not significantly different in the survival and death groups (Figure 2A, P=0.868), however, in patients with other IIPs (Figure 2B, P=0.005) and AE of CVD-IP (Figure 2C, P=0.039), CCI was significantly higher in the death group compared to the survival group.
Figure 2.
The clinical relevance of Charlson comorbidity index according to types of IP. In patients with AE of IPF, CCI was not significantly different in the survival and death groups (A, P=0.868), however, in patients with other IIPs (B, P=0.005) and AE of CVD-IP (C, P=0.039), CCI was significantly higher in the death group compared to the survival group. AE, acute exacerbation; CCI, Charlson comorbidity index; CVD-IP, collagen vascular disease-related interstitial pneumonia; IP, interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; IIPs, idiopathic interstitial pneumonias.
Comparison between high and low CCI
Table 5 shows a comparison of comorbidities in the groups with respectively high CCI (≥4) and low CCI (<4). The incidences of symptomatic chronic pulmonary disease (84% vs. 37%), diabetes without complications (37% vs. 14%), hemiplegia (11% vs. 0%), myocardial infarction (32% vs. 10%), congestive heart failure (32% vs. 6%), moderate or severe renal disease (16% vs. 2%), and second metastatic solid tumor (32% vs. 0%) were significantly higher in the group with a high CCI (P<0.05). The 3-month mortality rates in high CCI and low CCI groups were significantly different at 63.2% and 16.3%, respectively (P<0.001).
Table 5. Comparison of patients with high and low CCI.
| Comorbidity | High CCI (N=19), n [%] | Low CCI (N=49), n [%] | P |
|---|---|---|---|
| Myocardial infarction | 6 [32] | 5 [10] | 0.032 |
| Congestive heart failure | 6 [32] | 3 [6] | 0.005 |
| Peripheral vascular disease | 0 [0] | 2 [4] | 0.371 |
| Dementia | 1 [5] | 4 [8) | 0.169 |
| Chronic pulmonary disease (symptomatic) | 16 [84] | 18 [37] | <0.001 |
| Ulcer | 1 [5] | 2 [4] | 0.831 |
| Mild liver disease | 2 [11] | 1 [2] | 0.126 |
| Diabetes (without complications) | 7 [37] | 7 [14] | 0.039 |
| Cerebrovascular disease | 3 [16] | 3 [6] | 0.207 |
| Collagen disease | 2 [11] | 10 [20] | 0.338 |
| Diabetes with end-organ damage | 1 [5] | 0 [0] | 0.106 |
| Hemiplegia | 2 [11] | 0 [0] | 0.021 |
| Moderate or severe renal disease | 3 [16] | 1 [2] | 0.031 |
| Second solid tumor (non-metastatic) | 8 [42] | 10 [20] | 0.069 |
| Leukemia | 0 [0] | 0 [0] | – |
| Lymphoma, multiple myeloma | 3 [16] | 2 [4] | 0.097 |
| Moderate or severe liver disease | 1 [5] | 0 [0] | 0.106 |
| Second metastatic solid tumor | 6 [32] | 0 [0] | <0.001 |
| Acquired immunodeficiency syndrome | 0 [0] | 0 [0] | – |
| 3-month mortality rates, % | 63.2 | 16.3 | <0.001 |
CCI, Charlson comorbidity index; LDH, lactate dehydrogenase.
Composite scoring system for predicting 3-month mortality
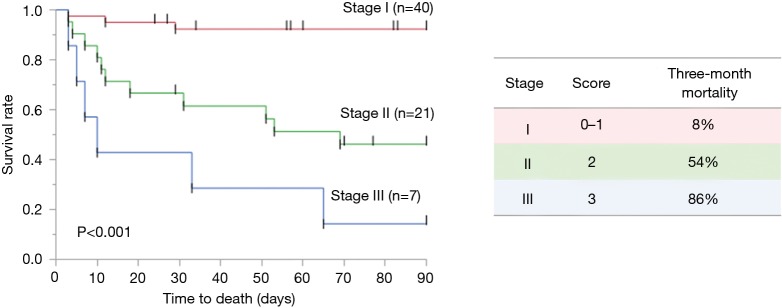
The composite score means the global score obtained by the score calculated from each parameter, ranging between 0 and 3: CCI (<4, 0; ≥4, 1), sex (female, 0; male, 1), serum LDH (<377, 0; ≥377, 1). Patients were categorized into three groups based on the composite score: stages I [0–1], II [2], and III [3]. In addition, we compared 3-month mortality rate among the three stages. The 3-month mortality rates of patients with the stages I, II, and III were 8%, 54% and 86%, respectively (P<0.001) (Figure 3). Moreover, the composite scoring system including CCI, serum LDH, and sex showed a higher bootstrap AUC and C-index than did scores of a single predictor or a combination of two predictors (Table 6).
Figure 3.
Comparison of patients of 3-month mortality according to the composite scoring system. The composite score is the global score obtained by the score calculated from each parameter, ranging between 0 and 3: CCI (<4, 0; ≥4, 1), sex (female, 0; male, 1), serum LDH (<377, 0; ≥377, 1). Patients were categorized into three groups based on the composite score: stages I [0–1], II [2], and III [3]. In addition, we compared 3-month mortality rate among the three stages. Three-month mortality rates of patients with the stages I, II, and III were 8%, 54% and 86%, respectively (P<0.001). CCI, Charlson comorbidity index; LDH, lactate dehydrogenase.
Table 6. The accuracy of the scoring system in predicting 3-month mortality.
| Variable | Bootstrap AUC | Bootstrap C-index |
|---|---|---|
| CCI | 0.752 | 0.542 |
| Serum LDH | 0.776 | 0.602 |
| Sex | 0.757 | 0.555 |
| CCI + serum LDH | 0.808 | 0.665 |
| CCI + sex | 0.805 | 0.651 |
| Serum LDH + sex | 0.813 | 0.668 |
| CCI + serum LDH + sex | 0.859 | 0.747 |
AUC, area under the receiver operating characteristic curve; CCI, Charlson comorbidity index; LDH, lactate dehydrogenase.
Discussion
The purpose of evaluating clinical biomarkers for patients with IP is to predict the risk of developing AE and the prognosis of patients with AE and to diagnose AE. For example, elevated serum KL-6 or serum D-dimer have been associated with the risk of developing AE (19,20). Serum HSP-47, serum procalcitonin, or arterial carboxyhemoglobin are helpful to diagnose AE (8,9,21). Although serum LDH, serum KL-6, PaO2/FiO2 ratio and the extent of abnormal HRCT findings are important for 3-month mortality predictors of AE-IPF (7), there are no multicenter study that examining biomarkers that can predict prognosis at the time of diagnosis of AE in the clinical setting. Therefore, we evaluated which of the clinical parameters of sex, age, diagnosis, blood biomarkers (LDH, SP-D, KL-6, and PaO2/FiO2 ratio), HRCT scores, and CCI might predict prognosis at the time of being diagnosed with an AE in a multicenter study.
Sex could be an important prognostic factor in patients with AE-IP (22-24). A retrospective study of factors related to the 1-month mortality of patients with AE-IP after lung cancer resection identified male sex as an independent prognostic factor (22,23). Another study investigated the ability of a scoring system to predict the long-term prognosis of patients with chronic interstitial lung disease (ILD) (ILD-GAP) (24). That system comprised ILD subtypes, sex, age and respiratory function including forced vital capacity and the diffusion capacity of the lungs for carbon monoxide. Here, we found that male sex is an important prognostic factor that also influenced 3-month mortality in patients with AE-IP.
Serum LDH could likewise be an important prognostic factor for patients with AE-IP, because elevated serum LDH reflects the extent of active lung inflammation and pulmonary cell damage in patients with ILD, and it can be a risk factor for the incidence of AE in patients with IPF (9,25,26). The present study found that serum LDH is an important prognostic factor influencing 3-month mortality in patients with AE-IP. Based on these findings, we consider that sex and serum LDH values are important clinical indicators in terms of the treatment and prognosis of patients with AE-IP.
We speculated that comorbidities would affect the prognosis of patients who are treated for AE-IP, but little is known about the relationship between prognosis and comorbidities in such patients. The CCI might serve as an important factor in predicting long-term prognosis in patients with ILD (25,27-29). A retrospective analysis of 224 patients with IP identified CCI as a prognostic factor for patients with chronic IP without honeycomb lung, although the prognosis of IPF was not affected by comorbidities (27). Others have associated CCI with post-discharge mortality among patients with acute respiratory worsening of fibrotic ILD (28). Cardiovascular disease and congestive heart failure are also useful for predicting AE onset (25,29). We found a significantly higher incidence of myocardial infarction and congestive heart failure among patients with a high CCI. We also found a significant difference in the frequency of secondary metastatic solid tumors between patients with high and low CCI. One study of 103 patients with IPF identified lung cancer as a complication that did not affect the prognosis after being diagnosed with IPF (30). In contrast, another study that investigated the influence of respiratory comorbidities on the mortality rates of hospitalized patients with IPF using the Japanese diagnostic procedure combined with a per-diem payment system database significantly related lung cancer complications with a poor prognosis among patients hospitalized with IPF (31). Although further studies are necessary to clarify these controversial results, having a history of complications including cardiac disease and malignant tumors, and evaluations of complications are probably extremely important when considering the treatment of patients with AE-IP.
Composite approaches have been developed using peripheral blood biomarkers, physiological, and radiographic measurements to provide more accurate prognostic information (32). Kishaba et al. reported the composite scoring system which was based on serum LDH (cut off value, 280 IU/L), KL-6 (cut off value, 1,000 IU/L), ratio of partial pressure of oxygen and fraction of inspiratory oxygen (cut off value, 100), and extent of abnormal HRCT findings, was a clinical prognostic factor associated with 3-month mortality in patients with AE-IPF (7). In the present research, we found that CCI is important in addition to sex and serum LDH for predicting 3-month mortality among patients and the composite scoring including these parameters could be useful for predicting the prognosis. Because CCI, serum LDH, and sex are all simple and objective parameters unlike HRCT findings, the composite scoring system including these parameters may be suitable to the clinical setting.
In the meantime, this retrospective study of a small patient cohort from two institutions has some limitations. First, our study is limited by the small number of patients and the absence of additional validation data sets. In order to verify the generalizability of our findings, large-scale, multi-institutional prospective collaborative research is essential. Second, the clinical diagnoses of the enrolled patients were heterogeneous. Therefore, the clinical relevance of CCI should be evaluated by histopathological diagnoses (for example, IPF alone), although IP subtypes were not significant predictors of 3-month mortality in this research.
Conclusions
We found that CCI, serum LDH, and sex were significant predictors of 3-month mortality in patients with acute or subacute IIPs and AE of CVD-IP requiring steroid pulse therapy. Moreover, the composite scoring system including CCI, serum LDH, and sex could be a useful mortality prediction tool for these patients.
Acknowledgments
None.
Ethical Statement: The Institutional Review Board at Yokohama City University Hospital approved this study (approval number B171100003). In all patients, consent for participation of this retrospective study was obtained by disclosing a clinical study including the description of opt-out (https://www.yokohama-cu.ac.jp/amedrc/ethics/ethical/fuzoku_optout.html).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi H, Kondoh Y. Acute and subacute idiopathic interstitial pneumonias. Respirology 2016;21:810-20. 10.1111/resp.12786 [DOI] [PubMed] [Google Scholar]
- 3.Collard HR, Moore BB, Flaherty KR, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. 10.1164/rccm.200703-463PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132:214-20. 10.1378/chest.07-0323 [DOI] [PubMed] [Google Scholar]
- 5.Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012;83:20-7. 10.1159/000329893 [DOI] [PubMed] [Google Scholar]
- 6.Arai T, Kagawa T, Sasaki Y, et al. Heterogeneity of incidence and outcome of acute exacerbation in idiopathic interstitial pneumonia. Respirology 2016;21:1431-7. 10.1111/resp.12862 [DOI] [PubMed] [Google Scholar]
- 7.Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014;192:141-9. 10.1007/s00408-013-9530-0 [DOI] [PubMed] [Google Scholar]
- 8.Kakugawa T, Yokota S, Ishimatsu Y, et al. Serum heat shock protein 47 levels are elevated in acute exacerbation of idiopathic pulmonary fibrosis. Cell Stress Chaperones. 2013;18:581-90. 10.1007/s12192-013-0411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara Y, Shinkai M, Kanoh S, et al. Arterial Carboxyhemoglobin Measurement Is Useful for Evaluating Pulmonary Inflammation in Subjects with Interstitial Lung Disease. Intern Med 2017;56:621-6. 10.2169/internalmedicine.56.7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11.Tominaga T, Nonaka T, Takeshita H, et al. The Charlson Comorbidity Index as an Independent Prognostic Factor in Older Colorectal Cancer Patients. Indian J Surg 2018;80:54-60. 10.1007/s12262-016-1544-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Leung LH, Wang J, et al. Association between Charlson comorbidity index score and outcome in patients with stage IIIB-IV non-small cell lung cancer. BMC Pulm Med 2017;17:112. 10.1186/s12890-017-0452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Núñez JE, Núñez E, Fácila L, et al. Prognostic value of Charlson comorbidity index at 30 days and 1 year after acute myocardial infarction. Rev Esp Cardiol 2004;57:842-9. [PubMed] [Google Scholar]
- 14.Ooi GC, Mok MY, Tsang KW, et al. Interstitial lung disease in systemic sclerosis. Acta Radiol 2003;44:258-64. [DOI] [PubMed] [Google Scholar]
- 15.Raghu G, Rochwerg B, Zhang Y, et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3-19. 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 16.Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig 2013;51:260-77. 10.1016/j.resinv.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 17.Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med 2003;168:952-8. 10.1164/rccm.200301-137OC [DOI] [PubMed] [Google Scholar]
- 18.Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 19.Ohshimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 2014;108:1031-9. 10.1016/j.rmed.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa G, Acquah SO, Salvatore M, et al. Elevated serum D-dimer level is associated with an increased risk of acute exacerbation in interstitial lung disease. Respir Med 2017;128:78-84. 10.1016/j.rmed.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Nagata K, Tomii K, Otsuka K, et al. Serum procalcitonin is a valuable diagnostic marker in acute exacerbation of interstitial pneumonia. Respirology 2013;18:439-46. 10.1111/resp.12018 [DOI] [PubMed] [Google Scholar]
- 22.Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-1611.e3. 10.1016/j.jtcvs.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. 10.1007/s11748-014-0487-6 [DOI] [PubMed] [Google Scholar]
- 24.Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723-8. 10.1378/chest.13-1474 [DOI] [PubMed] [Google Scholar]
- 25.Kakugawa T, Sakamoto N, Sato S, et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res 2016;17:79. 10.1186/s12931-016-0400-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeRemee RA. Serum lactic dehydrogenase activity and diffuse interstitial pneumonitis. JAMA 1968;204:1193-5. 10.1001/jama.1968.03140260033015 [DOI] [PubMed] [Google Scholar]
- 27.Ando K, Ohkuni Y, Makino H, et al. Relationship between the prognosis of interstitial pneumonia and its comorbidities. Nihon Kokyuki Gakkai Zasshi 2011;49:800-9. [PubMed] [Google Scholar]
- 28.Moua T, Westerly BD, Dulohery MM, et al. Patients with fibrotic interstitial lung disease hospitalized for acute respiratory worsening: A Large Cohort Analysis. Chest 2016;149:1205-14. 10.1016/j.chest.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 29.Collard HR, Yow E, Richeldi L, et al. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res 2013;14:73. 10.1186/1465-9921-14-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14:723-8. 10.1111/j.1440-1843.2009.01547.x [DOI] [PubMed] [Google Scholar]
- 31.Oda K, Yatera K, Fujino Y, et al. Respiratory comorbidities and risk of mortality in hospitalized patients with idiopathic pulmonary fibrosis. Respir Investig 2018;56:64-71. 10.1016/j.resinv.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 32.Hara Y, Shinkai M, Rubin BK. Biomarkers for staging and evaluating the therapy for idiopathic pulmonary fibrosis. Clin Pulm Med 2015;22:165-71. 10.1097/CPM.0000000000000111 [DOI] [Google Scholar]